Abstract
Objective
Although widely recommended, Lynch syndrome (LS) testing with tumor microsatellite instability (MSI) and/or immunohistochemistry (IHC) is infrequently performed in early-onset colorectal cancer (CRC), and CRC generally. Reasons are poorly understood. Hence, we conducted a national survey focusing on gastroenterologists, as they are frequently first to diagnose CRC, assessing testing barriers and which specialist is felt responsible for ordering MSI/IHC. Additionally, we assessed factors influencing timing of MSI/IHC ordering; testing on colonoscopy biopsy, opposed to post-operative surgical specimens, assists decisions on preoperative germline genetic testing and extent of colonic resection (ECR).
Methods
A 21-question web-based survey was distributed through an American College of Gastroenterology email listing.
Results
In total 509 completed the survey. 442 confirmed gastroenterologists were analyzed. Only 33.4% felt gastroenterologists were responsible for MSI/IHC ordering; pathologists were believed most responsible (38.6%). Cost, unfamiliarity interpreting results and unavailable genetic counseling most commonly prevented routine ordering (33.3%, 29.2%, 24.9%, respectively). In multivariable analysis, non-academic and rural settings were associated with cost and genetic counseling barriers. Only 46.1% felt MSI/IHC should always be performed on colonoscopy biopsy. Guideline familiarity predicted whether respondents felt surgical resection should be delayed until results returned given potential effect on ECR decisions.
Conclusion
Inconsistencies in who is felt should order MSI/IHC may lead to diffusion of responsibility, preventing consistent testing, including preoperatively. Assuring institutional universal testing protocols are in place, with focus on timing of testing, can optimize care. Strategies addressing cost barriers and genomic service availability in rural and non-academic settings can enhance testing. Greater emphasis on guideline familiarity is required.
Introduction
CRC is the third most common cancer in men and women1. It is the 3rd leading cause of cancer death in women and 2nd in men. Up to 4% of CRCs are attributed to Lynch Syndrome (LS), with penetrance for CRC development as high as 82%2,3. In early-onset cases, LS can be common with up to 17% unselected for family history being diagnosed4–7.
Guidelines recommend testing all CRCs, regardless of patient age, for microsatellite instability (MSI) and/or with immunohistochemistry (IHC) for mismatch repair proteins (MLH1, MSH2, MSH6, PMS2) as initial screen for LS2,8–10. Ideally, results should be available preoperatively to help facilitate surgical planning11. Studies demonstrate IHC on preoperative colonoscopic biopsy specimens correlates well with surgical resection specimens12,13. Abnormal MSI/IHC prompts germline genetic testing to identify a mutation, confirming LS.
LS identification is important as surveillance colonoscopy decreases mortality14. Additionally, extra-colonic cancer screening is performed2. Preoperative LS identification affects operative decisions regarding extended colonic resection (ECR). Observational studies demonstrate ECR (subtotal/total colectomy) decreases metachronous cancer development and can increase life expectancy, particularly in younger patients15–17. One study demonstrated with segmental resection, metachronous rates may be 16% at 10 years, 41% at 20 years and 62% at 30 years post-operatively, even with frequent post-operative colonoscopic surveillance, versus 0% in extended colectomy15. Hence, guidelines recommend ECR for cancer risk reduction18,19. Finally, LS identification guides germline testing in family members.
Despite benefits, MSI/IHC is infrequently performed. In a statewide population-based study of early-onset CRC patients, MSI and/or IHC was only performed in 23%20. Of those tested, results were available preoperatively in only 16.9%. This was felt to contribute to low subtotal/total colectomy rates, even with abnormal MSI and/or IHC, in this same early-onset population21. Low mismatch repair deficiency testing was recently demonstrated nationally with only 28.2% of CRC patients overall and 43.1% of early-onset patients tested22. Reasons for inadequate testing have not been well studied, but potentially include decreased understanding of testing importance, effect on management, unclear responsibility regarding who should order testing (gastroenterologist, pathologist, etc.) and lack of specialists (i.e., genetic counselors) to assist with testing/interpretation.
To explore LS testing practices and barriers, we conducted a national survey of physicians in 2017, the overwhelming majority of whom were gastroenterologists. Gastroenterologists are important as they frequently first diagnose CRC and can facilitate preoperative LS identification by helping assure MSI/IHC is performed on colonoscopy biopsy specimens. Although testing is recommended in patients of all ages, the survey focuses on early-onset CRC patients as they are often at highest risk of hereditary syndromes. Hence studying testing practices in this group may provide a best-case management scenario.
We asked the following questions.
1) Which specialist in the cancer care continuum do study participants feel is responsible for ordering MSI/IHC?
2) What barriers prevent ordering MSI/IHC?
3) What factors affect preoperative result availability?
4) In LS patients, what operative approach, ECR vs. segmental resection, is felt should be taken?
Methods
Survey overview
The Tulane Institutional Review Board approved this study. Study investigators with expertise in hereditary cancer syndromes developed survey questions (Supplemental Fig. 1). An opportunity to enter a $25 gift card raffle was given. Web-based survey items were presented in scenario/non-scenario formats using Qualtrics software.
The survey was distributed March 2017 through an American College of Gastroenterology (ACG) email listing. Reminders were sent 2 weeks later. Emails came from the ACG directly, including content explanation and embedded URL survey links. Submissions closed 4 weeks after the 2nd email. Given that some ACG members may be non-gastroenterologists, specific questions were also developed for surgeons, pathologists and other providers. However, given low responses from non-gastroenterologists, they were ultimately excluded from analysis.
Statistical analysis
Frequency tables were created for demographics and responses to decision-making questions. 10-year career stage intervals were chosen based on board certification/recertification intervals. For questions with multiple responses, comparisons were made using meaningfully pooled data. Multivariable logistic regression was employed to assess the association of responses with demographic factors. Chi-squared analysis was utilized. Odds ratios were reported with 95% confidence intervals. Analyses were performed using SAS 9.4 with p-values of 0.05 considered significant.
Results
Response rate
The survey link was distributed to 11,924 email addresses. Emails were opened, confirming receipt, in 4491 instances with links followed in 320 instances. Reminders were distributed 2 weeks later, with 3,508 opening it and 183 following the link. There were 5786 unique confirmed email recipients (2213 opened both the first and 2nd emails) yielding an overall response rate of 8.7%. Confirmed email receipts were used to calculate response rate versus all distribution emails as many accounts have potential to be out-of-date, infrequently used or members may potentially have multiple email addresses listed. The response rate was 4.2% if using the entire email list. Response rates to individual questions/items were >80% in all cases (80.6–97.7%).
In total 509 participants took the survey. 6 of 509 accessed surveys without receiving the ACG email directly. In total 505 answered whether they were a medical student or resident. 31 students/residents, 13 non-gastroenterologists and 19 respondents who never indicated their medical specialty were excluded (442 respondents analyzed).
