Abstract
BACKGROUND
Spinal tuberculosis (TB) is perhaps the most clinically important extrapulmonary form of the disease. Early recognition is therefore necessary to minimize residual spinal deformity and/or permanent neurological deficit. We defined the CT and MRI image morphology of spinal TB and correlated the imaging features of these two modalities.
METHODS
CT (29 patients) and MRI (11 patients) images were retrospectively analyzed in 30 patients with proved spinal TB. CT and MRI findings were compared in cases with both imaging tests (10 cases). The parameters assessed were the type and extent of bone and soft tissue involvement.
RESULTS
The majority of the 30 patients were males (n=18) in the 30–49 year age group (43%). The most common clinical presentation was backache (73.3%) followed by fever (63.3%) and malaise (36.6%). The lumbar spine was the commonest site of the disease (43.3%) followed by the thoracic region (36.6%). A fragmentary type of bone destruction was the most frequent CT feature of the disease (48.2%) followed by the lytic type (24.1%). Intervertebral disc destruction (72%) and paravertebral mass/abscess (65.5%) were other features. Of the 11 patients who had an MRI, contiguous vertebral disease with disc destruction was seen in 10 cases. In 4 patients, there was distant vertebral disease in addition to the disease at the symptomatic site.
CONCLUSIONS
MRI offers excellent visualization of the bone and soft tissue components of spinal tuberculosis and helps to identify disease at distant asymptomatic sites. CT is useful in assessing bone destruction, but is less accurate in defining the epidural extension of the disease and therefore its effect on neural structures. MR imaging clearly demonstrated the extent of soft tissue disease and its effect on the theca/cord and foramen in cases with doubtful CT findings.
Keywords: Spinal tuberculosis, computed tomography, magnestic resonance imaging, diagnosis
Tuberculosis is re-emerging globally. It is increasing in the developing countries and re-emerging in the developed ones, and more so in the immunocompromised population,1–5 the homeless and the expanding immigrant populations. Spinal TB is perhaps the most clinically important extrapulmonary form of tuberculosis, as it may produce serious neurological sequelae due to compression of spinal cord as a result of the disease itself, as well as the resultant deformity. Early recognition and prompt treatment are therefore necessary to minimize residual spinal deformity and/or permanent neurological deficit. CT and/or MRI are frequently used imaging modalities in evaluating spinal disorders. In this retrospective study we highlight and correlate the image morphology of spinal TB on CT and MRI.
Methods
CT and MRI case records of 30 patients with proven tuberculosis from the year 1985 to 2002 were retrospectively analyzed. Of these patients 29 had CT examination and 11 had MRI. Ten of these patients had both CT and MRI. Those patients who presented prior to 1996 had only CT (GE Medical System, CT 8800, USA) examination because of the non-availability of MRI. After January 1996 when the magnetic resonance (MR) scanner (Signa 1.5T GE Medical systems, USA) and a new CT machine ( GE ProSpeed Advantage, USA) was installed, patients had both CT and MR examinations. For CT scanning, axial images were obtained in the region of interest using a 4-mm slice thickness and a 4-mm table feed. 2D or 3D reformatted images were produced in some patients. Intravenous iodinated contrast agent was not routinely used. MR was performed with 1.5 Tesla or super-conducting units. All patients were studied in the coronal, sagittal and axial planes using standard T1-weighted and T2-weighted images over the region of interest with and without intravenous gadolinium (Omniscan, Nycomed, Ireland). MR was performed to obtain the diagnosis and in preoperative planning. The following parameters were assessed by CT and MRI : the presence and extent of vertebral involvement, vertebral body and/or posterior element involvement, type of bone destruction, and paravertebral mass and/or abscess. The scans were independently reviewed by two radiologists and any disagreement in findings was resolved by consensus. The diagnosis was established on the basis of at least one of the following criteria: histological evidence of caseating granuloma, histological demonstration of acid-fast bacilli in the lesion, growth of mycobacterium on culture of tissue or ascitic fluid, satisfactory therapeutic response to chemotherapy in patients with clinical, or radiological and operative evidence of spinal TB. The case records were analyzed with respect to age, sex, clinical presentation, and CT and/or MRI findings.
