Abstract
Experimental studies have suggested that Wingless-related integration site 5A (WNT5A) is a proinflammatory secreted protein that is associated with metabolic dysfunction in obesity. Impaired angiogenesis in fat depots has been implicated in the development of adipose tissue capillary rarefaction, hypoxia, inflammation, and metabolic dysfunction. We have recently demonstrated that impaired adipose tissue angiogenesis is associated with overexpression of antiangiogenic factor VEGF-A165b in human fat and the systemic circulation. In the present study, we postulated that upregulation of WNT5A is associated with angiogenic dysfunction and examined its role in regulating VEGF-A165b expression in human obesity. We biopsied subcutaneous and visceral adipose tissue from 38 obese individuals (body mass index: 44 ± 7 kg/m2, age: 37 ± 11 yr) during planned bariatric surgery and characterized depot-specific protein expression of VEGF-A165b and WNT5A using Western blot analysis. In both subcutaneous and visceral fat, VEGF-A165b expression correlated strongly with WNT5A protein (r = 0.9, P < 0.001). In subcutaneous adipose tissue where angiogenic capacity is greater than in the visceral depot, exogenous human recombinant WNT5A increased VEGF-A165b expression in both whole adipose tissue and isolated vascular endothelial cell fractions (P < 0.01 and P < 0.05, respectively). This was associated with markedly blunted angiogenic capillary sprout formation in human fat pad explants. Moreover, recombinant WNT5A increased secretion of soluble fms-like tyrosine kinase-1, a negative regulator of angiogenesis, in the sprout media (P < 0.01). Both VEGF-A165b-neutralizing antibody and secreted frizzled-related protein 5, which acts as a decoy receptor for WNT5A, significantly improved capillary sprout formation and reduced soluble fms-like tyrosine kinase-1 production (P < 0.05). We demonstrated a significant regulatory nexus between WNT5A and antiangiogenic VEGF-A165b in the adipose tissue of obese subjects that was linked to angiogenic dysfunction. Elevated WNT5A expression in obesity may function as a negative regulator of angiogenesis.
NEW & NOTEWORTHY Wingless-related integration site 5a (WNT5A) negatively regulates adipose tissue angiogenesis via VEGF-A165b in human obesity.
Keywords: obesity, angiogenesis, adipose tissue, Wingless-related integration site 5a, VEGF-A165b
obesity and its metabolic complications have emerged as major healthcare problems in the United States and worldwide (15, 18, 30). In particular, central adiposity and excess accumulation of visceral fat have been most closely associated with cardiometabolic risk (7, 10, 11, 13, 20, 24). Fat tissue dysfunction within the adipose microenvironment characterized by inflammation, hypoxia, vascular rarefaction, and adipokine dysregulation has been implicated in the pathogenesis of obesity-induced systemic diseases (15a, 25, 26). Experimental data suggest that reduced angiogenesis and vascularization within human fat domains may contribute to impaired adipose tissue perfusion and nutrient exchange and metabolic dysregulation (17, 24). We (21) recently demonstrated that tissue angiogenic dysfunction is associated with upregulation of an antiangiogenic isoform of VEGF-A, VEGF-A165b. VEGF-A165b antagonism can restore proliferative signaling and promote neovascularization; however, regulation of its expression is incompletely understood.
Our previous work (16) identified noncanonical Wingless-related integration site 5A (WNT5A) signaling as a key modulator of adipose tissue inflammation and metabolic dysfunction in experimental animals and human tissue. Furthermore, WNT5A can promote inflammation-driven VEGF-A165b expression, which has been shown to impair vascular collateralization in murine hindlimb ischemic models (21). These data support the hypothesis that aberrant regulation of noncanonical WNT5A signaling in the obese state may be an important but previously unrecognized mediator of obesity-linked vascular disease. To date, the link between WNT5A and VEGF-A165b activation and its associated angiogenic effects in fat have not been examined in human obesity. In the present study, we tested the hypothesis that obesity-induced upregulation of WNT5A controls adipose tissue angiogenesis via modulation of VEGF-A165b expression.
MATERIALS AND METHODS
Study subjects.
Consecutive obese men and women [body mass index (BMI) ≥ 35 kg/m2, age ≥ 18 yr] with severe long-standing obesity enrolled in the Boston Medical Center Bariatric Surgery Program were recruited into the study. Among our study participants, 53% were white, 32% were African-American, 2% were Hispanic, and 13% self-identified as multiracial. Samples of subcutaneous and visceral adipose tissue were collected intraoperatively from the lower abdominal wall and greater omentum during planned bariatric surgery, as previously described (14, 20). Each subject provided one biopsy specimen from the subcutaneous fat depot and one specimen from the visceral fat depot. Subjects with unstable medical conditions or pregnancy were not eligible for bariatric surgery and thus excluded. The study was approved by the Boston University Medical Center Institutional Review Board, and written consent was obtained from all participants.
