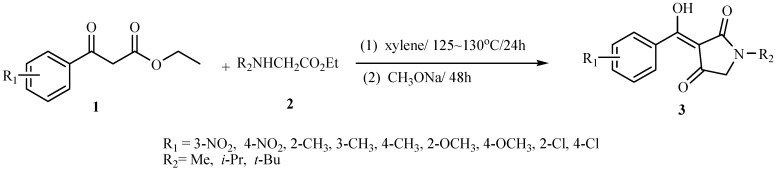
Abstract
In the search for better herbicides a series of 1-alkyl-3-(α-hydroxy-(un)substituted benzylidene)pyrrolidine-2,4-diones were prepared and their structure-activity relationships studied. All their structures have been confirmed by 1H-NMR and elemental analysis. The preliminary bioassay results indicated that some of them have high herbicidal activity against annual dicotyledonous and monocotyledonous plants.
Keywords: Pyrrolidine, herbicides, inhibition
Introduction
The inhibitors of 4-hydroxyphenylpyruvate dioxygenase (HPPD, EC 1.13.11.27) constitute a new kind of herbicides [1]. Generally, potent herbicides of this kind must possess the following structural features: 1) a tricarbonyl methane structure and one of the three carbonyl groups must be a subustituted benzoyl group; 2) the compound must be able to enolise so that the enolate is capable of inhibiting HPPD enzyme by competitive combination with Fe2+– the reaction center of HPPD enzyme [2]. Recently, we observed that the 3-acyltetramic acids form an expanding group of antibiotics and pigments derived from microorganisms [3]. They display a range of biological activities [4] and all compounds of this kind possess a tricarbonyl methane structure and can essentially enolise completely (the chemical shifts of their enolic hydroxyls were larger than 10). Tests of their antimicrobial activities indicated that the structure of the acyl substituent at the 3-position and possession of a 3-acyltetramic acid moiety as the tricarbonylmethane structure is important for many typical antibiotics [4,5]. These characteristics stimulated us to study this kind of compounds in the search for new herbicides. In our previous study [6], we synthesized a series of 1-benzyl-3-(α-hydroxy-(un)substituted benzylidene)-pyrrolidine-2,4-diones and found there existed to some extent structure-activity relationships between them. Furthermore and importantly, these compounds possessed both growth inhibiting and bleaching properties. The combination of both features could lead to the discovery of new potential herbicides. In this paper, we report the design and synthesis of the title compounds 3, based on the HPPD structure and its reactivity with chemical compounds [7,8,9], which were found to have herbicidal activities about 100 times those of the compounds reported before [6]. Their structure-activity relationships are discussed.
Results and Discussion
Synthesis and characterization of target compounds
The synthesis route is shown in Scheme 1.
Scheme 1.
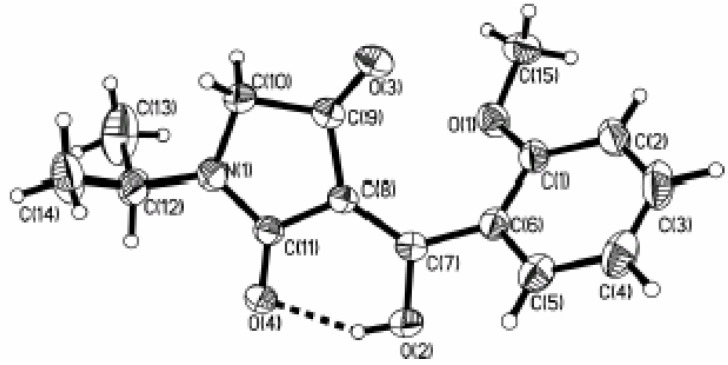
Compounds 3a~3w were identified by IR and 1H-NMR. The measured elemental analyses are also consistent with the corresponding calculated ones. In the IR spectra there are medium or weak absorption bands for the enol group (νO-H) at around 3400cm-1 and the three carbonyl groups show relatively strong absorption bands at around 1700cm-1, 1680 and 1580cm-1. In the 1H‑NMR spectra of these compounds, no signals are found for the proton of CH group connecting the three carbonyl groups; this is evidence that an enol isomer was formed and as a result of proton exchange, the enol proton peak was not observed. The enol form was also confirmed through the single crystal X-ray diffraction of compound 3k (whose molecular structure is shown in Figure 1) [10].
