Abstract
BACKGROUND
Primary breast lymphoma is a rare disease. The small number of patients and the paucity of data make large-series studies difficult. We conducted a pooled analysis to evaluate the treatment outcome and prognostic factors in patients with primary breast lymphoma.
METHODS
In a search of PUBMED and MEDLINE we found 7 observational studies with 93 patients that were eligible for inclusion. Treatments included single therapy or combined surgery, chemotherapy and radiotherapy. We analyzed the correlation between treatment protocols, tumor relapse and survival. Histopathology and cancer stage were analyzed to evaluate their significance in treatment outcome.
RESULTS
All 93 patients were female, with a mean age of 57 years. The histopathology of 63 patients (68%) was diffuse large cell lymphoma. According to Ann Arbor classification, 57% were stage I, 23% were stage II, 4% were stage III, and 16% were stage IV. Thirteen percent received surgery alone, 27% received chemotherapy alone, 7% received radiotherapy alone, 10% received surgery and chemotherapy, 10% received surgery and radiotherapy, 22% received chemotherapy and radiotherapy, and 11% received surgery combined with chemotherapy and radiotherapy. With a median follow-up duration of 34 months (mean, 53 months), 48% had relapse of disease, 50% had no relapse, while 2% had disease progression. The mean time to first tumor relapse after treatment was 20 months. The 3-year and 5-year overall survival rates were 70% and 56%, respectively. Radiotherapy was a significant prognostic factor predicting tumor relapse (P=0.044). Tumor stage was a significant prognostic factor affecting overall survival, disease-free survival and disease-specific survival (P=0.0231, 0.0015, 0.0124, respectively).
CONCLUSION
With a 3-year overall survival rate of 70%, the high relapse rate of 48% is a cause for concern. Patients who received chemotherapy and radiotherapy had better survival outcome and a lower relapse rate. We suggest that chemotherapy and radiotherapy be the initial treatment for patients with primary breast lymphoma.
Lymphomatous involvement in breast malignancies is uncommon, with an incidence of less than 5%. Most are caused by metastatic spread of a systemic lymphoma.1 However, primary malignant breast lymphoma, with no evidence of presentation elsewhere, is a rare disease, which accounts for 0.5%–1% of all malignant breast neoplasms,1–3 and 2%–3% of extranodal non-Hodgkin’s lymphoma.3–4 The most common histologic type of primary breast lymphoma is diffuse large B-cell lymphoma.2 There is still no standard universal management. Surgery, chemotherapy, and radiotherapy are the main choices of treatment. The rarity of the disease and the paucity of patient data make large-series studies difficult. We reviewed published reports of primary breast lymphoma, pooled individual patient data on treatment outcome and evaluated prognostic factors affecting survival.
Methods
This study was designed to assess the treatment outcome and evaluate the prognostic factors affecting survival of patients with primary breast lymphoma. With no language restriction, we used the terms “primary breast lymphoma” and “non-Hodgkin’s lymphoma of breast” to search in the titles of all published literature before October 2004 through two computerized searching systems, PUBMED (National Library of Medicine) and MEDLINE. All literature, including abstracts or full-texts of case reports and original articles, with the title containing the term “primary breast lymphoma” or “non-Hodgkin’s lymphoma of breast” were identified and reviewed. The following inclusion criteria were used to select studies for the analysis: report was published, full-text was available instead of abstract only, the lymphoma lesion of the breast had to be of primary origin, treatment was surgery alone or in combination with chemotherapy and radiotherapy in the treatment protocol, and there was sufficient published data for every individual patient, including histopathology, cancer stage, time and site of tumor relapse, duration of follow-up, survival status, and cause of death.
Fifteen potentially eligible reports were identified from searching PUBMED and MEDLINE. We excluded eight articles from the fifteen potentially eligible publications based on the inclusion criteria. Thus, there were seven studies that had sufficient data on every individual patient for the pooled analysis (Table 1).3,5–10 Reviewing the full-text of these seven articles, there were 101 patients in total with primary breast lymphoma. However, 7 of these patients did not have sufficient information of interest, and there was one male patient. These 8 patients were excluded from the study, leaving 93 patients. The data of interest included histopathology, cancer stage, time and site of tumor relapse, duration of follow-up, survival status, and cause of death. The definition of relapse was recurrence in primary involved breast, lymph nodes metastasis, or new lesions in other sites or organs, such as the contra-lateral breast, central nervous system (CNS), abdomen, and skin.
