Abstract
A new diacetyl triterpene lactone, drosericarpone (2), was isolated from the hexane extract of the herb Cleome droserifolia, together with buchariol (1, a sesquiterpene oxide, isolated for the first time from Cleome species) and stigmasterol glucoside (3). The structures of 1-3 were identified by spectroscopic means.
Keywords: Cleome droserifolia, Cleomaceae, buchariol, drosericarpone
INTRODUCTION
Cleome droserifolia (Forssk.) Del. belongs to Family Cleomaceae [1,2]. Cleome species are generally used in folk medicine as stomachics, rubefacients and in the treatment of scabies, rheumatic fever and inflammation [3,4,5,6]. The dried herb of C. droserifolia, locally known as Samwah, Afein, Reeh-El-Bard [7], is used by herbalists in Egypt as a hypoglycemic agent, and its decoction is widely used by the Bedouins of the southern Sinai for the treatment of diabetes [8]. Several studies were carried out to confirm the hypoglycemic effect of the decoction of this herb [8,9,10].
Flavonoids [7,11,12,13,14,15] and sesquiterpenes (carotol and an unidentified one) were isolated from C. droserifolia [22]. To date, several dammarane triterpenes have been isolated from genus Cleome, viz. C. amblyocarpa Barr. Et Murb. [16] (syn. C. arabica auct. Non L. and C. africana Botsch [17,18] and C. brachycarpa Vahl ex. DC (Punwar) [19,20,21], but nothing has been reported on the isolation of dammarane triterpenes from Cleome droserifolia. Therefore, the following study was carried out to isolate and identify the constituents of the hexane extract of the plant.
Results and Discussion:
The hexane fraction of the ethanolic extract of the powdered herb of C. droserifolia afforded three terpenoidal compounds 1-3. The identification of these compounds was accomplished by examination of their spectral data (1H-, 13C-NMR, COSY, HMQC, HMBC and EIMS) and supported by comparison with published data of related compounds [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30].
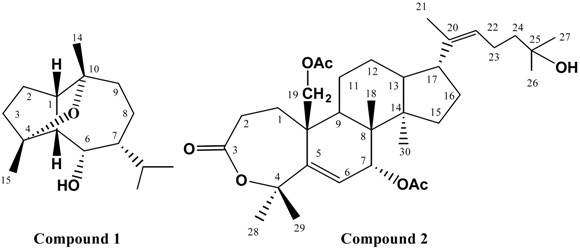 |
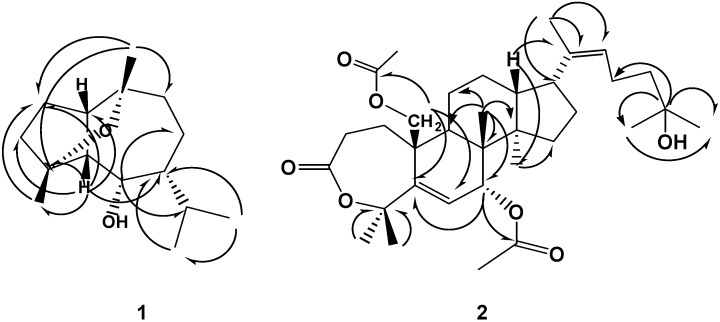
Compound 1, C15H26O2, EIMS, m/z 238 [M]+, was identified as buchariol, previously isolated from the herb Salvia bucharica, by comparing its spectral data (Table 1) with that reported for this compound [24]. The complete assignment of the 13C-NMR data of 1 was accomplished using 2D NMR spectra (HMQC and HMBC) and is reported here for the first time (Table 1 and Figure 1).
Table 1.
1H- and 13C-NMR data of compound 1 and 2
| Compound 1 | Compound 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Position No. |
δc | δH | Position No. |
δc | δH | Position No. |
δc | δH | |
| 1 | 53.3 d | 2.34 m | 1 | 34.6 t |
2.05
m
2.46 m |
16 | 29.3 t |
1.36
m
1.65 m |
|
Assignments were verified using the 2D-NMR (1H-1H COSY, HMQC and HMBC) experiments, and multiplicity was determined by DEPT experiments and J values are given in parenthesis.
Figure 1.