Figure 1.
Molecular structure for the compound (3k) with the atomic numbering scheme.
Biological evaluation
The herbicidal activities of the target compounds were determined with Brassica napus and Echinochloa crusgalli (L.) Beauv as samples of annual dicotyledonous and monocotyledonous plants, respectively, by the method reported by Yang et al. [11]. The results are summarized in Table 1 and they show that in these preliminary tests, most of the target compounds (such as 3l, 3m, 3n, 3o, 3s, 3t and 3u) have inhibiting, and to some extent, bleaching activity and exhibit better efficacy against Brassica napus and Echinochloa crusgalli (L.) Beauv on the whole, with inhibition rates against these species ranging between 74% to 83% at 100 ug/L.
Table 1.
The biological activity(%) of compounds 3a ~ 3w against Brassica napus and Echinochloa crusgalli (L.) Beauv
| Cpd No |
R1 ; R2 | Brassica napus(ug/mL, inhibition %) | Echinochloa crusgalli (L.) Beauv(ug/mL, inhibition %) | |||
|---|---|---|---|---|---|---|
| 10 | 100 | 10 | 100 | Bleaching result at 100 ug/mL * | ||
| 3a | 3-NO2 ; Me | 0 | 22.4 | 5.2 | 42.8 | + |
| 3b | 4-NO2 ; Me | 5.1 | 40.9 | 9.0 | 51.9 | + |
| 3c | 4-Cl ; Me | 15.8 | 90.5 | 9.4 | 50.1 | + |
| 3d | 2-CH3 ; Me | 0 | 44.4 | 31.2 | 78.3 | ++ |
| 3e | 3-CH3 ; Me | 14.0 | 64.0 | 25.3 | 68.3 | ++ |
| 3f | 4-OCH3 ; Me | 7.9 | 50.7 | 16.2 | 78.8 | ++ |
| 3g | 3-NO2 ; i-Pr | 10.2 | 70.3 | 0 | 42 | + |
| 3h | 4-NO2 ; i-Pr | 4.5 | 34.2 | 0 | 1.6 | - |
| 3i | 2-Cl ; i-Pr | 38.9 | 66.8 | 51 | 77.9 | ++ |
| 3j | 4-Cl ; i-Pr | 16 | 77.2 | 25.1 | 68 | ++ |
| 3k | 2-OCH3 ; i-Pr | 3.0 | 58.7 | 77.3 | 78.1 | +++ |
| 3l | 4-OCH3 ; i-Pr | 11.9 | 71 | 79.6 | 80 | +++ |
| 3m | 2-CH3 ; i-Pr | 15.2 | 62.4 | 76.9 | 77.3 | +++ |
| 3n | 3-CH3 ; i-Pr | 3.3 | 82.1 | 49.2 | 75.5 | +++ |
| 3o | 4-CH3 ; i-Pr | 8.1 | 86.4 | 76.8 | 78.4 | +++ |
| 3p | 3-NO2; t-Bu | 22.1 | 80.1 | 0 | 0.5 | - |
| 3q | 4-NO2; t-Bu | 1.8 | 48.1 | 0 | 9.8 | - |
| 3r | 4-Cl; t-Bu | 13.8 | 98 | 0.4 | 20.8 | - |
| 3s | 2-OCH3; t-Bu | 41.2 | 69.5 | 74.9 | 82.9 | ++ |
| 3t | 4-OCH3; t-Bu | 5.4 | 77.7 | 78.7 | 80.3 | ++ |
| 3u | 2-CH3; t-Bu | 21.1 | 74.9 | 63.6 | 74.4 | ++ |
| 3v | 3-CH3; t-Bu | 24.9 | 85.0 | 4.9 | 48.5 | + |
| 3w | 4-CH3; t-Bu | 13.5 | 88.1 | 36.3 | 48 | + |
| Sulcotrione** | 3.5 | 38.1 | 29.4 | 45.1 | +++ | |
* - no change ; + weak ; + + moderate ; + + + high; ** 2-(2-chloro-4-mesylbenzoyl)cyclohexane-1,3-dione
At the lower test concentration (10 ug/L), the compounds mentioned above showed higher biological activity against Echinochloa crusgalli (L.) Beauv and lower biological activity against Brassica napus. The highest inhibition rate against Echinochloa crusgalli (L.) Beauv is 79.6% at 10 ug/L (the rates are 100 times of that of these compounds previous reported [6]). This indicated that these compounds had better selectivity. The data in Table 1 also show that most of the target compounds have much higher inhibiting activity and slightly weaker bleaching activity than the standard sulcotrione under the test conditions.