Table 1.
Studies of primary breast lymphoma included in the pooled analysis.
| Study | Recruitment period | No. of patients | Median age (years) | Majority of treatment protocols (%) | Median follow-up duration (months) |
|---|---|---|---|---|---|
| Ribrag V3 | 1976–1999 | 20 | 50 | Chemotherapy and Radiotherapy (60%) | 54 |
| Kim SH5 | 1988–1998 | 7 | 58 | Surgery and Chemotherapy (29%) | 34 |
| Lyons JA6 | 1980–1996 | 17 | 62 | Chemotherapy alone (38%) | 48 |
| Au WY7 | 1974–1996 | 14 | 51 | Chemotherapy alone (50%) | 60 |
| Suzuki Y8 | 1990–1994 | 3 | 38 | Chemotherapy alone (67%) | 48 |
| Lee KC9 | 1996–1999 | 2 | 40 | Chemotherapy and Radiotherapy (100%) | 36 |
| Kuper-Hommel MJ10 | 1981–1999 | 38 | 65 | Surgery and Radiotherapy (20%) | 32 |
Surgery included lumpectomy, partial mastectomy, and modified radical mastectomy (MRM)
Survival estimations were performed using the method of Kaplan and Meier. Overall survival, disease-free survival, and disease-specific survival were defined by the following definitions. Overall survival was defined as the time from diagnosis to death resulting from any cause. Patients who were alive were classified as censored observations at the time of last follow-up for overall survival; disease-free survival was defined as the time from diagnosis to local failure, nodal failure, systemic failure or death resulting from any cause, whichever occurred first. Patients who were alive without local failure, nodal failure or systemic failure were classified as censored observations at the time of last follow-up for disease-free survival. Disease-specific survival was defined as the time from diagnosis to death from breast lymphoma. Patients who were alive were classified as censored observations at the time of last follow-up and patients dying from any cause other than breast lymphoma were classified as censored observations at the time of death for disease-specific survival.
Because there is no standard universal treatment of primary breast lymphoma, the correlations between treatment protocols, tumor relapse and survival were analyzed. Also, variables such as histopathology and cancer stage were analyzed to evaluate their significance affecting the treatment outcome. Both univariate and multivariate analysis were performed. Univariate analysis was performed using the log rank test and multivariate analysis using the Cox proportional hazards model.
Results
We found 93 patients diagnosed with primary breast lymphoma from 1974 to 1999. All received surgery, chemotherapy, and radiotherapy or a combined modality according to different treatment protocols of different institutes. All 93 patients were female. The mean age was 57 years (range from 19 to 92 years). The histopathology of 63 patients (68%) was diffuse large cell lymphoma, 5 patients (5%) had Burkitt’s lymphoma, 4 patients (4%) had follicular small cleaved cell lymphoma, 2 patients (2%) had mucosa-associated lymphoid tissue lymphoma, and 19 patients (21%) were in other classifications. According to Ann Arbor classification,11 53 patients (57%) were stage I, 21 patients (23%) were stage II, 4 patients (4%) were stage III, and 15 patients (16%) were stage IV (Table 2).
Table 2.
