Wolcott-Rallison syndrome, an autosomal recessive disease, was first described in 1972 by Wolcott and Rallison.1 Several other cases from diverse ethnic families were later reported in the world literature.2–12 However, six of the identified cases came from consanguineous Arab families.4,7,9,11,12 The disease phenotype includes infancy onset insulin-dependent diabetes mellitus, spondylo epiphyseo metaphyseal dysplasia, osteoporosis, and walking difficulties with painful joints. Hepatic and renal insufficiency, chronic neutropenia, tooth discolouration, skin dryness and pigmentations, seizure disorder, microcephaly and mental retardation, and cardiovascular abnormalities are among other reported manifestations. Recently, isolated central hypothyroidism was suggested as an additional feature of this syndrome.12 Chromosomal abnormalities have also been found in some patients. This syndrome might be under-diagnosed in the population of the Arab peninsula possibly due to the early death of diabetic infants and late expression of its full phenotype. We present a case of a Bedouin boy whose clinical and radiological findings are consistent with WRS. This case is an addition to cases previously reported from the Arabian peninsula.
Case
The patient, a male, was born in 1995 after an uncomplicated term pregnancy to a 21-year-old mother and a 24 year-old father, who were healthy first cousins. Both were Kuwaitis from a large Bedouin tribe, the lineage of which extended back to the eastern coast of Saudi Arabia. Pedigree analysis revealed the presence of adult onset diabetes mellitus on both sides of the family, and early infant deaths, the cause of which could not be clarified. The child developed insulin dependent diabetes mellitus at the age of 2 months. He was prescribed injectable Monotard, but later shifted to Mixtard insulin, 10 units in the morning and 2 units in the evening. However, at the ages of 10 months, 14 months and 2 1/2 years he developed gastroenteritis/upper respiratory tract infection with sever episodes of hepatitis with altered consciousness, jaundice and extremely high hepatic enzymes with hypoglycaemia (a Reyes-like syndrome). The liver biopsy showed severe post-necrotic type bridging fibrosis with early nodular parenchymal hyperplasia, pale staining hepatocytes, and minimal inflammation, suggesting a metabolic disorder. Skin fibroblast culture revealed mitochondrial cytopathy respiratory chain complex I deficiency. However, ultrasound and CT scan of the abdomen showed a normal liver, spleen, pancreas and kidneys. He was treated with daily ubiquinone 10 mg, thiamine 50 mg and vitamin K 2mg orally. He was put on a low-fat diet under the suspicion of having an underlying fatty acid oxidation defect, and with careful follow up and control for diabetes. His alpha-fetoprotein was normal. He was repeatedly negative for anti-islet cell antibodies, which in turn excludes autoimmune-related diabetes.
At the age of 3 1/2 years he was diagnosed with hypothyroidism because of his short stature and dry skin. The FT4 level was 5.5 pmol/L (NR 12–29) and the TSH level was 2.2 μIU/mL (normal range, 0.3–5.0). He was then treated with Eltroxin (thyroxine) 75 μg daily. Random growth hormone was within the normal range. Serum vitamin D assay showed slightly low levels of vitamin D2+3, and he was treated with One Alpha Drops (alfacalcidol). Renal function tests, a complete hologram, an echocardiogram and an ophthalmic examination were normal.
Clinical examination at the age of 7 years revealed a weight of 14 kg (< third percentile), a height of 92 1/2 cm (< third percentile) and a head circumference of 49 cm (< third percentile). He had microbrachycephaly, a depressed nasal bridge, hypertelorism, a high arched palate, protruding ears with abnormal auricles, discolored and decayed teeth, a short neck with hyper-pigmented dry skin. The child also had a short broad chest, a short trunk, kyphoscoliosis and protuberant abdomen with a 2 cm palpable, slightly firm liver. His hands were short with a broad middle and incurved 5th fingers. His feet were short and flat. He had genu valga, generalized stiffness of the joints, with limited abduction of hips. His external genitalia were normal. Neurological examination showed mild mental retardation and deficit speech with normal hearing.