Participant demographics
71.3% identified as general gastroenterologists, followed by advanced endoscopy and other subspecialties (Table 1). 85.6% practiced in urban counties. The most common practice settings were “single-specialty private practice” and “university/academic” (40.6%, 31.1% respectively). 52.0% were in practice >10 years, 33.9% between 0 and 10 years and 14.2% were in fellowship.
Table 1.
Demographics of survey respondents (N = 442)
| Demographic variable | Frequency | Percentage |
|---|---|---|
| Gastroenterology subspecialty | ||
| General GI | 308 | 71.3 |
| GI Oncologya | 19 | 4.4 |
| Hepatology | 20 | 4.6 |
| Inflammatory Bowel Disease (IBD) | 35 | 8.1 |
| Functional/Motility | 5 | 1.2 |
| Advanced Endoscopy | 45 | 10.4 |
| Urban/rural locationb | ||
| Urban | 374 | 85.6 |
| Rural | 63 | 14.4 |
| Practice setting | ||
| Multispeciality Private Practice | 57 | 13.0 |
| Hospital Employed | 52 | 11.9 |
| Single Specialty Private Practice | 178 | 40.6 |
| University/Academic Center | 136 | 31.1 |
| Veterans Affairs | 15 | 3.4 |
| Career Stage | ||
| Fellows in training | 61 | 14.2 |
| In practice from 0 to 10 years | 146 | 33.9 |
| In practice from 11 + years | 224 | 52.0 |
aGI Oncology was presented as a sub specialization to those respondents who previously identified as gastroenterologists to avoid confusion with medical oncologists
bThis study defines rural versus urban areas based upon the USDA’s 2013 “Rural-Urban Continuum Codes,” a classification scheme that distinguishes metro counties by population size and non-metro, or rural, areas by degree of urbanization and adjacency to metro areas. Survey takers were provided access to the continuum coded spreadsheet with instructions to help define the county in which they practiced
Specialist felt responsible for ordering MSI/IHC
No consensus exists among gastroenterologists regarding which specialist they feel should be responsible for ordering MSI/IHC (Fig. 1). Pathologists were believed most responsible (38.6%) followed by gastroenterologists (33.4%). Medical oncologists, surgeons and geneticists were felt to be responsible less frequently.
Fig. 1. Healthcare provider believed to be responsible for ordering MSI/IHC testing to screen for Lynch Syndrome in newly diagnosed CRC under the age of 50.
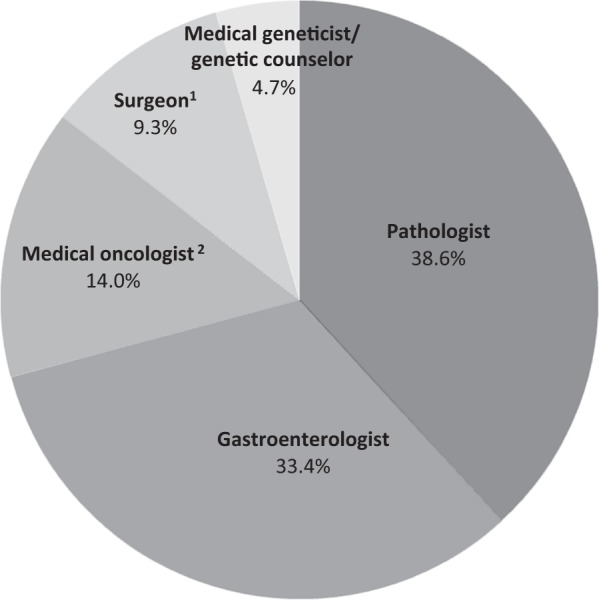
1For the purposes of this study, “Surgeon” refers to Colorectal Surgeon, General Surgeon, or Surgical Oncologist. 2“Medical Oncologist” refers to those physicians who have completed an Internal Medicine residency followed by a Hematology/Oncology Fellowship. Gastroenterologists who specialize in genetics and GI cancers are included above in the “Gastroenterologist” category
Barriers to MSI/IHC test ordering
Cost, unfamiliarity interpreting results and unavailable genetic counseling were the most common reasons preventing testing, Table 2 (22.4%, 18.5%, 15.9%, respectively). In multivariable analysis, non-academic or rural settings and being a GI fellow were associated with a higher perception of deficiencies in access to genetic counseling and germline testing and belief MSI/IHC cost was prohibitive. GI fellows, those in early-career stages and those in non-academic settings more commonly believed waiting for MSI/IHC results would delay colon resection and therefore negatively impact cancer outcomes, thus preventing initial ordering. Those in non-academic settings also felt waiting for germline testing results (if MSI/IHC were abnormal) would delay colonic resection and negatively impact outcomes.
Table 2.
