Abstract
BACKGROUND
Methicillin-resistant Staphylococcus aureus (MRSA) is an important agent of hospital-acquired infection. The mode of entry of MRSA in the hospital might be on admission of patients with MRSA infection or nasal colonization. The present study was undertaken to determine the prevalence of MRSA nasal colonization among patients on admission to hospital.
METHODS
Six hundred patients were screened for nasal colonization of MRSA on admission to hospital. Nasal swabs were cultured on salt mannitol agar and blood agar. Age, sex, previous admission to hospital and antibiotic therapy were recorded.
RESULTS
S. aureus was isolated from the nasal swabs of 122 patients (20.2%) on admission to hospital. MRSA was isolated from 7 patients (1.1%) and methicillin-sensitive S. aureus (MSSA) from 115 patients (19.1%). Nasal colonization of S. aureus was higher in younger and elderly patients and significantly higher colonization was observed among females. The MRSA strains isolated from nasal swabs had a different antibiotic susceptibility pattern than those isolated from patients having hospital-acquired MRSA infection. Previous admissions to hospital, underlying disease antibiotic therapy were not risk factors for MRSA nasal colonization.
CONCLUSION
MRSA nasal colonization of patients on admission to hospital is low in this region. The screening of every new admission would not be cost effective, but patients transferred form other institutions should be screened for MRSA. Standard infection control precautions should be strictly implemented to prevent the spread and control of MRSA infections.
MRSA is an important cause of hospital-acquired infections leading to high morbidity and mortality. MRSA infection places an additional burden on the patient care budget due to prolonged hospital stays. Further, it adds an inestimable human suffering, which could be avoided by taking the proper infection control precautions.1 According to a European Antimicrobial Resistance Surveillance System (EARSS) report, MRSA was responsible for 0.5% to 44% of cases of staphylococcal bacteremia in Europe, with the highest incidence of 44% in Greece and the lowest of 0.5% in Iceland.2 According to a National Nosocomial Infection Surveillance System (NNIS) report, 50% of hospital acquired infections in ICUs in the USA are due to MRSA.3 Apart from high transmissibility in the hospital, MRSA also carries certain virulence factors that are associated with toxic shock syndrome, necrotizing pneumonia and skin infections.4 Lately, community-acquired infections due to MRSA among children are a matter of concern because of high mortality. The community-acquired strains of MRSA often differ from hospital strains and arise independently in the community.5,6 Nasal colonization with MRSA is a significant risk factor for hospital- and community-acquired infections. Nasal colonization of MRSA among 1% to 3% of the outpatient population in the USA has been reported,7–9 whereas nasal colonization of MRSA was absent among the outpatient population in Turkey.10 A very high prevalence of nasal colonization (18.1%) among healthy community individuals has been reported from India.11 The prevalence of MRSA nasal colonization in the community needs to be determined before instituting measures to prevent the transmission of MRSA. There is no information available on the prevalence of MRSA nasal colonization among patients at the time of admission in Saudi Arabia. We determined the prevalence of MRSA and MSSA nasal colonization among patients at the time of admission to the hospital and examined possible risk factors involved in the nasal carriage of Staphylococcus aureus.
Methods
This study was conducted by the infection control department of the 500-bed King Fahad Hospital and Tertiary Care Center, Al Hofuf, Saudi Arabia, during the period of January to March 2004. Nasal, throat and axillary swabs were collected within 8 hours of admission from 600 patients admitted to the departments of medicine and surgery. The newly admitted patients were not isolated but standard infection control precautions were followed to prevent the transmission of MRSA in the hospital. Data on age, sex, underlying disease, history of previous hospitalization, and antibiotic therapy in the last 6 months was recorded. Of the patient population enrolled for the study, 279 were males and 321 were females with a mean age of 32.5 years (range 10–90 years). The swabs were cultured on salt mannitol agar (Oxoid Ltd, Basingstoke Hampshire, England) and 5% sheep blood agar. The plates were incubated at 35ºC for 24 hours. Swabs were also inoculated in nutrient broth containing 7.5% sodium chloride and subcultured on sheep blood agar after 24 hours of incubation at 35ºC. S. aureus was identified by the Gram stain, catalase and tube coagulase tests. Antibiotic susceptibility was tested by the disk diffusion technique according to the criteria of the National Committee for Clinical Laboratory Standards. Muller-Hinton agar with 4% sodium chloride was used for the antibiotic susceptibility test and the plates were incubated at 35ºC for 24 hours. Screening of MRSA was done by inoculating S. aureus on Mueller-Hinton agar with 4% sodium chloride and 6 μg/mL oxacillin.12 The Statistical Package for Social Sciences (SPSS, version 10.1) was used for data analysis. The chi-square test and Fisher exact tests were used for categorical variables and the Student t test for continuous variables for the univariate comparison. P<0.05 was considered statistically significant.
