Abstract
BACKGROUND
Limb anomalies rank behind congenital heart disease as the most common birth defects observed in infants. More than 50 classifications for limb anomalies based on morphology and osseous anatomy have been drafted over the past 150 years. The present work aims to provide a concise summary of the most common congenital limb anomalies on a morpho-etiological basis.
PATIENTS AND METHODS
In a retrospective study, 70 newborns with anomalies of the upper and/or lower limbs were ascertained through clinical examination, chromosomal analysis, skeletal surveys and other relevant investigations.
RESULTS
Fetal causes of limb anomalies represented 55.8% of the cases in the form of 9 cases (12.9%) with chromosomal aberrations (trisomy 13, 18 and 21, duplication 13q and deletion 22q) and 30 cases (42.9%) with single gene disorders. An environmental etiology for limb anomalies was diagnosed in 11 cases (15.7%) as amniotic band disruption, monozygotic twin with abnormal circulation, vascular disruption (Poland sequence, sirenomelia and general vascular disruption) and an infant with a diabetic mother. Twenty cases (28.5%) had limb anomalies as part of sporadic syndromes of unknown etiology.
CONCLUSIONS
The morpho-etiological work-up of limb anomalies adopted in the present study is valuable for detecting the cause of the anomaly and is crucial for its prevention. Prevention can be achieved by proper genetic counseling, which includes recurrence risk estimation and prenatal diagnosis.
Congenital limb malformations rank behind congenital heart disease as the most common birth defects observed in infants.1 One in 506 newborns has congenital malformation of the upper limb.2 These malformations can occur as isolated malformations, in combination with another hand and/or foot, or as part of a syndrome. The etiology can be divided into environmental and genetic causes. A well known example of an environmental cause is the wave of thalidomide-induced hand malformations that occurred in the 1960s.3
The field of congenital anomalies of the limbs has been inundated by many classifications. More than 50 classification schemes have been drafted over the past 150 years, each claiming some special merit not possessed by others.4 Currently, hand surgeons use the morphological classification first presented by Swanson in 19765 and adopted by both the American Society for Surgery of the Hand and the International Federation of Societies for Surgery of the Hand (IFSSH).6 IFSSH proposed a seven category classification based on the proposed classification of Swanson. These seven groups are 1) failure of formation; transverse (A), or longitudinal (B), 2) failure of differentiation, 3) polydactyly, 4) overgrowth, 5) undergrowth, 6) amniotic band syndrome, and 7) generalized skeletal syndromes. 7 Recently, the Japanese Society for Surgery of the Hand (JSSH) has proposed an extension/modification of the IFSSH classification.7
A workable classification should employ a simple, easily remembered and descriptive terminology. It should allow the recording of common clinical entities with minimal confusion, yet permit the full categorization of complex cases. An ideal classification should be made by grouping malformations according to morphogenesis or cause.8 In the present study, cases of congenital limb anomalies were diagnosed according to the widely used morphological classification of Swanson,5,7,9,10 then they were grouped together etiologically to highlight the genetic causes for the sake of genetic counseling, for proper recurrence risk estimation, and for providing suitable prenatal methods for early detection.
Patients and Methods
In this retrospective study, 70 newborns with anomalies of the upper and/or lower limbs diagnosed in the Kuwait Medical Genetics Centre during the period 1997 to 1999 were ascertained. All patients had undergone clinical evaluation, including personal history, consanguinity, and limb and general examination, and chromosomal studies using peripheral blood following the Hungerford technique11 and the Seabright technique for Giemsa-Trypsin (GTG-) banding.12 A minimum of 20 cells were examined for each patient. The cytogenetic findings were interpreted according to the International System for Human Cytogenetic Nomenclature (ISCN 1995).13 Patients had also undergone other relevant investigations such as biochemical and skeletal surveys, ultrasonography, echocardiography and CT of head. The 70 cases with limb anomalies were diagnosed following the morphological classification of Swanson,5,7,9,10 then grouped according to their etiology.14–17
Results
Limb anomalies in the 70 patients could be divided etiologically by fetal, environmental and sporadic (unknown) causes (Tables 1–3). Fetal causes were present in 39 (55.8%) patients (Table 1) in the form of chromosomal aberrations (12.9%) and single gene disorders (42.9%). The anomalies observed in patients with chromosomal abnormalities were pre- and postaxial polydactyly, and syndactyly (Figure 1, a–c). A wide range of limb anomalies due to single gene disorders, whether autosomal dominant (AD) or autosomal recessive (AR), was observed in this group of patients (Figure 2a–c, and Figure 3). Combinations of anomalies were observed in some patients where failure of separation was combined with 1) longitudinal limb deficiency to give split hand/foot syndrome, 2) limb duplication resulting in synpolydactyly syndrome (Figures 2c, 3a,b) and 3) transverse limb reduction defect (fingers amputation), forming acrosyndactyly.
Table 1.
Fetal causes of limb anomalies.
