Abstract
BACKGROUND
Kikuchi-Fujimoto disease (KFD) is a rare entity of uncertain cause that commonly presents as a benign self-limiting disease of unknown origin. The objective of this study was to document the clinical features, mode of presentation, histopathological and immunohistochemical (IHC) features of KFD at our institutions since little is known about this disease in our region.
METHODS
We reviewed the histopathological reports of all lymph nodes resected at or referred to King Abdulaziz University Hospital between 1990 and 2003 and King Faisal Specialist Hospital & Research Center, Jeddah, Kingdom of Saudi Arabia between 2000 and 2003. All cases diagnosed as KFD were identified and the histological slides and clinical data were reviewed. IHC was performed for the proliferative marker Ki-67 and the apoptosis-related markers Bcl-2 and p53.
RESULTS
In 2500 lymph node biopsies, 15 cases were diagnosed as KFD. The female to male ratio was 2.7:1. One patient presented with axillary lymphadenopathy and the others presented with cervical lymphadenopathy. Ages averaged 29 years and ranged from 13 to 46 years. There was no recurrence of the lymphadenopathy over 1 to 10 years of follow up. Bcl-2 and p53 were negative and Ki-67 was positive in 11 of 15 cases.
CONCLUSION
The results support earlier findings that KFD is a self-limiting disorder that requires no specific management. We suggest a clinical follow-up for several years. The female predominance was striking. Apoptosis-regulating proteins are not helpful in the diagnosis. KFD usually expressed the proliferation-associated nuclear antigen Ki-67. Increased awareness of KFD will minimize the risk of confusing this entity with malignant lymphoma or other serious conditions.
Though Kikuchi-Fujimoto disease (KFD) was first described in 19721,2 many clinicians and pathologists are still unaware of its existence. It affects cervical lymph nodes, predominantly in young females with persistently enlarged cervical lymph nodes unresponsive to antibiotic therapy. Although a self-limiting disease that usually follows a benign course, KFD has been repeatedly misdiagnosed as malignant lymphoma;3 hence, clinicians and pathologists alike need to be aware of this disease entity. It has been widely reported from Japan1,2,4–12 and sporadically from many other countries of the world including Saudi Arabia.13–16 KFD has to be differentiated also from infectious agents, particularly tuberculous lymphadenitis, which is the most common cause of necrotising inflammation of the lymph node in our country. Little information is known about KFD in the kingdom. We review the pathological, clinical and immunohistochemistry features of this interesting disease. KFD is characterized histologically by apoptosis so we examined the expression of the apoptosis-regulating proteins bcl-2 and p53 using immunohistochemistry. To our knowledge this study represent the largest series of this disease reported from the kingdom.
Methods
The study was carried out at King Abdulaziz University Hospital (KAUH) and King Faisal Specialist Hospital & Research Centre (KFSH&RC), Jeddah, Saudi Arabia, two main referral hospitals in the western region of Saudi Arabia. All cases diagnosed as KFD at KAUH between 1990 and 2003 and KFSH&RC between 2000 and 2003 were evaluated and the clinical data reviewed for age at presentation, sex, clinical features, diagnosis, treatment and outcome. All specimens were fixed in 10% neutral formalin. Paraffin-embedded blocks were resectioned and stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), and Ziel-Neelsen stains (ZN) and examined. The immunohistochemistry was performed using avidin-biotin complex technique (ABC) with appropriate positive and negative controls. The immunohistochemistry panel included CD45, CD3, CD20 CD68 and antibodies against proliferative marker (Ki-67) and apoptosis-related markers (Bcl-2 and p53). The proliferative and apoptotic markers were selected because KFD is characterized by extensive apoptosis.
Table 1.