Results
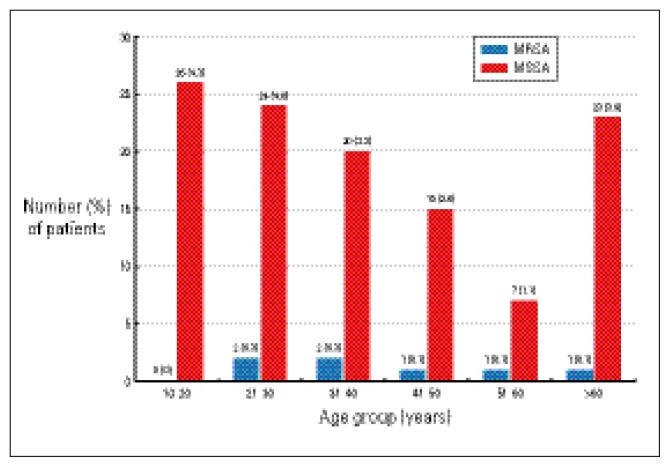
S. aureus was isolated from the nasal swabs of 122 of 600 patients (20.2%) at the time of admission. Of the 600, 7 (1.1%) had MRSA and 115 (19.1%) had MSSA colonization. S. aureus nasal colonization was significantly more common among female patients (11.6% vs. 8.6%) than male patients. Also, more female patients were nasal colonizers of MRSA than males (0.8% vs. 0.3%) (Table 1). S. aureus nasal colonization was higher among patients admitted to medical specialties (74/600, 12.3%) than surgical specialties (48/600, 7.9%). MRSA colonization was also higher among patients requiring admission to a medical specialty than those being admitted to surgical specialties (0.8% vs. 0.3%). S. aureus colonization was higher among patients 10 to 40 years of age and >60 years of age (Figure 1). MRSA nasal colonization was higher in the 20 to 40 year age group than elderly patients, but the difference was not statistically significant. There was no significant correlation between admission to the hospital during the last six months and nasal colonization (Table 2). S. aureus was isolated from 37 patients (6.1%) having a history of previous admission to the hospital and from 85 patients (14.1%) with no history of previous admission to the hospital. MRSA isolation was also higher (n=6, 1%) among patients who did not have a history of previous admission and among patients who were admitted to the hospital during the last six months (n=1, 0.1%). There was no relationship between the underlying disease, antibiotic therapy and the nasal carriage of MRSA and MSSA.
Table 1.
Methicillin-resisant (MRSA) and methicillin-sensitive Staphylococcus aureus (MSSA) nasal colonization at the time of admission to the hospital by gender.
| Male (n=279) | Female (n=321) | Total (n=600) | Odds ratio (95%CI) | P value | |
|---|---|---|---|---|---|
| MRSA | 2 (0.3) | 5 (0.8) | 7 (1.1) | 2.191 (1.268–3.817) | 0.004 |
| MSSA | 50 (8.6) | 65 (10.8) | 115 (19.1) | 1.163 (0.754–1.788) | 0.046 |
| Total | 52 (8.6) | 70 (11.6) | 122 (20.2) |
Numbers and percentages (in parenthesis) of patients
Figure 1.
Methicillin-resisant (MRSA) and methicillin-sensitive Staphylococcus aureus (MSSA) nasal colonization at the time of admission to the hospital by age group.
Table 2.
