Abstract
The primary role of eyelashes is to protect and maintain the health of the lid margin. However, the mechanisms to fulfill this role are not fully understood. Unraveling these mechanisms will stand to greatly improve the efficiency of eye care professionals’ interventions in anomalies of the eyelashes. The aim of this article is to provide a review on eyelashes including highlights and new avenues for research; the biology of both the lash and its follicle; the pathophysiology and management of lash anomalies by eye care professionals; and the effect of iatrogenic factors on lashes. Using the database of Ovid MEDLINE, we reviewed studies specifically directed on human/mammalian eyelashes and key articles on current trends in scalp hair methodologies that can be applicable to lash research. The eyelash morphology, pigmentation and growth rate have been documented using techniques ranging from lash imaging to follicle immunohistochemistry. Furthermore, studies have demonstrated that the lash follicle is sensitive to many factors of the external environment, a variety of systemic/topical medications and cosmetics. Recently, aerodynamic studies using a mammalian eye model confirmed that an optimal lash length was needed so that eyelashes serve a protective role in reducing the number of particles that can reach the eye. Despite recent advances in lash research, studies are still scarce, due to the limited availability of the human lid for sampling. This review brings awareness that further research is needed with respect to eyelashes and will hopefully reduce the gap with scalp hair research.
Keywords: Eyelash, Follicle, Hair, Eyelash pathophysiology, Lid margin
Resumen
La función principal de las pestañas es proteger y mantener la salud del margen palpebral. Sin embargo, los mecanismos de desempeño de esta función no se comprenden plenamente. Desentrañar estos mecanismos ayudará a mejorar la eficiencia de las intervenciones de los profesionales de cuidados oculares en cuanto a las anomalías de las pestañas. El objetivo de este artículo es aportar una revisión sobre las pestañas, incluyendo los aspectos más destacados y las nuevas aportaciones para la investigación, la biología de la pestaña y su folículo, la patofisiología y tratamiento de las anomalías de las pestañas por parte de los profesionales de cuidados oculares, y el efecto de los factores iatrogénicos sobre las pestañas. Utilizando la base de datos de Ovid MEDLINE, revisamos los estudios específicamente dirigidos a las pestañas humanas/de mamíferos, así como los artículos clave sobre las tendencias actuales en cuanto a las metodologías del cuero cabelludo, que pueden aplicarse a la investigación sobre las pestañas. Se han documentado la morfología de las pestañas, así como su pigmentación y tasa de crecimiento, utilizando técnicas que oscilan entre la imagen de las pestañas y la inmunohistoquímica del folículo. Además, los estudios han demostrado que el folículo de la pestaña es sensible a diversos factores del entorno externo, diversas medicaciones sistémicas/tópicas y cosméticos. Recientemente, los estudios aerodinámicos que han utilizando un modelo de ojo de mamífero, han confirmado que se precisaba una longitud de pestañas óptima para que éstas ejercieran su función protectora a la hora de reducir el número de partículas que pueden acceder al ojo. A pesar de los avances recientes de la investigación sobre las pestañas, los estudios son aún escasos, debido a la disponibilidad limitada de párpado humano para muestreo. Esta revisión sirve de concienciación acerca de la necesidad de investigación futura con respecto a las pestañas, que reducirá presumiblemente la brecha existente con respecto a la investigación sobre el cuero cabelludo.
Palabras clave: Pestañas, Folículo, Pelo, Patofisiología de las pestañas, Margen palpebral
Introduction
Little research has been done on the human eyelash on account that most of the attention has been directed to research on hair for people suffering from scalp hair loss. However, recent discoveries on the role of eyelashes and its distinctive characteristics have led to an increased scientific interest. Moreover, eyelashes are now considered an important aspect of the facial esthetic and are the object of various beauty treatments to enhance them.1, 2 Since eyelashes form a barrier between the external and internal environment of the eye, they are extremely sensitive to a variety of threats and irritants and are highly innervated to perform that function.3 Eyelashes are an integral part of the lid margin anatomy, much like the Meibomian glands, eyelid skin and biofilm, each contributing to the overall homeostasis of the ocular surface. As such, it is important to maintain their integrity. As a whole, the lid margin is responsible for the production of the tear film lipid layer and the protection of the eye from external trauma. Via the blink, it distributes the tears toward the nasolacrimal puncta found in the inner portion of the lid margin.4 If any part of the lid margin is inflamed, it can induce a tear film disturbance or instability which can, in turn, affect the ocular surface.5 Left untreated, this inflammatory cascade can develop into dry eye disease.5 Therefore, studying the eyelash and its pathophysiology is valuable for researchers and eye care professionals (ECPs) alike, to maintain ocular surface homeostasis.
An Ovid MEDLINE search for eyelash physiopathology/abnormalities/pathologies has led to 419 human and 59 non-human publications. The articles that were selected for this non-systematic review concentrated on the general biology of the human lash, the prevailing methods in lash research, lash anomalies and the resulting pathologies with their associated clinical management by an ECP. Also, we reviewed relevant articles on current trends in scalp hair research that can be applicable to eyelashes, and the iatrogenic factors that can affect the lashes, such as cosmetics.
The general biology of the human lash
The human lower lid contains 75–80 lashes dispersed in three to four rows, whereas the upper lid has 90–160 lashes scattered on five to six rows.3, 6, 7 The anatomy of the lash and hair has some similar characteristics.8 Both have a hair shaft (the visible part) that extends outside the skin, a root that is under the skin and a bulb, which is the enlarged terminal portion (Fig. 1). The inferior portion of the bulb is in direct contact with the dermal papilla, which brings key mesenchymal-epithelial interactions in follicle cycling.
Figure 1.
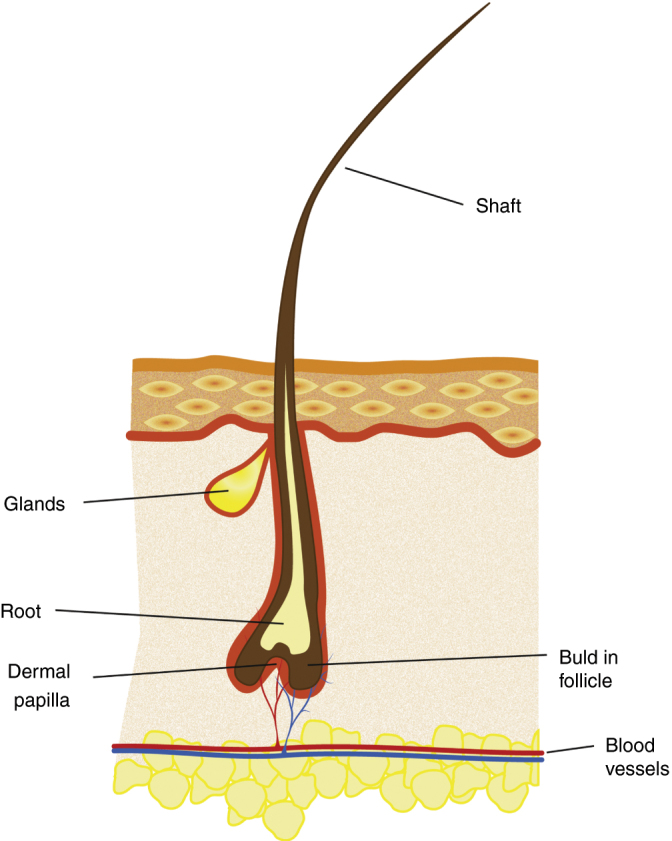
The general morphology of the eyelash and its surrounding skin.
The lash itself is made up of three structures that fit into one another (Fig. 2).6 The innermost structure, the medulla, consists of loose cells. A thicker cortex surrounds the medulla to ensure its strength and stability. The pigmentation of either the lash or hair is the result of the melanin contained in the cortex. Finally, the cuticle, composed of several cell layers, forms the outermost portion, offering protection to the internal structures by its impermeability.
Figure 2.