Barriers to ordering MSI/IHC testing, multivariable analysis
| Barriers to MSI/IHC Testing Ordering (Number of respondents indicating this as a barrier/total respondents answering this question; percentage) | Variables | Number of respondents answering yes (Percent respondents) | Odds ratio (95% Confidence interval) |
|---|---|---|---|
| Cost of MSI and/or IHC testing is prohibitive (85/379; 22.4%) | Gastroenterology subspecialty | ||
| General GI | 58 (21.5) | 1.00 | |
| Non-general GI | 27 (25.7) | 1.96 (1.07, 3.60) | |
| Urban/rural location | |||
| Urban | 65 (20.5) | 1.00 | |
| Rural | 20 (33.9) | 1.95 (1.01, 3.79) | |
| Practice Setting | |||
| Academic Centera | 19 (17.9) | 1.00 | |
| Non-academic Center | 66 (24.5) | 2.55 (1.24, 5.23) | |
| Career Stage | |||
| Fellows in training | 14 (29.8) | 2.97 (1.27, 6.93) | |
| In practice from 0 to 10 years | 34 (26.6) | 1.82 (1.02, 3.23) | |
| In practice from 11+ years | 36 (17.9) | 1.00 | |
| Familiarity with the guideline | |||
| Familiar | 29 (17.9) | 1.00 | |
| Unsureb | 25 (20.8) | 1.29 (0.70, 2.39) | |
| Unfamiliarc | 27 (32.9) | 2.05 (1.08, 3.88) | |
| Lack of familiarity interpreting and applying the results from MSI and/or IHC testing (70/379; 18.5%) | Gastroenterology subspecialty | ||
| General GI | 54 (20.0) | 1.00 | |
| Non-general GI | 16 (15.2) | 0.81 (0.40, 1.61) | |
| Urban/rural location | |||
| Urban | 54 (17.0) | 1.00 | |
| Rural | 15 (25.4) | 1.41 (0.69, 2.88) | |
| Practice Setting | |||
| Academic Centera | 19 (18.1) | 1.00 | |
| Non-academic Center | 51 (18.9) | 1.13 (0.54, 2.35) | |
| Career Stage | |||
| Fellows in training | 13 (27.7) | 1.99 (0.83, 4.80) | |
| In practice from 0 to 10 years | 23 (18.0) | 1.24 (0.66, 2.31) | |
| In practice from 11+years | 33 (16.4) | 1.00 | |
| Familiarity with the guideline | |||
| Familiar | 17 (10.6) | 1.00 | |
| Unsureb | 23 (18.9) | 2.04 (1.03, 4.04) | |
| Unfamiliarc | 28 (34.6) | 3.89 (1.94, 7.80) | |
| Lack of access to genetic counseling at my facility (60/378; 15.9%) | Gastroenterology subspecialty | ||
| General GI | 50 (18.5) | 1.00 | |
| Non-general GI | 10 (9.6) | 0.74 (0.33, 1.67) | |
| Urban/rural location | |||
| Urban | 42 (13.3) | 1.00 | |
| Rural | 18 (30.0) | 2.22 (1.09, 4.50) | |
| Practice Setting | |||
| Academic Centera | 6 (5.7) | 1.00 | |
| Non-academic Center | 54 (20.2) | 6.96 (2.31, 20.94) | |
| Career Stage | |||
| Fellows in training | 12 (25.5) | 5.70 (2.10, 15.51) | |
| In practice from 0 to 10 years | 17 (13.3) | 1.08 (0.54, 2.14) | |
| In practice from 11+ years | 30 (15.0) | 1.00 | |
| Familiarity with the guideline | |||
| Familiar | 20 (12.3) | 1.00 | |
| Unsureb | 19 (15.8) | 1.43 (0.70, 2.93) | |
| Unfamiliarc | 20 (25.0) | 2.16 (1.03, 4.53) | |
| Lack of access to germline genetic testing if MSI/IHC abnormal (51/381; 13.4%) | Gastroenterology (GI) subspecialty | ||
| General GI | 41 (15.1) | 1.00 | |
| Non-general GI | 10 (9.5) | 0.88 (0.39, 2.00) | |
| Urban/rural location | |||
| Urban | 35 (11.0) | 1.00 | |
| Rural | 16 (27.1) | 2.61 (1.26, 5.41) | |
| Practice Setting | |||
| Academic Centera | 7 (6.6) | 1.00 | |
| Non-academic Center | 44 (16.2) | 3.68 (1.32, 10.22) | |
| Career Stage | |||
| Fellows in training | 9 (18.8) | 3.14 (1.15, 8.63) | |
| In practice from 0 to 10 years | 18 (14.1) | 1.61 (0.80, 3.25) | |
| In practice from 11+ years | 23 (11.4) | 1.00 | |
| Familiarity with the guideline | |||
| Familiar | 17 (10.4) | 1.00 | |
| Unsureb | 17 (13.9) | 1.41 (0.67, 2.96) | |
| Unfamiliarc | 15 (18.5) | 1.60 (0.72, 3.55) | |
| Waiting for germline testing results (after initial MSI and/or IHC testing) would delay resection and therefore negatively impact the patient’s outcome (45/376; 12.0%) | Gastroenterology subspecialty | ||
| General GI | 37 (13.8) | 1.00 | |
| Non-general GI | 8 (7.7) | 0.71 (0.29, 1.75) | |
| Urban/rural location | |||
| Urban | 36 (11.4) | 1.00 | |
| Rural | 9 (15.8) | 1.16 (0.49, 2.74) | |
| Practice Setting | |||
| Academic Centera | 7 (6.7) | 1.00 | |
| Non-academic Center | 38 (14.2) | 3.91 (1.31, 11.66) | |
| Career Stage | |||
| Fellows in training | 7 (14.9) | 2.70 (0.90, 8.07) | |
| In practice from 0 to 10 years | 17 (13.3) | 1.83 (0.89, 3.75) | |
| In practice from 11+ years | 20 (10.1) | 1.00 | |
| Familiarity with the guideline | |||
| Familiar | 17 (10.6) | 1.00 | |
| Unsureb | 16 (13.3) | 1.36 (0.64, 2.86) | |
| Unfamiliarc | 10 (12.5) | 1.03 (0.43, 2.48) | |
| Waiting for MSI and/or IHC testing results would delay colon resection and therefore negatively impact the patient’s outcome (35/378; 9.3%) | Gastroenterology subspecialty | ||
| General GI | 30 (11.2) | 1.00 | |
| Non-general GI | 5 (4.8) | 0.52 (0.17, 1.61) | |
| Urban/rural location | |||
| Urban | 27 (8.5) | 1.00 | |
| Rural | 8 (13.6) | 1.36 (0.53, 3.48) | |
| Practice Setting | |||
| Academic Centera | 5 (4.7) | 1.00 | |
| Non-academic Center | 30 (11.2) | 5.38 (1.39, 20.83) | |
| Career Stage | |||
| Fellows in training | 6 (12.8) | 4.23 (1.23, 14.54) | |
| In practice from 0 to 10 years | 15 (11.7) | 2.78 (1.22, 6.34) | |
| In practice from 11+ years | 13 (6.5) | 1.00 | |
| Familiarity with the guideline | |||
| Familiar | 10 (6.2) | 1.00 | |
| Unsureb | 15 (12.5) | 2.29 (0.96, 5.45) | |
| Unfamiliarc | 8 (9.9) | 1.36 (0.48, 3.84) | |
| Ordering Testing may adversely affect a patient’s medical insurance status (35/376; 9.3%) | Gastroenterology subspecialty | ||
| General GI | 28 (10.4) | 1.00 | |
| Non-general GI | 7 (6.8) | 0.84 (0.33, 2.13) | |
| Urban/rural location | |||
| Urban | 27 (8.6) | 1.00 | |
| Rural | 8 (13.8) | 1.39 (0.55, 3.47) | |
| Practice Setting | |||
| Academic Centera | 8 (7.6) | 1.00 | |
| Non-academic Center | 27 (10.1) | 1.60 (0.58, 4.41) | |
| Career Stage | |||
| Fellows in training | 6 (12.8) | 1.76 (0.54, 5.67) | |
| In practice from 0 to 10 years | 12 (9.5) | 1.38 (0.62, 3.08) | |
| In practice from 11+ years | 16 (8.0) | 1.00 | |
| Familiarity with the guideline | |||
| Familiar | 12 (7.4) | 1.00 | |
| Unsureb | 11 (9.2) | 1.30 (0.55, 3.07) | |
| Unfamiliarc | 11 (13.8) | 1.70 (0.70, 4.18) | |
aAcademic center includes university/academic center; and non-academic center incluldes multispeciality private practice, hospital employed physician, single specialty private practice, and veterans affairs facility
bUnsure means they are not clear on whether they are aware of the applicable guidelines or not
cUnfamiliar means providers are not well versed with current guidelines
When barriers were stratified based on routine versus non-routine test ordering on colonoscopy, all became magnified (Table 3). Most common barriers in non-routine orderers included cost (33.3%), lack of familiarity interpreting/applying results (29.2%) and decreased genetic counseling access (24.9%).
