Abstract
Oral anticoagulants are being used in the postoperative period of hip arthroplasty to prevent of thromboembolic events, create doubts as to the effectiveness of thromboprophylaxis and reduce the risk of hemorrhage. This systematic revision is aimed at evaluating the use of oral anticoagulants in the prevention of thromboembolic events in the postoperative period of patients undergoing hip arthroplasty. Research with descriptors found on PubMed, BVS, and the CAPES portal for medical journal publications from September 2015 to June 2016, from the last ten years (2005–2015), complete, free, and written in Portuguese and in English were the methods used. The results of the studies showed some cases of pulmonary embolism, deep vein thrombosis, and bleeding; even then, the NOACs were effective in preventing thromboembolic events. There is no consensus regarding the prophylaxis method for these events, which is why the challenge is to obtain high levels of prevention while minimizing the adverse effects. The most studied oral anticoagulant was rivaroxaban (67%). The three drugs that were studied have shown to be effective in preventing thromboembolic events, but the best results were obtained with rivaroxaban 10 mg, one tablet daily; treatment duration ranged from 30 to 35 days with oral anticoagulants and from 28 to 42 days with anti-platelet drugs.
Keywords: Hip arthroplasty, Anticoagulants, Pulmonary embolism, Venous thrombosis/prevention & control
Resumo
Os anticoagulantes orais usados no pós-operatório de artroplastia de quadril para prevenção de eventos tromboembólicos geram dúvidas a respeito da efetividade tromboprofilática e da redução de riscos hemorrágicos. Para isso, esta revisão sistemática tem como objetivo avaliar o uso de anticoagulantes orais para prevenção de eventos tromboembólicos no pós-operatório de pacientes submetidos a artroplastia de quadril. Os métodos usados foram pesquisas nas bases de dados indexadas do PubMed, BVS e periódicos da Capes de setembro de 2015 a junho de 2016, dos últimos dez anos, completos, livres e nos idiomas inglês e português. Os resultados apresentaram alguns casos de embolia pulmonar, trombose venosa profunda e sangramentos; apesar disso, os NACOs foram considerados, pelos estudos citados, eficazes na prevenção de eventos tromboembólicos. Os três medicamentos estudados mostraram-se importantes na prevenção de eventos tromboembólicos, mas os melhores resultados profiláticos foram obtidos com Rivaroxaban 10 mg, uma vez ao dia, com duração entre 30 e 35 dias com anticoagulantes orais e 28 a 42 dias com antiagregante plaquetário.
Palavras-chave: Artroplastia de quadril, Anticoagulantes, Embolia pulmonar, Trombose venosa/prevenção & controle
Introduction
Total hip arthroplasty (THA) is one of the most successful orthopedic surgical procedures. In patients with degenerative hip joint cartilage pathologies (coxarthrosis), this technique offers significant pain relief, improved quality of life, and increased mobility in the medium- and long-terms.1
By 2030, the number of arthroplasties is projected to increase by 170% worldwide. An increase in the complication rates is expected to follow.2, 3 Thromboembolic events are considered to be common postoperative complications of hip arthroplasty. Nonetheless, their prophylaxis is still questioned in two ways: the need to prevent avoidable complications, such as deep venous thrombosis (DVT) and pulmonary embolism (PE), and to reduce the risk of hemorrhage.4 Oral anticoagulants aim to improve these indices, seeking efficacy in prophylaxis and to reduce the adverse effects through a more acceptable route of administration.5
Thromboembolic events are observed in 50–60% of the patients who undergo hip arthroplasty; the great majority of cases do not present clinical manifestations.4, 6 From a temporal standpoint, studies indicate the peak risk of DVT and PE to be between the third and fourth postoperative week, more specifically between the 18th and 21st days.4, 7
The high risk of postoperative complications has stimulated improvements in prevention so as not to increase the risk of hemorrhage. Oral anticoagulants are more often used, which in turn raises doubts as to the risk/benefit ratio of this treatment. Rivaroxaban, dabigatran, and Aspirin® are the main drugs referred to in the studies. Although aspirin is not an oral anticoagulant, but rather an antiplatelet agent, it has been used in the prophylaxis of thromboembolic events.8
Therefore, the present study will focus on the use of oral anticoagulants in the prevention of thromboembolic events in the postoperative period of hip arthroplasty.
The present study was aimed at assessing the use of oral anticoagulants in the prevention of thromboembolic events in the postoperative period of patients who underwent hip arthroplasty.
Methods
The systematic search was done in the journals indexed in the PubMed databases and CAPES journals, through the Rede de Comunidade Acadêmica Federada (Rede CAFe) and the Biblioteca Virtual em Saúde (BVS), from September 2015 to June 2016.
