Abstract
A new iso-amyl benzothiazolyl sulfoxide (ABSO) was synthesized and used in the extraction of Pd(II) from hydrochloric acid media. Pd(II) was extracted quantitatively from 0.1 M HCl with ABSO in benzene (0.5 M). Ammonia solution (2.0 M) could be used as stripping agent. ABSO and Pd(II) form a 2:1 adduct [Pd (ABSO)2Cl2] in the extraction. X-ray crystal structure determination revealed PdCl2(ABSO)2 is a square-planar complex in which ABSO acts as a neutral unidentate ligand coordinated with palladium(II) via the thiazolyl N atom.
Keywords: Iso-amyl benzothiazolyl sulfoxide, Palladium, Solvent extraction, Crystal structure
Introduction
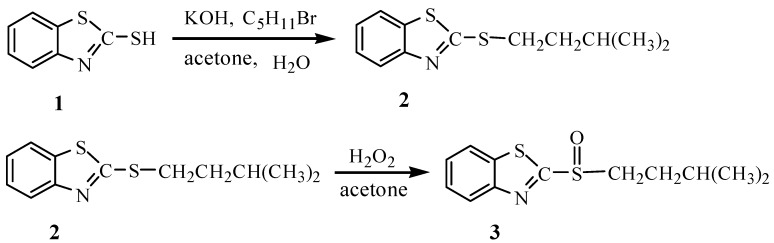
Palladium is a good catalyst and is widely used in hydrogenation and dehydrogenation reactions. Owing to its corrosion resistance properties and easy alloying, palladium and its alloys are also used in chemical industry, medical devices and jewelry manufacture [1,2]. As Pd(II) can form a number of complexes that are soluble in organic solvents [3], solvent extraction has become an effective technique in the recovery and separation of palladium from aqueous solutions [4,5,6,7]. One coordination chemistry property of Pd(II) is that it prefers to coordinate most strongly with polarizable atoms, a fact that has pushed the development of extractants bearing donor atoms such as sulfur, phosphorus and nitrogen. These ligands are ‘soft’ bases according to the empirical Pearson classification [9]. Sulfoxides are known to be highly selective for extraction of Pd(II), and have been widely used in the extraction of this species [10,11,12]. So far, most sulfoxides reported for this purpose are petroleum or dialkyl sulfoxides. In this work, a new sulfoxide extractant bearing a heterocyclic substituent, iso-amyl benzothiazolyl sulfoxide (ABSO, 3), was prepared by the oxidation of the corresponding iso-amyl benzothiazolyl sulfide ether (2, Figure 1). Its extraction behavior towards Pd(II) was also investigated and a Pd(II)-ABSO adduct – [PdCl2(ABSO)2] – was obtained in crystal form. The crystal structure of PdCl2(ABSO)2 showed that ABSO acts as a neutral unidentate ligand coordinated with Pd(II) via the benzothiazolyl N atom; this is quite different from general Pd(II) dialkyl sulfoxide complexes in which sulfoxides are coordinated with palladium via the O or S atoms of the ligand [13,14,15,16].
Figure 1.