Results
Of the 30 patients in this study more than half were males (60%) and the majority (43%) of these were in the 30-to 49-year age group. The clinical presentation included backache and local tenderness in 22 of the 30 patients (73.3%), fever in 19 patients (63.3%), malaise in 11 patients (36.6%), weight loss in 10 patients (30%) and neck stiffness in 2 patients (6.6%). Three patients (10%) presented with symptoms of neurological deficit or pain related to nerve compression. Table 1 summarizes the lesion distribution within the spine. The lumbar spine (43.3%) was the commonest site of the disease followed by disease in the thoracic region (36.6%). Isolated posterior element involvement was noted in 3 cases (10%), involving a lateral mass of the C1 vertebra in one case, the arch of C1 in one case and the transverse process of L5 in the third case.
Table 1.
Site of lesion in 30 cases of spinal tuberculosis.
| Site lesion | n = 30 | |
|---|---|---|
| Cervical | 2 | 6.6 |
| Thoracic | 11 | 36.6 |
| Lumbar | 13 | 43.3 |
| Thoroco/lumbar | 3 | 10.0 |
| Sacral | 1 | 3.3 |
CT findings
CT findings (Table 2) revealed vertebral destruction in all 29 cases (100%) involving the vertebral bodies in the majority (22 cases, 75.8%) of the cases. Vertebral body with disc destruction was noted in 21 cases (72%). Isolated posterior element disease was rare. Of the types of bone destruction (Table 3), the fragmentary type (Figure 1a) was the most common (48.2%) followed by the lytic type (24.1%, Figure 2). Paravertebral soft tissue mass and/or abscess were seen in 19 cases (65.5%) of which 6 cases had associated calcification. Epidural extension of the paraspinal mass was present in 16 cases, which were defined clearly only in three of these cases. In these three cases there was associated intra spinal calcification in 2 cases and bone fragments in 1 case. 2D- or 3D-reformatted images did not add any additional information to that of the axial images.
Table 2.
CT features in spinal tuberculosis (N=29).
| N | Percent | |
|---|---|---|
| Bone destruction | 29 | 100.0 |
| Vertebral body | 22 | 76.9 |
| Vertebral body and posterior element | 4* | 13.8 |
| Posterior element | 3 | 10.3 |