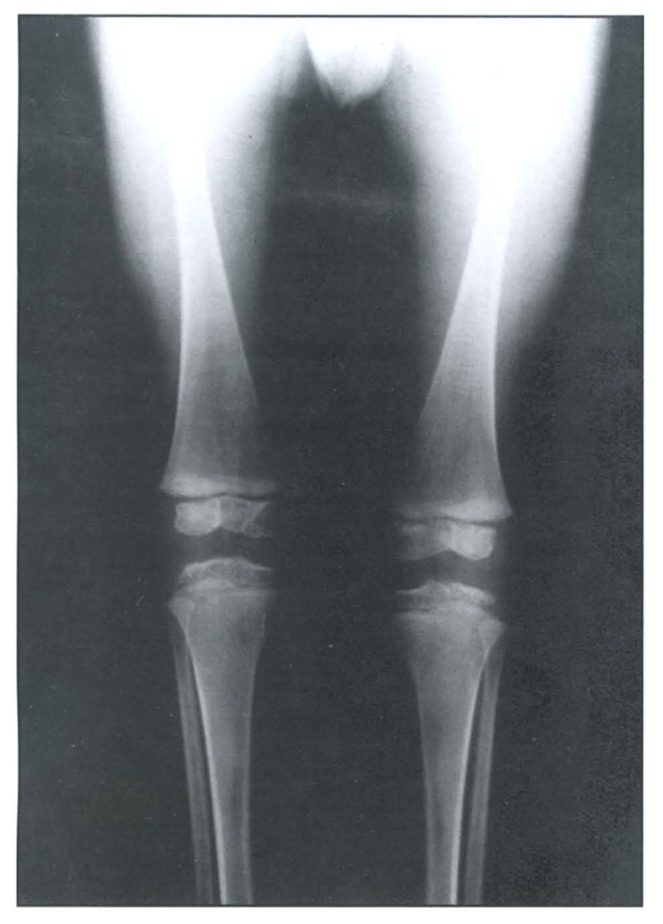
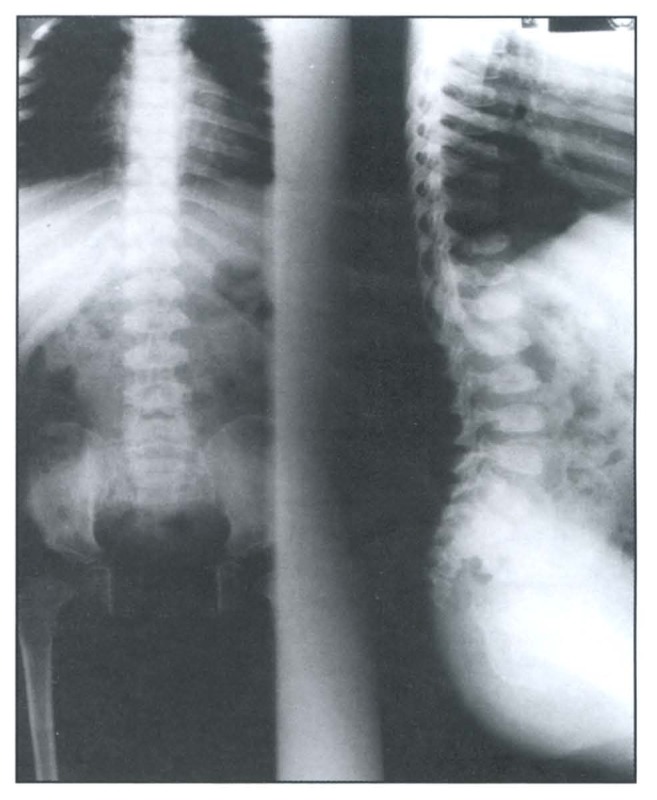
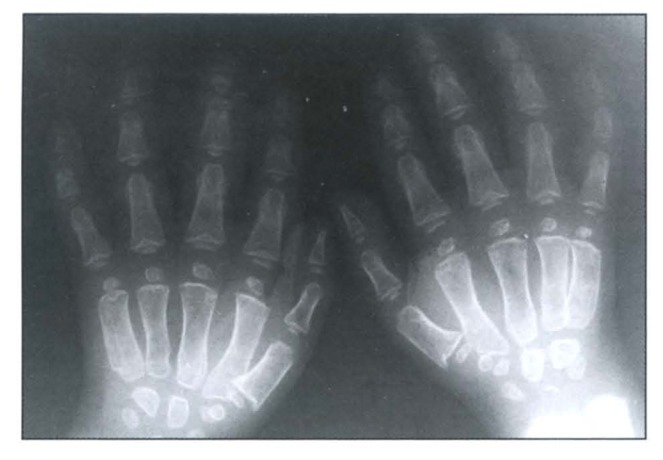
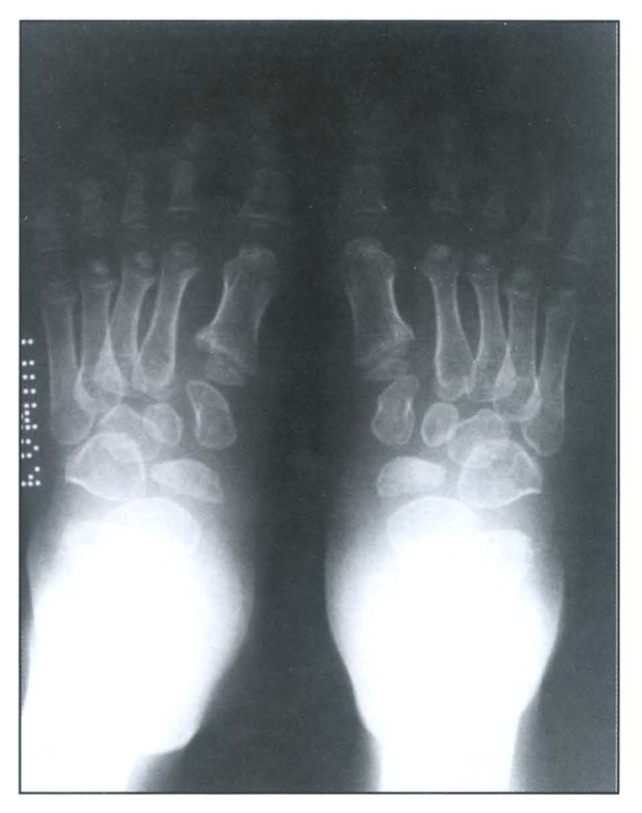
A skeletal survey revealed a generalized osteopenic texture, delayed bone age, multiple spondylo-epiphyseo-metaphyseal dysplasias (Figure 1 and Figure 2). The epiphysis showed irregularity and fragmentation of ossification centers especially of hips, shoulders and wrists. Hands and feet showed small irregular carpal and tarsal bones, respectively (Figure 3 and Figure 4). The phalanges were hypoplastic and cone-shaped. The metaphyses were enlarged with some flaring. Vertebrae showed platyspondyly with anterior tongue. His karyotype was 46, XY.
Figure 1.
Radiograph of the knees illustrating metaphyseal and epiphyseal changes.
Figure 2.
Anteroposterior and lateral radiograph of the spine showing platyspondyly of the dorsal and lumbar spines, narrow iliac wings and flaring of ribs.
Figure 3.
Hands radiograph showing cone-shaped deformities of the affected epiphyses.
Figure 4.
Feet radiograph showing hypoplastic middle and distal phalanges of toes.
Discussion
The patient fulfiled the diagnostic criteria for Wolcott-Rallison syndrome.1 Our case, a child from a consanguineous marriage, presented with two major and constant manifestations of this syndrome: permanent insulin-dependant infant-onset diabetes mellitus and multiple spondylo-epiphyseo-metaphyseal dysplasia in addition to other broad features such as hepatic insufficiency, short stature, microcephaly and mental retardation (Table 1). Recently bin-Abbas and colleagues12 suggested that central hypothyroidism is a feature associated with WRS. Our patient has this manifestation, for which he received a treatment. Our report supports their suggestion.
Table 1.
Clinical and radiological features of the present case compared to some cases from literature (modified from Al-Gazali et al4).
