Abstract
BACKGROUND
An association between HCV infection and lichen planus is uncertain because the prevalence of HCV infection in patients with lichen planus varies considerably from one geographic area to another. The purpose of this study was to determine the frequency of anti-HCV antibodies and its association with various clinical types of lichen planus in Mekkah, Saudi Arabia.
METHODS
A total of 114 cases of lichen planus were selected for the study. These were divided into four categories, including patients with skin lesions, skin and oral lesions, and oral or genital lesions alone. The sera of these patients were tested for HCV antibodies by means of a third-generation ELISA and serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were simultaneously determined. A group of 65 volunteers served as a control group.
RESULTS
Of the 114 patients with lichen planus, 30 had HCV antibodies (26.3%). In the 65 control group subjects anti-HCV antibodies were observed in 3 volunteers (4.6%). There was a significant difference between the two groups (P<0.0001). The ALT was raised in 22 patients and the AST level was elevated in 14 of the 114 cases of lichen planus. In the control group, the AST level was raised in 3 of the 65 controls while 2 had an elevated ALT level. The number of patients with an abnormal transaminase level also significantly differed in the two groups.
CONCLUSION
A high prevalence of HCV infection was detected in patients with lichen planus. These results support a possible relationship between lichen planus and hepatitis C.
Lichen planus is an immunologically mediated skin and mucous membrane disease, which has been described in patients with hepatitis C virus-related liver disease with variable frequency in several studies to date.1 Most of these reports, especially the larger series, were conducted in Europe. Oral lichen planus was reported in most studies. Chronic hepatitis C is associated with a variety of disorders, particularly dermatologic conditions. The most frequent of these are mixed cryoglobulinemia with leukocytoclastic vasculitis, porphyria cutanea tarda and lichen planus.1 Numerous cases of lichen planus in patients with hepatitis C virus (HCV) infection have been published and an association of chronic hepatitis with lichen planus has been established.2 However, an association between HCV infection and lichen planus is uncertain because the prevalence of HCV infection in patients with lichen planus varies considerably from one geographic area to another, ranging from 4% in northern France to 62% in Japan.3 Studies from Great Britain have failed to reveal any association. Similarly, another study from France found no difference with regard to HCV prevalence in patients with lichen planus and with other dermatoses.4 However, Rebora reported that the prevalence of HCV antibodies was 14% in 87 patients with lichen planus.5
This study was conducted at Alawi Tonsi Hospital, Makkah, Saudi Arabia, to determine the frequency of HCV antibodies in patients with lichen planus in the city of Makkah. Makkah has a Saudi as well as a large expatriate population belonging to multiple ethnic groups, because of its holy background.
Methods
This study was carried out in the Department of Dermatology, Alawi Tonsi Hospital, Makkah, Kingdom of Saudi Arabia from October 1999 to September 2001. The study involved 114 patients with lichen planus diagnosed on the basis of clinical features, and in some difficult cases, by specific histologic findings. Patients suspected of drug-induced lichenoid eruptions such as those taking beta-blockers, thiazide diuretics and chloroquine were not included. Sera from all 114 patients were tested for HCV antibodies by means of third-generation enzyme- linked immunosorbent assay (Bioelisa HCV, 08186Ll-1CA-D-AMUNT, Biokit SA, Barcelona, Spain). The sensitivity of the test was 99% while the specificity was 94%. The serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were simultaneously determined in all patients. As a control group, 65 volunteers were chosen randomly from a group of 135 relatives of patients. After informed written consent was provided, they agreed to undergo various blood investigations and also to possibly donate blood for their relatives undergoing various surgical procedures. The chi square test was used for statistical comparison of the two groups.
