Abstract
Background
Poor maternal nutrition during pregnancy is a leading modifiable risk factor associated with risks of adverse pregnancy outcomes (APO). Nevertheless, there is paucity of evidence if consumption of some food groups is associated with lower risk of APO, particularly in low-income settings. We aimed to determine whether consumption of some food groups is associated with lower risk of APOs such as: preterm birth (PTB), low-birth weight (LBW), and stillbirth in rural Central Ethiopia.
Methods
A multi-center (8 health centers) prospective cohort study, enrolling 432 pregnant women during their initial antenatal care visit, was employed. All mothers were then followed monthly (for a total of four visits) from enrollment to delivery. Midwives in respective health centers assessed dietary diversity using the Women’s individual dietary diversity score and evaluated birth outcomes following standard procedures. Logistic regression models were run to predict association of food groups with the APO.
Findings
Out of the 374 pregnant women who completed the study, one in five [74 (19.8%)] experienced at least one of the APO: 34 (9.1%) gave birth to LBW babies, 51(13.6%) had PTB and 17 (4.5%) experienced stillbirth. Poor or inconsistent consumption (<¾ assessments) of dark green leafy vegetables (adjusted odds ratio (AOR) = 2.01; 95% confidence interval (CI): 1.04–3.87), dairy products (AOR = 2.64; 95% CI: 1.11–6.30), and fruits and vegetables (AOR = 2.92; 95% CI: 1.49–5.67) were independently associated with higher APO risks. Whereas, being nonanemic at term (AOR = 0.24; 95% CI: 0.12–0.48) was independently associated with lower APO risks.
Conclusions
Poor or inconsistent consumption of dairy, dark green leafy vegetables and fruits were associated with higher risk of APOs. While community-based trials and mechanistic studies are needed to substantiate these findings, efforts to promote dietary diversity through increased consumption of fruits, vegetables and dairy may be beneficial in this and similar settings.
Introduction
In the last couple of decades, a substantial progress in child mortality was made worldwide1,2. Some of the world’s poorest countries have managed to cut child mortality rates by >50% in just a couple of decade’s time2. The mortality rate of under-five children has also declined substantially from 12.6 (12.4–12.8) in 1990 to 5.6 (5.4–6.0) million deaths in 20161,2. Despite these achievements, 5.6 million under five children continue to die every year mainly from preventable causes. Majority of child deaths occur during the neonatal period (first one month). About one million of neonates die on the first day and another close to one million die in the first week3.
Adverse outcomes of pregnancy, mainly: preterm birth (PTB), low-birth weight (LBW), and stillbirth continue to be major causes of neonatal and under five child deaths, worldwide4,5. In 2015 alone, close to 15 million premature (<37 completed weeks of gestation) babies were born; of which, more than a million died during the neonatal period due to complications, and those surviving often experienced lifetime of impairments5,6.
In Ethiopia, recent estimates suggest that prenatal death happens in 46 out of 1000 pregnancies and about 11% of newborns have LBW (7 and 8). These estimates rank Ethiopia among the ten top countries with the highest global burden of maternal (fifth) and neonatal (sixth) deaths, as well as stillbirth (seventh) incidence rates.
Poor nutrition of women during pregancy is among the leading modifiable risk factors associated with elevated APO risks10–15. Evidence is accumulating that maternal dietary patterns during pregnancy is associated with some APO risks. Studies from Europe16–19, New Zealand20, and Brazil21 showed that maternal adherence to certain dietary patterns reffered as “western”, “prudent”, or “traditional” diets were positively associated with birth weight, offspring bone size, bone mineral density, and forearm fractures; with additional protective effect against risks of having infants of small for gestational age. However, the associations between dietary diversity on APO risks remains unclear as some studies show heightened or null-effects22,23. Besides, many of the studies investigating such associations are mainly from high-income settings and hence studies from resource-poor settings are urgently needed. Therefore, in this study, we aimed to determine the association between consumption of some food groups with APO risks.
