Abstract
Vomiting is a complex autonomic reflex orchestrated by several neurological centres in the brain. Vagus, the cranial nerve plays a key role in regulation of vomiting. Kunjal Kriya (Voluntarily Induced Vomiting), is a yogic cleansing technique which involves voluntarily inducing vomiting after drinking saline water (5%) on empty stomach. This study was designed with an objective to understand the effect of voluntary induced vomiting (ViV) on pulmonary functions in experienced practitioners and novices and derive its possible therapeutic applications. Eighteen healthy individuals volunteered for the study of which nine had prior experience of ViV while nine did not. Pulmonary function tests were performed before and after 10 min of rest following ViV. Analysis of Covariance was performed adjusted for gender and baseline values. No significant changes were observed across genders. The results of the present study suggest a significant increase in Slow Vital Capacity [F(1,13) = 5.699; p = 0.03] and Forced Inspiratory Volume in 1st Second [p = 0.02] and reduction in Expiratory Reserve Volume [F(1,13) = 5.029; p = 0.04] and Respiratory Rate [F(1,13) = 3.244, p = 0.09]. These changes suggest the possible role of ViV in enhancing the endurance of the respiratory muscles, decreased airway resistance, better emptying of lungs and vagal predominance respectively. We conclude that ViV when practiced regularly enhances the endurance of the respiratory muscles and decreases airway resistance. These findings also indicate need for scientific understanding of ViV in the management of motion sickness and restrictive pulmonary disorders like bronchitis and bronchial asthma.
Keywords: Kunjala Kriya, Voluntarily induced vomiting, Pulmonary functions, Yoga, Kriya
1. Introduction
Yoga is a comprehensive lifestyle practice which involves practices for the body, mind and the intellect through physical postures (asanas), voluntary breath regulation (pranayama), cleansing practices (kriya) and meditation (dhyana). Yoga is being practiced in India since thousands of years. Studies have established the therapeutic benefit of Yoga practices irrespective of an individual being obese [1], hypertensive [2], [3], diabetic [4] or even suffering from cancer [5], [6]. Yoga practices are efficacious in not only regulating the autonomic nervous system but also beneficially regulating the gene expressions [7]. With all the available evidence to possibly suggest Yoga as a non-pharmacological intervention for several lifestyle diseases and non-communicable diseases, the basic underlying mechanism of several practices remains unexplored. This study aims to understand the physiological adaptation of pulmonary functions following Kunjal Kriya (voluntarily induced vomiting – ViV), a yogic cleansing technique in experienced and novice practitioners. There is no published scientific literature available documenting safety and psycho-physiological effects of ViV until date, making the present study as a novel effort. Despite the practice being observed as ‘involving considerable risk’ by the modern medical professionals, the safety of its practice is time tested and no complications have been reported.
Hatha Yoga Pradipika, an ancient Yoga scripture describes Kunjal Kriya (Voluntarily Induced Vomiting – ViV) as one of the six cleansing techniques to clean the body and regulate the mind [8]. Following practice of ViV, subjectively, an individual feels emptiness of stomach. Traditional practitioners suggest acute fever, visceral infection, hernia and cardiovascular disorders as contraindications for the practice. Gherenda Samhita, an ancient treatise on Yoga claims that this practice when performed everyday can cure ailments of liver and spleen [9].
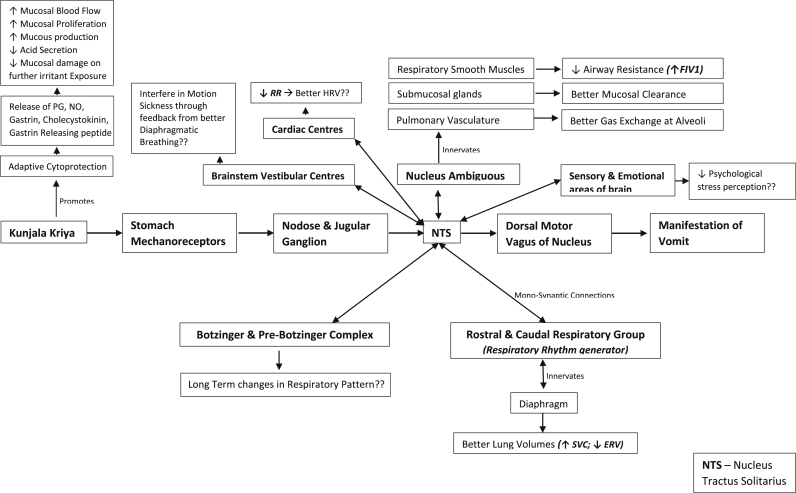
Vomiting, a survival mechanism conserved during evolution in humans and several organisms is understood to be one of the most complex autonomic reflex orchestrated by several neurological centres in the brain. The stimulus is manifested as an orderly response through excessive salivation, inhibition of normal gastric motility, retro-peristaltic movement, relaxation of lower esophageal sphincter, tachycardia, sweating, breath retention and contraction of abdominal and thoracic muscles. Despite vomiting being one of the most common clinical sign, understanding its neurobiology and relevance in maintaining is incomplete. Current understanding states, vagus as the key moderator of vomiting, manifested in strict co-ordination of nucleus tractus solitarius with area postrema, brainstem vestibular centres, sensory and emotional areas and several other areas of the brain [10].