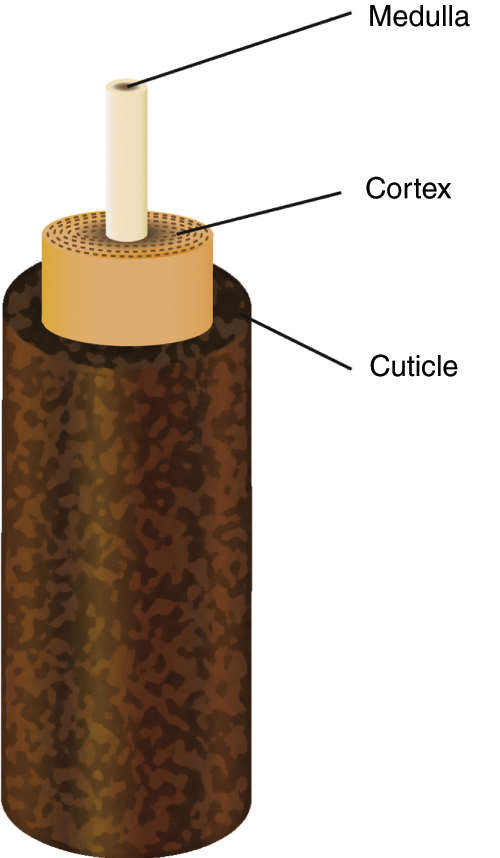
The anatomy of the eyelash.
The anatomy and physiology of the lash follicle are distinctive from other hair follicles. Consequently, the lash follicle is worthy of a detailed study and appreciation of its specific characteristics and surrounding skin; its influence on the lash life cycle, curvature, and pigmentation; and the age-related changes of the lashes.
The surrounding skin of the follicle and its features
To appreciate the main characteristics of the lash follicle and its surrounding skin, it is imperative to compare these structures to that of the scalp hair, which have been studied extensively. The scalp skin contains three layers: the epidermis (external), the dermis (middle) and the hypodermis (internal)8 whereas the skin of the lids has two layers: a thinner epidermis and a dermis.9 All follicles on the human body are rooted in their deepest skin layer, notably the hypodermis for the scalp and the dermis for the lid. Consequently, the lash follicle is shorter than the scalp hair follicle.9 Another major characteristic that distinguishes lash follicles from scalp hair follicles is that they have no arrector pili muscles, which are responsible for straightening the hair in response to cold or intense emotions,8 producing, what is commonly referred to as ‘goose bumps’. Therefore, lashes do not require individual mobility.3
Lash follicles are connected with two types of secretory glands: Zeiss and Moll. They produce different substances released through channels that flow into the follicle. The Zeiss glands use a holocrine mechanism of action, thus liberating their complete cell content, which is a sebum.10 It has antimicrobial and lubrication properties, just as it allows the transport of antioxidants, although the exact function of the sebum is unknown.10 The Moll glands, only found in the lids and active from birth, are apocrine glands that produce secretions by fragmentation from one side of their cells.11 Their secretions, which contain a variety of sugar components, might play a critical role in the defense against microorganisms.11
The life cycle, curvature and pigmentation
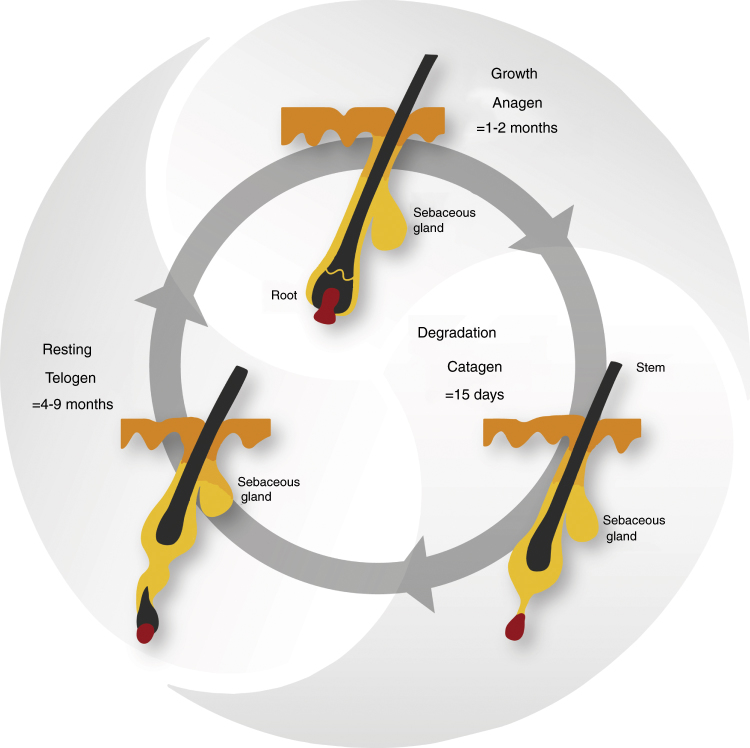
Lashes have a life cycle consisting of three phases: the growth phase (anagen), the degradation phase (catagen) and the resting phase (telogen).12 Following the telogen phase, the lash falls out and the life cycle begins again with a new lash in the anagen phase (Fig. 3).12 The daily growth rate of a lash is 0.12–0.14 mm.7, 9 The anagen phase duration varies from four to ten weeks and the complete life cycle is from four to eleven months.7, 9, 13 The lash length rarely exceeds 12 mm, as the growth rate and anagen phase duration are shorter than the ones observed in scalp hair research.9 The lash growth rate is influenced by several factors, including the topical prostaglandin analogs used to reduce the intraocular pressure in glaucoma patients.13 Nesher et al. demonstrated that the prostaglandin analog F2α receptors were expressed in several layers of the lash follicle during its anagen phase.14 Further studies are needed to confirm the expression of the prostaglandin analog F2α receptors with the use of prostaglandin analogs eyedrops.
Figure 3.
The life cycle of the eyelash.
The morphology of the eyelashes is such that they are curved in all individuals, regardless of ethnicity. This curvature is initiated at the bulb of the lash and continues until the tip of the shaft.15 Thibaut et al. have shown that several cell types of the lash follicle alongside the bulb location are asymmetric (i.e. certain sheaths along the concave side of the bulb are thicker than the ones on the convex side).9 While studying the biology of curly hair in vitro, the same phenomenon of asymmetry has been observed.15 Curly hair's markers are also found in the cuticle and cortex of the lash itself.9, 16 These studies offer possible explanations of the mechanisms involved in lash curvature. Nevertheless, the amplitude of the curvature can vary among individuals. Ethnic studies have identified a difference in the degree of lash curvature (or curl) revealing that the lift-up and curl-up angles are more pronounced among Caucasians as compared to Asians.7
The skin and all hair on the human body acquire their pigmentation mostly by melanogenesis, which is the synthesis of different types of melanin by the melanocytes,17 these cells being under the influence of several enzymes, including tyrosinase-related protein 2 (TRP-2).18 When it comes to lashes, their degree of pigmentation is defined by the quantity of melanocytes in the lash follicle structure.9 The lash becomes grayish at a very advanced age and rarely whitens.6 The maintenance of the lash pigmentation may be explained by a sustained expression of TRP-2.9 Further studies are needed to confirm if this association is a direct cause-effect relationship. In contrast, many studies have shown that scalp hair will progressively lose its pigmentation due to a gradual depletion of melanocytes.19 Several mechanisms have been demonstrated to explain this phenomenon: melanocyte apoptosis, oxidative stress and a reduced expression of certain enzymes, such as TRP-2.17, 20, 21
Age-related changes
There are limited studies about age-related changes on eyelashes. Procianoy et al. have studied women's lower lid lashes and found that the curvature of the lashes on the medial and central eyelid portion increased with age, while those of the lateral portion remained in the same direction.22 Glaser et al. reported that there was a reduction of length, thickness and pigmentation of the lashes with age.23
Research methods
Compared to scalp hair, little research has been dedicated to the study of eyelashes, namely due to the limitation inherent to the in vivo access of the lash follicle. This section explores the techniques employed in lash research and their challenges.