Methods
This is part of a larger study that aimed to investigate dietary patterns during pregnancy and several outcomes. Detailed information about the larger study and the methods applied are reported elsewhere24. Briefly, the study employed a prospective cohort follow-up study design enrolling 374 pregnant women from their initial antenatal care (ANC). All mothers were then followed monthly (for a total of four visits) from enrollment to delivery.
The study was conducted in eight health centers randomly selected from four rural districts of Oromia Regional State, Ethiopia. The four rural districts were selected purposively to represent all agro climatic areas and socio-cultural aspects of the zone. Pregnant women were enrolled to the study according to their exposure to diversify (exposed) and nondiversified (unexposed) diets.
We used the following criteria to include mothers to the study: permanent residence (weather she lived at least for 6 months in the area or not), apparently healthy (having no known medical, surgical, or obstetric problems), and mother willing to attend the monthly ANC visits.
We collected socio-economic data including: age, educational status, land ownership and others using questionnaire-based and face-to-face interviews. Midwives in each health center collected anthropometric data during enrollment and follow-up ANC visit. Weight was measured to the nearest 100 g using electronic scales (Salter Brecknell) with a weighing capacity of 10–140 kg following the standardized procedures recommended by World Medical Association25 and World Health Organization26. Portable devices equipped with calibrated and standardized height gauges (SECA 206 Body meter) were used to measure the height of mothers to the nearest mm during each visit. Using a nonstretch measuring, midwives also measured the Mid-upper arm circumference (MUAC).
Women dietary diversity scores (WDDS) was collected monthly using a four 24 h dietary recall from enrollment to delivery. According to FAO, the WDDS has nine food groups that include: (i) cereals, (ii) roots and tubers; (iii) vitamin A rich fruits and vegetables; (iv) other fruit and vegetables; (v) legumes and nuts; (vi) meat, poultry, and fish; (vii) fats and oils; (viii) dairy; and (ix) eggs.
A woman was considered to be either in the diversified (“consumers”) or a nondiversified (“nonconsumers”) group, based on data computed from the WDDS. Accordingly, if a woman completed the four monthly visits and remained in same group for at least three of the four visits, she was considered to be part of the group. Women who missed a visit or changed dietary diversity group more than once, were excluded from the analysis as shown in Fig. 1.
Fig. 1.
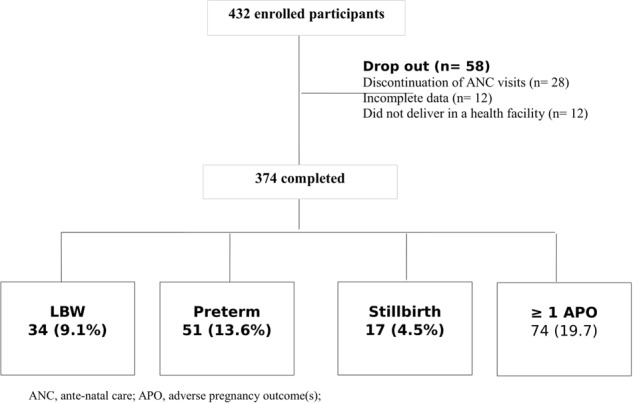
Study participant flowchart— prospective cohort of pregnant mothers, in rural Arsi, Central Ethiopia
Outcome assessment
The midwives were trained on how to take hemoglobin levels using a portable HemoCue (AB Leo Diagnostics, Helsinborg, Sweden). Accordingly, hemoglobin levels were taken twice at enrollment and at term for every women. The readings were adjusted for altitude20, and women whose altitude adjusted hemoglobin values below 11.0 g/dl were considered to be anemic26. They also recorded birth weight to the nearest 100 g, and assessed stillbirth (a baby born with no signs of life at birth after 24 completed weeks of gestation) as well as PTB (delivery < 37 weeks of gestation), immediately after delivery. The midwives have also estimated gestational age of pregnancy, by combing data from the women’s report of last menstrual period and performing fundal palpation27.