ViV is a common practice observed in patients suffering from bulimia apart from their laxative abuse, and diuretic abuse driven by distorted body-image perception. Complications of repeated ViV include dental erosion and discolouration of teeth. Due to the acidic contents coming in contact with the oesophagus, pharynx and oropharynx, symptoms of hoarseness, sore throat, dry cough, and difficulty in swallowing are reported [11].
ViV immediately after food has been viewed as a psychiatric illness. Ancient Yogic literature recommends practice of ViV following consumption of saline water in empty stomach to be therapeutically beneficial. Hence, this study was designed with an objective to document the safety of its practice and possibly explore a mechanism of action of ViV from the perspective of Yoga practices.
2. Materials and methods
A flyer was displayed in the classrooms of undergraduate naturopathy and yoga medical students regarding the study. Participants were recruited after obtaining a written informed consent. Eighteen volunteers were recruited into two groups: Novices and Experienced group. All participants were informed about the study and a written informed consent was obtained. All volunteers recruited for the study were reported to be healthy. Emergency medical treatment facility was available to address any unanticipated complications. The experimental and novices group consisted of nine individuals in each group with age of 19.2 ± 0.9 years and 19.6 ± 0.7 years respectively. Volunteers having experience of performing ViV for more than four times were recruited in the experienced group. There were 5 men and 4 women and 7 men and 2 women in the experienced and novices groups respectively.
The practice of ViV involves drinking warm saline water (5%) in the morning on an empty stomach, sitting in squatting position until the individual feels a sense of fullness or nausea. The individual is then recommended to stand and bend forward from the low back and voluntarily trigger vomiting by gently touching the root of the tongue and uvula. It is observed that with practice, triggering the vomit may be required once or not at all whereas, in novices, it is required to trigger three to four times until most of the consumed water is vomited out. The participants rested in supine position and voluntarily relaxed the entire body for 10 min following vomiting. Pulmonary function tests were performed as per standard guidelines [12] immediately before and after the practice of ViV using Schiller Spirovit SP-1 system.
Analysis of co-variance (ANCOVA) was performed to understand the between group changes, adjusted for the baseline values and gender. Body Mass Index (BMI) was not considered as a covariate in our study as all the volunteers in both groups were having a BMI of 20.1 ± 0.4 kg/m2. There was no significant difference observed amongst the genders.
3. Results
The slow vital capacity [F(1,13) = 5.699; p = 0.03] increased in experienced group as compared to the novices. Within group comparison showed a contrasting change with a significant increase in slow vital capacity (SVC) in experienced group (p = 0.01) as compared to the significant reduction observed in the novices (p = 0.02). Expiratory reserve volume (ERV) [F(1,13) = 5.029; p = 0.04] decreased significantly in novices as compared to a non-significant increase in experienced practitioners. Within group comparison indicated a significant reduction in ERV in novices (p = 0.04) while no change was observed in the experienced group. A reduction in respiratory rate (RR) was observed in both experienced (p = 0.01) and novices (p = 0.03), with the extent of reduction being greater in the experienced group [F(1,13) = 3.244, p = 0.09]. A significant increase in Forced Inspiratory Volume in first second (FIV1) was observed in experienced group (p = 0.02) (Table 1).
Table 1.