| Vertebral body and disc destruction | 72 | 72.4 |
| Paravertebral mass/abscess | 19 | 65.5 |
| Epidural extension | 16 | 55.1 |
| Soft tissue calcification | 6 | 13.0 |
Table 3.
Type of bone destruction seen on CT (N=29).
| N | Percent | |
|---|---|---|
| Fragmentary | 14 | 48.2 |
| Osteolytic | 7 | 24.1 |
| Subperiosteal | 5 | 17.2 |
| Sclerotic | 3 | 10.3 |
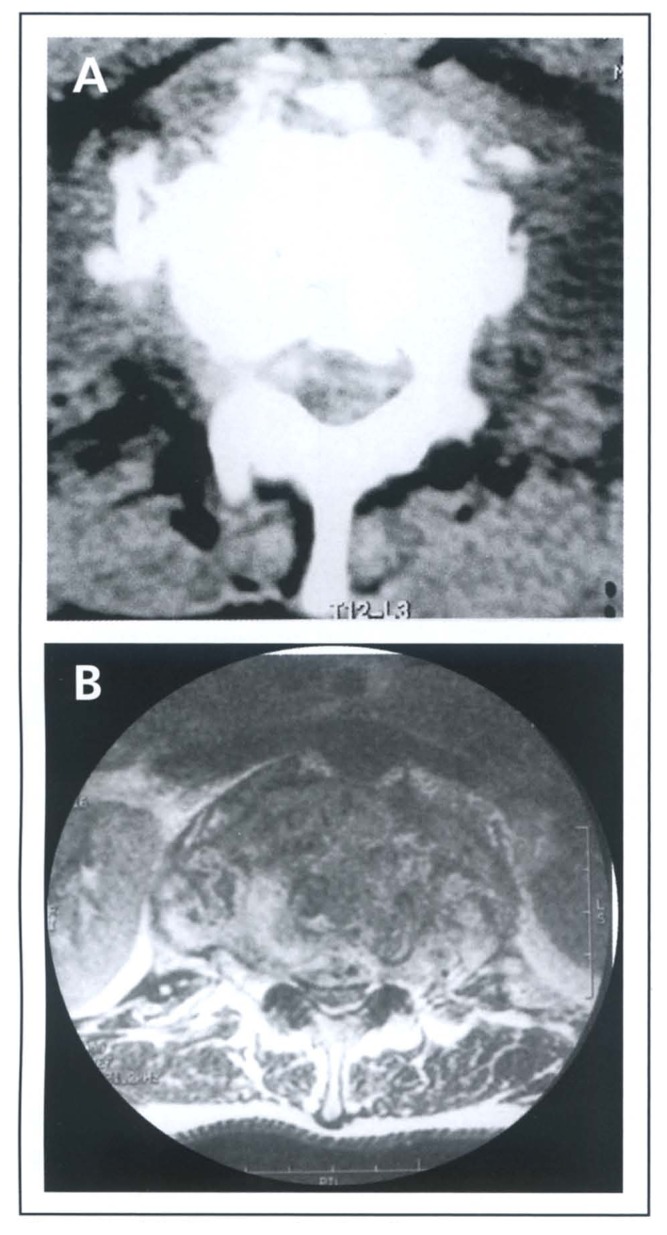
Figure 1.
(A) CT scan showing fragmentary type bone destruction. Note bone fragments within the canal. The effect on the theca is not clearly defined. (B) MRI showing significant compromise of the spinal canal and compression on the theca
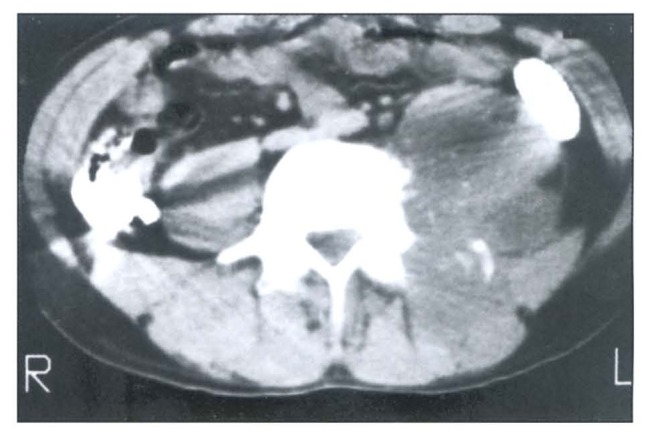
Figure 2.
CT scan showing osteolytic type destruction. Anterior and lateral parts of the vertebral body are destroyed. Note associated paravertebral and psoas abscess.
MR Imaging
Analysis of MRI findings in 11 patients showed contiguous vertebral body disease with disc destruction (osteitis and discitis) in 10 cases and vertebral body disease (osteitis) in one case (Figure 3). Skip lesion with disease at a distant site was noted in 4 cases, which involved the vertebral body in all the cases (Figure 4). Paravertebral abscess (osteitis, discitis and abscess) was present in 7 cases and all the patients had subligamental and epidural extension of the abscess and granulation tissue (Figures 3, 4). Five of these 7 patients showed significant thecal/cord compression (more than 50% compromised spinal canal). These patients were neurologically asymptomatic. Two of the 5 patients elicited neurological deficit on examination. One patient with no symptoms or signs at presentation developed acute neurological symptoms requiring emergency surgery. The signal characteristics on MRI are shown in Table 4. The abscesses showed low signal on T1WI and high signal on T2 images. Gadolinium enhanced images showed diffuse enhancement of the diseased vertebra and the paraspinal granulation tissues and rim enhancement in cases of abscesses. Meningeal enhancement suggesting associated arachnoiditis adjacent to the site of the disease was noted in 5 cases (Figure 3). Myelomalacia was not seen in any of the cases.
