Abstract
Mitochondrial damage is often overlooked in acute lung injury (ALI), yet most of the lung’s physiological processes, such as airway tone, mucociliary clearance, ventilation-perfusion (Va/Q) matching, and immune surveillance require aerobic energy provision. Because the cell’s mitochondrial quality control (QC) process regulates the elimination and replacement of damaged mitochondria to maintain cell survival, we serially evaluated mitochondrial biogenesis and mitophagy in the alveolar regions of mice in a validated Staphylococcus aureus pneumonia model. We report that apart from cell lysis by direct contact with microbes, modest epithelial cell death was detected despite significant mitochondrial damage. Cell death by TdT-mediated dUTP nick-end labeling staining occurred on days 1 and 2 postinoculation: apoptosis shown by caspase-3 cleavage was present on days 1 and 2, while necroptosis shown by increased levels of phospho- mixed lineage kinase domain-like protein (MLKL) and receptor-interacting serine/threonine-protein kinase 1 (RIPK1) was present on day 1. Cell death in alveolar type I (AT1) cells assessed by bronchoalveolar lavage fluid receptor for advanced glycation end points (RAGE) levels was high, yet AT2 cell death was limited while both mitochondrial biogenesis and mitophagy were induced. These mitochondrial QC mechanisms were evaluated mainly in AT2 cells by localizing increases in citrate synthase content, increases in nuclear mitochondrial biogenesis regulators nuclear respiratory factor-1 (NRF-1) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), and increases in light chain 3B protein (LC3-I)/LC3II ratios. Concomitant changes in p62, Pink 1, and Parkin protein levels indicated activation of mitophagy. By confocal microscopy, mitochondrial biogenesis and mitophagy were often observed on day 1 within the same AT2 cells. These findings imply that mitochondrial QC activation in pneumonia-damaged AT2 cells promotes cell survival in support of alveolar function.
Keywords: mitophogy, quality control, acute lung injury, alveolar epithelial cells, mitochondria
Pathogenic Staphylococcus aureus bacterial species cause acute necrotizing pneumonia in humans (5), commonly as a complication of influenza. S. aureus is notorious for the capacity to rapidly develop antibiotic resistance and for the expression of virulence factors that act as immune evasion molecules (5, 12). Virulence factors, including super-antigens (SAgs), target innate immune cells, such as neutrophils and macrophages, for destruction or loss-of-function, counteracting the host defenses necessary for S. aureus clearance (41). SAgs can induce massive inflammation and cell death, causing the severe lung parenchymal destruction associated with this pneumonia (13, 39). For instance, α-hemolysin toxin activates the nucleotide-binding domain and leucine-rich repeat (LRR)-containing gene family, pyrin domain containing 3 (NLRP3) inflammasome to induce interleukin-1β, and programmed necrosis (necroptosis) (17). The production of α-hemolysin and other S. aureus toxins also triggers necroptosis through the receptor-interacting serine/threonine-protein kinase 1 (RIPK1)/RIPK3/mixed lineage kinase domain-like protein (MLKL) signaling pathway, which amplifies damage in resident lung cells and alveolar macrophages (18).
Phagocytosis of S. aureus by alveolar macrophages can activate NLRP3 in the lung through innate mechanisms involving mitochondria and release of danger molecules such as ATP from leaky or dying cells (8). In the imperiled cell, phagolysosomal rupture causes free calcium mobilization that can induce mitochondrial pore transition and activate the NLRP3 inflammasome (24). These events increase mitochondrial reactive oxygen species production as well as other inflammasome-activating mitochondrial products, such as the mitochondrial antiviral signaling protein (33) and oxidized mitochondrial DNA (mtDNA) (32, 42). NLRP3 activators induce the release of the mitochondrial membrane lipid cardiolipin, which binds the LRR region of NLRP3 in parallel with potassium efflux (15). This allows NLRP3 activation in macrophages independently of reactive oxygen species production. Together these findings implicate mitochondrial damage as a critical factor in NLRP3 activation in numerous cell types (8).
The assembled NLRP3 inflammasome promotes caspase-1 activation, which drives IL-1β and IL-18 production, thereby leading to interferon-γ secretion and macrophage activation as additional mechanisms for infiltration of immune cells that sustain the inflammatory response (8). Caspase-related activation of proteolysis results in cell death by pyroptosis. Moreover, accumulation of dysfunctional mitochondria and the appearance of cytosolic mtDNA are potent triggers for caspase-1 activation and secretion of both interleukins (25). The implication that mitochondrial damage leads to unregulated inflammasome activation in lung parenchymal cells would predispose to loss of alveolar surface, chronic lung injury, and chronic inflammation after pneumonia.