In Table 1, as far as the relationships between the structure and the activity is concerned, it also shows that when R1 is the same and R2 is changed, the inhibition ranking is Me < t-Bu ≤ i-Pr, indicating that alkyl groups both larger and smaller in size than an iso-propyl group do not favor the target compounds’ combination with Fe2+– the reaction center of HPPD; when R2 is same, and R1 is changed, the result that the inhibition ranking is electron-withdrawing group < electron-donating group shows that electron-donating groups are propitious to the enol-isomer form, and furthermore enhance the target compounds’ chance of combining with the center of HPPD. The above phenomena are more obvious and shows that these target compounds not only have bleaching activity, but they also have inhibiting activity.
Conclusions
Twenty-three novel title compounds containing a tricarbonylmethane unit have been synthesized. Their structures have been verified by 1H-NMR and IR spectral data, crystal x-ray diffraction and elemental analysis. Under the test conditions most of them show better inhibition activity and some of them show a similar degree of bleaching activity as a sulcotrione standard, and compared to the commercial agent the herbicidal activities of this type of compounds are encouraging. It is possible that the combination of the two action modes lead to their better herbicidal activity. Further work is in progress.
Experimental
Materials and instruments
Melting points were determined using a Yanaco MP-241 apparatus and are uncorrected. Infrared spectra were recorded on a Shimadzu IR-435 spectrophotometer as thin films or potassium bromide tablets. 1H-NMR spectra were measured on a Bruker AC-P500 instrument (300MHz) using tetra-methylsilane as an internal standard and deuterochloroform as solvent. Elemental analyses were performed on a Yanaco MT-3CHN elemental analyzer. All agents were on analytical grade and were used without further purification. The crystal structure determination of compound 3k and its crystal structure were described in reference [10]. The intermediates 1 were prepared according to the literature method [6]. All of the related data were listed in that reference. The intermediates 2 were prepared according to the literature [12] (R2= i-Pr, 40%, 51~53°C/4.5mmHg, nd25=1.4170; R2= t-Bu, 65.4%, 59~61/°C/2 mmHg, nd25=1.4225).