Relationship between previous hospital admission and MRSA and MSSA nasal colonization at the time of admission to the hospital.
| Previous admission during last 6 months (n=149) | No admission during last 6 months (n=451) | Total | Odds ratio (95% CI) | P value | |
|---|---|---|---|---|---|
| MRSA | 1 (0.1) | 6 (1.0) | 7 (1.1) | 0.501 (0.213–4.223) | 0.834 |
| MSSA | 36 (6.1) | 79 (13.1) | 115 (19.1) | 0.667 (0.417–1.068) | 0.095 |
| Total | 37 (6.1) | 85 (14.1) | 122 (20.2) |
Numbers and percentages (in parenthesis) of patients
Nasal swabs were positive for MRSA in 7 patients while the throat and axillary swabs were positive in 5 and 3 patients, respectively. Salt mannitol agar detected all S. aureus-positive strains. All 7 MRSA strains isolated from the nasal swabs of patients at the time of admission were susceptible to cephalothin, clindamycin, co-trimoxazole, rifampicin, and vancomycin, while they were resistant to penicillin, erythromycin, oxacillin, tetracycline, fucidic acid, Augmentin, cefotaxime, chloramphenicol, gentamicin, imepenem, ciprofloxacin and piperacillin. Nineteen strains of MRSA isolated from the clinical samples of hospitalized patients having hospital-acquired infection during the same period were sensitive to chloramphenicol, rifampicin and vancomycin, and resistant to other antibiotics. None of the MRSA or MSSA strains isolated from the nasal swabs of patients at the time of admission or strains isolated from hospitalized patients having hospital-acquired infection had resistance to vancomycin.
Discussion
Nasal colonization by MRSA is significant risk factor and an important initial step in hospital-acquired infections due to this organism.1,13 Although MRSA is a well recognized pathogen in hospital-acquired infections, lately it is recognized as an emerging etiological agent for skin and soft tissue infections and fatal bacteremia among persons in the community who have never had contact with health care settings.6,14 MRSA and MSSA nasal colonization of 2% and 38%, respectively, in the community has been reported from Hawaii. A higher rate of MSSA nasal colonization was observed among people under 18 years of age, whereas MRSA colonization was higher in people more than 18 years of age with a history of previous hospitalization.7 In the present study, a slightly higher prevalence of MRSA and MSSA was observed among the 10 to 40 year age groups and in elderly patients (>60 years), but the difference was not statistically significant. A study from San Francisco reported nasal colonization rates in outpatients of 22.8% and 2.8% for MSSA and MRSA, respectively. Higher nasal colonization was reported among urban poor in overcrowded living conditions.15
MRSA and MSSA nasal colonization in another study from the USA was observed in 3.0% and 21.7% of outpatients, respectively. MRSA colonization was independently associated with previous admission to a hospital or nursing home.8 No such correlation was observed in the present study. In the USA, MRSA nasal carriage at the time of admission to the hospital was observed among 2.6% of patients, and diabetes mellitus, antibiotic therapy and previous admission to nursing homes were the major risk factors.16 In the present study, none of the underlying diseases at the time of admission was a risk factor for nasal colonization of MRSA.
A higher prevalence (14.6%) of MRSA nasal carriage at the time of admission of elderly patients to geriatric wards has been reported from France. This higher nasal carriage was associated with repeated hospitalization in geriatric wards, and with bedsores.17 In the Netherlands, a very low prevalence (0.03%) of MRSA nasal carriage at the time of admission to the hospital was observed, although the MSSA nasal carriage rate was 24%. This low prevalence of MRSA has been related to the national policy of ‘search and destroy’ and restrictive antibiotic prescribing.18 Low rates nasal carriage, 1.5% for MRSA and 23% for MSSA, have been reported from the UK among the healthy adult population from the community.19 In Saudi Arabia, S. aureus nasal carriage rates of 26.1% and 25.4% among healthy adults from the community and hospital personnel, respectively, have been reported from the Abha area. MRSA nasal carriage rates among these community and hospital personnel were 1.3% and 4.7%, respectively. Higher antibiotic resistance in the strains isolated from hospital personnel was observed than in the strains isolated from the community.20
In the present study, S. aureus nasal carriage was observed among 20.2% of the patients at the time of admission, which is lower than that reported from other countries and from the Abha region of Saudi Arabia.13,16–20 Nasal swabs yielded more MRSA positivity than throat or axillary swabs. Culture on salt mannitol agar detected all S. aureus strains, suggesting that culture of nasal swabs on salt mannitol agar is a better method for identifying patients having MRSA colonization. MRSA nasal carriage was observed among 1.1% of the patients at the time of admission to the hospital, which is much lower than that reported in most locales5–9,16 and higher than the 0.03% reported from Netherlands.10 The MRSA nasal colonizers were isolated and standard infection control precautions were followed to prevent its transmission to other patients.
