Abstract
The mechanisms of aging that are involved in the development of idiopathic pulmonary fibrosis (IPF) are still unclear. Although it has been hypothesized that the proliferation and activation of human lung fibroblasts (hLFs) are essential in IPF, no studies have assessed how this process works in an aging lung. Our goal was to elucidate if there were age-related changes on primary hLFs isolated from IPF lungs compared with age-matched controls. We investigated several hallmarks of aging in hLFs from IPF patients and age-matched controls. IPF hLFs have increased cellular senescence with higher expression of β-galactosidase, p21, p16, p53, and cytokines related to the senescence-associated secretory phenotype (SASP) as well as decreased proliferation/apoptosis compared with age-matched controls. Additionally, we observed shorter telomeres, mitochondrial dysfunction, and upon transforming growth factor-β stimulation, increased markers of endoplasmic reticulum stress. Our data suggest that IPF hLFs develop senescence resulting in a decreased apoptosis and that the development of SASP may be an important contributor to the fibrotic process observed in IPF. These results might change the existing paradigm, which describes fibroblasts as aberrantly activated cells, to a cell with a senescence phenotype.
Keywords: idiopathic pulmonary fibrosis, fibroblasts, senescence, aging, telomeres, mitochondria, TGF-β, collagen
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is the most common of all the idiopathic interstitial lung diseases (33). IPF is a chronic, progressive, and irreversible restrictive lung disease affecting individuals generally over the age of 60. There is still a lack of complete knowledge of its pathogenesis (33), and although Pirfenidone and Nintedanib have been approved as IPF therapies, these drugs only slow down the progression of the disease (3, 28). Therefore, no definitive treatment other than lung transplantation exists.
Because IPF is a disease affecting the elderly, our efforts have been focused on the study of their different hallmarks of aging (8, 22, 40) within the context of the disease to clarify the link between aging and IPF. Although the complete mechanisms are unknown, telomere shortening (2, 4, 7) and mitochondrial dysfunction (11) have been found to be linked to the disease.
Our group is interested in studying the consequences of age, in particular on lung fibroblasts and the significance as effector cells. It is thought that IPF fibroblasts and myofibroblasts are responsible for the increased deposition of collagen and other extracellular matrix compounds, resulting in lung remodeling and a decline in lung function (33). However, the mechanisms of aging that influence the abnormal activation of fibroblasts are still unknown. Recently, some authors have reported that cellular senescence of fibroblasts could mediate the development of IPF (42). However, it is still not clear what mechanisms link the different hallmarks of aging and fibrosis. Additionally, although animal models have been useful in the study of lung fibrosis, they still lack the ability to mimick IPF. Here, we studied primary human lung fibroblasts (hLFs) from IPF and age-matched normal controls, looking for the contribution of aging in the characteristics of IPF fibroblasts. We demonstrated that as a consequence of shorter telomere length and dysfunctional mitochondria, hLFs isolated from IPF patients present an increase in senescence compared with age-matched controls. As a result of senescence, proliferation and apoptosis are reduced. On the other hand, a profibrotic senescence-associated secretory phenotype (SASP) is observed in IPF hLFs, which, especially in a niche enriched with transforming growth factor-β (TGF-β), may be responsible of the fibrotic process observed in IPF.
MATERIALS AND METHODS
Isolation and growth of fibroblasts.
Collection of human lung tissue was approved by the Institutional Review Board and the Committee for Oversight of Research and Clinical Training Involved Decedents of the University of Pittsburgh. Lung tissue from explanted IPF lungs and age-matched normal donors was collected from the lower lobes (Table 1) to find age and sex of the lines included in the experiments. Enzymatic digestion (0.05% trypsin; GIBCO) was used to isolate hLFs, and later fibroblasts were grown with F12 nutrient Ham Medium (GIBCO) containing 10% of fetal bovine serum (Atlanta Biologicals) and 1% of antimycotic-antibiotic (GIBCO). Cells were cultured at 37°C, 5% CO2 and then expanded to select a homogeneous fibroblast population (experiments were realized with lines between passages 5 and 9).
Table 1.
Information of lines used in the different experiments hLFs
| Control Lines |
IPF Lines |
||||
|---|---|---|---|---|---|
| Experiment | Size, n | Average age, yr (SD) | Size, n | Average age, yr (SD) | P Value Age |
| Actin stain | 3 | 68 (5) | 4 | 65 (7) | 0.63 |
| Proliferation assay | 11 | 64 (6) | 8 | 63 (8) | 0.86 |
| Telomere length | 5 | 64 (4) | 5 | 66 (6) | 0.61 |
| β-Galactosidase activity | 4 | 66 (5) | 4 | 68 (4) | 0.55 |
| mRNA expression senescence (nonstimulated) | 5 | 64 (4) | 9 | 61 (8) | 0.36 |
| Protein expression senescence markers | 4 | 66 (5) | 3 | 62 (6) | 0.41 |
| mRNA expression senescence markers, collagen 1, α-SMA, BiP, and XBP1 (TGF-β stimulated) | 4 | 66 (5) | 4 | 65 (7) | 0.96 |
| SASP mRNA expression | 3 | 65 (6) | 3 | 69 (5) | 0.41 |
| OCAR_ECAR (nonstimulated) | 3 | 64 (5) | 3 | 62 (10) | 0.78 |
| OCAR_ECAR (TGF-β stimulated) | 3 | 67 (4) | 4 | 71 (3) | 0.23 |
| Mitotracker green | 3 | 64 (5) | 4 | 62 (8) | 0.63 |
| ATP production | 3 | 64 (4) | 3 | 71 (3) | 0.07 |
| Electron microscopy | 3 | 64 (5) | 4 | 62 (8) | 0.63 |
| Apoptosis (TUNEL assay) | 4 | 66 (5) | 4 | 68 (4) | 0.55 |
hLFs, human lung fibroblasts; IPF, idiopathic pulmonary fibrosis; α-SMA, α-smooth muscle actin; OCR, oxygen consumption rate; SASP, senescence-associated secretory phenotype; ECAR, extracellular acidification rate; TGF-β, transforming growth factor-β; TUNEL, terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling.
Proliferation.
Eleven IPF and eight control fibroblasts were seeded in 96-well plates in a density of 500 cells/well and triplicate of each line per plate. Half of the volume of the media was changed every 2 days, and cells were collected and frozen every 3 days for 12 days. Subsequently, fluorescence was measured (CyQUANT cell proliferation assay kit; Molecular Orobes) and normalized against DNA content.
β-Galactosidase assay.
Fibroblasts were grown on six-well cell culture plates nonstimulated after 24 h of TGF-β stimulation (5 ng/ml). The activity of β-galactosidase was assessed according to the manufacturer’s instructions (Cell Signaling). Posteriorly, five photos per line in different fields were taken at ×10 magnification and quantification of blue cells (β-galactosidase positive) over total cells was calculated.
