Abstract
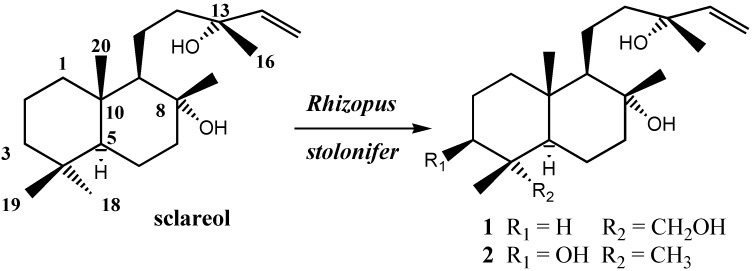
Incubation of sclareol with Rhizopus stolonifer affords in high yield a mixture of triols with 18-hydroxy-sclareol as the main component.
Keywords: Sclareol, Rhizopus stolonifer, 3β-hydroxy-sclareol, 18-hydroxy-sclareol
Introduction
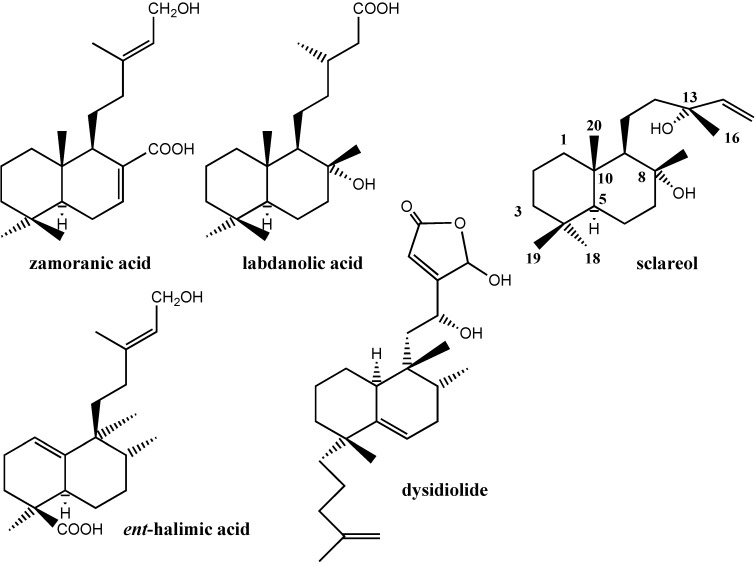
For a few years we have been involved in studying the transformations of the major components of plants of our region, such as labdanolic [1], zamoranic [2] or ent-halimic [3] acids (Figure 1), into biologically active compounds or with odourant properties like Ambrox®.
Figure 1.
Recently we have been involved in the transformation of sclareol (Figure 1), a diterpenoid which is easily isolated from Salvia sclarea [4], into biologically active compounds such as (-)-hyrtiosal [5], prehispanolone analogs [6] or 9-11-secoespongianes [7]. Other groups have transformed sclareol into very interesting compounds as well [8]. We were interested in the transformation of sclareol into 18-hydroxysclareol, which would be an excellent precursor for obtaining new analogues, in order to do structure activity studies as we have been successful in the transformation of ent-halimic acid into analogues of dysidiolide [3a] (Figure 1) that increase the anticancer potency of this last compound.
Microbial hydroxylation of sclareol has been carried out by several groups [9]. The best results for the compound of interest were reported by Prof. McChesney’s group, who obtained 18-hydroxysclareol in 50% yield by incubation of sclareol with Cunninghanella species NRRL 5695 [9a]. As we wanted to increase this yield for extension of the south side chain in order to synthesize analogues of dysidiolide, we examined the incubation of sclareol with Rhizopus stolonifer.
Results and Discussion
Our results show that incubation of sclareol with a growing culture of Rhizopus stolonifer (Scheme 1) affords a mixture of diols 1 and 2, that improve the yield reported before (Table 1). The products were characterized by comparison with the spectral data reported in the literature ([9g] for 1 and [9a] for 2, see references in [9] as well). As it can be seen, the best results are obtained after 5 days by following procedure B as described in the Experimental section. Longer reaction times led to an increase in the transformation of compound 1 into degradation products.
Scheme 1.
Table 1.
| Conditions* | Time | Transformation of sclareol % | 1 (%) | 2 (%) |
|---|---|---|---|---|
| A | 5 days | 21 | ||
| B | 5 days | 87 | 74 | 9 |
| B | 8 days | 98 | 68 | 20 |
| C | 5 days | 88 | 17 |
* See Experimental.
Conclusions
We have described a microbial oxidation of sclareol by Rhizopus stolonifer that provides an easy route to 18-hydroxysclareol (1), that could subsequently be transformed into more active compounds following synthetic sequences similar to those used for zamoranic, labdanolic or ent-halimic acid.