Statistical analyses
Data entry and cleaning was conducted using Epi-data and SPSS (version 20.0) statistical software (3.1). Kolmogorov–Smirnov test was used to test normality of continuous variables. A log-binomial model was run to adjust baseline group differences of key variables including MUAC, educational level, hemoglobin, age, height, and gestational age.
We reported Adjusted ORs with 95% CI values for regression models run. Differences with p values < 0.05 were considered statistically significant. A bivariate and multivariate logistic regression analyses were to test the association of food groups consumed and APOs.
Furthermore, false discovery rate adjustment was performed using the Benjamini–Hochberg (B–H) procedure to reduce the inflation of type 1 error due to multiple comparisons. The following formula was used to calculate B–H critical values for each p value:
Benjamini–Hochberg (B–H) = (i/m)Q; where,
i = the individual p value’s rank, m = total number of tests, and Q = the false discovery rate (set at 15%).
We then compared our original p values to the critical B–H values, and all p values were < 0.05, with the highest value obtained for dark green leafy vegetables (p = 0.043).
Results
We enrolled a total of 432 (216 from each group) eligible pregnant women who fulfilled the inclusion criteria. Amongst of these, 374 completed the study. This yielded an overall dropout rate of 13.4%, which was balanced across groups. The main reasons for dropout were: discontinuation of ANC visits (n = 28), shift from study group (n = 12), and delivery at home or away from index health facility (n = 18) (Fig. 1).
Close to 1 in every 5 (19.8%) women, of those who remained in the study, experienced at least 1 of the APOs. As such, 51 (13.6%) gave birth to premature baby, 34 (9.1%) had a LBW baby, and 17 (4.5%) had a stillbirth babies who died at, or immediately after birth, Fig. 1.
Table 1 presents selected socio-demographic, nutritional and anthropometric characteristics of pregnant women by their APO experience. Likewise, only close to half (n = 159; 42.5%) of the women completed primary education, and majority (n = 147; 39.3%) were in the early 20s20–24 by age category, and 241 (65.1%) had ≥4 ANC visits. A sizable proportion 166(44.9%) of women owned two or more hectares of land. Hematologic investigations showed that more than two-third (71.4 % at enrollment and 67.9 % at term) were nonanemic.
Table 1.
Selected socio-demographic, anthropometric, and nutritional characteristics of pregnant women in rural Ethiopia, stratified by adverse pregnancy outcomes (n = 374)
| Selected characteristics | All (n = 374) | LBW (n = 34) | Preterm (n = 51) | Stillbirth (n = 17) | APO (n = 74) |
|---|---|---|---|---|---|
| Maternal age (y) | |||||
| <20 | 34 (9.1) | 1 (2.9) | 1 (2) | 0 | 2(2.7) |
| 20–24 | 147 (39.3) | 12 (35.5) | 13 (25.5) | 2 (11.8) | 23 (31.1) |
| 25–29 | 130 (34.8) | 15 (44.1) | 27 (52.9) | 8 (47.1) | 33 (44.6) |
| ≥30 | 63 (16.8) | 6 (17.6) | 10 (19.6) | 7 (41.2) | 16 (21.6) |