Sampling and organ culture of the lash follicle
There are only a few studies on human lash follicle sampling because it is limited to cadaveric studies and eyelid surgeries, such as ectropion repairs.3, 9, 14 It was in 1969 that the first research on the histology and cytochemistry of human lids and lashes was published.3 The function and microscopic anatomy of the follicle cells were studied post mortem following sudden accidental deaths. Other studies that have succeeded in preserving a portion of the human lid have used immunohistochemistry to perform analysis.9, 14 This technique consists of detecting certain antigens (proteins) in the cells of the tissue, using antibodies that bind to them specifically. This technique allowed researchers to describe the morphology, curvature and pigmentation of the lash follicles,9 and the presence and characteristics of some prostaglandin receptors in the follicles.14
As sampling on human lash follicles is limited, it is even more complex for scientists to perform lash follicle cultures, hence existing publications on that topic are limited to animal studies. Yet, human scalp hair cultures are common and have been successfully achieved for the past several decades, whereby tissues are obtained during face-lift procedures.12, 24, 25 A research guide has been proposed to standardize the scalp hair follicle culture, which is of interest to researchers in different fields: experimental dermatology, genetics, developmental biology and endocrinology.26 It is even possible to observe human hair follicles in vivo with xenotransplantation (the follicles in their anagen phase or full-thickness skin portions are removed from the human scalp and transplanted on the back of mice).27, 28, 29 All of these studies highlight how hair research has progressed as compared to eyelash research. To overcome this gap, other mammals had to be considered. In the past, primate tissues have been used due to their resemblance with respect to anatomy and physiology. However, animal protection laws have evolved and primates have a restricted accessibility.30 Previous hair studies have demonstrated that both human and porcine hair follicles are anatomically close.31, 32 Consequently, preliminary trials on cultivated porcine lash follicles were performed as recently as 2016, whereby follicles from pigs intended for human consumption were used for organ culture assays.13 An elongation of the follicles was obtained within a few days. Hopefully, this study and future ones will enhance our knowledge on the lash and help to reduce the gap between studies on lashes and hair.
Imaging of lashes
Imaging is a minimally invasive technique to quantify various parameters of human lashes. Observational studies with different age categories demonstrated that lashes change with age.22, 23 Characteristics such as the orientation, length, thickness and pigmentation were measured from photographs of the lower and upper lids.22, 23 Human and mouse eyelash life cycle phases have been estimated with photographs in several studies.7, 9, 13, 33 However, the measures were variable from one study to another due to non-standardized methodologies. To improve accuracy, Thibault et al. observed preselected lashes over a nine-month period by measuring their length each week.9 Once again, while imaging of lashes estimates the parameters of the life cycle, more advanced techniques described above, such as xenotransplantation and follicle cultures, are routinely used to measure the same parameters for hair.29
Imaging techniques have also been utilized for ethnic differences between the lashes of Caucasian and Asian women.7 Digital photographs were used to calculate the lift-up and curl-up angles of the upper lashes and phototrichogram (allows in vivo study of the growth cycle)34 for the number, length and thickness of lashes.7 Smaller lash details such as the cuticle textures and layer density were observed ex vivo with electron microscopy.7
Study on aerodynamics
No in vivo studies of human lash aerodynamics exist to date in the literature. Only one in vitro study exists using a model of a mammalian eye to study the aerodynamics of particles around eyelashes.35 The biophysics research team designed a wind tunnel in which air flow, that could affect the ocular surface, was recreated. The model had dimensions of an adult human eye, with a depth of 4 mm and a diameter of 20 mm, and was made with an aluminum dish. Water filled in the dish was used to mimic the tear film and a mesh was chosen to represent the lashes, after obtaining the same results with commercially available lashes made with human hair. Measurements of water evaporation on the ocular surface and calculation of particle deposition on the lashes were obtained using various mesh lengths. Optimal lash lengths that decreased evaporation and particle deposition were determined. Using fluid mechanic principles, the flow on the model eye's surface, as well as around and through lashes, were calculated. The optimal lash length determined was compared with other mammalian eyelash lengths, obtained by photographs from phylogenetically diverse preserved mammalian heads. They established that the optimal lash length was one-third of the width of the eye. Aerodynamic analysis confirmed that this was the optimal length, because it reduced tear evaporation and deposition of particles on lashes by half. Short lashes created a zone of airflow stagnation above the ocular surface, while long lashes pushed the airflow toward the ocular surface. These aerodynamic studies highlighted the important role of lashes in the protection of the eye. In vivo studies should be carried out to validate these results on humans, but the wind tunnel effect would most undoubtedly induce an increased frequency of reflex blinking to protect the ocular surface. Based on this study, any alterations of lash length, be it with certain pharmaceuticals or cosmetic procedures (i.e. lash extensions), would have an impact on the protective effect of the ocular surface. Further studies will be needed to validate this impact. Although the study provided an interesting insight into the role that lashes play in the aerodynamics at the front surface of the eye, there remained limitations. Some examples of those include the plane surface used in the model, instead of a convex surface, and water as the chosen composition to represent the tear film, which is more complex in mammals. Studying the protective role of lashes was unexplored before this study, and the authors have paved the way for future investigations in the aerodynamic role of in vivo human lashes.
Pathophysiology of the lashes and their management
Definitions of lash anomalies
During an evaluation of the anterior segment of the eye, ECPs need to assess the normalcy of several structures including eyelashes. There are several terms in the literature that define anomalies of hair anywhere on the body, however some are specific for eyelashes. Table 1 summarizes the different types of hair anomalies and their associated pathologies.
Table 1.
Hair anomalies and their associated pathologies.
| Anomaly | Pathological condition | Lashes only | Description |
|---|---|---|---|
| Pigmentation | Poliosis | Decreased/absence of pigmentation in hair from any area of the body,36 by decreased/absence of melanin and/or hair follicle melanocytes37 | |
| Direction and position | Primary trichiasis: misdirected lash, by misdirection of the hair shaft38 | ||
| Trichiasis | X | Secondary trichiasis: secondary to entropion, normal orientation of the hair shaft38 | |
| Distichiasis | X | Abnormal row of lashes near or in the Meibomian glands; rare condition39 | |
| Growth | Hypotrichosis | Reduced hair density in any area of the body40 | |
| Milphosis | X | Lash loss41 | |
| Madarosis | Lash and/or eyebrow loss41 | ||
| Mixed | Hypertrichosis | An increased of hair in any area of the body, beyond the normal variation for age, sex or race42 | |
| Trichomegaly | Increased lash (>12 mm) and/or eyebrow length, curl, stiffness, pigmentation and thickness43, 44, 45 | ||
There is a consensus on most definitions on eyelash anomalies with the exception of hypertrichosis and trichomegaly. The term hypertrichosis is commonly misinterpreted as a synonym for trichomegaly, even though they have distinct definitions. Hypertrichosis is defined as an increase in hair in any part of the body, whereas trichomegaly is specific for eyelashes and eyebrows.43, 44, 45 The definitions of hypertrichosis and trichomegaly adopted for this review reflect the ones used in recent publications.43, 44, 45 Also, the terms madarosis and milphosis are often confounded. In a clinical setting, the term madarosis is used to describe lash loss, however milphosis is the more appropriate term.
Management of lash anomalies
The etiology and management of poliosis, trichiasis, milphosis and trichomegaly have been summarized from the written literature, mainly from case reports (Table 2). Depending on the etiology, a single pathology or several lash pathologies may be present simultaneously. As a result of the lack of substantive clinical trials on lash pathologies, Table 2 emphasizes the most frequently reported management strategies, and as such, is non-exhaustive.
Table 2.