The synthetic route of iso-amyl benzothiazolyl sulfoxide
Results and Discussion
Extraction behavior of ABSO towards Pd(II)
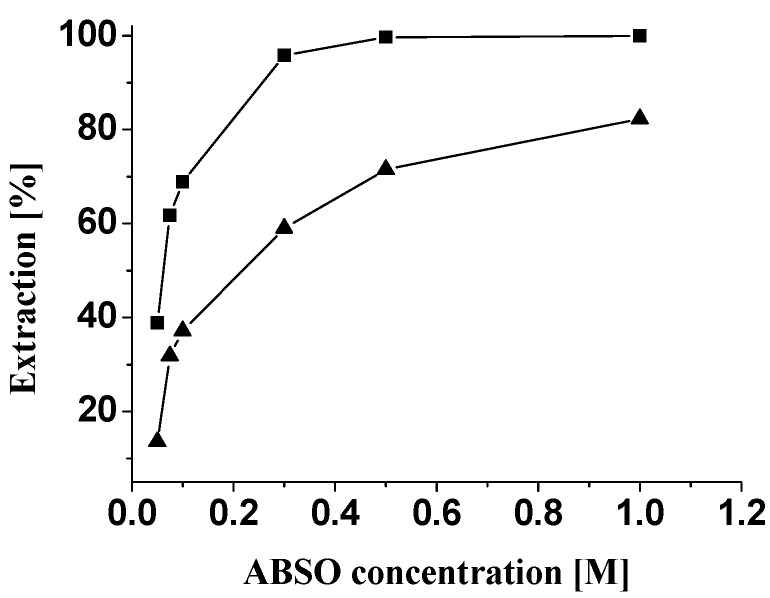
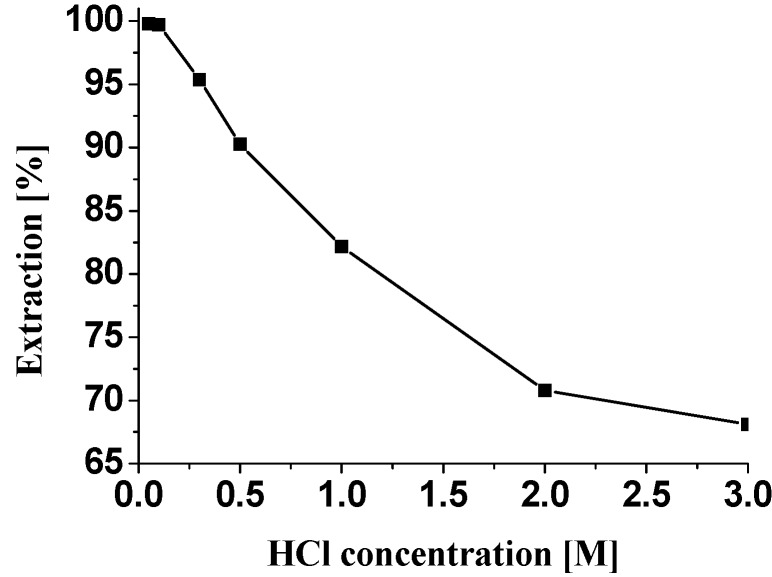
Extractions were carried out by shaking equal volumes (10 mL) of the Pd(II) aqueous solution and ABSO dissolved in benzene with the extractant concentration varying from 5x10-2 ~ 1.0 M. It was found the percentage extraction of Pd(II) increases as the extractant concentration increases, and 0.5 M ABSO was needed for quantitative extraction of Pd(II) from a 0.1 M HCl aqueous solution containing 100 mg/L palladium (Figure 2). The effect of HCl concentration on the extraction of Pd(II) is shown in Figure 3. The extraction curve indicated the percentage of extraction of Pd(II) decreased drastically with the increase of HCl concentration. Quantitative extraction of palladium occurred at 0.1 M HCl. Therefore, 0.1 M HCl was adopted in all subsequent experiments. Equilibrium time was determined by measuring the metal content in the aqueous phase as a function of time until the metal concentration in the aqueous solution did not vary. The two phases were shaken for a period ranging from 3 to 30 min. The minimum period of equilibration required for the quantitative extraction of palladium was found to be about 15 min. Palladium loaded in the organic phase was stripped with various stripping agents, such as HCl, HNO3, NaOH, NH3·H2O and Na2SO3 (Table 1). The stripping was observed only with NaOH, NH3·H2O and Na2SO3 as stripping agents. It was found the stripping was less than 40% when NaOH solution was used as stripping agent. The stripping was quantitative when 2.0 M ammonia solution was used. Hence, 2.0 M ammonia solution can be used as the effective stripping agent.
Figure 2.
Effect of extractant concentration on the percentage extraction of palladium. [Pd(II)] = 100 mg·L-1; ■: 0.1 M HCl; ▲: 2.0 M HCl.
Figure 3.
Effect of HCl concentration on the percentage extraction of palladium. [Pd(II)] = 100 mg·L-1; Organic phase: 0.5 M ABSO in benzene.
Table 1.