Clinical features of Kikuchi-Fujimoto disease and immunohistochemistry results in 15 patients.
| Case | Age (year) | Sex | Location | P53 | BCL-2 | Ki-67 |
|---|---|---|---|---|---|---|
| 1 | 15 | F | Cervical | − | − | + |
| 2 | 17 | F | Cervical | − | − | + |
| 3 | 40 | F | Cervical | − | − | − |
| 4 | 23 | F | Cervical | − | − | + |
| 5 | 28 | F | Cervical | − | − | − |
| 6 | 35 | M | Cervical | − | − | + |
| 7 | 31 | F | Cervical | − | − | + |
| 8 | 32 | F | Cervical | − | − | + |
| 9 | 34 | F | Cervical | − | − | + |
| 10 | 38 | M | Cervical | − | − | + |
| 11 | 40 | F | Cervical | − | − | − |
| 12 | 46 | F | Axillary | − | − | + |
| 13 | 16 | F | Cervical | − | − | − |
| 14 | 13 | M | Cervical | − | − | + |
| 15 | 30 | M | Cervical | − | − | + |
Results
A total of 15 cases were diagnosed as KFD out of 2500 lymph node biopsies received at our institutions during that period. There were 11 females and 4 males. One patient presented with axillary lymphadenopathy while all the others presented with cervical lymphadenopathy. Ages averaged 29 years and ranged from 13 to 46 years. There was no recurrence of the lymphadenopathy over a period of 1 to 10 years follow up. All cases showed the classic pathological features, which include variable amounts of nuclear debris, usually extracellular, transformed lymphoid cells of medium and large size (immunoblasts), an absence of inflammatory and granulomatous reaction features, preserved sinuses in the uninvolved areas, and the presence of benign histiocytes with moderate to marked nuclear irregularities and scanty to moderate cytoplasm with crescent-shaped nuclei (Figures 1a, b, c, d). Neither acid-fast bacilli nor fungal organisms were found on special stains. Immunohistochemistry showed that the majority of cells in the affected foci were a mixture of CD3+ cells T-lymphocytes and CD68+ cells histiocytes with very few B cells. Immunostaining for the apoptosis-regulating proteins bcl-2 and p53 were negative in all cases. In 11 of 15 cases, numerous cells expressed the proliferation-associated nuclear antigen Ki-67 in the areas with relatively preserved cellularity around the necrotic areas.
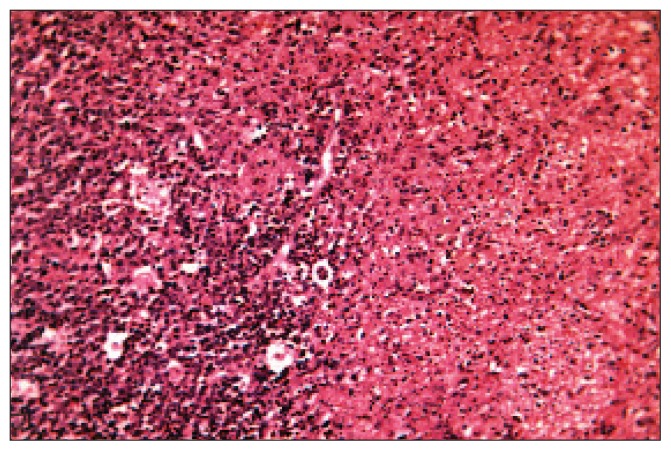
Figure 1a.
Section from a case of Kikuchi-Fujimoto disease showing variable amounts of nuclear debris with benign histiocytes and moderate-to-marked nuclear irregularities; also shown is scanty-to-moderate cytoplasm with crescent-shaped and an absence of inflammatory and granulomatous reaction features. (haematoxylen and eosin stain, original power x400)
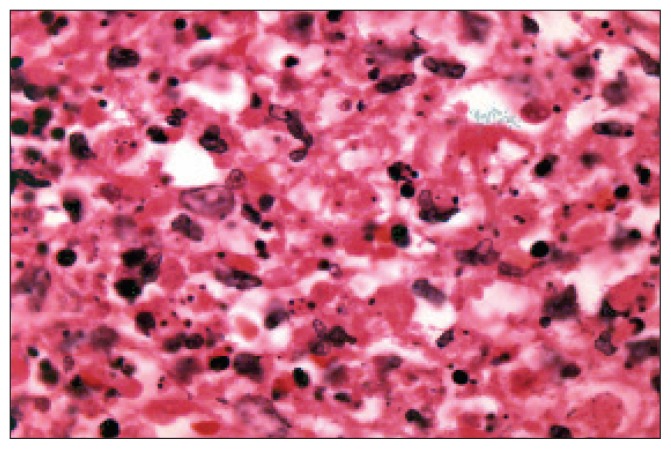
Figure 1b.