| Ethnicity | |||||
| Oromo | 301 (80.5) | 27 (79.4) | 33 (64.7) | 13 (76.5) | 53 (71.6) |
| Amhara | 52 (13.9) | 5 (14.7) | 10 (19.6) | 2 (11.8) | 11 (14.9) |
| Guraghe | 17 (4.5) | 2 (5.9) | 8 (15.7) | 29 (11.8) | 10 (13.5) |
| Other | 4 (1.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Educational status | |||||
| Unable to read and write | 120 (32.1) | 7 (20.6) | 18 (35.3) | 8 (47.1) | 25 (33.8) |
| Read and write only | 95 (25.4) | 17 (50) | 16 (31.4) | 5 (29.1) | 26 (35.1) |
| Primary education | 31 (8.3) | 2 (5.9) | 7 (13.7) | 2 (11.8) | 7 (9.5) |
| Secondary or above | 128 (34.2) | 8 (23.5) | 6 (11.7) | 2 (11.8) | 16 (21.6) |
| Land size (hectare) | |||||
| <One | 166 (44.9) | 8 (23.5) | 27 (52.9) | 9 (52.9) | 30 (40.5) |
| One–two | 136 (36.4) | 19 (55.9) | 18 (35.3) | 4 (23.5) | 33 (44.6) |
| >Two | 70 (18.7) | 7 (20.6) | 6 (11.8) | 4 (23.5) | 11(14.9) |
| Hemoglobin (Baseline) | |||||
| Anemic (Hb < 110 g/l) | 107 (28.6) | 22 (64.7) | 28 (54.9) | 8 (47.1) | 39 (52.6) |
| Nonanemic | 267 (71.4) | 12 (64.3) | 23 (45.1) | 9 (52.9) | 35 (47.3) |
| Hemoglobin (term) | |||||
| Anemic (Hb < 110 g/l) | 121 (32.1) | 27 (79.4) | 33 (64.7) | 11 (64.7) | 48 (64.8) |
| Nonanemic | 253 (67.9) | 7 (20.6) | 18 (35.3) | 6 (35.3) | 26 (55.2) |
| MUAC (cm) | |||||
| Malnourished (<23) | 173 (46.3) | 26 (76.5) | 35 (68.6) | 11 (64.7) | 49 (66.2) |
| Well-nourished (≥23) | 201 (53.9) | 8 (23.5) | 16 (31.4) | 6 (35.3) | 25 (33.8) |
MUAC mid-upper arm circumference
Bivariate analyses results showed that pregnant women who had poor consumption of dark green leafy vegetables, vitamin A-rich foods, other fruits and vegetables, milk and milk products, experienced a higher risk of APO, compared to those women who reported better consumption. Nevertheless, dark green leafy vegetables and dairy products remained to be independent predictors of APO risks in the final multivariate logistic regression analysis, Table 2. As such, pregnant women with poor consumption of dark green leafy vegetables had a 92% (AOR = 1.92; 95% CI: 1.04–3.58) added risk of experiencing at least one of the APO relative to consumers. Similarly, those pregnant women who had poor consumption of dairy products were >3× more (AOR = 3.35; 95% CI: 1.48–7.60) at risk of APO compared to those who consumed dairy products more regularly (Table 2).
Table 2.
Association between consumption of specific food groups with adverse pregnancy outcomes in Central Arsi, rural Ethiopia (n = 374)a
| Food groups consumed | Number (%) | Adverse pregnancy outcome (APO) | |||
|---|---|---|---|---|---|
| COR (95% CI) | AOR (95% CI) | p Values | (i/m)Qb | ||
| All types of fruits and vegetables | |||||
| Yes | 212 (56.7) | 1 | 1 | 0.010 | 0.015 |
| No | 162 (43.3) | 3.24 (1.89, 5.54)b | 2.92 (1.49, 5.67)b | ||
| Milk and products | |||||
| Yes | 113 (30.2) | 1 | 1 | 0.023 | 0.030 |
| No | 261 (69.8) | 3.35 (1.65, 6.79)b | 2.64 (1.11, 6.30)b | ||
| Dark green leafy vegetables | |||||
