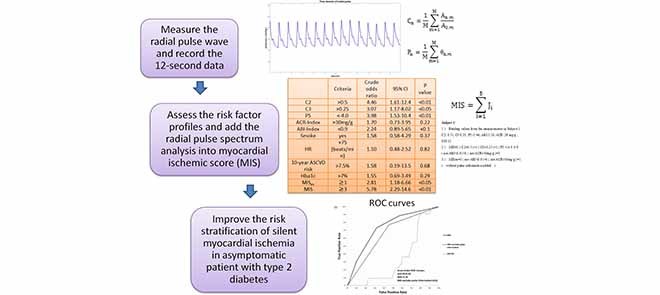
Abstract
Diabetic patients with silent myocardial ischemia (SMI) have elevated rates of morbidity and mortality and need intensive care and monitoring. An early predictor of SMI may lead to early diagnosis and medical treatment to prevent progression and adverse clinical events. Therefore, this paper was aimed to evaluate the radial pulse spectrum as risk markers to improve the risk stratification of SMI in type-2 diabetic patients; 195 diabetic patients at high-risk of SMI were enrolled. All patients underwent myocardial perfusion imaging and radial pressure wave measurement. The spectrum analysis of the radial pressure wave was calculated and transformed into Fourier series coefficients Cns and Pns. The risk of SMI (odds ratio: 4.46, 95%, C.I. 1.61–12.4, 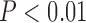 ) was raised in diabetic patients classified high-risk group by C2. Multivariable regression analysis showed that C2 (
) was raised in diabetic patients classified high-risk group by C2. Multivariable regression analysis showed that C2 (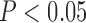 ) and ankle–brachial index [(ABI)
) and ankle–brachial index [(ABI) 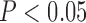 )] were related to SMI (
)] were related to SMI (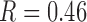 and
and 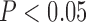 ). The myocardial ischemic score (MIS), combining C2, C3, and P5, the albumin-to-creatinine ratio (ACR), and ABI, presented an excellent risk stratification performance in enrolled patients (odds ratio: 5.78, 95%, C.I. 2.29–14.6,
). The myocardial ischemic score (MIS), combining C2, C3, and P5, the albumin-to-creatinine ratio (ACR), and ABI, presented an excellent risk stratification performance in enrolled patients (odds ratio: 5.78, 95%, C.I. 2.29–14.6, 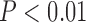 ). The area under receiver operating characteristic curves for C2, C3, P5, ABI, ACR, and MIS were 0.66, 0.60, 0.68, 0.51, 0.56, and 0.74, respectively, in identifying SMI. This paper demonstrated that C2 was independently associated with the extent of SMI in multivariable regression analysis. Odds ratio and chi-square tests reflected that C2 could be an important marker for the risk stratification of SMI. Furthermore, MIS, adding radial pulse spectrum analysis to ACR and ABI, could significantly improve the risk stratification of SMI in type-2 diabetic patients compared to any single risk factor.
). The area under receiver operating characteristic curves for C2, C3, P5, ABI, ACR, and MIS were 0.66, 0.60, 0.68, 0.51, 0.56, and 0.74, respectively, in identifying SMI. This paper demonstrated that C2 was independently associated with the extent of SMI in multivariable regression analysis. Odds ratio and chi-square tests reflected that C2 could be an important marker for the risk stratification of SMI. Furthermore, MIS, adding radial pulse spectrum analysis to ACR and ABI, could significantly improve the risk stratification of SMI in type-2 diabetic patients compared to any single risk factor.
Keywords: Radial pulse spectrum, harmonic analysis, silent myocardial ischemia, pulse wave analysis
This study aimed to evaluate the radial pulse spectrum as risk markers to improve the risk stratification of silent myocardial ischemia in type-2 diabetic patients. This report demonstrated that second harmonic of radial pulse could be an important marker for silent myocardial ischemia. Furthermore, MIS score, adding radial pulse spectrum analysis to albumin-to-creatinine ratio and ankle-brachial index, could significantly improve the detection of silent myocardial ischemia in type 2 diabetic patients.
I. Introduction
Silent Myocardial Ischemia (SMI) is an issue of public health that leads to heart attack and significantly influences the mortality rate in patients with type 2 diabetes. SMI is myocardial ischemia without chest discomfort and other angina symptoms. The incidence rate of SMI in diabetic patients was 2.2 times higher than the incidence rate of SMI in non-diabetic patients [1]. SMI had been investigated and confirmed with a 6%–23% prevalence in diabetic patients using Myocardial Perfusion Imaging (MPI) and invasive coronary angiography [2], [3]. SMI can lead to acute myocardial infarction, adverse cardiac events, and poor prognosis outcomes, that are severe in diabetic care [4]–[7]. Therefore, it is quite important to have an early predictor of SMI that can feasibly screen diabetic patients and give a risk stratification of heart ischemia and may prevent many diabetic patients from sudden cardiac death or adverse cardiovascular events.
Holter devices, the ambulatory electrocardiography (ECG), has proven to be a useful tool to detect patients at high risk of SMI [8]–[10]. However, Holter devices need to monitor the patients over the course of 24 hours or even up to 72 hours to detect abnormal electrical signals from the electrocardiogram. This long-term measurement makes the Holter less effective for screening the whole population of high cardiovascular risk group such as diabetic groups. Besides the ambulatory ECG, low ankle-brachial index (ABI) [11] and microalbuminuria, the albumin-to-creatinine ratio (ACR) between 30mg/g to 300mg/g, have also been investigated in the detection of SMI [12]–[14].
Another potential method of detecting SMI is using arterial pulse spectrum analysis. The spectrum of arterial pulse wave reflects the loading condition of the arterial system, which has been investigated [15], modeled [16], applied [17], and interpreted [18] in many clinical studies. According to Lin’s model [16], radial pulse spectrum analysis can reveal the arterial-ventricular function by its harmonics change [16], [18]. Chen et al. [19] validated this concept and proved that the specific characteristic of radial pulse spectrum changed from the resting state to the onset of acute, uncomplicated myocardial infarction state, and gradually shifted to other resting characteristics a week after surgery. Furthermore, the cross-sectional study showed that the harmonics of the radial pulse spectrum were correlated with the ischemic heart disease [20], [21]. To summarize results from those studies, the ventricular-arterial coupling system distributed the pressure pulse wave to different organs in proportions of harmonics according to the system state [16], [22]. Therefore, the pattern of harmonic components could reveal the blood flow condition of organs [17], [23], and more specifically, reveal the condition of myocardial perfusion.
However, there is still a lack of direct statistic evidence quantifying the correlation between harmonics of the radial pulse wave and myocardial perfusion, and validating whether the harmonics of the pulse spectrum contains the information in identifying SMI. Hence, the objective of this study was to statistically validate the degree of confidence that the harmonics of radial pulse spectrum and myocardial perfusion were correlated, using receiver operating characteristic curve (ROC) and multivariable linear regression. This report chose type 2 diabetic patients because of their high-risk prevalence for SMI. We included the patients without any angina pectoris history, at high risk of SMI, and suitable for performing MPI. We further investigated the relationship between SMI and different risk factors. In the end, this report analyzed the different risk factor profiles to propose an effective and efficient method for early SMI diagnosis.