Etiologies and management of lash conditions.
| Pathological condition | Etiology | Management |
|---|---|---|
| Poliosis | Congenital syndromes46 | No medication approved to restore lash pigmentation |
| Acquired conditions36, 47, 48, 49: melanocytic lesions, inflammatory systemic disorders, blepharitis and vernal keratoconjunctivitis (VKC) | If ophthalmic-related: treat underlying etiology54 with results varying from no change to full recovery of the pigmentation47, 51, 53 | |
| Less common: systemic/ophthalmic drug-induced (side effect of all topical prostaglandin analogs)50, 51, 52, 53 | If systemic implication54: referred to the patient's primary care doctor | |
| Trichiasis | Ophthalmic39: lid margin scars from trauma/surgery; lid margin inflammation; conjunctival diseases; conjunctival burns | Initial episode: epilation of the affected lash(es) and soft contact lenses for short-term relief of induced conjunctival or corneal irritation55 |
| Various skin diseases39 | Recurrent episode: refer to an ophthalmologist for an ablation procedure (depends on the severity): laser argon ablation,56, 57 electrolysis,58 trephination,59 direct internal lash bulb extirpation,38 folliculectomy,60 cryotherapy,61 entropion surgery62 | |
| If skin disease suspected: refer to a dermatologist | ||
| Milphosis (loss of lashes) | Ophthalmic41: lid infestation (staphylococcal spp., Demodex folliculorum, trachoma) and/or lid inflammation (posterior blepharitis, ocular rosacea, seborrheic blepharitis) | Reversible follicle damage64: lash(es) regrowth possible; consider pharmaceutical approach to increase the regrowth rate (bimatoprost ophthalmic solution 0.03% is the only approved drug in patients with healthy lashes and hypotrichosis)65, 66, 67, 68 |
| Associated with eyebrow madarosis63: several dermatological diseases and inherited conditions | Irreversible follicle damage: no lash regrowth | |
| Associated with eyebrow and hair madarosis63: Extensive list of systemic and drug-induced conditions | Severe irreversible milphosis: refer to an oculoplastic surgeon for lash grafting consideration63 | |
| Trichomegaly | Congenital syndromes69: (Oliver-McFarlane and Cornelia de Lange) | Trimming of the affected lashes if visual disturbance or smudging with inner surface of eyeglass lens70, 71, 72 |
| Familial | With concomitant trichiasis55: refer to trichiasis management (above) | |
| Acquired44: Allergic rhinitis, atopic dermatitis, HIV infection, uveitis and VKC | ||
| Drug-induced44: Topical prostaglandin analogs and epidermal growth factor receptor (EGFR) inhibitors used in oncology | ||
It is essential for ECPs to first determine the etiology of the lash pathology to select the appropriate management. In the particular case of milphosis without a clear etiology, ECPs must consider a psychiatric disorder called trichotillomania as a possible causal factor, regardless of the concomitance of eyebrow and/or hair madarosis. Trichotillomania is characterized by an uncontrollable urge to pull out hair from any part of the body73 and a referral for a psychological evaluation should be considered. This condition can be managed by investigating the cause of the behavior and, in some cases, pharmacotherapy is recommended.74
The etiology of the lash pathology also affects the appearance of the lashes, which may affect the patient's esthetic appearance. For example, common causes of trichomegaly, such as HIV infection and allergic rhinitis, will induce the regrowth of long and smooth lashes, while epidermal growth factor receptor (EGFR) inhibitors make them rougher and more dispersed.44 The appearance of eyelashes also has a major impact on the quality of life, as hair loss can cause various degrees of psychological distress.75 Dunnill et al. have demonstrated that the most distressing side effect of chemotherapy is the loss of hair.76 Other systemic diseases that induce hair loss can be stressful for the affected person, such as alopecia areata, which is characterized by a patchy loss of scalp hair. This disease can progress to other parts of the body, including eyelashes.77 Although the exact pathophysiological mechanism is not established yet, it is thought to be caused by an autoimmune attack on hair follicles.78 Patients with alopecia areata have poor health-related quality life scores similar to what is established in other chronic skin diseases, as demonstrated in two systematic studies.77, 79
As seen in Table 2, the management of lash pathologies will be influenced by their causal agent. When the lash pathology severity ranges from mild to moderate, this can be managed by an ECP in most cases. To date, there is only one pharmaceutical option approved for eyelash loss (lash hypotrichosis), consisting of a topical application of bimatoprost ophthalmic solution 0.03% (Latisse, Allergan) along the superior lash base. Its mode of action is to prolong the anagen phase of the lash life cycle,65 hence resulting in an increased lash length, thickness and pigmentation.65, 66, 67, 68 Before using this product, patients should always seek the advice of an ECP. If adverse events occur, such as discomfort and skin pigmentation, discontinuation of the product will typically reverse the effects. Some cases of poliosis have been reported following prostaglandin analogs use.51 To date, there is no medication approved to restore the pigmentation of lashes.
Severe lash pathologies must be referred to specialists for an ablation procedure,57, 58, 59, 60 eyelash grafts,63 lid reconstruction80, 81 or systemic therapies.78 Promising therapies are emerging for hair loss, which can lead to the discovery of new treatments for eyelashes specifically. Dainichi and Kabashima have reviewed all current therapies and treatments that have an effect on hair regrowth in alopecia areata and universalis (subtype of alopecia that also affect the lashes).78 In alopecia universalis, hair regrowth has been observed with medications such as topical immunotherapy and corticosteroids.78 However, these studies focused on scalp hair regrowth and did not collect data on the regrowth rate of lashes. Data from scalp hair studies may guide future therapies for eyelashes.
Lid margin microflora
The inherent microflora of the lid margin also plays a vital role in maintaining the lashes free of anomalies. The lid margin typically contains a microflora composed of commensal bacteria and parasites. A disruption of the balance of this microflora can lead to various types of blepharitis.82 Lee et al. have demonstrated that the most common bacteria found in lash samples were Propionibacterium, Staphylococcus, Streptophyta, Corynebacterium and Enhydrobacter.83 The variety of the lash microflora is unique to each individual. The study further highlighted that when blepharitis was diagnosed, Staphylococcus, Streptophyta, Corynebacterium, and Enhydrobacter were increased whereas Propionibacterium was decreased. Due to the constant contact of the lids with the ambient air, the lid microflora is particularly influenced by environmental factors, such as pollen, dust, and soil particles. Furthermore, touching our lids with our fingers can inoculate other varieties of the microflora, contributing to the dynamic nature of the lid margin microflora.
Bacterial overpopulation can be observed clinically with the presence of debris on the lashes. Acute Staphylococcal blepharitis is identified with collarettes, which are hard crusts on lashes, and with other non-specific signs (squame/scale, telangiectasia vessels, Meibomian gland dysfunction).84 Chronic signs are madarosis, trichiasis, poliosis, tylosis or scars on the lid margin.82, 84 Blepharitis can also be caused by other micro-organisms, such as parasites. Three types of parasites, all arthropods, can inhabit the lid margin: Demodex folliculorum, Demodex brevis and Phthirus pubis.85 Demodex parasites are part of the natural eye microflora82 and their number increases with age, so much so that one study has reported 100% prevalence in people over 70 years old.86 D. folliculorum is mostly found in the lash follicle and other parts of the body such as eyebrows, scalp, nose and ears. D. brevis is found mainly in the Meibomian glands and other sebaceous glands, such as the face.87 The anatomy of the Demodex parasite consists of a head with four pairs of legs on either side and an elongated body/tail that contains the digestive system. The parasite has no anus, hence all the ingested material remains in the gut, along with its own microflora of Streptococcus spp., Staphylococcus spp. and Bacillus oleronius.88 At the end of its life cycle, approximately 15–18 days in length, the parasite bursts and releases its content, which can provoke an inflammatory response.87, 88 An overpopulation of the parasite is called a demodicosis, and on the lids, this would be termed blepharitis secondary to Demodex. Since Demodex has the potential to affect both the anterior (lashes) and posterior (Meibomian glands) portion of the lid margin, some89 have proposed the term marginal blepharitis to describe a demodicosis of the lid margin. Clinical signs of demodicosis include follicular hypertrophy,87 gelatinous debris surrounding the base of the lashes termed cylindrical dandruff,90 and non-specific blepharitis signs as stated above. Cylindrical dandruff is a pathognomonic sign for Demodex.90 On the other hand, the parasite Phthirus pubis is not found in the normal lid microflora, as it originates in pubic hair. It can be transmitted by sexual contact and less often through linens and bedding.91 Transfer of the parasite from the infested region to the hand can reach any hair on the body, including the eyelashes.92 The resulting blepharitis is termed Phthiriasis palpebrarum and its clinical presentation, in one or both eyes, includes the translucent parasite's body firmly attached to lashes, brown scales corresponding to the parasite's feces, and multiple nits (unhatched parasites) fixed on the lashes and the lid margin.91, 92 Symptoms include itchiness and irritation of the lid margin.91
Finally, blepharitis can be the result of seborrheic dermatitis, a dermatological condition affecting the skin and in some cases, the lid margin. Many etiologies have been proposed and researchers are still looking for the exact mechanism.93 An imbalance in the skin micro-organisms might be the cause.93 Other than the non-specific signs of blepharitis detailed above, ECPs can observe some flaky scales on the lids that can fall on the lashes.94 When the condition affects the anterior portion of the lid, it is called seborrheic blepharitis.
