Human papillomavirus (HPV) is a small (55 nm) non-enveloped virus that is known to cause a variety of lesions such as skin warts, mucosal papillomas and lesions in the anogenital area called condylomas (Figure 1).1–3 By far the most serious and clinically significant lesions however are the cervical carcinoma and precancerous intraepithelial lesions of the cervix.4 In recent years, because of availability of sensitive molecular techniques, significant strides have been made in our understanding of the nature of the virus and the various pathways involved in carcinogenesis. This knowledge has also contributed to the development of new strategies for prevention and treatment of these serious complications of HPV infection.1,5
Figure 1.
High magnification electron micrograph featuring large numbers of non-enveloped viral structures.
Epidemiology and Natural History
Cervical cancer is the second most common cancer among women after breast cancer. Worldwide approximately 466 000 new cases are diagnosed every year and around 1.4 million women are living with invasive cervical cancer, most of whom are in developing countries. Cervical cancer is a leading cause of death among women in these countries. In the developing countries, the precancerous intraepithelial lesion is recognized early due to mass screening programs, and this has lead to a dramatic decrease in the prevalence of invasive cervical cancer.6
Cervical cancer and its precursor lesions are encountered in women who have had sexual intercourse. A prerequisite for the development of cervical cancer is infection by HPV, which is a sexually transmitted disease.1,7 There are more than 120 types of HPV with approximately 40 anogenital types which are usually divided into lowand high-risk groups based on their oncogenic potential. The common types in the low risk group are 6, 11 and occasionally 42, 43, 44 and 54. Most of the benign lesions such condylomas and genital warts are caused by these types. These types may also be involved in low-grade intraepithelial lesions of the cervix. The high-risk group consists of 16, 18, 31, 33, 39, 45, 51, 52, 54, 56, 58, 59, 66, and 68. This group is generally involved in low- and high-grade intraepithelial cervical lesions and invasive carcinoma of the cervix. Type 16 is the most commonly encountered HPV type in invasive carcinoma. Worldwide, more than eighty percent of invasive cervical carcinomas are caused by 16, 18, 31, and 45. Mixed infections by more than one type may also be encountered in these cases.1,6
The male sexual partner acts as the carrier for HPV infection.8 The penile skin hosts the virus although it is only rarely involved in carcinoma. Male circumcision may reduce the incidence of infection probably due to better hygiene. During sexual intercourse an infected male may transmit the infection to the female partner. The most vulnerable site of infection in the anogenital region is the transformation zone of the cervix.1,9 The virus initially infects the cells in the basal layer where the presence of integrins act as receptors. Once inside the cell the virus can exist within the cell in two distinct biological states. In the latent form, a small number of viral copies may continue to reside inside the nucleus of the basal cell in a free circular form called an episome. The cells with latent HPV infection appear normal and the detection of virus within these cells requires sensitive molecular techniques. The second mode of viral infection is the productive form in which viral replication is independent of the host chromosomal DNA synthesis, resulting in a large number of virions. The presence of large numbers of virions within the cell results in a cytopathic effect, which is recognizable cytologically and histologically as acanthosis, cytoplasmic vacuolation, koilcytosis, multinucleation and nuclear atypia.
Human papilloma virus seems to evade successfully the immune system of the body10,11 A significant antibody response to infection is only seen in approximately 50% of infected individuals. These antibodies are directed against the capsid proteins of the virus. The response however takes several months to develop. An existing high titer of neutralizing antibody may successfully prevent infection by HPV. Delayed-type hypercellularity may also develop and may lead to regression of lesions. This involves a cytotoxic T-cell response encompassing natural killer cells and antigen-specific cytotoxic T cells. That the role of cell-mediated immunity is important in control of established infections is consistent with an increased prevalence of HPV infection and cervical intraepithelial lesions in immunocompromised patients.