General procedure for the synthesis of compounds 3a~3w: Preparation of 1-methyl-3-(α-hydroxy-3’-nitrobenzylidene)pyrrolidine-2,4-dione (3a)
A mixture of 1 (R1=3-NO2, 4.11 mmol) and ethyl benzylamino acetate (2, R2= Me) (4.30 mmol) in dry xylene (15 mL) was heated at 125-130 ºC with stirring for 20 hours. The cooled solution was added to methanolic CH3ONa, prepared from Na metal (0.10 g, 4.35 mmol) and methanol (10mL) at room temperature with stirring. After the above mixture was stirred at room temperature for 48 hours, water (30 mL) was added to the reaction mixture and the organic layer was separated and extracted twice with water. The original water layer and the extracts were combined and acidified to pH 2-3 with 2N HCl under cooling. The acidic solution was extracted three times with chloroform (30 mL) and the extracts were washed with saturated brine and then dried over Na2SO4. The solvent was removed under reduced pressure to give crude product 3a, which was purified by flash column chromatography on silica gel, using 1:1 (v:v) ethyl acetate-petroleum ether as the eluent to afford the target product as white crystals (1.0g, 92.8%) with m.p. 145~146 ºC; IR (KBr) cm-1 3443(O-H), 1678, 1575, 1520(C=O); 1H-NMR δ: 3.06 (s, 3H, N-CH3), 3.78 (s, 2H, C-CH2-N), 8.22-8.33 (m, 4H, C6H4); Anal. Calc. for C12H10N2O5 (262.21) C 54.96, H 3.84, N 10.68; Found: C 55.01, H 3.89, N 10.51. The twenty two other title compounds 3b~3x were synthesized in the same manner.
1-methyl-3-(α-hydroxy-4’-nitrobenzylidene)pyrrolidine-2,4-dione (3b): Yield 67.0%; m.p. 167~169ºC; IR (KBr) cm-1 3450(O-H), 1690, 1585, 1520 (C=O); 1H-NMR δ: 3.06 (s, 3H, N-CH3), 3.78 (s, 2H, C-CH2-N), 8.22-8.33(m, 4H, C6H4); Anal. Calc. for C12H10N2O5 (262.21) C 54.96, H 3.84, N 10.68; Found: C 54.83, H 3.99, N 10.72.
1-methyl-3-(α-hydroxy-4’-chlorobenzylidene)pyrrolidine-2,4-dione (3c). Yield 57.3%; m.p. 104~105 ºC; IR (KBr) cm-1 3463(O-H), 1712, 1670,1598(C=O); 1H-NMR δ: 3.10 (s, 3H, NCH3), 3.81 (s, 2H, CCH2N), 7.46, 7.49, 8.21, 8.24 (d-d, 4H, J=9.04 Hz, C6H4); Anal. Calc. for C12H10ClNO3 (251.66) C 57.27, H 4.00, N 5.57; Found C 57.21, H 4.07, N 5.57.
1-methyl-3-(α-hydroxy-2’-methylbenzylidene)pyrrolidine-2,4-dione (3d). Yield 52.2%; yellow liquid; IR (KBr) cm-1 3422 (O-H), 1719, 1657, 1620 (C=O); 1H-NMR δ: 2.32 (s, 3H, ArCH3), 3.02 (s, 3H, N‑CH3), 3.67 (s, 2H, CCH2N), 7.13-7.42 (m, 4H, C6H4); Anal. Calc. for C13H13NO3 (231.24) C 67.52, H 5.67, N 6.06; Found C 67.42, H 5.51, N 6.01.
1-methyl-3-(α-hydroxy-3’-methylbenzylidene)pyrrolidine-2,4-dione (3e). Yield 86.5%; red liquid; IR (KBr) cm-1 3435 (O-H), 1720, 1654, 1621 (C=O); 1H-NMR δ: 2.36 (s, 3H, ArCH3), 3.02 (s, 3H, N‑CH3), 3.72 (s, 2H, CCH2N), 7.23-7.39, 7.83-8.03 (m, 4H, C6H4); Anal. Calc. for C13H13NO3 (231.24) C 67.52, H 5.67, N 6.06; Found C 67.60, H 5.59, N 6.05.
1-methyl-3-(α-hydroxy-4’-methoxybenzylidene)pyrrolidine-2,4-dione (3f). Yield 62.9%; m.p. 96~97 ºC; IR (KBr) cm-1 3419 (O-H), 1717, 1652, 1616 (C=O); 1H-NMR δ: 3.00 (s, 3H, NCH3), 3.71 (s, 2H, CCH2N), 3.81 (s, 3H, OCH3), 6.88, 6.99, 8.28, 8.31 (d-d, 4H, J=9.04 Hz, C6H4); Anal. Calc. for C13H13NO4 (247.24) C 63.15, H 5.30, N 5.67; Found C 63.14, H 5.43, N 5.78.