Effect of Stripping Agents on the Recovery of Palladium(II)
| Stripping Agents | % Recovery | ||||
|---|---|---|---|---|---|
| 0.5 M | 1 M | 2 M | 3 M | 5 M | |
| HCl | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| HNO3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| NaOH | 35.8 | 32.5 | 31.4 | 30.7 | 27.3 |
| NH3·H2O | 97.6 | 99.2 | 99.5 | 99.6 | 99.6 |
| Na2SO3 | 12.4 | 10.6 | 9.8 | 7.5 | 4.4 |
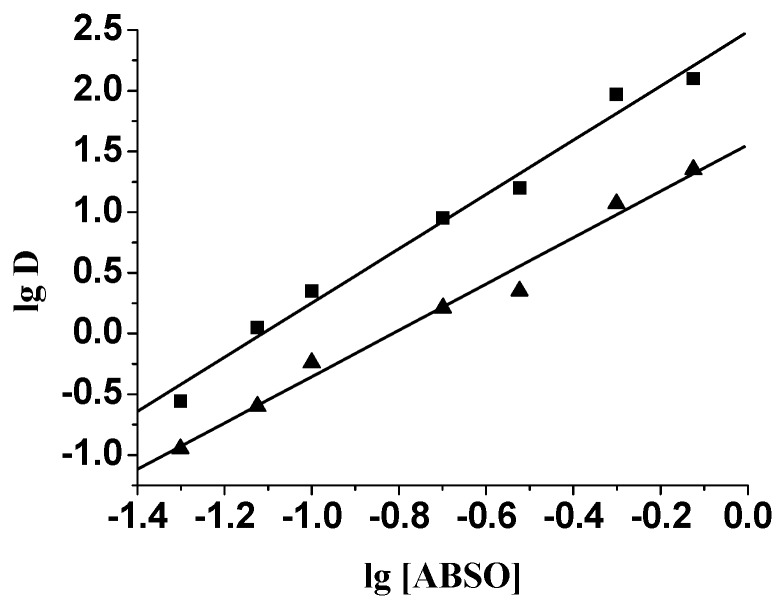
The ratio of metal ion extractant in the extracted species can be determined by plotting log D (D: distribution ratio) versus log [extractant] at constant pH [17,18]. As can be seen in Figure 4, Plots of log D versus log [ABSO] for the extraction of Pd(II) at 0.1 and 1.0 M HCl yield slopes of 2.13 and 1.91 respectively. This indicates that two ABSO molecules were involved in the extracted Pd(II)-ABSO adduct. Furthermore, we have studied the dependence of Pd(II) extraction upon the acidity. [H+] was varied from 0.1 M to 2.0 M while maintaining a constant ionic strength (Cl- = 2.0 M) by adding NaCl. Experimental results showed the variation of [H+] did not affect the distribution ratio. This is similar to petroleum sulfoxide and dialkyl sulfoxide cases in the extraction of palladium(Ⅱ) at low acidity [19,20,21]. So the extraction of Pd(II) by ABSO may be depicted as:
| PdCl42-(aq) + 2[ABSO](org) ⇔ [PdCl2(ABSO)2](org) + 2Cl-(aq) |
Figure 4.
Plot of Log D versus Log [ABSO]. [Pd(II)] = 100 mg·L-1; ■: 0.1 M HCl; ▲: 1.0 M HCl.
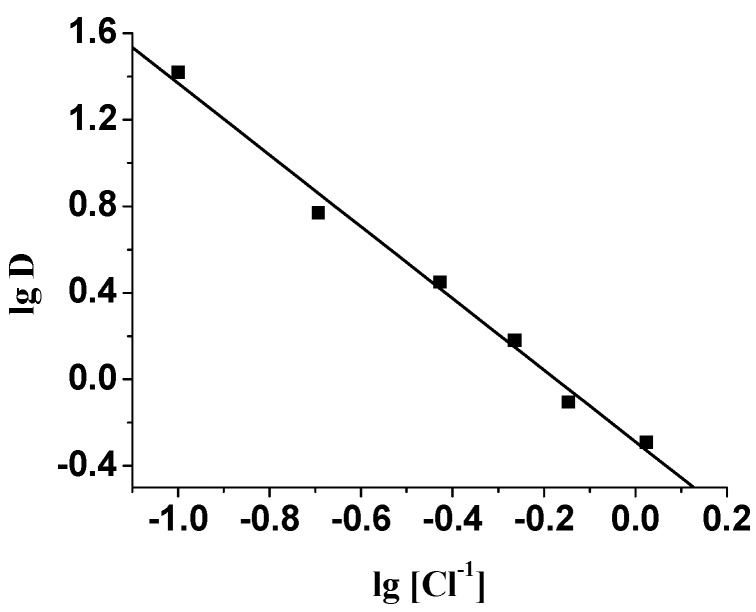
Since the extraction percentage of Pd (II) decreased drastically with the increasing of hydrochloric acid concentration, while the extraction was independent on acidity, an increase in Cl- concentration should have a negative effect on the extraction of Pd (II). Such a tendency was actually observed by adding NaCl to the extraction system. A plot of logD versus log[Cl-] yielded a straight line with a slope of about -2.0 (Figure 5), showing that two Cl- ions were released during the extraction of Pd (II).