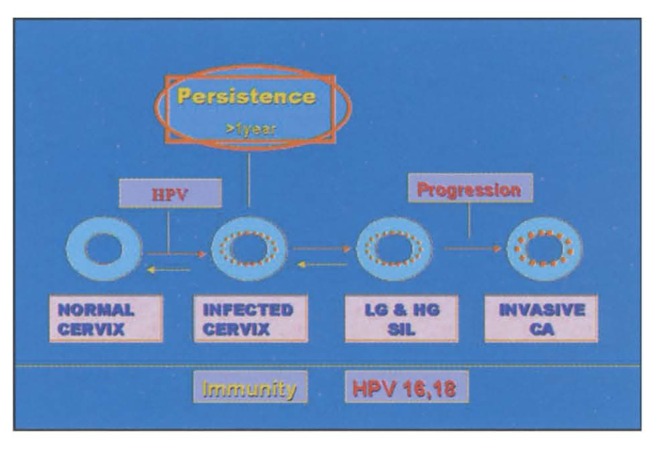
The natural history of HPV infection is that most sexually active women are exposed to the virus after initiating sexual activity. The risk of infection is much greater in women who have multiple sex partners.1,5 Most of the women develop transient HPV infections, which last for several months but are eventually eliminated so that most of these HPV-infected women become HPV negative. Acquisition of HPV infection and its eventual clearance is a dynamic process in which several additional factors come into play and determine the final outcome. Thus women who have multiple partners may become infected repeatedly. Immunity may also play an important role in elimination of the virus. Furthermore, the viral elimination is much more rapid in infections by low-grade types such as 6 and 11. Only a small proportion of women become persistently infected and continue to have detectable HPV DNA in genital epithelium one year after infection (Figure 2). It is these persistently infected women who are at risk of developing intraepithelial neoplastic lesions and subsequently developing carcinoma.12 High-grade intraepithelial lesions and cervical cancer almost exclusively develop in women carrying infection by high-risk HPV types, especially type 16. The transformation from high-grade intraepithelial lesion to invasive carcinoma takes approximately ten years. The lesions caused by persistent infection by low-risk HPV are self-limiting and ultimately undergo regression.
Figure 2.
A diagram depicting the natural history of human papillomavirus.
Development of cervical carcinoma and precancerous intraepithelial lesions is a complex multifactorial process in which infection by high-risk HPV plays a pivotal role. In addition several co-factors are also thought to contribute significantly towards development of malignancy13 These include smoking, concomitant infections by chlamydia and HSV2, prolonged use of contraceptives and immunosuppression by HIV infection.
Molecular Biology
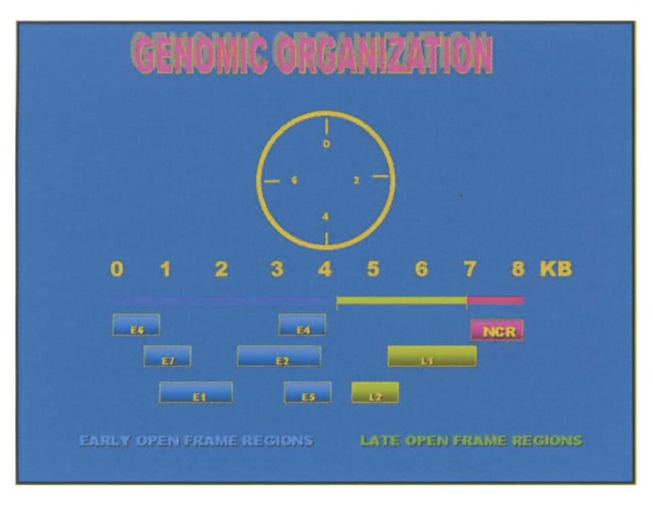
Understanding the process of malignant transformation of cells infected by HPV requires some understanding of the genomic organization of HPV and the interaction between the host and the proteins encoded by the virus DNA. HPV is a small (55 nm) non-enveloped virus with a circular double stranded DNA virus consisting of approximately 7.9 kilo base pairs.1,14 The genome is made up of early and late open reading frames (ORFs) and a noncoding region called the upstream regulatory region (UPR), also termed the long central region (LCR), that is vital for viral replication because it contains a complex array of transcriptional activators and repressors. The early region ORFs encode for proteins required for viral replication. Six different ORFs have been identified in the early region. These are E1, E2, E4, E5, E6, and E7. The late region of HPV is downstream of the early region and contains two ORFs, termed L1 and L2, which encode viral capsid proteins (Figure 3). These may also give rise to virus-like particles (VLPs), which are composed of capsid proteins without the viral DNA.
Figure 3.
Human papillomavirus genome consists of several regions encoding a series of proteins (modified from Figure 2 in reference 1).