Table represents the Mean ± SD of the lung volumes measured in experienced and novices before and 10 min after ViV.
| Variables | Experienced Group |
Novices Group |
F | Sig. (ANCOVA) p value | Partial Eta Squared | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | p value | Pre | Post | p value | ||||
| SVC | 2.61 ± 0.6 | 3.04 ± 0.4 | 0.017a | 3.02 ± 0.3 | 2.56 ± 0.3 | 0.027a | 5.699 | 0.033x | 0.305 |
| ERV | 0.87 ± 0.2 | 0.97 ± 0.5 | 0.588 | 0.86 ± 0.1 | 0.57 ± 0.4 | 0.044a | 5.029 | 0.043x | 0.279 |
| IRV | 0.91 ± 0.4 | 0.87 ± 0.4 | 0.679 | 0.93 ± 0.3 | 0.93 ± 0.5 | 0.996 | 2.029 | 0.178 | 0.135 |
| FVC | 2.61 ± 0.5 | 2.71 ± 0.5 | 0.533 | 2.71 ± 0.5 | 2.47 ± 0.7 | 0.208 | 1.042 | 0.326 | 0.074 |
| PEF | 6.63 ± 1.9 | 6.64 ± 1.6 | 0.990 | 7.62 ± 1.4 | 6.98 ± 1.8 | 0.170 | 0.172 | 0.685 | 0.013 |
| FIVC | 2.52 ± 0.5 | 2.58 ± 0.5 | 0.579 | 2.81 ± 0.3 | 2.65 ± 0.3 | 0.116 | 0.390 | 0.543 | 0.029 |
| FIV1 | 2.36 ± 0.4 | 2.49 ± 0.5 | 0.021a | 2.75 ± 0.4 | 2.55 ± 0.3 | 0.131 | 1.268 | 0.280 | 0.089 |
| PIF | 4.10 ± 1.4 | 4.34 ± 1.2 | 0.561 | 4.83 ± 1.2 | 4.44 ± 1.4 | 0.075 | 1.163 | 0.300 | 0.082 |
| RR | 15.28 ± 4.3 | 10.69 ± 2.7 | 0.01b | 16.36 ± 5.0 | 13.44 ± 2.3 | 0.032a | 3.244 | 0.095 | 0.200 |
| TV | 0.82 ± 0.1 | 0.99 ± 0.4 | 0.179 | 0.72 ± 0.1 | 0.87 ± 0.4 | 0.264 | 0.041 | 0.843 | 0.003 |
Levels of significance as understood from within group comparison using paired t test: a p ≤ 0.05; b p ≤ 0.01; c p ≤ 0.001.
Levels of significance as understood from between group comparison using Analysis of Covariance adjusted for Gender and baseline differences: x p ≤ 0.05; y p ≤ 0.01; z p ≤ 0.001.
SVC – Slow Vital Capacity; ERV – Expiratory Reserve Volume; IRV – Inspiratory Reserve Volume; FVC – Forced Vital Capacity; PEF – Peak Expiratory Flow; FIVC – Forced Inspiratory Vital Capacity; FIV1 – Forced Inspiratory Volume in 1st Second; PIF – Peak Inspiratory Flow; RR – Respiratory Rate; TV – Tidal Volume.
4. Discussion
The present study was designed with an objective to understand the role of ViV on pulmonary function. All the volunteers were healthy and did not report any clinical symptom following the intervention the entire day suggesting the safety of the intervention. This work is the first study conducted to understand the physiology of (ViV) as a standalone intervention in healthy human participants.
The results of the present study suggest a significant increase in SVC and FIV1 in the experienced group as compared to the significant reduction in the SVC and ERV in the novice group. The increase in SVC shall be attributed to better functioning of the diaphragm [13]. These changes suggest the possible role of ViV in enhancing the endurance of the respiratory muscles, decreased airway resistance, better emptying of lungs and vagal predominance respectively with practice suggesting adaptation of the pulmonary system. Reduced lung volume in the novices group shall be attributed to the psychological stress involved in practicing ViV for the first time. Both groups reported significant reduction in the respiratory rate. Post hoc analysis of our study showed a statistical power of 0.75, with an effect size of 1.32 and critical t = 2.119 indicating a strong evidence.
Vagus, the tenth cranial nerve orchestrates the vomiting reflex. Two distinct vagal afferent mechanoreceptors from the stomach: intra-ganglionic laminar ending (IGLE) and intramuscular array (IMA) respond to distension and smooth muscle contractions and also function as tension receptors [14]. These vagal afferents carry the mechanical information to the nucleus tractus solitarius (NTS) from stomach through jugular and nodose ganglion [15]. The NTS, located inside the blood brain barrier apart from its connections with mechanoreceptor vagal afferents from the stomach, also has intense neurological connections with areas for control of respiration [16], sensory and emotional areas of brain, and the brainstem vestibular centres [17]. NTS is also connected with area postrema that serves as the chemosensor, detecting any chemical change in the blood.