The MRSA strains isolated from the nasal swabs of patients at the time of admission had distinctly different antibiotic susceptibility patterns compared with MRSA strains isolated from the hospitalized patients during the same period. All the MRSA strains isolated at this center during the previous 4 years were susceptible only to chloramphenicol, vancomycin and rifampicin, while the MRSA strains isolated from the nasal swabs of patients at the time of admission were susceptible to cephalothin, clindamycin, cotrimoaxzole, vancomycin and rifampacin. This suggests that the MRSA strains in the community are different from the strains isolated from hospitalized patients having hospital-acquired infections. Recently, it has been reported that community-acquired strains of MRSA are often clonally different from hospital-acquired strains as these strains arise independently in the community.5,6 Similar observations on differences in antibiotic resistance among MRSA strains isolated from the community and hospital-acquired strains were reported from the Abha region of Saudi Arabia.20 Resistance to vancomycin was not observed among strains of S. aureus at this center. The differences in susceptibility patterns suggest that MRSA strains arising in the community perhaps have lower transmissibility than strains causing hospital-acquired infections. Previous admission to hospital/nursing homes, elderly age, and antibiotic therapy have been described as major risk factors for the nasal colonization of MRSA in the adult community population.6–8,17 In the present study, none of these risk factors were associated with nasal colonization of MRSA among patients at the time of admission, which is similar to observations reported by others.3,10,19 There is a lower prevalence of nasal carriage of MRSA among patients at the time of admission to hospital in this region than reported from other countries16,17 and the strains associated with colonization at the time of admission are different from the ones responsible for hospital-acquired infections.
The nasal colonization of MRSA at the time of admission of patients is very low in this region. Patients acquire MRSA infection with the locally prevalent strain during a stay in the hospital. Surveillance of every patient for MRSA colonization at the time of admission may not be cost effective, but the screening of patients transferred from other institutions should be helpful in controlling entry of MRSA. Standard infection control practices should be strictly implemented to prevent and control MRSA infections in the hospital.
References
- 1.Duckworth G. Controlling Methicillin resistant Staphylococcus aureus. BMJ. 2003;327:1177–1178. doi: 10.1136/bmj.327.7425.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiemersma EW, Bronzwear LAM, Lyytikainen O, Degener JE, Schrijnemakers P, Bruinsma N, Monan J, Witte W, Grundmaun H. Methicillin resistant Staphylococcus aureus in Europe 1999–2002. Emerg Infect Dis. 2004;9:1627–1634. doi: 10.3201/eid1009.040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salgado CD, Farr BM, Calfee DP. Community acquired Methicillin resistant Staphylococcus aureus: A Meta analysis of prevalence and risk factors. Clin Infect Dis. 2003;36:131–139. doi: 10.1086/345436. [DOI] [PubMed] [Google Scholar]
- 4.Dufour P, Gillet J, Bes M, Litta G, Vandenesch F, Floret D. Community acquired Methicillin resistant Staphylococcus aureus infection in France: emergence of a single clone that produces Panton-Valentine Leukocidin. Clin Infect Dis. 2002;35:819–824. doi: 10.1086/342576. [DOI] [PubMed] [Google Scholar]
- 5.Mongkolrattanothai K, Boyle S, Khanna MD, Daum RS. Severe Staphylococcus aureus infections caused by clonally related community acquired Methicillin susceptible and Methicillin resistant isolates. Clin Infect Dis. 2003;37:1050–1058. doi: 10.1086/378277. [DOI] [PubMed] [Google Scholar]