Anthropometric and biochemical measures.
During a single outpatient visit before planned bariatric surgery, clinical characteristics including blood pressure, height, weight, and waist circumference were measured along with recording of cardiovascular risk factors. Fasting blood was taken via an antecubital vein for biochemical parameters including lipids, glucose, insulin, glycosylated hemoglobin (HbA1c), and high-sensitivity C-reactive protein. Homeostasis model assessment was used as the index of insulin sensitivity. All biochemical analyses were performed by the Boston Medical Center clinical chemistry laboratory.
Angiogenesis assay of adipose tissue.
The fat pad angiogenesis assay was performed as previously described (24). Briefly, freshly collected fat samples removed during bariatric surgery were immediately placed in sterile endothelial basal media (EBM-2; catalog no. CC3156, Lonza). Fat pads were finely minced and enzymatically digested with 1 mg/ml collagenase type II (catalog no. LS004176, Worthington Biochemical) for 30 min at 37°C, and digested tissue was passed through 100-μm nylon filter and washed in EBM-2. Pieces of ~1 mm2 were embedded in 150 μl/well growth factor-depleted Matrigel (catalog no. 354230, Corning) on a 48-well plate placed on ice and incubated for 30 min at 37°C. After polymerization of Matrigel, wells were covered with 500 μl EBM-2 supplemented with growth factors (EGM-MV; catalog no. CC4143, Lonza) and cultured at 37°C for 7 days. For samples treated with recombinant human (rh)WNT5A (catalog no. 645-WN-010, R&D Systems), 500 ng rhWNT5A was added to the media initially and during each media change. Samples treated with rhWNT5A and VEGF-A165b-neutralizing antibody (clone 56-1, catalog no. MAB3045, R&D Systems), 500 ng/ml rhWNT5A, and 10 μg/ml VEGF-A165b-neutralizing antibody were added in the media at the beginning and during each media change. In addition, for samples treated with rhWNT5A and secreted frizzled-related protein 5 (SFRP5; catalog no. 6266-SF, R&D Systems), 500 ng/ml both rhWNT5A and SFRP5 were added to the media initially and during each media change. Half of the media was removed and replaced with fresh media every other day. Sprouts growing from the adipose tissue explant were counted along the perimeter of each sample under ×100 magnification by an investigator who was blinded to the outcome of the experiment.
Western immunoblot analyses.
VEGF-A165b (catalog no. MAB3045-100) and WNT5A (catalog no. ab72583) antibodies for Western blot analysis were purchased from R&D Systems and Abcam, respectively. Proteins were extracted from adipose tissue by homogenizing in liquid nitrogen followed by the addition of ice-cold lysis buffer [50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and 0.25% SDS] supplemented with protease inhibitor cocktail and phosphatase inhibitors 2 and 3. Samples were assayed for protein content using the Bradford method. Twenty to thirty micrograms of protein were subjected to electrophoresis in a 12% SDS-polyacrylamide gel under reducing conditions, blotted to a polyvinylidene difluoride membrane using the Bio-Rad Trans-Blot Turbo Transfer system, blocked with Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE), and then incubated overnight with the respective anti-human primary antibodies at 4°C. The amount of protein loaded was the same for both depots for each specific subject. Membranes that were immunostained for VEGF-A165b and WNT5A were incubated with infrared dye-conjugated secondary antibodies and visualized and quantified using the LI-COR Odyssey system.
Secretion of VEGF-A165b and soluble fms-like tyrosine kinase-1.
Sprout media were collected on day 2 for VEGF-A165b quantification and day 7 for soluble fms-like tyrosine kinase-1 (sFlt-1) measurement for each experimental condition. For VEGF-A165b quantification, samples were concentrated at 1:100 dilution using StrataClean Resin (catalog no. 400714, Agilent Technologies). Samples were subsequently subjected to Western blot analysis under reducing conditions as described above. Total protein was adjusted by staining with the Pierce Reversible Protein Stain Kit (catalog no. 24580, Thermo Scientific). Secretion of sFlt-1 in the sprout media was quantified using an ELISA kit from R&D Systems (catalog no. DVR100B) according to the manufacturer’s instructions.
Endothelial cell isolation from adipose tissue.