II. Methods
A. Patients
The study population consisted of 195 type 2 diabetic patients without any angina pectoris history, aged 63±11 years, selected from patients who visited the Division of Endocrinology & Metabolism of Zhongxiao Branch of Taipei City Hospital. The enrolled group was investigated between February 2017 and November 2017. Both oral and written information about the study was given to the patients. Informed consent was obtained from all patients after receiving approval from the Institutional Review Board of Taipei City Hospital (IRB number: ISRCTN14306167).
Patients were included if they meet one of the following criteria:
-
1.
The patients with two or more risk factors present [24], [25].
The risk factors includes (i) lipid disorders (total cholesterol240mg/dl, low-density lipoprotein (LDL) 160mg/dl, or high-density lipoprotein (HDL)35 mg/dl), (ii) Blood pressure >140/90 mmHg, (iii) Family history of premature coronary heart disease, (iv) age 70 years, and (v) newly discovered resting ECG abnormalities).
-
2.
The patients had ABI of less than 0.9 on one side or ABI between the range of 0.9 and 1 on both sides.
-
3.
The patients had macroalbuminuria (ACR 300 mg/g) or microalbuminuria (30mg/g< ACR<300mg/g).
Patients with the end-stage renal disease, with angina related symptoms, or with a history of coronary disease were excluded.
B. Study Protocol
This study aimed to evaluate the relationship between the radial pressure waveform and SMI. At first, semi-quantitative 20-segment analysis of myocardial perfusion single-photon emission computed tomography (GE Infinia, USA) was applied to all enrolled patients. The analysis of MPI yielded a summed stress score (SSS), which was negatively correlated with myocardial perfusion condition [26]. ABI was then carried out for each enrolled patient using a vascular screening system (VaSera VS-1500N, Japan).
After the ABI assessment was completed, the measurement of the radial pressure wave and harmonic analysis were performed. Each subject was asked to lie down in a supine position and rest for 5 minutes before the measurement. The pulse pressure was then recorded on the left-hand radial artery with a pulse wave analyzer TD01C (MII-ANN Technology, Taiwan), which has proven its intrinsic reliability using the artificial pulse generator [27]. The intra-observer and inter-observer reliabilities of TD01C were also demonstrated in the clinical study [28]. The consecutive pressure pulses were obtained during a 12-second period. The pressure pulse data were collected with a sampling rate of 400 data points per second. These data were then converted into harmonic amplitude components (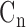 ) and harmonic phases (
) and harmonic phases (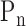 ) using Fourier series calculation [29], [30].
) using Fourier series calculation [29], [30]. 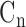 and
and 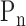 are defined by the following equation:
are defined by the following equation:
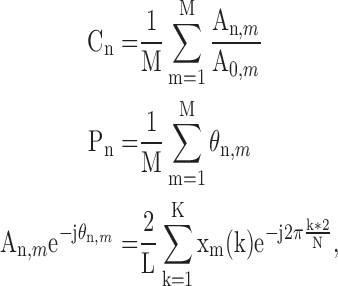 |
where 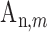 and
and 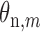 are the amplitude and phase of the
are the amplitude and phase of the 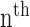 harmonic of the
harmonic of the 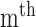 radial pulse within one measurement.
radial pulse within one measurement. 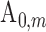 is the mean value of the
is the mean value of the 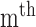 radial pulse.
radial pulse. 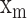 (k) is the
(k) is the 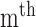 pulse signal, and n is the
pulse signal, and n is the 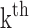 discrete sampling data point in the
discrete sampling data point in the 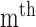 pulse signal. K is the number of data points within
pulse signal. K is the number of data points within 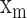 (k), and M is the total number of pulses within one measurement. [30]
(k), and M is the total number of pulses within one measurement. [30]
Second, we analyzed markers of medical records such as age, body mass index, blood test, electrocardiogram, urine examination, ABI, and harmonics of the radial wave, which we considered important characteristics of the enrolled patients. The ten-year Atherosclerotic Cardiovascular Disease (ASCVD) risk score was calculated by the “ASCVD Risk Estimator Plus” using the website from the American College of Cardiology. The ACR-Index was defined as 1 for ACR>30 mg/g (microalbuminuria), and 0 for others; ABI-Index was defined 1 for ABI<0.9, and 0 for others.
We enrolled the patients without angina pectoris and divided those patients into two groups: Group 1 included the patients with non-SMI to mild SMI according to the stress MPI (SSS:0~8, < 10% ischemic myocardium, and without angina pectoris). Group 2 included patients with moderate SMI to severe SMI (SSS9, 11% ischemic myocardium, and without angina pectoris). We compared the risk factors between Group 1 and Group 2 to evaluate the feasibility of identifying patients with moderate to severe SMI from combined groups of non-angina patients with type 2 diabetes.
C. Statistics
At first, we assessed the association between the risk variables and SMI through the baseline characteristics. Variables from medical records and from the spectrum analysis of the radial pressure wave were described as mean±SD (Table 1) both in Group 1 and Group 2. We compared the means of those functional variables of the two groups using the Student’s t-test or chi test. Second, the harmonics of the radial pressure wave were used for the chi-squared test to examine the independent association between the harmonics and SMI. (Table 2)
TABLE 1. Clinical Characteristics of the 195 Diabetic Patients at High Risk of SMI and With No Chest Discomfort or Angina Symptoms Enrolled in the Study, Divided Into Group 1 and Group 2 According to the Silent Ischemic Myocardium (11% Ischemic Myocardium) or Not.
| Group 1 (SSS:0~8, <10 % ischemic myocardium) | Group 2 (SSS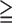 9, 9, 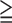 11% ischemic myocardium) 11% ischemic myocardium) |
|
|---|---|---|
| N | 169 | 26 |
| Male (%) | 50.3 | 73.1** |
| Age (year) | 64±11 | 61±12* |
| BMI (kg/m2) | 28.0±4.8 | 27.9±4.3 |
| SBP (mmHg) | 129±12 | 129±14 |
| DBP (mmHg) | 75±7 | 75±10 |
| Ischemicmyocardium (%) | 3.1±2.8 | 16.7±8.2*** |
| HbA1C (%) | 7.1±1.2 | 7.5±1.4** |
| LDL (mg/dl) | 86±32 | 74±25** |
| HDL (mg/dl) | 52±17 | 47±10 |
| TG (mg/dl) | 138±84 | 137±73 |
| Duration of diabetes (years) | 12±7 | 13±7 |
| HR (beats/min) | 73±11 | 78±13** |
| C2 | 0.51±0.12 | 0.58±0.14*** |
| C3 | 0.24±0.08 | 0.28±0.10** |
| P5 | −4.04±1.28 | −4.48±0.70** |
| 10-year ASCVD risk | 27.0±20.7 | 17.1±8.1* |
| ACR>30 mg/g (%) | 30.2 | 42.3 |
| ABI<0.9 (%) | 16.6 | 30.8* |
| Smokers (%) | 16.0 | 23.1 |
| Hypertension (%) | 52.7 | 46.2 |
| Polyneuropathy (%) | 6.5 | 15.4 |
SBP=Systolic blood pressure, DBP=Diastolic blood pressure, LDL=low-density lipoprotein cholesterol, HDL=high-density lipoprotein cholesterol, TG=triglycerides, HR=heart rate, ASCVD=Atherosclerotic Cardiovascular Disease. Single, double, and triple asterisks indicate that the means of variables in Group 2differ significantly (p < 0.1, p < 0.05, and p <0.01 respectively) from Group 1using the Student’s t-test or chi-square test for appropriate use.