1-i-propyl-3-(α-hydroxy-3’-nitrobenzylidene)pyrrolidine-2,4-dione (3g). Yield 74.8%; m.p. 136~137 ºC; IR(KBr) cm-1 3453 (O-H), 1688, 1595, 1532 (C=O); 1H-NMR δ: 1.21(d, 6H, J=6.78Hz, C(CH3)2), 3.72 (s, 2H, C-CH2-N), 4.38-4.68 (m,1H, J=6.78Hz, CH); 7.51-7.70, 8.28-8.60, 9.02-9.13 (m, 4H, J=8.29 Hz, C6H4); Anal. Calc. for C14H14N2O5 (290.26) C 57.93, H 4.86, N 9.65; Found: C 57.96, H 5.08, N 9.75.
1-i-propyl-3-(α-hydroxy-4’-nitrobenzylidene)pyrrolidine-2,4-dione (3h). Yield 69.2%; m.p. 146~147 ºC; IR (KBr) cm-1 3463 (O-H), 1693, 1610, 1553 (C=O); 1H-NMR δ: 1.22 (d, 6H, J=6.78Hz, C(CH3)2), 3.71 (s, 2H, C-CH2-N), 4.47-4.57 (m,1H, J= 6.78Hz,CH), 7.91-7.94 , 8.23-8.31 (m, 4H, J=9.04 Hz, C6H4); Anal. Calc. for C14H14N2O5 (290.26) C 57.93, H 4.86, N 9.65; Found C 57.94, H 4.73, N 9.65.
1-i-propyl-3-(α-hydroxy-2-chlorobenzylidene)pyrrolidine-2,4-dione (3i). Yield 37.2%; m.p. 103~104 ºC. IR (KBr) cm-1 3418(O-H), 1726,1656 1561(C=O). 1H-NMR δ: 1.27-1.29 (d, 6H, J=6.78Hz, C(CH3)2), 3.71 (s, 2H, CCH2N), 4.50-4.74 (m, 1H, J=6.78Hz, CH), 7.31-7.62 (m, 4H, J=9.04 Hz, C6H4). Anal. Calc. for C14H14ClNO3 (279.71) C 60.11, H 5.04, N 5.01; Found C 60.22, H 5.09, N 5.11.
1-i-propyl-3-(α-hydroxy--4’-chlorobenzylidene)pyrrolidine-2,4-dione (3j). Yield 73.1%; m.p. 134~135 ºC; IR (KBr) cm-1 3405 (O-H), 1694 1587, 1544 (C=O); 1H-NMR δ: 1.27 (d, 6H, J=6.78Hz, C(CH3)2), 3.76 (s, 2H, CCH2N), 4.54-4.66 (m, 1H, J=6.78Hz, CH), 7.46-7.48, 8.21-8.28 (m, 4H, C6H4); Anal. Calc. for C14H14ClNO3 (279.71) C 60.11, H 5.04, N 5.01; Found C 60.22, H 5.09, N 5.11.
1-i-propyl-3-(α-hydroxy-2’-methoxybenzylidene)pyrrolidine-2,4-dione (3k). Yield 79.9%; m.p. 99~100 ºC; IR (KBr) cm-1 3441 (O-H), 1741, 1600, 1559 (C=O); 1H-NMR δ: 1.25 (d, 6H, J=6.78Hz, C(CH3)2), 3.66 (s, 2H, CCH2N), 3.86 (s, 3H, Ar-OCH3), 4.57-4.62 (m, 1H, J=6.78Hz, CH), 7.00-7.06, 7.45-7.53 (m, 4H, C6H4); Anal. Calc. for C15H17NO4 (275.29) C 65.44, H 6.22, N 5.09; Found C 65.45, H 6.27, N 4.98.