Management of anterior blepharitis
The aim in blepharitis management is to restore the normal microflora.95 A complete eradication of Phthiriasis palpebrarum is desired and can be achieved by mechanical removal of the parasites and nits.85, 96 Blepharitis secondary to the imbalance of the microflora are chronic conditions. Hence, they are not expected to be completely eradicated. First line therapies of blepharitis include warm compresses to soften the debris and eyelid hygiene to manually dislodge them.97 Targeted lid margin therapies should be adopted by ECPs, including antibacterial for Staphylococcal infestation87 and anti-parasitic formulations for Demodex parasites,98, 99 in order to limit lid margin anomalies that come with chronic blepharitis. To target properly seborrheic blepharitis, a reference to dermatology is required to establish the diagnosis of seborrheic dermatitis and treat the affected skin with the appropriate pharmaceutical.93
ECP assessment of lashes
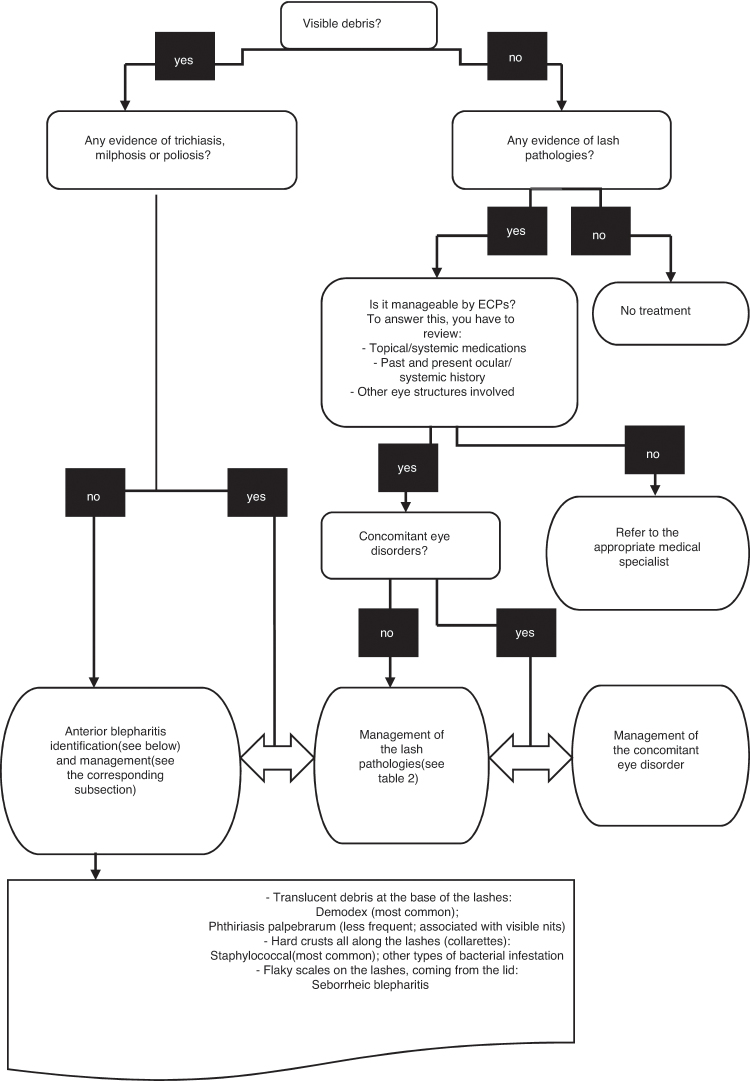
Fig. 4 summarizes in a flowchart the management of debris on eyelashes. This tool can be used when teaching students or residents to identify and localize debris on lashes and select the appropriate management.
Figure 4.
Eyelash debris assessment by eyecare professionals.
Iatrogenic factors
The appearance of eyelashes is an increasingly growing preoccupation in many people who are seeking an esthetic enhancement. Cosmetics have been used since biblical times to improve the esthetic of the eye and the thickness, length and color of eyelashes.100 Lash tints and extensions are newer and popular, surpassing expectations in the cosmetic marketplace. Over $55 million in 2014 were dedicated to eyelash extension and adhesives alone in the US.101 Synthetic lashes are glued individually on the natural lashes with different adhesives, which have been linked to ocular problems such as keratoconjunctivitis.102 In most cases, the glue and removing agents have induced inflammation on the eye, by direct contact with the ocular surface. Furthermore, the vapors associated with the application, or the dissolving, of the glue afterwards have been reported as ocular irritants. Lash tinting is done with dyes that contain p-phenylenediamine, a sensitizer that can provoke an allergic reaction and contact dermatitis.103, 104 Water-based mascara is made of several waxes, types of pigment and resins dissolved in water, whereas solvent-based mascara, known as waterproof, has its pigments and waxes added in petroleum distillates.100 Fukami et al. found a positive correlation between the frequency of mascara use and the degree of cracking in the lash cuticles.105 Also, long-term use of mascara led to milphosis, possibly due to the rubbing, with fingers and water only, by the users.106 Needless to say, eye cosmetics in general can have an impact on the lids, lashes, tear film and ocular surface.107 Consequently, patients need to be educated appropriately about the application, removal, shelf life and associated precautions of cosmetic use.
Conclusion
This review brings a deeper awareness on the eyelashes and their follicles. The current literature has numerous cases of how lashes are affected by systemic/eye diseases, pharmaceuticals and cosmetics, and how they change the lash morphology. In addition, an imbalance of the lash microenvironment can lead to a variety of blepharitis and impact negatively the adjoining lid margin. Many studies have demonstrated that lashes do not serve only a cosmetic function, but also a protective role on the lid margin and the ocular surface. The lash follicle structures are mostly studied with ex vivo techniques, which are limited by the poor availability of the human lid sampling. This review further highlights the challenges when studying the lash follicle and the need to develop newer techniques. Increasing lash research will certainly improve the efficiency of ECP's interventions in lash anomalies.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
SA has received honorarium for conferences/consulting from Allergan and Shire. EB has received funding from Alcon, Allergan, Canadian Optometric Trust Fund, I-Med Pharma Inc, Shire and honorarium for conferences/consulting from Akorn, Alcon, Allergan, American Academy of Optometry, Canadian Association of Optometry, CooperVision, Labtician, Jobson Publishing, Novartis, Santen, Shire.
Acknowledgement
A special thanks to Micheline Gloin for her graphic assistance.