TABLE 2. Chi-Square Test for Second Harmonic (C2) of the Radial Pressure Wave and SMI (N=195).
Group 2 (SSS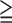 9, 9, 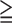 11% ischemic myocardium) 11% ischemic myocardium) |
Group 1 (SSS:0~8, <10 % ischemic myocardium) | Row sum | |
|---|---|---|---|
| C2>0.50 | 21 | 82 | 103 |
| C2<0.50 | 5 | 87 | 92 |
| Column sum | 26 | 169 | 195 (total) |
The chi-square statistic is 9.40. P-value<0.01.
The odds ratios of risk variables in identifying moderate to severe SMI were assessed. The receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to test the capability of harmonics and other markers to identify the patients with moderate to severe SMI diagnosed by the stress MPI. The significant difference of area under the curve of ROC between two risk markers was tested by the DeLong test. [31]
A multivariable linear regression model was performed to assess the relationship between all risk factors and SMI. All the statistical analyses were performed using the Matlab software (MathWorks Inc, USA).
D. Risk Factor Profiles and Myocardial Ischemic Score (MIS)
Out of all risk indicators, we chose five risk factors with higher odds ratios in identifying moderate to severe SMI (Table 3). These factors are ACR, ABI, C2, C3, and P5. We defined the myocardial ischemic score (MIS) as the sum of the votes of the five risk factors mentioned above, with equal weighting. The MIS was defined as:
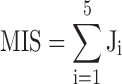 |
where 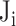 is the vote from the ith risk factor (
is the vote from the ith risk factor (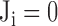 if the ith risk factors determined a low-risk group of myocardial ischemia;
if the ith risk factors determined a low-risk group of myocardial ischemia; 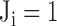 if the ith risk factors determined a high-risk group of myocardial ischemia, according to the criteria of risk factors.) If criteria “MIS3” is reached, we defined this as a patient with positive test result (at high risk of moderate to severe SMI)
if the ith risk factors determined a high-risk group of myocardial ischemia, according to the criteria of risk factors.) If criteria “MIS3” is reached, we defined this as a patient with positive test result (at high risk of moderate to severe SMI)
TABLE 3. The Odds Ratio of Risk Markers (N=195) for Predicting Moderate to Severe SMI (SSS9) in Type 2 Diabetic Patients Without Any Angina Symptoms.
| Criteria | Crude odds ratio | 95% CI | P value | |
|---|---|---|---|---|
| C2 | >0.5 | 4.46 | 1.61–12.4 | <0.01 |
| C3 | >0.25 | 3.07 | 1.17–8.02 | <0.05 |
| P5 | <−4.0 | 3.98 | 1.53–10.4 | <0.01 |
| ACR-Index | >30mg/g | 1.70 | 0.73–3.95 | 0.22 |
| ABI-Index | <0.9 | 2.24 | 0.89–5.65 | <0.1 |
| Smoke | yes | 1.58 | 0.58–4.29 | 0.37 |
| HR | >75 (beats/min) | 1.10 | 0.48–2.52 | 0.82 |
| 10-year ASCVD risk | >7.5% | 1.58 | 0.19–13.5 | 0.68 |
| Hba1c | >7% | 1.55 | 0.69–3.49 | 0.29 |
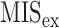 |
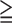 1 1 |
2.81 | 1.18–6.66 | <0.05 |
| MIS |
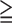 3 3 |
5.78 | 2.29–14.6 | <0.01 |
HR=heart rate, 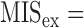 MIS excludes the pulse information
MIS excludes the pulse information
The five criteria for the high-risk group stratified by 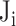 were:
were:
-
1)
ACR-Index vote: 1 for ACR>30 mg/g, and 0 for others
-
2)
ABI-Index vote: 1 for ABI<0.9, and 0 for others
-
3)
C2 vote: 1 for >0.5, and 0 for others
-
4)
C3 vote: 1 for >0.25, and 0 for others
-
5)
P5 vote: 1 for <−4.0, and 0 for others
Taking two subjects as examples to calculate MIS and MISex (myocardial ischemic score excluding pulse information):
-
Subject 1:
-
1)Reading values from the measurements in Subject 1:C2: 0.51, C3:0.31, P5:−5.46, ABI:1.10, ACR: 28 mg/g, SSS:11
-
2)MIS=1 (C2>0.5)+1 (C3>0.25)+1 (P5 <−4.0)+0 (not ABI<0.9)+0 (not ACR>30mg/g)=3
-
3)MISex=0 (not ABI<0.9)+0 (not ACR > 30mg/g)=0 (without pulse information added)
-
1)
-
Subject 2:
-
1)Reading values from the measurements in Subject 2:C2: 0.39, C3:0.18, P5:−3.72, ABI:0.86, ACR: 9 mg/g, SSS:2
-
2)MIS = 0 (not C2>0.5)+0 (not C3>0.25)+0 (not P5<−4.0)+1 (ABI<0.9)+0 (not ACR>30mg/g) = 1
-
3)MISex = 1 (ABI<0.9)+0 (not ACR>30mg/g) = 1 (without pulse information added)
-
1)
III. Results
We explored the relationship between risk variables and SMI (SSS>=9) in type 2 diabetic patients without any symptoms of angina pectoris. The baseline characteristics of Group 1 and Group 2 were shown in Table 1. Both groups of enrolled patients had similar population means of systolic pressure, diastolic pressure, HDL, and triglyceride (TG), which t-test showed non-significant differences in mean variables between the two groups. In contrast, the results showed that Group 2 has higher ischemic myocardium, slightly lower age (P<0.1), lower LDL (P<0.0.5),higher glycated hemoglobin (Hba1c, P<0.05), and mildly higher heart rate (P<0.01) comparing to Group 1. The results also showed that Group 2 also had a higher prevalence of peripheral artery disease, microalbuminuria, and polyneuropathy comparing to the Group 1.