The following Descriptors in Health Sciences (DeCs) were used: “hip arthroplasty,” “anticoagulants,” “postoperative care,” “prevention and control,” “pulmonary embolism,” and “venous thrombosis.” The following Medical Subject Headings (MeSHs) were used: “Arthroplasty, Replacement, Hip,” “Anticoagulants,” “Prevention & Control,” “Pulmonary Embolism,” and “Venous Thrombosis.” Aiming to broaden the search of studies, all the aforementioned terms were used and, subsequently, terms were removed, allowing the retrieval of a greater number of eligible articles.
The inclusion criteria were: studies published in the past ten years (2006–2016), with free complete text, conducted in human beings, and written either in English or Portuguese. Systematic/literature reviews, meta-analyses, articles focusing on heart diseases, and those that did not contemplate the objective of the present review were excluded.
The articles were first assessed by title and abstract, aiming to refine the sample through the inclusion and exclusion criteria, and later the articles were read in their entirety. For the results, data on the use of injectable anticoagulants, knee arthroplasty, and previous thromboembolic event were disregarded. If data on oral anticoagulants could not be assessed without the interference of these factors, the study was excluded.
This review followed the recommendations from the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist.9
Results
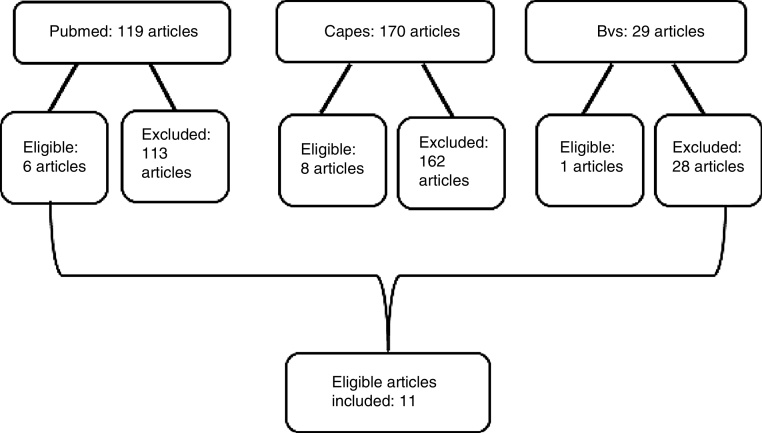
A total of 318 articles were retrieved from the literature search. Of these, 303 were excluded based on their title, abstract, and/or keywords, and 15 were considered eligible. After duplicates were excluded and the full text of the remaining articles were assessed by two reviewers (and, in the case of conflicts, by a third reviewer), 11 articles met the inclusion criteria (Fig. 1).
Fig. 1.
Flowchart of article search in the chosen databases.
The studies presented different results in all variables considered. Table 110, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 demonstrates that the most studied oral anticoagulant was rivaroxaban, followed by the antiplatelet agent Aspirin® and dabigatran.
Table 1.
Results of the database search.
| Author/year | Type of study | N | Oral anticoagulant/dosage | Time | Results |
|---|---|---|---|---|---|
| Eriksson et al., 200610 | Intervention | 128 142 140 143 142 |
Rivaroxaban 5 mg Rivaroxaban 10 mg Rivaroxaban 20 mg Rivaroxaban 30 mg Rivaroxaban 40 mg |
7 days |
DVT 14.9%; major bleeding 2.3% DVT 10.6%; major bleeding 0.7% DVT 8.5%; major bleeding 4.3% DVT 13.5%; major bleeding 4.9% DVT 6.4%; major bleeding 5.1% Venous Doppler. No case of PE. Effective and safe in preventing PE/DVT. |
| Eriksson et al., 200811 | Intervention | 2209 | Rivaroxaban 10 mg | 35 days | Major bleeding: 6 (0.3%) cases. Minor bleeding: 128 (2.1%) cases. PE: 4 (0.3%) cases. Deep venous thromboembolism: 4 (0.2%) DVT: 12 (0.8%) cases. Low incidence of thrombosis and safe to use in thromboprophylaxis. |
| Kanan et al., 200812 | Intervention | 33 | Rivaroxaban 10 mg | 32–36 days | 3 cases of DVT (phlebography 32–36 PO). No cases of PE or major bleeding. Effective. |
| Anderson et al., 201313 | Intervention | 380 | Aspirin® 81 mg | 28 days | Proximal DVT 1 (0.3%) case. Major bleeding: 8 (2.1%) cases. No cases of PE. No cases of major bleeding. Effective and safe in prolonged thromboprophylaxis after THA. |
| Ozler et al., 201514 | Intervention | 60 60 |
Rivaroxaban 10 mg Dabigatran 220 mg |
30 days 30 days |
Minor bleeding: 3 patients (5%). Major bleeding: 1 patient (1.6%); minor bleeding: 2 patients (3.3%). Absence of DVT (Doppler). |
| Clave et al., 201215 | Observational | 70 | Rivaroxaban 10 mg | 30 days | Absence of thromboembolic complications (at hospital discharge and 3 months after). 4 patients transfused. |
