Primary neuroendocrine carcinoma of the skin is a rare tumour. Primary neuroendocrine carcinoma of the vulva is an extremely unusual occurrence with less than 10 cases reported in the literature so far.1 It is usually seen in the 7th to 8th decades of life1,2 and the occurrence in the younger age groups is uncommon. There are around 10 proved cases reported in the literature of primary neuroendocrine carcinoma of the vulva. There have been rare reports of vaginal small cell carcinoma mimicking Bartholin’s gland abscess.3 However, vulval Merkel cell carcinoma (MCC) mimicking the clinical presentation of Bartholin’s gland abscess has not been reported so far. This remarkably rare entity of a vulval primary neuroendocrine carcinoma occurring in a young lady mimicking Bartholin’s gland abscess is presented here along with a review of literature.
Case
A 35-year-old lady, para 3, was referred to the Obstetrics and Gynaecolgy A&E with painful swelling of the vulva and a purulent discharge of one week’s duration. She had attended other clinics with the above complaints and was on antibiotics and analgesics at the time of presentation, but had only marginal relief from these. She was afebrile and appeared to be in acute pain. A brief systemic examination revealed no positive findings. Local examination revealed an extremely tender, firm 5 to 6 centimeter swelling of the left labium majus from which was exuding purulent discharge. In view of the excruciating pain, a speculum and bimanual examination were deferred. A provisional diagnosis of Bartholin’s gland abscess was made. Treatment was changed to systemic broad-spectrum antibiotics after taking a swab from the purulent discharge for culture and sensitivity. Baseline investigations like complete blood count and urine examination were done on an urgent basis and were found to be within normal limits. She was posted for examination under anaesthesia.
Under anaesthesia, after the pus was drained, plenty of friable tissue was found extruding from the stoma. On palpation a firm to hard mass of 6 × 4 centimeters size involving the whole of the left labium majus was found. The above findings gave rise to a suspicion of malignant aetiology with possible secondary infection. Speculum examination showed a normal healthy looking cervix and vagina. Bimanual examination also showed a normal sized uterus with free fornices. Examination of the inguinal region revealed a large 3 to 4 centimeter, hard fixed lymph node on the left side. No other lymph nodes were palpable. The accessible part of the tumour tissue was excised and the excised tissue was sent for histopathological examination.
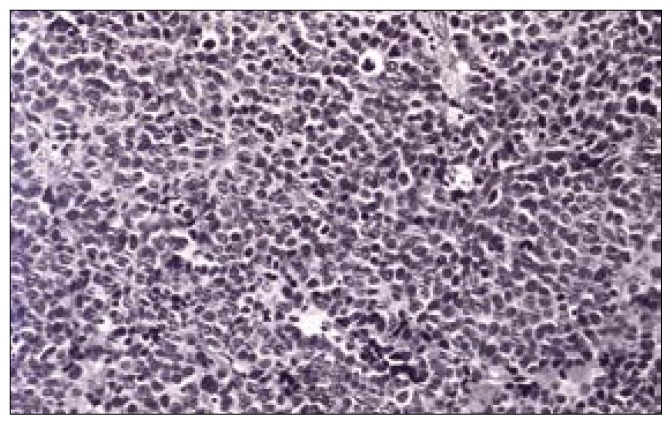
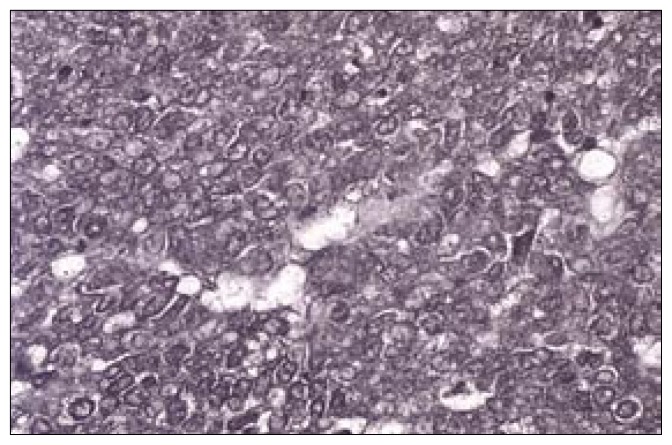
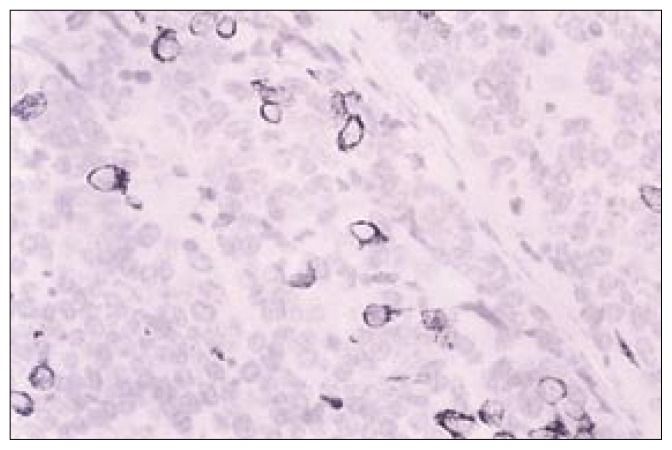
Histopathological examination revealed sections of the tumour composed of sheets and trabeculae of cells intersected by thin fibro-vascular septa. The cells showed scant cytoplasm and round-to-oval nuclei with coarse granular chromatin, exhibiting increased mitotic activity (>20/10 HPF in areas) and karyorrhexis (Figure 1). Occasional pseudo-rosetting of tumor cells was present. There were foci of necrosis and recent haemorrhage. Fragments of stratified squamous epithelium with underlying scanty connective tissue were also present. Immunohistochemistry showed positivity for neuron-specific enolase (Figure 2), enomysial antibody, chromogranin (Figure 3) and focal positivity for keratin. Staining for S100, and synaptophysin was negative. The light microscopic features and immunostaining results were consistent with neuroendocrine carcinoma. Electron microscopic examination of the tissue showed very poor preservation of the ultrastructure and therefore was noncontributory.
Figure 1.
Photomicrograph of the surgical specimen [H&E stain X20] showing sheets of cells with scant cytoplasm and dark, round nuclei, and increased mitotic figures.