Morphology assessment.
Fibroblasts were grown on chamber slides, fixed with 2% paraformaldehyde, permeabilized with 0.25% triton (Sigma), and incubated with Actin-Red (Molecular Probes) according to manufacturer’s instructions. With the use of fluorescence microscopy, pictures of the stained fibroblasts were taken at ×20 magnification. With the use of Zeiss pro, thearea of hLFs was measured.
Telomere length.
Two-million cells per line were subjected to flow cytometry and fluorescence in situ hybridization (flowFISH) as previously described (10). Each hybridized sample was run in triplicate, and the mean intra-assay coefficient of variation was 3.5% (range, 0.7–7.8).
Protein extraction and quantification.
Fibroblasts were grown on a 15-cm cell culture dish until 80% confluence, and cells were washed three times with PBS (Sigma) and 100 μl of RIPA buffer (Sigma) containing 1% of protease. Phosphatase inhibitors were added to each dish and after scraping, and the lysate was collected and frozen. At the moment of the assay, protein concentration was determined (Pierce BCA Protein Assay Kit) at 562-nm absorbance on a plate reader (incubation at 37°C during 30 min).
Electrophoresis of proteins and Western blot.
With the use of 4–12% Bis-Tris Gels and between 10 and 30 μg protein per well, proteins were separated and subsequently transferred to a nitrocellulose membrane. After transfer, the membrane was blocked for 1 h at room temperature using either 5% nonfat milk or 5% BSA in PBS-Tween (0.001%). Next, it was washed quickly with PBS, and primary antibodies against p16 (BD Cat. No. 551153) (18), p21 (Santa Cruz Biotechnology Cat. No. SC-397) (43), p53 (Santa Cruz Biotechnology Cat. No. SC-126) (15), and β-actin (Santa Cruz Biotechnology Cat. No. SC-47778) (29) were incubated overnight at 4°C. Later, after the membrane had been washed with PBS-Tween, the secondary antibodies were incubated at room temperature for 1 h. Afterwards, chemiluminescence substrate (Perkin Elmer kit) was added to the membranes and exposed at different time points to an X-ray film, which was then developed and scanned. With the use of ImageJ, the bands representing the target protein were quantified and then normalized against β-actin.
RNA extraction and quantification.
Fibroblasts lysate was collected and frozen in 700 μl QiAZOL (Qiagen) when the lines reached 80% confluence. In the corresponding case, cells were stimulated with 5 ng/ml of TGF-β during 6 and 24 h previous collected in QiAZOL. Subsequently, RNA was extracted according to the manufacturer’s instructions (miRNeasy mini kit; Qiagen) and the RNA concentration was read using cell plate reader. Extracted RNA was stored at −80°C.
Quantitative RT-PCR.
TaqMan primers from Thermo Fisher [p21, p53, Col 1, and α-smooth muscle actin (α-SMA)] and Integrated DNA Technologies [binding immunoglobulin protein (BiP), X-box binding protein 1 (XBP1), IL-6, IL-1β, and FGF2] were bought and real-time PCR was run using RNA to CT one-step kit (Applied Biosystems). Twenty-five nanograms of RNA were used per reaction, and each sample was run in triplicates. Ct values were normalized with the endogenous control GAPDH. ΔCt (target-endogenous control), ΔΔCt (target-normal control), and fold change (2^-ΔΔCt) were also calculated. The list of the primers is shown in detail on Table 2.
Table 2.
Primers used for RT-PCR
| Gene Symbol | Name | Assay | Company |
|---|---|---|---|
| ACTA2 | Actin; alpha 2; smooth muscle | Hs00426835_g1 | Thermo Fisher |
| COL1A1 | Collagen type I alpha 1 | Hs00164004_m1 | Thermo Fisher |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A (p21) | Hs00355782_m1 | Thermo Fisher |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A (p16) | Hs00923894_m1 | Thermo Fisher |
| TP53 | Tumor protein p53 | Hs01034249_m1 | Thermo Fisher |
| IL-6 | Interleukin-6 | Hs.PT.56a.40226675 | Integrated DNA Technologies (IDT) |
| IL-1β | Interleukin-1β | Hs.PT.56a. 1518186 | Integrated DNA Technologies (IDT) |
| FGF2 | Fibroblast growth factor 2 | Hs.PT.56a.24613308 | Integrated DNA Technologies (IDT) |
| XBP1 | X-box binding protein 1 | Hs.PT.58.1903847 | Integrated DNA Technologies (IDT) |
| BiP | Binding immunoglobulin protein (BiP) | Hs.PT.58.22715160 | Integrated DNA Technologies (IDT) |
ATP production.
IPF fibroblasts and age-matched controls were grown in six-well plates until 80% confluence was reached. Luminescence of luciferase activity in the cells (this requires ATP to generate light through its substrate luciferin) was measured according to the manufacturer’s instructions (Molecular Probes), and results were normalized by protein content.
Oxygen consumption rate and extra cellular acidification rate analysis.
Twenty thousand fibroblasts per well were seeded on a 96-well Seahorse plate. Samples were plated in quadruplicate. Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured as previously described (11). Analysis was done at basal conditions as well as after 4 h of stimulation with 5 ng/ml of TGF-β (PeproTech).
Mitochondria morphology.
Fibroblasts were grown to 80% confluence and fixed in 2.5% gluteraldehyde for 5 days. Sections were prepared and images of representative areas were taken under transmission electronic microscopy (×25,000 and ×100,000 magnification lens).
Mitochondrial mass.
MitoTracker green was performed according to the manufacturer’s instructions (Molecular Probes). The cells were incubated for a half an hour in 200 nM Mito-tracker green dye before being analyzed by optical reader.
Apoptosis assay.
Five-thousand fibroblasts were seeded in two-chamber slides, and eight lines were evaluated (4 age-matched controls and 4 IPF). When 80% confluence was reached, cells were fixed with 1% paraformaldehyde. Subsequently, terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling assay was performed according to the manufacturer’s instructions (Millipore). Finally, using a fluorescence microscope, photos were taken at ×20 magnification and percentage of apoptotic cells was calculated using Excel.
Statistical analysis.
Two-way ANOVA, mixed model analysis, Mann-Whitney test, and t-test were used for the statistical analysis as appropriate. Significance was calculated when P < 0.05.
RESULTS
Diminished proliferative capacity of IPF hLFs suggests senescent phenotype.