Characteristics of patients with primary breast lymphoma (n=93)
| n | % | |
|---|---|---|
| Mean age 57 years (range 19 to 92) | 93 | 100 |
| Ann Arbor classification | ||
| Stage I | 53 | 57 |
| Stage II | 21 | 23 |
| Stage III | 4 | 4 |
| Stage IV | 15 | 16 |
| Histopathology | ||
| Diffuse large cell lymphoma | 63 | 68 |
| Burkitt’s lymphoma | 5 | 5 |
| Follicular small cleaved cell lymphoma | 4 | 4 |
| Mucosa-associated lymphoid tissue lymphoma | 2 | 2 |
| Other classifications | 19 | 21 |
| Initial treatment | ||
| Surgery alone | 12 | 13 |
| Chemotherapy alone | 25 | 27 |
| Radiotherapy alone | 8 | 7 |
| Surgery and chemotherapy | 9 | 10 |
| Surgery and radiotherapy | 9 | 10 |
| Chemotherapy and radiotherapy | 20 | 22 |
| Surgery, chemotherapy and radiotherapy | 10 | 11 |
| Survival status | ||
| Alive | 51 | 55 |
| Expire | 42 | 45 |
| Relapse | 45 | 48 |
| Sites of relapse | ||
| CNS | 5 | 11 |
| Lymph nodes | 5 | 11 |
| Contra-lateral breast | 3 | 7 |
| Others | 32 | 71 |
Twelve patients (13%) received surgery alone (lumpectomy, partial mastectomy, modified radical mastectomy (MRM), 25 patients (27%) received chemotherapy alone, 8 patients (7%) received radiotherapy alone, 9 patients (10%) received surgery and chemotherapy, 9 patients (10%) received surgery and radiotherapy, 20 patients (22%) received chemotherapy and radiotherapy, and 10 patients (11%) received surgery combined with chemotherapy and radiotherapy. Surgery meant lumpectomy, partial mastectomy and MRM, without inclusion of excisional biopsy. The chemotherapy mainly consisted of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) or CHOP-like regimens, and the course ranged from 3 to 8 cycles. The median radiation dose of radiotherapy was 40Gy (ranged from 36Gy to 50Gy).
With the median follow-up duration of 34 months (mean, 53 months, range from 3 to 226 months), 45 patients (48%) had relapse of disease, 46 patients (50%) showed no relapse, while 2 patients (2%) had disease progression. Among the 45 patients with tumor relapse, 5 patients (11%) had relapse in CNS, 5 patients (11%) in lymph nodes, 3 patients (7%) in contra-lateral breast, 3 patients (7%) in bone marrow, 2 patients (4%) in liver, and 2 patients (4%) in skin. The mean time of the first tumor relapse after treatment was 20 months (range from 3 to 71 months).
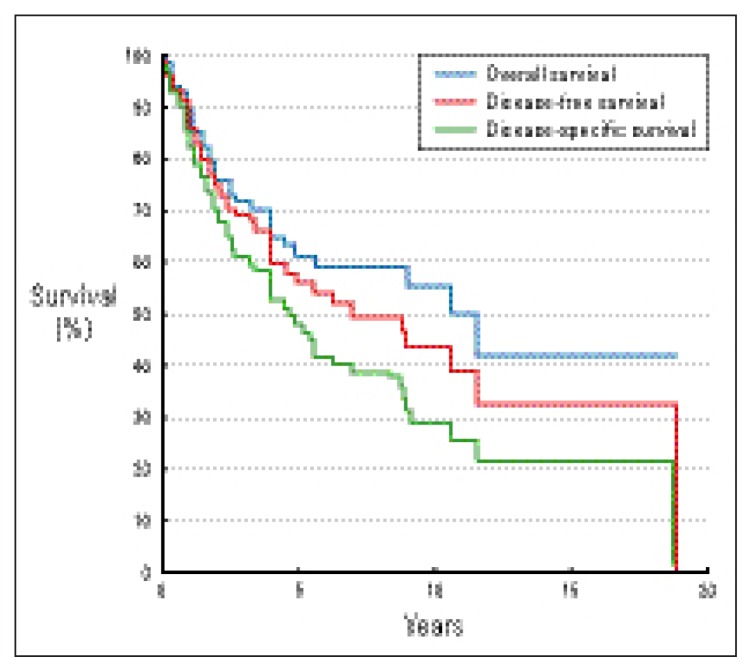
With the median follow-up duration of 34 months, 51 patients (55%) were alive, and 42 patients (45%) expired. Using the method of Kaplan and Meier for survival estimations, the 3-year and 5-year overall survival rates were 70% and 56%, respectively. The 3-year and 5-year disease-free survival rates were 61% and 48%, respectively. The 3-year and 5-year disease-specific survival rates were 71% and 60%, respectively (Figure 1).
Figure 1.
Survival curves from pooled data on 93 patients with primary breast lymphoma.
In a univariate analysis, radiotherapy was a significant prognostic factor predicting tumor relapse, favoring patients with treatment of radiotherapy (P=0.044). Forty-seven patients received radiotherapy, and only 20 patients (43%) had disease relapse. In 46 patients without radiotherapy, 28 patients (57%) had disease relapse, and even 2 patients had disease progression.