The disposal of damaged mitochondria in the cell by selective autophagy (mitophagy) has thus been proposed as an important cell defense (6). If mitophagy fails, i.e., from the loss or oxidation of functional autophagic proteins, the cell will die by necroptosis. The necroptotic pathway can be activated in lung cells, yet there is little information on persistent mitochondrial damage in specific cell types that survive after S. aureus pneumonia (6). This subcritical mitochondrial damage would imply prolonged inflammation in the alveolar region and delays in pneumonia resolution. Here we undertook to localize mitochondrial damage, mitophagy, and changes in mitochondrial biogenesis in alveolar cells in a standard murine model of S. aureus pneumonia (1). We focused on alveolar type II (AT2) cells, which are enriched in mitochondria and involved in acute lung injury (ALI) resolution. We postulated that alveolar cells during pneumonia would show compromise of mitochondrial density in regions undergoing alveolar disruption, while surviving cells would show mitophagy and mitochondrial biogenesis.
METHODS
Mice.
C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Male mice at 14–18 wk of age and weighing 25 to 30 g were used for this study. Mouse studies were approved by the Duke University Medical Center Institutional Animal Care and Use Committee (IUCAC No. A1301606). They were carried out strictly in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All efforts were made to minimize suffering.
Animals.
Mice were anesthetized using 0.3 mg xylazine and 2.5 mg/kg ketamine ip and gently inoculated intranasally with S. aureus, ensuring no loss of dose. Each mouse then received vancomycin (6 mg/kg ip) 6 h later to prevent death. The mice were monitored daily for weight loss and signs of respiratory distress and euthanized in an isoflurane chamber at days 0, 1, 2, and 3 after inoculation.
Bacteria. S. aureus (Ssp. aureus, No. 25923; American Type Culture Collection) was reconstituted according to the manufacturer’s specifications and inoculated onto trypticase soy agar slants (BD Diagnostic Systems). The slants were incubated for 18 h at 37°C to achieve adequate log-phase growth. Bacteria were harvested sterilely and centrifuged, and the pellets were suspended in sterile NaCl solution. Suspensions were quantified on a spectrophotometer (550 nm), and stock solutions of 1 × 1010 viable colony-forming units per milliliter (CFU/ml) were generated. For this study, dilutions of 5 × 108 CFU were prepared for administration to mice.
Lung mechanics.
Mice were anesthetized and then paralyzed with sequential intraperitoneal injection of 70–90 mg/kg pentobarbital sodium (American Pharmaceutical) and 0.8 mg/kg pancuronium bromide (Baxter) for lung mechanics measurements including resistance and compliance by the single oscillation technique performed by standard protocol on a computer-controlled small animal ventilator (FlexiVent System; Scireq).
Bronchoalveolar lavage.
Bronchoalveolar lavage fluid (BALF) was obtained in mice immediately upon euthanasia. The trachea and bronchi were exposed through a midline incision. The left main bronchus was ligated, and the trachea cannulated with a sterile 22-gauge Abbocath-T catheter (Abbott Laboratories). Unilateral, right-sided BAL was performed by instilling three 0.3-ml aliquots of sterile PBS. BALF of 0.7–0.9 ml was retrieved per mouse. Cellular BALF (30–50 μl) was stained with trypan blue, and the total cell count was manually obtained using a Neubauer hemocytometer (Reichert, Buffalo, NY). The remaining BALF was centrifuged at 1,800 g for 5 min at 4°C, and the supernatant was centrifuged a second time at 12,000 g for 10 min at 4°C. The supernatant was collected and used to determine total protein by bicinchoninic acid protein assay (Pierce, Rockford, IL).
Hematoxylin and eosin staining.
Tissue sections were deparaffinized, rehydrated, and incubated with hematoxylin staining reagent (Cat. No. S3302; Dako) for 4 min followed by an eosin secondary counter-stain (Cat. No. 3801600; Leica Microsystems) for 1 min. Slides were cleared by xylene, mounted with mounting medium (Cat. No. 4111; Thermo Fisher Scientific), and examined on a Nikon E100 microscope.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay.
Thin paraffin-embedded lung sections were used for the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay commercial kit (Cat. No. G3250; Promega) using the manufacturer’s instructions. Positive and negative control sections were prepared with either label solution only or by preinduction of strand breakage using recombinant DNase I. The reactions were terminated, and the slides were stained for surfactant protein C (SP-C; SC-13979; Santa Cruz Biotechnology) and treated with primary antibody followed by Alexa fluorescent secondary antibody (Alexa 488). Nuclei were counterstained with DAPI. Sections from four to five lungs in each group were stained, and at least five unique fields per section were quantified in each experiment by a blinded observer using Nikon image software (NIS element BR 3.2).
Western blotting and immunoprecipitation.