Subcutaneous fat tissue samples collected during surgery were placed immediately in cold DMEM and used to isolate endothelial cells as previously described (20). Briefly, tissue was cut into small pieces, minced, and digested in cocktail of collagenase type I and Dispase I (catalog nos. 234153 and D4818, respectively, Sigma-Aldrich) for 1 h in a 37°C water bath at 100 rpm rotation. To remove undigested tissue, cells were passed through a 100-μm filter and then centrifuged at 600 rpm at 4°C for 10 min to separate adipocytes. Red blood cells were lysed using 1× red blood cell lysis buffer (catalog no. WL1000, R&D Systems), and the remaining cells were labeled with CD31 microbeads (catalog no. 130-091-935, Miltenyi Biotech) before being loaded into the autoMACS Pro Separator. Isolated CD31-positive endothelial cells were plated on fibronectin (catalog no. NC0702888, Fisher Scientific)-coated eight-well chamber slides. Cells were then treated with 500 ng rhWNT5A for 48 h, fixed, and stored at −80°C for immunofluorescence analysis.
Endothelial cell quantitative immunofluorescence.
We quantified VEGF-A165b protein expression of isolated endothelial cells in response to rhWNT5A treatment as previously described (20). Briefly, fixed samples were rehydrated with 50 mmol/l glycine, permeabilized with 0.1% Triton X-100, and blocked with 0.5% BSA. Slides were incubated for overnight at 37°C with primary antibodies against VEGF-A165b (catalog no. MAB3045, R&D Systems) and CD31 (catalog no. MA5-13188, Thermo Fisher Scientific) to select endothelial cells. We used analogous Alexa fluor 488 and Alexa fluor 594 antibodies (catalog nos. A11001 and A11012, respectively, Invitrogen) for the secondary antibodies. Cells were mounted under glass coverslips with VECTASHIELD (catalog no. H-1500, Vector) containing 4′,6′-diamidino-2-phenylindole (DAPI) to identify nuclei. Slides were imaged using a fluorescent microscope (×20 magnification, Nikon Eclipse TE2000E, Nikon Instruments, Melville, NY), and digital images were captured using a Photometrics CoolSNAP HQ2 Camera (Photometrics, Tucson, AZ). Exposure time was kept constant, and fluorescent intensity (corrected for background fluorescence) was quantified by NIS-Elements AR software (Nikon Instruments). To control for batch to batch staining variability, fluorescence intensity for each sample was normalized to the intensity of human aortic endothelial cell staining performed simultaneously. Data are expressed in arbitrary units calculated by dividing the average fluorescence intensity of the subject sample by the intensity of the human aortic endothelial cell sample multiplied by 100, as previously described and validated (14).
Statistics.
Clinical characteristics of subjects were analyzed using SPSS 20.0 and presented as means ± SD or percentages. Other analyses were performed using GraphPad Prism 6.0 software. The area under the curve of the plot for cumulative angiogenic growth (quantified as capillary sprout count) over the time period of 7 days was examined using GraphPad Prism 6.0. Differences between rhWNT5A treatment alone and vehicle control and treatments with rhWNT5A and VEGF-A165b-neutralizing antibody or SFRP5 were analyzed using one-way ANOVA with post hoc analysis. Spearman correlation analysis was performed to examine associations between VEGF-A165b and WNT5A protein expression. Values of P < 0.05 were considered significant. Graphic data are presented as means ± SE unless otherwise indicated.
RESULTS
Clinical characteristics.
A total of 38 obese subjects (BMI: 44 ± 7 kg/m2, age: 37 ± 11 yr) were recruited for the study. Clinical characteristic of subjects are shown in Table 1. As expected and consistent with bariatric surgery populations, subjects were predominantly women with metabolic abnormalities and evidence of insulin resistance and systemic inflammation. Categories for medication use are shown in Table 2.
Table 1.
Clinical characteristics
| Parameter | Value |
|---|---|
| Number of subjects | 38 |
| Age, yr | 37 ± 11 |
| Women, % | 89.5 |
| Men, % | 11.5 |
| Body mass index, kg/m2 | 44 ± 7 |
| Waist circumference, cm | 117 ± 15 |
| Weight, kg | 123 ± 23 |
| Insulin, mIU/ml | 17 ± 10 |
| Glucose, mg/dl | 108 ± 44 |
| HbA1c, % | 6.1 ± 1.6 |
| Homeostasis model assessment of insulin resistance | 4.7 ± 3.6 |
| High-sensitivity C-reactive protein, mg/dl | 9.3 ± 5.8 |
| Triglycerides, mg/dl | 129 ± 82 |
| HDL-cholesterol, mg/dl | 47 ± 11 |
| LDL-cholesterol, mg/dl | 121 ± 33 |
| Systolic blood pressure, mmHg | 128 ± 12 |
| Diastolic blood pressure, mmHg | 77 ± 14 |
| Diabetes mellitus, % | 29 |
| Hypertension, % | 37 |
| Hypercholesterolemia, % | 24 |
Data are means ± SD.