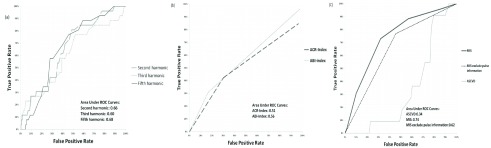
The means of C2, C3, and P5 of arterial pulse were significantly different in Group 2 (C2:0.58±0.14; C3:0.28±0.10; P5:−4.48±0.70) than in Group 1 (C2:0.51±0.12, P<0.01; C3:0.24±0.08, P<0.05; P5:−4.04±1.28, P<0.05, respectively). Table 2 showed that C2 was associated with the degree of severity of SMI using the chi-squared test (C2: P <0.01). Table 3 revealed that the risks of SMI in patients with type 2 diabetes were higher with the raised C2 value (odds ratio: 4.46, 95% C.I. 1.61–12.4, P<0.01). Furthermore, the ROC curves of C2, C3, and P5 were 0.66, 0.60, and 0.68 respectively, implying the potential of those harmonics in identifying patients with SMI (Figure 1). Multivariable regression analysis also showed that C2 (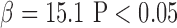 ) and ABI (
) and ABI (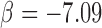 , P<0.05) had the independent effects on SMI (R=0.46, P<0.05).
, P<0.05) had the independent effects on SMI (R=0.46, P<0.05).
FIGURE 1.
ROC curves for model 2(N=195)with (a) first(solid line), second(dashed line), and third(dotted line) harmonic amplitude of radial pressure wave, with (b) Hba1c(solid line), ACR-Index(dashed line), and ABI-Index(dotted line), and with (c)myocardial ischemic score(MIS, solid line) to predict patients with more than 11% silent ischemic myocardium determined by the stress MPI (SSS9).
In addition, we built up the MIS score to combine the five risk markers including C2, C3, P5, ACR-Index, and ABI-Index, to evaluate the risk of SMI. The ROC curve showed that MIS could further improve the capability of identifying the SMI (area under ROC: 0.74, Figure 1). The odds ratio revealed that risk of SMI (Odds ratio: 5.78, P<0.01, Table 3) was increased along with the increase of MIS score in type 2 diabetic patients.
IV. Discussion
This study may have found an efficient and effective way to screen suspected SMI patients. SMI is a serious issue in type 2 diabetic patients, which may lead to silent myocardial infarction and serious prognosis outcome [32]. The traditional way of identifying SMI patients uses an ambulatory electrocardiogram to monitor suspected patients [33]. Ambulatory electrocardiograms, taking 24 hours or longer to detect abnormal signals, are only suitable for monitoring a few highly suspected SMI patients. Therefore, the need to identify SMI patients by other indicators still exists. This report further explored the capability of harmonics of the radial pulse wave to discriminate SMI patients from type 2 diabetic patients without angina-related symptoms.
The results showed that type 2 diabetic patients without angina symptoms had similar physiology conditions between Group 1 and Group 2 in population means of systolic pressure, diastolic pressure, duration of diabetes, body mass index (BMI), HDL level, TG level, and in the prevalence of hypertension. There were only slight differences of population means of age and heart rate (HR) between Group 1 and Group 2 (Table 1).The group mean of the 10-year ASCVD score in Group 2 was even lower than that in Group 1.Thus, clinical evaluation based on the risk factors mentioned above would be unlikely to identify the actual risk of SMI. In contrast, Group 2 had higher Hba1c, and higher prevalence of peripheral artery disease (ABI<0.9) and microalbuminuria (ACR>30mg/g) than Group 1 (Table 1), which could help stratify the high-risk and low-risk SMI patients. The below-average LDL level in both Group 2 and Group 1was due to the intensive lipid therapy [34]–[36] provided by the Division of Endocrinology & Metabolism of Zhongxiao Branch of Taipei City Hospital.
This report then compared the odds ratios of harmonics with odds ratios of ACR and ABI to test the diagnostic performance of each marker. The results showed that C2, C3, and P5 provided more information in discriminating patients with SMI when compared to ACR and ABI (Table 3). LDL and 10-year ASCVD showed limited capability in stratifying the risk of SMI in type 2 diabetic patients because the LDL levels were usually well controlled by the intensive lipid therapy.
MIS score is a proposed score with a combination of the five risk markers, including C2, C3, P5, ACR-Index, and ABI-Index to further evaluate the risk of SMI. The results of the ROC curve showed that MIS (area under ROC: 0.74) outperformed the conventional ASCVD score (area under ROC: 0.34, Figure 1) in identifying moderate to severe SMI. The area under the curve of ROC in identifying SMI using MIS was significantly larger than those using ABI-Index (P=0.01), ACR-Index (P<0.01), ASCVD score (P<0.01), and MIS score excluded pulse information (P=0.06). Therefore, we concluded that the radial pulse spectrum could give additional information in stratifying the risk of moderate to severe SMI.
The odds ratio revealed that the risk of SMI (odds ratio: 5.78, P<0.01, Table 3) was increased along with the increase of MIS score in type 2 diabetic patients. Furthermore, C2, C3, and P5 were the essential risk markers in MIS score to identify SMI precisely. WhenC2, C3, and P5 were excluded, the MIS had fewer efficacies in identifying SMI (area under ROC: 0.62, Figure 2). MIS excluded the pulse information had lower odds ratios in the diagnostic test (odds ratio: 2.81, Table 3). Therefore, the study demonstrated that radial pulse spectrum analysis gave additional diagnostic information compared to traditional coronary heart disease risk factors such as ABI and ACR. We also showed that each of C2, C3, and P5 had superior single diagnostic performance in stratifying SMI than the performance of ASCVD score in type 2 diabetic patients, using odds ratio and ROC curve. The cause of 10-year ASCVD showing limited efficacy in identifying SMI may be due to the active medication in controlling the traditional risk factors such as systolic pressure and LDL level as part of the routine practice for type 2 diabetic patients. This also implies the need for other more relevant markers to improve risk stratification for SMI for type 2 diabetic patients.
Table 4 showed the Positive Predictive Value (PPV) of all risk markers. The enrolled patients had a high prevalence of SMI (66% mild SMI and 13% moderate to severe SMI).In the routine clinical practice of the diabetes management program of the study hospital, the ABI and ACR inclusion criteria have been used in the detection of SMI. Patients deemed at high-risk of SMI will be further evaluated. The prevalence of all SMI (79%) has proven the usefulness of clinical screening procedures for selecting the patients who needed the medical treatment to lower the rate of myocardial infarction and cardiac death [26], [37]. However, the low probability of moderate to severe SMI (13%) doesn’t justify the further use of stress test to identify the candidates of the suspected CAD for revascularization [26], [37], [38].
TABLE 4. The Positive Predictive Value of Risk Markers (N=195) for Predicting all SMI (SSS1) and Moderate to Severe SMI (SSS9) in Type 2 Diabetic Patients Without Any Angina Symptoms.