- 6.Melish M, Arpon R, Coon P, Kim M, Slavish S. Community associated Methicillin resistant Staphylococcus aureus infections in Pacific Islanders Hawaii. MMWR. 2004;53:767–770. [PubMed] [Google Scholar]
- 7.Kenner J, O’Conner T, Piatanida N, Fishbain J, Eberly B, Viscount H, Uyehara C, Hospenthal D. Rates of carriage of Methicillin resistant and Methicillin susceptible Staphylococcus aureus in an out patient population. Infect Control Hosp Epidemiol. 2003;24:234–444. doi: 10.1086/502229. [DOI] [PubMed] [Google Scholar]
- 8.Jernigan JA, Pullen AL, Flowers L, Bell M, Jarvis WR. Prevalence of and risk factors for colonization with Methicillin resistant Staphylococcus aureus in an outpatient clinic population. Infect Control Hosp Epidemiol. 2003;24:445–450. doi: 10.1086/502223. [DOI] [PubMed] [Google Scholar]
- 9.Leman R, Alvarado RF, Pocock S, Barg N, Kellum M, McAllister S, Cheek J, Kuehnert M. Nasal carriage of Methicillin resistant Staphylococcus aureus in an American Indian population. Infect Control Hosp Epidemiol. 2004;25:121–125. doi: 10.1086/502361. [DOI] [PubMed] [Google Scholar]
- 10.Erdenizmenli M, Yaper N, Senger SS, Ozdemir S, Yuce A. Investigation of colonization with Methicillin resistant and Methicillin susceptible Staphylococcus aureus in outpatient population in Turkey. Jpn J Infect Dis. 2004;57:172–175. [PubMed] [Google Scholar]
- 11.Saxena S, Singh K, Talwar V. Methicillin resistant Staphylococcus aureus prevalence in community in the East Delhi. Jpn J Infect Dis. 2003;56:54–56. [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. NCCLS Document, M2-A6. Wyne, PA, USA: 2002. Performance standards for antimicrobial susceptibility testing. 12th Informational Supplement. [Google Scholar]
- 13.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 14.Baggett HC, Hennassy TW, Lemen R. An outbreak of community onset Methicillin resistant Staphylococcus aureus skin infections in Southwestern Alaska. Infect Control Hosp Epidemiol. 2003;24:397–402. doi: 10.1086/502221. [DOI] [PubMed] [Google Scholar]
- 15.Cherlebois ED, Bangshbers DR, Moss NJ, Moore MR, Moss AR, Chambers HF. Population based community prevalence of Methicillin resistant Staphylococcus aureus in the urban poor of San Francisco. Clin Infect Dis. 2002;34:425–433. doi: 10.1086/338069. [DOI] [PubMed] [Google Scholar]
- 16.Troillet N, Carmeli Y, Samore MH, Dakos J, Eichelberger K, DeGirolami PC, Karchmer AW. Carriage of Methicillin resistant Staphylococcus aureus at hospital admission. Infect Control Hosp Epidemiol. 1998;19:181–185. doi: 10.1086/647791. [DOI] [PubMed] [Google Scholar]
- 17.Eveillard M, Ernst C, Cuviller S, Lescure FX, Malpaux M, Defouilloy J. Prevalence of Methicillin resistant Staphylococcus aureus carriage at the time of admission in two acute geriatric wards. Hosp Infect. 2002;50:122–126. doi: 10.1053/jhin.2001.1152. [DOI] [PubMed] [Google Scholar]
- 18.Wertheim HF, Voss MC, Boeleus HA, Voss A, Vandenbroucke CM, Meester MH, Kluytmans JA, Van Kenlen RH, Verbrugh HA. Low prevalence of Methicillin resistant Staphylococcus aureus at hospital admission in the Netherlands: the value of search and destroy and restrictive antibiotic use. J Hosp Infect. 2004;56:321–325. doi: 10.1016/j.jhin.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Abudu L, Blair I, Fraise A, Cheng KK. Methicillin resistant Staphylococcus aureus: a community based prevalence survey. Epidemiol Infect. 2001;126:351–356. doi: 10.1017/s0950268801005416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alghaithy AA, Bilal NE, Gedebon M, Weily AH. Nasal carriage and antibiotic resistance of Staphylococcus aureus isolated from hospital and non hospital personnel in Abha, Saudi Arabia. Trans R Soc Trop Med Hyg. 2000;94:504–705. doi: 10.1016/s0035-9203(00)90066-x. [DOI] [PubMed] [Google Scholar]