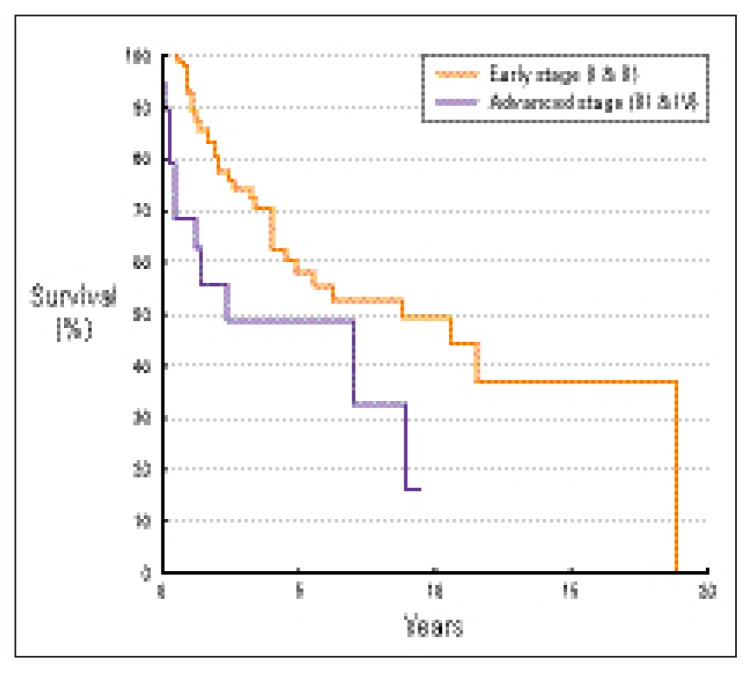
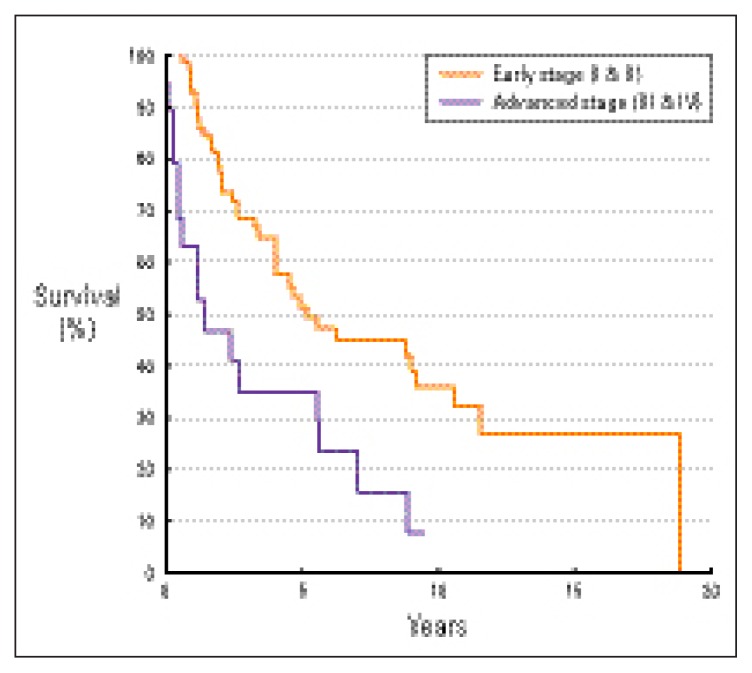
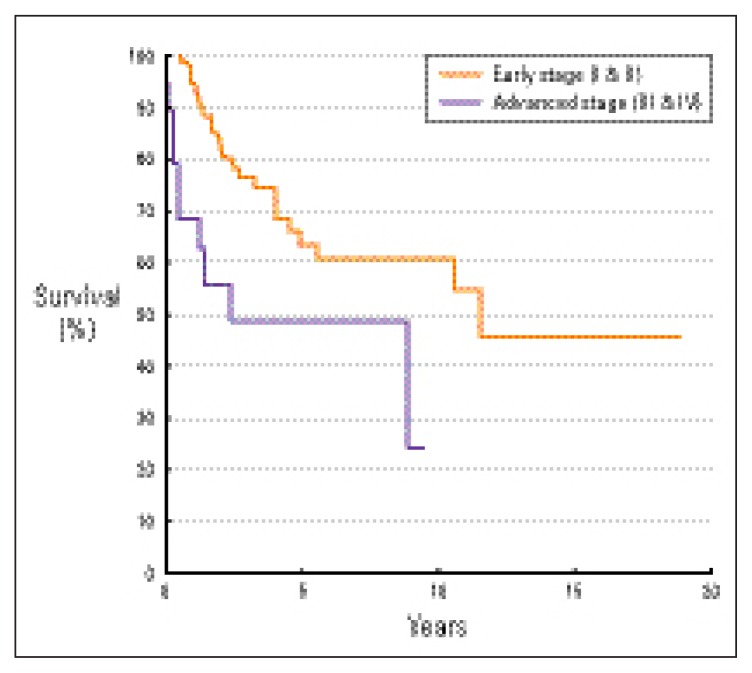
Tumor stage was a significant prognostic factor affecting overall survival, disease-free survival and disease-specific survival, favoring patients with early stages (P=0.0231, 0.0015, 0.0124, respectively). The 5-year overall survival rates of patients with early (stage I and II) and advanced (stage III and IV) stages were 58% and 49%, respectively (Figure 2). The 5-year disease-free survival rates of patients with early and advanced stages were 51% and 35%, respectively (Figure 3). The 5-year disease-specific survival rates of patients with early and advanced stages were 63% and 49%, respectively (Figure 4).
Figure 2.
Overall survival by stage (Ann Arbor classification11).
Figure 3.
Disease-free survival by stage (Ann Arbor classification11).
Figure 4.
Disease-specific survival by stage (Ann Arbor classification11).
Discussion
Among non-Hodgkin’s lymphomas, primary extranodal lymphoma accounts for 25% to 40%, and the gastrointestinal tract is the most common site of primary extranodal lymphoma.5 The incidence of primary extranodal non-Hodgkin’s lymphoma of the breast was 2%–3%.3–4 Primary breast lymphoma was first reported and described as early as 1893.12 Usually, the disease was unilateral at the initial time of diagnosis, and right side predominance was described although cases of bilateral breast involvement have been reported.1,6,13 Diagnosis is made by breast excision or aspiration biopsy. The major histologic type of primary breast lymphoma is diffuse large cell lymphoma; B-cell phenotype is the most common, while some are of the T-cell phenotype.1–2,12,14 In our study, for the definition of primary breast lymphoma, we used a modification of the original