Figure 2.
Photomicrograph—Immunohistochemistry [NSE X40] Diffuse strong membrane cytoplasmic positivity for neuron-specific enolase.
Figure 3.
Photomicrograph—Immunohistochemistry [CK20 X 40] Occasional tumour cells showing strong cytoplasmic positivity.
After the histopathological diagnosis was made, a thorough examination was done again to look for a primary lesion in the cervix or the skin or for secondary metastases elsewhere, both of which were negative. CT scans and MRI scans could not be done in this case since the patient could not afford them. Postoperative antibiotics and analgesics were continued. The swab culture showed growth of Staphylococcus aureus and the patient was changed to appropriate antibiotics. The patient left for her home country and initial attempts to determine her condition suggested that she was receiving radiotherapy. We acknowledge that the treatment offered to this patient was inadequate. However, the patient had left the country and further follow-up was not available save the information that she received radiotherapy.
Discussion
Primary neuroendocrine carcinoma of the skin (Merkel’s cell carcinoma) was first described by Toker in 1972.4–6 These rare tumours are mostly seen in the 7th to 9th decades of life7 though cases have been reported in the younger age group.2,8,9 More than 50% of these tumours arise in the head and neck region and a further 35% on extremities.4,6,10 Only about 15% occur in the other regions of the body. Ultraviolet light has been indirectly implicated in its development.7
Vulval primary neuroendocrine carcinoma is an extremely rare entity with less than ten cases reported in the literature. The usual clinical presentation of a Merkel cell carcinoma is a raised, purple to red, firm nodule without any overlying skin ulceration.11 They are highly malignant tumours with an aggressive behavior.10,12 The malignant potential of vulval MCC is reported to be greater than MCC in other sites1,4,13 with a 100% rate of inguinal node metastasis and 100% distant metastasis and rapid fatality. It has a great tendency for local recurrence,6 regional lymph node metastases and also for distant metastases. 14 MCC of the skin, in general, has been reported to develop metastases in the regional lymph nodes in 48% and distant metastases in 15% at the time of presentation.10
The possible histogenesis of these neoplasms is controversial. Histologically, the Merkel cell carcinoma is dermal in location.4,15 They are difficult to identify by light microscopy alone. The tumour is composed of sheets, nests, cords or trabeculae of small uniform cells with scant cytoplasm and hyperchromatic nuclei. By light microscopy alone, these tumours are difficult to distinguish from melanomas, lymphomas and metastatic oat cell carcinomas.7,16 Therefore, additional techniques like electron microscopy for neurosecretory granules or immunochemical staining for neuron specific enolase and cytokeratin are essential for diagnosis. The expression of low molecular weight cytokeratin, chromogranin and neuron specific enolase is an almost constant feature and decisive in the diagnosis in majority of the cases.1,7,12 An ultrastructural study using electron microscopy is also helpful in making a definitive diagnosis. The main ultrastructural finding is the presence of dense membrane bound neurosecretory granules.2,6,15 Histopathological confirmation of primary neuroendocrine carcinoma of skin warrants detailed examination for nodules in skin in other body sites and to look for lymph node involvement, as was done here. Investigations to rule out secondaries are essential and include liver function tests, abdominal ultrasound and CT scan of the chest, abdomen, pelvis and spine. Because of the rarity of these neoplasms involving the vulva, information regarding the sensitivity to radiation and chemotherapy are extrapolated from the management of these neoplasms at other sites.6