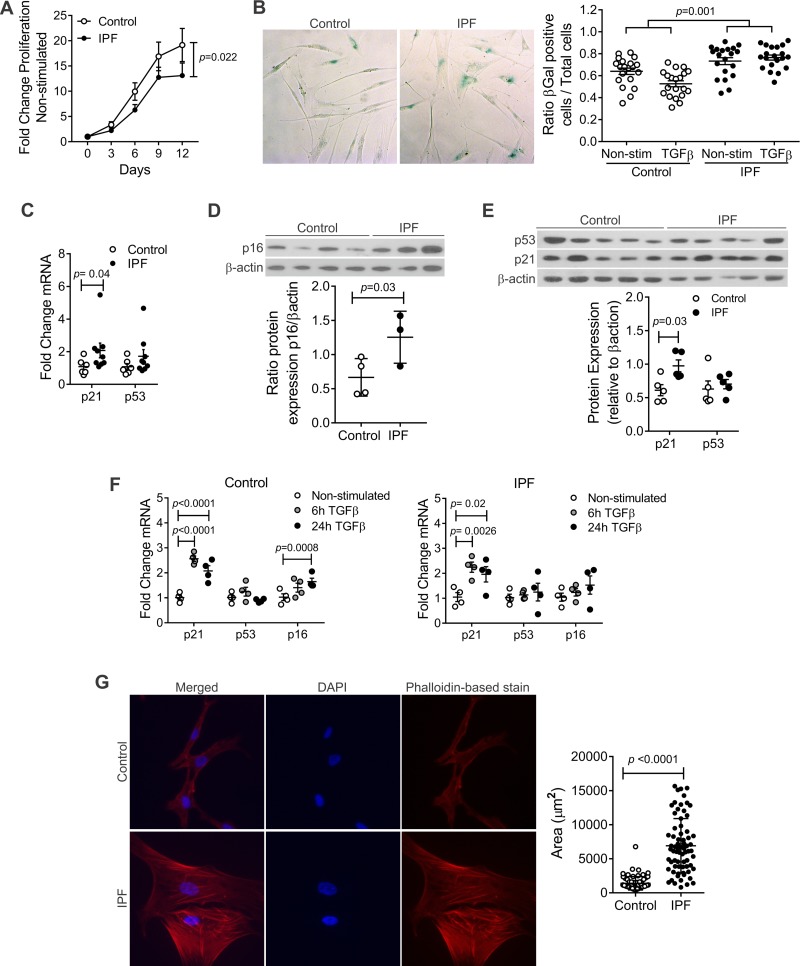
When culturing the cells, we noticed that the growth rate of hLFs from IPF was lower than controls; thus we decided to assess the proliferative capacity of hLFs. For that purpose, 500 cells were seeded in triplicates on 96-well cell culture plates and grown for a period of 12 days. Fifty percent of each well’s media was replaced every 2 days, and every 3 days a 96-well cell culture plate (representing a time point) was frozen. After 12 days, proliferation was measured through DNA content and fold change of proliferation was calculated. Our findings demonstrated statistically significant lower proliferation in the IPF group compared with age-matched controls (Fig. 1A).
Fig. 1.
Increased senescence in idiopathic pulmonary fibrosis (IPF) human lung fibroblasts (hLFs). A: fold change of proliferation in hLFs, showing statistical significant decreased proliferation in IPF hLFs (P = 0.02; n = 11 control and 8 IPF hLFs). Repeated-measures analysis (mixed model) was applied to test the interaction between time and IPF group as a measure of difference between the 2 groups across time. B: IPF hLFs have higher β-galactosidase (β-gal) expression compared with controls in response to transforming growth factor-β (TGF-β) stimulation (P = 0.001 for interaction between IPF and TGF-β stimulation). The difference of IPF vs. control in response to TGF-β stimulation was analyzed using repeated-measures analysis (mixed effect models in which multiple optical reads from the same line were treated as correlated observations). Alternatively, if we use the average ratio from multiple measures in each subject, the corresponding p for the difference in TGF-β response is 0.041 (n = 4 lines per group). C: fold change of mRNA expression of senescence markers n = 6 in control and 9 in IPF hLFs (means ± SE). Error bars represent SE, and P value was calculated by unpaired t-test. D: protein expression of p16 normalized against β-actin (n = 4 control and 3 in IPF hLFs P = 0.03). Error bars represent SE, and P value was calculated by unpaired t-test. E: protein expression of p21 and p53 normalized against β-actin (n = 5 per group). Error bars represent SE and p-value was calculated by unpaired t-test. F: fold change of mRNA expression of senescence markers upon TGF-β stimulation (n = 4 per group). Bars represent SE. G: phalloidin-based stain and quantification of area of fibroblasts (means ± SE) using Zeuss pro (n = 68 cells in IPF hLFs and n = 71 cells in control hLFs, corresponding to 3 lines in the control group and 4 in the IPF).
Expression of markers of cell senescence in IPF hLFs.
Once we demonstrated the decreased proliferation in IPF hLFs, we intended to determine the effect of aging on IPF hLFs, exploring the possibility of an increase in cell senescence. It has been determined that increased β-galactosidase activity and upturned expression of p21, p16, and p53 proteins suggest senescence (24). We observed this fact when measured the activity of β-galactosidase on control lung fibroblasts isolated form young donors (mean age 18 yr) and old donors (mean age 66 yr) (data not shown, P < 0.0001). Then, we demonstrated that there is a significant increase in the activity of β-galactosidase in nonstimulated IPF hLFs compared with age-matched controls upon TGF-β stimulation (Fig. 1B). Additionally, we found a significant increase in the mRNA expression of p21 and an upward trend in the expression of p53 in nonstimulated IPF hLFs compared with controls (Fig. 1C). Protein expression of the senescence markers p21 and p16 was statiscally significantly increased, and p53 showed an upward trend in the IPF hLFs compared with age-matched controls (Fig. 1, D and E). Subsequently, due to the fact that TGF-β has been implicated in senescence (23), fibroblasts were stimulated with 5 ng/ml of TGF-β for 6 and 24 h to assess its role on senescence. Our results show a statistically significant increase in gene expression of p21 and an upward trend in p16 and p53 in both groups upon TGF-β stimulation (Fig. 1F).
IPF hLFs show changes in morphology.
Once demonstrated that IPF hLFs had increased senescence-like phenotype compared with their age-matched controls, we decided to correlate this finding with differences in size and morphology. To explore these findings, hLFs isolated from explanted IPF lungs and from normal age-matched control lungs were grown and then seeded on chamber slides. After fixation, phalloidin-based stain was used to assess the morphology of the fibroblasts. IPF hLFs have a larger area than the controls, with a morphology more similar to that described for senescent cells and lack the characteristic spindle shape (42) (Fig. 1G).
IPF hLFs have shorter telomeres than age-matched normal controls.