After distention of the stomach, NTS signals dorsal motor nucleus of vagus to initiate vomiting. The neuronal firing of vagal afferents decrease, resulting in relaxation of gastric wall tone and reduction of acid production [18], [19]. NTS signals to increase the diaphragmatic functions through its mono-synaptic connections with the rostral and caudal ventral respiratory group [20]. Simultaneously, NTS signals the respiratory smooth muscles, sub-mucosal glands and pulmonary vasculature [21] through nucleus ambiguous to alleviate airway resistance, facilitate better expiration, mucosal clearance and better oxygen diffusion into the vasculature [22]. Interestingly, integration of the cardiac function also occurs at the nucleus ambiguous [10] – indicating a probable influence of ViV on the cardiac autonomic functions. As there are no chemicals sensed by the retro-trapezoid nucleus and the area postrema, the evolutionary survival mechanism of chemicals triggering vomiting will be conserved.
Studies on motion sickness suggest diaphragmatic breathing as an effective non-pharmacological intervention [23]. We speculate that the regular practice of ViV may enable the individual to control motion sickness associated symptoms through better diaphragmatic breathing. The results from the novices of the present study also indicate that vomiting might be an evolution conserved response to relieve the organism from an adverse stimuli (emotional, psychological shock or loss of equilibrium while at constant motion) and facilitate relaxation by promoting vagal predominance.
Earlier studies show that administering mild irritants like 2–5% sodium chloride increased the secretion of prostaglandins and other factors like nitric oxide, leptin, ghrelin, cholecystokinin and gastrin releasing peptide and facilitate ‘adaptive cytoprotection’ to protect gastric mucosa [24], [25]. Treatment with 5% sodium chloride enhanced mucosal blood flow, mucous secretion, mucosal proliferation and decreased acid secretion [26]. Also, the DNA content in the gastric juice reduced indicating decreased mucosal damage and cell shredding [27]. Interestingly, following exposure to sodium chloride, histologically visible mucosal necrosis and plasma protein leakage into the gastric lumen were observed [28]. These distinct findings also point towards Kunjal Kriya as a potent ulcer protecting agent. However, studies on representative human participants are required to authenticate these preliminary findings.
Understanding the impact of ViV practice on biochemical changes, teeth, pharynx and oro-pharynx is beyond the scope of the present study and requires to be documented in the future studies. Further detailed studies are required to ascertain the psycho-physiological and biochemical changes following practice and the frequency for safe practice requires to be established.
5. Conclusion
From the above findings, we have conceptualized the probable mechanism of action of ViV and possible future directions for research (Fig. 1). Based on the findings from this study, we conclude that ViV when practiced regularly is expected to be a technique to enhance the endurance of the respiratory muscles and decrease the airway resistance. These findings also indicate the possibility of using the practice of ViV in the management of motion sickness and restrictive pulmonary disorders like bronchitis and bronchial asthma.
Fig. 1.
Probable mechanism of action of voluntarily induced vomiting (Kunjala Kriya).
Sources of funding
S-VYASA University.
Conflict of interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Jain S.C., Uppal A., Bhatnagar S.O.D., Talukdar B. A study of response pattern of non-insulin dependent diabetics to yoga therapy. Diabetes Res Clin Pract. 1993;19(1):69–74. doi: 10.1016/0168-8227(93)90146-v. [DOI] [PubMed] [Google Scholar]
- 2.Gokal R., Shillito L., Maharaj S.R. Positive impact of yoga and pranayam on obesity, hypertension, blood sugar, and cholesterol: a pilot assessment. J Altern Complement Med. 2007;13(10):1056–1058. doi: 10.1089/acm.2007.0679. [DOI] [PubMed] [Google Scholar]
- 3.Patel C., North W.R.S. Randomised controlled trial of yoga and bio-feedback in management of hypertension. Lancet. 1975;306(7925):93–95. doi: 10.1016/s0140-6736(75)90002-1. [DOI] [PubMed] [Google Scholar]
- 4.Bijlani R.L., Vempati R.P., Yadav R.K., Ray R.B., Gupta V., Sharma R. A brief but comprehensive lifestyle education program based on yoga reduces risk factors for cardiovascular disease and diabetes mellitus. J Altern Complement Med. 2005;11(2):267–274. doi: 10.1089/acm.2005.11.267. [DOI] [PubMed] [Google Scholar]