1-i-propyl-3-(α-hydroxy-4’-methoxybenzylidene)pyrrolidine-2,4-dione (3l). Yield 85.4%; m.p. 145~146 ºC ; IR (KBr) cm-1 3463 (O-H), 1695, 1590, 1546 (C=O); 1H-NMR δ: 1.17 (d, 6H, J=6.78Hz, C(CH3)2), 3.65 (s, 2H, C-CH2-N), 3.82 (s, 3H, Ar-OCH3), 4.45-4.55 (m,1H, J=6.78Hz, CH), 6.89, 6.92 , 8.27, 8.30 (d-d, 4H, J=9.04Hz, C6H4); Anal. Calc. for C15H17NO4 (275.29) C 65.44, H 6.22, N 5.09; Found C 65.39, H 6.25, N 5.07.
1-i-propyl-3-(α-hydroxy-2’-methylbenzylidene)pyrrolidine-2,4-dione (3m). Yield 90.8%; m.p. 79~80 ºC ; IR (KBr) cm-1 3427 (O-H), 1719, 1646, 1601 (C=O); 1H-NMR δ: 1.37 (d, 6H, J=6.78Hz, C(CH3)2); 2.42 (s, 3H, Ar-CH3), 3.70 (s, 2H, CCH2N), 4.56-4.66 (m, 1H, J=6.78Hz, CH), 7.28-7.49 (m, 4H, C6H4); Anal. Calc. for C15H17NO3 (259.29) C 69.48, H 6.61, N 5.40; Found C 69.56, H 6.58, N 5.35.
1-i-propyl-3-(α-hydroxy-3’-methylbenzylidene)pyrrolidine-2,4-dione (3n). Yield 75.7%; m.p. 64~65 ºC; IR (KBr) cm-1 3477 (O-H), 1745, 1685, 1591 (C=O); 1H-NMR δ: 1.18 (d, 6H, J=6.78Hz, C(CH3)2), 2.24 (s, 3H, Ar-CH3), 3.68 (s, 2H, CCH2N), 4.48-4.55 (m, 1H, J=6.78Hz, CH), 7.19-7.95 (m, 4H, C6H4); Anal. Calc. for C15H17NO3 (259.29) C 69.48, H 6.61, N 5.40; Found C 69.35, H 6.45, N 5.48.
1-i-propyl-3-(α-hydroxy-4’-methylbenzylidene)pyrrolidine-2,4-dione (3o). Yield 85.3%; m.p. 101~102 ºC; IR (KBr) cm-1 3378 (O-H), 1692, 1652, 1590 (C=O); 1H-NMR δ: 1.37 (d, 6H, J=6.78Hz, C(CH3)2), 2.45 (s, 3H, Ar-CH3), 3.74 (s, 2H, CCH2N), 4.57-4.62 (m, 1H, J=6.78Hz, CH), 7.28-7.49 (m, 4H, C6H4); Anal. Calc. for C15H17NO3 (259.29) C 69.48, H 6.61, N 5.40; Found C 69.69, H 6.65, N 5.56.
1-t-butyl-3-(α-hydroxy-3’-nitrobenzylidene)pyrrolidine-2,4-dione (3p). Yield 35.1%; m.p. 168~169ºC. IR (KBr) cm-1 3463(O-H), 1698, 1651,1557(C=O). 1H-NMR δ: 1.55 (s, 9H, C(CH3)3), 3.91 (s, 2H, C-CH2-N), 7.61-7.85, 8.35-8.67, 9.07-9.20 (m, 4H, J=8.29 Hz, C6H4); Anal. Calc. for C15H16N2O5 (304.29) C 59.20, H 5.30, N 9.31; Found C 59.31, H 5.28, N 9.20.