| Yes | 146 (39) | 1 | 1 | 0.043 | 0.045 |
| No | 228 (61) | 2.31 (1.29, 4.31)b | 2.01 (1.04, 3.87)b | ||
| Starchy staples | |||||
| Yes | 374 (100) | – | – | ||
| No | 0 (0) | – | – | ||
| Meat and fish | |||||
| Yes | 17 (4.5) | 1 | 1 | 0.054 | 0.060 |
| No | 357 (95.5) | 1.89 (0.42, 8.47) | 0.50 (0.07, 3.84) | ||
| Organ meat | |||||
| Yes | 23 (6.1) | 1 | 1 | 0.067 | 0.075 |
| No | 351 (93.9) | 2.71 (0.62, 11.82) | 1.21 (0.20, 7.27) | ||
| Vitamin A rich foods | |||||
| Yes | 103 (27.5) | 1 | 1 | 0.112 | 0.090 |
| No | 271 (72.5) | 2.01 (1.05, 3.84)b | 1.23 (0.57, 2.65) | ||
| Other fruits and vegetables | |||||
| Yes | 83 (22.2) | 1 | 1 | 0.230 | 0.105 |
| No | 291 (77.8) | 2.75 (1.26, 5.99)b | 1.48 (0.57, 3.84) | ||
| Legumes | |||||
| Yes | 333 (89) | 1 | 1 | 0.340 | 0.120 |
| No | 41 (11) | 1.16 (0.53, 2.55) | 1.39 (0.56, 3.49) | ||
| Eggs | |||||
| Yes | 71 (19) | 1 | 1 | 0.551 | 0.135 |
| No | 303 (81) | 1.88 (0.89, 3.99) | 0.59 (0.21, 1.66) | ||
| Animal source foods (ASF) c | |||||
| Yes | 141 (37.7) | 1 | 1 | 0.643 | 0.150 |
| No | 233 (62.3) | 2.85 (1.54, 5.25)b | 1.46 (0.69, 3.06) | ||
aLogistic regression adjusted for baseline MUAC and hemoglobin concentrations; APO, adverse pregnancy outcomes
bBenjamini–Hochberg critical value *P < 0.05
cAll ASF (meat, dairy, eggs, and fish) are included
Among maternal nutritional characteristics run the multivariate logistic regression analysis, only anemia at term was found to be independently associated with higher APO risks. Accordingly, nonanemic women whose altitude adjusted hemoglobin level was higher than 11 mg/dl at term had a 77% reduced risk (AOR = 0.23; 95% CI: 0.11–0.45) of experiencing APO compared to those who were anemic (Table 3).
Table 3.
Association between anthropometric measures and hemoglobin concentration with adverse pregnancy outcomes in central Arsi, rural Ethiopia
| Nutritional Characteristics | Number (%) | Adverse Pregnancy Outcome (APO) | |
|---|---|---|---|
| COR(95% CI) | AOR (95% CI) | ||
| Weight gained (kg) | |||
| <6 | 33 (8.8) | 1 | 1 |
| 6–9 | 155 (41.4) | 0.98 (0.41, 2.37) | 1.23 (0.46, 3.32) |
| 9.1–12.5 | 147 (39.3) | 1.64 (0.66, 4.07) | 1.78 (0.64, 4.99) |
| >12.5 | 39 (10.4) | 2.80 (0.76, 10.37) | 2.60 (0.63, 10.78) |
| MUAC (cm) | |||
| <21 | 90 (24.1) | 1 | 1 |
| 21–23 | 171 (45.7) | 0.31 (0.15, 0.64) | 1.39 (0.72, 2.70) |
| 23+ | 113 (30.2) | 0.61 (0.31, 1.21) | 1.72 (0.76, 3.86) |
| Hemoglobin (g/dl) - at term | |||
| <11 | 121 (32.4) | 1 | 1 |
| ≥11 | 253 (67.6) | 0.17 (0.10, 0.30) | 4.56 (2.25, 9.25)a* |
alogistic regression adjusting for baseline hemoglobin concentrations; *P < 0.05
Discussion
We employed a prospective cohort design to examine the associations between dietary patterns during pregnancy with APO risks in a resource-limited setting of rural central Ethiopia. Anemia at term and poor consumption of dairy, fruits, and vegetables (dark green leafy vegetables) were associated with higher risk of experiencing at least one of the APOs: LBW, preterm, and stillbirth.