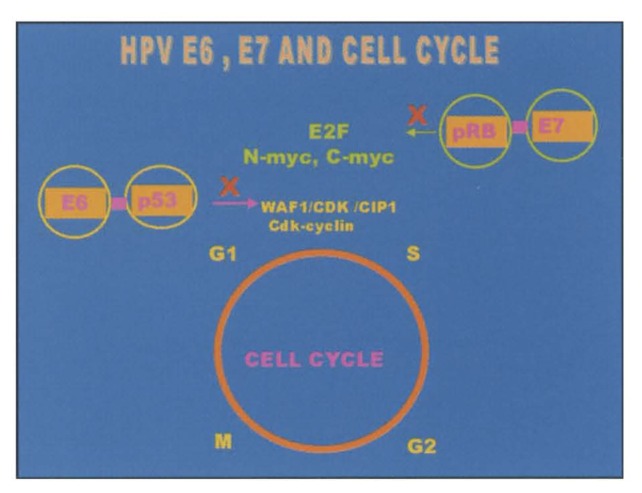
Expression of E6 and E7 proteins encoded by high-risk HPV such as 16 and 18 in established tissue culture cell lines causes the cells to become completely transformed. Such an effect is not evident with low-risk HPV. In addition to having in vitro transforming activity, both E6 and E7 are always actively transcribed in cervical cancer, suggesting that unregulated expression of these genes plays a significant role in carcinogenesis and maintenance of the malignant phenotype. These proteins interact with cellular proteins such as p53 and the product of retinoblatoma gene (pRb), which is normally responsible for controlling key steps in the cell cycle. P53 is a key cellular regulatory gene that acts as a transcriptional activator and has characteristics of a tumor suppressor gene. In non-infected cells, p53 levels increase in response to cellular or DNA damage or aberrant cellular proliferation signals. High levels of p53, by interacting with WAF1/cipl and cdk-cyclin, induce the cell to undergo growth arrest in the G1 phase of the cell cycle, which provides an opportunity for the cell to either repair DNA damage or be eliminated through programmed cell death (apoptosis). In HPV-infected cells E6 protein from high-risk HPVs binds to p53 thus causing loss of its crucial role in controlling the cell cycle.15 pRb also plays a crucial role in controlling the transition from G1 to the S phase of the cell cycle by interacting with other cell cycle proteins including E2F, c-myc and n-myc. E7 encoded by high-risk HPVs binds with pRb, blocking its ability to interact with E2F and unregulated proliferation occurs.16 On the other hand, E6 and E7 from low-risk HPVs have a lower affinity for p53 and pRB and therefore are not oncogenic (Figure 4).
Figure 4.
Diagram depicting interaction between cell cycle proteins (p53 and pRb) and HPV encide proteins E6 and E7.
Prevention Strategies
Cervical cytology screening has been significantly responsible for a declining incidence of cervical cancer in large parts of the world.17 Mass screening plays a preventive role by identifying precursor lesions, which can be treated effectively thus reducing the prevalence of invasive cervical carcinoma. Since HPV infection is sexually transmitted, a change in sexual habits and behavior may also have a protective influence by preventing infection not only of HPV but also by other infectious agents such as HIV, HSV2 and Chlamydia trachomatis, which may act as co-factors for development of cervical cancer. Modulation of other co-factors such as smoking and long-term use of contraceptive hormones may also contribute toward prevention of malignant transformation. It is also important to provide public education to both men and women regarding the HPV infection and the steps that may be taken to prevent serious complications.
The most promising and exciting method for prophylaxis of HPV infection and its complications is vaccination.6,18 In recent years significant strides have been made toward making vaccination for HPV a reality in the not too distant future.19,20 If successful, vaccination for HPV will have a dramatic impact on the control of cervical cancer, the magnitude of which may far exceed that seen as a result of cervical screening.
There are still many technical and practical problems and issues that need to be resolved before safe, effective and cost-effective vaccines become available for use in the general population.6 An important issue, which is still being discussed, is which of the 30 or more oncogenic types of HPV should be included in these vaccines. At present almost 80% of the cervical cancers are caused by HPV 16, 18, 31 and 45. There is a concern that when vaccines against the common HPV types are used extensively the uncommon types may become more prevalent. There is still no consensus on the most suitable route of administration of vaccines although nasal and oral routes may be preferable. The target population should of course be girls but a case can be made for vaccination of boys since male partners are the usual source of infection. The vaccination may be administered during infancy or before the start of sexual activity. The cost of production and administration of vaccines to the general public may also determine ultimate success or failure of the project. The problem seems to be greater for the therapeutic vaccines although their production has moved into an industrial phase.