1-t-butyl-3-(α-hydroxy-4’-nitrobenzylidene)pyrrolidine-2,4-dione (3q). Yield 39.6%; m.p. 142~144 ºC; IR (KBr) cm-1 3463 (O-H), 1698, 1651, 1557 (C=O); 1H-NMR δ: 1.46 (s, 9H, C(CH3)3), 3.81 (s, 2H, C-CH2-N), 8.15-8.38 (m, 4H, C6H4); Anal. Calc. for C15H16N2O5 (304.29) C 59.20, H 5.30, N 9.31; Found C 61.50, H 4.85, N 11.28.
1-t-butyl-3-(α-hydroxy--4’-chlorobenzylidene)pyrrolidine-2,4-dione (3r). Yield 73.1%; m.p. 134~135 ºC; IR (KBr) cm-1 3405(O-H), 1694 1587,1544(C=O). 1H-NMR δ : 1.27 (d, 6H, J=6.78Hz, C(CH3)2), 3.76 (s, 2H, CCH2N), 4.54-4.66 (m, 1H, J=6.78Hz, CH), 7.46-7.48, 8.21-8.28 (m, 4H, C6H4); Anal. Calc. for C14H14ClNO3 (279.71) C 60.11, H 5.04, N 5.01; Found C 60.22, H 5.09, N 5.11.
1-t-butyl-3-(α-hydroxy-2’-methoxybenzylidene)pyrrolidine-2,4-dione (3s). Yield 30%; m.p. 66~67 ºC; IR (KBr) cm-1 3381 (O-H), 1748, 1711, 1624 (C=O); 1H-NMR δ: 1.52 (s, 9H, C(CH3)3), 3.88 (s, 3H, Ar-OCH3), 3.78 (s, 2H, C-CH2-N), 6.99-7.06, 7.44-7.48 (m, 4H, J=9.04Hz, C6H4); Anal. Calc. for C16H19NO4 (289.32) C 66.42, H 6.62, N 4.84; Found C 66.35, H 6.57, N 5.01.
1-t-butyl-3-(α-hydroxy-4’-methoxybenzylidene)pyrrolidine-2,4-dione (3t). Yield 40.7%; m.p. 117~118 ºC; IR (KBr) cm-1 3431 (O-H), 1743, 1683, 1594 (C=O); 1H-NMR δ: 1.43 (s, 9H, C(CH3)3), 3.80 (s, 3H, Ar-OCH3), 3.75 (s, 2H, C-CH2-N), 6.88, 6.91, 8.25, 8.28 (d-d, 4H, J=9.04Hz, C6H4); Anal. Calc. for C16H19NO4 (289.32) C 66.42, H 6.62, N 4.84; Found C 66.32, H 6.67, N 5.04.
1-t-butyl-3-(α-hydroxy-2’-methylbenzylidene)pyrrolidine-2,4-dione (3u). Yield 60.4%; m.p. 69~70 ºC; IR (KBr) cm-1 3430 (O-H), 1718, 1651, 1611 (C=O); 1H-NMR δ: 1.45 (s, 3H, NCH3), 2.33 (s, 2H, CCH2N), 3.72 (s, 3H, OCH3), 7.16-7.38 (m, 4H, J=6.028 Hz, C6H4); Anal. Calc. for C16H19NO3 (273.32) C 70.31, H 7.01, N 5.12; Found C 70.16, H 6.98, N 5.08.
1-t-butyl-3-(α-hydroxy-3’-methyl benzylidene)pyrrolidine-2,4-dione (3v). Yield 53.8%; m.p. 137~138 ºC; IR (KBr) cm-1 3412 (O-H), 1708, 1648, 1564 (C=O); 1H-NMR δ: 1.53 (s, 3H, NCH3), 2.44 (s, 2H, CCH2N), 3.84 (s, 3H, OCH3), 7.32-8.04 (m, 4H, C6H4); Anal. Calc. for C16H19NO3 (273.32) C 70.31, H 7.01, N 5.12; Found C 70.32, H 7.08, N 5.15.