Table 3.
Barriers to MSI/IHC ordering stratified by those routinely and non-routinely ordering testing on colonoscopy biopsy specimens
| Barrier to MSI/IHC test ordering | Non-routinely orderinga | Routinely orderinga | Odds ratio (95% CI) |
|---|---|---|---|
| Cost of MSI and/or IHC testing is prohibitive | 33.3% | 11.7% | 3.79 (2.19, 6.53) |
| Lack of familiarity interpreting and applying the results from MSI and/or IHC testing | 29.2% | 6.2% | 6.23 (3.15, 12.35) |
| Lack of access to genetic counseling at my facility | 24.9% | 5.6% | 5.56 (2.72, 11.39) |
| Lack of access to germline genetic testing if MSI/IHC abnormal | 20.0% | 6.2% | 3.82 (1.89, 7.72) |
| Waiting for germline testing results (after initial MSI and/or IHC testing) would delay resection and therefore negatively impact the patient’s outcome | 18.2% | 5.7% | 3.72 (1.78, 7.77) |
| Waiting for MSI and/or IHC testing results would delay colon resection and therefore negatively impact the patient’s outcome | 14.1% | 4.5% | 3.50 (1.54, 7.92) |
| Ordering testing may adversely affect a patient’s medical insurance status | 12.0% | 6.2% | 2.08 (0.98, 4.40) |
aThe definition of routinely vs. non-routinely ordering is based on the survey question of “What percentage of the time will you plan to perform MSI and/or IHC testing for LS on tumor biopsies taken during colonoscopy?” Non-routinely ordering are the respondents who answer “0%”, “25%”, “50%” or “75%” of the time, and the routinely ordering are those respondents who answer “100%” of the time
Timing of MSI and/or IHC testing
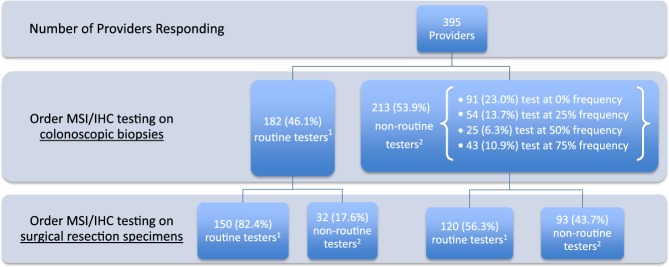
Only 46.1% felt MSI/IHC should be routinely (100% of the time) performed on colonoscopy biopsy specimens (Fig. 2). In the 53.9% that do not routinely (0–75% of the time) order MSI/IHC on colonoscopy biopsy, 43.7% also do not believe that MSI/IHC should be performed routinely on surgical resection specimens. In those routinely performing MSI/IHC on colonoscopy biopsy, 82.4% believe testing should also be performed concurrently on surgical resection specimens. Participants familiar with guidelines, in practice from 0 to 10 years, GI subspecialists (including GI oncology) and those working in academic or urban settings were most likely to believe testing should be performed on preoperative colonoscopy biopsy (Table 4).
Fig. 2. MSI/IHC ordering practices on colonoscopy biopsy and surgical resection specimens.
1“Routine tester” is defined as a provider indicating a frequency of ordering MSI/IHC testing in 100% of cases of CRC in patients < 50 years old. This definition applies to both testing performed on colonoscopic biopsies or surgical resection specimens. 2 “Non-routine tester” is defined as a provider indicating a frequency of ordering MSI/IHC testing in “0%”, “25%”, “50%” or “75%” of the time in CRC patients < 50 years old. This definition likewise applies to both testing performed on colonoscopic biopsies or surgical resection specimens
Table 4.
Analysis of subgroup opinions regarding routine performance of MSI/IHC testing on colonoscopy biopsy specimens, surgical resection specimens or both
| Performance of MSI/IHC testing | Gastroenterologist Subgroups | Number of participants who answered “100% of the time” (%) | |
|---|---|---|---|
| Will plan to perform MSI/IHC testing on PRE-SURGICAL tumor biopsies taken during colonoscopy | Gastroenterology (GI) subspecialty | ||
| General GI | 125 (43.4) | ||
| GI Oncology | 10 (55.6) | ||
| All other GI Specializations | 55 (55.6) | ||
| Urban/rural location | |||
| Urban | 172 (50.0) | ||
| Rural | 19 (31.2) | ||
| Practice setting | |||
| Academic Center | 64 (52.5) | ||
| Non-academic Center | 127 (44.9) | ||
| Career stage | |||
| Fellows in training | 25 (47.2) | ||
| In practice 0–10 years | 75 (54.7) | ||
| In practice 11+ years | 91 (42.5) | ||
| Familiarity with the guideline | |||
| Familiar | 86 (53.8) | ||
| Unsurea | 58 (46.8) | ||
| Unfamiliarb | 30 (36.1) | ||
| Expect MSI/IHC testing performed on POST-SURGICAL specimens | Gastroenterology (GI) subspecialty | ||
| General GI | 186 (65.3) | ||
| GI Oncology | 17 (89.5) | ||
| All other GI Specializations | 74 (76.3) | ||
| Urban/rural location | |||
| Urban | 240 (70.6) | ||
| Rural | 36 (59.0) | ||
| Practice setting | |||
| Academic Center | 86 (72.9) | ||
| Non-academic Center | 192 (67.6) | ||
| Career stage | |||
| Fellows in training | 32 (59.3) | ||
| In practice 0–10 years | 90 (66.2) | ||
| In practice 11+ years | 155 (73.5) | ||
| Familiarity with the guideline | |||
| Familiar | 115 (73.3) | ||
| Unsurea | 81 (67.5) | ||
| Unfamiliarb | 48 (57.1) | ||
| Plan to perform MSI/IHC testing both on colonoscopy and post-surgical specimens | Gastroenterology (GI) subspecialty | ||
| General GI | 92 (33.2) | ||
| GI Oncology | 9 (50.0) | ||
| All other GI Specializations | 46 (47.9) | ||
| Urban/rural location | |||
| Urban | 132 (39.9) | ||
| Rural | 15 (25.0) | ||
| Practice setting | |||
| Academic Center | 49 (42.2) | ||
| Non-academic Center | 100 (36.2) | ||
| Career stage | |||
| Fellows in training | 21 (40.4) | ||
| In practice 0–10 years | 51 (38.6) | ||
| In practice 11+ years | 76 (36.7) | ||
| Familiarity with the guideline | |||
| Familiar | 67 (43.5) | ||
| Unsurea | 44 (37.0) | ||
| Unfamiliarb | 22 (26.8) | ||
aUnsure means they are not clear on whether they are aware of the applicable guidelines or not
bUnfamiliar means providers are not well versed with current guidelines
Impact of MSI/IHC results on timing of surgery and extent of resection
Physicians in academic centers were 2.41 times as likely as non-academic counterparts to agree abnormal testing results (MSI/IHC and/or germline) could impact decision processes regarding ECR (Table 5). Guideline familiarity was associated with the opinion surgery should not proceed until MSI/IHC results return as this could effect decisions regarding ECR. Only GI oncology sub-specialization and urban practice were associated with guideline familiarity (Supplementary table 1).