All SMI (SSS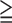 1) 1) |
Moderate to severe SMI (SSS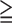 9) 9) |
||
|---|---|---|---|
| Pre-test probability | 79% | 13% | |
| Criteria | PPV for all SMI (SSS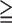 1) 1) |
PPV for all SMI (SSS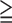 9) 9) |
|
| C2 | >0.5 | 84% | 20% |
| C3 | >0.25 | 84% | 19% |
| P5 | <−4.0 | 85% | 22% |
| ACR-Index | >30mg/g | 78% | 18% |
| ABI-Index | <0.9 | 86% | 22% |
| HR | >75 (beats/min) | 78% | 16% |
| 10-year ASCVD risk | >7.5% | 73% | 12% |
| Hba1c | >7% | 85% | 16% |
 |
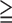 1 1 |
81% | 20% |
| MIS |
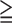 3 3 |
92% | 32% |
PPV= postive predivtive value, HR=heart rate, 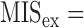 MIS excludes the pulse information
MIS excludes the pulse information
The PPV for all SMI and moderate to severe SMI using the criteria of MIS3 were 92% and 32% respectively (Table 4). Compared with the prevalence of SMI in the enrolled group, using MIS score made an improvement in the risk stratification of the enrolled patients. Compared with the PPVs of SMI using ABI and ACR (MIS excluding pulse information), the PPVs of SMI using MIS score were better. (Table 4) This confirmed the additional diagnostic value of radial pulse spectrum for the risk stratification of SMI.
The patients with moderate to severe SMI (SSS9, more than 11% ischemic myocardium) has a higher prevalence of CAD and need revascularization to lower the rate of myocardial infarction and cardiac death [26], [37]. Compared with the all enrolled patients, the patients with positive test of MIS score were at higher risk of moderate to severe SMI. 32% of patients with positive test had moderate to severe myocardial perfusion defect. The results justify the reason for further stress tests such as exercise ECG or stress MPI [39].
Out of 195 enrolled patients in this study, 4 patients with moderate to severe SMI and 5 patients with more than 50% of coronary stenosis were detected by MIS score system, while other risk factors showed negative results. Furthermore, 12 patients without moderate to severe SMI were determined negative by MIS score system, while the criteria of ABI, ACR, and ASCVD gave positive testing results. In summary, the MIS score may reduce the number of patients with the false-positive results and identify more patients who need further stress test.
There are limitations to the radial pulse spectrum or the MIS score system. First, the PPV of SMI using C2, C3, or P5 was similar to the PPV either using ABI-Index or using ACR-Index. Table 3 and Table 4 showed that the MIS score, the combination of above five risk markers had better performance in the risk stratification of SMI. This study proved that the radial pulse spectrum gave additional diagnostic values to the existing clinical screening procedures in Zhongxiao Branch of Taipei City Hospital. Second, the inclusion and exclusion criteria for enrolled patients constrained MIS score system in screening the risk of the more general population such as all asymptomatic patients with type 2 diabetes. However, this is not suitable to perform myocardial perfusion image in those patient at low or medium risk of heart disease. Therefore, for the whole asymptomatic patients, we have carried out a prospective follow-up study. We investigated the radial pulse spectrum as risk factors for new onset of symptoms or signs of myocardial ischemia, coronary artery diseases, left ventricular dysfunction, and adverse cardiac events in asymptomatic patients with type 2 diabetes. The preliminary results proved that the C2 is independently associated with the extent of coronary stenosis(P<0.05) in diabetic patients treated with coronary revascularization [40] and that C3 is correlated with new onset of myocardial ischemia and left ventricular dysfunction [21]. The above results are consistent with results of this cross-sectional study, showing C2 and C3 as risk factors for cardiovascular events in asymptomatic patients with type 2 diabetes. Third, the C2 and HR values in Group 2 are both higher than those in Group 1. It is necessary to confirm whether the higher values of C2 and HR in patients with moderate to severe SMI are from the same mechanism. We examined the linear correlation between heart rate and C2 throughout the enrolled patient and found that there was no significant association between C2 and heart rate in this study (R2 =0.02). Thus, we had no clear evidence to interpret the C2 and HR from the same mechanism. The higher C2 and higher HR in patients with silent myocardial ischemia might cause from the different reasons. Forth, only a few clinical studies of cardiovascular risk had performed radial pulse analysis [19]–[21], [40], which is not widely used in the clinical practice. Radial pulse wave measurement needs standard protocol and training to maintain its reliability [28]. There are some researches focused on the pulse wave analysis using carotid applanation tonometry to assess the risk of cardiovascular disease [41]–[43]. Although the relationship between radial pulse waveform and carotid pulse waveform existed [44], [45], more studies are needed to validate the effectiveness of radial pulse analysis in assessing the cardiovascular risk.
In addition, the proportion of mild SMI was higher in female patients (29 mild cases out of 36 total SMI cases, or 81%) than the proportion of mild SMI in male patients (30 mild cases out of 49 total SMI cases, or 61%). Mild SMI was defined by 4SSS8. Other researchers also found that female patients had more proportion of mild SMI and may lead to silent myocardial infarction or lead to more adverse prognosis [46]–[48]. In diabetic patients, the relative risk for fatal coronary heart disease is higher in women than in men [49]. Further investigation found that women with acute myocardial infarction were more likely to have normal coronary arteries without or with only mild stenosis [50]. The above phenomenon may be attributed to myocardial microvascular dysfunction [51]. Microvascular dysfunction could change the loading condition of myocardial tissue and thus affect radial pulse waveforms, where a similar phenomenon was demonstrated as a resonance effect [15]. Nonetheless, the detailed interaction mechanism and principle between SMI and pressure pulse spectrum remain unclear. We anticipate this assay to be a starting point for discovering the mechanisms underlying myocardial-perfusion-mediated change of pressure pulse and for finding more indicators for SMI.
Atherosclerosis of coronary artery is one of the most important factors that increase the risk of SMI. Coronary stenosis constrained the maximum blood flow in the coronary artery. In mild atherosclerosis of coronary arteries, the endothelial function plays an important role in the auto-regulation of coronary blood flow and compensates the increasing need of oxygen consumption by a substantial decline in coronary vascular resistance [52], [53]. In most of the time, coronary microvascular resistance adapts to match coronary blood flow during the higher oxygen consumption of myocardium [54], [55]. In a more severe case of coronary stenosis, the heterogeneous nature of coronary vasculature and myocardial perfusion, and reaching the optimal limit of coronary vasodilation create the potential risk of regional perfusion defect [53], [56]. The regional perfusion defect and the imbalance between myocardial oxygen demand and supply, usually called myocardial ischemia, resulted in the angina related symptoms. However, a large proportion of diabetic patients with coronary heart disease are “silent” until the onset of the adverse cardiovascular events or sudden cardiac death [57], with the absence of angina symptoms. Studies showed that asymptomatic diabetic patients have 10–67% prevalence of SMI according to different study settings [58], [59]. The silent effect may be caused by the subclinical neuropathy in patients with type 2 diabetes. Compared with the symptomatic patients, patients with type 2 diabetes and SMI have more significant evidence of autonomic impairment [60].