Figure 3.
(A) Midsagittal T1-weighted image before and (B) after gadolinium contrast enhancement showing contiguous vertebral body and disc disease. Note inflamed vertebral elements and the epidural mass showing low signal changes on T1W1 and intense enhancement in the post-contrast sequence. Also note enhancement of the theca due to arachnoiditis.
Figure 4.
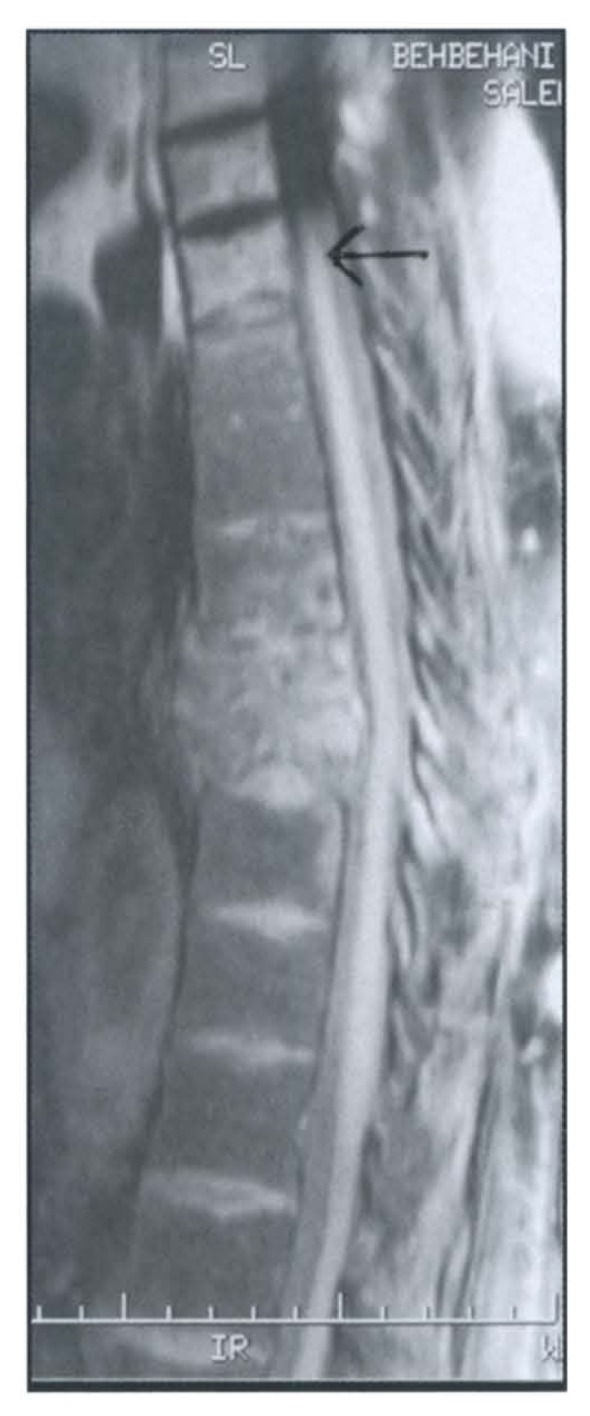
T2 with fat saturation image showing in-homogeneous high signal intensity of the affected vertebra with disc destruction. There are high signal changes in the vertebral body above (arrow) representing disease at distant site (skip lesion).
Table 4.
MRI features of spinal tuberculosis.
| MRI pattern | n | V body | Disc | Abscess | |||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | T1 | T2 | ||
| Osteitis | 1 | D | I | N | I | A | A |
|
| |||||||
| Osteitis and discitis | 3 | D | I | N | I | A | A |
|
| |||||||
| Osteitis, discitis, abscess | 7 | D | I | D | I | D | I |
T1: T1-weighted image, T2: T2-weighted image, D: decreased signal intensity, I: increased signal intensity, A: absent, N: normal
Comparison of the imaging modalities demonstrated vertebral body, disc and paraspinal disease (Table 5). CT was less accurate and underestimated the extent of epidural and foraminal extension of the disease and its effect on the neural structures which MRI clearly defined (Figure 1). MRI only demonstrated arachnoiditis. CT defined the various type of bone destruction and demonstrated bone fragments and calcification in the epidural space, which MRI failed to demonstrate.