| Etiology/Syndrome | Number of cases | Presentation | |
|---|---|---|---|
| Limb anomalies | Associated anomalies | ||
|
| |||
| Chromosomal aberrations | 9 | ||
|
| |||
| Trisomy 13 | 5 | Postaxial polydactyly | Holoprosencephaly, microcephaly, cleft lip/palate |
|
| |||
| Duplication 13 (13q+) | 1 | Postaxial polydactyly | Congenital heart, blepharophimosis, long philtrum |
|
| |||
| Trisomy 18 | 1 | Preaxial polydactyly | Micognathia, clenched hands, protruded occiput, malformed ears, overlapping fingers |
|
| |||
| CATCH 22 (22q−) | 1 | Postaxial polydactyly (F) | Congenital heart, abnormal face, thymus hypoplasia, cleft palate, hypocalcaemia |
|
| |||
| Trisomy 21 | 1 | Syndactyly 4th, 5th fingers | Upward slanting palpebral fissures, low-set ears, depressed nasal bridge, hypotonia, mental retardation |
|
| |||
| Single gene disorders | 30 | ||
|
| |||
| Isolated polydactyly (AD) | 12 | Polydactyly (F and/or T) | |
|
| |||
| Synpolydactyly (AD) | 5 | Synpolydactyly (F or T) | |
|
| |||
| Meckel-Gruber synd. (AR) | 3 | Polydactyly (F and/or T) | Anencephaly, polycystic kidney, cleft palate |
|
| |||
| Apert syndorome (AD) | 2 | Acrosyndactyly (F) | Craniosynostosis, flat face, down slanting palpebral fissures |
|
| |||
| Radial ray defect (AD or AR) | 2 | Absent thumb, radial hypoplasia | Ventricular septal defect |
|
| |||
| Fraser syndrome (AD) | 1 | Acrosyndactyly (F and T) | Eyes covered by forehead skin, no eye lids, anal atresia |
|
| |||
| Bardet-Biedl syndrome (AR) | 1 | Postaxial polydactyly (F and T) | Obesity, retinal pigmentation, ventricular septal defect |
|
| |||
| Syndactyly (AD) | 2 | Syndactyly (F or T) | |
|
| |||
| Split hand/foot syndrome (AD) | 1 | Absent middle finger, absent second toe, syndactyly 3rd and 4th toes | |
|
| |||
| Triphalangeal thumb polydactyly syndrome (AD) | 1 | Triphalangeal thumb, preaxial poldactyly | |
|
| |||
| Total cases | 39 | ||
F=fingers, T=toes
Table 2.
Environmental causes of limb anomalies.
| Etiology/Syndrome | Number of Cases | Presentation | |
|---|---|---|---|
| Limb anomalies | Associated anomalies | ||
|
| |||
| Intrauterine constraint: | |||
|
| |||
| Extrinsic | |||
|
| |||
| Amniotic band disruption | 4 | Syndactyly with amputation of fingers and toes and constriction ring | Anencephaly, cleft lip and palate, ear malformations |
|
| |||
| Monozygotic twins with abnormal circulation | 1 | Gangrene of both lower limbs and muscle atrophy | Microcephaly, small ears (one of a triplet) |
|
| |||
| Intrinsic | |||
|
| |||
| Vascular disruption | |||
|
| |||
| Poland sequence | 2 | Syndactyly (Fingers) | Unilateral absence of pectoralis major |
|
| |||
| Sirenomelia | 2 | Fused lower limbs | Absent external genitalia, imperforate anus |
|
| |||