Figure 5.
Plot of Log D versus Log [Cl-]. [Pd(II)] = 100 mg·L-1; HCl = 0.1 M; Organic phase: 0.5 M ABSO in benzene.
IR spectra of extracted Pd(II)-ABSO adduct
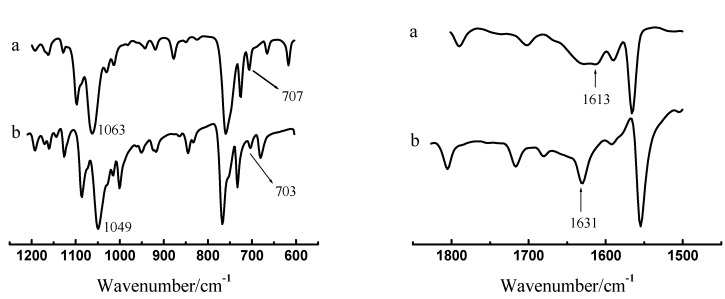
Extracted Pd(II)-ABSO adduct may be prepared by following procedure: 0.5 M ABSO in benzene was shaken with Pd(Ⅱ) aqueous solution (1.0 g·L-1 in 0.1 M HCl) many times until a saturated extraction organic phase was obtained. After removing benzene by distillation, a yellowish solid complex was obtained and dried in vacuum. Figure 6 shows the IR spectra of ABSO and Pd(II)-ABSO complex. The υS-O of free ABSO ligand appears as a strong absorption at 1049 cm-1. In the extracted complex, the characteristic υS-O absorption appears at 1063 cm-1 and it does not shift remarkably as compared to υS-O of a wide variety of metal-sulfoxide complexes [22,23,24]. This suggested that ABSO is not coordinated with palladium through the O or S atoms. In addition, the peak at 704 cm-1 attributed to the absorption of υC-S-C of the benzothiazolyl ring [25] appears at 707 cm-1 in the Pd(II)-ABSO complex, indicating that the S atom is not coordinated with the metal ion too. The C=N stretching vibration observed at 1631 cm-1 for ABSO is shifted to 1613 cm-1 in the Pd-ABSO complex, and the shift extent is similar to the Pd(II)–imidazole complex [26,27]. These facts indicated that ABSO is coordinated with Pd (II) via nitrogen atom on the thiazole ring, which is similar to the binding of benzothiazole derivatives to a number of other metals reported previously [28,29,30,31,32].
Figure 6.
Infrared spectra of Pd(II)-ABSO complex (a) and ABSO (b)
X-Ray Crystallography of PdCl2(ABSO)2 [33]
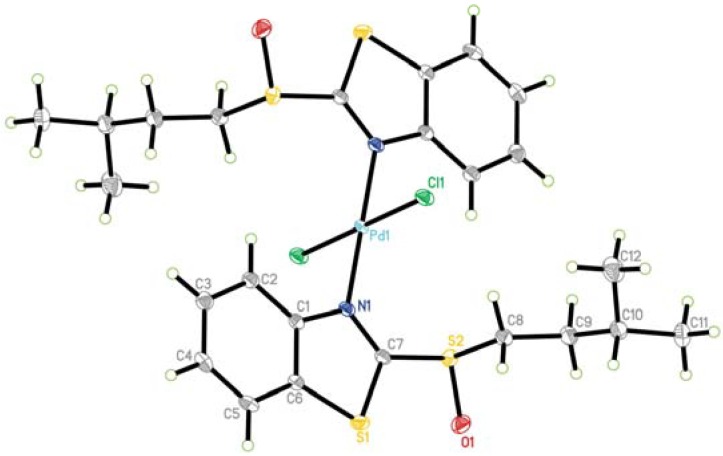
The crystal structure of PdCl2(ABSO)2 is shown in Figure 7. Crystallographic data and some experimental conditions used to obtain the intensity data are given in Table 2. As expected, in Pd(II)-ABSO complex, ABSO acts as neutral unidentate ligand coordinating to palladium via the thiazolyl N atom. The complex is of trans-conformation and Pd(II) is coordinated by two Cl atoms and two ABSO at opposite position. Two opposite Pd-N and Pd-Cl bond distances are equivalent respectively. The Pd-atom and two N-atoms are coplanar ( N(1)-Pd(1)-N(1)# = 180.00° ). Thus, the palladium-ABSO complex as a whole is in complete symmetry with palladium atom at the symmetry center of the square-planar complex. This is similar to bis[2-(2-benzoxazolyl)phenol-N]dichloro- palladium(II) [34].