Results
The 114 patients with lichen planus comprised 70 males (61.4%) and 44 females (38.5%) with a mean age of 49.2 years in males and 40.1 in females. Thirty patients (26.3%) tested positive for HCV antibodies. Of those 30 patients, 12 (40%) were Saudis and 18 (60%) were non-Saudi expatriates, including 14 (77.7%) from the Indian subcontinent (Pakistan, India, Bangladesh and Burmese origin), 3 (16.6%) from the Far East and 1(5.5%) from Egypt (Africa). The sex distribution and type of lesions in the 30 patients are shown in Table 1.
Table 1.
Sex distribution of various clinical types of lichen planus in 30 hepatitis C virus antibody-positive patients.
| No of Patients | Skin/oral lesions | Skin lesions | Oral lesions | Genital lesions |
|---|---|---|---|---|
| Males (n=20) | 9 | 4 | 4 | 3 |
| Females (n=10) | 5 | 2 | 3 | 0 |
| Total (n=30) | 14 | 6 | 7 | 3 |
Of the 65 volunteers in the control group, 60 were males aged 30 to 50 years (mean 38.4 years) and 5 were females aged 25 to 45 years (mean 35.5 years). Only 3 patients in this group had HCV antibodies (4.6%). The difference in the number of patients with HCV antibodies between the two groups was highly significant (P<0.001; χ2=15.4). Of the 114 patients with lichen planus, ALT was raised in 22 and 14 also had an elevated AST level, while in the control group the AST level was elevated in 3 of the 65 patients while 2 had raised ALT levels. The patients with lichen planus were 10 years older on average than the controls for the males and 5 years older than the controls for the females.
Discussion
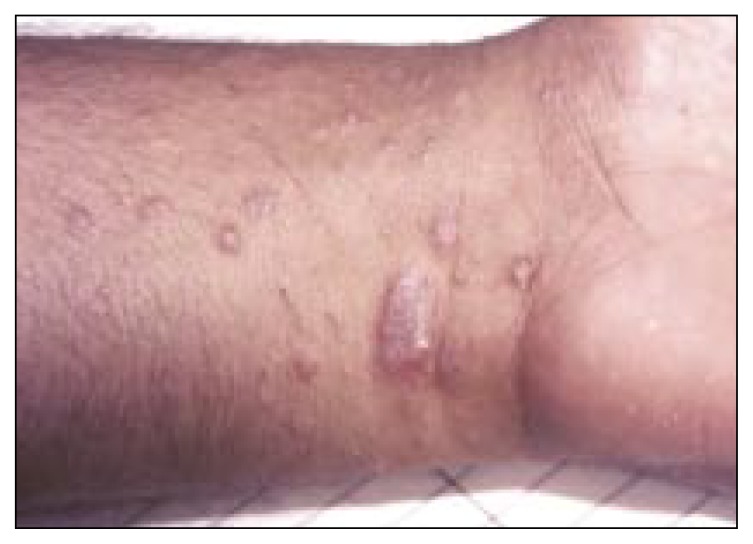
Lichen planus is a pruritic, papular eruption characterized by its violaceous color and polygonal shape. It is most commonly found on the flexor surface of the upper and lower extremities (Figures 1 and 2), genitalia, and on the mucous membranes. This condition is most likely an immunologically mediated reaction. More than 50 per cent of cutaneous lesions resolve within 6 months and about 85 per cent of cases resolve within 18 months. However, the oral lesions have a mean duration of about 5 years.
Figure 1.
Typical lichen planus lesion with violaceous color and polygonal shape.
Figure 2.
Characteristic lesions of lichen planus in a 22-year-old patient.
Lichen planus was reportedly associated with liver disorders, particularly chronic active hepatitis, in several well-executed studies that predate the discovery of hepatitis C virus (HCV).2 HCV infection is present in other diseases of altered immunity, such as ulcerative colitis, vitiligo, alopecia aerata, morphea, and lichen sclerosis. HCV was not seriously considered a precipitating factor of lichen planus until serologic tests for HCV became available in 1990. In our study we found HCV antibodies in 30 of 114 cases of lichen planus. Our results support a possible relationship between lichen planus and hepatitis C.