After lung dissection to remove conducting airways, lung parenchyma was divided and snap frozen for protein and mRNA extraction or used at once for preparation of lung nuclei or mitochondria (n = 6 per group). Mitochondria were separated by differential centrifugation using a tissue mitochondria isolation kit (Cat. No. 78510; Thermo Fisher). Nuclei fractions were isolated using CellLytic Nuclear Extraction Kit (Cat. No. NXTRACT-1KT; Sigma-Aldrich). These methods are heavily weighted for the respiratory zone, but we did not exclude the smaller airways. Total proteins, mitochondrial proteins, or nuclear proteins were analyzed by Western blotting (35). The proteins were resolved after fractionation by SDS-PAGE on 4–20% gradient or 14% gels (Thermo Fisher), transferred to polyvinylidene difluoride membranes (Millipore), blocked with 4% nonfat dry milk in Tris-buffered saline with Tween 20, and probed overnight at 4°C with antibodies against nuclear respiratory factor-1 (NRF-1; 1:2,000, developed in our laboratory); peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α; 1:1,000; Cat. No. ab54481; Abcam); mitochondrial transcription factor A (Tfam; 1:1,000; developed in our laboratory); Pink1 (1:500; cat. no. ab23707; Abcam); PARK2 (1:500; Cat. No. sc-32282; Santa Cruz Biotechnology); light chain 3B protein (LC3I/II) and NLRP3 (1:1,000; Cat. No. 41085 and 15101; Cell Signaling); Sqstm1/p62 (1:1,000; Cat. No. 8025; Cell Signaling); Toll-like receptor-2 (TLR2), TLR9, IL-18, TTF1, and caspase 3 (Cat No. sc-16237, sc-25468, sc-133127, sc-13040, and sc-271759; Santa Cruz Biotechnology); RIPK1 (Ab23707); pMLKL (ab196436); dynamin-1-like protein (Drp1; Cat. No. 85705; Cell Signaling); mitochondrial fission factor (Mff; ab129075)l; mitofusin-1 and -2 (Mfn1 and Mfn2; developed in house); IL-1β; and receptor for advanced glycation end points (RAGE; Cat. No. ab3611; Abcam). Protein loading was confirmed by nuclear Lamin B protein (sc-374015; Santa Cruz Biotechnology), tubulin (1:1,000; Cat. No. T-5168; Sigma-Aldrich), or mitochondrial VDAC (1:500; Cat. No. sc-8829; Santa Cruz Biotechnology) and/or Coomassie blue staining. After incubation with primary antibody, membranes were treated for 60 min with horseradish peroxidase-conjugated secondary antibody, goat anti-rabbit, or anti-mouse IgG conjugated to horseradish peroxidase (1:5,000~8,000) and developed by enhanced chemiluminescence (Luminol; Santa Cruz Biotechnology). Protein bands were quantified on digitized images in the mid-dynamic range using Quantity One (Bio-Rad).
Immunoprecipitation.
Lung tissue lysate (0.5 mg) was precleaned with 20 μl A/G plus agarose (Cat. No. sc-2003; Santa Cruz Biotechnology) and precipitated by 20 μl and LC3II at 4°C overnight. Following further precipitation with 20 μl A/G plus agarose for 2 h, samples were washed three times using cold PBS, solubilized with 40 μl 2× SDS sample buffer (Cat. No. S3401–1VL; Sigma-Aldrich) and analyzed by Western blot using anti-TFAM antibodies.
Quantitative PCR.
After lung dissection to remove conducting airways, lung parenchyma was collected for RNA isolation by RNAqueous-4 PCR kit (Thermo Fisher Scientific) using the manufacturer’s instructions. Samples were treated with DNase I (4 units) for 2 h at 37°C that was then inactivated, and cDNA was prepared with a high-capacity cDNA archive kit (Applied Biosystems) by the manufacturer’s protocol. The quantitatve PCR primers for glutathione peroxidase 4 (Gpx4; Mm0051041), heme oxygenase-1 (HO-1; Mm00516006), peroxyredoxin 3 (Prdx3; Mm00545848), SOD2 (Mm00449726), and 18S (AIY9XUS) from ThermoFisher were used with the manufacturer’s protocol. All reactions were conducted on StepOnePlus for 40 cycles. Results were analyzed using the 2−ΔΔCt method after normalized to the 18S level in each sample.
mtDNA copy number.