| Wollcot and Rallison1 | Goumy et al2 | Al-Gazali et al4 | bin-Abbas et al12 | Present case | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 1 | 2 | 1 | 2 | ||
|
| ||||||||||
|
| ||||||||||
| Sex | M | F | M | F | M | M | M | F | M | M |
|
| ||||||||||
| Consanguinity | + | + | + | + | + | |||||
|
| ||||||||||
| Clinical features | ||||||||||
|
| ||||||||||
| Onset of diabetes | 6w | 8w | 6w | 4w | 3w | 8w | 2w | 2m | 2m | 2m |
|
| ||||||||||
| Microcephaly/ developmental delay/ Mental Retardation | + | + | + | + | + | + | + | |||
|
| ||||||||||
| Seizures | + | + | ||||||||
|
| ||||||||||
| Renal insufficiency | + | + | + | + | ||||||
|
| ||||||||||
| Hepatitis/ Hepatic fibrosis/ encephalopathy | + | + | + | + | ||||||
|
| ||||||||||
| Cardiac murmer | + | + | + | |||||||
|
| ||||||||||
| Skin pigmentation | + | + | + | + | ||||||
|
| ||||||||||
| Teeth discolouration | + | + | + | |||||||
|
| ||||||||||
| Upward slanting palpebral fissures | + | + | ||||||||
|
| ||||||||||
| Depressed nasal bridge | + | + | + | + | ||||||
|
| ||||||||||
| High arched palate | + | + | + | |||||||
|
| ||||||||||
| Short neck | + | |||||||||
|
| ||||||||||
| Short stature | + | + | ||||||||
|
| ||||||||||
| Hypothyroidism | + | + | + | |||||||
|
| ||||||||||
| Radiology | ||||||||||
|
| ||||||||||
| Osteoporosis | + | + | + | + | ||||||
|
| ||||||||||
| Irregular/fragmented epiphyses | + | + | + | + | + | + | + | + | + | + |
|
| ||||||||||
| Platyspondyly | + | + | + | + | ||||||
|
| ||||||||||
| Narrow or abnormal iliac wings | + | + | + | |||||||
|
| ||||||||||
| Small femoral epiphyses or laterally displaced | + | + | + | + | + | |||||
|
| ||||||||||
| Small and irregular carpal bones | + | + | + | + | + | |||||
|
| ||||||||||
| Cone-shaped epiphyses of fingers | + | + | ||||||||
|
| ||||||||||
| Small irregular tarsal bones | + | + | ||||||||
|
| ||||||||||
| Hypoplastic middle and distal phalanges of the toes | + | + | ||||||||
M: male, F: female, w: week, m: month.
The presence of infant deaths in this family raises doubt about diagnosis similar to this genetic disease. Because of late radiological manifestations of WRS, these patients could remain unrecognized for some time. For such patients, emphasis should be put on their radiological follow up to diagnose the skeletal abnormalities.
In our patient, chromosomal analysis revealed no abnormalities involving chromosomes 2p or 15q. No uniparental disomy of chromosome 6q was detected, as was reported in some infants with transient neonatal diabetes.5,13–18 However, abnormalities on the genetic level were suspected and a DNA sample was kept, with permission, for future mutation screening of the candidate gene. A potential gene for WRS is postulated to reside in the chromosomal region 15q11-q12 following the identification of a mosaic del (15) (q11-q12) in a 4-year-old sporadically affected girl.5 PAX4 gene on chromosome 7q31.3, involved in pancreatic islet beta cells developed in the mouse, was excluded as a possible candidate gene for this novel syndrome.7 Moreover, WRS was recently mapped to a region on chromosome 2p12. The PERK gene encoding the pancreatic eukaryotic translation initiation factor 2-alphakinase 3 (EIF2AK3) lies in the same interval. Two different pathogenic mutations in EIF2AK3 were identified in two unrelated families. This in turn raises the possibility of this gene being the potential gene for WRS8. Supportive evidence was given by Zhang et al19, who reported the presence of a phenotype found in PERK −/− mice similar to that of the human WRS, the loss of critical domains of the PERK gene resulted in the complex phenotype in the affected mice.
Because of the previous reports of WRS in Arab children from the Arabian Peninsula, we speculate the presence of a quite large number of potential gene carriers in members of some highly inbred families from tribal origin in countries of the Gulf area. Therefore, a DNA sample should be banked from infants with insulin-dependant diabetes mellitus and a family history of unclear infant deaths for future tests for when the candidate gene is identified. In addition, there is a possibility of a common founder gene effect leading to WRS in apparently unrelated families in this region.