References
- 1.Jones D. Enhanced eyelashes: prescription and over-the-counter options. Aesthet Plast Surg. 2011;35:116–121. doi: 10.1007/s00266-010-9561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Draelos Z.K. Eye cosmetics. Dermatol Clin. 1991;9:1–7. [PubMed] [Google Scholar]
- 3.Montagna W., Ford D.M. Histology and cytochemistry of human skin 3. The eyelid. Arch Dermatol. 1969;100:328–335. [PubMed] [Google Scholar]
- 4.Willcox M.D.P., Argueso P., Georgiev G.A. TFOS DEWS II Tear Film Report. Ocul Surf. 2017;15:366–403. doi: 10.1016/j.jtos.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig J.P., Nichols K.K., Akpek E.K. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15:276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Liotet S., Riera M., Nguyen N.H. Les cils. Physiologie, structure, pathologie. Arch Ophtalmol (Paris) 1977;37:697–708. [PubMed] [Google Scholar]
- 7.Na J.I., Kwon O.S., Kim B.J. Ethnic characteristics of eyelashes: a comparative analysis in Asian and Caucasian females. Br J Dermatol. 2006;155:1170–1176. doi: 10.1111/j.1365-2133.2006.07495.x. [DOI] [PubMed] [Google Scholar]
- 8.Marieb E. Le système tégumentaire. In: ERPi, editor. Anatomie et physiologie humaines. 3rd ed. 2005. pp. 156–208. [Google Scholar]
- 9.Thibaut S., De Becker E., Caisey L. Human eyelash characterization. Br J Dermatol. 2010;162:304–310. doi: 10.1111/j.1365-2133.2009.09487.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith K.R., Thiboutot D.M. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res. 2008;49:271–281. doi: 10.1194/jlr.R700015-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Stoeckelhuber M., Stoeckelhuber B.M., Welsch U. Human glands of Moll: histochemical and ultrastructural characterization of the glands of Moll in the human eyelid. J Invest Dermatol. 2003;121:28–36. doi: 10.1046/j.1523-1747.2003.12328.x. [DOI] [PubMed] [Google Scholar]
- 12.Kloepper J.E., Sugawara K., Al-Nuaimi Y., Gaspar E., van Beek N., Paus R. Methods in hair research: how to objectively distinguish between anagen and catagen in human hair follicle organ culture. Exp Dermatol. 2010;19:305–312. doi: 10.1111/j.1600-0625.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- 13.Paus R., Burgoa I., Platt C.I., Griffiths T., Poblet E., Izeta A. Biology of the eyelash hair follicle: an enigma in plain sight. Br J Dermatol. 2016;174:741–752. doi: 10.1111/bjd.14217. [DOI] [PubMed] [Google Scholar]
- 14.Nesher R., Mimouni M., Elnaddaf H., Nemet A., Kidron D. Characterization of prostaglandin F2alpha receptors in human eyelids. Eur J Ophthalmol. 2015;25:81–84. doi: 10.5301/ejo.5000519. [DOI] [PubMed] [Google Scholar]
- 15.Thibaut S., Gaillard O., Bouhanna P., Cannell D.W., Bernard B.A. Human hair shape is programmed from the bulb. Br J Dermatol. 2005;152:632–638. doi: 10.1111/j.1365-2133.2005.06521.x. [DOI] [PubMed] [Google Scholar]
- 16.Thibaut S., Barbarat P., Leroy F., Bernard B.A. Human hair keratin network and curvature. Int J Dermatol. 2007;46:7–10. doi: 10.1111/j.1365-4632.2007.03454.x. [DOI] [PubMed] [Google Scholar]
- 17.Commo S., Gaillard O., Thibaut S., Bernard B.A. Absence of TRP-2 in melanogenic melanocytes of human hair. Pigment Cell Res. 2004;17:488–497. doi: 10.1111/j.1600-0749.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- 18.Schallreuter K., Slominski A., Pawelek J.M., Jimbow K., Gilchrest B.A. What controls melanogenesis? Exp Dermatol. 1998;7:143–150. doi: 10.1111/j.1600-0625.1998.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 19.Commo S., Gaillard O., Bernard B.A. Human hair greying is linked to a specific depletion of hair follicle melanocytes affecting both the bulb and the outer root sheath. Br J Dermatol. 2004;150:435–443. doi: 10.1046/j.1365-2133.2004.05787.x. [DOI] [PubMed] [Google Scholar]
- 20.Tobin D.J. Human hair pigmentation – biological aspects. Int J Cosmet Sci. 2008;30:233–257. doi: 10.1111/j.1468-2494.2008.00456.x. [DOI] [PubMed] [Google Scholar]
- 21.Arck P.C., Overall R., Spatz K. Towards a “free radical theory of graying”: melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006;20:1567–1569. doi: 10.1096/fj.05-4039fje. [DOI] [PubMed] [Google Scholar]
- 22.Procianoy F., Mendonca T.B., Bins C.A., Lang M.P. Characterization of normal mediolateral angular direction of lower eyelid eyelashes in different age groups. Ophthalmic Plast Reconstr Surg. 2015;31:332–333. doi: 10.1097/IOP.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 23.Glaser D.A., Jones D., Carruthers J. Epidemiologic analysis of change in eyelash characteristics with increasing age in a population of healthy women. Dermatol Surg. 2014;40:1208–1213. doi: 10.1097/DSS.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 24.Harries M.J., Meyer K., Chaudhry I. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle's epithelial stem cell niche. J Pathol. 2013;231:236–247. doi: 10.1002/path.4233. [DOI] [PubMed] [Google Scholar]
- 25.Philpott M.P., Green M.R., Kealey T. Human hair growth in vitro. J Cell Sci. 1990;97(Pt 3):463–471. doi: 10.1242/jcs.97.3.463. [DOI] [PubMed] [Google Scholar]
- 26.Langan E.A., Philpott M.P., Kloepper J.E., Paus R. Human hair follicle organ culture: theory, application and perspectives. Exp Dermatol. 2015;24:903–911. doi: 10.1111/exd.12836. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto T., Kazama T., Ito M. Histologic and cell kinetic studies of hair loss and subsequent recovery process of human scalp hair follicles grafted onto severe combined immunodeficient mice. J Invest Dermatol. 2000;115:200–206. doi: 10.1046/j.1523-1747.2000.00063.x. [DOI] [PubMed] [Google Scholar]
- 28.Gilhar A., Keren A., Paus R. A new humanized mouse model for alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S37–S38. doi: 10.1038/jidsymp.2013.11. [DOI] [PubMed] [Google Scholar]
- 29.Oh J.W., Kloepper J., Langan E.A. A guide to studying human hair follicle cycling in vivo. J Invest Dermatol. 2016;136:34–44. doi: 10.1038/JID.2015.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weatherall D., Munn H. Moving the primate debate forward. Science. 2007;316:173. doi: 10.1126/science.1142606. [DOI] [PubMed] [Google Scholar]
- 31.Debeer S., Le Luduec J.B., Kaiserlian D. Comparative histology and immunohistochemistry of porcine versus human skin. Eur J Dermatol. 2013;23:456–466. doi: 10.1684/ejd.2013.2060. [DOI] [PubMed] [Google Scholar]
- 32.Mangelsdorf S., Vergou T., Sterry W., Lademann J., Patzelt A. Comparative study of hair follicle morphology in eight mammalian species and humans. Skin Res Technol. 2014;20:147–154. doi: 10.1111/srt.12098. [DOI] [PubMed] [Google Scholar]
- 33.Tauchi M., Fuchs T.A., Kellenberger A.J., Woodward D.F., Paus R., Lutjen-Drecoll E. Characterization of an in vivo model for the study of eyelash biology and trichomegaly: mouse eyelash morphology, development, growth cycle, and anagen prolongation by bimatoprost. Br J Dermatol. 2010;162:1186–1197. doi: 10.1111/j.1365-2133.2010.09685.x. [DOI] [PubMed] [Google Scholar]
- 34.Dhurat R., Phototrichogram Indian J Dermatol Venereol Leprol. 2006;72:242–244. doi: 10.4103/0378-6323.25795. [DOI] [PubMed] [Google Scholar]