| Jobski et al., 201416 | Observational | 206 | Rivaroxaban 10 mg | 35 days | TEV 1%. |
| Turpie et al., 201417 | Observational | 8778 | Rivaroxaban 10 mg | 33 days | Thromboembolic events: 0.89%. Major bleeding: 0.4%. Effective and safe in preventing PE/DVT. |
| Cossetto et al., 201218 | Observational | 50 | Aspirin® 100 mg | 42 days | DVT: 3 patients (USG - 5 PO). No cases of PE. |
| Vulcano et al., 201219 | Observational | 887 | Aspirin® 325 mg (2×/daily) | 45 days | Thromboembolic events: 18.8 (1.2%), PE: 5.6 (0.36%), pDVT: 7.0 (0.45%), dDVT: 5.6 (0.36%). Major bleeding: 5 (0.3%). |
| Bonarelli et al., 201520 | Observational | 211 | Dabigatran 220 mg | 35 days | Absence of symptomatic DVT or EP. |
Regarding prophylaxis duration, 90.9% of the studies maintained it for 28 days or more and only one (9.1%) investigated the effects of the use of oral anticoagulants in a shorter time.
These studies observed cases of PE, DVT, and bleeding, but these drugs were considered effective in the prevention of thromboembolic events due to their low occurrence. For DVT, the use of rivaroxaban 10 mg and dabigatran 220 mg for 30–35 days was more satisfactory; similar results were observed for prevention of PE and bleeding, which can also be achieved with Aspirin® at the dose of 81 and 100 mg from 28 to 42 days.
Discussion
As a protocol, oral anticoagulants have been used in several hospitals and orthopedic services worldwide. In Brazil, most services use the following oral anticoagulants: rivaroxaban, dabigatran, and Aspirin®, although the latter is not an anticoagulant, but an antiplatelet agent.
Postoperative thromboprophylaxis can be accomplished with different dosages and duration. In its 2011 guidelines, the American Academy of Orthopedic Surgeons (AAOS) suggests that the surgeon and patient discuss treatment duration in order to individualize it.21 In large orthopedic surgeries, the American College of Chest Physicians (ACCP) guidelines recommend long-term antithrombotic therapy with anticoagulants for 35 days after the day of surgery.22, 23 Some hospitals’ internal protocols recommend prophylaxis for ten days to five weeks.24
The efficacy of rivaroxaban has been demonstrated by several studies: in one study, 33 patients took, the drug for 32–36 days, and only three cases of DVT were observed; rivaroxaban was shown to be important in the prevention of thromboembolic events.12 In a second study, conducted in 2012 with 70 patients who used rivaroxaban for 30 days postoperatively, no cases of DVT or PE were observed.15 A similar finding was also observed by a later study, in which the same medication was used for a similar period (35 days); the study had a larger sample (206 patients) and only 1% of thromboembolic events.16
In a study with 8778 patients, Turpie et al.17 found a rate of 0.89% symptomatic thromboembolism and 0.4% greater bleeding with rivaroxaban 10 mg, once daily.
Other authors used rivaroxaban in different dosages (5, 10, 20, 30, and 40 mg) in 695 subjects for a considerably shorter period (seven days) and observed DVT rates of 14.9%, 10.6%, 8.5%, 13.5%, and 6.4%, respectively, checked by bilateral venous Doppler one day after the last dose. No cases of PE were diagnosed in the study. Moreover, in the analysis of safety and efficacy, oral rivaroxaban 10 mg once daily was shown to be the best option among the doses tested.10
The higher number of cases of DVT found in this last study is noteworthy; it can be explained by a prophylaxis duration lower than that is currently recommended.
Kwong25 presented data that corroborates the aforementioned authors, suggesting that the use of rivaroxaban is safe, without cardiovascular or hepatic effects, and without a statistically significant increase in severe hemorrhage or any bleeding.
