Abstract
This review article discusses the reaction of nitrilimines and nitrile oxides with hydrazines, hydrazones, and oximes. Three reaction modes were observed. The article mainly covers our work published over the last fifteen years, in which interesting heterocyles such as oxadiazoles, triazoles, and tetrazines were synthesized and fully characterized.
Keywords: Nitrilimines, nitrile Oxides, hydrazines, hydrazones, oximes
Introduction
The concept of 1,3-dipoles was first introduced by Huisgen in 1963 [1]. Since that time, a lot of work has been done on their reactions and the mechanism of 1,3-dipolar cycloaddition. A comprehensive review discussing the chemistry of 1,3-dipoles appeared in 1984 [2]. Numerous significant papers have been published over the last two decades on the reaction of nitrilimines and nitrile oxides with substituted hydrazines, hydrazones and oximes. Different aspects of these reactions will be outlined in this short review.
Nitrilimines and nitrile oxides
Nitrilimines 2, also called nitrile imides, are transient intermediates in solution. The most common method for their generation is dehydrohalogenation of hydrazonoyl halides 1 in the presence of triethylamine [2,3].
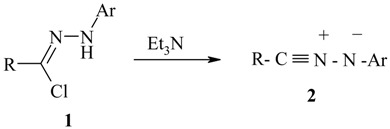 |
Likewise, nitrile oxides 4 are usually generated and trapped in situ; the most common method for their generation is dehydrohalogenation of α-chloro-oximes 3 upon reaction with a base (usually triethylamine). Nitrile oxides dimerize easily and it is usually beneficial to generate them slowly at low temperature, in presence of the trapping agent, so their concentration remains low [2].
 |
Both nitrilimines 2 and nitrile oxides 4 are widely used in the synthesis of different heterocycles. Three modes of reaction were observed for their reaction with different dipolarophiles [3]; namely:
-
i)
Replacement reactions with nucleophiles (Nu) leading to acyclic adducts:

-
ii)Cycloaddition reactions with multiple bonds leading to five-membered heterocycles. The reactions proceed with almost complete stereochemical control and a remarkable regiochemical control in many cases.

-
iii)Cyclocondensation reactions with nucleophiles incorporating suitably located electrophilic centers leading to five- or six-membered heterocyclic rings. An example is the reaction of nitrilimines generated in situ from the respective hydrazonoyl halides 1 with α-amino acid esters 5 leading to 4,5-dihydro-1,2,4-triazin-6-ones 6 [4].

All the above modes of reaction were observed for the reaction of nitrilimines 2 and nitrile oxides 4 with differently substituted hydrazines, hydrazones and oximes.
Reactions of Nitrilimines and Nitrile Oxides with Hydrazines
Reaction with phenyl-, acetyl- and benzoylhydrazines
Nitrilimines undergo 1,3-additions with phenyl-, acetyl- and benzoylhydrazines 7 leading to the acyclic adducts 8a or 8b [5].
 |
The reaction of nitrile oxides with hydrazine hydrate was similarly reported to give hydrazidoximes [6].
 |
Reaction with methylhydrazine
The reaction with methylhydrazine occurs at the N-Me group, rather than the NH2 group, leading to the acyclic adducts 10 [7].
 |
A similar reaction was reported for the reaction of nitrile oxides with methylhydrazine to yield 11 [6,8].
 |
Reaction with methoxycarbonylhydrazine
The reaction of C-acetyl-N-aryl nitrilimines with methoxycarbonylhydrazine (12) afforded the acyclic adduct 1-methoxycarbonyl-2-[1-arylhydrazono-propane-2-one]hydrazine (13). Refluxing compound 13 with charcoal in toluene for six hours gave the oxidized product 3-acetyl-1-methoxy-carbonyl-5-arylformazan (14) in high yield. No other cyclic products were observed [9].
 |
Similarly, nitrile oxides react with methoxycarbonylhydrazine (12) to give the acyclic adducts 15, albeit in poor yield (about 20%).
 |
Reaction with 1-ethoxycarbonyl-1-methylhydrazine
This reaction gave the acyclic product 1-ethoxycarbonyl-1-methyl-2(1-arylhydrazonopropane-2-one)hydrazine (17). Thermal oxidative cyclization of compound 17 gave unexpected products, as these were found to be the s-tetrazines 18 rather than the expected tetrazinones 19. Structural assignment of compounds 16 was based on elemental analysis, mass spectra, 1H- 13C- and 2D-NMR spectral data, including HMQC and HMBC experiments. These compounds exist as a pair of tautomers in solution. This reaction was carried out with C-acetyl [9], C-benzoyl- and C-2-naphthoyl nitrilimines [11].