Table 2.
Prevalence of medication use
| Class of Medication | Prevalence, % |
|---|---|
| Antihypertensive | 37 |
| Lipid lowering | 13 |
| Hypoglycemic | 13 |
Correlation between VEGF-A165b and WNT5A protein expression in adipose tissue.
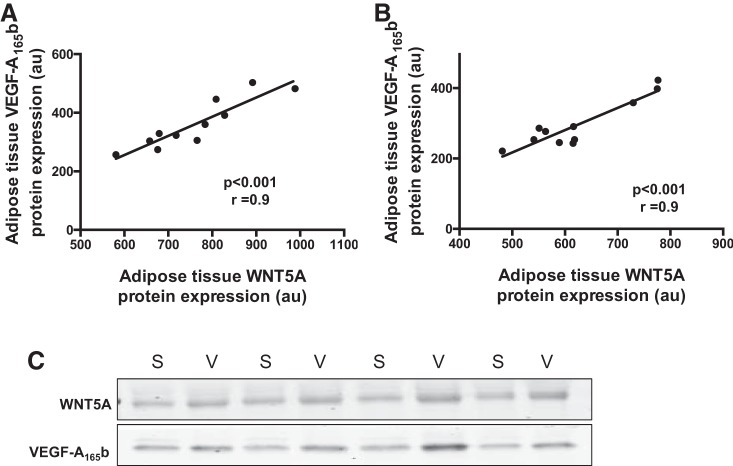
We examined WNT5A and VEGF-A165b protein levels in subjects that provided paired adipose tissue samples simultaneously from both their subcutaneous and visceral fat. In both fat depots, there were strong correlations between WNT5A and VEGF-A165b expression (n = 11, P < 0.001, r = 0.9; Fig. 1, A and B). Representative Western blots for both proteins are shown in Fig. 1C.
Fig. 1.
Correlation between VEGF-A165b and Wingless-related integration site 5A (WNT5A) protein in adipose tissue. VEGF-A165b protein expression was positively associated with WNT5A in subcutaneous adipose tissue (A; n = 11, P < 0.001, r = 0.9) and visceral adipose depot (B; n = 11, P < 0.001, r = 0.9). au, Arbitrary units. C: representative Western blots for VEGF-A165b and WNT5A. S, subcutaneous; V, visceral.
Effect of rhWNT5A on adipose tissue angiogenesis.
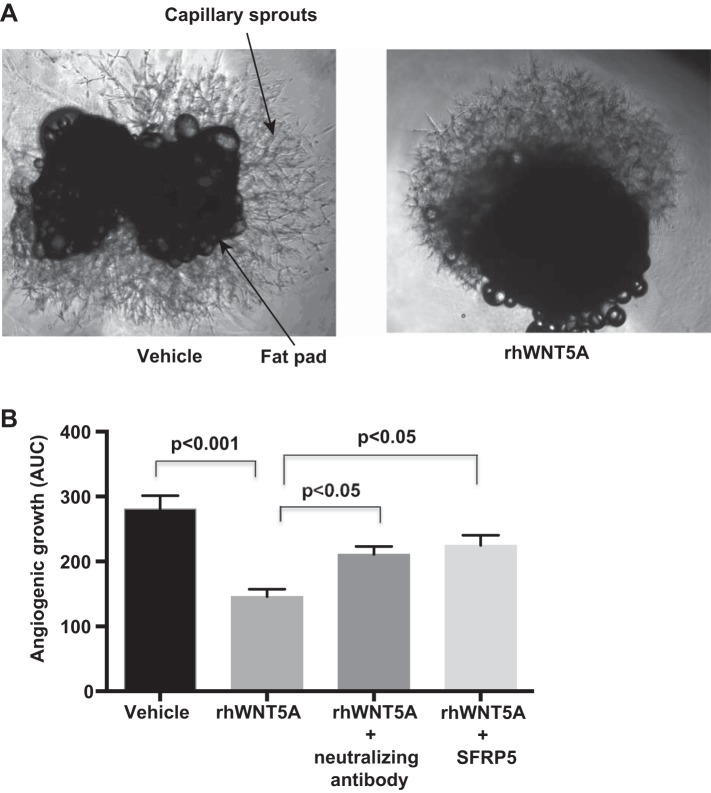
Given the strong associations above, we examined whether WNT5A negatively modulates adipose tissue angiogenesis via VEGF-A165b. We focused on the subcutaneous depot where previous data demonstrated differentially higher angiogenic capacity than visceral fat (24). As shown in Fig. 2, A and B, ex vivo capillary sprouting from the central fat pad was significantly blunted in adipose tissue samples exposed to recombinant human WNT5A compared with vehicle control (n = 19, P < 0.001). The inhibitory effect of rhWNT5A on angiogenesis was rescued by treatments with either VEGF-A165b-neutralizing antibody (n = 8, P < 0.05) or SFRP5 (n = 8, P < 0.05).