| General | 1 | Lower limb amputation, absent thumb, index finger, radial hypoplasia | Absent external genitalia, pulmonary & liver infarctions |
|
| |||
| Drugs, Toxins, Maternal illness, etc | |||
|
| |||
| Infant of diabetic mother | 1 | Radial and femoral hypoplasia | Spina bifida, hydrocephalus, cleft lip, renal anomalies |
|
| |||
| Total cases | 11 | ||
Table 3.
Sporadic syndromes associated with limb anomalies
| Etiology/Syndrome | Number of cases | Presentation | |
|---|---|---|---|
| Limb anomalies | Associated anomalies | ||
|
| |||
| Acheiria (transverse limb defect) | 5 | Ectrodactyly (rudimentary hand and fingers) | |
|
| |||
| Thumb duplication | 2 | Bifid thumb | |
|
| |||
| VATER association | 2 | Preaxial polydactyly, syndactyly, radial hypoplasia | Vertebral anomalies, anal atresia, tracheoesophageal fistula, ear anomalies |
|
| |||
| Proteus syndrome | 1 | Macrodactyly (F) | Cerebroid gyriform configuration of the sole, café au-lait spots, subcutaneous lipomata |
|
| |||
| Coffin-Siris syndrome | 1 | Hypoplasia 5th finger and 5th toe | Coarse facies, anteverted nares, sparse scalp hair |
|
| |||
| Ulnar ray defect | 2 | Ulnar hypoplasia | |
|
| |||
| Aarskog (Facio-digito-genital syndrome) | 1 | Brachysyndactyly | Rounded face, hypertelorism, short anteverted nose, long philtrum, ptosis, shawl scrotum |
|
| |||
| Tetraperomelia | 1 | Four limb amputation | |
|
| |||
| Synpolydactyly with cardiac malformation | 1 | Synpolydactyly (F &T), Polydactyly (F&T) | Congenital heart |
|
| |||
| Klippel-Trenaunay-Weber syndrome | 1 | Postaxial polydactyly (F&T) | Hemangioma on the trunk and upper and lower limbs, unilateral hypertrophy of the leg |
|
| |||
| Polydactyly with polycystic kidney | 2 | Postaxial polydactyly (F& T) | Polycystic kidney, hepatomegaly |
|
| |||
| Polydactyly with diaphragmatic hernia | 1 | Postaxial polydactyly (F) | Large diaphragmatic hernia, lung atelectasis |
|
| |||
| Total Cases | 20 | ||
F=fingers, T=toes
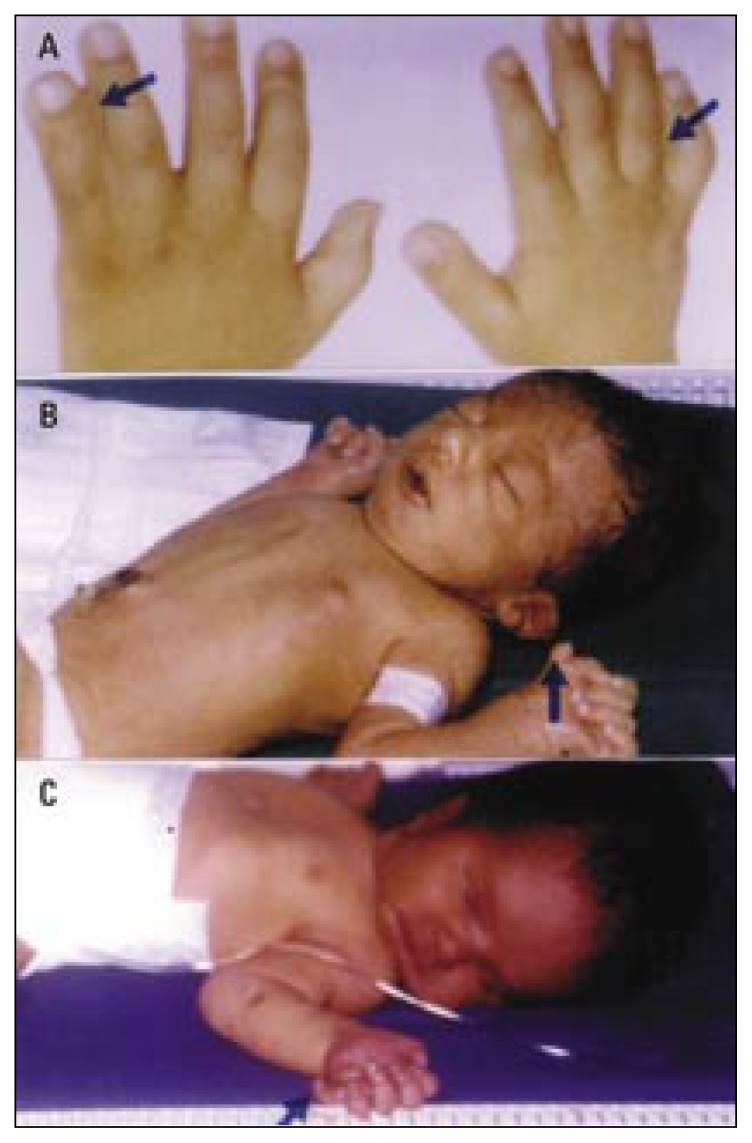
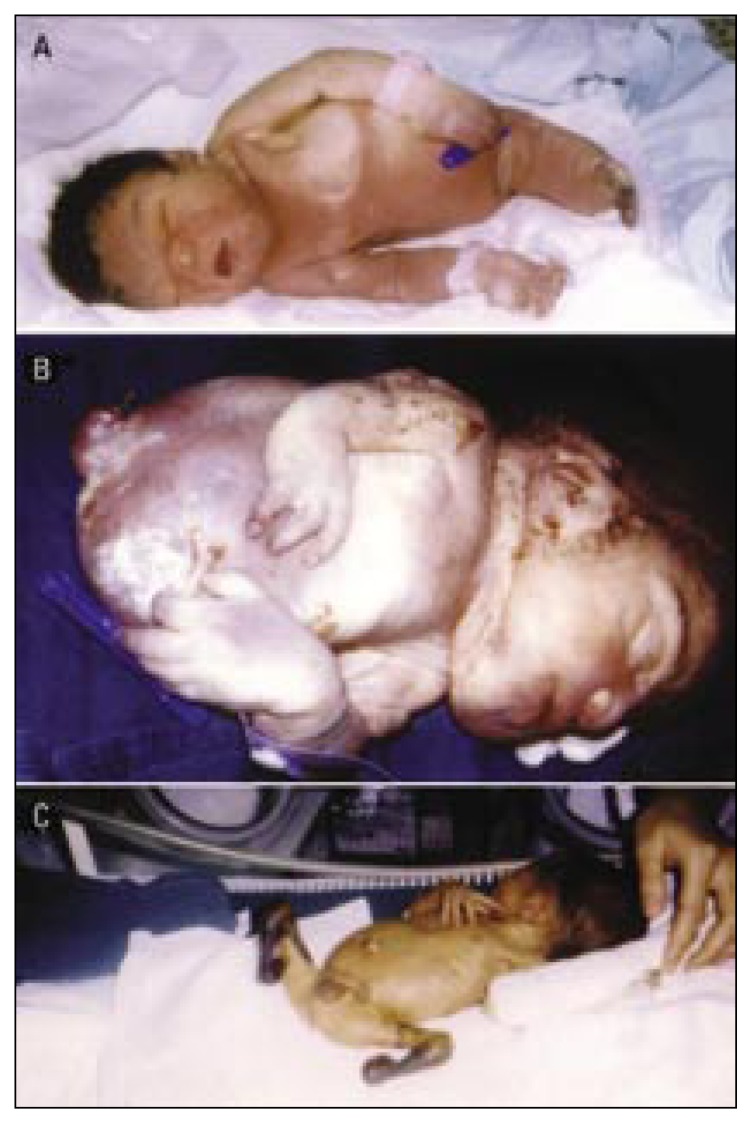
Figure 1.
Limb anomalies due to fetal causes (chromosomal aberrations).
a) Bilateral cutaneous syndactyly of the little and ring fingers (arrow) and right little finger clinodactyly (arrow) in a short broad hand of a Down syndrome child (trisomy 21).
b) Left preaxial polydactyly (arrow) in a newborn male with Edward’s syndrome (trisomy 18). Note the malformed low-set and posteriorly rotated ears, the micrognathia, the clenched hand, and the overlapping fingers.
c) Left postaxial polydactyly (arrow) in a newborn male with partial trisomy 13 (13q+). Other dysmorphic features include bulging forehead, blepharophimosis, deep-set eyes, depressed nasal bridge, long philtrum, thin upper lip, and pectus excavatum.