criteria defined by Wiseman and Liao:15 presence of an adequate biopsy, a close histological association of mammary tissue and malignant lymphomatous infiltrate, the breast being the site of initial manifestation of the lymphoma. A prior manifestation of a malignant lymphoma was not allowed.
Due to the rarity of the disease and the paucity of patient data, there is no standard universal approach toward management for primary breast lymphoma. Surgery, chemotherapy, and radiotherapy are the main choices of treatment. However, either single therapy alone or a combined modality therapy may be found in different research series. The optimal treatment for primary breast lymphoma is at issue. Also, prognostic factors affecting the outcome and survival should be evaluated. For this reason we decided to perform a pooled study to gather the data from the small number of patients with primary breast lymphoma. We evaluated several variables that might affect the treatment outcome, including treatment protocols and patient characteristics.
In our literature review we found that the stage of disease, tumor size, histologic characteristics and age were regarded as prognostic factors in primary breast lymphoma.16–20 However, in recent reports, the stage of disease was the only significant factor for overall survival in primary breast lymphoma.5,12,21 Similarly, our analysis revealed that the stage of disease was a significant prognostic factor affecting overall survival, disease-free survival and disease-specific survival. Surgery, on the other hand, did not make a significant impact on survival. Forty patients (43%) received surgery (lumpectomy, partial mastectomy or MRM) as the only treatment or in a combined modality, but surgery was not an important prognostic factor affecting survival outcome.