References
- 1.Wolcott CD, Rallison ML. Infancy-onset diabetes mellitus and multiple epiphyseal dysplasia. J Pediatr. 1972;80:292–297. doi: 10.1016/s0022-3476(72)80596-1. [DOI] [PubMed] [Google Scholar]
- 2.Goumy O, Maroteux P, Stanescu V, Stanescu R, Labbe A, Menut G. Syndrome de transmission récessive autosomeique anociant un diabète congénital et des désordres de la croissance des épiphyses. Arch Franc Pediatr. 1980;37:323–328. [PubMed] [Google Scholar]
- 3.Stoss H, Pesch HJ, Pontz B, Otten A, Spranger J. Wolcott-Rallison syndrome: diabetes mellitus and spondyloepiphyseal dysplasia. Eur J Pediatr. 1982;138:120–129. doi: 10.1007/BF00441137. [DOI] [PubMed] [Google Scholar]
- 4.Al-Gazali LI, Makia S, Azzam A, Hall CM. Wolcott-Rallison syndrome. Clin Dysmorph. 1995;4:227–233. [PubMed] [Google Scholar]
- 5.Stewart FJ, Carson DJ, Thomas PS, Humphreys M, Thornton M, Nevin NC. Wolcott-Rallison syndrome associated with congenital malformations and a mosaic deletion 15q11-12. Clin Genet. 1996;49:152–155. doi: 10.1111/j.1399-0004.1996.tb03275.x. [DOI] [PubMed] [Google Scholar]
- 6.Thornton CM, Carson DJ, Stewart FJ. Autopsy findings in the Wolcott-Rallison syndrome. Pediatr Pathol & Lab Med. 1997;17(3):487–496. [PubMed] [Google Scholar]
- 7.Bonthorn DT, Dunlop N, Barr DGD, El Sanousi AA, Al-Gazali LI. Organization of the human PAX4 gene and its exclusion as a candidate for the Wolcott-Rallison syndrome. J Med Genet. 1998;35:288–292. doi: 10.1136/jmg.35.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deléphine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2- kinase3, is mutated in patients with Wolcott-Rallison syndrome. Nature Genet. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 9.Abdelrahman S, Bin-Abbas B, Al-Ashwal A. Wolcott-Rallison syndrome in a Saudi Infant. Curr Pediat Res. 2000;1:51–52. [Google Scholar]
- 10.Castelnau P, Le Lerrer M, Diatloff-Zito C, Marquis E, Tete M-J, Robert J-J. Wolcott-Rallison syndrome: a case with endocrine and exocrine pancreatic deficiency and pancreatic hypertrophy. Eur J Pediatr. 2000;159(8):631–633. doi: 10.1007/pl00008394. [DOI] [PubMed] [Google Scholar]
- 11.Bin-Abbas B, Shabib S, Hainu B, Al-Ashwal A. Wolcott-Rallison syndrome: clinical, radiological and histological finding in a Saudi child. Ann Saudi Med. 2001;2:73–74. doi: 10.5144/0256-4947.2001.73. [DOI] [PubMed] [Google Scholar]
- 12.Bin-Abbas B, Al-Mulhim A, Al-Ashwal A. Wolcott-Rallison syndrome in two siblings with isolated central hypothyroidism. Am J Med Genet. 2002;111(2):187–190. doi: 10.1002/ajmg.10495. [DOI] [PubMed] [Google Scholar]
- 13.Temple IK, Gardner RJ, Robinson DO, Kibirige MS, Ferguson AW, Baum JD, Barber JC, James RS, Shield JP. Further evidence for an imprinted gene for neonatal diabetes localized to chromosome 6q22-q23. Hum Mol Genet. 1996;8:1117–1121. doi: 10.1093/hmg/5.8.1117. [DOI] [PubMed] [Google Scholar]
- 14.Arthur EI, Zlotogora J, Lerer I, Dagan J, Marks K, Abeliovich AD. Transient neonatal diabetes mellitus in a child with in VDUP (6)(q22q23) of paternal origin. Eur J Hum Genet. 1997;6:417–419. [PubMed] [Google Scholar]
- 15.Whiteford ML, Narendra A, White MP, et al. Paternal uniparental disomy for chromosome 6 causes transient neonatal diabetes. J Med Genet. 1997;34:167–168. doi: 10.1136/jmg.34.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner RJ, Robinson DO, Lamont L, Shield JR, Temple IK. Paternal uniparental disomy for chromosome 6 and transient neonatal diabetes. J Med Genet. 1998;6:522–526. doi: 10.1111/j.1399-0004.1998.tb03774.x. [DOI] [PubMed] [Google Scholar]
- 17.Gardner RJ, Mungall AJ, Dunham I, et al. Localization of a gene for transient neonatal diabetes mellitus to an 18.7 cR3000 (approximately 5.4 Mb) interval on chromosome 6q. J Med Genet. 1999;3:192–196. [PMC free article] [PubMed] [Google Scholar]
- 18.Marquis E, Robert JJ, Bouvattier C, Bellanné-Chantelot C, Junien C, Diatloff-Zito C. Major difference in aetiology and phenotypic abnormalities between transient and permanent neonatal diabetes. Letter. J Med Genet. 2002;39:370–374. doi: 10.1136/jmg.39.5.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang P, McGareth B, Li S, et al. The PERK Eukaryotic initiation Factor 2 Kinase Is Required for the Development of the Skeletal System, Postnatal Growth, and the Function and Viability of the Pancreas. Mol Cell Biol. 2002;22(11):3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]