MPI can identify myocardial perfusion defects during vasodilator stress and indicate the flow limiting coronary lesions [61], [62]. Hence, we used the SSS score derived from MPI as an endpoint of the significant perfusion defects in the myocardium, which had manifested its ability to detect SMI [39], [63]. Performing the chi-test with the criteria of C2>0.50 showed that C2 was strongly correlated with SMI (Table 2). Furthermore, the odds ratio showed that groups with exposure to higher C2, higher C3, and lower P5 values tend to have a higher risk of SMI (Table 3). In addition, the multivariable regression analysis proved C2 and ABI had a significant independent influence on SMI.
Since C2, C3, P5, and ABI were all physical attributes of the blood pulse wave, the results proved that an interaction existed between the blood pulse and the myocardial perfusion. One of the reasons for correlations between radial pulse spectrum and SMI may be due to coronary atherosclerosis and auto-regulation of coronary blood flow. In the development of coronary atherosclerosis, coronary microvascular resistance reduces to meet the myocardial oxygen need [64] Hemodynamic change of the loading condition and vascular characteristics was reflected on arterial blood pulse. This explains why the aortic pulse is associated with cardiovascular risk and predicts the future adverse cardiovascular events [41]–[43]. The recent studies manifested that harmonics of the radial pulse could also be risk factors for coronary artery stenosis, myocardial ischemia, and the decrease of heart function [20], [21]. This result was consistent with Wang’s studies and “resonance theory” in hemodynamics [15], [19], [23]. In addition, this report found that the enrolled patients with moderate to severe SMI had higher resting heart rates. The higher heart rate will increase the oxygen consumption of myocardium and cause the decrease of micro-vascular resistance by auto-regulation [64], [65]. The coronary microvascular dilation at rest will also make an influence on resting radial pulse spectrum. Elevated resting heart rate is also a risk factor for cardiac events [66]. Heart rate reduction by medical treatment can improve the cardiovascular outcome [66]. More studies were needed to reveal the detailed interaction mechanism and principle between SMI and pressure pulse spectrum.
V. Conclusion
In this cross-sectional study, we proposed and proved significant associations between potential risk markers (C2, C3, and P5) and SMI through the t-test of baseline characteristic, chi-test, odds ratios, and multivariable regression. Furthermore, to the best of our knowledge, this report was the first article to combine the harmonics of the radial pulse wave, ABI-index, and ACR-index to give an MIS score, where the diagnostic performance of MIS demonstrated superior capability in stratifying risk of SMI compared to 10-year ASCVD score and to any single risk markers using odds ratio analysis. In MIS score, C2 C3, and P5 were essential and provided additional crucial information in identifying SMI. All of the above five markers are safe enough to facilitate in routine clinical practice and also are cost-effective to repeat within months. Thus, periodic screening for radial pulse spectrum combined with ABI, and urine examination may allow early identification and better stratification of SMI in type 2 diabetic patients. A positive MIS test score may signify the need for further investigations of SMI using stress imaging such as stress myocardial perfusion imaging or stress echocardiography. Conclusively, pulse spectrum information combining with ABI and ACR manifested excellent performance in stratifying risk of SMI in patients with type 2 diabetes.
VI. List of Abbreviations
SMI: silent myocardial ischemia, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, LDL: low-density lipoprotein cholesterol, HDL: high-density lipoprotein cholesterol, TG: triglycerides, HR: heart rate, ASCVD: Atherosclerotic Cardiovascular Disease, BMI: Body Mass Index, Hba1c: Glycated hemoglobin, ABI: Ankle–brachial pressure index, ACR: Albumin Creatinine Ratio, SSS: Summed Stress Score, MPI: Myocardial perfusion imaging, MIS: myocardial ischemia score.
References
- [1].Naka M.et al. , “Silent myocardial ischemia in patients with non-insulin-dependent diabetes mellitus as judged by treadmill exercise testing and coronary angiography,” Amer. Heart J., vol. 123, no. 1, pp. 46–53, 1992. [DOI] [PubMed] [Google Scholar]
- [2].Koistinen M. J., “Prevalence of asymptomatic myocardial ischaemia in diabetic subjects,” BMJ, vol. 301, no. 6743, pp. 92–95, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zellweger M. J., “Prognostic significance of silent coronary artery disease in type 2 diabetes,” Herz Kardiovaskuläre Erkrankungen, vol. 31, no. 3, pp. 240–245, 2006. [DOI] [PubMed] [Google Scholar]
- [4].Nademanee K., Intarachot V., Josephson M. A., Rieders D., Mody F. V., and Singh B. N., “Prognostic significance of silent myocardial ischemia in patients with unstable angina,” J. Amer. College Cardiol., vol. 10, no. 1, pp. 1–9, 1987. [DOI] [PubMed] [Google Scholar]
- [5].Cohn P. F., “Silent myocardial ischemia: Classification, prevalence, and prognosis,” Amer. J. Med., vol. 79, no. 3A, pp. 2–6, 1985. [DOI] [PubMed] [Google Scholar]
- [6].Zednícek L. and Hrubá J., “Silent myocardial ischemia in diabetics,” Sbornik Lekarsky, vol. 91, nos. 11–12, pp. 339–345, 1989. [PubMed] [Google Scholar]
- [7].Passa P., Paillole C., Paycha F., and Leblanc H., “Silent myocardial ischemia in diabetics. Detection–prognostic and therapeutic implications,” Diabete Metabolisme, vol. 15, no. 4, pp. 206–208, 1989. [PubMed] [Google Scholar]
- [8].Abrate M.et al. , “Detection with the Holter method of asymptomatic arrhythmias and silent ischemias in myocardial infarct patients transferred from the coronary unit,” Boll. Della Soc. Italiana Cardiol., vol. 25, no. 8, pp. 883–886, 1980. [PubMed] [Google Scholar]
- [9].Saccomanno G., Marini M., Tomassini P. F., and Paciaroni E., “Prognostic value of ECG changes detected by Holter monitoring in silent ischemic heart disease in the aged,” Minerva Cardioangiol., vol. 43, no. 10, pp. 409–417, 1995. [PubMed] [Google Scholar]
- [10].Nakao Y. M.et al. , “Holter monitoring for the screening of cardiac disease in diabetes mellitus: The non-invasive Holter monitoring observation of new cardiac events in diabetics study,” Diabetes Vascular Disease Res., vol. 12, no. 6, pp. 396–404, 2015. [DOI] [PubMed] [Google Scholar]