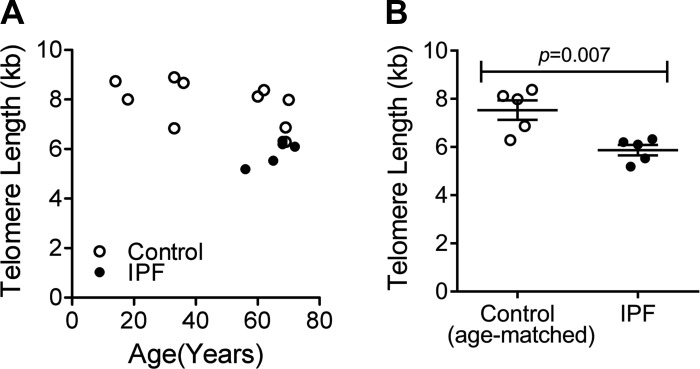
Different groups (2, 5, 13, 16, 34) have reported the association between telomere length and lung fibrosis. Short telomeres (below 10th percentile) have been found in 25–50% of sporadic IPF and nearly half of familial cases (2, 13, 37), and mutations in the telomerase complex have been implicated as an underlying risk factor of the disease (36). We used flow cytometry and FISH to measure telomere length data in a Clinical Laboratory Improvement Amendments-certified laboratory and analyzed the data relative to age-matched controls. We found that, as expected, telomeres get shorter with age. However, the striking fact is that the shortest telomeres were found in IPF fibroblasts compared with their age-matched normal controls (Fig. 2, A and B). This is consistent with a global and germline defect in telomere maintenance previously seen in epithelial, lymphocyte, and granulocyte telomere length in IPF patients (13).
Fig. 2.
Telomere length in hLFs. By flow cytometry and fluorescence in situ hybridization, telomere length was measured on hLFs, demonstrating shorter telomeres in IPF hLFs compared with controls. A: average telomere length (kb) in control and IPF hLFs (n = 10 control group and n = 5 in IPF). B: average telomere length (kb) in age-matched control and IPF hLFs (n = 5 per group). Error bars represent SE, and P value was calculated by unpaired t-test.
Mitochondrial function and energy production in fibroblasts.
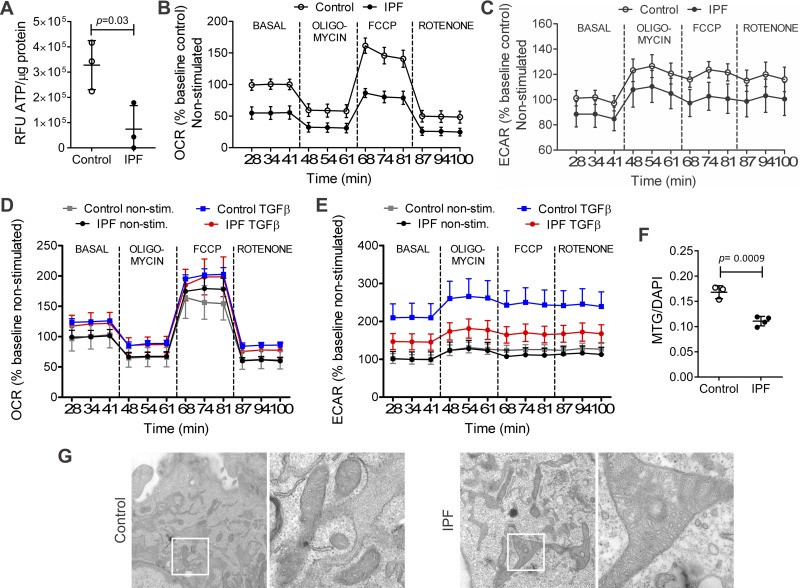
To explain the decrease in proliferation found in IPF hLFs, the energy production of fibroblasts was studied. ATP was significantly decreased in the IPF hLFs compared with controls (Fig. 3A). Next, to correlate the decrease in ATP production with mitochondrial function, OCR was measured and the bioenergetic profile of nonstimulated hLFs was analyzed (Fig. 3B). The first three time points represent the basal respiration. Oligomycin was then used to inhibit ATPase activity, causing an expected drop in OCR in both groups. After that, the FCCP was used to dissipate the membrane potential and the maximal uncoupled response of hLFs was assessed. Finally, rotenone, a complex I inhibitor, was used to inhibit mitochondrial respiration. IPF hLFs showed a statistically significant decrease in basal, ATP-dependent, and maximal mitochondrial respiration (OCR) compared with controls, indicating poor mitochondrial function. The ECAR, an indicator of glycolysis, showed a tendency to lower rates in the IPF hLFs, although no significant differences between the groups were found (Fig. 3C). Statistical analysis is shown in Table 3.
Fig. 3.
Mitochondrial function and structure in hLFs. IPF hLFs show lower energy production (ATP content), lower mitochondrial function measured by oxygen consumption rate (OCR), lower glycolytic flow measured by extracellular acidification rate (ECAR), and altered mitochondrial structure compared with controls. A: basal content of ATP was measured in control and IPF hLFs, showing statistically significant decrease in ATP in IPF hLFs compared with controls. Results were normalized by protein concentration. Bars represent SE (P = 0.03; n = 3 per group). B: OCR bioenergetic profile of nonstimulated control and IPF hLFs showing a significant decrease in basal and maximal respiration in IPF hLFs compared with controls (n = 3 per group; P = 0.001). C: %baseline ECAR of nonstimulated control and IPF hLFs showing no differences in glycolysis across the bio profile in IPF hLFs compared with controls (n = 3 per group. P = 0.2). D: OCR bioenergetic profile of TGFβ stimulated hLFs, showing no differences upon TGF-β (n = 3 in control and n = 4 in IPF group; P = 0.12). E: %baseline ECAR of TGF-β stimulated control and IPF hLFs showing a significant decrease in glycolysis in IPF hLFs compared with controls (n = 3 in control and 4 in IPF hLFs; P < 0.001). F: mitochondrial mass using MitoTracker green/DAPI (n = 3 control hLFs and 4 IPF hLFs). Bars represent SE. G: representative image of electronic microscopy of control and IPF hLFs at ×25 and ×100 magnification camera showing mitochondrial structure. For detailed statistical analysis of figures B to E, refer to Tables 3–5.
Table 3.
Statistical analysis of OCR and ECAR at basal conditions
| Time | P Value Between Two Traces (OCR) | P Value Between Two Traces (ECAR) |
|---|---|---|
| All | 0.001 | 0.2 |
| Basal | 0.001 | 0.3 |
| FCCP | <0.001 | 0.1 |
| Oligomycin | 0.033 | 0.3 |
| Rotenone | 0.035 | 0.3 |
Effect of TGF-β in mitochondrial function.