There may be an important association between hepatitis C and lichen planus, especially in those countries with a high incidence of hepatitis C infection. Liver abnormalities are frequently reported in patients with lichen planus.6,7 Chronic active hepatitis, especially due to hepatitis C virus, is suspected to be a contributing factor. The significant association of erosive lichen planus with chronic hepatitis was demonstrated in a case-controlled study.8 More recently there have been reports suggesting that these two conditions might be interrelated. One study found a high prevalence of HCV positive patients in Pakistan.9 A similar study of high incidence has been reported from Iran.10 Another study conducted in Spain reported a statistically significant association between erosive lichen planus and HCV infection suggesting that a high prevalence of HCV-RNA in lichen planus provided some evidence that HCV infection might have a role in the pathogenesis of lichen planus.11 The results of our study are in line with the findings in the above mentioned studies.
Studies conducted in western countries with a low incidence of hepatitis infection have not found an association between these two diseases.12 A study in 180 English patients with oral lichen planus failed to show any significant association with liver dysfunction.13 Similarly, a study in Turkey found no relation between lichen planus and hepatitis C infection, but the authors suggested that the virus might be playing a potential pathogenic role by replicating in cutaneous tissue and triggering lichen planus in genetically susceptible HCV-infected patients.14
The prevalence of HCV in patients with lichen planus varies considerably from one geographic area to another, ranging from 4% in France17 to 62% in Japan.3 The wide geographical location of patients and their origin may be important factors in the relationship between the pathogenesis of these two conditions. In our study, there was a strong association between lichen planus and HCV infection. The HCV prevalence of 26.3% vs. 4.6% in the controls is not a chance association suggesting that lichen planus and HCV infection may be etiologically related. We also found that the incidence of HCV infection was higher in the expatriate patients. The explanations for this observation could be that expatriates are from countries in which a higher incidence of HCV infection and lichen planus has been reported.9,10
Our study has limitations as regards the control group. Because of the holy environment and conservative society not many females volunteered to participate in the study. The mean age of the control group was also much lower than the patient group. Unfortunately, this was the best control group we could get from the restricted number of relatives who agreed to participate in the study.
Lichen planus may represent a mucosal reaction to a variety of factors, including hepatitis C virus (HCV) infection. One of the aims of this study was to compare the prevalence of HCV infection in patients with lichen planus of the oral mucosa. The results in this study showed interesting findings in that of 10 cases of oral lichen planus, 7 patients tested positive for HCV. These findings are quite different from the results observed in other studies especially from countries with a low incidence of hepatitis C infections reported from Europe.15,16
Lichen planus can be the first presentation of HCV. Because of HCV’s the significant morbidity and mortality associated with HCV, it is important for clinicians to actively look for HCV infection in patients with lichen planus. Lichen planus appears to be related to the pattern of immune dysregulation induced by HCV, probably in a host with an underlying susceptibility for autoimmune disease. It would be worth doing a more board-based multi-centre trial involving cases of lichen planus and hepatitis C diagnosed clinically from different geographical areas and ethnic groups to determine the association between these two conditions.