- 35.Amador G.J., Mao W., DeMercurio P. Eyelashes divert airflow to protect the eye. J R Soc Interface. 2015;12 doi: 10.1098/rsif.2014.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sleiman R., Kurban M., Succaria F., Abbas O. Poliosis circumscripta: overview and underlying causes. J Am Acad Dermatol. 2013;69:625–633. doi: 10.1016/j.jaad.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Meyer K.C., Brzoska T., Abels C., Paus R. The alpha-melanocyte stimulating hormone-related tripeptide K(D)PT stimulates human hair follicle pigmentation in situ under proinflammatory conditions. Br J Dermatol. 2009;160:433–437. doi: 10.1111/j.1365-2133.2008.08872.x. [DOI] [PubMed] [Google Scholar]
- 38.Dutton J.J., Tawfik H.A., DeBacker C.M., Lipham W.J. Direct internal eyelash bulb extirpation for trichiasis. Ophthalmic Plast Reconstr Surg. 2000;16:142–145. doi: 10.1097/00002341-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira I.S., Bernardes T.F., Bonfioli A.A. Trichiasis. Semin Ophthalmol. 2010;25:66–71. doi: 10.3109/08820538.2010.488580. [DOI] [PubMed] [Google Scholar]
- 40.Law S.K. Bimatoprost in the treatment of eyelash hypotrichosis. Clin Ophthalmol. 2010;4:349–358. doi: 10.2147/opth.s6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vij A., Bergfeld W.F. Madarosis, milphosis, eyelash trichomegaly, and dermatochalasis. Clin Dermatol. 2015;33:217–226. doi: 10.1016/j.clindermatol.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Wendelin D.S., Pope D.N., Mallory S.B. Hypertrichosis. J Am Acad Dermatol. 2003;48:161–182. doi: 10.1067/mjd.2003.100. [DOI] [PubMed] [Google Scholar]
- 43.Almagro M., del Pozo J., Garcia-Silva J., Martinez W., Castro A., Fonseca E. Eyelash length in HIV-infected patients. AIDS. 2003;17:1695–1696. doi: 10.1097/00002030-200307250-00015. [DOI] [PubMed] [Google Scholar]
- 44.Paul L.J., Cohen P.R., Kurzrock R. Eyelash trichomegaly: review of congenital, acquired, and drug-associated etiologies for elongation of the eyelashes. Int J Dermatol. 2012;51:631–646. doi: 10.1111/j.1365-4632.2011.05315.x. 643-634, 646. [DOI] [PubMed] [Google Scholar]
- 45.Santmyire-Rosenberger B.R., Albert M. Acquired trichomegaly with topiramate. J Am Acad Dermatol. 2005;53:362–363. doi: 10.1016/j.jaad.2005.01.121. [DOI] [PubMed] [Google Scholar]
- 46.Dessinioti C., Stratigos A.J., Rigopoulos D., Katsambas A.D. A review of genetic disorders of hypopigmentation: lessons learned from the biology of melanocytes. Exp Dermatol. 2009;18:741–749. doi: 10.1111/j.1600-0625.2009.00896.x. [DOI] [PubMed] [Google Scholar]
- 47.Tabbara K.F. Reversal of poliosis and vitiligo following Vogt-Koyanagi-Harada disease. Arch Ophthalmol. 2012;130:394–396. doi: 10.1001/archopthalmol.2011.1520. [DOI] [PubMed] [Google Scholar]
- 48.Moorthy R.S., Inomata H., Rao N.A. Vogt-Koyanagi-Harada syndrome. Surv Ophthalmol. 1995;39:265–292. doi: 10.1016/s0039-6257(05)80105-5. [DOI] [PubMed] [Google Scholar]
- 49.Kumar S., Al Khars W. Vitiligo in association with vernal keratoconjunctivitis. Saudi J Ophthalmol. 2016;30:128–129. doi: 10.1016/j.sjopt.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakakura S., Yamamoto M., Terao E. Prostaglandin-associated periorbitopathy in latanoprost users. Clin Ophthalmol. 2015;9:51–56. doi: 10.2147/OPTH.S75651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen C.S., Wells J., Craig J.E. Topical prostaglandin F(2alpha) analog induced poliosis. Am J Ophthalmol. 2004;137:965–966. doi: 10.1016/j.ajo.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 52.Ozyurt S., Cetinkaya G.S. Hypertrichosis of the malar areas and poliosis of the eyelashes caused by latanoprost. Actas Dermosifiliogr. 2015;106:74–75. doi: 10.1016/j.ad.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Waheed K., Laganowski H. Bilateral poliosis and granulomatous anterior uveitis associated with latanoprost use and apparent hypotrichosis on its withdrawal. Eye (Lond) 2001;15:347–349. doi: 10.1038/eye.2001.116. [DOI] [PubMed] [Google Scholar]
- 54.Jalalat S.Z., Kelsoe J.R., Cohen P.R. Alopecia areata with white hair regrowth: case report and review of poliosis. Dermatol Online J. 2014;20 [PubMed] [Google Scholar]
- 55.Chiou A.G.Y., Florakis G.J., Kazim M. Management of conjunctival cicatrizing diseases and severe ocular surface dysfunction. Surv Ophthalmol. 1998;43:19–46. doi: 10.1016/s0039-6257(98)00005-8. [DOI] [PubMed] [Google Scholar]
- 56.Yung C.W., Massicotte S.J., Kuwabara T. Argon laser treatment of trichiasis: a clinical and histopathologic evaluation. Ophthalmic Plast Reconstr Surg. 1994;10:130–136. doi: 10.1097/00002341-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Basar E., Ozdemir H., Ozkan S., Cicik E., Mirzatas C. Treatment of trichiasis with argon laser. Eur J Ophthalmol. 2000;10:273–275. doi: 10.1177/112067210001000401. [DOI] [PubMed] [Google Scholar]
- 58.Benson A. On the treatment of partial trichiasis by electrolysis. Br Med J. 1882;2:1203–1204. doi: 10.1136/bmj.2.1146.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCracken M.S., Kikkawa D.O., Vasani S.N. Treatment of trichiasis and distichiasis by eyelash trephination. Ophthalmic Plast Reconstr Surg. 2006;22:349–351. doi: 10.1097/01.iop.0000229872.81219.9e. [DOI] [PubMed] [Google Scholar]
- 60.Tirakunwichcha S., Tinnangwattana U., Hiranwiwatkul P., Rohitopakarn S. Folliculectomy: management in segmental trichiasis and distichiasis. J Med Assoc Thai. 2006;89:90–93. [PubMed] [Google Scholar]
- 61.Elder M.J., Bernauer W. Cryotherapy for trichiasis in ocular cicatricial pemphigoid. Br J Ophthalmol. 1994;78:769–771. doi: 10.1136/bjo.78.10.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ballen P.H. A simple procedure for the relief of trichiasis and entropion of the upper lid. Arch Ophthalmol. 1964;72:239–240. doi: 10.1001/archopht.1964.00970020239019. [DOI] [PubMed] [Google Scholar]
- 63.Kumar A., Karthikeyan K. Madarosis: a marker of many maladies. Int J Trichol. 2012;4:3–18. doi: 10.4103/0974-7753.96079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khong J.J., Casson R.J., Huilgol S.C., Selva D. Madarosis. Surv Ophthalmol. 2006;51:550–560. doi: 10.1016/j.survophthal.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Barron-Hernandez Y.L., Tosti A. Bimatoprost for the treatment of eyelash, eyebrow and scalp alopecia. Expert Opin Investig Drugs. 2017;26:515–522. doi: 10.1080/13543784.2017.1303480. [DOI] [PubMed] [Google Scholar]
- 66.Zaleski-Larsen L.A., Ruth N.H., Fabi S.G. Retrospective evaluation of topical bimatoprost and iris pigmentation change. Dermatol Surg. 2017 doi: 10.1097/DSS.0000000000001208. [DOI] [PubMed] [Google Scholar]
- 67.Smith S., Fagien S., Whitcup S.M. Eyelash growth in subjects treated with bimatoprost: a multicenter, randomized, double-masked, vehicle-controlled, parallel-group study. J Am Acad Dermatol. 2012;66:801–806. doi: 10.1016/j.jaad.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;3:39–48. doi: 10.2147/ccid.s5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaur S., Mahajan B.B. Eyelash trichomegaly. Indian J Dermatol. 2015;60:378–380. doi: 10.4103/0019-5154.160484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lacouture M.E., Boerner S.A., Lorusso P.M. Non-rash skin toxicities associated with novel targeted therapies. Clin Lung Cancer. 2006;8(suppl 1):S36–S42. doi: 10.3816/clc.2006.s.012. [DOI] [PubMed] [Google Scholar]
- 71.Robert C., Soria J.C., Spatz A. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005;6:491–500. doi: 10.1016/S1470-2045(05)70243-6. [DOI] [PubMed] [Google Scholar]