Although 60% of preterm births6, 98% of stillbirths5 and 96.5% of LBW infants28 occur in low-income settings, mainly in Africa and South Asia, there is a critical shortage of evidence investigating the association of APO with dietary patterns during pregnancy. Most of the existing studies in Ethiopia are cross-sectional7–9; and hence, ill-fitted to relate outcomes with dietary patterns. However, another study in Ethiopia showed that dietary diversity ≥4 (out of 9 food groups) has been correlated with lower risk of maternal anemia, preterm birth, and low birth weight babies24, but which food groups are associated with reduced risk remained unknown.
In the present analysis, our multivariate analysis suggests that poor intake of dairy and dark green leafy vegetables during pregnancy were found to be independent predictors of higher APO risks. Pregnant women who had poor consumption of dairy, fruits and vegetables had nearly two to four fold increased risks of APO, compared to those who consumed either of these food items. This is in line with the Iranian29, Spanish30, and Indian31 prospective studies that enrolled and followed pregnant women all showing reduced risk of APOs with increasing consumption of dairy, green leafy vegetables, and fruits.
Many factors can explain the role of dairy consumption in reducing the risk of APO. First, dairy is a good source of essential nutrients, including calcium. The role of calcium in preventing preeclampsia, a leading contributor to APO, is well-documented32. Second, proteins and growth hormones provided by dairy can support fetal growth and thus may increase birth weight. Beyond its nutritional contribution, milk can also promote anabolism and serve as an endocrine signaling system for postnatal growth by activating the nutrient-sensitive kinase mTORC133,34, thus increasing gestational age, placental, and fetal weight. On the other hand, fruits and vegetables have been found to have a protective role against APO. Perhaps, this role can be conferred by micronutrients and antioxidants found in these foods, which contribute to optimal immune and placental functions, and besides contribute to fetal growth35,36
The study had limitations that require curiosity and consideration when interpreting the findings. Firstly, unlike preconception nutrition is associated with pregnancy outcomes, we were only able to follow the women starting from their second trimester, mainly related to maternal habit of late ANC onset in the country7. Secondly, in spite of our efforts to adjust for baseline differences computing APOs risks, the effect of hidden factors in the model may not be completely controlled. Our study was not powered enough to evaluate associations of consumption of each food groups with individual APOs. Besides, given that this is a health-facility based study, our sampling might be subject to favoring women with better access to health facilities.
Notwithstanding the above limitations, our study highlights that consistent consumption of dairy products, fruits, and vegetables (dark green leafy vegetables) were found to be independently associated with APO risks. While community-based trials and mechanistic studies are needed to establish causal relationships, promotions of the consumption of these food groups in Ethiopia and other similar settings may be beneficial.
Acknowledgments
We would like to acknowledge graduate school of Addis Ababa University, Center for Food Science and Nutrition, the African Population and Health Research Center and the International Development Research Center for financing the project. We also thank the Oromia Regional Health Bureau and the Arsi Zone Health Office.
Source of support
This research was supported by the graduate program of the Addis Ababa University. The donors had only a funding role, and thus were not involved in any of the research design, execution, and interpretation.
Author’s contribution
T.A.Z. and E.P. designed research; T.A.Z. and K.B. conducted research; T.A.Z. and K.B. analyzed data; T.A.Z. and K.B. wrote the paper and E.P. reviewed all versions; T.A.Z. had primary responsibility for final content. All authors read and approved the final manuscript
Dedication
This work is dedicated to the late Dr. Melaku Umeta for his guidance and involvement in the study as an advisor.
Ethical consideration
After a detailed and face to face explanation of the purpose and methods of the study to all women, informed verbal consent was obtained from in the presence of local administrators. All the study procedures were performed in accordance with the Helsinki Declaration25. The study protocol was approved by the institutional review boards of the College of Natural Sciences, Addis Ababa University and ethics review committee of Oromia Regional Health Bureau.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Taddese A. Zerfu, Phone: +251911062646, Phone: +254799748589, Email: tadalzerfu@gmail.com
Kaleab Baye, Email: kaleabbaye@gmail.com.
References
- 1.World Health Organization. Countdown to 2015. Fulfilling the Health Agenda for Women and Children: Rwanda Country Profile. 2014.