The initial mode of treatment is always surgery followed by radiotherapy. Tumour tissue should be radically excised either by hemi-vulvectomy or vulvectomy with ipsilateral lymphadenectomy similar to vulvar melanoma.17 Surgery alone carries a recurrence rate of 70% to 75% which is reduced by wide field radiation.14 Therefore, combination chemotherapy has been recommended in those with nodal or distant metastasis. Adjuvant chemotherapy would be recommended because of the possibility of early distant metastasis and the morphological similarity between MCC and small cell cancer of the lung. Therefore, combination chemotherapy has been recommended in those cases with nodal or distant metastasis. Regimens like cyclophosphamide, doxorubicin and vincristine or cisplatin/carboplatin and etoposide have been recommended.1,2,17,18
Because of the proximity of Bartholins’ gland to the vulval skin, primary neuroendocrine carcinoma of Bartholin’s glands needs to be considered in the differential diagnosis. But differentiation between the two will be difficult since small cell cancer frequently contains evidence of multidirectional differentiation, including glandular, squamous and neuroendocrine features which have led to the hypothesis that many of these neoplasms derive from pluripotent stem cells.19 Hence prognosis is likely to be similar.
Due to their aggressive behaviour they carry an extremely poor prognosis. Besides the stage of the disease, other factors influencing prognosis are tumour size, depth of invasion, histological differentiation and lymph node involvement.20,21 At other sites adjuant chemotherapy and/or radiotherapy does improve survival. Overall mortality is as high as 50% within 2 years of diagnosis11 and 5-year survival was reported to be 14%.16
Interestingly in rare instances, there are certain reports of spontaneous regression of MCC in other regions in elderly women.1 We acknowledge that surgery done in this patient was inadequate. Recommended surgery could not be done in the absence of informed consent and proper planning. Further the follow-up was limited as the patient left this country and has received radiotherapy in her own country.
The Bartholin’s gland abscess is a relatively common occurrence. However, in rare instances, primary neuroendocrine carcinoma of the vulva with secondary infection, usually Staphylococcus aureus, may mimic Bartholin’s gland abscess. If it is not diagnosed in time with clinicopathologic studies then prognosis may be much worse and the patient may not receive appropriate treatment.
References
- 1.Gil-Moreno A, Garcia-Jimenez A, Gonzalez-Bosquet J, Estell M, Castellivi Vives J, Martinez palones JM, Xercavins J. Merkel cell carcinoma of the vulva. Gynecol Oncol. 1997;64(3):526–32. doi: 10.1006/gyno.1996.4575. [DOI] [PubMed] [Google Scholar]
- 2.Husseinzadeh N, Wesseler T, Newman N, Shbaro I, Ho P. Neuroendocrine (Merkel cell) carcinoma of the vulva. Gynecol Oncol. 1988;29(1):105–12. doi: 10.1016/0090-8258(88)90154-0. [DOI] [PubMed] [Google Scholar]
- 3.Mirhashemi R, Kratz A, Weir MM, Molpus KL, Goodman A. Vaginal small cell carcinoma mimicking a Bartholin’ gland abscess: a case report. Gynecol Oncol. 1998;3:297–300. doi: 10.1006/gyno.1998.4959. [DOI] [PubMed] [Google Scholar]
- 4.Karl TK, Chen MD. Merkel’s cell (neuroendocrine) carcinoma of the vulva. Cancer. 1994;73(8):2186–91. doi: 10.1002/1097-0142(19940415)73:8<2186::aid-cncr2820730825>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Rilke Franco, Pilotti Silvana. Histopathological classification and Typing of the Solid Tumours. In: Michaelpeckham Herbert Pineddo, Veronesi Umberto., editors. Oxford Textbook of Oncology. Vol. 1. 1995. p. 321. [Google Scholar]