Long range correlations observed in the HMBC spectra of 1 and 2
Compound 2 was isolated as an oily residue and had a molecular formula of C34H52O7 (13C-NMR data and DEPT experiment). Analysis of the 1H- and 13C NMR spectra of 2 with the aid of 2D-NMR spectra (1H-1H COSY, HMQC and HMBC) revealed features characteristic of a triterpene unit containing a 7-membered lactone ring (IR, 1730 cm-1) as compared with related compounds isolated from other Cleome species [20,21]. The 1H-NMR spectrum of 2 showed seven methyl singlet at δH 1.15, 1.26, 1.41, 1.47, 1.53, 1.56, and 2.18; which directly correlated with 13C-NMR signals at δc 18.3, 29.2, 27.2, 23.1, 25.9, 16.2, and 31.5, respectively, an olefinic methyl (δH 2.1), nine methylenes [including one attached to an acetyl group (δH 4.56 d and 4.92 d, δc 61.8)], six methines [including one oxymethine at δc 72.9 (δH 5.15 d) and two olefinic methines at δc 126.8 (δH 4.92 t) and 128.2 (δH 5.22 d)], and ten quaternary carbons [including three carbonyl carbons (δc 177.6, 171.8 and 171.0), two olefinic carbons (δc 135.0 and 136.1), and a carbon bearing OH group at (δc 69.8)]. The spectra also revealed the presence of two acetyl groups (δH 2.0/ δc 22.8 and 2.03/ δc 21.2). HMBC correlations of 2 (Figure1) confirmed the gross structure of 2 to be a diacetyl triterpene lactone. The relative stereochemistry at C-7 was confirmed to be 7β-H [7α-H should appear as singlet or br s near δH 4.7 and H-6 should appear as d (J=1.5 Hz) near δH 5.9 as confirmed by ROESY [25], while H-7 appears at δH 5.15 as d (J=10.5 Hz) and H-6 appears at δH 5.22 as d (J=10.5 Hz)]. The chemical shifts of C-17 and C-21 were found comparable with those of a related compound with 17β-H (C-17 δH 2.61 dd / δc 60.5 and C-21 δH 2.24/ δc 31.2) [26], suggested the β-orientation of H-17, compared with the data of related compounds with 17α-H [25,26,27,28,29]. The stereochemistry of the double bond at C-20 (22) was proposed to be a Z-type; since the signal for C-21 was observed at δc 31.5 (C-21 of the E- type is usually observed near δc 13-15 [25]). From the above data, the structure of 2 was inferred to be as proposed and it was given the name drosericarpone. This compound is reported here for the first time from family Cleomaceae and from nature.
Compound 3, C35H58O6, was obtained as fine colorless needles (ethyl acetate), mp, 284˚C, API-MS (positive ion mode) m /z 613.5 [(M+H)+Na]+. Its structure was identified as stigmasterol glucoside from comparison of its spectral data, (1H- and 13C-NMR) with those previously reported [30].
Experimental
General
M.p. was measured on a Gallekamp melting point apparatus and was uncorrected. 1D-1H-(500MHz) and 13C-(125MHz) NMR spectra were recorded at 25°C using (benzene-d6) as solvent and TMS as internal standard on a JEOL 500 Spectrophotometer. 2D-NMR spectra were obtained on a Bruker Avance DRX 400 Spectrophotometer. EI-MS was obtained on Shimadzu PQ-5000 (70 eV) and Bruker Autoflex (Bruker Daltonics, Germany) mass spectrometers. Atmospheric pressure ionization mass spectra (API-MS) were recorded using a PE SCIEX API III bimolecular mass analyzer. Silica gel 60 (70-230 mesh) and Silica gel RP-8 (both from Merck) were used for column chromatography and silica gel 60 H was employed for VLC technique. Centrifugal accelerated radial TLC was performed on a Chromatotron, model no.7924 (Harrison Research Inc. Palo, Alto, Calif., USA). TLC were conducted on precoated silica gel 60 F254 plates (0.25 mm thickness, Merck), developed with the solvent system MeOH-CHCl3 (5:95). The TLC plates were visualized by spraying with p-anisaldehyde reagent followed by heating at 110°C.
Plant material
Plant material was collected from the Suez-Cairo desert road, Egypt, in March 2002 and was kindly identified by Dr. M. Gebali (Plant Taxonomy and Egyptian Flora Department, National Research Center, Giza, Egypt). A voucher specimen has been deposited in the herbarium of the Faculty of Pharmacy, Cairo University.