Total cellular DNA was extracted using the Sigma-Aldrich DNA isolation kit. The mtDNA copy number was quantified with real-time PCR on StepOnePlus Sequence Detector System (Applied Biosystems). Primers were designed for cytochrome b (cyt b) with ABI Probe Design software (Applied Biosystem), and amplifications were performed on 10 ng total mtDNA using PCR primers (cyt b-sense [cyt b-s] and cyt b-antisense [cyt b-as]). One copy of linearized pGEMT-cyt b vector was used for standard mtDNA quantification (34). The cyt b probe, 5′ FAM-ttcctccacgaaacaggatcaaa-TAMRA 3′, contained 6-carboxy-fluorescein (FAM) at the 5′-end as a fluorescent reporter dye and 6-carboxy-tetramethyl-rhodamine (TAMRA) at the 3′-end as a quencher dye selected from a highly conserved region of mouse cyt b gene. Serial dilutions of 1 × 105 to 1 × 1010 copies of standard cyt b plasmid were prepared for a standard curve. Samples were tested for mtDNA at 1:100 and 1:1,000 dilutions. Samples were analyzed in triplicate; mtDNA copy number per nanograms of DNA was determined relative to the standards of known mtDNA copies per dilution.
Microscopy and immunostaining.
Inflation-fixed lung samples were paraffin-embedded, cut into 5-μm sections, and mounted on slides. Four channel immunofluorescence staining for mitochondrial citrate synthase (CS; primary antibody; 1:200; GeneTex), AT2 cell marker TTF1 (sc-13040), NRF-1, mitophagy marker LC3II (sc-54237), Pink1 (ab23707), or Park2 (sc-32282). The nuclei were stained with DAPI (Molecular Probes). Alexa-Fluor-coupled secondary antibodies (Invitrogen) were used at 1:400. Stained sections were examined on a Zeiss LSM 710 laser-scanning confocal microscope to identify and localize mitophagosomes. The colocalization was assessed by four-channel fluorescence: green (for CS), red (for LC3II, Pink1, or Park2), magenta (for NRF-1 or TTF1), and blue (DAPI).
Statistical analysis.
Data are expressed as means ± SE. Statistical analyses were performed with Sigma plot 12.0 statistics software. ANOVA and Student’s Newman-Keuls test for post hoc comparisons were used to determine differences between control and experimental groups. Changes in values over time were evaluated by a one-way ANOVA. P < 0.05 was accepted as significant.
RESULTS
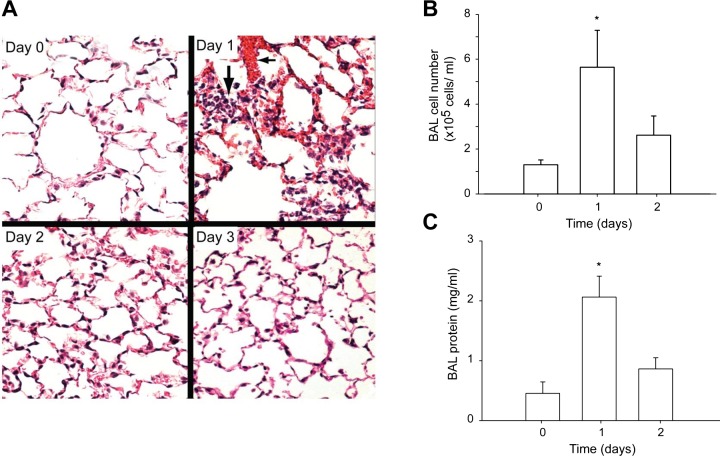
Administration of 5 × 108 CFU of S. aureus (SA) by nasal inoculation followed 6 h later by vancomycin produced diffuse pneumonia with no mortality or distal abscess formation. Lung inoculation led to significant physiological loss of compliance and increased resistance detected by Flexivent measurements (Table 1). Lung system compliance fell by ~30% (P < 0.05) while resistance increased by 56% on postinoculation day 1. The degree of postpneumonia lung inflammation was evaluated by assessment of random hematoxylin and eosin histological sections of lung and by BAL fluid analysis for protein and cell number, as shown in Fig. 1. The inflammatory responses were greatest histologically and by BALF at day 1 and showed almost complete resolution by day 3.
Table 1.
Lung function by single frequency forced oscillation in mice pre-/postinoculation
| Function | Control | Pneumonia |
|---|---|---|
| Resistance, cmH2O⋅s/ml | 0.553 ± 0.0483 | 0.868 ± 0.0286* |
| Compliance, ml/cm H2O | 0.0442 ± 0.001 | 0.0311 ± 0.007* |
Values are means ± SE.
P < 0.05; n = 4 per group.
Fig. 1.
Staphylococcus aureus pneumonia and alveolar pathology in C57/BL mice. A: hematoxylin and eosin (H&E)-stained sections of inflation-fixed lung in C57/BLWT mice at 0, 1, 2, and 3 days postinoculation with 5 × 108 colony-forming units (CFU) S. aureus (200 ×). The inflammatory response is markedly enhanced at day 1. In that photomicrograph, loss of alveolar membrane integrity is indicated by red blood cell and plasma leakage (short arrow) and alveolar neutrophil infiltration (long arrow). B: bronchoalveolar lavage (BAL) fluid cell number (cells/ml × 105) plotted against days postinfection. BALF cell number increased significantly at day 1 (*P < 0.05) and subsided by day 2. C: total BAL fluid protein concentration plotted against days of infection. Protein increased by day 1 and subsided by day 2. Bars are means ± SE for n = 6/group; *P < 0.05 vs control by one-way ANOVA.