Experimental
General
Unless otherwise stated, all chemicals were purchased as the highest purity commercially available and were used without further purification. Sclareol was purchased from Aldrich, ref. 35,799-5. Melting points were determined with a Kofler hot stage melting point apparatus and are uncorrected. IR spectra were recorded on a BOMEM 100 FT IR spectrophotometer. 1H and 13C-NMR spectra were recorded in deuterochloroform and referenced to the residual peak of CHCl3 at δ 7.26 ppm and δ 77.0 ppm for 1H and 13C, respectively, on a Bruker WP-200 SY and a BRUKER DRX 400 MHz instrument. Chemical shifts are reported in δ, ppm and coupling constants (J) are given in Hz. MS were performed in a VG-TS 250 spectrometer at 70 eV ionizing voltage. Mass Spectra are presented as m/z (% rel. int.). HRMS were recorded in a VG Platform spectrometer using Electronic Impact (EI) or Fast Atom Bombardment (FAB) technique. Optical rotations were determined in a Perkin-Elmer 241 polarimeter in 1 dm cells. Diethyl ether, was distilled from sodium, under argon. Rhizopus stolonifer CECT 2672, obtained from the Colección Española de Cultivos Tipo (Valencia, Spain), was maintained and sporulated on agar slants (1 % yeast extract, 1 % glucose, 0.1 bactopeptone and 2 % agar). 250 mL Erlenmeyer flasks, containing 100 mL of liquid medium (glucose, 10 g/ L; K2HPO4, 2.5 g/L; NH4NO3, 2.5 g/L; MgSO4, 0.25 g/L; CaCl2·6H2O, 10 –4 M; FeSO4, 1.5x10 –5 M; MnCl2, 10 –5 M) were sterilized to 121 ºC for 30 min. Next, the flasks were inoculated with an aqueous spore suspension and incubated at 30 ºC with orbital shaking (200 rpm). When the growth of mycelium was complete (36-40 h), the medium was filtered and the pellets (1-3 mm) were washed with sterilized water. The mycelium (0.5 g dry weight/L) was added to a new 250 mL Erlemeyer flask containing the secondary liquid medium (100mL), which contained: In case A: glucose, 10 g/L; K2HPO4, 2.5 g/L; NH4NO3, 2.5 g/L; MgSO4, 0.25 g/L; CaCl2 6·H2O, 10 –4 M; FeSO4, 1.5x10 –5 M; MnCl2, 10 –5 M and 100 mg of sclareol dissolved in ethanol (3-5 mL). In case B: K2HPO4, 2.5 g/L; NH4NO3, 2.5 g/L; MgSO4, 0.25 g/L; CaCl2 ·6H2O, 10 –4 M; FeSO4, 1.5x10 –5 M; MnCl2, 10 –5 M and 100 mg of sclareol dissolved in ethanol (3-5 mL). In case C: yeast extract (3 g/L); glucose (3 g/L) and 100 mg of sclareol dissolved in ethanol (3-5 mL). Finally the sclareol and the fungi were incubated during 5-8 days at 30 ºC and 200 rpm. The mycelial mass was removed, washed thoroughly with water and squeezed. The aqueous washings were mixed with the aqueous filtrate and extracted with EtAcO (3 x 500 mL). The organic extract was washed with H2O, dried and concentrated in vacuo to give a residue that was chromatographed on silica gel, eluting with mixures of hexane-EtOAc of increasing polarity. After isolation of the compounds, compound 1 was isolated in the fraction eluted with 3:2 hexane-EtOAc, and compound 2 was isolated in the fraction eluted with 1: 1 hexane-EtOAc,. Their structures were established by spectroscopic methods by comparison with literature data (see references [9a], [9g] and in general references [9]) and the optical rotation for all compounds.
Acknowledgements
The authors thank the CICYT (BQU2001-1034) for financial support.
Footnotes
Sample Availability: Available from the authors.
References and Notes
- 1. Urones J. G., Marcos I. S., Basabe P., González J. L., Sexmero M. J., Lithgow A. M. Ambergris Compounds from Labdanolic acid. Tetrahedron. 1992;48:9991–9998. Lithgow A. M., Marcos I. S., Basabe P., Sexmero M. J., Diez D., Gomez A, Estrella A., Urones J. G. Labdanolic acid: synthetic precursor of tricyclic diterpenes. Nat. Prod. Lett. 1995;6:291–294. and references cited therein.
- 2. Urones J. G., Díez D., Gomez P. M., Marcos I. S., Basabe P., Moro R. F. Chemistry of Zamoranic acid. Part 10 Homochiral hemysinthesis of Pereniporin A. J. Chem. Soc. Perkin Trans. 1997:1815–1818. and references cited therein. Marcos I. S., Moro R. F., Carballares S., Urones J. G. An efficient total synthesis of isodrimeninol from zamoranic acid. Synlett. 2000;541
- 3. Marcos I. S., Pedrero A. B., Sexmero M. J., Díez D., Basabe P., Hernández F. A., Broughton H. B., Urones J. G. Synthesis and absolute Configuration of the supposed Structure of Cladocoran A and B. Synlett. 2002:105–109. Marcos I. S., Hernández F. A., Sexmero M. J., Díez D., Basabe P., Pedrero A. B., García N., Sanz F., Urones J. G. Synthesis and Absolute Configuration of (-)-Chettaphanin II. Tetrahedron Lett. 2002;43:1243–1245. doi: 10.1016/S0040-4039(01)02400-5.