Isolation
The air-dried powdered herb of C. droserifolia (600 g) was extracted with 70% ethanol. The residue left after distillation of the solvent (75 g), was successively fractionated with hexane, chloroform and methanol. The hexane extract (3.2 g.) was chromatographed on a VLC column (14 cm L x 4 cm D) of silica gel H (50g), eluted with hexane, CHCl3, EtOAc and MeOH, in increasing proportions untill 5 % MeOH-EtOAc, in fractions, each of 200 mL. Fraction A: 980 mg, eluted with CHCl3, showed a major violet spot with the spray reagent, Rf = 0.75. This fraction was further purified by CC (18 cm L x 3 cm D) on silica 60 eluted with 5% MeOH-CHCl3, in fractions, each of 5 mL, which gave: Fraction A-1 (combined frs.7-12, 760 mg) was further purified twice on a Chromatotron, eluted with 4% MeOH/CHCl3, in fractions of 2 mL each, to give compound 1 (50 mg, oily residue, Rf = 0.35). Fraction A-2 (frs. 40-66, 50 mg) was further purified twice on a SiO2 CC, eluted with benzene, CHCl3 → 20% MeOH-CHCl3, to gave a fraction, which was purified on CC/RP-18 (eluted with water/ MeOH) to give compound 2 (10 mg, oily residue, Rf =0.52). Fraction B (290 mg, eluted with EtOAc) was further purified on a Chromatotron, eluted with 8% MeOH-CHCl3, in fractions, each of 2 mL, to give compound 3 (14 mg, fine colorless needles, Rf = 0.6).
Buchariol (1), oily residue, C15H26 O2, [24]; EI -MS (m /z ): 238 ( M+, base peak) , 220 ( M+ - 18), 195 ( M+-43, C3H7) , 177 (M+- H2O - C3H7), 159 (M+- 2H2O - C3H7), 141, 81. 1H- and 13C-NMR spectral data (CDCl3) see Table 1.
Drosericarpone (2), oily residue, C34H52O7; EI -MS (m /z): 446 [(M+H) - C8H15O] +, 388 [446 – Me2CO]+, 328 (388 – CH3COOH), 286, 268 (388 – 2 x CH3COOH), 225, 135, 127, 121; IR νmax (KBr) cm-1: 3440 (OH), 1720, 1730 (carbonyl); 1H- and 13C-NMR spectral data, see Table 1.
Stigmasterol glucoside (3), fine colorless needles (from ethyl acetate), mp. 284 ˚C; C35H58O6; API-MS (positive ion mode) m /z 613.5 {( M+H)+ Na}+, 569.5 {( M+H)+ Na – C3H8, 44}+, 525.5 {(M+H)+ Na – 2 C3H8}+, 481.5 {( M+H)+ Na– 3 C3H8}+, 413.5 {( M+H) - 162}+, 393.5 ( base peak ), 349.5, 243, 295.5, 245.5, 133; IR υmax (KBr) cm-1: 3400 (OH), 2960-2850, 1640, 1465, 1380; 1H-NMR (500 MHz, C6D6): δ 5.35 (1H, t, J = 4.7 & 1.7 Hz, H-6), 5.21 (1H, dd, J = 15.2 & 8.8 Hz, H-22), 5.05 (1H, dd, J = 15.2 & 8.8 Hz, H-23), 4.89 (1H, d, J = 7.9 Hz, H-1`), 4.42 (1H, dd, J = 2.4 & 11.7 Hz, H-6`a ), 4.23 (1H, dd, J = 5.3 & 11.7 Hz, H-6`b ), 4.12 (1H, m, H-3`), 4.08 (1H, m, H-4`) 3.89 (1H, t, J = 7.9 & 8.8 Hz, H-2`), 3.84 (1H, m, H-5`), 2.62 (1H, dd, J = 2.6 & 12.3 Hz), 2.37 (1H, t, J = 11.3 & 11.5 Hz, H-7a ), 2.0 (1H, m, H-8), 1.9 (1H, m, H-7b), 0.98 (3H, d, J = 6.4 Hz, Me-21), 0.92 (3H, s, Me- 19), 0.88 (3H, d, J = 6.8 Hz, Me-26), 0.86 (3H, d, J = 6.8 Hz, Me-27), 0.81 (3H, t, J = 7 Hz, Me-29), 0.67 (3H, s, Me-18); 13C-NMR (125 MHz, C6D6): δc 37.4 (C-1 , t) 28.5 (C-2 , t), 78.1 (C-3, d), 39.2 (C-4, t), 140.8 (C-5, s), 121.9 (C-6, d), 32.1 (C-7, t), 32.0 (C-8, d), 50.3 (C-9, d), 36.9 (C-10, s), 21.2 (C-11, t), 39.9 (C-12, t), 42.5 (C-13, s), 56.8 (C-14, d), 24.5 (C-15, t), 29.4 (C-16, t), 56.2 (C-17, d), 12.0 (C-18, q), 19.4 (C-19, q), 36.4 (C-20, d), 19.0 (C-21, q), 137.3 (C-22, d), 128.3 (C-23, d), 46.0 (C-24, d), 26.3 (C-25, d), 20.0 (C-26, q), 19.2 (C-27, q), 29.9 (C-28, t),12.2 (C-29, q), sugar carbons, 102.4 (C-1`, d), 75.0 (C-2`, d), 78.2 (C-3`, d), 71.5 (C-4`, d), 78.1 (C-5`, d), 62.7 (C-6`, t).