Fig. 2.
A: representative image of angiogenic capillary sprouting in subcutaneous adipose fat pads with and without (vehicle) recombinant human (rh)WNT5A treatment. B: quantitative analysis demonstrating reduced angiogenic growth in rhWNT5A-treated samples (n = 19, P < 0.001) compared with vehicle control. Angiogenic growth improved with either VEGF-A165b-neutralizing antibody or SFRP5 (n = 8, P < 0.05). Data are presented as means ± SE. AUC, area under the curve.
Effect of rhWNT5A on VEGF-A165b and sFlt-1 production.
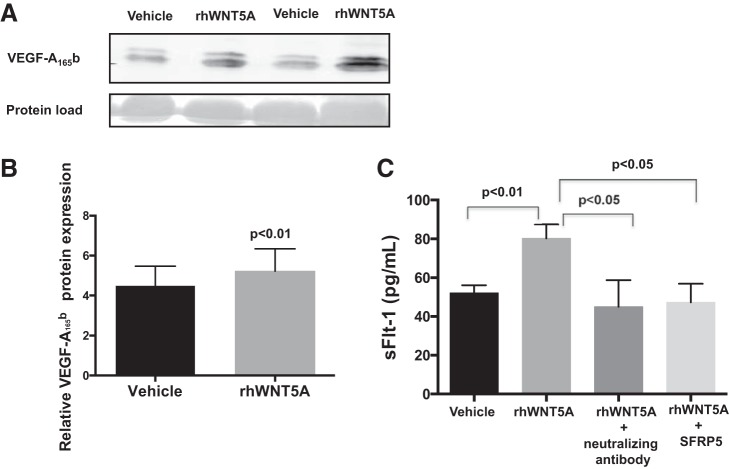
We quantified VEGF-A165b release into the sprout media after 48 h of rhWNT5A treatment. As shown in Fig. 3, A and B, rhWNT5A significantly increased VEGF-A165b levels in treated samples compared with control (n = 11, P < 0.01). It has been reported that WNT5A exerts antiangiogenic effects via splice variant of the receptor sFlt-1 (28). In our samples, we observed a significant effect of rhWNT5A in upregulating sFlt-1 concentrations released into the media (n = 24, P < 0.01; Fig. 3C). Both VEGF-A165b-neutralizing antibody and SFRP5 reduced sFlt-1 release into the media (n = 8, P < 0.05; Fig. 3C).
Fig. 3.
rhWNT5A increases VEGF-A165b release into the culture media. A: representative immunoblot demonstrating VEGF-A165b production with and without (vehicle) rhWNT5A treatment. B: VEGF-A165b secretion is increased with rhWNT5A treatment compared with vehicle control (n = 14; P < 0.01). C: soluble fms-like tyrosine kinase-1 (sFlt-1) secretion was increased with rhWNT5A treatment compared with vehicle control (n = 24, P < 0.01). sFlt-1 release was reduced with either VEGF-A165b-neutralizing antibody or secreted frizzled-related protein 5 (SFRP5; n = 8, P < 0.05). Data are presented as means ± SE.
Effect of rhWNT5A on VEGF-A165b protein expression in isolated endothelial cells.
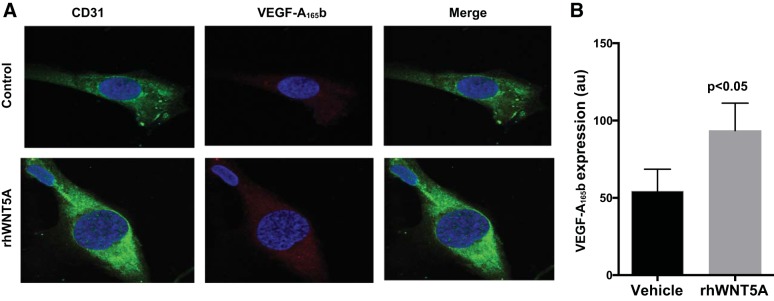
To provide evidence for cell-autonomous functional defects at the level of the endothelium, we developed methods to isolate endothelial cells from human adipose tissue and characterized their functional properties using quantitative immunofluorescence for VEGF-A165b. As shown in Fig. 4A, treatment with rhWNT5A significantly increased endothelial cell VEGF-A165b protein expression compared with control, as measured by immunofluorescence (n = 7, P < 0.05). The relative quantitative effects are shown in Fig. 4B.
Fig. 4.