Studies have shown that TGF-β has a negative effect on mitochondrial function (35), so mitochondrial function was assessed upon TGF-β stimulation. OCR was analyzed after 4 h of stimulation with TGF-β (5 ng/ml). The OCR bioenergetic profile after 4 h of stimulation shows that TGF-β has no effect on OCR in both groups (Fig. 3D), but a statistically significant effect on ECAR, an indicator of glycolysis, was identified upon TGF-β stimulation. We found that ECAR was decreased in IPF compared with control hLFs (Figs. 3E), indicating poor compensation through glycolysis. The statistical analysis is shown in Tables 4 and 5.
Table 4.
Statistical Analysis of OCR and ECAR on TGF-β-stimulated hLFs
| Time | P Value Between of TGF-β and Trace (OCR) | P Value Between of TGF-β and Trace (ECAR) |
|---|---|---|
| All | 0.12 | <0.001 |
| Basal | 0.7 | 0.002 |
| FCCP | 0.2 | 0.010 |
| Oligomycin | 0.8 | 0.001 |
| Rotenone | 0.3 | 0.008 |
Table 5.
Statistical analysis of the effect of TGF-β on hLFs OCR and ECAR
| TGF-β Effect (P Value) in IPF and Control (OCR)* |
TGF-β Effect (P Value) in IPF and Control (ECAR) |
|||||
|---|---|---|---|---|---|---|
| Time | IPF | Control | Difference* | IPF | Control | Difference* |
| All | 18.0 (<0.001) | 28.0 (<0.001) | −9.9 (0.12) | 52.4 (<0.001) | 120.4 (<0.001) | −68.0 (<0.001) |
| Basal | 20.0 (0.008) | 24.8 (0.004) | −4.8 (0.7) | 45.9 (<0.001) | 109.8 (<0.001) | −63.9 (0.002) |
| FCCP | 16.8 (0.14) | 41.5 (0.008) | −24.7 (0.2) | 56.8 (<0.001) | 120.5 (<0.001) | −63.7 (0.010) |
| Oligomycin | 19.2 (0.001) | 21.8 (0.001) | −2.6 (0.8) | 52.5 (<0.001) | 135.5 (<0.001) | −83.0 (0.001) |
| Rotenone | 16.4 (0.001) | 23.7 (<0.001) | −7.2 (0.3) | 54.5 (<0.001) | 115.9 (<0.001) | −61.3 (0.008) |
After-before TGF-β in IPF – after-before TGF-β in control.
Mitochondrial mass and structure.
By comparing the fluorescence of Mito-tracker green dye (normalized to nuclear DAPI), mitochondrial mass was assessed. This showed a statistically significant decrease in mitochondrial mass in IPF hLFs compared with controls (Fig. 3F). Additionally, the mitochondrial structure was analyzed using transmission electron microscopy, revealing disrupted membranes and alteration of cristae in the mitochondria of IPF hLFs, which look shorter and are occasionally absent (Fig. 3G).
Endoplasmic reticulum stress in hLFs.
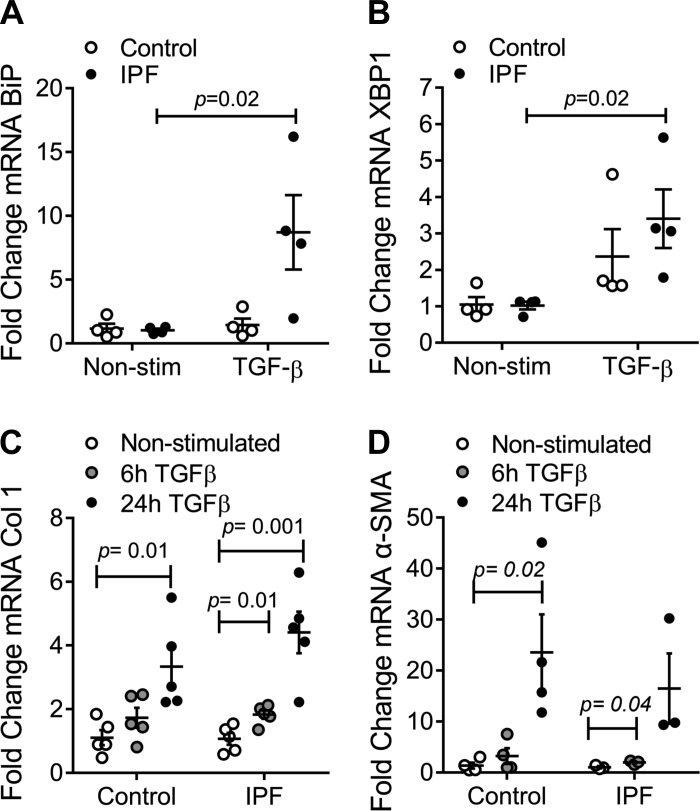
Alterations in the cross talk between mitochondria and the endoplasmic reticulum (ER) have been identified in epithelial cells in IPF (11). Additionally, a profibrotic role for ER stress in fibroblasts has also been described (20). Upon constant stressors, the ER activates the unfolded protein response to restore the ER balance. This mechanism is initiated by BiP, which in turn activates three pathways (IRE-1, PERK/eIF2α, and AFT), which ultimately contribute to decreasing the burden on the ER (19). In this way, evaluation of ER stress was assessed. Our results showed a statistically significant increase in mRNA expression of BiP and XBP1 in IPF hLFs upon TGF-β stimulation, suggesting a role for ER stress in the disease (Fig. 4, A and B).
Fig. 4.
Endoplasmic reticulum (ER) stress, collagen, and α-smooth muscle actin (α-SMA) expression in hLFs. A: fold change of mRNA expression of binding immunoglobulin protein (BiP) in IPF and control hLFs upon TGF-β stimulation (n = 4 per group). Bars represent SE (P = 0.02). B: fold change of mRNA expression of X-box binding protein 1 (XBP1) in IPF and control hLFs upon TGF-β stimulation (n = 4 per group). Bars represent SE (P = 0.02). C: fold change of mRNA expression of collagen 1 in nonstimulated control and IPF hLFs and after a 6- and 24-h stimulation with TGF-β (5 ng/ml) (n = 5 per group). D: fold change of mRNA expression of α-SMA of nonstimulated and TGF-β stimulated hLFs (n = 4 control n = 3 IPF hLFs).
IPF hLFs expressed higher collagen 1 after stimuli with TGF-β than controls.
Increased collagen deposition in the extracellular matrix is the characteristic finding in the lung parenchyma of IPF. Fibroblasts are thought to be responsible for its production, especially in the presence of TGF-β (17, 26, 27). To assess this hypothesis, IPF and control fibroblasts were stimulated with 5 ng/ml of TGF-β for 6 and 24 h, and mRNA expression of collagen was measured. IPF hLFs showed a higher expression of collagen 1 than controls after TGF-β stimulation (Fig. 4C).