Table 5.
Comparison of CT and MRI results (N= 10).
| CT | MRI | |
|---|---|---|
| V body with or without disc destruction | 10 | 10 |
| Paraspinal mass/abscess | 6 | 6 |
| Epidural and/or neural foramen encroachment | 3* | 5 |
| Subligamental spread | 0 | 6 |
| Meningeal enhancement | 0 | 5 |
| Calcification | 3 | 0 |
Suggestive but not clearly defined.
Discussions
Tuberculous spondylitis is defined as an infection by mycobacterium tuberculosis involving one or more components of the spine, namely the vertebra, intervertebral discs, paraspinal soft tissues and the epidural space.6 It is one of the most common manifestations of musculoskeletal TB.7 Though generally considered a disease of childhood in the developing countries and of the elderly in the USA,8 the average age is reported to be around 50 years in a number of studies involving both developed and developing nations,8–10 with wide variation in male:female ratio ranging from 1:3 to 4: 1.9–11 In our study the average age was 30 years with a male: female ratio of 4:3.
The thoracic and the thoracolumbar spine are the most common areas involved, comprising 48% to 67% of the lesions8,11,13 and the lumbar spine about 27%.7,14 The cervical spine and other sites are less frequently involved.11,13–15 In our study the lumbar spine was most commonly involved (43.3%) followed by the thoracic spine (36.6%).
Spinal infection is usually the result of hematogenous seeding of the vertebral body and the diagnosis often remains elusive because of the indolent nature of the infection. The problem is further compounded with atypical presentations. The role of the radiologist is not only in recognizing the imaging characteristics of the disease, but also being alert to the more atypical presentations.16,17 CT remains the preferred imaging modality for the assessment of osteomyelitis showing cortical bone destruction and sequestration formation, and for guided aspiration cytology in patients in whom the diagnosis is uncertain.18,19 Such a dilemma is usually seen in the atypical form of the disease, which is reported to range from 2% to 7%.12,14,16, 19 Of the 30 cases in our study, 3 patients had isolated posterior element involvement. The use of intravenous iodinated contrast agents defines the extent of inflammatory tissue in the paraspinal region. Axial CT scans readily demonstrate the pattern of bone destruction of the vertebrae, as was observed in our series, the fragmentary type being the most common. The osteolytic type is less common.6, 11 Migration of the bony fragments to the surrounding structures including the spinal canal is shown well by CT. Associated soft tissue masses usually are larger than the extent of bone destruction calcification within or surrounding these abscesses and is reported to be pathognomonic for tuberculosis.6,21 The paraspinal soft tissue masses may extend anterolaterally, or directly posteriorly with or without symptoms of neurological compression. In patients with neurological deficit, CT can define the presence and extent of epidural compression and is especially useful for detection of bone fragments within the spinal space.22,23 In our study, CT underestimated the compression in the neural structures especially in those who were neurologically asymptomatic.