Table 5.
Multivariable analysis of subgroup opinions relevant to the impact of MSI/IHC results on timing of surgery and extent of colonic resection
| Statement for Evaluation | Demographic variables | Respondents answering “True” (Percentage) | Odds Ratio (95% confidence interval) |
|---|---|---|---|
| “Abnormal MSI/IHC/Germline testing results can affect extent of colonic resection.” | Gastroenterology (GI) subspecialty | ||
| General GI | 175 (64.8%) | 1.00 | |
| GI Oncology Specializations | 12 (80.0%) | 1.04 (0.26, 4.25) | |
| All other GI Specializations | 59 (71.1%) | 0.98 (0.55, 1.74) | |
| Urban/rural location | |||
| Urban | 210 (67.5%) | 0.93 (0.50, 1.74) | |
| Rural | 36 (63.2%) | 1.00 | |
| Practice setting | |||
| Academic Center | 80 (80.8%) | 2.41 (1.25, 4.64) | |
| Non-academic Center | 168 (62.7%) | 1.00 | |
| Career Stage | |||
| Fellows in training | 35 (76.1%) | 1.33 (0.60, 2.97) | |
| In practice from 0 to 10 years | 88 (72.7%) | 1.57 (0.94, 2.61) | |
| In practice from 11+ years | 122 (60.7%) | 1.00 | |
| Familiarity with the guideline | |||
| Familiar | 115 (70.6%) | 1.30 (0.73, 2.33) | |
| Unsurea | 77 (62.6%) | 0.91 (0.50, 1.66) | |
| Unfamiliarb | 56 (65.9%) | 1.00 | |
| “If MSI/IHC testing is ordered on CRC biopsy, surgery should wait to perform resection until after results have returned” | Gastroenterology (GI) subspecialty | ||
| General GI | 80 (29.0%) | 1.00 | |
| GI Oncology Specializations | 8 (53.3%) | 1.54 (0.49, 4.89) | |
| All other GI Specializations | 29 (31.9%) | 1.04 (0.59, 1.83) | |
| Urban/rural location | |||
| Urban | 107 (33.0%) | 1.72 (0.84, 3.54) | |
| Rural | 11 (18.6%) | 1.00 | |
| Practice setting | |||
| Academic Center | 39 (36.8%) | 1.39 (0.77, 2.51) | |
| Non-academic Center | 79 (28.6%) | 1.00 | |
| Career stage | |||
| Fellows in training | 13 (27.7%) | 0.70 (0.31, 1.57) | |
| In practice from 0 to 10 years | 44 (34.1%) | 1.26 (0.76, 2.07) | |
| In practice from 11+ years | 60 (29.0%) | 1.00 | |
| Familiarity with the guideline | |||
| Familiar | 65 (40.1%) | 2.36 (1.25, 4.45) | |
| Unsurea | 35 (28.0%) | 1.49 (0.76, 2.91) | |
| Unfamiliarb | 17 (20.0%) | 1.00 | |
aUnsure means they are not clear on whether they are aware of the applicable guidelines or not
bUnfamiliar means providers are not well versed with current guidelines
Physician opinion on ECR
Despite guidelines recommending ECR in LS, only 59.4% felt this was the preferred operation in patients < 50 (Table 6). 37.4% believed ECR was preferred in patients > 50. Academic practice settings were associated with belief ECR should be performed in young LS patients (74.8% opting for ECR).
Table 6.
Multivariable analysis of factors associated with preference for performing total colectomy vs. segmental resection in Lynch Syndrome patients under age 50a
| Variables | Number of respondents who prefer total colectomy with ileorectal anastomosis or proctocolectomy in the case of rectal cancer (percentage within each category) | Odds ratio (95% Confidence Interval) |
|---|---|---|
| Gastroenterology (GI) subspecialty | ||
| General GI | 153 (57.3) | 1.00 |
| GI Oncology Specializations | 12 (80.0) | 1.35 (0.33, 5.45) |
| All other GI Specializations | 52 (61.9) | 0.95 (0.55, 1.65) |
| Urban/rural location | ||
| Urban | 189 (61.2) | 1.23 (0.68, 2.23) |
| Rural | 28 (49.1) | 1.00 |
| Practice setting | ||
| Academic Center | 74 (74.8) | 2.20 (1.20, 4.03) |
| Non-academic Center | 144 (53.9) | 1.00 |
| Career stage | ||
| Fellows in training | 33 (71.7) | 1.50 (0.70, 3.24) |
| In practice from 0 to 10 years | 77 (63.6) | 1.44 (0.89, 2.34) |
| In practice from 11+ years | 108 (54.0) | 1.00 |
| Familiarity with the guideline | ||
| Familiar | 109 (67.3) | 1.62 (0.92, 2.85) |
| Unsureb | 64 (51.6) | 0.90 (0.50, 1.61) |
| Unfamiliarc | 46 (55.4) | 1.00 |
a59.4% of total respondents surveyed prefer total colectomy or proctocolectomy for LS patients <50 years old, whereas 37.4% prefer the same operation in those age >50 years old (p<0.0001)
bUnsure means they are not clear on whether they are aware of the applicable guidelines or not
cUnfamiliar means providers are not well versed with current guidelines
Discussion
We have uncovered barriers to LS screening which has provided insight into historically low MSI/IHC testing rates, particularly preoperatively. Understanding barriers is critical as failure of LS screening places both patients and family members at-risk. Study results reflect opinions of, and barriers experienced by, gastroenterologists. Gastroenterologists are important as they are frequently first to identify CRC during colonoscopy and can help facilitate LS identification, including preoperatively to assist surgical decision-making.