 |
On the other hand, 1-ethoxycarbonyl-1-methylhydrazine (16) readily reacts with the nitrile oxide generated by the action of triethylamine on benzohydroxamoyl chloride, yielding the acyclic adduct 2‑benzohydroxamoyl-1-ethoxycarbonyl-1-methylhydrazine (20) in moderate yield. The latter cyclizes almost quantitatively to the corresponding novel 4,5-dihydro-,1,2,4,5-oxatriazin-6-one (21) upon stirring with excess sodium hydride in dry tetrahydrofuran for 30 min. at room temperature [11].
 |
Reaction with 1-acetyl- and 1-formyl-1-methylhydrazine
C-acetyl-, C-benzoyl- and C-(2-naphthoyl)nitrilimines react also with 1-acetyl- and 1-formyl-1-methylhydrazines 22 to give the acyclic adducts 23. Thermal cyclization of the latter adducts gave tetrahydro-1,2,4,5-tetrazines 24. Dihydro-1,2,4,5-tetrazines 25 were also obtained upon elimination of formaldehyde from compounds 24 [12].
 |
Reaction of nitrilimines with 1,1-dimethylhydrazine and 1-methyl-1-phenylhydrazine
C-acetyl-, C-benzoyl- and C-(2-naphthoyl)-nitrilimines react with 1,1-dimethylhydrazine and 1-methyl-1-phenylhydrazine 26 giving the amidrazones 27 [12].
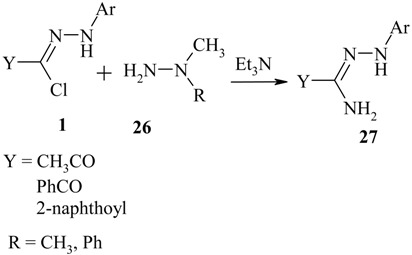 |
Reaction of nitrilimines with 1-ethoxycarbonyl-2-phenylhydrazine
This reaction gave the acyclic adducts 28. Refluxing 28 with charcoal in either toluene or xylene for several hours gave no reaction and the starting material was recovered unchanged [9].
 |
Reaction with ethyl hydrazinoacetate
Reaction of C-methoxycarbonyl nitrilimines with ethylhydrazino acetate (30) under mild conditions gave 4-amino-1-aryl-3-methoxycarbonyl-6-oxo-1,4,5,6-tetrahydro-1,2,4-triazines 31 [13].
 |
On the other hand, the reaction of nitrile oxides with ethyl hydrazine acetate gave the acyclic adducts 32, which resulted from nucleophilic addition through the terminal NH2 followed by oxidation [14].

Reaction with hydrazones
Reaction with substituted simple hydrazones
Simple hydrazones derived from aliphatic aldehydes and ketones 33 reacted with C-acetyl- and C-methoxycarbonyl nitrilimines at ambient temperature to furnish the acyclic products 34. Attempts to cyclize the latter by heating in tetrahydrofuran or ethanol were unsuccessful. However, treatment of solutions of these acyclic adducts with Pd-C at room temperature brought about oxidative cyclization to the orange-red coloured 1,6-dihydro-s-tetrazines 36 [15].
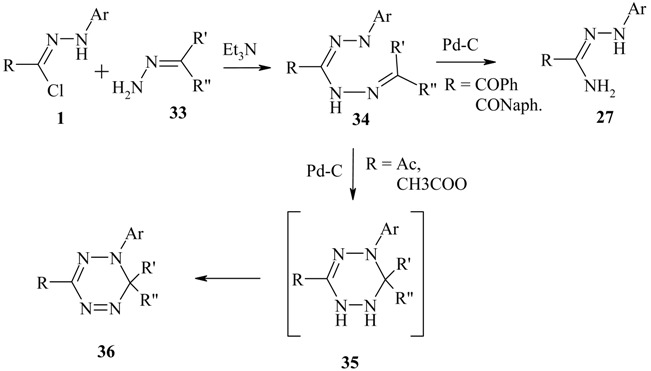 |
Attempts to isolate the tetrahydrotetrazine intermediate 35 were generally unsuccessful. C-benzoyl and C-2-naphthoyl nitrilimines were also found to give acyclic adducts 34. However, these adducts gave a mixture of complicated products upon heating with Pd-C from which amidrazones 27 were separated as the major products.