A: representative immunofluorescence images of isolated endothelial cells from subcutaneous adipose tissue demonstrating increased VEGF-A165b with rhWNT5A treatment compared with control. Red, VEGF-A165b; green, CD31; blue, DAPI. B: quantification of VEGF-A165b expression with and without rhWNT5A treatment in endothelial cells isolated from subcutaneous fat (n = 7, P < 0.05). Data are presented as au and expressed as means ± SE.
DISCUSSION
In the present study, we demonstrated that WNT5A regulates angiogenic capacity in part via upregulation of an antiangiogenic VEGF-A isoform VEGF-A165b. Although interregulatory mechanisms of these proteins have not been extensively studied in the context of human adiposity, we observed coordinate upregulation and strong associations between WNT5A and VEGF-A165b protein expression in both subcutaneous and visceral fat depots of obese subjects. We showed that recombinant WNT5A impairs adipose tissue angiogenic capacity, increases endothelial cell VEGF-A165b expression, and stimulates sFlt-1 release. Consistently, pharmacological antagonism with VEGF-A165b-neutralizing antibody or SFRP5 rescued the impaired angiogenesis phenotype. These findings are salient because, to our knowledge, they have not been previously described in human adipose tissue and prompt recognition that WNT5A may play an important role in regulating vascularization.
Our previous data showed that selective overexpression of VEGF-A165b in visceral obesity inhibits angiogenic proliferation by competitive interference with proangiogenic VEGF-A isoforms (24). The regulatory control of VEGF-A165b, which results from alternative VEGF isoform splicing, is a poorly understood yet potentially important process that could be clinically significant. Among a broad cadre of candidate regulators and proinflammatory cytokines that are overexpressed in obesity, we recently identified noncanonical WNT5A signaling as a key mediator of insulin resistance, inflammation, and vascular dysfunction (16, 21). Wnt proteins consist of secreted molecules that play fundamental roles in regulating cell proliferation, differentiation, and body axis patterning during embryonic development. More recently, they have also been implicated in critical aspects of clinical disease in adults (1, 5). There are 19 WNT family members in mammals that typically act in an autocrine/paracrine fashion to activate a number of signaling pathways, generally classified as either canonical (β-catenin-dependent), typified by WNT1, WNT3a, and WNT10b, or noncanonical (β-catenin-independent), mainly WNT5A and WNT11. WNTs play important roles in adipose tissue homeostasis, and cellular, animal, and human studies from our combined laboratories have recently provided evidence that WNT5A-mediated noncanonical signaling promotes adipose tissue inflammation and contributes to obesity-associated insulin resistance (16).
Angiogenesis, the generation of new blood vessels, plays an important role in tissue expansion and remodeling, and adequate vascular support appears critical for normal homeostatic and metabolic functions of adipose tissue (8). Clinical and animal studies have suggested that fat mass expansion in obesity may lead to relative vascular insufficiency and pseudohypoxic conditions within the adipose milieu that are associated with inflammation and metabolic dysfunction (26). Experimental animal studies have shown that stimulating neovascularization in fat improves whole body metabolism, whereas antagonizing vascular proliferation has the opposite negative effect (12, 27, 29). This suggests that tissue vascularity may play a key role in shaping metabolic phenotypes; however, regulatory mechanisms are incompletely understood.
In the present study, we demonstrated that WNT5A governs neovascularization within the human fat pad, which conceptually builds on our recent vascular findings in murine models (21). We reported that antiangiogenic VEGF-A165b is upregulated in mice with leptin deficiency, diet-induced obesity, genetic ablation of the anti-inflammatory adipokine SFRP5, and transgenic overexpression of WNT5A in myeloid cells (21). In these conditions, regenerative angiogenesis is impaired in the skeletal muscle of mice after surgical hindlimb ligation, and this is linked to elevated VEGF-A165b expression. One mechanism connecting WNT5A to VEGF-A165b may be via sFlt-1, which results from alternative splicing of the VEGF receptor as Flt-1 that can act as an endogenous inhibitor of angiogenesis by sequestering VEGF (28). Our findings that WNT5A increases both VEGF-A165b and sFlt-1 support this hypothesis. Furthermore, we observed that antagonism of WNT5A by SFRP5 or VEGF-A165b-neutralizing antibody restores capillary growth. Taken together, these data suggest that perturbations in the WNT5A regulatory system can contribute to defective vascularization.