References
- 1.Bonkovsky HL, Mehta S. Hepatitis C: a review and update. J Am Acad Dermatol. 2001;44:150–2. doi: 10.1067/mjd.2001.109311. [DOI] [PubMed] [Google Scholar]
- 2.Gruppo Italiano Sudi Epidermiologici in Dermatologia (GISED) Lichen planus and liver disease: a multicentre case-controlled study. BMJ. 1990;300:227–30. doi: 10.1136/bmj.300.6719.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagao Y, Sata M, Tanikawa K, et al. Lichen planus and hepatitis C virus in northern region of Japan. Eur J Clin Invest. 1995;25:910–4. doi: 10.1111/j.1365-2362.1995.tb01966.x. [DOI] [PubMed] [Google Scholar]
- 4.Cribier B, Gamier C, Laustriat D, Heid E. Lichen planus and hepatitis C virus infection: an epidemiologic study. J Am Acad Dermatol. 1994;31:1070–2. doi: 10.1016/s0190-9622(09)80092-3. [DOI] [PubMed] [Google Scholar]
- 5.Rebora A. Lichen planus and the liver. Int J Dermatol. 1992;3:392–5. doi: 10.1111/j.1365-4362.1992.tb02665.x. [DOI] [PubMed] [Google Scholar]
- 6.Doutre MS. Hepatitis C virus-related skin disease. Arch Dermatol. 1999;135:1401–3. doi: 10.1001/archderm.135.11.1401. [DOI] [PubMed] [Google Scholar]
- 7.Tanei R, Watanabe K, Nishiyama S. Clinical and histopathologic analysis of the Relationship between lichen planus and chronic hepatitis C. J Dermatol. 1995;22:316–23. doi: 10.1111/j.1346-8138.1995.tb03395.x. [DOI] [PubMed] [Google Scholar]
- 8.Mignogna MD, Muzio LL, Favia G, et al. Oral lichen planus and HCV infection: a clinical evaluation of 263 cases. Int J Dermatol. 1998;37:575–8. doi: 10.1046/j.1365-4362.1998.00510.x. [DOI] [PubMed] [Google Scholar]
- 9.Mahboob A, Haroon TS, Iqbal Z, et al. Frequency of anti-HCV antibodies in patients with lichen planus. J Coll Physicians Surg Pak. 2003;13(5):248–251. [PubMed] [Google Scholar]
- 10.Ghodsi SZ, Daneshpazhooh M, Shahi M, et al. Lichen Planus and Hepatitis C: a case controlled study. BMC Dermatol. 2004;4(1):6. doi: 10.1186/1471-5945-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez-Perez J, De Castro M, Buezo GF, et al. Lichen Planus and hepatitis C virus: prevalence and clinical presentation of patients with lichen planus and hepatitis C virus infection. Br J Dermatol. 1996;134(4):715–9. doi: 10.1111/j.1365-2133.1996.tb06977.x. [DOI] [PubMed] [Google Scholar]
- 12.Tucker SC, Coulson IH. Lichen planus is not associated with hepatitis C virus infection in patients from north west England. Acta Derm Venerol. 1999;79(5):378–9. doi: 10.1080/000155599750010328. [DOI] [PubMed] [Google Scholar]
- 13.El-Kabir M, Scully C, Porter S, et al. Liver function in UK patients with oral lichen planus. Clin Exp Dermatol. 1993;18:12–16. doi: 10.1111/j.1365-2230.1993.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 14.Erkek E, Bozdogan O, Olut AI. Hepatitis C virus infection prevalence in lichen planus: examination of lesional and normal skin of hepatitis C virusinfected patients with lichen planus for the presence of hepatitis C virus RNA. Clin Exp Dermatol. 2001;26(6):540–4. doi: 10.1046/j.1365-2230.2001.00885.x. [DOI] [PubMed] [Google Scholar]
- 15.Van der Meij EH, Van der Meij Waal I. Hepatitis C virus infection and oral lichen planus: a report from the Netherlands. J Oral Pathol Med. 2000 Jul;29(6):256–8. doi: 10.1034/j.1600-0714.2000.290603.x. [DOI] [PubMed] [Google Scholar]
- 16.Grote M, Reichart PA, Hopf U. Increased incidence of of oral lichen planus in hepatitis C infection. Mund Kiefer Gesichtschir. 1999 Jan;3(1):30–3. doi: 10.1007/s100060050089. [DOI] [PubMed] [Google Scholar]
- 17.Jubert C, Pawlotsky JM, Pouget F, et al. Lichen planus and hepatitis C virus-related chronic active hepatitis. Arch Dermatol. 1994;130:73–6. [PubMed] [Google Scholar]