On the other hand, aryl nitrile oxides undergo 1,3-dipolar cycloaddition with alkanone hydrazones 33 to give the cycloaddition product 4-amino-3-aryl-5,5-dialkyl-4,5-dihydro-1,2,4-oxadiazole (37). The reaction with alkanal hydrazones gave, however, a complex mixture of intractable products, from which the oxadiazole 38 was the major product [17].
 |
Reaction of hydrazones of aryl aldehydes and ketones 39 with nitrile oxides gave, however, the corresponding acyclic adducts 40, formed through nucleophilic addition of the hydrazones to nitrile oxides. 1,3-Dipolar cycloaddition across azomethine л-bond of the hydrazones was not observed here [18].
 |
It is worth mentioning that acylation of the 4-amino-4,5-oxadiazoles 37 in refluxing toluene or treatment of the acyl derivatives of these compounds 41 with trifluoroacetic anhydride brings up ring transformation to the aromatic 1,3,4-oxadiazoles 42 [18].
 |
Similarly, 4-amino-4,5-dihydro-1,2,4-oxadiazole 37 are transformed into the corresponding 2-arylamino-1,3,4-oxadiazoles (44, X = O) or thiadiazoles (44, X = S) via reaction with phenyl-isocyanate or phenylisothiocyanate, followed by brief treatment of the resulting adducts 43 with trifluoroacetic anhydride at ambient temperature [19].
 |
Reaction of nitrilimines and nitrile oxides with substituted methylhydrazones
The interaction between methylhydrazones of aliphatic alkanones and alkanals 45 provided a direct synthetic route to 1,2,3,4-tetrahydro-1,2,4,5-tetrazines 46 [20].
 |
The reaction with methylhydrazones of aromatic aldehydes and ketones 47 gave “ring-chain” tautomerism, where the tautomeric ratio was found to be dependent on the steric and electronic effects of the substituent at C-3, C-6 and N-4 [21].
 |
The reaction of nitrile oxides with methylhydrazones 45 in chloroform was found to constitute a convenient synthetic route to the novel 4,5-dihydro-6H-1,2,4,5-oxatriazines 49 [6].
 |
Risitano and coworkers obtained the triazoles directly from the appropriate monomethylhydrazones of aryl aldehydes in refluxing ether for 2 hours [22].
 |
1,2,3,4-tetrahydro-1,2,4,5-tetrazines 49, derived from methylhydrazones of aryl aldehydes, under-went ring contaction, via elimination of H2O, to yield the respective 1H-1,2,4-triazoles 51. This transformation was envisaged to proceed via the ring-opened (E)-hydrazonoximes which then suffered dehydrative cyclization. The process is acid-catalyzed and was thermally induced. Apparently, the driving force for this transformation is linked to the aromaticity of the triazole product [8].
 |
The reaction of nitrile oxides with 1,1-dimethylhydrazones 52 yields the cycloaddition products, the N,N-dimethylamino-oxadiazolines 53.
 |
Reaction of nitrilimines with hydrazones carrying electron withdrawing groups
Hydrazonoyl halides reacted with alkanone and cycloalkanone alkoxycarbonylhydrazones 54 to give the cycloaddition products 4,5-dihydro-1,2,4-triazoles 55, rather than the tetrazine cyclocondensation products 56 [23].
 |
The 1H-NMR spectra showed a signal at 6.5 – 7.0 ppm characteristic for the N-H of the five membered ring compounds 55. The N-H of the six membered ring structure 56 is expected to appear at 4-5 ppm [1]. Signal doubling is observed both in the 1H- and 13C-NMR spectra of compound 55f containing the 4-methylcyclohexane moiety due to tautomeric isomerism. The 13C-NMR spectra display the characteristic signals of the suggested structures. The signal for C5 (quaternary or spiro carbon) appears in the range of 80 – 90 ppm. This was similar to reported values of quaternary and spiro carbons flanked by two nitrogens in five-membered heterocycles. This provided strong evidence in support of structures 55, rather than the six-membered heterocyclic structure 56, which was expected to have a C6 signal at about 70 ppm.
 |
Similarly, alkanone and cycloalkanone hydrazones 57 carrying electron withdrawing groups (OCOCH3, COCH3, COPh) react with C-benzoyl- and C-2-naphthoyl nitrilimines to give the cycloaddition triazole products 58. IR, 1H-NMR, 13C-NMR and mass spectral data are consistent with the assigned triazole ring system. Compounds 58 having an acetyl group, showed signal doubling in their 13C-NMR spectra, apparently, owing to their presence as two different mesomeric structures [24]. Ferwanah et al. also reported the synthesis of another series of these triazoles 60 from the reaction C-acetyl-N-arylnitrilimines (2) with acetaldehyde, alkanone and cycloalkanone benzoylhydrazones 59. Intersting spiro compounds containing heteroatoms were prepared from this reaction [25].