On MR images, vertebral changes (intraosseous abscesses), paraspinal soft tissue abscesses, discitis, scoliosis and kyphosis, skip lesions, spinal canal encroachment, and root distortion are all readily detected on routine sagittal, axial and coronal images using T1- and T2-weighted sequences.22,24 MRI is shown to be as sensitive as bone scintigraphy for detecting infections of vertebrae and hence can detect the disease early. With the multiplanar capability of imaging, the disease is readily shown at distant sites in cases with skip lesions.5,22,25,26 As was observed in our series, T1-weighted images usually show decreased signal within the affected vertebral marrow. On T2-weighted images a relative increase in signal intensity is noted within the diseased tissues.17,24,27 The high contrast resolution greatly improves the detection of intraosseous abscesses, subligamental spread and epidural extension of infection, which can be useful even in asymptomatic cases, like in 5 patients in our study. Enhanced MR studies are particularly useful for demonstrating enhancement of the meninges (signifying ongoing inflammation),28 and rim enhancement around intraosseous and paraspinal soft tissue abscesses, which are rarely seen in nontubercular abscesses.24,27
CT and MR imaging are useful for delineating the features of tuberculous spinal disease, but MRI is recommended as it is highly accurate in detecting disease at distant sites and for assessment of soft tissue components of spinal tuberculosis. MRI is especially important in the evaluation of spinal cord and the nerve root integrity in the asymptomatic patient. CT is useful in accessing the type and extent of bone destruction but is less accurate in defining the epidural and foraminal extension of the disease.
References
- 1.Garst RJ. Tuberculosis of the spine: a review of 236 operated cases in an under developed region from 1954 to 1964. J Spinal Disord. 1992;5:286–300. [PubMed] [Google Scholar]
- 2.Leibert E, Schluger NW, Bonk S, Rom WN. Spinal tuberculosis in patients with human immunodeficiency virus infection: Clinical presentation, therapy and outcome. Tuber Lung Dis. 1996;77:329–334. doi: 10.1016/s0962-8479(96)90097-0. [DOI] [PubMed] [Google Scholar]
- 3.Parsons LM, Driscoll JR, Taber HW, Salfinger M. Drug resistance in tuberculosis. Infect Dis Clin North Am. 1997;11:905–928. doi: 10.1016/s0891-5520(05)70397-4. [DOI] [PubMed] [Google Scholar]
- 4.Ventura G, Caudda R, Paliotta VF, Colosimo C, Lucia MB. Pott’s Disease of the cervico occipital junction in an AIDS patient. Tuber Lung Dis. 1996;77:188–190. doi: 10.1016/s0962-8479(96)90037-4. [DOI] [PubMed] [Google Scholar]
- 5.Sharif HS, Aideyan OA, Clark DC, et al. Brucellar antuberculous spondylitis: Comparative imaging features. Radiology. 1989;171:419–425. doi: 10.1148/radiology.171.2.2704806. [DOI] [PubMed] [Google Scholar]
- 6.Sharif HS, Morgan JL, Al-Shahed MS, et al. Role of CT and MR imaging in the management of tuberculous spondylitis. Radiol Clin North Am. 1995;33:787–804. [PubMed] [Google Scholar]
- 7.Coppola J, Muller NL, Connell DG. Computed tomography of musculoskeletal tuberculosis. Can Assoc Radiol J. 1987;38:199–203. [PubMed] [Google Scholar]
- 8.Gorse GJ, Pais MJ, Kasske JA, Cesario TC. Tuberculos spondylitis: a report of six cases and review of the literature. Medicine. 1983;62:178–193. [PubMed] [Google Scholar]
- 9.Rezai AR, Lee M, Cooper PR, et al. Modern management of spinal tuberculosis. Neurosurgery. 1995;36:87–804. doi: 10.1227/00006123-199501000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Chan WH, Liu JS, Hownig SL. Tuberculos spondylitis: a clinical analysis. Kao Hsiung I Hsueh Tsa Chih. 1996;6:428–434. [PubMed] [Google Scholar]