Acknowledgments
I am grateful to Prof. Dr. Meselhy R. Meselhy (Faculty of Pharmacy, Cairo University, Egypt) for NMR measurements and for valuable discussions. Also, to Prof. Dr. Essam Abdel Sattar (Faculty of Pharmacy, Cairo University, Egypt) and Prof. Dr. Ahmed A. Ahmed (Department of Chemistry, El-Minia University, El-Minia, Egypt) for their valuable comments on the spectral data analysis.
Footnotes
Sample Availability: Contact the author.
References
- 1.Täckholm V. Students` Flora of Egypt. 2nd Ed. Cairo University Press; Cairo, Egypt: 1974. p. 167. [Google Scholar]
- 2.Zohary M. Flora Palaestina. vol. I. The Israel Academy of Sciences and Humanities; Jerusalem: 1966. p. 245. [Google Scholar]
- 3.Boulos L. Medicinal Plants of North Africa. Reference Publication Inc.; Michigan, USA: 1983. p. 52. [Google Scholar]
- 4.Boulos L. Flora of Egypt. Al Hadara Publishing; Cairo, Egypt: 1999. p. 177. [Google Scholar]
- 5.Ghazanfar S.A. Handbook of Arabian Medicinal Plants. CRC Press; Boca Raton-London-Tokyo: 1994. p. 76. [Google Scholar]
- 6.Chopra R.N., Chopra I.C., Handa K.L., Kapur L.D. Chopra`s Indigenous Drugs of India. Dhur & Sons, Private Limited; Calcutta, India: 1972. pp. 321, 669. [Google Scholar]
- 7.Yang S.S., Mabry T.J., El-Fishawy A.M., El-Kashoury E.A., Abdel-Kawy M.A., Soliman F.M. Flavonoids of Cleome droserifolia (Forssk.) Del. Egypt. J. Pharm. Sci. 1990;31:443. [Google Scholar]
- 8.Abdel-Hady N.M. MS Thesis. Faculty of Pharmacy (Girls), Al-Azhar University; Cairo: 1998. Pharmacognostical Investigation and Biological Verification of Some Recipes and Preparations of Natural Origin for the Treatment of Diabetes. [Google Scholar]
- 9.Yaniv Z., Dafni A., Friedman J., Palevitch D. Plants used for the treatment of Diabetes in Israel. J. Ethnopharmacol. 1987;19:145. doi: 10.1016/0378-8741(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 10.Nocola W.G., Ibrahim K.M., Mikhail T.H., Girgis R.B., Khadr M.E. Role of the hypoglycemic plant extract Cleome droserifolia in improving glucose and lipid metabolism and its relation to insulin resistance in fatty liver. Boll. Chim. Farm. 1996;135:507. [PubMed] [Google Scholar]
- 11.Seif El-Din A.A., Darwish F.A., Abou-Donia A.A. Flavonoids from Cleome droserifolia (Forssk.) Del. Growing in Egypt. Egypt. J. Pharm. Sci. 1987;28:313. [Google Scholar]
- 12.Seif El-Nasr M.M., Youssef M.M., Helmy M. Flavonoids of Cleome droserifolia (Forssk.) Del. Fitoterapia. 1984;55:231. [Google Scholar]
- 13.Abdel-Kawy M.A., El-Deib S., El-Khyat Z., Mikhail Y.A. Chemical and biological studies of Cleome droserifolia (Forssk.) Del. Part-I. Egypt. J. Biomed. Sci. 2000;6:204. [Google Scholar]
- 14.Fushiya S., Kishi Y., Hattori K., Batkhuu J., Takano F., Singab A.N.B., Okuyama T. Flavonoids from Cleome droserifolia Suppress NO Production in Activated Macrophages in Vitro. Planta Med. 1999;65:404. doi: 10.1055/s-1999-14084. [DOI] [PubMed] [Google Scholar]