The expression of α-SMA is similar in IPF hLFs and controls.
Due to the differences in morphology found in IPF hLFs, we looked at mRNA expression of α-SMA, a marker of myofibroblasts. No differences were found in the expression of α-SMA between IPF and control hLFs at the mRNA level when nonstimulated, suggesting that our two populations (IPF and control hLFs) could be comparable. Additionally, we assessed changes in the expression of α-SMA using stimulation with 5 ng/ml of TGF-β during 6 and 24 h, which showed a lower response of α-SMA in IPF hLFs after TGF-β stimulation (Fig. 4D).
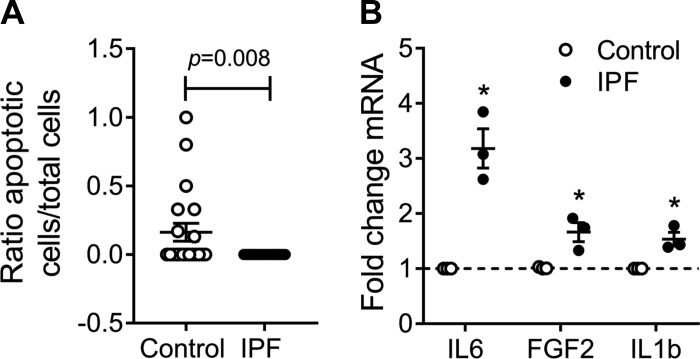
IPF hLFs have decreased apoptosis compared with controls.
As apoptosis is associated with senescene (12), we assessed it on hLFs. IPF hLFs showed decreased apoptosis compared with controls at basal conditions (Fig. 5A). This correlates with data previously published by our group, where fibroblasts with IPF do not go through apoptosis, even under starvation conditions, compared with age-matched controls, which went through apoptosis after 24 h of starvation (30).
Fig. 5.
Apoptosis and senescence-associated secretory phenotype (SASP) in hLFs. A: ratio apoptotic cells to total cells of nonstimulated hLFs (n = 4) in both groups. Bars represent SE P = 0.008. B: fold change of mRNA expression of IL-6, FGF2, and IL-1β (n = 3 per group; P < 0.05).
IPF hLFs have a secretory phenotype.
Once it was demonstrated that IPF hLFs show features of senescent cells, we decided to assess their secretory capacity, since this secretory phenotype forms part of the characteristics of senescence (12). Transcript levels of the SASPs were measured at nonstimulated conditions, revealing statistically significant higher levels of IL-6, IL-1β, and FGFb in IPF hLFs compared with controls (Fig. 5B).
DISCUSSION
The aberrant proliferation and activation of fibroblasts have been hypothesized as a mechanism that drives the fibrotic process, in a similar way to the neoproliferative process observed in cancer (41). Contrary to this hypothesis, we have demonstrated that cellular senescence, a key hallmark of aging, is increased in hLFs from IPF patients compared with control individuals of a similar age range (Fig. 1). These findings are concordant with a recent publication that also observed that cellular senescence contributes to the lung fibrotic process (32). Thus the characteristic exaggerated accumulation of collagen, fibronectin, and other extracellular matrix compounds, which lead to changes in lung architecture and a decrease in lung function in IPF, does not seem to be caused by the aberrant activation of hLFs in view of our results. In addition to a decreased apoptosis, which may contribute to a higher accumulation of fibroblasts (Fig. 5), the presence of a SASP (Fig. 5) with a secretome that involves different cytokines and chemokines, growth factors, and profibrotic mediators (12), may be the mechanism that explains the reason why senescent fibroblasts could contribute to lung fibrosis. In accordance with the markers of cell senescence, the proliferative capacity of fibroblasts is decreased in the IPF group, which is consistent with the limited replicative senescence (6). The decrease in epithelial proliferation and senescence has been attributed to TGF-β (23) and senescence-associated miRNAs (14). Its effect on fibroblasts has been related to a decrease in autophagy (25), an important stress-mediator mechanism also linked to mitochondrial dysfunction and senescence (30, 35). In fact, it has been hypothesized that when senescent cells are not efficiently cleared from the tissues and the mechanisms of regeneration are not fully available, a senescence state occurs, which results in fibrosis and organ dysfunction (24).
In addition to these findings, we observed that, morphologically, IPF fibroblasts look different from those of controls, with a larger area and broader shape than the characteristic spindle-shape found in control fibroblasts. This observation is also supported by Yanai et al. (42). Furthermore, we measured mRNA expression of α-SMA, a myofibroblast marker, and found no differences in its expression between IPF and control hLFs when nonstimulated. This lack of difference suggests that both populations could be comparable.
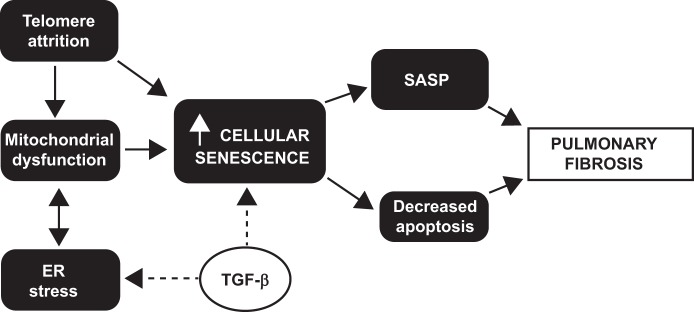
The results of our study are concordant with the current view of IPF as an aging disease. Recently the nine hallmarks of aging have been described (22), and many of these hallmarks end in a common path, leading to cell senescence. In Fig. 6 we have summarized how the hallmarks of aging assessed in our study are interrelated and linked to pulmonary fibrosis. Important data have shown a link between telomere shortening and pulmonary fibrosis (1, 2, 13, 36). Short telomeres have been documented in alveolar epithelial cells of IPF patients (2) and have been linked to alveolar stem cell cellular senescence in animal models (1). Our results show that telomere shortening is also found in hLFs of IPF patients, confirming the pervasive nature of the telomere defects in IPF (2). This result translate to lung fibroblasts observations from other groups that telomere length is abnormally short in more than half of sporadic IPF patients. It has been demonstrated that in all leukocyte subsets and alveolar epithelial cells (2). It is well known that telomere length shortens with age. It is thus critical to compare telomere length of IPF patients to age-matched controls. In a recent publication, the authors suggest that telomere shortening is not a common finding in IPF (21); their data do not show age correction or mention the ages of the IPF patients and controls, in contrast to our study. When measuring telomere length, the method is critical, we use flow cytometry and FISH (10). This method has been reported to be superior with an extremely low coefficient of variation. In contrast, other methods like PCR and telomere restriction fragment/Southern blot are more variable, lack internal standard controls, and are altered depending on the DNA quality.