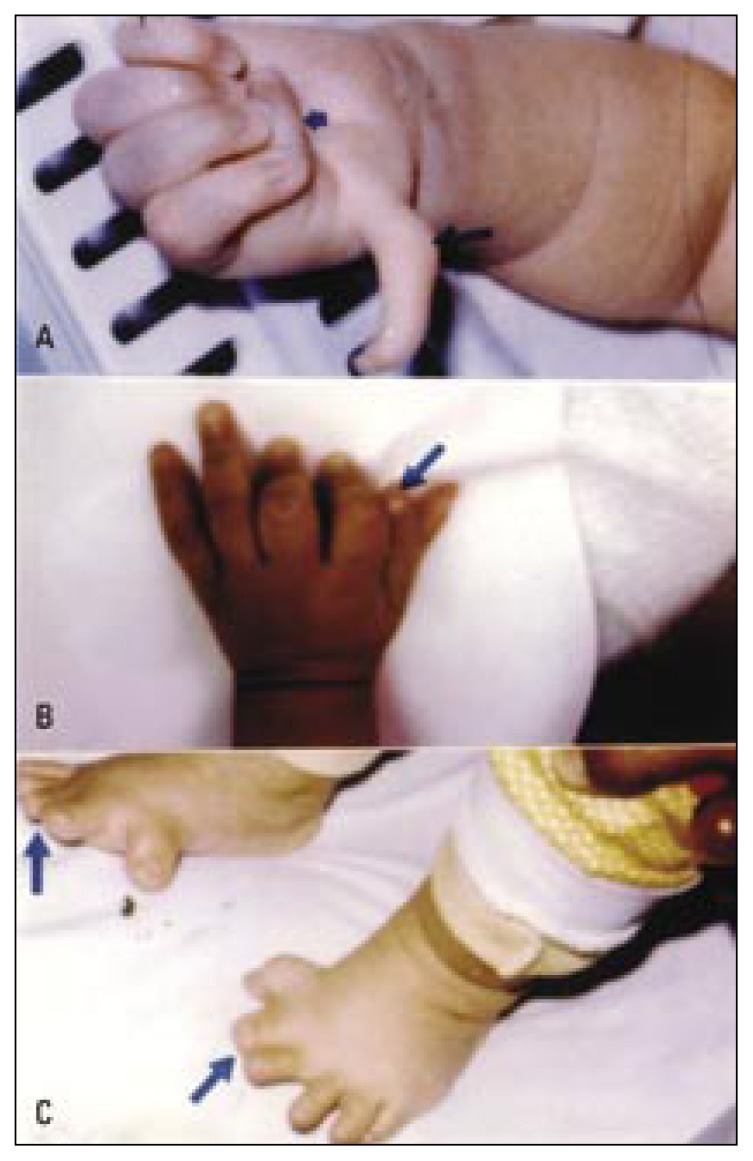
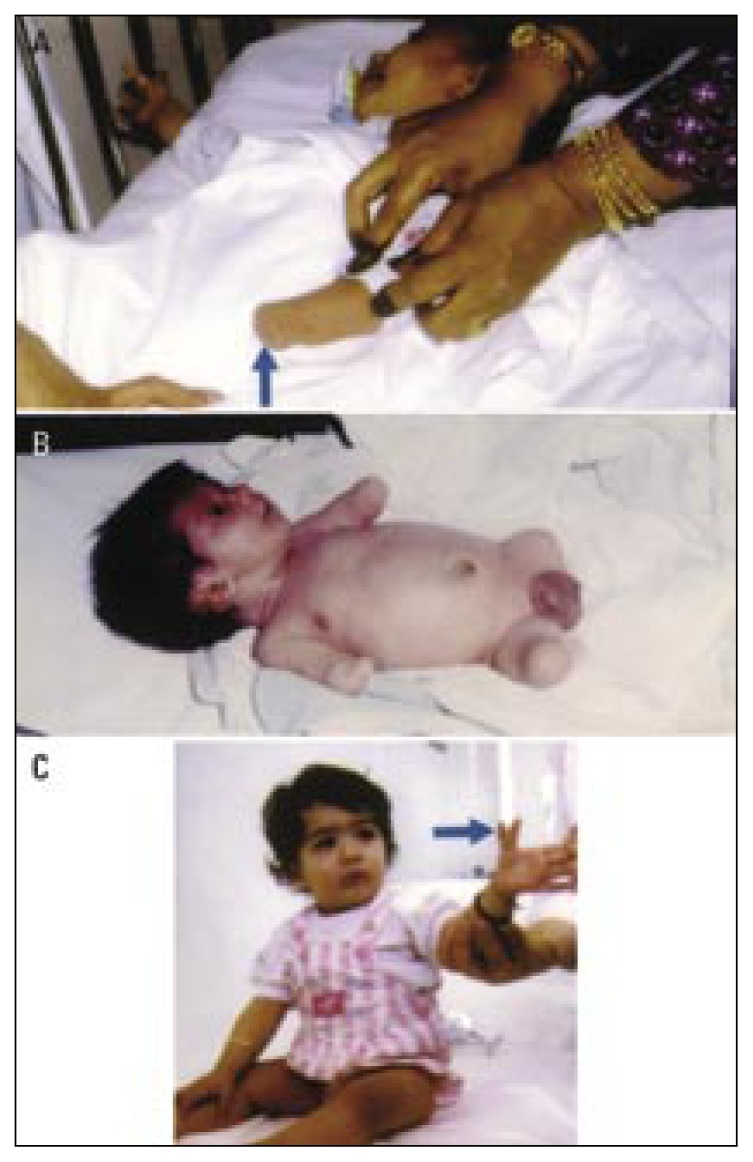
Figure 2.
Showing limb anomalies due to fetal causes (single gene disorders).
a) Triphalangeal and distally displaced left thumb (short arrow) with thenar muscles hypoplasia, and preaxial polydactyly (long arrow) in an infant with the autosomal dominant triphalangeal thumb polydactyly syndrome.
b) Isolated preaxial polydactyly (arrow).
c) Bilateral preaxial autosomal dominant synpolydactyly. The second toe is duplicated and fused (arrows).
Figure 3 (a,b).
Limb anomalies due to fetal causes (single gene disorders).
A newborn boy with bilateral postaxial synpolydactyly of the ring finger (short arrows) and bilateral preaxial synpolydactyly of the big toe (long arrow).