- 15.Diab L. MS Thesis. Faculty of Pharmacy (Boys) Al-Azhar University; Cairo, Egypt: 1992. Pharmacognostical Study of Certain Cleome Species Growing in Egypt. [Google Scholar]
- 16.Harraz F.M., Ulubelen A., Osuz S., Tan N. Dammarane triterpenes from Cleome amblyocarpa. Phytochemistry. 1995;39:175. [Google Scholar]
- 17.Nagaya H., Tobita Y., Nagae T., Itokawa H., Takeya K., Halim A.F., Abdel-Halim O.B. Cytotoxic triterpenes from Cleome africana. Phytochemistry. 1997;44:1115. doi: 10.1016/s0031-9422(96)00681-4. [DOI] [PubMed] [Google Scholar]
- 18.Tsichritzis F., Abdel-Mogip M., Jakupovic J. Dammarane triterpenes from Cleome africana. Phytochemistry. 1993;33:423. doi: 10.1016/0031-9422(93)85532-V. [DOI] [Google Scholar]
- 19.Ahmad V.U., Qazi S., Bin Zia N., Xu C., Clardy J. Cleocarpone, A triterpenoid from Cleome brachycarpa. Phytochemistry. 1990;29:670. [Google Scholar]
- 20.Ahmad V.U., Alvi K.A. Deacetoxybrachycarpone, A trinortriterpenoid from Cleome brachycarpa. Phytochemistry. 1987;26:315. [Google Scholar]
- 21.Ahmad V.U., Alvi K.A. The Molecular Structure and Absolute Configuration of Brachycarpone, A new trinortriterpenoid dilactone from Cleome brachycarpa. J. Nat. Prod. 1986;49:249. [Google Scholar]
- 22.Hussein N.S., Ahmed A.A., Darwish F.M. Sesquiterpenes from Cleome droserifolia. Pharmazie. 1994;49:76. [Google Scholar]
- 23.Jente R., Jakupovic J., Olatunji G.A. A Cembranoid Diterpene from Cleome viscosa. Phytochemistry. 1990;29:666. [Google Scholar]
- 24.Ahmad VU., Zahid M., Ali M.S., Jassbi A.R., Abbas M., Ali Z., Iqbal M.Z. Bucharioside and buchariol from Salvia bucharica. Phytochemistry. 1999;52:1319. doi: 10.1016/s0031-9422(99)00389-1. [DOI] [PubMed] [Google Scholar]
- 25.Dou D., Chen Y., Liang L., Pang F., Shimizu N., Takeda T. Six new dammarane-type trirepene saponins from the leaves of Panax ginseng. Chem. Pharm. Bull. 2001;49:442. doi: 10.1248/cpb.49.442. [DOI] [PubMed] [Google Scholar]
- 26.El-Askary H. Pregnene glycoside and monoterpene derivative from Solenostemma argel Hayne. Bull. Fac. Pharm. Cairo Univ. 2003;41:131. [Google Scholar]
- 27.Zou K., Zhu S., Meselhy R., Tohda C., Cai S., Komatsu K. Dammarane-type saponins from Panax japonicus and their neurite outgrowth activity in SK-N-SH cells. J. Nat. Prod. 2002;65:1288. doi: 10.1021/np0201117. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura N., Kojima S., Aura Y., Meselhy R., Hattori M., Gupta M., Correa M. Dammarane-type triterpenes from Cordia spinescens. Phytochemistry. 1997;46:1139. doi: 10.1016/S0031-9422(97)00407-X. [DOI] [Google Scholar]
- 29.Rouf A.S., Ozaki Y., Rashid M.A., Rui J. Dammarane derivatives from the dried fruits of Forsythia suspensa. Phytochemistry. 2001;56:815. doi: 10.1016/s0031-9422(01)00028-0. [DOI] [PubMed] [Google Scholar]
- 30.Goad L.J., Akihisa T. Analysis of Sterols. Blackie Academic & Professional; Glasgow, UK: 1997. p. 380. [Google Scholar]