- 11.Jain R, Sawhney S, Berry M. Computed tomography of vertebral tuberculosis : patterns of bone destruction. Clin Radiol. 1993;47:659–664. doi: 10.1016/s0009-9260(05)81162-6. [DOI] [PubMed] [Google Scholar]
- 12.Brandt-Zawadzki M, Burke V, Brooke-Jeffrey R. CT in the evaluation of spine infection. Spine. 1983;8:358. doi: 10.1097/00007632-198305000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Weaver P, Lifeso RM. The radiological diagnosis of tuberculosis of the adult spine. Skeletal Radiol. 1984;12:178–86. doi: 10.1007/BF00361084. [DOI] [PubMed] [Google Scholar]
- 14.Nael SL, Kearns MJ, Seeliq JM, Harris JP. Manifeststions of potts disease in the head and neck. Laryngoscope. 1986;96:494–497. doi: 10.1288/00005537-198605000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Patankar T, Krishnan A, Patkar D, Kale H, Prasad S, Shah J, Castillo M. Imaging in isolated sacral tuberculosis: a review of 15 cases. Skeletal Radiology. 2000;29:392–396. doi: 10.1007/s002560000229. [DOI] [PubMed] [Google Scholar]
- 16.Pande KC, Babhulkar SS. Atypical Spinal Tubercu-losis. Clinical Orthopaedics and Related Research. 2002;398:67–74. doi: 10.1097/00003086-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Narlawar RS, Shah JR, Pimple MK, Patkar DP, Patankar T, Castillo M. Isolated tuberculosis of posterior elements of spine: magnetic resonance imaging findings in 33 patients. Spine. 2002;27:275–281. doi: 10.1097/00007632-200202010-00015. [DOI] [PubMed] [Google Scholar]
- 18.Francis IM, Das DK, Luthra UK, Sheikh Z, Sheikh M, Bashir M. Value of radiologically guided fine needle aspiration cytology (FNAC) in the diagnosis of Spinal tuberculosis: a study of 29 cases. Cytopathology. 1999;10:390–340. doi: 10.1046/j.1365-2303.1999.00206.x. [DOI] [PubMed] [Google Scholar]
- 19.Shaniey DJ. Tuberculosis of the spine: imaging features. AJR-Am J Roentgenol. 1995;164:659–664. doi: 10.2214/ajr.164.3.7863889. [DOI] [PubMed] [Google Scholar]
- 20.Yalniz E, Pekindil G, Aktas S. Atypical tuberculosis of the spine. Yonsei Med J. 2000;41:657–661. doi: 10.3349/ymj.2000.41.5.657. [DOI] [PubMed] [Google Scholar]
- 21.Modic M, Feiglin D, Piraino D, et al. Vertebral Osteomyelitis-assessment using MR. Radiology. 1985;157:157–166. doi: 10.1148/radiology.157.1.3875878. [DOI] [PubMed] [Google Scholar]
- 22.Zamiati W, Jiddane M, El Hassani MR, Chakir N, Boukhrissi N. Contribution of spiral CT scan and MRI in spinal tuberculosis (Spanish). J of Neuroradiology. J de Neuroradiologie. 1999;26:27–34. [PubMed] [Google Scholar]
- 23.Srivastva S, Sanghavi NG. Non traumatic paraparesis: aetiological clinical and radiological profile. J of the assoc of physicians of India. 2000;10:988–990. [PubMed] [Google Scholar]
- 24.Omari B, Robertson JM, Nelson RJ, et al. Pott’s disease: A resurgent challenge to the thoracic surgeon. Chest. 1989;95:145–150. doi: 10.1378/chest.95.1.145. [DOI] [PubMed] [Google Scholar]
- 25.Magid D. Computed Tomographic imaging of the musculoskeletal system-current status. Radiol Clin North Am. 1994;32:255–274. [PubMed] [Google Scholar]
- 26.Sharif H. Role of imaging in the Management of spinal infections. AJR-Am J Roentgenol. 1992;158:1333–1345. doi: 10.2214/ajr.158.6.1590137. [DOI] [PubMed] [Google Scholar]
- 27.Sharif HS, Clark DC, Aabed MY, Haddad MC, Al Deeb SM, et al. Granulomatous Spinal Infections: MR Imaging. Radiology. 1990;177:101–107. doi: 10.1148/radiology.177.1.2399306. [DOI] [PubMed] [Google Scholar]
- 28.Sharma A, Goyal M, Mishra NK, Gupta V, Gaiwak SB. MR Imaging of Tubercular Spinal Arachnoiditis. AJR-Am J Roentgenol. 1997;168:807–812. doi: 10.2214/ajr.168.3.9057539. [DOI] [PubMed] [Google Scholar]