Environmental causes of limb anomalies were diagnosed in 11 patients (15.7%, Table 2). All the environmental causes resulted in limb reduction defects such as finger and toe amputations and constriction rings in the amniotic band disruption (Figure 4), finger syndactyly in Poland’s anomaly, fused lower limbs in sirenomelia (Figure 5a), lower limb amputation in addition to absent thumb, index finger and radial hypoplasia in the case with general vascular disruption (Figure 5b), and lower limb gangrene in the monozygotic twin with abnormal circulation (Figure 5c).
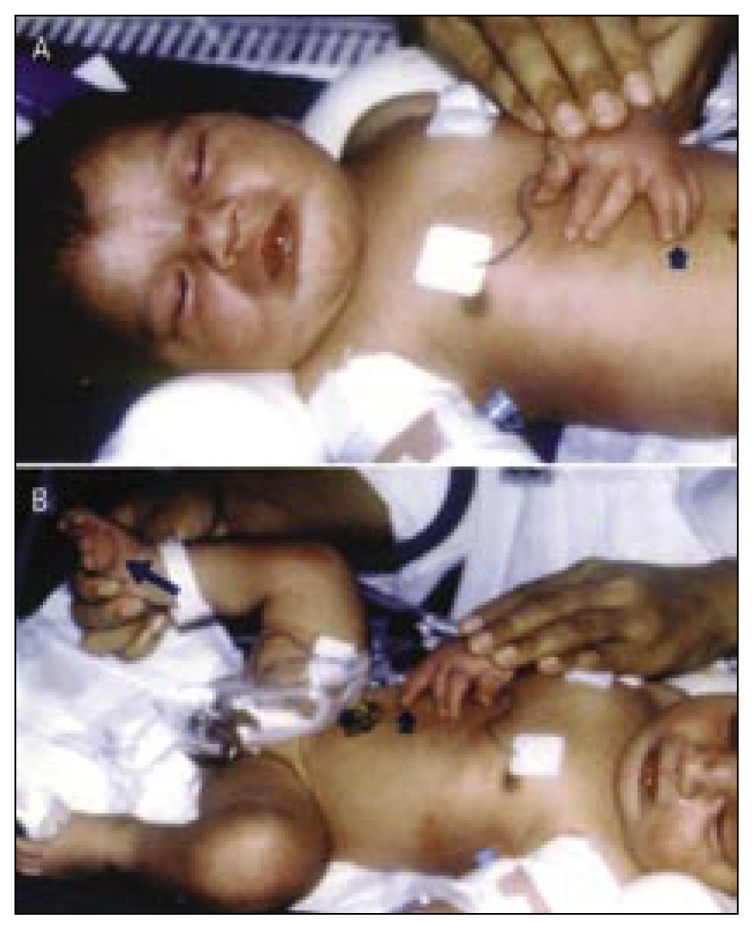
Figure 4 (a,b).
Limb anomalies due to environmental causes.
Limb anomalies due to amniotic band disruption: a) constriction rings (arrow), syndactyly, and ectrodactyly or terminal phalanges amputations; b) Transverse limb defect, i.e. amputation of both feet.
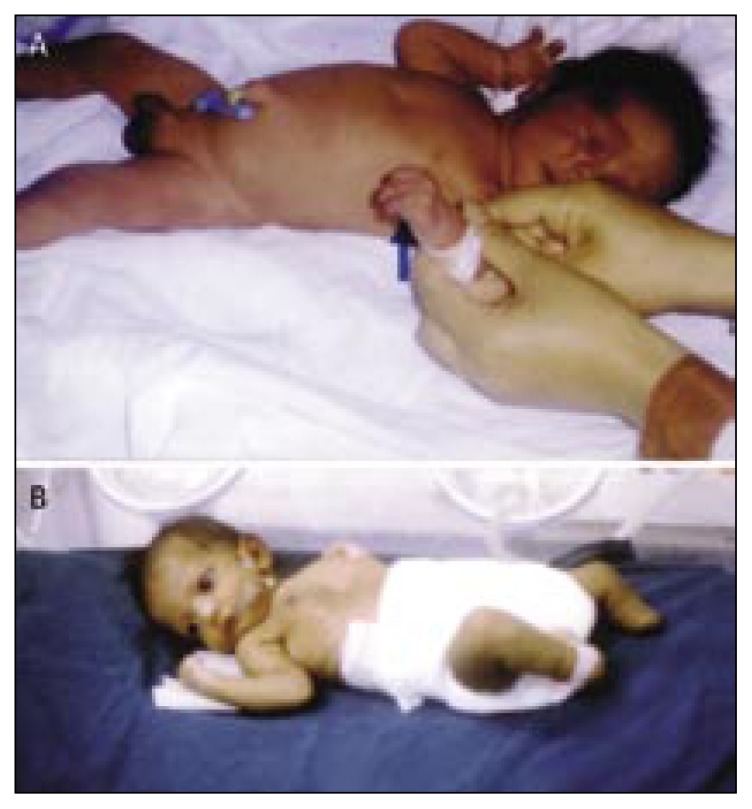
Figure 5.
Limb anomalies due to environmental causes.
a) A newborn with sirenomelia (due to vascular disruption) where both lower limbs and feet are fused, absent external genitalia and imperforate anus.
b) A newborn with general vascular disruption presented with bilateral limb amputation (transverse reduction defect), absent right thumb and index finger, right radial hypoplasia (radial ray defect), absent external genitalia, and blue colored skin.
c) Bilateral gangrene of the leg and foot due to abnormal circulation in one of a triplet.
Twenty cases (28.5%) had limb anomalies as part of sporadic syndromes of unknown etiology (Table 3). A wide variety of limb anomalies are present in this group as rudimentary hand and fingers in acheiria (Figure 6a), amputation of both upper and lower limbs in tetraperomelia (Figure 6b), and bifid thumb in the thumb duplication syndrome (Figure 6c).
Figure 6.
Limb anomalies due to sporadic causes.
a) Acheiria (transverse limb defect) in a newborn boy where the left palm is absent and the fingers are rudimentary and presented by digital buds (arrow), b) Tetraperomelia in a newborn boy (amputation in both upper and lower limbs), c) Bifid thumb.