- 4.Ruzicka L., Janot M.M. Höhere Terpenverbindungen L. Zur Kenntnis des Sclareol. Helv. Chim. Acta. 1931;14:645. doi: 10.1002/hlca.19310140162. [DOI] [Google Scholar]
- 5. Basabe P., Diego A., Díez D., Marcos I.S., Urones J.G. Synthesis and Absolute Configuration of (-)-Hyrtiosal. Synlett. 2000:1807–1809. Basabe P., Diego A., Díez D., Marcos I.S., Mollinedo F., Urones J.G. Synthesis and Absolute Configuration of (-) Hyrtiosal. Synthesis. 2002:1523–1529. doi: 10.1055/s-2002-33332.
- 6.Basabe P., Estrella A., Marcos I.S., Díez D., Lithgow A. M., White A. J. P., Williams D. J., Urones J.G. Prehispanolone Analogs: Stereochemistry Control at C-5 in the Preparation of Oxaspiro(4,5)decane Fused Systems and Related Compounds. Synlett. 2001:153–155. [Google Scholar]
- 7.Basabe P., Gomez A., Marcos I.S., Díez D., Broughton H. B., Urones J.G. Towards the synthesis of 9,11-secoespongianes. Tetrahedron Lett. 1999;40:6857–6860. doi: 10.1016/S0040-4039(99)01384-2. [DOI] [Google Scholar]
- 8. Barrero A. F., Cortes M., Mazaneda E. A., Cabrera E., Chahboun R., Lara M., Rivas A. R. Synthesis of 11,12-epoxydrim-8,12-en-11-ol, 11,12-diacetoxydrimae and warburganal from (-)-sclareol. J. Nat. Prod. 1999;62:1488–1491. doi: 10.1021/np990140q. Barrero A. F., Mazaneda E. A., Chahboun R., Paiz M. C. A new enantiospecific route toward monocabocyclic terpenoids: syntheis of (-)-caparrapi oxide. Tetrahedron Lett. 1998;39:9543–9544. doi: 10.1016/S0040-4039(98)02119-4. Muller M., Schroder J., Magg C., Seifert K. Synthesis of (+)-coronarin E. Tetrahedron Lett. 1998;39:4655–4656. doi: 10.1016/S0040-4039(98)00861-2. Barrero A. F., Mazaneda E. A., Chahboun R. Synthesis of the Wiedendiol-A and Wiedendiol-B from Labdane Diterpenes. Tetrahedron. 1998;54:5635–5650. and references cited therein. Jung M., Seokjoon L., Byunghee Y. Conversion of Sclareol into (+)-Galanolactone and (+)-Labdienedial. Tetrahedron Lett. 1997;38:2871–2874. doi: 10.1016/S0040-4039(97)00485-1. Barton D. H. R., Taylor D. K., Tse C-L. An improved synthesis of (-)-dodecahydro-3a,6,6,9a-tetramethylnaphtol[2,1,-b]furan via ozonolysis of (-)-sclareol. Tetrahedron Lett. 1994;35:9505–9508. Barton D. H. R., Taylor D. K., Tse C-L. An efficient synthesis of (-)-dodecahydro-3a,6,6,9a-tetramethylnaphtol[2,1,-b]furan from (-)-sclareol. Tetrahedron Lett. 1994;35:5801–5804.
- 9. Kouzi S. A., McChesney J. D. Microbial metabolism of the Diterpene sclareol: oxidation of the A ring by septomyxa affinis. Helv. Chim. Acta. 1990;73:2157–2164. doi: 10.1002/hlca.19900730811. Kouzi S. A., McChesney J. D. Microbial models of mammalian metabolism: fungal metabolism of the diterpene sclareol by cunninghamella species. J. Nat. Prod. 1991;54:483–490. doi: 10.1021/np50074a021. Abraham W-R. Microbial hydroxylation of Sclareol. Phytochemistry. 1994;36:1421–1424. Farooq A., Tahara S. Biotransformation of two cytotoxic terpenes α-santonin and sclareol by botrytis cinerea. J. Biosci. 2000;55:713–717. doi: 10.1515/znc-2000-9-1008. Hanson J. R., Hitchcock, Nasir H., Truneh A. The Biotransformation of the diterpenoid, Sclareol, by Cephalosporium aphidicola. Phytochemistry. 1994;36:903–906. Aranda G., Lallemand J-Y, Hammoumi A., Azerad R. Microbial hydroxylation of Sclareol by Mucor plumbeus. Tetrahedron Lett. 1991;32:1783–1786. Aranda G, El Kortbi M. S., Lallemand J-Y, Neuman A., hammoumi A., Facon I., Azerad R. Tetrahedron. 1991;39:8339–8350.