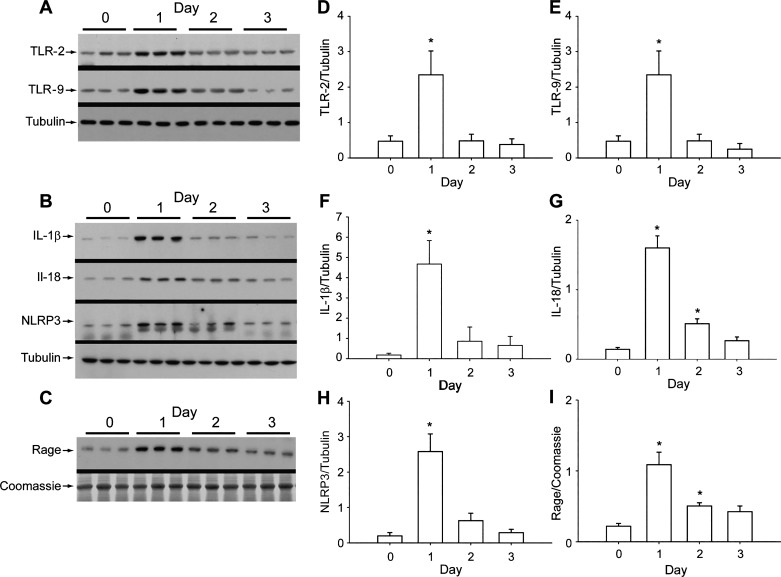
The innate immune response of the lung was activated by S. aureus pneumonia as determined by changes in total lung protein levels by Western analysis for relevant toll-like proteins (TLR2, TLR9) and for NLRP3, IL-1β, IL-18, and BAL fluid levels of epithelial RAGE (Fig. 2). The lung’s proinflammatory protein responses reached a peak at day 1 but had largely subsided by day 3, except for BAL fluid levels of RAGE, which were more persistently elevated than inflammasome protein levels. RAGE is highly expressed in the distal lung, especially by alveolar AT1 epithelial and vascular endothelial cells, and it is useful as a marker for loss of alveolar fluid clearance (16).
Fig. 2.
Toll-like receptor-2 (TLR2) and -9 expression and nucleotide-binding domain and leucine-rich repeat-containing gene family, pyrin domain containing 3 (NLRP3) inflammasome activation in pneumonia. A: Western analysis of TLR2 and TLR9 protein levels in total lung homogenate in mice. TLR protein expression showed a robust increase at day 1 then returned to baseline at day 3 postinfection. B: Western analysis of NLRP3 and IL-1β proteins. Tubulin is loading control. C: receptor for advanced glycation end points (RAGE) protein in BAL fluid determined by Western analysis. Coomassie staining is loading control. Release of RAGE indicates alveolar injury. D: mean immunoblot densities for TLR2 compared with corresponding tubulin. E: TLR9 histogram relative to tubulin. F: relative densities of IL-1β relative to tubulin. G: IL-18 protein levels. H: NLRP3 protein levels relative to tubulin. I: RAGE protein levels in BAL fluid. Each bar in D–I represents means ± SE for each group; n = 6; *P < 0.05 by one-way ANOVA.