Little is known about the role of VEGF-A165b in human disease, although it appears to be expressed ubiquitously in different tissues, including the eyes, muscle, vascular endothelium, and skin, among others (2, 3, 19, 23). Its pathogenic role has been described in systemic sclerosis in which VEGF-A165b-neutralizing antibody improves angiogenesis of microvascular endothelial cells (23). Conversely, VEGF-A165b is downregulated in diabetic retinopathy and some cancers such as renal cell carcinoma and malignant melanoma and has thus been discussed as a potential treatment strategy in oncological diseases (4). We (24) recently showed that VEGF-A165b is overexpressed in adipose tissue and circulating blood and associated with reduced adipose tissue angiogenic capacity in severely obese individuals. Interestingly, weight loss lowers blood concentrations of both VEGF-A165b and WNT5A (9, 24); however, direct vascular effects of these changes are unknown.
Our study has several limitations. First, the study consisted of a severely obese population undergoing planned bariatric surgery. Although this allowed for a pragmatic approach to secure human adipose tissue samples, the findings may not be applicable to the general population. Second, the experimental model involved ex vivo studies that may not fully recapitulate in vivo conditions, although studies were performed under conditions that approximate the physiological state. Third, the majority of study participants were women, which reflects the general clinical practice and known sex differences in populations that seek bariatric surgery treatments (6, 22). Fourth, we did not identify the main cellular source of VEGF-A165b in human fat, which will be a direction for future studies. Fifth, we did not find any correlations between common clinical variables and adipose tissue protein expressions, which was likely due to a relatively small sample size and limited power with <30% of subjects with diabetes. Finally, we examined responses within the adipose tissue microvasculature, which may not reflect processes at the level of other systemic organs or the coronary circulation.
In conclusion, we demonstrate a regulatory connection between WNT5A and antiangiogenic VEGF-A165b in the adipose tissue of obese subjects that was linked to angiogenic dysfunction. WNT5A overexpression in obesity and conditions of metabolic stress may be a negative regulator of angiogenesis.
GRANTS
N. Gokce is supported by National Heart, Lung, and Blood Institute (NHLBI) Grants HL-081587, HL-114675, and HL-126141. S. Karki is supported by NHLBI Grant T32-HL-07224. K. Walsh is supported by NHLBI Grants HL-120160, HL-126141, HL-131006, and HL-132564. M. G. Farb is supported by NHLBI Grant K23-HL-135394.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.K. conceived and designed research; S.K. and D.T.M.N. performed experiments; S.K. and D.T.M.N. analyzed data; S.K. and D.T.M.N. interpreted results of experiments; S.K. prepared figures; S.K. drafted manuscript; S.K., D.T.M.N., M.G.F., S.Y.P., S.M.S., N.M.H., B.C., D.T.H., K.W., and N.G. edited and revised manuscript; N.G. approved final version of manuscript.
REFERENCES
- 1.Arnold AC, Robertson D. Defective Wnt signaling: a potential contributor to cardiometabolic disease? Diabetes : 3342–3344, 2015. doi: 10.2337/db15-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba T, McLeod DS, Edwards MM, Merges C, Sen T, Sinha D, Lutty GA. VEGF 165 b in the developing vasculatures of the fetal human eye. Dev Dyn : 595–607, 2012. doi: 10.1002/dvdy.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res : 4123–4131, 2002. [PubMed] [Google Scholar]
- 4.Bates DO, Harper SJ. Therapeutic potential of inhibitory VEGF splice variants. Future Oncol : 467–473, 2005. doi: 10.2217/14796694.1.4.467. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt PM, Malgor R. WNT5A: a player in the pathogenesis of atherosclerosis and other inflammatory disorders. Atherosclerosis : 155–162, 2014. doi: 10.1016/j.atherosclerosis.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigornia SJ, Farb MG, Tiwari S, Karki S, Hamburg NM, Vita JA, Hess DT, Lavalley MP, Apovian CM, Gokce N. Insulin status and vascular responses to weight loss in obesity. J Am Coll Cardiol : 2297–2305, 2013. doi: 10.1016/j.jacc.2013.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation : e837–e841, 2011. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov : 107–115, 2010. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- 9.Catalán V, Gómez-Ambrosi J, Rodríguez A, Pérez-Hernández AI, Gurbindo J, Ramírez B, Méndez-Giménez L, Rotellar F, Valentí V, Moncada R, Martí P, Sola I, Silva C, Salvador J, Frühbeck G. Activation of noncanonical Wnt signaling through WNT5A in visceral adipose tissue of obese subjects is related to inflammation. J Clin Endocrinol Metab : E1407–E1417, 2014. doi: 10.1210/jc.2014-1191. [DOI] [PubMed] [Google Scholar]