Fig. 6.
Cellular senescence in IPF fibroblasts. The proposed mechanisms involved in aging, senescence, and IPF and how they are interconnected are illustrated.
Furthermore, when the mitochondrial structure and function were assessed, our results show not only alteration in morphology and a decrease in mitochondrial mass but also a decrease in respiration and glycolysis in IPF hLFs compared with controls. These findings draw attention to the fact that mitochondrial dysfunction has been implicated in the increase of profibrotic factors (11). The decrease in respiration was confirmed with the significantly lower production of ATP in the IPF group compared with controls. Senescent fibroblasts have been shown to present low levels of ATP as a result of a diminished glycolytic capacity and abnormalities in oxidative phosphorylation. Interestingly, short telomere length has also been linked to mitochondrial dysfunction in animal models (31), and our data suggest an association in the setting of IPF.
On the other hand, studies in the ER, the structure responsible for the folding and sorting of proteins, have shown that ER stress also plays a role in IPF and aging (11, 38, 39). Studies carried out not only on fibroblasts (9, 20) but also on alveolar epithelial cells (11) highlight the fact that ER stress targets the unfolded protein response and apoptosis and that ER stress is upregulated in IPF. We found elevated mRNA expression of ER stress markers, especially upon TGF-β stimuli, suggesting a role for ER in the abnormal activation of fibroblasts.
The senescence phenotype that we described of lung fibroblasts in IPF lungs is consistent with recent published observations (42). Senescence cells are metabolically active and with a robust secretory capacity known as SASP that includes components of the extracellular matrix and proinflammatory mediators (12). In addition, senescence cells are resistant to apoptosis. However, the diminished proliferative capacity reported here for the IPF lung fibroblasts is inconsistent with the proliferative process suggested in the fibroblast foci. Whether these differences represent diverse stages of the disease have not been established and will require further examination. The changes on the phenotype of IPF hLFs are the result of a predisposition toward short telomeres and poor mitochondrial function, which contribute to cellular senescence in IPF fibroblasts. As novel therapeutic approaches influencing senescence are currently available (32, 44), the potential use of these therapies in IPF seems to be an option to consider in future research.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-123766, R01-HL-131789, and R01-HL-119476 and the Commonwealth Foundation (to M. Armanios).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.A. and M.R. conceived and designed research; D.A., N.C., M.B., C. Corey, V.S.H., Y.P., H.D., J. Sembrat, S. Shanker, and C. Caufield performed experiments; D.A., N.C., M.N., and M.R. analyzed data; D.A., J. Sellares, S. Shiva, M.A., A.L.M., and M.R. interpreted results of experiments; D.A. and M.B. prepared figures; D.A., J. Sellares, and M.R. drafted manuscript; D.A., J. Sellares, S. Shiva, M.A., A.L.M., and M.R. edited and revised manuscript; D.A., N.C., J. Sellares, M.B., C. Corey, V.S.H., Y.P., H.D., J. Sembrat, M.N., S. Shanker, C. Caufield, S. Shiva, M.A., A.L.M., and M.R. approved final version of manuscript.
REFERENCES
- 1.Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, Tuder RM, Hogan BL, Mitzner W, Armanios M. Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci USA 112: 5099–5104, 2015. doi: 10.1073/pnas.1504780112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, Phillips JA 3rd, Lansdorp PM, Loyd JE, Armanios MY. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA 105: 13051–13056, 2008. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Álvarez D, Levine M, Rojas M. Regenerative medicine in the treatment of idiopathic pulmonary fibrosis: current position. Stem Cells Cloning 8: 61–65, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutat Res 730: 52–58, 2012. doi: 10.1016/j.mrfmmm.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armanios M. Telomeres and age-related disease: how telomere biology informs clinical paradigms. J Clin Invest 123: 996–1002, 2013. doi: 10.1172/JCI66370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet 13: 693–704, 2012. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev 88: 557–579, 2008. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 8.Aunan JR, Watson MM, Hagland HR, Søreide K. Molecular and biological hallmarks of ageing. Br J Surg 103: e29–e46, 2016. doi: 10.1002/bjs.10053. [DOI] [PubMed] [Google Scholar]
- 9.Baek HA, Kim DS, Park HS, Jang KY, Kang MJ, Lee DG, Moon WS, Chae HJ, Chung MJ. Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. Am J Respir Cell Mol Biol 46: 731–739, 2012. doi: 10.1165/rcmb.2011-0121OC. [DOI] [PubMed] [Google Scholar]
- 10.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH). Nat Protoc 1: 2365–2376, 2006. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- 11.Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, Duncan SR, Rojas M, Shiva S, Chu CT, Mora AL. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest 125: 521–538, 2015. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campisi J. Cellular senescence and lung function during aging. yin and yang. Ann Am Thorac Soc 13, Suppl 5: S402–S406, 2016. doi: 10.1513/AnnalsATS.201609-703AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cronkhite JT, Xing C, Raghu G, Chin KM, Torres F, Rosenblatt RL, Garcia CK. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med 178: 729–737, 2008. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Disayabutr S, Kim EK, Cha SI, Green G, Naikawadi RP, Jones KD, Golden JA, Schroeder A, Matthay MA, Kukreja J, Erle DJ, Collard HR, Wolters PJ. miR-34 miRNAs regulate cellular senescence in type ii alveolar epithelial cells of patients with idiopathic pulmonary fibrosis. PLoS One 11: e0158367, 2016. doi: 10.1371/journal.pone.0158367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Majada V, Welz PS, Ermolaeva MA, Schell M, Adam A, Dietlein F, Komander D, Büttner R, Thomas RK, Schumacher B, Pasparakis M. The tumour suppressor CYLD regulates the p53 DNA damage response. Nat Commun 7: 12508, 2016. doi: 10.1038/ncomms12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George G, Rosas IO, Cui Y, McKane C, Hunninghake GM, Camp PC, Raby BA, Goldberg HJ, El-Chemaly S. Short telomeres, telomeropathy, and subclinical extrapulmonary organ damage in patients with interstitial lung disease. Chest 147: 1549–1557, 2015. doi: 10.1378/chest.14-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasaneen NA, Cao J, Pulkoski-Gross A, Zucker S, Foda HD. Extracellular matrix metalloproteinase inducer (EMMPRIN) promotes lung fibroblast proliferation, survival and differentiation to myofibroblasts. Respir Res 17: 17, 2016. doi: 10.1186/s12931-016-0334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huebbers CU, Adam AC, Preuss SF, Schiffer T, Schilder S, Guntinas-Lichius O, Schmidt M, Klussmann JP, Wiesner RJ. High glucose uptake unexpectedly is accompanied by high levels of the mitochondrial ß-F1-ATPase subunit in head and neck squamous cell carcinoma. Oncotarget 6: 36172–36184, 2015. doi: 10.18632/oncotarget.