Figure 7.
Molecular structure of PdCl2(ABSO)2 and the atomic labeling
Table 2.
Crystal data and structure refinement details
| Empirical formula | C26H32Cl8N2O2PdS4 | Density (calculated) | 1.658 g/mL |
| Formula weight | 922.78 | Absorption coefficient | 1.334 mm-1 |
| Temperature | 100(2) K | F(000) | 928 |
| Wavelength | 0.71073 Å | Crystal size | 0.25 x 0.08 x 0.08 mm3 |
| Crystal system | Monoclinic | θ range for data collection | 2.53 to 28.29° |
| Space group | P2(1)/c | Reflections collected | 13380 |
| Unit cell dimensions | a = 18.0079(15) Å, α= 90° | Independent reflections | 4358 [R(int) = 0.0367] |
| b = 6.0692(5) Å, β= 116.5450(10)° |
Final R indices [I>2sigma(I)] |
R1 = 0.0364, wR2 = 0.0811 | |
| c = 18.9080(16) Å, γ= 90° | Goodness-of-fit on F2 | 1.051 | |
| Volume | 1848.7(3) Å3 | R indices (all data) | R1 = 0.0494, wR2 = 0.0862 |
| Z | 2 | Largest diff. peak and hole | 1.350 and -0.811 e.Å-3 |
The Pd-N and Pd-Cl distances are 2.015(2) and 2.2919(6) Å. These values are a little shorter than the values expected from the sum of Shannon’s ionic radii [35] (Pd-N = 2.10; Pd-Cl = 2.45 Å). The Pd-N bond length is longer than the values reported for bis[2-(2-benzoxazolyl)phenol-N]dichloro palladium (Pd-N 2.002 Å) [34]. Bond angles around the central Pd atom show small deviations from the idealized value of 90° ( N(1)-Pd(1)-Cl(1) = 90.69°, N(1)-Pd(1)-Cl(1)# = 89.31° ).
Almost all C-C-C bond angles of ABSO phenyl ring are about 120°, except that the angles of C(1)-C(2)-C(3), C(4)-C(5)-C(6) are a little smaller (117.9° and 117.1°). The bond lengths and angles in the thiazole unit are essentially similar to those reported for thiazole units in [Rh{bis(benzothiazol-2-yl)ethane}(CO)2]BF4 [29], [ReOCl3(2-(2-pyridyl)benzthiazole)] [31], [cis-dichloro(2-pyrid-2-ylbenzothiazole)platinum(Ⅱ)] [32]. The N(1)-C(7) bond length (1.306(3) Å) are between the single and double bond (according to Pauling’s presentation, the bond lengths of C-N and C=N are 1.47 and 1.29 Å respectively [36]), which may result from the fact that the benzothiazole-2-thiolate group has the following resonance structure (Figure 8) [37], namely, the negative charge of S(2) is partially delocalized to the N atom. S-O bond length (1.487(2) Å) is consistent with a considerable double bond character and similar to the lengths of uncoordinated sulfoxides [38].
Figure 8.