 |
C-methoxycarbonyl hydrazonoyl halides 2 were also found to react with substituted hydrazones of alkanone, cycloalkanone and heterocyclic ketones 57 to give the cycloaddition products 3-methoxycarbonyl-4,5-dihydro-1,2,4-triazoles 61 [26].
 |
Reaction with Oximes
Acetone oxime 62 readily reacted with nitrilimines yielding the unexpected 3-acetyl-4,5-dihydro-5,5-dimethyl-1H-triazoles 63 in moderate yields. The 4-hydroxytriazoles 64 were not observed. Structural assignment of the resulting triazoles was based on elemental analysis, and spectral data including MS, IR, 1H- and 13C-NMR spectal data. Further evidence was obtained from 15N-NMR spectra, which displays a doublet for the NH at 284.26 ppm relative to nitromethane (1JN-H = 85Hz; 3JN-CH3 = 2.5Hz) [27].
 |
Nitrilimines reacted similarly with acetophenone oxime to afford the corresponding triazaoles 63. Similarly, the reaction with 1-methyl-4-piperidone oxime 65 yields the respective spiro triazoles 66 [28].
 |
The reaction with cycloalkanone oximes 67 gave the heterocyclic spiro triazoles 68 in moderate yields [29].
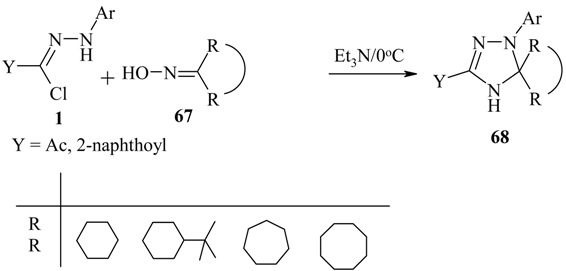 |
Reaction of triazoles 61 with acetyl chloride or acetic anhydride gave the ring transformation product 1-Aryl-3-acetyl-5-methyl-1H-1,2,4-triazole 70 instead of the expected N-acetyl derivative 69. This ring transformation was believed to start with N-acylation of the dihydrotriazole to form the corresponding N-acetyl derivative, which extruded the acetone via a four-membered ring intermediate and recyclizes to the aromatic triazole suggesting the conversion sequence (61) → (69) → (70) [29].
 |
On the other hand, the reaction of nitrile oxides with oximes 62 was reported to give the 4-hydoxy-4,5-dihydro-1,2,4-oxadiazolines 71 [30].
 |
Conclusions
Nitrilimines and nitrile oxides react with hydrazines giving acyclic adducts. The acyclic adducts resulting from the reaction of 1-substituted-1-methylhydrazines with nitrilimines cyclize to give s‑tetrazines, and those resulting from the reaction of nitrile oxides with 1-ethoxycarbonyl-1-methylhydrazine cyclize to oxatriazinones upon stirring with sodium hydride. The reaction of nitrilimines with hydrazones give the acyclic adducts which also cyclize to s-tetrazines. The reaction with nitrile oxides gives, however, the 1,3-dipolar cycloaddition oxadiazole derivatives. s-Tetrazines and oxatriazines were obtained from the reaction of methylhydrazones with nitrilimines and nitrile oxides, respectively. Hydrazones carrying electron withdrawing groups react with nitrilimunes affording the respective cycloaddition triazole products. Ketooximes react with nitrilimines yielding the respective triazoles.