Importantly, there appears to be no consistent specialist who gastroenterologists feel is responsible for ordering MSI/IHC. Only 33.4% felt gastroenterologists should order MSI/IHC. Pathologists were believed most responsible followed by medical oncologists and surgeons. Such “diffusion of responsibility”, a sociopsychological phenomenon in which inaction may occur when multiple people are involved in decision-making, could potentially prevent testing, particularly preoperatively, and may help explain historically low testing rates23. Unless there are clear institutional protocols, with closed-loop communication between different specialists, respective physicians may incorrectly assume others are ordering testing. Previous research demonstrated that reflex (automatic) IHC and MSI testing institutional protocols are frequently not in place, particularly in community settings24. This could potentially allow diffusion of responsibility to negatively impact testing rates. Furthermore, a recent provider study, most of whom were non-gastroenterologists, revealed inconsistent LS identification and ad hoc testing practices with IHC ordered by a variety of providers25. Prior research demonstrated that even when institutional universal (all CRC cases) IHC testing was recommended, some might still not undergo testing26. Furthermore, if results were abnormal, genetic testing referral was inconsistent. This highlights assuring clear lines of provider responsibility for ordering, interpreting and acting on test results.
The most common testing barriers we uncovered were cost and lack of familiarity interpreting/applying MSI/IHC results. With regard to the later, pathology laboratories often provide interpretations of MSI/IHC results, which can help overcome this barrier. Nevertheless, lack of familiarity interpreting/applying results correlated with decreased guideline familiarity and highlights needs to enhance genomics education. Pertaining to cost, in many cases this may be only a perceived barrier as tumor testing is frequently, but not always (particularly in older patients), covered by insurance27,28. Furthermore, routine LS screening by MSI and IHC has been demonstrated as cost-effective with benefits for CRC patients and relatives29. Interestingly, a CRC patient survey revealed very positive attitudes toward LS tumor testing but a common barrier was cost concerns of additional testing and surveillance30. Clearly, testing coverage needs to be understood in each patient as out-of-pocket expense can prevent reliable testing. Insurance carriers not covering testing should be encouraged to do so given multiple benefits.
Lack of germline testing access and genetic counseling were also barriers. Non-academic and rural settings were associated with these barriers as well as with cost barriers. Suboptimal availability of genomic resources in these settings is a target for intervention. Decreased access to genetic specialists is a significant barrier to genetic testing and diagnosing LS26. Rural location was demonstrated as a barrier to genetics evaluation for hereditary breast cancer for multiple reasons including lack of awareness and distance of services31. Importantly, many genetic testing companies offer detailed interpretation of results and counseling services, which can help overcome these barriers. Furthermore, DNA sequencing cost has decreased in recent years32.
Timing of MSI/IHC result availability is important. Preoperative results effect decisions regarding germline testing and ECR. LS patients have increased risk of developing a 2nd CRC if colonic resection is limited. According to the U.S. Multi-Society Task-Force on Colorectal Cancer, to facilitate surgical planning, tumor testing should be performed on preoperative biopsy specimens, if possible11. According to American Society of Clinical Oncology, expediency in reporting biomarker results is important and need for evaluation is compounded by patient need to receive complete understanding of diagnosis and treatment plans going forward33. Additional benefit of preoperative testing is potential to perform prophylactic hysterectomy/bilateral salpingo-oophorectomy at time of CRC surgery in confirmed LS patients34.
Despite benefits of performing preoperative MSI/IHC, only 46.1% felt testing should be routinely conducted on colonoscopy specimens. If testing is performed only on surgical specimens, abnormal results return after the operation has been performed. In those non-routinely recommending preoperative testing, 43.7% also believed testing did not need to be routine on surgical resection specimens, which increases chances testing will never be performed. Many respondents, particularly routine colonoscopy specimen testers, also felt testing should be done concurrently on surgical specimens. Reasons for this are not entirely clear as this practice is not guideline supported. However, a potential explanation may be desire to assure concordance between colonoscopy and surgical resection specimens. It is important to address why providers might suggest MSI/IHC testing inconsistently (25–75% versus never or 100% of time). This may potentially reflect differences in patient-related factors (age, CRC family history, insurance coverage etc.) or practice settings (the same physician may work in multiple clinical settings with varying testing access).
Physicians in academic and urban settings were more likely to understand the importance of preoperative result availability. Guideline familiarity was associated with belief surgery should not proceed until MSI/IHC results return given potential affect on surgical decision-making. Guideline familiarity itself was associated with urban location and GI oncology sub-specialization. These findings likely reflect clustering of specialized services and potentially educational activities that may be available more frequently in academic and urban settings. Genomic educational programs in rural and non-academic settings can help overcome this issue.
GI fellows, those in early-career stages and those in non-academic settings felt waiting for MSI/IHC results would delay colon resection and therefore negatively impact outcome, thus preventing initial test ordering. Practitioners in non-academic settings believed waiting for germline testing results (if abnormal MSI/IHC) would delay resection and therefore negatively impact patient outcome. These findings potentially reflect a perceived or actual higher turnaround time for results in certain settings. This may also imply the complex multistep process (MSI/IHC followed by germline testing) can be a significant barrier.
Our survey also allowed assessment of opinions on colonic resection extent. Despite guidelines, only 59.4% felt in LS patients <50, ECR should be performed. Academic practice settings were positively associated with belief ECR should be undertaken. Assuring providers understand risks of synchronous and metachronous cancer is necessary. Notably, other factors may impact surgical resection decision-making including patient preference and potential effects on functional outcome35. However, a survey of colorectal surgeons revealed if pre-operative testing in early-onset CRC indicated LS, 84.9% would perform total colectomy36.
A potential study limitation is our survey response rate of 8.7%. Importantly, however, physician-based surveys may be less affected by non-response bias compared with other surveys due to higher homogeneity of knowledge, training, attitudes and behaviors than other groups37. Additionally, sensitive topics (in our survey, potentially adequacy of high-stakes genomics screening and assessing knowledge gaps) have been demonstrated to depress response rates, even in anonymous surveys38. Although we assessed which specialist gastroenterologists feel is responsible for ordering MSI/IHC, we do not have information on actual testing rates. Hence, another specialist may be ordering testing. However, given prior studies have shown low testing rates, this may not be occurring frequently. Finally, we do not have information on whether universal or reflex testing protocols are established at specific practices.
Study strengths include responses representing opinions from providers in multiple practice settings and career stages from across the U.S., which can increase generalizability compared to studies limited to specific settings. Furthermore, the distribution of academic versus non-academic settings was similar to distributions measured by the Association of American Medical Colleges, in which 19.5% and 12.3% reported current or prior academic faculty appointments respectively39. Additionally, the study focused on gastroenterologists who are important as they are often first to diagnosis CRC and can help identify LS preoperatively.
In conclusion, novel information has been obtained allowing better understanding of previously demonstrated low MSI/IHC testing rates and why results are infrequently available preoperatively. Testing cost, decreased availability of genomic services in rural and non-academic settings, guideline familiarity deficiencies and diffusion of responsibility regarding test ordering may be key barriers. Educating providers that testing costs have been decreasing, insurance frequently covers testing and that companies often provide result interpretation and counseling services can help circumvent many of these barriers. Importantly, a focus on underserved populations is required in which barriers, including financial/insurance coverage and access to specialized care, may be more pronounced.