The projects and plans for the testing and production of HPV vaccines are exciting and there is great optimism that it will be ultimately successful, but it may be 10 to 15 years before these vaccines are available for use on the general public. In the meantime it is important that current methods of surveillance and screening for cervical cancer and its precursor lesion be continued.
References
- 1.Garland SM. Human papillomavirus update with a particular focus on cervical disease. Pathology. 2002;34:213–224. doi: 10.1080/00313020212469. [DOI] [PubMed] [Google Scholar]
- 2.Galloway DA. Biology of genital human papilloma viruses. In: Holmes KK, Mardh PA, Sparling PF, et al., editors. Sexually transmitted diseases. 3rd edition. New York: Mcgraw Hill; 1999. pp. 335–346. [Google Scholar]
- 3.Somers GR, Tabrizi SN, Borg AJ, Garland SM, Chow CW. Juvenile laryngeal papillomatosis in a paediatric population: a clinicopathologic study. Pediatr Pathol Lab Med. 1997:53–64. [PubMed] [Google Scholar]
- 4.Holoway P, Miller AB, Rohan T, To T. Natural history of dysplasia of uterine cervix. J Natl Cancer lnst. 1999;91:252–258. doi: 10.1093/jnci/91.3.252. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman M, Castle PE. Human Papillomavirus: Epidemiology and Public Health. Arch Pathol Lab Med. 127:930–934. doi: 10.5858/2003-127-930-HPEAPH. [DOI] [PubMed] [Google Scholar]
- 6.Stewart BW, Klienhues P. World Cancer Report. Lyon France: IARC Press; 2003. Human papillomavirus vaccination; pp. 148–150. [Google Scholar]
- 7.Walboomers JMM, Jacobs MV, Manos MM, et al. Human papilloma virus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Franceschi S, Castellsague X, Dal Maso L, et al. Prevalence and determinants of human papilloma virus genital infection in men. Br J Cancer. 2002;86:705–711. doi: 10.1038/sj.bjc.6600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garland SM, Faulkner-Jones B, Fortune DF, Quin M. Cervical cancer-what role for human papillomavirus? Med J Aust. 1992;156:204–212. doi: 10.5694/j.1326-5377.1992.tb139710.x. [DOI] [PubMed] [Google Scholar]
- 10.Coleman N, Birley HD, Renton AM, et al. Immunological events in regressing genital warts. Am J Clin Pathol. 1994;102:768–774. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa N, Stites D, Patel S. Persistence of human papillomavirus 16 infection is associated with lack ofcytotoxicT lymphocyte response to e6 antigen. JInf Dis. 2000;182:595–598. doi: 10.1086/315706. [DOI] [PubMed] [Google Scholar]
- 12.Woodman CBJ, Collins S, Winter R, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357:183–186. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 13.Hildescheim A, Herrero R, Castle PE, et al. HPV co-factors related to the development of cervical cancer: results from a population-based study in Costa Rica. Brit J Cancer. 2001;84:1219–1226. doi: 10.1054/bjoc.2001.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubbard RA. Human papillomavirus testing methods. Arch Pathol Lab Med. 2003;127:940–945. doi: 10.5858/2003-127-940-HPTM. [DOI] [PubMed] [Google Scholar]
- 15.Werness BA, Levine AL, Howley PM. Association of human papillomavirus type 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 16.Dayson N, Howley PM, Munger K, Harlow E. The human papillomavirus-16 E7 oncoprotein is able to bind to retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 17.Pontem J, Adami HO, Bergstrom R, et al. Strategies for global control of cervical cancer. Int J Cancer. 1995;60:1–26. doi: 10.1002/ijc.2910600102. [DOI] [PubMed] [Google Scholar]
- 18.Lowy DR, Schiller JT. Papilloma virus and cervical cancer: pathogenesis and vaccine development. J Natl Cancer Inst Mononogr. 1998;23:27–30. doi: 10.1093/oxfordjournals.jncimonographs.a024169. [DOI] [PubMed] [Google Scholar]
- 19.Harm CD, Pang YY, Roden RB, et al. Safety and immunogenicity trial in adult volunteers of a human papilloma virus 16 L1 virus-like, particles vaccine. J Ntal Cancer Inst. 2001;93:284–292. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- 20.Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Eng JMed. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]