- 72.Dueland S., Sauer T., Lund-Johansen F., Ostenstad B., Tveit K.M. Epidermal growth factor receptor inhibition induces trichomegaly. Acta Oncol. 2003;42:345–346. doi: 10.1080/02841860310006038. [DOI] [PubMed] [Google Scholar]
- 73.Christenson G.A., Popkin M.K., Mackenzie T.B., Realmuto G.M. Lithium treatment of chronic hair pulling. J Clin Psychiatry. 1991;52:116–120. [PubMed] [Google Scholar]
- 74.Johnson J., El-Alfy A.T. Review of available studies of the neurobiology and pharmacotherapeutic management of trichotillomania. J Adv Res. 2016;7:169–184. doi: 10.1016/j.jare.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Phillips T.G., Slomiany W.P., Allison R. Hair loss: common causes and treatment. Am Fam Physician. 2017;96:371–378. [PubMed] [Google Scholar]
- 76.Dunnill C.J., Al-Tameemi W., Collett A., Haslam I.S., Georgopoulos N.T. A clinical and biological guide for understanding chemotherapy-induced alopecia and its prevention. Oncologist. 2017 doi: 10.1634/theoncologist.2017-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu L.Y., King B.A., Craiglow B.G. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806–812. doi: 10.1016/j.jaad.2016.04.035. e803. [DOI] [PubMed] [Google Scholar]
- 78.Dainichi T., Kabashima K. Alopecia areata: what's new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3–12. doi: 10.1016/j.jdermsci.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 79.Rencz F., Gulacsi L., Pentek M., Wikonkal N., Baji P., Brodszky V. Alopecia areata and health-related quality of life: a systematic review and meta-analysis. Br J Dermatol. 2016;175:561–571. doi: 10.1111/bjd.14497. [DOI] [PubMed] [Google Scholar]
- 80.Khafagy A., Mostafa M.M., Fooshan F. Management of trichiasis with lid margin split and cryotherapy. Clin Ophthalmol. 2012;6:1815–1817. doi: 10.2147/OPTH.S35678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choo P.H. Distichiasis, trichiasis, and entropion: advances in management. Int Ophthalmol Clin. 2002;42:75–87. doi: 10.1097/00004397-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 82.Nicholls S.G., Oakley C.L., Tan A., Vote B.J. Demodex species in human ocular disease: new clinicopathological aspects. Int Ophthalmol. 2017;37:303–312. doi: 10.1007/s10792-016-0249-9. [DOI] [PubMed] [Google Scholar]
- 83.Lee S.H., Oh D.H., Jung J.Y., Kim J.C., Jeon C.O. Comparative ocular microbial communities in humans with and without blepharitis. Invest Ophthalmol Vis Sci. 2012;53:5585–5593. doi: 10.1167/iovs.12-9922. [DOI] [PubMed] [Google Scholar]
- 84.Bernardes T.F., Bonfioli A.A. Blepharitis. Semin Ophthalmol. 2010;25:79–83. doi: 10.3109/08820538.2010.488562. [DOI] [PubMed] [Google Scholar]
- 85.Padhi T.R., Das S., Sharma S. Ocular parasitoses: a comprehensive review. Surv Ophthalmol. 2017;62:161–189. doi: 10.1016/j.survophthal.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 86.Roth A.M. Demodex folliculorum in hair follicles of eyelid skin. Ann Ophthalmol. 1979;11:37–40. [PubMed] [Google Scholar]
- 87.Liu J., Sheha H., Tseng S.C. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol. 2010;10:505–510. doi: 10.1097/ACI.0b013e32833df9f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Szkaradkiewicz A., Chudzicka-Strugala I., Karpinski T.M. Bacillus oleronius and Demodex mite infestation in patients with chronic blepharitis. Clin Microbiol Infect. 2012;18:1020–1025. doi: 10.1111/j.1469-0691.2011.03704.x. [DOI] [PubMed] [Google Scholar]
- 89.Duncan K., Jeng B.H. Medical management of blepharitis. Curr Opin Ophthalmol. 2015;26:289–294. doi: 10.1097/ICU.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 90.Gao Y.Y., Di Pascuale M.A., Li W. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46:3089–3094. doi: 10.1167/iovs.05-0275. [DOI] [PubMed] [Google Scholar]
- 91.Anane S., Malek I., Kamoun R., Chtourou O. Phthiriasis palpebrarum: diagnosis and treatment. J Fr Ophtalmol. 2013;36:815–819. doi: 10.1016/j.jfo.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 92.Turgut B., Kurt J., Catak O., Demir T. Phthriasis palpebrarum mimicking lid eczema and blepharitis. J Ophthalmol. 2009;2009:803951. doi: 10.1155/2009/803951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paulino L.C. New perspectives on dandruff and seborrheic dermatitis: lessons we learned from bacterial and fungal skin microbiota. Eur J Dermatol. 2017;27:4–7. doi: 10.1684/ejd.2017.3038. [DOI] [PubMed] [Google Scholar]
- 94.Wolf R., Orion E., Tuzun Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131–140. doi: 10.1016/j.clindermatol.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 95.Nicholls S.G., Oakley C.L., Tan A., Vote B.J. Demodex species in human ocular disease: new clinicopathological aspects. Int Ophthalmol. 2016 doi: 10.1007/s10792-016-0249-9. [DOI] [PubMed] [Google Scholar]
- 96.Dagdelen S., Aykan U., Cetinkaya K. Phthriasis palpebrarum can resemble tick larva infestation in an eyelid. J AAPOS. 2013;17:440–442. doi: 10.1016/j.jaapos.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 97.Lindsley K., Matsumura S., Hatef E., Akpek E.K. Interventions for chronic blepharitis. Cochrane Database Syst Rev. 2012:CD005556. doi: 10.1002/14651858.CD005556.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao Y.Y., Di Pascuale M.A., Li W. In vitro and in vivo killing of ocular Demodex by tea tree oil. Br J Ophthalmol. 2005;89:1468–1473. doi: 10.1136/bjo.2005.072363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ngo W., Jones L., Bitton E. Short-term comfort responses associated with the use of eyelid cleansing products to manage Demodex folliculorum. Eye Contact Lens. 2017 doi: 10.1097/ICL.0000000000000415. [DOI] [PubMed] [Google Scholar]
- 100.Draelos Z.D. Special considerations in eye cosmetics. Clin Dermatol. 2001;19:424–430. doi: 10.1016/s0738-081x(01)00204-8. [DOI] [PubMed] [Google Scholar]
- 101.Statista . 2017. Annual sales of the leading false eyelashes and adhesives in the United States in 2014 (in million U.S. dollars) [Google Scholar]
- 102.Amano Y., Sugimoto Y., Sugita M. Ocular disorders due to eyelash extensions. Cornea. 2012;31:121–125. doi: 10.1097/ICO.0b013e31821eea10. [DOI] [PubMed] [Google Scholar]
- 103.Teixeira M., de Wachter L., Ronsyn E., Goossens A. Contact allergy to para-phenylenediamine in a permanent eyelash dye. Contact Dermatitis. 2006;55:92–94. doi: 10.1111/j.0105-1873.2006.00883.x. [DOI] [PubMed] [Google Scholar]
- 104.Ali L., Foulds J.S., Abdul Ghaffar S. Severe eyelid allergic contact dermatitis secondary to eyelash tint: two case reports. Contact Dermatitis. 2017;77:59–60. doi: 10.1111/cod.12749. [DOI] [PubMed] [Google Scholar]
- 105.Fukami K., Inoue T., Kawai T., Takeuchi A., Uesugi K., Suzuki Y. Internal structure changes of eyelash induced by eye makeup. J Cosmet Sci. 2014;65:217–224. [PubMed] [Google Scholar]
- 106.Kadri R., Achar A., Tantry T.P., Parameshwar D., Kudva A., Hegde S. Mascara induced milphosis, an etiological evaluation. Int J Trichol. 2013;5:144–147. doi: 10.4103/0974-7753.125611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ng A., Evans K., North R.V., Jones L., Purslow C. Impact of eye cosmetics on the eye adnexa, and ocular surface. Eye Contact Lens. 2016;42:211–220. doi: 10.1097/ICL.0000000000000181. [DOI] [PubMed] [Google Scholar]