Ozler et al.14 studied rivaroxaban 10 mg/day and dabigatran 220 mg/day, both administered orally, once daily, for 30 days after hospital discharge. In their study, thromboprophylaxis was carried out in 60 patients using rivaroxaban and in another 60 patients using dabigatran. Doppler ultrasonography was performed six weeks after surgery; no cases of DVT or PE were observed. There was no statistical difference between the groups regarding postoperative bleeding. The efficacy of dabigatran was confirmed in the case series by Bonarelli et al.,20 in which none of the 211 patients in whom this medication was administered at a dose of 220 mg for 35 days in postoperative hip arthroplasty presented DVT or PE.
Another option analyzed in this study was Aspirin®. Vulcano et al.19 analyzed 887 patients who received Aspirin 325 mg twice daily for 45 days after THA. On the 90th postoperative day, cases of thromboembolic events (1.2%), DVT (0.81%), and PE (0.36%) were documented. Apart from the low number of cases, that study has limitations, as it only included low-risk patients in the aspirin group. In that same year, another study analyzed 50 patients with a hip prosthesis, who received 100 mg aspirin once daily for 42 days postoperatively; these authors observed only three cases of DVT and no cases of PE.18
The use of aspirin had been a point of disagreement between the AAOS and ACCP guidelines, as it was recommended by the former and not by the latter group. In 2012, a consensus was reached and both guidelines started to recommend Aspirin® as a post-THA prophylactic agent.9, 26 In 2014, the Surgical Care Improvement Project (SCIP), which aims to standardize medical and hospital protocols in the United States, included Aspirin® among the prophylactic agents acceptable for thromboembolism in THA, total knee arthroplasty (TKA), and femoral fractures. In 2015, the ACCP23 guidelines maintained Aspirin® as a recommended agent for this prophylaxis. However, conclusive studies are still needed to indicate the best, safest, and most effective dose to be used.27
Final considerations
There is still no consensus regarding the proper use of oral anticoagulants in the postoperative period of hip arthroplasty. Effectiveness is sought in the prevention of thromboembolic events and in the reduction of adverse events.
The three drugs studied presented good results in the prevention of DVT and PE; the best prophylactic results were obtained between 30 and 35 days with oral anticoagulants, which is in agreement with the guidelines suggested by the ACCP, and between 28 and 42 days with antiplatelet agents.
Dabigatran 220 mg once daily was shown to be a good option. However, even with similar complication rates, rivaroxaban 10 mg once daily was the drug that presented the best prophylactic results.
Aspirin was the last drug added to the lists approved drugs for thromboprophylaxis after large orthopedic surgeries. However, the dose to be administered has not been standardized. In addition to the scientific results found, the use of oral anticoagulants is also ethically and legally justified, protecting both physicians and patients.
Conflicts of interest
The authors declare to have no conflicts of interest.
Footnotes
Study conducted at Instituto de Ortopedia e Traumatologia (IOT), Passo Fundo, RS, Brazil.
References
- 1.Learmonth I.D., Young C., Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508–1519. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 2.Iorio R., Robb W.J., Healy W.L., Berry D.J., Hozack W.J., Kyle R.F. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598–1605. doi: 10.2106/JBJS.H.00067. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 4.Leme L.E., Sguizzatto G.T. Prophylaxis of venous thromboembolism in orthopaedic surgery. Rev Bras Ortop. 2015;47(6):685–693. doi: 10.1016/S2255-4971(15)30023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brieger D. Anticoagulation: a GP primer on the new oral anticoagulants. Aust Fam Physician. 2014;43(5):254–259. [PubMed] [Google Scholar]
- 6.Falck-Ytter Y., Francis C.W., Johanson N.A., Curley C., Dahl O.E., Schulman S. American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl.):e278S–e325S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweetland S., Green J., Liu B., Berrington de González A., Canonico M., Reeves G. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ. 2009;339:b4583. doi: 10.1136/bmj.b4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazo-Langner A., Rodger M.A. Overview of current venous thromboembolism protocols in hip reconstruction. Orthop Clin North Am. 2009;40(3):427–436. doi: 10.1016/j.ocl.