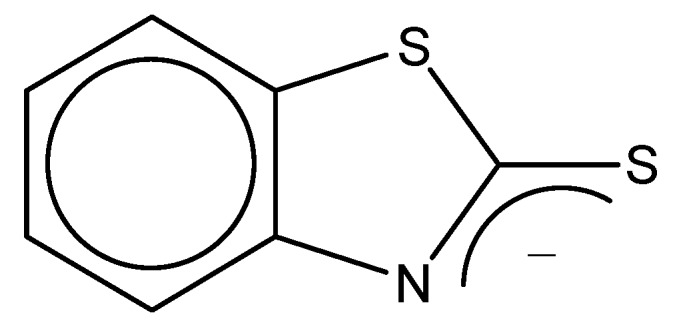
Resonance structure of benzothiazole-2-thiolate group
Conclusions
A new sulfoxide extractant, iso-amyl benzothiazolyl sulfoxide (ABSO) was synthesized and used in the solvent extraction of Pd (II) from hydrochloric acid media. The extraction of Pd (II) was quantitative from 0.1 M HCl with 0.5 M ABSO in benzene. The pH of aqueous solution did not affect the extraction process of Pd (II) when [H+] ≤2 M. IR and X-ray crystal structure analysis of Pd(II)-ABSO extraction adduct indicated that ABSO acts as neutral unidentate ligand coordinated with palladium via the thiazole N atom, which is quite different from general alkyl sulfoxides which is coordinated with Pd (Ⅱ) via O or S atoms of the ligands.
Experimental
General
The concentration of palladium in the aqueous phase was determined with a Shimadzu Model AA-680 atomic absorption spectrophotometer. IR spectra were obtained for KBr pellets using a VECTOR33 IR spectrophotometer (Bruker). 1H-NMR spectra were performed on an AVANCE Digital 400 MHz NMR Apparatus (Bruker). FAB-MS spectra were obtained with Finigan-2000 mass apparatus.
Preparation of iso-amyl benzothiazolyl sulfide (2)
2-Mercaptobenzothiazole (250.9 g, 1.5 mol), acetone (500 mL), water (50 mL) and KOH (120 g, 2.1 mol) were put in a round-bottom flask fitted with a mechanical stirrer and condenser and the mixture was heated for about 30 min. Iso-amyl bromide (302.1 g, 2.0 mol) was then added gradually with stirring through a dropping funnel and the reaction mixture was refluxed for 5 hours. The residual solid was filtered after cooling down and the acetone was removed by distillation. The organic phase was diluted with ether (50 mL), washed with water three times and dried with anhydrous Na2SO4. The ether was evaporated and 281.3 g (yield: 79%) of a brown liquid product was obtained. FT-IR (KBr): 3062.7 (=CH), 2994.80, 2958.66, 2931.01, 2871.73 (-CH2-, -CH3), 1559.57, 1459.58 (C=C), 1427.75, 1365.58 (-C(CH3)2), 1237.42, 1183.39 (C-S-C), 886.77 (C-N), 757.48, 727.05 cm-1.
Iso-amyl benzothiazolyl sulfoxide (ABSO, 3):
Iso-amyl benzothiazolyl sulfide ether (2, 142.4 g, 0.6 mol) was placed in a round-bottomed flask fitted with a mechanical stirrer and condenser and a mixture of acetic acid (100 mL) and acetone (100 mL) were added. H2O2 (30%, 150 mL) was added gradually through a dropping funnel and the reaction mixture was stirred for 2 hour at room temperature, then it was poured into ice-water, the organic phase was collected and the acetone was further removed by distillation. The yellow crude product was crystallized from ethanol and 125.4 g of colorless crystals were obtained (yield: 82.5%). FT-IR (KBr): 3061 (=CH), 2952, 2924, 2913, 2869 (-CH2-, -CH3), 1631 (-C=N-), 1555, 1471, 1455 (C=C), 1425, 1385 (-C(CH3)2), 1049 (S=O), 845 (C-N), 767, 733cm-1; 1H-NMR (CDCl3): δ (ppm) 8.04-8.02 (1H, =CH), 7.97-7.95 (1H), 7.54-7.50 (1H), 7.47-7.43 (1H), 3.27-3.09 (2H, CH2-C), 1.86-1.65 (2H, C-CH2-C), 1.58-1.52 (1H, -CH-), 0.91-0.88 (6H, 2CH3); FAB-MS (M+1)/z: 254.1.