References
- 1.Huisgen R. Angew. Chem. Intern. Ed. Engl. 1963;2:256. [Google Scholar]
- 2.Padwa A., editor. 1,3-Dipolar Cycloaddition. Wiley-Interscience; New York: 1984. [Google Scholar]
- 3.(a) Shawali A. S. Heterocycles. 1983;20:2239. [Google Scholar]; (b) Shawali A. S. Chem Rev. 1993;93:2731. [Google Scholar]
- 4.El-Abadelah M. M., Saleh S. A., Awadallah A. M. Asian J. Chem. 1997;9:474. [Google Scholar]
- 5.Hegarty A. F., Aylwand J. B., Scott F. L. J. Chem. Soc. (C) 1967:2587. [Google Scholar]
- 6.Hussein A. Q., El_Abadelah M.M., Hodali H. H., Kamal M. R., Aouf M. Heterocycles. 1987;26:2199. doi: 10.3987/R-1987-08-2199. [DOI] [Google Scholar]
- 7.Hassaneen H. M., Mousa H. A., Nosrat K. M. Heterocycles. 1988;27:695. [Google Scholar]
- 8.El-Abadelah M.M., Hussein A. Q., Nazer M. Z., Musa O. M., Rademacher P., Bandmann H. Heterocycles. 1993;36:455. [Google Scholar]
- 9.El-Haddad M., Ferwanah A. R. S., Awadallah A. M. J. Prakt. Chem. 1998;340:623. [Google Scholar]
- 10.Awadallah A. M., Ferwanah A. R. S., El-Sawi E. A., Dalloul H. M. Heterocyclic Commun. 2002;8:369. [Google Scholar]
- 11.Ferwanah A. R. S., Awadallah A. M. Asian J. Chem. 1998;10:180. [Google Scholar]
- 12.Ferwanah A. R. S., Awadallah A. M., El-Sawi E. A., Dalloul H. M. Synth. Commun. 2003;33:1245. [Google Scholar]
- 13.El-Abadelah M. M., Nazer M., El-Abadlah N. S., Meier H. J. Prakt. Chem. 1997;339:90. [Google Scholar]
- 14.Awadallah A. M. unpublished data.
- 15.Hussein A. J. Chem. Res (S) 1996;174 J. Chem. Res (M), 1996, 949. [Google Scholar]
- 16.El-Sawi E. A., Awadallah A. M., Ferwanah A. R. S., Dalloul H. M. Asian J. Chem. 2002;14:1225. [Google Scholar]
- 17.El-Abadelah M. M., Hussein A. Q., Awadallah A. M. Heterocycles. 1989;29:1957. [Google Scholar]
- 18.El-Abadelah M. M., Nazer M. Z., Hussein A. Q., Awadallah A. M., Rademacher P., Woydt M. J. Heterocycl. Chem. 1991;28:1229. doi: 10.1002/jhet.5570280513. [DOI] [Google Scholar]
- 19.Hussein A. Q., El-Abadelah M. M., Nazer M. Z., Awadallah A. M., Rademacher P., Bandman H. Heterocycles. 1994;38:981. [Google Scholar]
- 20.El-Abadelah M. M., Hussein A. Q., Kamal M. R., Al-Adhami K. H. Heterocycles. 1988;27:917. [Google Scholar]
- 21.(a) Hussein A. Q., El-Abadelah M. M., Al-Adhami K., Abushamla A. S. Heterocycles. 1988;29:1136. [Google Scholar]; (b) El-Abadelah M. M., Hussein A. Q., Abushamla A. S. J. Prakt. Chem. 1991;333:61. [Google Scholar]; (c) El-Abadelah M. M., Hussein A. Q., Saadeh H. A. Heterocycles. 1991;32:1063. [Google Scholar]; (d) Boese R., El-Abadelah M. M., Hussein A. Q., Abushamleh A. S. Heterocyclic Chem. 1994;31:505. [Google Scholar]
- 22.Risitano F., Grassi G., Fote F. J. Chem Res. (S) 1981;65 J. Chem.. Res. (M), 1981, 831. [Google Scholar]
- 23.Awad B. M., Ferwanah A. R. S., Awadallah A. M., El-Halabi N. M. Asian J. Chem. 2002;14:1235. [Google Scholar]
- 24.Awadallah A. M., El-Sawi E. A., Ferwanah A. R. S., Dalloul H. M. Asian J. Chem. 2002;14:1230. [Google Scholar]
- 25.Ferwanah A. R. S. Synth. Commun. 2003;33:243. [Google Scholar]
- 26.Awadallah A. M., Ferwanah A. R. S., El-Haddad M. R., Schrader W. Asian J. Chem. 2004;16:1691. [Google Scholar]
- 27.Ferwanah A. R. S. Asian J. Chem. 1999;11:480. [Google Scholar]
- 28.Ferwanah A. R. S., Awadallah A. M., Khafaja N. A. Asian J. Chem. 2001;13:1203. [Google Scholar]
- 29.Ferwanah A. R. S., Kandile N. G., Awadallah A. M., Miqdad O. A. Synth. Commun. 2002;32:2017. [Google Scholar]
- 30.Morrocchi S., Ricca A. Chim Ind. (Milan) 1967;49:629. [Google Scholar]