Discussion
Chromosomal aberrations lead to limb anomalies due to duplication/deficiency of the genes involved in limb development. Trisomy 13 or duplication of a specific region (q21-q32) on the long arm of chromosome 13 is usually associated with postaxial polydactyly. 18 Limb deficiencies such as ectrodactyly and oligodactyly have also been reported in trisomy 13.19 Aplasia of the radius, usually associated with absence or hypoplasia of the first metacarpal and thumb, is the most prevalent reduction malformation in trisomy 18 infants.20 Brachydactyly, fifth finger clinodactyly and hypoplasia of the middle phalanx, syndactyly, and a wide gap with a deep crease between the first and second toes are frequent but non-specific findings in patients with Down syndrome.21–23 Nine patients in the present study had different forms of limb anomalies such as polydactyly (limb duplication), syndactyly (failure of separation), and radius aplasia and hypoplasia (limb deficiency) due to chromosomal aberrations. These findings warrant the need for performing karyotyping as a routine investigation in cases of congenital limb anomalies.
Limb anomalies can arise due to mutations in many genes, including the “homebox” genes and fibroblast growth factors and their receptor genes.10 The prevalence of polydactyly with or without associated malformations varies between 5 and 7 per 10 000 live births.24 Polydactyly of the little finger is often seen in the African-American population (as frequent as 1 in 100) owing to an AD trait and is usually present as an isolated finding.25 Relative proportions of the preaxial and postaxial forms of polydactyly vary largely among racial groups.14 In the present study postaxial polydactyly was seen in 23 of the 30 (76.7%) cases of upper limb polydactyly. Triphalangeal thumb is a rare preaxial deformity with a variety of presentations. Its percentage in the present study was 1.4%. The prevalence of this deformity is reported to be one in 25 000.26 It may be seen as a sporadic disorder but is most frequently noticed as an AD trait. The gene locus is on chromosome 7q36.6
Syndactyly (fusion of adjacent digits) is the most common congenital anomaly in Caucasians as it is noticed in 14% of newborns.27,28 It often presents with a positive family history when isolated. In addition, syndactyly is frequently seen in generalized disorders such as Poland and Apert syndromes.29 In the present study isolated syndactyly was observed in 2 cases (2.9%) and as part of a syndrome in another 11 cases (15.7%). Synpolydactyly is usually inherited as an AD trait.25,30 The synpolydactyly gene is mapped to a locus on chromosome 2q31.31 In the present study, synpolydactyly was observed in 6 cases (8.6%). Five cases had isolated synpolydactyly while the sixth one also had congenital heart. His diagnosis was unknown.
Limb reduction anomalies have a frequency of 8–49 per 10 000 births.32,33 Longitudinal absence defects are much rarer than transverse ones.34 Radial ray defect has an incidence of 1:100 000 and may be uni- or bilateral29 or part of a large associated disorder.27 Cardiac or gastrointestinal anomalies are frequent in these patients. A detailed study of limb reduction defects could lead to a better understanding of clinical presentation and to an etio-pathogenic diagnosis to control risk factors.32 Radial ray defect may be inherited as AD, AR or present with an unknown etiology.6 The two cases with radial ray defect (2.9%) encountered in the present study had ventricular septal defect in addition, pointing to the diagnosis of Holt-Oram syndrome and the AD mode of inheritance. In one case, the father was also affected so his risk of having other affected children is 50% if he is heterozygous (having one mutant gene). In the other case, both parents were normal, indicating a new mutation origin of the disease and the very low recurrence risk. Split hand/foot malformation, also termed ectrodactyly, is characterized by absence of the central digital rays, a deep median cleft, and syndactyly of the remaining digits.35 Its incidence varies between one in 10 000 and one in 90 000.36 Split hand/foot malformation is genetically heterogenous (caused by many genes). Only one case with this malformation was detected in the present study.
Many environmental events can lead to limb anomalies in humans. Approximately one in 2000 neonates has birth defects associated with rupture of the amnion.6 In the present study 4 cases (5.7%) had amniotic band disruption causing finger and toe amputations with constriction bands in addition to craniofacial anomalies. Rupture of the amnion during early pregnancy may result in the formation of amniotic bands or strands that can adhere to the embryo or the fetus. With fetal growth, the bands can tighten and compromise developing areas and lead to resorptive necrosis. The bands most often affect the limbs (leading to amputations or hypoplastic digits) and the craniofacial and abdominal areas. Careful examination of the placenta may reveal the bands and remnants of amnion.27 Al-Qattan37 has reviewed the associated anomalies and the pathogenesis of 31 upper limbs with congenital amputations due to constriction rings in 20 patients. He classified the pattern of amputation into three types; namely proximal upper limb amputation (type I), digital amputation associated with “coning” or “superimposition” of the digits (II), and multiple-digit amputation (IIIA), and single-digit amputation (IIIB).
Vascular disruption can also lead to limb anomalies as in hypoplasia of the pectoralis muscles with syndactyly or short digits (Poland sequence) due to a decreased blood supply in the embryonic subclavian artery or its branches. Al-Qattan38 has reviewed 20 cases with Poland sequence and classified the hand anomalies into seven types according to severity of the deformity. Sirenomelia (vitelline artery steal) is another example of vascular disruption leading to abnormal caudal development.24 Five cases (7.1%) in the present study suffered from limb anomalies due to vascular disruption in the embryonic blood vessels. Vascular anastomoses and discordant placental blood flow in twins with a common placenta predispose them to vascular disruptions. In approximately 1% of monozygotic twins, intrauterine death of one twin may result in release of thromboplastin or emboli to the surviving co-twin leading to ischemia in localized areas.17 One patient in the present study had gangrene and muscle atrophy of both lower limbs. He was one of a triplet. Other causes of vascular disruption include placental infarction, maternal hypotension, vasoactive drugs, agents like cocaine and certain medications.17 The prenatal diagnostic technique, chorionic villus sampling (CVS), has also been associated with limb reduction defect, with anoxia of distal fetal structures as a postulated etiology.39
Infants of diabetic mothers have 6% risk of developing congenital malformations. The risk may be over 20% if there is poor control during the first trimester. The malformations involve the heart (transposition of great vessels), anencephaly, spina bifida, holoprosencephaly, cleft lip and/or palate, renal anomalies and caudal regression sequence.17 One case with caudal regression in the present study was an infant of an uncontrolled diabetic mother.