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, Weaver TE, Guenther A. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 178: 838–846, 2008. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenna S, Trojanowska M. The role of endoplasmic reticulum stress and the unfolded protein response in fibrosis. Curr Opin Rheumatol 24: 663–668, 2012. doi: 10.1097/BOR.0b013e3283588dbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu T, Ullenbruch M, Young Choi Y, Yu H, Ding L, Xaubet A, Pereda J, Feghali-Bostwick CA, Bitterman PB, Henke CA, Pardo A, Selman M, Phan SH. Telomerase and telomere length in pulmonary fibrosis. Am J Respir Cell Mol Biol 49: 260–268, 2013. doi: 10.1165/rcmb.2012-0514OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 153: 1194–1217, 2013. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, Kawaishi M, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L391–L401, 2011. doi: 10.1152/ajplung.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15: 482–496, 2014. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 25.Patel AS, Lin L, Geyer A, Haspel JA, An CH, Cao J, Rosas IO, Morse D. Autophagy in idiopathic pulmonary fibrosis. PLoS One 7: e41394, 2012. doi: 10.1371/journal.pone.0041394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel AS, Song JW, Chu SG, Mizumura K, Osorio JC, Shi Y, El-Chemaly S, Lee CG, Rosas IO, Elias JA, Choi AM, Morse D. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis. PLoS One 10: e0121246, 2015. doi: 10.1371/journal.pone.0121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulichino AM, Wang IM, Caron A, Mortimer J, Auger A, Boie Y, Elias JA, Kartono A, Xu L, Menetski J, Sayegh CE. Identification of transforming growth factor beta1-driven genetic programs of acute lung fibrosis. Am J Respir Cell Mol Biol 39: 324–336, 2008. doi: 10.1165/rcmb.2007-0186OC. [DOI] [PubMed] [Google Scholar]
- 28.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, Johkoh T, Martinez FJ, Myers J, Protzko SL, Richeldi L, Rind D, Selman M, Theodore A, Wells AU, Hoogsteden H, Schünemann HJ; American Thoracic Society; European Respiratory society; Japanese Respiratory Society; Latin American Thoracic Association . An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 192: e3–e19, 2015. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 29.Riethmueller S, Somasundaram P, Ehlers JC, Hung CW, Flynn CM, Lokau J, Agthe M, Düsterhöft S, Zhu Y, Grötzinger J, Lorenzen I, Koudelka T, Yamamoto K, Pickhinke U, Wichert R, Becker-Pauly C, Rädisch M, Albrecht A, Hessefort M, Stahnke D, Unverzagt C, Rose-John S, Tholey A, Garbers C. Proteolytic origin of the soluble human IL-6R in vivo and a decisive role of N-glycosylation. PLoS Biol 15: e2000080, 2017. doi: 10.1371/journal.pbio.2000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero Y, Bueno M, Ramirez R, Álvarez D, Sembrat JC, Goncharova EA, Rojas M, Selman M, Mora AL, Pardo A. mTORC1 activation decreases autophagy in aging and idiopathic pulmonary fibrosis and contributes to apoptosis resistance in IPF fibroblasts. Aging Cell 15: 1103–1112, 2016. doi: 10.1111/acel.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahin E, Colla S, Liesa M, Moslehi J, Müller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470: 359–365, 2011. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H, LeBrasseur NK. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8: 14532, 2017. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selman M, Pardo A. Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. An integral model. Am J Respir Crit Care Med 189: 1161–1172, 2014. doi: 10.1164/rccm.201312-2221PP. [DOI] [PubMed] [Google Scholar]
- 34.Snetselaar R, van Moorsel CH, Kazemier KM, van der Vis JJ, Zanen P, van Oosterhout MFM, Grutters JC. Telomere length in interstitial lung diseases. Chest 148: 1011–1018, 2015. doi: 10.1378/chest.14-3078. [DOI] [PubMed] [Google Scholar]
- 35.Sosulski ML, Gongora R, Danchuk S, Dong C, Luo F, Sanchez CG. Deregulation of selective autophagy during aging and pulmonary fibrosis: the role of TGFβ1. Aging Cell 14: 774–783, 2015. doi: 10.1111/acel.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanley SE, Armanios M. The short and long telomere syndromes: paired paradigms for molecular medicine. Curr Opin Genet Dev 33: 1–9, 2015. doi: 10.1016/j.gde.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stella GM, Balestro E, Lacedonia D, Baraldo S. Telomeropathies: an emerging spectrum of disorders with important implications for patients with interstitial lung disease. Minerva Med 107, Suppl 1: 9–14, 2016. [PubMed] [Google Scholar]
- 38.Tanjore H, Blackwell TS, Lawson WE. Emerging evidence for endoplasmic reticulum stress in the pathogenesis of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 302: L721–L729, 2012. doi: 10.1152/ajplung.00410.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanjore H, Lawson WE, Blackwell TS. Endoplasmic reticulum stress as a pro-fibrotic stimulus. Biochim Biophys Acta 1832: 940–947, 2013. doi: 10.1016/j.bbadis.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thannickal VJ, Murthy M, Balch WE, Chandel NS, Meiners S, Eickelberg O, Selman M, Pardo A, White ES, Levy BD, Busse PJ, Tuder RM, Antony VB, Sznajder JI, Budinger GR. Blue journal conference. Aging and susceptibility to lung disease. Am J Respir Crit Care Med 191: 261–269, 2015. doi: 10.1164/rccm.201410-1876PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vancheri C. Idiopathic pulmonary fibrosis: an altered fibroblast proliferation linked to cancer biology. Proc Am Thorac Soc 9: 153–157, 2012. doi: 10.1513/pats.201203-025AW. [DOI] [PubMed] [Google Scholar]
- 42.Yanai H, Shteinberg A, Porat Z, Budovsky A, Braiman A, Ziesche R, Fraifeld VE. Cellular senescence-like features of lung fibroblasts derived from idiopathic pulmonary fibrosis patients. Aging (Albany NY) 7: 664–672, 2015. doi: 10.18632/aging.100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang P, Elabd S, Hammer S, Solozobova V, Yan H, Bartel F, Inoue S, Henrich T, Wittbrodt J, Loosli F, Davidson G, Blattner C. TRIM25 has a dual function in the p53/Mdm2 circuit. Oncogene 34: 5729–5738, 2015. doi: 10.1038/onc.2015.21. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15: 428–435, 2016. doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]