Pd (II) stock solution (1.0 g·L-1):
A weighed portion of palladium metal was dissolved in aqua regia (40 mL). When the metal was completely dissolved, the solution was evaporated to approximately 5 mL. Residual HNO3 was removed by adding concentrated HCl (12 M, 50 mL) and evaporating to approximately 5 mL again, and this was repeated 3 times. Then the solution was evaporated to dryness. The solid was dissolved and diluted to 1000 mL with 0.1 M HCl. Working solutions were prepared by further dilution and standardized HCl solutions were added to adjust the H+ concentration to the desired value. All the chemicals used were of analytical reagent grade. Double-distilled water was used throughout.
General extraction procedure
Experiments were carried out by shaking equal volumes (10 mL) of the aqueous and organic phases in stoppered glass tubes. The aqueous phase containing 100 mg·L-1 palladium(II) was equilibrated for 15 min with 0.5 M ABSO in benzene. The two phases were then allowed to settle and separate and an aliquot was taken from the aqueous phase for analysis tof he metal concentration by atomic absorption spectrophotometer at 247.6 nm. The metal concentration in the organic phase was determined by mass balance of the metal before and after extraction. All experiments were performed at a controlled temperature of 22±1℃.
X-ray crystal structure determination
The crystal of extracted Pd(Ⅱ)-ABSO complex was grown by slow evaporation of CH3Cl and hexane mixed solutions at room temperature. A single crystal with approximate dimensions of 0.25 x 0.08 x 0.08 mm3 was selected and sealed in a thin-walled glass capillary for structural analysis. Diffraction data were collected at 100K on a Bruker Apex CCD Area Detector, by using graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å). Semi-empirical absorption correction was applied. The structure was solved by using Bruker SHELXTL (Version 6.10) program package. All non-hydrogen atoms were refined anisotropically, hydrogen atoms were introduced at calculated positions and refined via a riding model.
Acknowledgements
This work was supported by Natural Science Foundation of China (29971011, NSFC/HKUST43) and SRF for ROCS, SEM.
Footnotes
Sample availability: Samples are available from the corresponding author.
References
- 1.Lokhande T.N., Anuse M.A., Chavan M.B. Talanta. 1998;46:163. doi: 10.1016/s0039-9140(97)00270-1. [DOI] [PubMed] [Google Scholar]
- 2.Sahu R., Sondhi S.M., Gupta B. Talanta. 1995;42:401. doi: 10.1016/0039-9140(95)01423-9. [DOI] [PubMed] [Google Scholar]
- 3.Olesehuk R.D., Chow A. Talanta. 1998;45:1235. doi: 10.1016/s0039-9140(97)00232-4. [DOI] [PubMed] [Google Scholar]
- 4.Al-bazi S.J., Freiser H. Inorg. Chem. 1989;28:417. [Google Scholar]
- 5.Mathew V.J., Khopkar S.M. Talanta. 1997;44:1699. doi: 10.1016/S0039-9140(97)00013-1. [DOI] [PubMed] [Google Scholar]
- 6.Baba Y., Inoue K. Ind. Eng. Chem. Res. 1988;27:1613. doi: 10.1021/ie00081a010. [DOI] [Google Scholar]
- 7.Wisniewski M. J. Radioanal. Nucl. Chem. 2000;256:693. doi: 10.1023/A:1006760316499. [DOI] [Google Scholar]
- 8.Fujii T., Yamana H., Watanabe M., Moriyama H. J. Radioanal. Nucl. Chem. 2001;247:435. doi: 10.1023/A:1006794727162. [DOI] [Google Scholar]
- 9.Pearson R.G. J. Am. Chem. Soc. 1963;85:3533. [Google Scholar]