- 2.You D, et al. Global, regional, and national levels and trends in under-5 mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet. 2015;386:2275–2286. doi: 10.1016/S0140-6736(15)00120-8. [DOI] [PubMed] [Google Scholar]
- 3.You D., Hug L., Ejdemyr S., Beise J. Levels and trends in child mortality. Report 2015. Estimates developed by the UN Inter-agency Group for Child Mortality Estimation.
- 4.Liu L, et al. Global, regional, and national causes of under 5 mortality in 2000–2015: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blencowe H, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. The Lancet Glob. Health. 2016;4:e98–e108. doi: 10.1016/S2214-109X(15)00275-2. [DOI] [PubMed] [Google Scholar]
- 6.Blencowe H, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod. Health. 2013;10:S2. doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CSA I. Ethiopia Demographic and Health Survey. (Central Statistics Agency and ICF international: USA, Addis Ababa, Ethiopia and Calverton, Maryland, 2011). 2012.
- 8.Adane AA, Ayele TA, Ararsa LG, Bitew BD, Zeleke BM. Adverse birth outcomes among deliveries at Gondar University hospital, Northwest Ethiopia. BMC Pregnancy Childbirth. 2014;14:90. doi: 10.1186/1471-2393-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assefa N, Berhane Y, Worku A. Wealth status, mid-upper arm circumference (MUAC) and antenatal care (ANC) are determinants for low birth weight in Kersa, Ethiopia. PLoS One. 2012;7:e39957. doi: 10.1371/journal.pone.0039957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi BH, et al. Predictors of stillbirth in sub-saharan Africa. Obstet. Gynecol. 2007;110:989–997. doi: 10.1097/01.AOG.0000281667.35113.a5. [DOI] [PubMed] [Google Scholar]
- 11.Stein RE, Siegel MJ, Bauman LJ. Are children of moderately low birth weight at increased risk for poor health? A new look at an old question. Pediatrics. 2006;118:217–223. doi: 10.1542/peds.2005-2836. [DOI] [PubMed] [Google Scholar]
- 12.Yuan B, et al. What interventions are effective on reducing inequalities in maternal and child health in low- and middle-income settings? A systematic review. BMC Public Health. 2014;14:634. doi: 10.1186/1471-2458-14-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan AI. Effects of pre- and postnatal nutrition interventions on child growth and body composition: the MINIMat trial in rural Bangladesh. Glob. Health Action. 2013;6:22476. doi: 10.3402/gha.v6i0.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu-Saad K, Fraser D. Maternal nutrition and birth outcomes. Epidemiol. Rev. 2010;32:5–25. doi: 10.1093/epirev/mxq001. [DOI] [PubMed] [Google Scholar]
- 15.Lawn JE, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016;387:587–603. doi: 10.1016/S0140-6736(15)00837-5. [DOI] [PubMed] [Google Scholar]
- 16.Mikkelsen TB, et al. Association between a Mediterranean‐type diet and risk of preterm birth among Danish women: a prospective cohort study. Acta Obstet. Gynecol. Scand. 2008;87:325–330. doi: 10.1080/00016340801899347. [DOI] [PubMed] [Google Scholar]
- 17.Englund-Ogge L, et al. Maternal dietary patterns and preterm delivery: results from large prospective cohort study. Br. Med. J. 2014;348:g1446. doi: 10.1136/bmj.g1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen MA, Maslova E, Halldorsson TI, Olsen SF. Characterization of dietary patterns in the Danish national birth cohort in relation to preterm birth. PLoS One. 2014;9:e93644. doi: 10.1371/journal.pone.0093644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grieger JA, Grzeskowiak LE, Clifton VL. Preconception dietary patterns in human pregnancies are associated with preterm delivery. J. Nutr. 2014;144:1075–1080. doi: 10.3945/jn.114.190686. [DOI] [PubMed] [Google Scholar]