- [11].Mostaza J. M.et al. , “Prevalence of carotid stenosis and silent myocardial ischemia in asymptomatic subjects with a low ankle-brachial index,” J. Vascular Surg., vol. 49, no. 1, pp. 104–108, 2009. [DOI] [PubMed] [Google Scholar]
- [12].Rutter M. K., McComb J. M., Brady S., and Marshall S. M., “Silent myocardial ischemia and microalbuminuria in asymptomatic subjects with non–insulin-dependent diabetes mellitus,” Amer. J. Cardiol., vol. 83, no. 1, pp. 27–31, 1999. [DOI] [PubMed] [Google Scholar]
- [13].Demirbaǧ R., “Assessment of the relationship between silent myocardial ischemia, microalbuminuria, and left ventricular function in asymptomatic subjects with non-insulin dependent diabetes mellitus,” Turk Kardiyoloji Dernegi Arsivi, vol. 37, no. 2, pp. 98–100, 2009. [PubMed] [Google Scholar]
- [14].Giovacchini G., “Microalbuminuria predicts silent myocardial ischaemia in type 2 diabetes patients,” Eur. J. Nucl. Med. Mol. Imag., vol. 40, no. 4, pp. 548–557, 2013. [DOI] [PubMed] [Google Scholar]
- [15].Wang Y. Y., Chang S. L., Wu Y. E., Hsu T. L., and Wang W. K., “Resonance. The missing phenomenon in hemodynamics,” Circulation Res., vol. 69, no. 1, pp. 246–249, 1991. [DOI] [PubMed] [Google Scholar]
- [16].Wang Y.-Y. L.et al. , “The ventricular-arterial coupling system can be analyzed by the eigenwave modes of the whole arterial system,” Appl. Phys. Lett., vol. 92, no. 15, p. 153901, 2008. [Google Scholar]
- [17].Wang Y.-Y. L., Hsu T.-L., Jan M.-Y., and Wang W.-K., “Theory and applications of the harmonic analysis of arterial pressure pulse waves,” J. Med. Biol. Eng., vol. 30, no. 3, pp. 125–131, 2010. [Google Scholar]
- [18].Wang Y.-Y. L. and Wang W.-K., “Anatomy of arterial systems reveals that the major function of the heart is not to emit waves associated with the axial blood motion,” J. Physiol., vol. 592, no. 3, p. 409, Jan. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen C.-Y., Wang W.-K., Kao T., Yu B. C., and Chiang B. C., “Spectral analysis of radial pulse in patients with acute, uncomplicated myocardial infarction,” Jpn. Heart J., vol. 34, no. 2, pp. 131–143, 1993. [DOI] [PubMed] [Google Scholar]
- [20].Chang C.-W., Liao K.-M., Chen Y. C., Wang S. H., Jan M. Y., and Chang C. W., “Radial pulse spectrum may be a predictor of ischemic heart disease in patients with type 2 diabetes,” in Proc. Int. Diabetes Fed. Congr., 2017, p. P-0641. [Google Scholar]
- [21].Chang C.-W., Liao K.-M., Chen Y. C., Wang S. H., Jan M. Y., and Chang C. W., “Harmonics of the radial pulse could be risk factors for myocardial ischemia and decrease of heart function in patients with type 2 diabetes,” in Proc. Int. Diabetes Fed. Congr., 2018, p. P-0089. [Google Scholar]
- [22].Wang Y.-Y. L.et al. , “Examining the response pressure along a fluid-filled elastic tube to comprehend Frank’s arterial resonance model,” J. Biomech., vol. 48, no. 6, pp. 907–910, 2015. [DOI] [PubMed] [Google Scholar]
- [23].Wang Y.-Y. L. and Wang W.-K., “Why the cardiovascular studies should start with the radial oscillation of arterial wall rather than from axial flow motion of blood,” Int. J. Cardiol., to be published. [Online]. Available: https://www.sciencedirect.com/science/article/pii/S0167527318335794 [DOI] [PubMed]
- [24].American Diabetes Association, “Consensus development conference on the diagnosis of coronary heart disease in people with diabetes: 10–11 February 1998, Miami, Florida,” Diabetes Care, vol. 21, no. 9, pp. 1551–1559, 1998. [DOI] [PubMed] [Google Scholar]
- [25].Fihn S. D.et al. , “2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease,” J. Amer. College Cardiol., vol. 60, no. 24, pp. e44–e164, 2012. [DOI] [PubMed] [Google Scholar]
- [26].Hachamovitch R.et al. , “Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: Differential stratification for risk of cardiac death and myocardial infarction,” Circulation, vol. 97, no. 6, pp. 535–543, 1998. [DOI] [PubMed] [Google Scholar]
- [27].Chang C.-W. and Wang W.-K., “Reliability assessment for pulse wave measurement using artificial pulse generator,” J. Med. Eng. Technol., vol. 39, no. 3, pp. 177–184, 2015. [DOI] [PubMed] [Google Scholar]
- [28].Chang C.-W., Chen J.-M., and Wang W.-K., “Development of a standard protocol for the harmonic analysis of radial pulse wave and assessing its reliability in healthy humans,” IEEE J. Transl. Eng. Health Med., vol. 3, 2015, Art. no. 2900206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].O’rourke M. F., “Vascular impedance in studies of arterial and cardiac function,” Physiol. Rev., vol. 62, no. 2, pp. 570–623, 1982. [DOI] [PubMed] [Google Scholar]
- [30].Katznelson Y., An Introduction to Harmonic Analysis. Cambridge, U.K.: Cambridge Univ. Press, 2004. [Google Scholar]
- [31].DeLong E. R., DeLong D. M., and Clarke-Pearson D. L., “Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach,” Biometrics, vol. 44, no. 3, pp. 837–845, 1988. [PubMed] [Google Scholar]
- [32].Kannel W. B., “Silent myocardial ischemia and infarction: Insights from the Framingham Study,” Cardiol. Clin., vol. 4, no. 4, pp. 583–591, 1986. [PubMed] [Google Scholar]
- [33].Gibson C. M.et al. , “Diagnostic and prognostic value of ambulatory ECG (Holter) monitoring in patients with coronary heart disease: A review,” J. Thrombosis Thrombolysis, vol. 23, no. 2, pp. 135–145, 2007. [DOI] [PubMed] [Google Scholar]
- [34].Baigent C.et al. , “The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): A randomised placebo-controlled trial,” Lancet, vol. 377, no. 9784, pp. 2181–2192, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jha V.et al. , “Chronic kidney disease: Global dimension and perspectives,” Lancet, vol. 382, no. 9888, pp. 260–272, 2013. [DOI] [PubMed] [Google Scholar]
- [36].Norgren L.et al. , “Inter-society consensus for the management of peripheral arterial disease (TASC II),” Eur. J. Vascular Endovascular Surg., vol. 33, no. 1, pp. S1–S75, 2007. [DOI] [PubMed] [Google Scholar]
- [37].Hachamovitch R., Hayes S. W., Friedman J. D., Cohen I., and Berman D. S., “Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography,” Circulation, vol. 107, no. 23, pp. 2900–2907, 2003. [DOI] [PubMed] [Google Scholar]
- [38].American Diabetes Association, “9. Cardiovascular disease and risk management: standards of medical care in diabetes–2018,” Diabetes Care, vol. 41, no. 1, pp. S86–S104, 2018. [DOI] [PubMed] [Google Scholar]