A higher power shows the nuclear debris and abnormal shaped histiocytes. (haematoxylen and eosin stain, original power x1000-Oil)
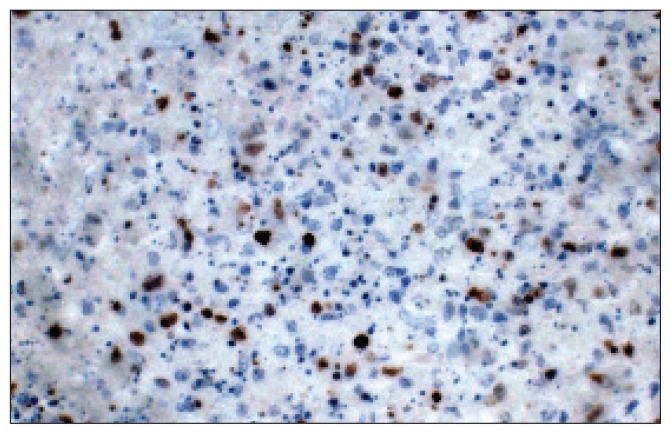
Figure 1c.
Immunohistochemistry stain for ki-67 shows some cells with positive staining.
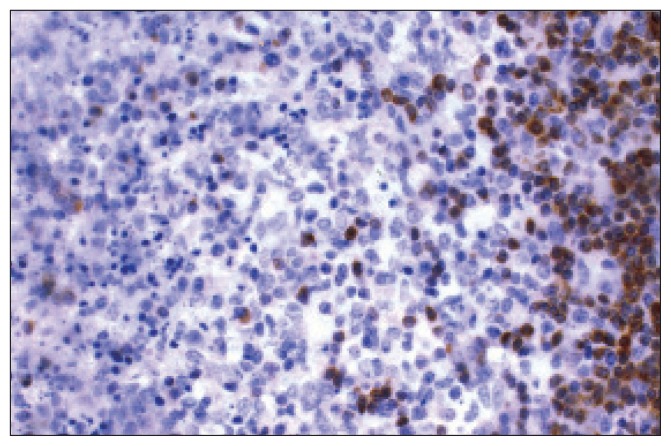
Figure 1d.
Immunohistochemistry stain for Bcl-2 shows negative staining in the affected area and positively stained cells in the surrounding interfollicular areas.
Discussion
While most common in Southeast Asia, where KFD was initially described, this lesion has been reported in patients of all ages, gender, and ethnic background from a variety of geographic locations, including Saudi Arabia. Although the disease commonly affects females, four of our patients were male. In 14 of 15 cases, the patients presented with cervical lymphadenopathy. One of our patients presented with axillary lymphadenopathy, which is an unusual presentation, but it has been reported.17–20 KFD presenting as bilateral hilar lymphadenopathy has also been reported.21 KFD in Saudi patients has been reported in different provinces of the kingdom.13–16 We saw typical histological features, namely patchy paracortical lymphohistiocytic aggregates, variable karyorrhexis and an absence of granulocytic infiltration. There were activated cells with numerous phagocytic cells with crescent-shaped nuclei. Those features favor this diagnosis over a lymphoma. Three of four cases sent to our institution for consultation had been misdiagnosed initially as lymphoma or identified as suggestive of lymphoma; the fourth case was reported as suggestive of tuberculosis. Recognition of the entity is important for it may be mistaken for other infective lymphadenitis or lymphoma.
A histological pattern similar to KFD has been described in patients with systemic lupus erythematosus (SLE), and there is evidence of an association between KFD and SLE.22 A flare-up of lupus disease activity frequently accompanies the onset of KFD. The simultaneous occurrence of these two diseases indicates that they are not independent events.22 It has been speculated that Kikuchi disease may be one of the manifestations of SLE.22 None of our patients had KFD associated with SLE.