- 10.Lews P.A., Morris D.F.C., Short E.L., Waters D.N. J. Less-Common Metals. 1976;45:193. [Google Scholar]
- 11.Gu G., Cheng F., Zhang Z., Zeng F., Long T. In: Solvent Extraction in the Process Industries. Logsdail D.H., Slater M.J., editors. Society of Chemical Industry; London: 1993. p. 196. [Google Scholar]
- 12.Rizvi G.H., Natarajan P.R. Fresenius J. Anal. Chem. 1990;336:498. doi: 10.1007/BF00323122. [DOI] [Google Scholar]
- 13.Bancroft D.P., Cotton F.A., Verbruggen M. Acta Crystallogr. 1989;C45:1289. [Google Scholar]
- 14.Langs D.A., Hare C.R., Little R.G. Chem. Commun. 1967:1080. [Google Scholar]
- 15.Vicente J., Arcas A., Borrachero M.V., Molins E., Miravitlles C. J. Organomet. Chem. 1989;359:127. [Google Scholar]
- 16.Bennett M.J., Cotton F.A., Weaver D.L., Williams R.J., Watson W.H. Acta Crystallogr. 1967;23:788. [Google Scholar]
- 17.Lokhande T.N., Anuse M.A., Chavan M.B. Talanta. 1998;46:163. doi: 10.1016/s0039-9140(97)00270-1. [DOI] [PubMed] [Google Scholar]
- 18.Gholivand M.B., Nozari N. Talanta. 2000;52:1055. doi: 10.1016/s0039-9140(00)00476-8. [DOI] [PubMed] [Google Scholar]
- 19.He Z.Q., Long T.W., Gu G.B. Chemical Metallurgy. 1986;7:46. (in Chinese) [Google Scholar]
- 20.Preston J.S., du Preez A. C. Solv. Extr. Ion Exch. 2002;20:359. doi: 10.1081/SEI-120004810. [DOI] [Google Scholar]
- 21.Wu S.P., Meng S.Y., Gu G.B. T. Nonferr. Metal Soc. 2004;14:202. [Google Scholar]
- 22.Cotton F.A., Francis R., Horrocks W.D. J. Phys. Chem. 1960;64:1534. [Google Scholar]
- 23.Kitching W., Moore C.J., Doddrell D. Inorg. Chem. 1970;9:541. [Google Scholar]
- 24.Price J.H., Williamson A.N., Schramm R.F., Wayland B.B. Inorg. Chem. 1972;11:1280. [Google Scholar]
- 25.Simons W.W. The Sadtler handbook of infrared spectra. Sadtler Research Laboratories Inc.; Pennsylvania: 1978. p. 341. [Google Scholar]
- 26.Lane T.J., Nakagawa I., Walter J.L., Kandathil A.J. Inorg. Chem. 1962;1:267. [Google Scholar]
- 27.Furuhashi A., Inayoshi T., Ouchi A. Bull. Chem. Soc. Jpn. 1987;60:3207. doi: 10.1246/bcsj.60.3207. [DOI] [Google Scholar]
- 28.Zhang L.X., Zhou X.G., Huang Z.E., Cai R.F., Huang X.Y. Polyhedron. 1999;18:1533. [Google Scholar]
- 29.Julius G.R., Cronje S., Neveling A., Esterhuysen C., Raubenheimer H.G. Helv. Chim. Acta. 2002;85:3737. doi: 10.1002/1522-2675(200211)85:11<3737::AID-HLCA3737>3.0.CO;2-8. [DOI] [Google Scholar]
- 30.West D.X., Szczepura L.F., Giesen J.M., Kaminsky W., Kelley J., Goldberg K.I. J. Mol. Struct. 2003;646:95. [Google Scholar]
- 31.Gangopadhyay J., Sengupta S., Bhattacharyya S., Chakraborty I., Chakravorty A. Inorg. Chem. 2002;41:2616. doi: 10.1021/ic011064t. [DOI] [PubMed] [Google Scholar]
- 32.He X.F., Vogels C.M., Decken A., Westcott S.A. Polyhedron. 2004;23:155. [Google Scholar]
- 33.CCDC 262327 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk
- 34.Ito M., Furuhashi A., Shimoi M. Polyhedron. 1997;16:1889. [Google Scholar]
- 35.Shannon R.D. Acta Crystallogr. 1976;A32:751. [Google Scholar]
- 36.Pauling L. The Nature of the Chemical Bond. 3rd ed. Cornell Univ. Press; New York: 1960. p. 246. [Google Scholar]
- 37.Ciriano M.A., Sebastian S., Oro L.A., et al. Angew. Chem. 1988;100:406. [Google Scholar]
- 38.Calligaris M., Carugo O. Coord. Chem. Rev. 1996;153:83. [Google Scholar]