- 5.Nicole Culos-Reed S., Carlson L.E., Daroux L.M., Hately-Aldous S. A pilot study of yoga for breast cancer survivors: physical and psychological benefits. Psycho-Oncology. 2006;15(10):891–897. doi: 10.1002/pon.1021. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee B., Vadiraj H.S., Ram A., Rao R., Jayapal M., Gopinath K.S. Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integr Cancer Ther. 2007;6(3):242–250. doi: 10.1177/1534735407306214. [DOI] [PubMed] [Google Scholar]
- 7.Himani S., Palika D., Archna S., Sudip S., Narendra K.B., Vinod K. Gene expression profiling in practitioners of Sudarshan Kriya. J Psychosomatic Res. 2008;64:213–218. doi: 10.1016/j.jpsychores.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Muktibhodananda S. Yoga Publications Trust; Munger, Bihar, India: 1998. Hatha yoga Pradipika – a light on hatha yoga. [Google Scholar]
- 9.Niranjananda S. Yoga Publications Trust; Munger, Bihar, India: 2013. Gherenda Samhitha. [Google Scholar]
- 10.Babic T., Browning K.N. The role of vagal neurocircuits in the regulation of nausea and vomiting. Eur J Pharmacol. 2014;722:38–47. doi: 10.1016/j.ejphar.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown C.A., Mehler P.S. Medical complications of self-induced vomiting. Eat Disord. 2013;21(4):287–294. doi: 10.1080/10640266.2013.797317. [DOI] [PubMed] [Google Scholar]
- 12.Miller M.R., Hankinson J.A.T.S., Brusasco V., Burgos F., Casaburi R., Coates A. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 13.Wade O.L., Gilson J.C. The effect of posture on diaphragmatic movement and vital capacity in normal subjects. Thorax. 1951;6(2):103–126. doi: 10.1136/thx.6.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips R.J., Powley T.L. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Rev. 2000;34(1):1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 15.Sengupta J.N., Gebhart G.F. Gastrointestinal afferent fibers and sensation. In: Johnson L.R., editor. Physiology of the gastrointestinal tract. 3rd ed. Raven; New York: 1994. pp. 483–519. [Google Scholar]
- 16.Ezure K., Otake K., Lipski J., She R.B.W. Efferent projections of pulmonary rapidly adapting receptor relay neurons in the cat. Brain Res. 1991;564(2):268–278. doi: 10.1016/0006-8993(91)91463-b. [DOI] [PubMed] [Google Scholar]
- 17.Smith J.C., Abdala A.P., Borgmann A., Rybak I.A., Paton J.F. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 2013;36(3):152–162. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iggo A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955;128(3):593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iggo A. Gastro-intestinal tension receptors with unmyelinated afferent fibres in the vagus of the cat. Q J Exp Physiol Cogn Med Sci. 1957;42(1):130–143. doi: 10.1113/expphysiol.1957.sp001228. [DOI] [PubMed] [Google Scholar]
- 20.Alheid G.F., Jiao W., McCrimmon D.R. Caudal nuclei of the rat nucleus of the solitary tract differentially innervate respiratory compartments within the ventrolateral medulla. Neuroscience. 2011;190:207–227. doi: 10.1016/j.neuroscience.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kc P., Martin R.J. Role of central neurotransmission and chemoreception on airway control. Respir Physiol Neurobiol. 2010;173(3):213–222. doi: 10.1016/j.resp.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman J.L., Del Negro C.A. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7(3):232–241. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stromberg S.E., Russell M.E., Carlson C.R. Diaphragmatic breathing and its effectiveness for the management of motion sickness. Aerosp Med Hum Perform. 2015;86(5):452–457. doi: 10.3357/AMHP.4152.2015. [DOI] [PubMed] [Google Scholar]
- 24.Robert A., Nezamis J.E., Lancaster C., Davis J.P., Field S.O., Hanchar A.J. Mild irritants prevent gastric necrosis through “adaptive cytoprotection” mediated by prostaglandins. Am J Physiol Gastrointest Liver Physiol. 1983;245(1):G113–G121. doi: 10.1152/ajpgi.1983.245.1.G113. [DOI] [PubMed] [Google Scholar]
- 25.Brzozowski T., Konturek P.C., Konturek S.J., Brzozowska I., Pawlik T. Role of prostaglandins in gastroprotection and gastric adaptation. J Physiol Pharmacol. 2005;56:33. [PubMed] [Google Scholar]
- 26.Maiti R., Goel R. Effect of mild irritant on gastric mucosal offensive and defensive factors. Indian J Physiol Pharmacol. 1999;44(1999):185–191. [PubMed] [Google Scholar]
- 27.Goel R.K., Bhattacharya S.K. Gastroduodenal mucosal defence and mucosal protective agents. Indian J Exp Biol. 1991;29(8):701–714. [PubMed] [Google Scholar]
- 28.Wallace J.L. Increased resistance of the rat gastric mucosa to hemorrhagic damage after exposure to an irritant: role of the “mucoid cap” and prostaglandin synthesis. Gastroenterology. 1988;94(1):22–32. doi: 10.1016/0016-5085(88)90605-1. [DOI] [PubMed] [Google Scholar]