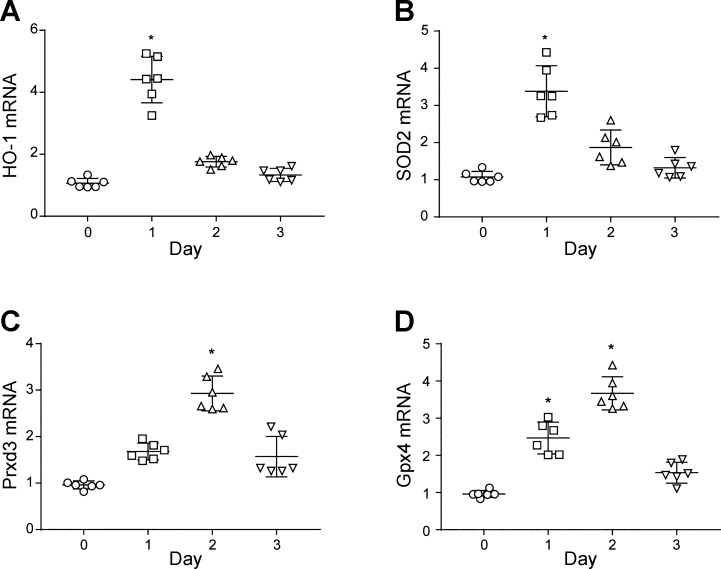
The pneumonia had an important effect on the antioxidant milieu in the distal lung as measured by the expression of key antioxidant enzyme genes including heme oxygenase-1 (HO-1), superoxide dismutase 2 (SOD2), peroxyredoxin 3 (Prdx3), and glutathione peroxidase 4 (Gpx4) (Fig. 3). Each of these gene products produces an important mitochondrial protectant enzyme. Moreover, two of them, HO-1 and SOD2 responded in the first day, while Gpx4 and Prdx3 mRNA levels peaked later at day 2. Thus Fig. 3 indicates that protective lung cell mitochondrial antioxidant enzymes represent the presence of a high risk of oxidative stress during pneumonia. We surmised that pneumonia had produced cellular changes that affected redox signaling and, therefore, might eventually lead to the increased expression of nuclear regulatory proteins for mitochondrial biogenesis (1).
Fig. 3.
Lung cellular antioxidant defenses in S. aureus pneumonia. Antioxidant enzyme mRNA levels for were measured in total purified RNA by quantitative RT-PCR and expressed as means ± SE. A: lung heme oxygenase-1 (HO-1) mRNA levels in inoculated mice peaked at day 1. B: SOD2 mRNA transcript expression increased by day 1. C: peroxyredoxin 3 (Prdx3) mRNA transcript, the mitochondrial isoform, increases significantly at day 2. D: glutathione peroxidase 4 (Gpx4) mRNA transcript levels, the mitochondrial isoform, increases significantly at days 1 and 2 of pneumonia. *P < 0.05 vs. time 0 by one-way ANOVA; n = 6 mice per group.
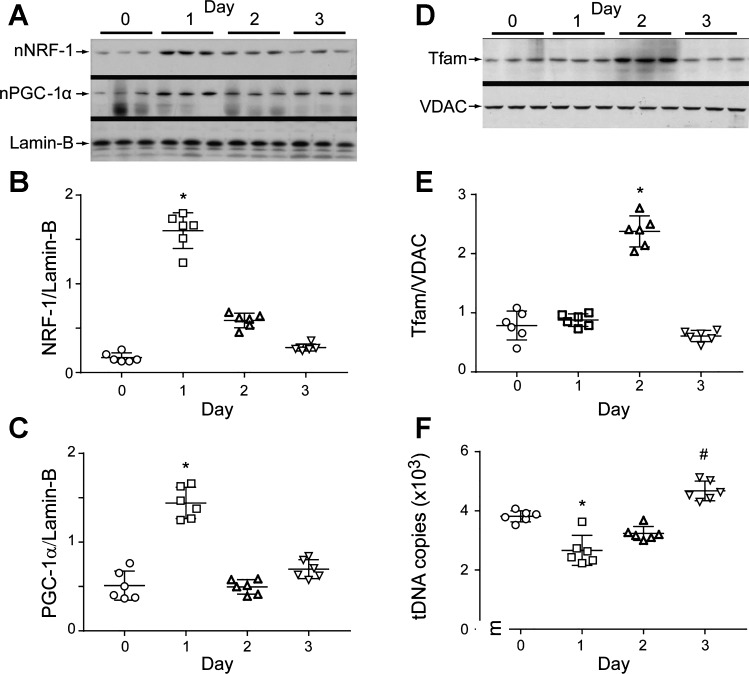
Induction of mitochondrial biogenesis by the redox pathway is a cell survival function in severe sepsis; hence, we evaluated pneumonia mice for changes in key transcriptionally active proteins involved in mitochondrial biogenesis. We selected NRF-1 and PGC-1α, which are necessary to upregulate Tfam and other nuclear-encoded, mitochondrial proteins (NEMPs) involved in mtDNA transcription and replication. We detected approximately fourfold increases in nuclear NRF-1 levels along with PGC-1α, its nuclear binding partner in lung parenchyma postinfection (Fig. 4). These nuclear proteins were prominently elevated by day 1 and associated with subsequent Tfam upregulation. Simultaneously, a depression in overall mtDNA copy number was observed that had recovered by day 3. Figure 4 demonstrates the induction of mitochondrial biogenesis and recovery of mtDNA copy number postinoculation (30).
Fig. 4.