Another problem was tumor relapse. From the literature review, there was 13% to 30% relapse rate in patients with primary breast lymphoma, even with a complete remission rate of 74% to 91%.3,12,22–23 The sites of relapse included the involved breast, contra-lateral breast, skin, and central nervous system (CNS).3,7,12,21–23 In our study, 45 patients had relapse of disease. The relapse rate was 48%. The majority of relapse sites were CNS (11%) and lymph nodes (11%). The mean time at which the first tumor relapse occurred after treatment was 20 months (range from 3 to 71 months). In the univariate analysis, radiotherapy was the only prognostic factor predicting the relapse of disease (P=0.044). In 47 patients treated with radiotherapy, only 20 patients (43%) had disease relapse. On the contrary, in 46 patients without radiotherapy, 28 patients (57%) had disease relapse, and 2 patients had disease progression.
The CNS, in particular, was a major site of relapse. Among the 45 patients with tumor relapse, 5 patients (11%) had relapse in the CNS. Four of these 5 patients (80%) presented with CNS relapse as part of systemic dissemination. One (20%) presented as a single relapse with CNS involvement. As for incidence of CNS relapse related to the stage at diagnosis of primary breast lymphoma, 3 patients (60%) were stage II, 1 (20%) was stage I, and 1 (20%) was stage IV. The median time to the CNS relapse was 6 months (range, 3 to 8 months). The high incidence of CNS dissemination in primary breast lymphoma seemed to differ from localized nodal lymphomas, but in other types of extranodal lymphoma, i.e., testicular lymphoma, CNS relapses were also frequent.3 Some authors suggest that primary CNS prophylaxis should be considered in primary breast lymphoma with high-grade disseminated disease.7
In summary, primary breast lymphoma is a rare disease. Due to the rarity of the disease and the paucity of patient numbers, no sufficient database, such as a large-series study, is available for analysis. Even with a 3-year overall survival rate of 70%, the high relapse rate of 48% should be noted and is a cause for concern. In the study, patients treated with chemotherapy and radiotherapy had a better survival outcome and a lower relapse rate. We suggest that chemotherapy and radiotherapy be the initial treatment for patients with primary breast lymphoma.
References
- 1.Shapiro CM, Mansur D. Bilateral primary breast lymphoma. American Journal of Clinical Oncology. 2001;24:85–6. doi: 10.1097/00000421-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Pinheiro RF, Colleoni GW, Baiocchi OC, Kerbauy FR, Duarte LC, Bordin JO. Primary breast lymphoma: an uncommon but curable disease. Leukemia and Lymphoma. 2003;44:149–51. doi: 10.1080/1042819021000040369. [DOI] [PubMed] [Google Scholar]
- 3.Ribrag V, Bibeau F, El Weshi A, Frayfer J, Fadel C, Cebotaru C, et al. Primary breast lymphoma: a report of 20 cases. British Journal of Haematology. 2001;115:253–6. doi: 10.1046/j.1365-2141.2001.03047.x. [DOI] [PubMed] [Google Scholar]
- 4.Michetti M, Minelli M, Berni C, Bellini V, La Porta A, Manente L, et al. A case of primary non-Hodgkin lymphoma of the breast. La Clinica Terapeutica. 1999;150:307–10. [PubMed] [Google Scholar]
- 5.Kim SH, Ezekiel MP, Kim RY. Primary lymphoma of the breast: breast mass as an initial symptom. American Journal of Clinical Oncology. 1999;22:381–3. doi: 10.1097/00000421-199908000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Lyons JA, Myles J, Pohlman B, Macklis RM, Crowe J, Crownover RL. Treatment of prognosis of primary breast lymphoma: a review of 13 cases. American Journal of Clinical Oncology. 2000;23:334–6. doi: 10.1097/00000421-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Au WY, Chan AC, Chow LW, Liang R. Lymphoma of the breast in Hong Kong Chinese. Hematological Oncology. 1997;15:33–8. doi: 10.1002/(sici)1099-1069(199702)15:1<33::aid-hon595>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y, Tokuda Y, Okumura A, Saito Y, Ohta M, Kubota M, et al. Three cases of malignant lymphoma of the breast. Japanese Journal of Clinical Oncology. 2000;30:33–6. doi: 10.1093/jjco/hyd008. [DOI] [PubMed] [Google Scholar]