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson B.I., Borris L.C., Dahl O.E., Haas S., Huisman M.V., Kakkar A.K. ODIXa-HIP Study Investigators. A once-daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59-7939), for thromboprophylaxis after total hip replacement. Circulation. 2006;114(22):2374–2381. doi: 10.1161/CIRCULATIONAHA.106.642074. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson B.I., Borris L.C., Friedman R.J., Haas S., Huisman M.V., Kakkar A.K. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358(26):2765–2775. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 12.Kanan P.S., Schwartsmann C.R., Boschin L.C., Conrad S., Silva M.F. Estudo comparative entre rivaroxaban e enoxaparina na profilaxia de tromboembolismo venoso profundo em pacientes submetidos à artroplastia total do quadril. Rev Bras Ortop. 2008;43(8):319–328. [Google Scholar]
- 13.Anderson D.R., Dunbar M.J., Bohm E.R., Belzile E., Kahn S.R., Zukor D. Aspirin versus low-molecular-weight heparin for extended venous thromboembolism prophylaxis after total hip arthroplasty: a randomized trial. Ann Intern Med. 2013;158(11):800–806. doi: 10.7326/0003-4819-158-11-201306040-00004. [DOI] [PubMed] [Google Scholar]
- 14.Özler T., Uluçay Ç., Önal A., Altıntaş F. Comparison of switch-therapy modalities (enoxaparin to rivaroxaban/dabigatran) and enoxaparin monotherapy after hip and knee replacement. Acta Orthop Traumatol Turc. 2015;49(3):255–259. doi: 10.3944/AOTT.2015.14.0219. [DOI] [PubMed] [Google Scholar]
- 15.Clavé A., Fazilleau F., Dumser D., Lacroix J. Efficacy of tranexamic acid on blood loss after primary cementless total hip replacement with rivaroxaban thromboprophylaxis: a case–control study in 70 patients. Orthop Traumatol Surg Res. 2012;98(5):484–490. doi: 10.1016/j.otsr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Jobski K., Enders D., Amann U., Suzart K., Wallander M.A., Schink T. Use of rivaroxaban in Germany: a database drug utilization study of a drug started in hospital. Eur J Clin Pharmacol. 2014;70(8):975–981. doi: 10.1007/s00228-014-1697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turpie A.G., Haas S., Kreutz R., Mantovani L.G., Pattanayak C.W., Holberg G. A non-interventional comparison of rivaroxaban with standard of care for thromboprophylaxis after major orthopaedic surgery in 17,701 patients with propensity score adjustment. Thromb Haemost. 2014;111(1):94–102. doi: 10.1160/TH13-08-0666. [DOI] [PubMed] [Google Scholar]
- 18.Cossetto D.J., Goudar A., Parkinson K. Safety of peri-operative low-dose aspirin as a part of multimodal venous thromboembolic prophylaxis for total knee and hip arthroplasty. J Orthop Surg (Hong Kong) 2012;20(3):341–343. doi: 10.1177/230949901202000315. [DOI] [PubMed] [Google Scholar]
- 19.Vulcano E., Gesell M., Esposito A., Ma Y., Memtsoudis S.G., Gonzalez Della Valle A. Aspirin for elective hip and knee arthroplasty: a multimodal thromboprophylaxis protocol. Int Orthop. 2012;36(10):1995–2002. doi: 10.1007/s00264-012-1588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonarelli S., Bacchin M.R., Frugiuele I., Feoli M.A., Facchini F., Altimari V. Dabigatran etexilate and LMWH for the prevention of venous thromboembolism in 532 patients undergoing hip surgery. Eur Rev Med Pharmacol Sci. 2015;19(5):897–903. [PubMed] [Google Scholar]
- 21.Jacobs J.J. Recommendations from the American Academy of Orthopedic Surgeons (AAOS) on DVT prophyalxis after orthopedic procedures. Am Acad Orthop Surg (AAOS) Clinical Guideline. 2011 [Google Scholar]
- 22.Guyatt G.H., Akl E.A., Crowther M., Gutterman D.D., Schuünemann H.J. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl.):7S–47S. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falck-Ytter Y., Francis C.W., Johanson N.A., Curley C., Dahl O.E., Schulman S. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl.):e278S–e325S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Protocolo de Profilaxia de Tromboembolismo Venoso em Pacientes Internados. São Paulo: Hospital Sírio Libanês; 2013. Available from: https://www.hospitalsiriolibanes.org.br/institucional/gestao-da-qualidade/Documents/protocolo-profilaxia-tromboembolismo.pdf
- 25.Kwong L.M. Therapeutic potential of rivaroxaban in the prevention of venous thromboembolism following hip and knee replacement surgery: a review of clinical trial data. Vasc Health Risk Manag. 2011;7:461–466. doi: 10.2147/VHRM.S4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geerts W.H., Heit J.A., Clagett G.P., Pineo G.F., Colwell C.W., Anderson F.A., Jr. Prevention of venous thromboembolism. Chest. 2001;119(1 Suppl.):132S–175S. doi: 10.1378/chest.119.1_suppl.132s. [DOI] [PubMed] [Google Scholar]
- 27.Mckee J. SCIP VTE measures changing in 2014 – new measures go into effect on Jan. 1, 2014. AAOS Now. November 2013 Available from: http://www.aaos.org/news/aaosnow/nov13/cover2.asp [Accessed 12 October 2015] [Google Scholar]