- 10.Coutinho T, Goel K, Corrêa de Sá D, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol : 1877–1886, 2011. doi: 10.1016/j.jacc.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 11.de Jongh RT, Serné EH, IJzerman RG, Jørstad HT, Stehouwer CD. Impaired local microvascular vasodilatory effects of insulin and reduced skin microvascular vasomotion in obese women. Microvasc Res : 256–262, 2008. doi: 10.1016/j.mvr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Elias I, Franckhauser S, Ferré T, Vilà L, Tafuro S, Muñoz S, Roca C, Ramos D, Pujol A, Riu E, Ruberte J, Bosch F. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes : 1801–1813, 2012. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farb MG, Ganley-Leal L, Mott M, Liang Y, Ercan B, Widlansky ME, Bigornia SJ, Fiscale AJ, Apovian CM, Carmine B, Hess DT, Vita JA, Gokce N. Arteriolar function in visceral adipose tissue is impaired in human obesity. Arterioscler Thromb Vasc Biol : 467–473, 2012. doi: 10.1161/ATVBAHA.111.235846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farb MG, Tiwari S, Karki S, Ngo DT, Carmine B, Hess DT, Zuriaga MA, Walsh K, Fetterman JL, Hamburg NM, Vita JA, Apovian CM, Gokce N. Cyclooxygenase inhibition improves endothelial vasomotor dysfunction of visceral adipose arterioles in human obesity. Obesity (Silver Spring) : 349–355, 2014. doi: 10.1002/oby.20505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA : 491–497, 2012. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 15a.Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res : 1786–1807, 2016. doi: 10.1161/CIRCRESAHA.115.306885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuster JJ, Zuriaga MA, Ngo DT, Farb MG, Aprahamian T, Yamaguchi TP, Gokce N, Walsh K. Noncanonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes : 1235–1248, 2015. doi: 10.2337/db14-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran KV, Straubhaar J, Nicoloro S, Czech MP, Thompson M, Perugini RA, Corvera S. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation : 186–194, 2011. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society . 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol : 2985–3023, 2014. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Jones WS, Duscha BD, Robbins JL, Duggan NN, Regensteiner JG, Kraus WE, Hiatt WR, Dokun AO, Annex BH. Alteration in angiogenic and anti-angiogenic forms of vascular endothelial growth factor-A in skeletal muscle of patients with intermittent claudication following exercise training. Vasc Med : 94–100, 2012. doi: 10.1177/1358863X11436334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karki S, Farb MG, Ngo DT, Myers S, Puri V, Hamburg NM, Carmine B, Hess DT, Gokce N. Forkhead box O-1 modulation improves endothelial insulin resistance in human obesity. Arterioscler Thromb Vasc Biol : 1498–1506, 2015. doi: 10.1161/ATVBAHA.114.305139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kikuchi R, Nakamura K, MacLauchlan S, Ngo DT, Shimizu I, Fuster JJ, Katanasaka Y, Yoshida S, Qiu Y, Yamaguchi TP, Matsushita T, Murohara T, Gokce N, Bates DO, Hamburg NM, Walsh K. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med : 1464–1471, 2014. doi: 10.1038/nm.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longitudinal Assessment of Bariatric Surgery (LABS) Consortium Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med : 445–454, 2009. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manetti M, Guiducci S, Ibba-Manneschi L, Matucci-Cerinic M. Impaired angiogenesis in systemic sclerosis: the emerging role of the antiangiogenic VEGF(165)b splice variant. Trends Cardiovasc Med : 204–210, 2011. doi: 10.1016/j.tcm.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Ngo DT, Farb MG, Kikuchi R, Karki S, Tiwari S, Bigornia SJ, Bates DO, LaValley MP, Hamburg NM, Vita JA, Hess DT, Walsh K, Gokce N. Antiangiogenic actions of vascular endothelial growth factor-A165b, an inhibitory isoform of vascular endothelial growth factor-A, in human obesity. Circulation : 1072–1080, 2014. doi: 10.1161/CIRCULATIONAHA.113.008171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasarica M, Rood J, Ravussin E, Schwarz JM, Smith SR, Redman LM. Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J Clin Endocrinol Metab : 4052–4055, 2010. doi: 10.1210/jc.2009-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes : 718–725, 2009. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu I, Aprahamian T, Kikuchi R, Shimizu A, Papanicolaou KN, MacLauchlan S, Maruyama S, Walsh K. Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Invest : 2099–2112, 2014. doi: 10.1172/JCI71643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stefater JA 3rd, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, Fan J, Ajima R, Molkentin JD, Williams BO, Wills-Karp M, Pollard JW, Yamaguchi T, Ferrara N, Gerhardt H, Lang RA. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature : 511–515, 2011. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sung HK, Doh KO, Son JE, Park JG, Bae Y, Choi S, Nelson SM, Cowling R, Nagy K, Michael IP, Koh GY, Adamson SL, Pawson T, Nagy A. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab : 61–72, 2013. doi: 10.1016/j.cmet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet : 815–825, 2011. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]