Twenty cases (28.6%) in the present study had limb anomalies as one of the manifestations of sporadic syndromes (syndromes with unknown etiology). Acheiria (rudimentary hand and fingers) and aphalangia (transverse limb anomalies) are probably caused by death of the mesenchymal cells in the limb bud region.40 Ruiter et al41 have described absent left hand (a terminal transverse reduction defect) in a boy with mosaic trisomy 22. They observed that this type of limb anomalies has never been reported in association with this chromosomal aberration. This finding points out the importance of genetics in the work-up in sporadic cases of limb anomalies.
Watson42 stressed that management of congenital limb anomalies has to include classification and etiology, incidence, diagnosis before birth, and counseling of parents. In a similar study done by Holder-Espinasse et al43 on 107 cases of limb anomalies, the diagnosis was made in 78%, including isolated, syndromic, or chromosomal anomalies with the conclusion that prenatal multidisciplinary assessment is fundamental to assist with counseling, as is the postnatal follow-up of the infant. The diagnosis, if made, will obviously influence the information that will be given to the parents and the management of the malformation, and guide recurrence risks.
In conclusion, the etiological study, including the genetic work-up, of morphologically classified cases with congenital limb anomalies is helpful in the prevention of many disorders causing limb defects. Prevention can be achieved via proper genetic counseling, which includes information on the etiology of the disorder (genetic or environmental), prenatal detection of limb anomalies and risk estimation for later siblings.
References
- 1.O’Quinn JR, Hennekam RC, Jorde LB, Bamshad M. Syndromic ectrodactyly with severe limb, ectodermal, urogenital, and palatal defects maps to chromosome 19. Am J Hum Genet. 1998;62(1):130–5. doi: 10.1086/301687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giele H, Giele C, Bower C, Allison M. The incidence and epidemiology of congenital upper limb anomalies: a total population study. J Hand Surg [Am] 2001;26(4):628–34. doi: 10.1053/jhsu.2001.26121. [DOI] [PubMed] [Google Scholar]
- 3.Zguricas J, Bakker WF, Heus H, Lindhout D, Heutink P, Hovius SE. Genetics of limb development and congenital hand malformations. Plast Reconstr Surg. 1998;101(40):1126–35. doi: 10.1097/00006534-199804040-00039. [DOI] [PubMed] [Google Scholar]
- 4.Mathes SJ, Kerley SM, Manske PR, Upton JIII. Symposium: changing concepts in the management of congenital hand anomalies. Contemp Orthop. 1993;27:481–04. [Google Scholar]
- 5.Swanson AB. A classification for congenital limb malformations. J Hand Surg. 1976;1:8–22. doi: 10.1016/s0363-5023(76)80021-4. [DOI] [PubMed] [Google Scholar]
- 6.Smith JG, Weiss AC, Weiss YS. Congenital anomalies of the hand. Clin Pediatr. 1998;37:459–68. doi: 10.1177/000992289803700801. [DOI] [PubMed] [Google Scholar]
- 7.De Smet L. Classification for congenital anomalies of the hand: the IFSSH classification and the JSSH modification. Genet Couns. 2002;13(3):331–8. [PubMed] [Google Scholar]
- 8.Stoll C, Duboule D, Holmes L, Spranger J. Classification of limb defects. Am J Med Genet. 1998;77:439–41. [PubMed] [Google Scholar]
- 9.Luijsterburg AJ, van Huizum MA, Impelmans BE, Hoogeveen E, Vermeij-Keers C, Hovius SE. Classification of congenital anomalies of the upper limb. J Hand Surg [Br] 2000;25(1):3–7. doi: 10.1054/jhsb.1999.0336. [DOI] [PubMed] [Google Scholar]
- 10.Gurrieri F, Kjaer KW, Sangiorgi E, Neri G. Limb anomalies: Developmental and evolutionary aspects. Am J Med Genet. 2002;115(4):231–44. doi: 10.1002/ajmg.10981. [DOI] [PubMed] [Google Scholar]
- 11.Hungerford GA. Leukocytes cultured from small inoculo, the whole blood and the preparation of metaphase chromosomes by treatment with hypotonic potassium chloride. Stain Technol. 1965;40:320–6. doi: 10.3109/10520296509116440. [DOI] [PubMed] [Google Scholar]
- 12.Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971;ii:97–01. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- 13.Mitelman F. ISCN- An International System for Human Cytogenetic Nomenclature. Basle, Switzerland: Karger; 1995. [Google Scholar]
- 14.McKusick VA. Mendelian inheritance in man. 11th ed. Baltimore: The Johns Hopkins University Press; 1996. Web site http://www.ncbi.nlm.nih.gov (updated weekly) [Google Scholar]
- 15.Mueller RF, Young ID. Emery’s elements of medical genetics. 10th edition. Churchill Livingstone; 1998. [Google Scholar]
- 16.Jones KL. Smith’s recognizable patterns of human malformations. 4th edition. WB Saunders; 1988. [Google Scholar]
- 17.Seashore MR, Wappner RS. Genetics in primary care & clinical medicine. Appleton & Lange; NJ: 1996. Congenital malformations. [Google Scholar]
- 18.Flatt AE. The care of congenital hand anomalies. St Louis: Quality Medical Publisher; 1994. Genetics and inheritance. [Google Scholar]