Lung mitochondrial biogenesis during S. aureus pneumonia. A: Western analyses of lung nuclear respiratory factor 1 (NRF-1) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) proteins showing nuclear accumulation of both proteins at day 1 postinfection. B and C: scatterplots of densitometry analysis of NRF-1 and PGC-1α. D: mitochondrial transcription factor A (Tfam) levels triple at day 2 postinoculation, and normalize by day 3. The Tfam reference is outer mitochondrial membrane protein VDAC. E: scatterplots of densitometry values for lung mitochondrial Tfam analysis. F: scatterplots for mitochondrial DNA (mtDNA) copy number in lung parenchyma after S. aureus infection. mtDNA copy number falls on day 1, recovers, and has increased by day 3. Bars show means ± SE for each group and time point; n = 6 mice per group; *P < 0.05 vs. day 0 control and #P < 0.05 vs. day 1 by one-way ANOVA.
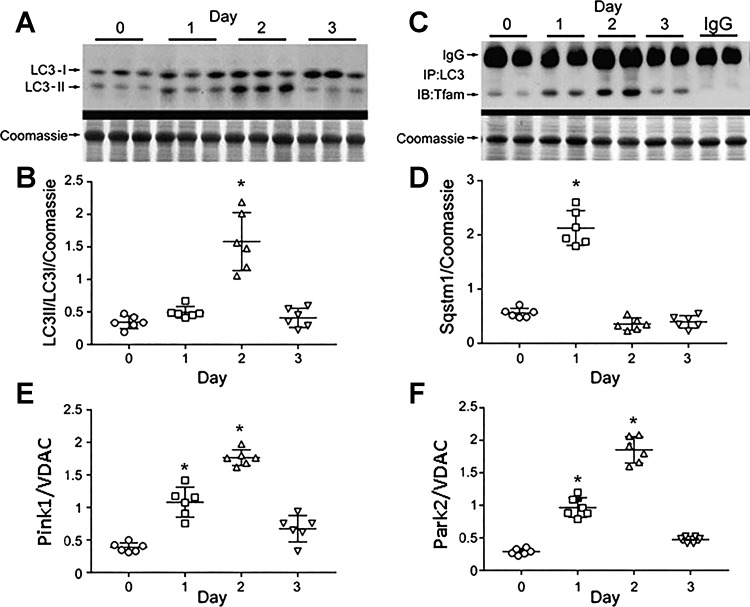
The early vigorous antioxidant response, fall-off in mtDNA copy number, induction of mitochondrial biogenesis, and subsequent restoration of mtDNA copy number implicate vigorous activation of mitochondrial quality control (QC) after the infection. The QC cycle also encompasses selective autophagy of mitochondria (mitophagy), which eliminates damaged mitochondria from the cells. Hence, we evaluated the responses of major mitophagy and mitophagy regulatory proteins postinfection. Figure 5 demonstrates the cleavage of microtubule-associated protein 1A/1B light chain 3B protein (LC3-I) into its short product LC3-II by Western analysis using a single antibody method (Fig. 5A). The ratio of LC3-II/LC3-I shown in Fig. 5B indicates an increase in autophagy at day 2 of pneumonia. Evidence for mitophagy by lung protein coimmunoprecipitation studies of alveolar autophagosome membrane protein LC3 with Tfam in mitochondria is shown in Fig. 5C. Tfam is an abundant mtDNA binding protein that participates in mtDNA transcription and replication and protects the genome. Tfam coprecipitation with LC3 provides specific evidence of active mitophagy. In order for mitophagy to be completed, mitochondrial decorator proteins such as Sequestosome-1 (Sqstm1; p62), E3 ubiquitin-protein ligase Parkin (Park2), and the serine/threonine-protein kinase Pink1 are required. In Fig. 5, D–F, it is seen that all three protein levels are increased significantly in the lungs after S. aureus inoculation. Peak Sqstm1 level was observed on day 1 followed by a sharp decline on days 2 and 3. Meanwhile, Park2 levels rose on days 1 and 2 as predicted by the protein’s direct ubiquitination of Sqstm1, which promotes its proteasomal degradation independently of ATG5. Parkin also prevents Pink1 egress from the mitochondria (28). Activation of mitophagy was caused by the infection and not by the use of the vancomycin antibiotic.
Fig. 5.
Mitophagy in S. aureus pneumonia. A: light chain 3B protein (LC3-I) cleavage to LC3II by Western blot pre-/post-S. aureus inoculation. B: scatterplots of lung autophagic index (LC3II/LC3I) pre-/post-S. aureus inoculation. The index increased at day 2. *P < 0.05 for n = 5 per group by two-way ANOVA. C: far Western immunoprecipitation of lung protein with anti-LC3 probed against Tfam. S. aureus inoculation enhances Tfam association with LC3. D: Western blot of lung Sqstm1 (p62) protein. S. aureus pneumonia enhances Sqstm1 levels at day 1 consistent with onset of mitophagy. E: scatterplots for Pink1 relative to VDAC pre-/postinoculation show higher Pink1 levels at days 1 and 2. F: scatterplots for Park2 mitophagy protein relative to VDAC pre-/postinoculation show higher Park2 (Parkin) at days 1 and 2. For scatterplots in D–F, bars are means ± SE for n = 6 per time point. *P < 0.05 vs. time 0 by one-way ANOVA.