An viral etiologic agent, human herpesvirus 6 (HHV-6), has been implicated in KFD. Dominguez et al showed that all cases (n=32) of KFD had elevated titers for HHV-6 and tested positive for HHV-6 by polymerase chain reaction.23,24 George et al showed that HHV-8 and EBV do not play causative roles.20 KFD has been described in association with certain disorders such as haemophagocytic syndrome,25 psoriasis vulgaris,26 antiphospholipid syndrome,27 aseptic meningitis,12 parotiditis viral infection,28 and Brucella melitensis infection.19 However, none of the above conditions was a constant feature in any reported series on KFD. Immunohistochemistry showed that the majority of the affected foci represent a mixture of CD3+ cells (T-lymphocytes) and CD68+ cells (histiocytes) with very few B cells. In the current study immunostaining for the apoptosis-regulating proteins bcl-2 and p53 was negative. Takakuwa et al also showed that bcl-2, bax, c-myc and p53 were not involved in KFD disease.7 However, some authors showed that bcl-2 was seen significantly more frequently in KFD than in Hodgkin’s disease. Krueger et al demonstrated that biopsy samples from patients with KFD did not express p53.29 In the areas with relatively preserved cells around the necrotic zones, numerous cells expressed the proliferation-associated nuclear antigen Ki-67, which is in keeping with the findings of others.30,31 KFD has been reported in most of the Arab countries. The largest series reported from Arab countries was from Egypt by Helal et al, where they reported 10 cases having the classic histological pattern of KFD.32 The lymphadenopathy usually resolved without medical treatment within 6 months after a definite diagnosis with no recurrence.33,34
In our society, biopsy of lymph nodes with extensive areas of necrosis should be interpreted very carefully because tuberculosis is the most common cause of lymph node necrosis in this area of the world. However, other classic features of KFD will be sufficient to avoid misdiagnosis with TB in the majority of cases. Despite its usually benign course, several cases with fatal outcomes have been reported, particularly when associated with SLE.35 To avoid a fatal outcome, early and intensive immunosuppressive treatment may be the only option for patients who develop very aggressive forms of KFD. The results of our study reveal that the clinical features and pathological pattern of this disease in Saudi patients is similar to those reported in the other countries. The study supports the theory that KFD is a self-limiting disorder that does not require any specific management, but we suggest that patients be followed up for several years. The female predominance was striking as it is in the studies in western countries, but this finding contrasts with reports from China.34 The combination of disease awareness among clincians, clinical information, careful microscopic examination, special stains and immunohistochemistry is helpful in differentiating it from malignant lymphoma and infectious lesions, particularly TB. Apoptosis-regulating proteins do not appear to be involved in KFD and are not helpful in the diagnosis. The mechanism of apoptosis in KFD is still unclear and more studies are encouraged to explore the exact pathogenesis of this rare entity.
References
- 1.Fujimoto Y, Kozima Y, Yamagouchi K. Cervical subacute necrotizing lymphadenitis. A new clinicopathologic entity. Naika. 1972;20:920–27. [Google Scholar]
- 2.Kikuchi M. Lymphadenitis showing focal reticulum cell hyperplasia with nuclear debris and phagocytes: A clinicopathological study. Nippon Ketsueki Gakkai Zasshi. 1972;35:379–80. [Google Scholar]