- 19.Martinez-Frias ML, Villa A, de Pablo RA, Ayala A, Calvo MJ, Bermejo E, Rodriguez L. Limb deficiencies in infants with trisomy 13. Am J Med Genet. 2000;93(4):339–41. doi: 10.1002/1096-8628(20000814)93:4<339::aid-ajmg15>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 20.Sepulveda W, Treadwell MC, Fisk NM. Prenatal detection of preaxial upper limb reduction in trisomy 18. Obst Gynae. 1995;85(5):847–50. doi: 10.1016/0029-7844(94)00284-k. [DOI] [PubMed] [Google Scholar]
- 21.Kava MP, Tullu MS, Muranjan MN, Girisha KM. Down syndrome: Clinical profile from India. Arch Med Res. 2004;35(1):31–5. doi: 10.1016/j.arcmed.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Stempfle N, Huten Y, Fredouille C, Brisse H, Nessmann C. Skeletal abnormalities in fetuses with Down’s syndrome: a radiographic post-mortum study. Pediatr Radiol. 1999;29(9):682–8. doi: 10.1007/s002470050675. [DOI] [PubMed] [Google Scholar]
- 23.Tongsong T, Wanapirak C, Sirichotiyakul S, Sirivatanapa P. Prenatal sonographic markers of trisomy 21. J Med Assoc Thai. 2001;84(2):274–80. [PubMed] [Google Scholar]
- 24.Sesgin MZ, Stark RB. The incidence of congenital defects. Plast Reconstr Surg. 1961;27:261–7. doi: 10.1097/00006534-196103000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Packard DS, Levinsohn EM, Hootnick DR. Extent of duplication in lower-limb malformations suggests the time of the teratogenic insult. Pediatrics. 1993;91(2):411–3. [PubMed] [Google Scholar]
- 26.Zguricas J, Snijders PJ, Hovius SE, Heutink P, Oostra BA, Lindhout D. Phenotypic analysis of triphalangeal thumb and associated hand malformations. J Med Genet. 1994;31:462–7. doi: 10.1136/jmg.31.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hensinger RN, Jones ET. Neonatal orthopaedics. New York: Grune & Stratton Inc; 1981. pp. 191–09. [Google Scholar]
- 28.Stelling F. The upper extremity. In: Ferguson AB Jr, editor. Orthopaedic surgery in infancy and childhood. 5th ed. Baltimore: Williams and Wilkins; 1981. pp. 709–45. [Google Scholar]
- 29.Dobyns JH, Doyle JR, Von Gillern TL, Cowen NJ. Congenital anomalies of the upper extremity. Hand Clinics. 1989;5:321–42. [PubMed] [Google Scholar]
- 30.Temtamy S, McKusick V. The genetics of hand malformations. Birth defects. 1978;14:1–23. [PubMed] [Google Scholar]
- 31.Qin W, Shu AL, Xing QH, Yang MS, Feng GY, He L. Genetic analysis of a Chinese pedigree with congenital synpolydactyly. Yi Chuan Xue Bao. 2003;30(10):973–7. [PubMed] [Google Scholar]
- 32.Riano Galan I, Fernandez Toral J, Garcia Lopez E, Moro Bayon C, Mosquera Tenreiro C. Limb reduction defects in Asturias (1986–1997): prevalence and clinical presentation. An Esp Pediatr. 2000;52(4):362–8. [PubMed] [Google Scholar]
- 33.Dastgiri S, Stone DH, Le-Ha C, Gilmour WH. Prevalence and secular trend of congenital anomalies in Glasgow, UK. Arch Dis Child. 2002;86(4):257–63. doi: 10.1136/adc.86.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon NP, Simon MW. Congenital absence of the 5th digital ray and its proximal segmental structures. Am J Perinatol. 2003;20(1):7–10. doi: 10.1055/s-2003-37954. [DOI] [PubMed] [Google Scholar]
- 35.Safarazi M, Akarsu AN, Sayli BS. Localization of the syndactyly type II (synpolydactyly) locus to 2q31 region and identification of tight linkage to HOXD8 intragenic marker. Hum Mol Genet. 1995;4:1453–8. doi: 10.1093/hmg/4.8.1453. [DOI] [PubMed] [Google Scholar]
- 36.Louis DS, Tsai E. Congenital hand and forearm anomalies. Curr Opin Ped. 1996;8:61–4. doi: 10.1097/00008480-199602000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Al-Qattan MM. Classification of the pattern of intrauterine amputations of the upper limb in constriction ring syndrome. Ann Plast Surg. 2000;44(6):626–32. doi: 10.1097/00000637-200044060-00008. [DOI] [PubMed] [Google Scholar]
- 38.Al-Qattan MM. Classification of hand anomalies in Poland’s syndrome. Br J Plast Surg. 2001;54(2):132–6. doi: 10.1054/bjps.2000.3505. [DOI] [PubMed] [Google Scholar]
- 39.Thomas M, Chitayat D, Babul R, Wyatt P, Waye JS, O’Brien K, Chui DHK, Conacher S, Silver M. Alpha thalassemia homozygotes and limb reduction defects. Am J Hum Genet. 1994;55(3):A94. [Google Scholar]
- 40.Ogino T. Congenital anomalies of the hand: The Asian perspective. Cli Orth Rel Res. 1996;323:12–21. doi: 10.1097/00003086-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Ruiter EM, Toorman J, Hochstenback R, De Vries BB. Mosaic trisomy 22 in a boy with terminal transverse limb reduction defect. Clin Dysmorphol. 2004;13(2):99–02. [PubMed] [Google Scholar]
- 42.Watson S. The principles of management of congenital anomalies of the upper limb. Arch Dis Child. 2000;83(1):10–7. doi: 10.1136/adc.83.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holder-Espinasse M, Devisme L, Thomas D, Boute O, Vaast P, Fron D, Herbaux B, Puech F, Manouvrier-Hanu S. Pre-and postnatal diagnosis of limb anomalies: a series of 107 cases. Am J Med Genet A. 2004;124(4):417–22. doi: 10.1002/ajmg.a.20359. [DOI] [PubMed] [Google Scholar]