- [39].Wackers F. J. T.et al. , “Detection of silent myocardial ischemia in asymptomatic diabetic subjects,” Diabetes Care, vol. 27, no. 8, pp. 1954–1961, 2004. [DOI] [PubMed] [Google Scholar]
- [40].Chen Y.-C., Wang S.-H., Wang G.-C., Chang C.-W., and Liao K.-M., “Glycated haemoglobin, ankle-brachial index, radial pulse spectrum and risk of coronary artery diseases with and without the angina symptoms in type 2 diabetic patients,” in Proc. Int. Diabetes Fed. Congr., 2018, p. P-0153. [Google Scholar]
- [41].Chirinos J. A.et al. , “Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease,” Hypertension, vol. 45, no. 5, pp. 980–985, 2005. [DOI] [PubMed] [Google Scholar]
- [42].Nurnberger J., Keflioglu-Scheiber A., Saez A. M. O., Wenzel R. R., Philipp T., and Schäfers R. F., “Augmentation index is associated with cardiovascular risk,” J. Hypertension, vol. 20, no. 12, pp. 2407–2414, 2002. [DOI] [PubMed] [Google Scholar]
- [43].Chirinos J. A.et al. , “Arterial wave reflections and incident cardiovascular events and heart failure: MESA (multiethnic study of atherosclerosis),” J. Amer. College Cardiol., vol. 60, no. 21, pp. 2170–2177, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pauca A. L., O’rourke M. F., and Kon N. D., “Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform,” Hypertension, vol. 38, no. 4, pp. 932–937, 2001. [DOI] [PubMed] [Google Scholar]
- [45].Takazawa K., Kobayashi H., Shindo N., Tanaka N., and Yamashina A., “Relationship between radial and central arterial pulse wave and evaluation of central aortic pressure using the radial arterial pulse wave,” Hypertension Res., vol. 30, no. 3, p. 219, 2007. [DOI] [PubMed] [Google Scholar]
- [46].Omerovic E.et al. , “Silent myocardial infarction in women with type II diabetes mellitus and microalbuminuria,” Therapeutics Clin. Risk Manage., vol. 4, no. 4, pp. 705–712, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tan Y. C., Sinclair H., Ghoorah K., Teoh X., Mehran R., and Kunadian V., “Gender differences in outcomes in patients with acute coronary syndrome in the current era: A review,” Eur. Heart J., Acute Cardiovascular Care, vol. 5, no. 7, pp. 51–60, 2016. [DOI] [PubMed] [Google Scholar]
- [48].Anand S. S.et al. , “Differences in the management and prognosis of women and men who suffer from acute coronary syndromes,” J. Amer. College Cardiol., vol. 46, no. 10, pp. 1845–1851, 2005. [DOI] [PubMed] [Google Scholar]
- [49].Huxley R., Barzi F., and Woodward M., “Excess risk of fatal coronary heart disease associated with diabetes in men and women: Meta-analysis of 37 prospective cohort studies,” BMJ, vol. 332, no. 7533, pp. 73–78, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dey S.et al. , “Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: The global registry of acute coronary events,” Heart, vol. 95, no. 1, pp. 20–26, 2009. [DOI] [PubMed] [Google Scholar]
- [51].Shaw L. J., Bugiardini R., and Merz C. N. B., “Women and ischemic heart disease: Evolving knowledge,” J. Amer. College Cardiology, vol. 54, no. 17, pp. 1561–1575, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zeiher A. M., Drexler H., Wollschläger H., and Just H., “Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis,” Circulation, vol. 84, no. 5, pp. 1984–1992, 1991. [DOI] [PubMed] [Google Scholar]
- [53].Weber K. T. and Janicki J. S., “The metabolic demand and oxygen supply of the heart: Physiologic and clinical considerations,” Amer. J. Cardiol., vol. 44, no. 4, pp. 722–729, 1979. [DOI] [PubMed] [Google Scholar]
- [54].Bache R. J. and Schwartz J. S., “Effect of perfusion pressure distal to a coronary stenosis on transmural myocardial blood flow,” Circulation, vol. 65, no. 5, pp. 928–935, 1982. [DOI] [PubMed] [Google Scholar]
- [55].Fokkema D. S., van Teeffelen J. W. G. E., Dekker S., Vergroesen I., Reitsma J. B., and Spaan J. A. E., “Diastolic time fraction as a determinant of subendocardial perfusion,” Amer. J. Physiol.-Heart Circulatory Physiol., vol. 288, no. 5, pp. H2450–H2456, 2005. [DOI] [PubMed] [Google Scholar]
- [56].van de Hoef T. P.et al. , “Coronary pressure-flow relations as basis for the understanding of coronary physiology,” J. Mol. Cellular Cardiol., vol. 52, no. 4, pp. 786–793, 2012. [DOI] [PubMed] [Google Scholar]
- [57].Jouven X., Lemaître R. N., Rea T. D., Sotoodehnia N., Empana J.-P., and Siscovick D. S., “Diabetes, glucose level, and risk of sudden cardiac death,” Eur. Heart J., vol. 26, no. 20, pp. 2142–2147, 2005. [DOI] [PubMed] [Google Scholar]
- [58].Wackers F. J., “Detection of silent myocardial ischemia in asymptomatic diabetic subjects: The DIAD study,” Diabetes Care, vol. 27, no. 8, pp. 1954–1961, 2004. [DOI] [PubMed] [Google Scholar]
- [59].Zellweger M. J.et al. , “Progression to overt or silent CAD in asymptomatic patients with diabetes mellitus at high coronary risk: Main findings of the prospective multicenter BARDOT trial with a pilot randomized treatment substudy,” JACC, Cardiovascular Imag., vol. 7, no. 10, pp. 1001–1010, 2014. [DOI] [PubMed] [Google Scholar]
- [60].Marchant B., Umachandran V., Stevenson R., Kopelman P. G., and Timmis A. D., “Silent myocardial ischemia: Role of subclinical neuropathy in patients with and without diabetes,” J. Amer. College Cardiol., vol. 22, no. 5, pp. 1433–1437, 1993. [DOI] [PubMed] [Google Scholar]
- [61].Branch K. R.et al. , “Myocardial computed tomography perfusion,” Cardiovascular Diagnosis Therapy, vol. 7, no. 5, p. 452, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].London B., Cardiology: An Illustrated Textbook, vol. 2 Bengaluru, India: Jaypee Brothers, Medical, 2018. [Google Scholar]
- [63].Young L. H.et al. , “Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: The DIAD study: A randomized controlled trial,” JAMA, vol. 301, no. 15, pp. 1547–1555, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Heusch G., “Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: Benefit from selective bradycardic agents,” Brit. J. Pharmacol., vol. 153, no. 8, pp. 1589–1601, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Tune J. D., Gorman M. W., and Feigl E. O., “Matching coronary blood flow to myocardial oxygen consumption,” J. Appl. Physiol., vol. 97, no. 1, pp. 404–415, 2004. [DOI] [PubMed] [Google Scholar]
- [66].Fox K. M. and Ferrari R., “Heart rate: A forgotten link in coronary artery disease?” Nature Rev. Cardiol., vol. 8, no. 7, p. 369, 2011. [DOI] [PubMed] [Google Scholar]