Institutional Quality Assurance leadership should ensure systems-based universal testing protocols focusing on provider responsibility and timing of testing are implemented. Ultimately, the responsible specialist (pathologist, gastroenterologist, etc.) may depend on specific factors including expertise level interpreting results and the physician-patient relationship. However, roles should be clearly defined with closed-loop communication between different specialists to confirm testing is completed and results acted upon. Reflex (automatic) testing protocols, infrequently in place based on prior studies, can be beneficial, as this would prevent specific providers from being responsible for test ordering. However, the provider acting on results must be clearly designated particularly given that prior study demonstrated those with abnormal mismatch repair are inconsistently referred for germline testing. In any testing scenario, emphasis must be placed on institutional workflow protocols to assure both tumor analysis and germline testing are processed expeditiously and results available preoperatively to allow risk/benefit discussions on ECR. By removing ordering steps from the process, reflex testing may help streamline result availability. Similarly, requesting testing companies expedite germline analysis in the setting of a pending cancer operation can be beneficial.
Future studies are needed addressing testing/management practices in non-gastroenterologists, including surgeons, pathologists, oncologists, and geneticists. Furthermore, the divergent practice patterns we have demonstrated underscore needs to investigate other approaches to LS screening. New technologies including single up-front tumor next-generation sequencing are currently under investigation40. Another recent potential approach, which is discussed in the National Comprehensive Cancer Network guidelines specifically, includes performing multipanel (LS and other genes associated with hereditary CRC) germline testing in early-onset cases or those with a strong family history41. However, with adoption of any new technologies or approaches, well-defined protocols still need to be instituted assuring clear lines of responsibility regarding test ordering and interpretation, and efforts will be necessary to facilitate preoperative result availability.
Study Highlights
What is current knowledge
Colorectal cancer tumor testing with microsatellite instability (MSI) and/or immunohistochemistry (IHC) to help identify Lynch syndrome (LS) is not performed routinely.
Preoperative MSI/IHC testing on colonoscopy biopsy assists with germline genetic testing decisions and extent of colonic resection but is inconsistently conducted.
Testing practices of gastroenterologists and barriers to testing, including preoperatively, are poorly understood.
What is new here
MSI/IHC testing barriers include cost, lack of familiarity interpreting results and genetic counseling access deficiencies.
Testing barriers are clustered in rural and non-academic practice settings.
No consensus exists among gastroenterologists regarding which specialist (gastroenterologist, pathologist etc.) should order MSI/IHC, which can prevent consistent testing.
Testing on preoperative colonoscopy biopsy specimens is not routinely recommended which prevents availability of information to assist surgical decision-making.
Electronic supplementary material
Financial support
None.
Guarantor of the article
Jordan J. Karlitz, MD.
Specific authors' contributions
Study concept: Jordan J. Karlitz; Study design: Jordan J. Karlitz, Alan Noll, and Parth Parekh. Drafting of manuscript: Jordan J. Karlitz and Alan Noll. All authors performed the analysis and interpretation of the data and critical revision of the manuscript. All the authors have approved the final draft submitted.
Potential competing interests
Dr. Dennis Ahnen-Speakers bureau for Ambry Genetics and a scientific advisor for Cancer Prevention Pharmaceuticals. Dr. Jordan Karlitz-Speaker’s bureau for Myriad Genetics.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
The online version of this article (10.1038/s41424-018-0047-y) contains supplementary material, which is available to authorized users.
References
- 1.American Cancer Society Cancer Facts & Figures (American Cancer Society: Atlanta, GA, USA). https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf (2017). Accessed 11 Sept 2017.
- 2.National Comprehensive Cancer Network. Colorectal Cancer Screening (Version 2.2017) https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf Accessed 16 Sept 2017
- 3.Jasperson KW, et al. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Southey MC, et al. Use of molecular tumor characteristics to prioritize mismatch repair gene testing in early-onset colorectal cancer. J. Clin. Oncol. 2005;23:6524–6532. doi: 10.1200/JCO.2005.04.671. [DOI] [PubMed] [Google Scholar]
- 5.Aaltonen LA, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N. Engl. J. Med. 1998;338:1481–1487. doi: 10.1056/NEJM199805213382101. [DOI] [PubMed] [Google Scholar]
- 6.Pearlman R, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 2017;3:464–471. doi: 10.1001/jamaoncol.2016.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yurgelun MB, et al. Cancer susceptibility gene mutations in individuals with colorectal cancer. J. Clin. Oncol. 2017;35:1086–1095. doi: 10.1200/JCO.2016.71.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ligtenberg MJ, et al. EPCAM deletion carriers constitute a unique subgroup of Lynch syndrome patients. Fam. Cancer. 2013;12:169–174. doi: 10.1007/s10689-012-9591-x. [DOI] [PubMed] [Google Scholar]
- 9.Sehgal R, et al. Lynch syndrome: an updated review. Genes. 2014;5:497–507. doi: 10.3390/genes5030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evaluation of Genomic Application in Practice and Prevention (EGAPP). Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet. Med.;11:35–41 (2009). [DOI] [PMC free article] [PubMed]
- 11.Giardello FM, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US multi-society task force of colorectal cancer. Am. J. Gastroenterol. 2014;109:1159–1179. doi: 10.1038/ajg.2014.186. [DOI] [PubMed] [Google Scholar]
- 12.Kumarasinghe AP, De boer B, Bateman AC, Kumarasinghe MP. DNA mismatch repair enzyme immunohistochemistry in colorectal cancer: a comparison of biopsy and resection material. Pathology. 2010;42:414–420. doi: 10.3109/00313025.2010.493862. [DOI] [PubMed] [Google Scholar]
- 13.Shia J, et al. Immunohistochemical staining for DNA mismatch repair proteins in intestinal tract carcinoma: how reliable are biopsy samples? Am. J. Surg. Pathol. 2011;35:447–454. doi: 10.1097/PAS.0b013e31820a091d. [DOI] [PubMed] [Google Scholar]
- 14.Jarvinen HJ, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/S0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 15.Parry S, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut. 2011;60:950–957. doi: 10.1136/gut.2010.228056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Win AK, et al. Risk of metachronous colon cancer following surgery for rectal cancer in mismatch repair gene mutation carriers. Ann. Surg. Oncol. 2013;20:1829–1836. doi: 10.1245/s10434-012-2858-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vos tot Nederveen Cappel WH, et al. Decision analysis in the surgical treatment of colorectal cancer due to a mismatch repair gene defect. Gut. 2003;52:1752–1755. doi: 10.1136/gut.52.12.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzig DO, et al. Clinical Practice Guidelines for the Surgical Treatment of Patients With Lynch Syndrome. Dis. Colon Rectum. 2017;60:137–143. doi: 10.1097/DCR.0000000000000785. [DOI] [PubMed] [Google Scholar]
- 19.Syngal S, et al. ACG clinical guideline: genetic testing and management of hereditary GI cancer syndromes. Am. J. Gastroenterol. 2015;110:223–262. doi: 10.1038/ajg.2014.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlitz JJ, et al. Population-based lynch syndrome screening by microsatellite instability in patients ≤50: prevalence, testing determinants, and result availability prior to colon surgery. Am. J. Gastroenterol. 2015;110:948–955. doi: 10.1038/ajg.2014.417. [DOI] [PubMed] [Google Scholar]
- 21.Karlitz Jordan J, Sherrill Meredith R, DiGiacomo Daniel V, Hsieh Mei-chin, Schmidt Beth, Wu Xiao-Cheng, Chen Vivien W. Factors Associated With the Performance of Extended Colonic Resection vs. Segmental Resection in Early-Onset Colorectal Cancer: A Population-Based Study. Clinical and Translational Gastroenterology. 2016;7(4):e163–e163. doi: 10.1038/ctg.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaikh Talha, Handorf Elizabeth A., Meyer Joshua E., Hall Michael J., Esnaola Nestor F. Mismatch Repair Deficiency Testing in Patients With Colorectal Cancer and Nonadherence to Testing Guidelines in Young Adults. JAMA Oncology. 2018;4(2):e173580. doi: 10.1001/jamaoncol.2017.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penn State 424 Psych. http://sites.psu.edu/aspsy/2014/02/18/diffusion-of-responsibility. Accessed 4 Jan 18.