1-t-butyl-3-(α-hydroxy-4’-methylbenzylidene)pyrrolidine-2,4-dione (3w). Yield 32.4%; m.p. 124~125 ºC; IR (KBr) cm-1 3378 (O-H), 1709, 1665, 1588 (C=O); 1H-NMR δ: 1.53 (s, 3H, NCH3), 2.44 (s, 2H, CCH2N), 3.84 (s, 3H, OCH3), 7.26-8.14 (m, 4H, C6H4); Anal. Calc. for C16H19NO3 (273.32) C 70.31, H 7.01, N 5.12; Found C 70.30, H 6.99, N 5.15.
Acknowledgements
This work was supported by the National Key Project for Basic Research (2003CB114400).
References
- 1.Wu Y. C., Hu F. Z., Yang H. Z. Research and develoment on the inhibitors of 4-hydroxyphenyl-pyruvate dioxygenase. Nongyaoxue Xuebao. 2001;3:1–10. (in Chinese) [Google Scholar]
- 2.Zhu Y. Q., Hu F. Z., Yang H. Z. Reviews on 4-hydroxyphenylpyruvate dioxygenase enzyme and the structure-activity relationships of its inhibitors. Huaxue Tongbao. 2004;67:w018/1–W018/7. (in Chinese) [Google Scholar]
- 3.Jones R. C. F., Begley M. J., Peterson G. E., Sumaria S. Acylation of pyrrolidine-2,4-dione. J. Chem. Soc., Perkin Trans. 1990;1:1959–67. [Google Scholar]
- 4.Rinehart K. L., MacKellar F. A., Grostic M. F., Olson E. C., Wnuk R. J., Branfman A. R., Tirandamycin I. Structure assignment. J. Am. Chem. Soc. 1971;93:4943–4945. doi: 10.1021/ja00748a067. [DOI] [PubMed] [Google Scholar]
- 5.Matsuo K., Kimura M., Kinuta T., Tarkai N., Tanaka K. Syntheses and antimicrobial activities of 3-acyltetramic acid derivatives. Chem. Pharm. Bull. 1984;32:4197–4204. doi: 10.1248/cpb.32.4197. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y. Q., Hu F. Z., Zou X. M., Yao C. S., Li Y. H., Yang H. Z. Synthesis of 1-benzyl-3-(α-hydroxy-(un)substituted enzylidene)pyrrolidine-2,4-diones and biological activity. Chin. J. Org. Chem. (in press) [Google Scholar]
- 7.Kakidani H., Hirai K. Three-dimentional modeling of plant 4-hydroxyphenylpyruvate dioxygenase, a molecular target of triketone-type herbicides. J. Pestic. Sci. 2003;28:409–415. [Google Scholar]
- 8.Fritze I. M., Linden L., Freigang J., Auerbach G., Huber R., Steinbacher S. The Crystal structures of Zea mays and Arabidopsis 4-hydroxyphenylpyruvate dioxygenase. Plant Physiol. 2004;134:1388–1400. doi: 10.1104/pp.103.034082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C. S., Huang J. L., Sun Y. S., Yang D. Y. Mode of action of 4-hydroxyphenylpyruvate dioxygenase inhibition by triketone-type inhibitors. J. Med. Chem. 2002;45:2222–2228. doi: 10.1021/jm010568y. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y. Q., Song H. B., Yao C.S., Gao Y., Hu F. Z., Zou X. M., Yang H. Z. 3-(a-Hydroxy-2-methoxylbenzylidene)-1-isopropylpyrrolidine-2,4-dione. Acta Cryst. 2004;E60:o599–o601. [Google Scholar]
- 11.Yang X.F. Study on bioassay methods for determining activities of sulfonyurea herbicides. Acta Scient. Nat.Univ. Nankaniensis. 1994:88–93. (in Chinese) [Google Scholar]
- 12.Speziale A. J., Jaworski E.G. N-Substituted glycinate and alaninate eaters. J. Org. Chem. 1960;25:728–732. doi: 10.1021/jo01075a014. [DOI] [Google Scholar]