- 3.Chamulak GA, Brynes RK, Nathwani BN. Kikuchi-Fujimoto disease mimicking malignant lymphoma. Am J Surg Pathol. 1990;14:514–23. doi: 10.1097/00000478-199006000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Mugnaini EN, Watson T, Guccion J, Benator D. Kikuchi disease presenting as a flu-like illness with rash and lymphadenopathy. Am J Med Sci. 2003;325:34–7. doi: 10.1097/00000441-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Takano Y, Saegusa M, Okudaira M. Pathologic analyses of non-overt necrotizing type Kikuchi and Fujimoto’s disease. Acta Pathol Jpn. 1993;43:635–45. doi: 10.1111/j.1440-1827.1993.tb02547.x. [DOI] [PubMed] [Google Scholar]
- 6.Kato K, Ohshima K, Anzai K, Suzumiya J, Kikuchi M. Elevated serumsoluble Fas ligand in histiocytic necrotizing lymphadenitis. Int J Hematol. 2001;73:84–6. doi: 10.1007/BF02981907. [DOI] [PubMed] [Google Scholar]
- 7.Takakuwa T, Ohnuma S, Koike J, Hoshikawa M, Koizumi H. Involvement of cell-mediated killing in apoptosis in histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto disease) Histopathology. 1996;28:41–8. doi: 10.1046/j.1365-2559.1996.267310.x. [DOI] [PubMed] [Google Scholar]
- 8.Ohshima K, Kikuchi M, Sumiyoshi Y, et al. Proliferating cells in histiocytic necrotizing lymphadenitis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;61:97–100. doi: 10.1007/BF02890410. [DOI] [PubMed] [Google Scholar]
- 9.Iguchi H, Sunami K, Yamane H, et al. Apoptotic cell death in Kikuchi’s disease: a TEM study. Acta Otolaryngol Suppl. 1998:250–3. [PubMed] [Google Scholar]
- 10.Ura H, Yamada N, Torii H, Imakado S, Iozumi K, Shimada S. Histiocytic necrotizing lymphadenitis (Kikuchi’s disease): the necrotic appearance of the lymph node cells is caused by apoptosis. J Dermatol. 1999;26:385–9. doi: 10.1111/j.1346-8138.1999.tb03494.x. [DOI] [PubMed] [Google Scholar]
- 11.Yasukawa K, Matsumura T, Sato-Matsumura KC, et al. Kikuchi’s disease and the skin: case report and review of the literature. Br J Dermatol. 2001;144:885–9. doi: 10.1046/j.1365-2133.2001.04151.x. [DOI] [PubMed] [Google Scholar]
- 12.Sato YKH, Oizumi K. Histiocytic necrotizing lymphadenitis (Kikuchi’s disease) with aseptic meningitis. J Neurol Sci. 1999;163:187–91. doi: 10.1016/s0022-510x(99)00037-4. [DOI] [PubMed] [Google Scholar]
- 13.Louis N, Hanley M, Davidson NM. Kikuchi-Fujimoto disease: a report of two cases and an overview. J Laryngol Otol. 1994;108:1001–4. doi: 10.1017/s0022215100128749. [DOI] [PubMed] [Google Scholar]
- 14.Kutty MK, Anim JT, Sowayan S. Histiocytic necrotising lymphadenitis (Kikuchi-Fujimoto disease) in Saudi Arabia. Trop Geogr Med. 1991;43:68–75. [PubMed] [Google Scholar]
- 15.Chavis PS, Fallata A, Al-Hussein H, Clunie D, Huaman A. Lacrimal gland involvement in Kikuchi-Fujimoto disease. Orbit. 1998;17:113–117. doi: 10.1076/orbi.17.2.113.2763. [DOI] [PubMed] [Google Scholar]
- 16.Al-Nazer MA, Al-Hadad AM, Al-Aithan SA, Al-Salem AH, Al-Faraj AA, Al-Saeed HH. Kukuchi-Fujimito disease. Saudi Med J. 2002;23:405–8. [PubMed] [Google Scholar]
- 17.Adhikari RC, Sayami G, Lee MC, Basnet RB, Shrestha PK, Shrestha HG. Kikuchi-Fujimoto disease in Nepal: a study of 6 cases. Arch Pathol Lab Med. 2003;127:1345–8. doi: 10.5858/2003-127-1345-KDINAS. [DOI] [PubMed] [Google Scholar]
- 18.Thongsuksai P, Kayasut K. Histiocytic necrotizing lymphadenitis (Kikuchi’s disease): clinicopathologic characteristics of 23 cases and literature review. J Med Assoc Thai. 1999;82:812–8. [PubMed] [Google Scholar]
- 19.Charalabopoulos K, Papalimneou V, haralabopoulos A, Bai MAN. Brucella melitensis infection stimulates an immune response leading to Kikuchi-Fujimoto disease. In Vivo. 2003;17:51–3. [PubMed] [Google Scholar]