- 6.Copeland LJ, Cleary K, Sneige N, Edwards CL. Neuroendocrine (Merkel cell) carcinoma of the vulva—a case report and review of the literature. Gynecol Oncol. 1985;22(3):367–78. doi: 10.1016/0090-8258(85)90053-8. [DOI] [PubMed] [Google Scholar]
- 7.Fefull David, Carruci John A. Cancer of the Skin. In: De Vita Vincent T, Jr, Hellman Samuel, Rosenberg Steven A., editors. Cancer Principles & Practice of Oncology Vol II. 6th Edition. 2001. pp. 1991–1992. [Google Scholar]
- 8.Chandeying V, Sutthijumroon S, Tungphaisal S. Merkelcell carcinoma of the vulva: a case report. Asia Oceania J Obstet Gynaecol. 1989;15(3):261–5. doi: 10.1111/j.1447-0756.1989.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 9.Waibel M, Richter K, Von Lengerken W, Neidobitek F. Merkel Cell tumor (neuroendocrine carcinoma of the skin) in an unusual location. Immunohistochemical a lectin histochemical findings. Zentralbl Pathol. 1991;137(2):140–50. [PubMed] [Google Scholar]
- 10.Bottles K, Lacey CG, Goldberg J, Lanner-Cusin K, Hom J, Miller TR. Merkel cell carcinoma of the vulva. Obstet Gynecol. 1984;63(3-Suppl):61S–65S. [PubMed] [Google Scholar]
- 11.Elner Victor, Bardenstein David, Litcher Allen S. Eye, Orbit & Adenexal structures. In: Abeloff Martin D, Armitage James O, Litcher Allen S, Neiderhuber John., editors. Clinical Oncology. II edition. 2000. pp. 1228–29. [Google Scholar]
- 12.Ferenczy . Pathology of malignant tumours of the vulva and vagina. In: Coppleson Malcolm, Monaghan John, Paul Morrow C, Tattersall Martin HN., editors. Gynaecologic oncology—Fundamental principles and clinical practice. 2nd edition. I. 1992. [Google Scholar]
- 13.Loret de Mola JR, Hudock PA, Steinetz C, Jacobs G, Macfee Abdul-karim FW. Merkel cell carcinoma of the vulva. Gynecol Oncol. 1993;51(1):272–6. doi: 10.1006/gyno.1993.1286. [DOI] [PubMed] [Google Scholar]
- 14.Burmenster BH, Smithers BM, Poulsen MG, Kennedy D, Thomson DB. Skin and Melanoma cancer. In: Pollock Raphael E, Doroshow James H, Geraghty James G, Khayat David, Kim Jin-Pok, O’Sullivan Brian., editors. Manual of Clinical Oncology. 7th Edition. 1993. pp. 328–33. [Google Scholar]
- 15.Hierro I, Blanes A, Matilla A, Munoz S, Vicioso L, Nogales F. Merkelcell (neuoroendocrine) carcinoma of the vulva case report with immunohistochemical and ultrastructural findings and review of literature. Pathol Res Pract. 2000;196(7):503–9. doi: 10.1016/S0344-0338(00)80052-7. [DOI] [PubMed] [Google Scholar]
- 16.Di Souza Philip J, Creasman William T. Invasive Vulval Cancer. In: Di Souza Philip J, Creasman William T., editors. Clinical Gynaecologic Oncology. 5th edition. 1997. pp. 63–64. [Google Scholar]
- 17.Fonyad G, Rosta A, Toth EK, Szaleczky E, Katona C, Sapi Z, Ungar L, Poller I. Merkel cell tumor. Orv Heetil. 1997;138(26):1695–7. [PubMed] [Google Scholar]
- 18.Minna JD, Higgins GA, Glatstein EJ. Cancer of the Lung. In: Devita Vt, Jr, Hellman S, Rosenberg SA., editors. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott; 1989. pp. 666–687. [Google Scholar]
- 19.Alexander CB. Small Cell Cancer of the Oesophagus. Evidence for a unified histogenesis. Human Pathology. 1984;15:460–468. doi: 10.1016/s0046-8177(84)80081-7. [DOI] [PubMed] [Google Scholar]
- 20.Philips GL, Bundy BN, Okagaki T, Kucera PR, Stehman FB. Malignant melanoma of the vulva treated by radical hemivulvectomy: A prospective study of the Gynecology Oncology Group. Cancer. 1994;73:2626–2632. doi: 10.1002/1097-0142(19940515)73:10<2626::aid-cncr2820731026>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Hitchcock CL, Bland KI, Laney RG, III, Franzini D, Harris B, Copeland EM., III Neuroendocrine (Mercel Cell) Carcinoma of the skin: Its natural history, diagnosis and treatment. Ann Surg. 1988;207:201–207. doi: 10.1097/00000658-198802000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]