- 9.Lee KC, Chang HT, Chen CJ, Mok KT. Primary non-Hodgkin’s lymphoma of the breast: breast conservation therapy in two patients. Zhonghua Yi Xue Za Zhi (Taipei) 1999;62:633–8. [PubMed] [Google Scholar]
- 10.Kuper-Hommel MJ, Snijder S, Janssen-Heijnen ML, Vrints LW, Kluin-Nelemans JC, Coebergh JW, et al. Treatment and survival of 38 female breast lymphomas: a population-based study with clinical and pathological reviews. Annals of Hematology. 2003;82:397–404. doi: 10.1007/s00277-003-0664-7. [DOI] [PubMed] [Google Scholar]
- 11.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. AJCC Cancer Staging Manual. 2nd edn. New York: Springer-Verlag; 2002. pp. 395–397. [Google Scholar]
- 12.Ha CS, Dubey P, Goyal LK, Hess M, Cabanillas F, Cox JD. Localized primary non-Hodgkin lymphoma of the breast. American Journal of Clinical Oncology. 1998;21:376–80. doi: 10.1097/00000421-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Sabate JM, Gomez A, Torrubia S, Camins A, Roson N, De Las Heras P, et al. Lymphoma of the breast: clinical and radiologic features with pathologic correlation in 28 patients. Breast Journal. 2002;8:294–304. doi: 10.1046/j.1524-4741.2002.08509.x. [DOI] [PubMed] [Google Scholar]
- 14.Tan PH, Sng IT. Breast lymphoma—a pathologic study of 14 cases. Annals of the Academy of Medicine, Singapore. 1996;6:783–90. [PubMed] [Google Scholar]
- 15.Wiseman C, Liao KT. Primary lymphoma of the breast. Cancer. 1972;29:1705–12. doi: 10.1002/1097-0142(197206)29:6<1705::aid-cncr2820290640>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Hugh JC, Jackson FI, Hanson J, Poppema S. Primary breast lymphoma. An immunohistologic study of 20 new cases. Cancer. 1990;66:2602–11. doi: 10.1002/1097-0142(19901215)66:12<2602::aid-cncr2820661224>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Dixon JM, Lumsden AB, Krajewski A, Elton RA, Anderson TJ. Primary lymphoma of the breast. British Journal of Surgery. 1987;74:214–6. doi: 10.1002/bjs.1800740322. [DOI] [PubMed] [Google Scholar]
- 18.Giardini R, Piccolo C, Rilke F. Primary non-Hodgkin’s lymphomas of the female breast. Cancer. 1992;69:725–35. doi: 10.1002/1097-0142(19920201)69:3<725::aid-cncr2820690320>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.Cohen PL, Brooks JJ. Lymphomas of the breast. A clinicopathologic and immunohistochemical study of primary and secondary cases. Cancer. 1991;67:1359–69. doi: 10.1002/1097-0142(19910301)67:5<1359::aid-cncr2820670515>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Ariad S, Lewis D, Cohen R, Bezwoda WR. Breast lymphoma. A clinical and pathological review and 10-year treatment results. South African Medical Journal. 1995;85:85–9. [PubMed] [Google Scholar]
- 21.Wong WW, Schild SE, Halyard MY, Schomberg PJ. Primary non-Hodgkin lymphoma of the breast: The Mayo Clinic Experience. Journal of Surgical Oncology. 2002;80:19–25. doi: 10.1002/jso.10084. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira A, Guimaraes T, Bento MJ, Viseu F, Silva I. Primary non-Hodgkin’s lymphoma of the breast. Annals of Oncology. 2000;11:103. [Google Scholar]
- 23.Mirimanoff RO, Taghian A, Epelbaum R, Poortmans P, Zwahlen D, Amsler B. Outcome and patterns of failure in primary breast lymphoma: a multicenter rare cancer network study. International Journal of Radiation, Oncology, Biology and Physics. 2002;54:300–1. [Google Scholar]
- 24.Kuper-Hommel MJ, Snijder S, Janssen-Heijnen ML, Vrints LW, Kluin-Nelemans JC, Coebergh JW, et al. Treatment and survival of 38 female breast lymphomas: a population-based study with clinical and pathological reviews. Annals of Hematology. 2003;82:397–404. doi: 10.1007/s00277-003-0664-7. [DOI] [PubMed] [Google Scholar]