- 20.Thompson JM, et al. Maternal dietary patterns in pregnancy and the association with small-for-gestational-age infants. Br. J. Nutr. 2010;103:1665–1673. doi: 10.1017/S0007114509993606. [DOI] [PubMed] [Google Scholar]
- 21.Coelho NDLP, Cunha DB, Esteves AP, Lacerda EM, ThemeFilha MM. Dietary patterns in pregnancy and birth weight. Rev. Saude Publ. 2015;49:62. doi: 10.1590/S0034-8910.2015049005403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haugen M, et al. Mediterranean-type diet and risk of preterm birth among women in the Norwegian Mother and Child Cohort Study (MoBa): a prospective cohort study. Acta Obstet. Gynecol. Scand. 2008;87:319–324. doi: 10.1080/00016340801899123. [DOI] [PubMed] [Google Scholar]
- 23.Saunders L, et al. Effect of a mediterranean diet during pregnancy on fetal growth and preterm delivery: results from a French Caribbean Mother–Child Cohort Study (TIMOUN) Paediatr. Perinat. Epidemiol. 2014;28:235–244. doi: 10.1111/ppe.12113. [DOI] [PubMed] [Google Scholar]
- 24.Zerfu TA, Umeta M, Baye K. Dietary diversity during pregnancy is associated with reduced risk of maternal anemia, preterm delivery, and low birth weight in a prospective cohort study, in rural Ethiopia. Am. J. Clin. Nutr. 2016;103:1193–1194. doi: 10.3945/ajcn.115.116798. [DOI] [PubMed] [Google Scholar]
- 25.WMA General Assembly. World Medical Asssociation Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. (World Medical Association, 2013).
- 26.WHO. Iron Deficiency Anaemia: Assessment, Prevention, and Control: A Guide for Programme Managers. (World Health Organization, 2001).
- 27.White LJ, et al. Estimation of gestational age from fundal height: a solution for resource-poor settings. J. R. Soc. Interface. 2012;9:503–510. doi: 10.1098/rsif.2011.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Black R. E. Global prevalence of small for gestational age births. In Low-Birth weight Baby: Born Too Soon or Too Small. Vol. 81, pp. 1–7. (Karger Publishers, 2005). [DOI] [PubMed]
- 29.Akbari Z, Mansourian M, Kelishadi R. Relationship of the intake of different food groups by pregnant mothers with the birth weight and gestational age: Need for public and individual educational programs. J. Educ. Health Promot.4, 23 (2015). 10.4103/2277-9531.154109. [DOI] [PMC free article] [PubMed]
- 30.Hrolfsdottir L, et al. Maternal diet, gestational weight gain, and inflammatory markers during pregnancy. Obesity. 2016;24:2133–2139. doi: 10.1002/oby.21617. [DOI] [PubMed] [Google Scholar]
- 31.Rao S, et al. Intake of micronutrient-rich foods in rural Indian mothers is associated with the size of their babies at birth: Pune Maternal Nutrition Study. J. Nutr. 2001;131:1217–1224. doi: 10.1093/jn/131.4.1217. [DOI] [PubMed] [Google Scholar]
- 32.Omotayo MO, et al. Calcium supplementation to prevent preeclampsia: translating guidelines into practice in low-income countries. Adv. Nutr. 2016;7:275–278. doi: 10.3945/an.115.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melnik BC, John SM, Schmitz G. Milk is not just food but most likely a genetic transfection system activating mTORC1 for postnatal growth. Nutr. J. 2013;12:103. doi: 10.1186/1475-2891-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melnik BC, John SM, Schmitz G. Milk consumption during pregnancy increases birth weight, a risk factor for the development of diseases of civilization. J. Transl. Med. 2015;13:13. doi: 10.1186/s12967-014-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramón R, et al. Vegetable but not fruit intake during pregnancy is associated with newborn anthropometric measures. J. Nutr. 2009;139:561–567. doi: 10.3945/jn.108.095596. [DOI] [PubMed] [Google Scholar]
- 36.Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv. Nutr. 2012;3:506–516. doi: 10.3945/an.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]