- 24.Beamer L, et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for lynch syndrome among US cancer programs and follow-up of abnormal results. J. Clin. Oncol. 2012;30:1058–1063. doi: 10.1200/JCO.2011.38.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bombard Y, et al. Universal tumor screening for Lynch syndrome:health-care providers’ perspectives. Genet. Med. 2017;19:568–574. doi: 10.1038/gim.2016.150. [DOI] [PubMed] [Google Scholar]
- 26.Brennan B, et al. Universal molecular screening does not effectively detect lynch syndrome in clinical practice. Ther. Adv. Gastroenterol. 2017;10:361–371. doi: 10.1177/1756283X17690990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ASCO. Genetic Testing Coverage & Reimbursement. https://www.asco.org/practice-guidelines/cancer-care-initiatives/genetics-toolkit/integrating-clinical-cancer-genetics-2 (2018). Accessed 14 July 18.
- 28.Graf MD, Needham DF, Teed N, Brown T. Genetic testing insurance coverage trends: a review of publicly available policies from the largest US payers. Pers. Med. 2013;10:235–243. doi: 10.2217/pme.13.9. [DOI] [PubMed] [Google Scholar]
- 29.Leenen CH, et al. Cost-effectiveness of routine screening for Lynch syndrome in colorectal cancer patients up to 70 years of age. Genet. Med. 2016;18:966–973. doi: 10.1038/gim.2015.206. [DOI] [PubMed] [Google Scholar]
- 30.Hunter JE, Zepp JM, Gilmore MJ. Universal tumor screening for Lynch Syndrome: assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121:3281–3289. doi: 10.1002/cncr.29470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koil CE, Everett JN, Hoechstetter L. Differences in physician referral practices and attitudes regarding hereditary breast cancer by clinical practice location. Genet. Med. 2003;5:364–369. doi: 10.1097/01.GIM.0000086477.00766.C9. [DOI] [PubMed] [Google Scholar]
- 32.Wetterstrand K. A. DNA Sequencing Costs: data from the NHGRI Genome Sequencing Program (GSP). www.genome.gov/sequencingcostsdata. Accessed 15 July 2018.
- 33.Sepulveda AR, et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology and the American Society of Clinical Oncology. J. Clin. Oncol. 2017;35:1453–1486. doi: 10.1200/JCO.2016.71.9807. [DOI] [PubMed] [Google Scholar]
- 34.Lynch HT, Casey JM. Prophylactic surgery prevents endometrial and ovarian cancer in Lynch syndrome. Nat. Clin. Pract. Oncol. 2007;4:672–673. doi: 10.1038/ncponc1002. [DOI] [PubMed] [Google Scholar]
- 35.Haanstra JF, et al. Quality of life after surgery for colon cancer in patients with Lynch syndrome: partial versus subtotal colectomy. Dis. Colon Rectum. 2012;55:653–659. doi: 10.1097/DCR.0b013e31824f5392. [DOI] [PubMed] [Google Scholar]
- 36.Warrier SK, et al. Results from an American Society of Colon and Rectal Surgeons survey on the management of young-onset colorectal cancer. Tech. Coloproctol. 2014;18:265–272. doi: 10.1007/s10151-013-1052-5. [DOI] [PubMed] [Google Scholar]
- 37.Kellerman SE, Herold J. Physician response to surveys. A review of the literature. Am. J. Prev. Med. 2001;20:61–67. doi: 10.1016/S0749-3797(00)00258-0. [DOI] [PubMed] [Google Scholar]
- 38.Cunningham, C. T. et al. Exploring physician specialist response rates to web-based surveys. BMCMed. Res. Methodol. 15 (2015). [DOI] [PMC free article] [PubMed]
- 39.Full-Time Faculty-Appointment Status at U.S. Medical Schools for Residents Who Completed Residencies, by Specialty. https://www.aamc.org/data/448498/c7table.html. Accessed on September 16, 2017.
- 40.Hampel H. et al. Assessment of tumor sequencing as a replacement for lynch syndrome screening and current molecular tests for patients with colorectal cancer. JAMA Oncology. Published online 3/29/18. [DOI] [PMC free article] [PubMed]
- 41.Provenzale Dawn, Gupta Samir, Ahnen Dennis J., Markowitz Arnold J., Chung Daniel C., Mayer Robert J., Regenbogen Scott E., Blanco Amie M., Bray Travis, Cooper Gregory, Early Dayna S., Ford James M., Giardiello Francis M., Grady William, Hall Michael J., Halverson Amy L., Hamilton Stanley R., Hampel Heather, Klapman Jason B., Larson David W., Lazenby Audrey J., Llor Xavier, Lynch Patrick M., Mikkelson June, Ness Reid M., Slavin Thomas P., Sugandha Shajanpeter, Weiss Jennifer M., Dwyer Mary A., Ogba Ndiya. NCCN Guidelines Insights: Colorectal Cancer Screening, Version 1.2018. Journal of the National Comprehensive Cancer Network. 2018;16(8):939–949. doi: 10.6004/jnccn.2018.0067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.