- 20.George TI, Jones CD, Zehnder JL, Warnke RA, Dorfman RF. Lack of human herpesvirus 8 and Epstein-Barr virus in Kikuchi’s histiocytic necrotizing lymphadenitis. Hum Pathol. 2003;34:130–5. doi: 10.1053/hupa.2003.11. [DOI] [PubMed] [Google Scholar]
- 21.Abba AA, Afzal M, Almoharab FI, Baez-Giangreco A. Kikuchi’s disease presenting as bilateral hilar lymphadenopathy. Respir Med. 1995;89:701–3. doi: 10.1016/0954-6111(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 22.Chen YH, Lan JL. Kikuchi disease in systemic lupus erythematosus: clinical features and literature review. J Microbiol Immunol Infect. 1998;31:187–92. [PubMed] [Google Scholar]
- 23.Dominguez DC, Torres ML, Antony S. Is human herpesvirus 6 linked to kikuchi-fujimoto disease? The importance of consistent molecular and serologic analysis. South Med J. 2003;96:226–33. doi: 10.1097/01.SMJ.0000054420.01333.B0. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez JN, Aguayo DM, Elizalde J, et al. Kikuchi-Fujimoto disease associated with acute infection by herpesvirus 6. Sangre. 1996;41:387–90. [PubMed] [Google Scholar]
- 25.Kelly J, Kelleher K, Khan MK, Rassam SM. A case of haemophagocytic syndrome and Kikuchi-Fujimoto disease occurring concurrently in a 17-year-old female. Int J Clin Pract. 2000;54:547–9. [PubMed] [Google Scholar]
- 26.Kato A, Kono T, Ishii M, Wakasa K, Taniguchi S. Spontaneous clearance of psoriasis during the course of Kikuchi-Fujimoto disease. J Am Acad Dermatol. 2002;47:S287–8. doi: 10.1067/mjd.2002.109253. [DOI] [PubMed] [Google Scholar]
- 27.Papaioannou G, Speletas M, Kaloutsi V, Pavlitou-Tsiontsi A. Histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto disease) associated with antiphospholipid syndrome: case report and literature review. Ann Hematol. 2002;81:732–5. doi: 10.1007/s00277-002-0562-4. [DOI] [PubMed] [Google Scholar]
- 28.Gomez Garcia AM, Martinez Hurtado E, Ruiz Ribera I. [Parotiditis viral infection associated with Kikuchi-Fujimoto disease. Review of 1 case]. An Med Interna. 2004;21:135–7. doi: 10.4321/s0212-71992004000300009. [Article in Spanish] [DOI] [PubMed] [Google Scholar]
- 29.Krueger GR, Buja LM, Rojo J, Lasch J, Koch B, Leyssens N. [Apoptosis and cell proliferation in HHV-6 infections. Regulatory mechanisms of p53/bcl-2/ras interactions]. Pathologe. 1995;16:120–7. doi: 10.1007/s002920050084. [DOI] [PubMed] [Google Scholar]
- 30.Krueger GR, Huetter ML, Rojo J, Romero M, Cruz-Ortiz H. Human herpesviruses HHV-4 (EBV) and HHV-6 in Hodgkin’s and Kikuchi’s diseases and their relation to proliferation and apoptosis. Anticancer Res. 2001;3:2155–61. [PubMed] [Google Scholar]
- 31.Rivano MT, Falini B, Stein H, et al. Histiocytic necrotizing lymphadenitis without granulocytic infiltration (Kikuchi’s lymphadenitis). Morphological and immunohistochemical study of eight cases. Histopathology. 1987;11:1013–27. doi: 10.1111/j.1365-2559.1987.tb01842.x. [DOI] [PubMed] [Google Scholar]
- 32.Helal TA, Talaat W, Danial MF. Kikuchi histiocytic necrotizing lymphadenitis: clinicopathological and immunohistochemical study. East Mediterr Health J. 2001;7:153–62. [PubMed] [Google Scholar]
- 33.Mamoon N, Haroon A, Luqman M, Jamal S. Kikuchi’s disease of lymph nodes. J Coll Physicians Surg Pak. 2003;13:138–42. [PubMed] [Google Scholar]
- 34.Lin HC, Su CY, Huang CC, Hwang CF, Chien CY. Kikuchi’s disease: a review and analysis of 61 cases. Otolaryngol Head Neck Surg. 2003;128:650–3. doi: 10.1016/S0194-59980223291-X. [DOI] [PubMed] [Google Scholar]
- 35.Quintas-Cardama A, Fraga M, Cozzi SN, Caparrini A, Maceiras F, Forteza J. Fatal Kikuchi-Fujimoto disease: the lupus connection. Ann Hematol. 2003;82:186–8. doi: 10.1007/s00277-003-0611-7. [DOI] [PubMed] [Google Scholar]