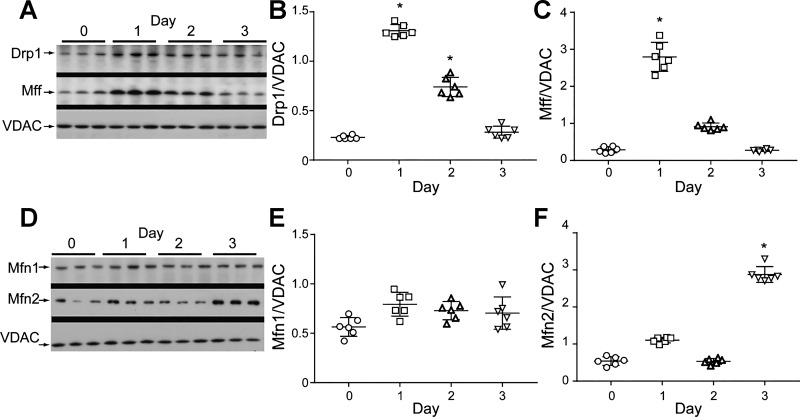
Mitophagy also produces changes in the levels of proteins involved in mitochondrial dynamics (Fig. 6). We examined fission and fusion proteins to establish the patterns of change in dynamics for dynamin-1-like protein (Drp1; Fig. 6A), mitochondrial fission factor (Mff; Fig. 6B), mitofusin-1 (Mfn1; Fig. 6C), and mitofusin-2 (Mfn2; Fig. 6, A and D). The proteins Drp1 and Mff were upregulated during the first 2 days postinoculation, while Mfn1protein expression did not change and Mfn2 showed a sharp increase only at day 3 postinfection. Together, these data support that mitochondrial fission is activated primarily in distal lung cell mitochondria on day 1 postinoculation by Drp1 binding to Mff. This implies that the induction of mitophagy has occurred at approximately the same time as the induction of mitochondrial biogenesis (9).
Fig. 6.
Mitochondrial network protein expression. A: lung dynamin-1-like protein (Drp) and mitochondrial fission factor (Mff) protein levels by Western analysis. These mitochondrial fission proteins compared with outer membrane protein VDAC respond at days 1 and 2 postinoculation. B: scatterplots for Drp1 showing postinoculation effect. C: scatterplots for Mff showing an increase at day 1. D: mitofusin-1 and -2 (Mfn1 and Mfn2) expression by Western analysis relative to VDAC. E: scatterplots for Mfn1 showing stability after inoculation. F: scatterplots for Mfn2 showing a response at day 3 postinfection. Data expressed as protein density relative to VDAC. For scatter plots in B, C, E, and F, bars are means ± SE for n = 6 per time point; *P < 0.05 vs. time 0 by one-way ANOVA.
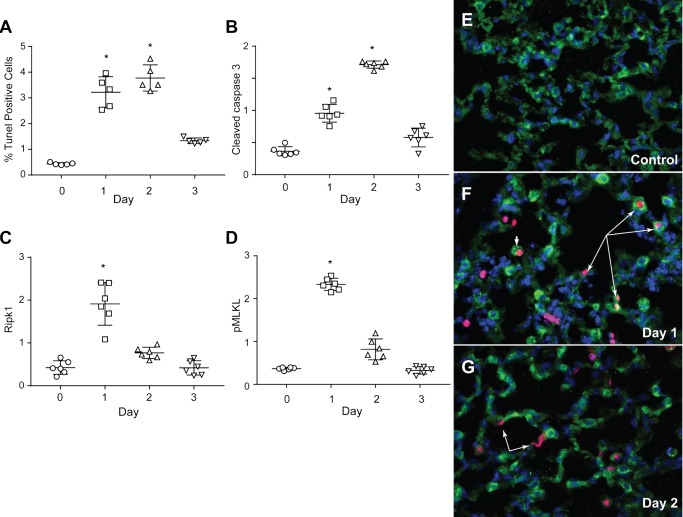
The activation of these mitochondrial QC mechanisms would be expected to favor lung cell survival during pneumonia. We assessed lung cell death by counting the numbers of immunochemical-stained, TUNEL-positive cells in fixed sections of distal lung. The levels of cell death were most pronounced at days 1 and 2 (Fig. 7A). Since TUNEL staining lacks high specificity for apoptosis, semiquantitative analysis of caspase 3 cleavage was also performed (Fig. 7B). This staining indicated enhanced caspase 3 cleavage by day 2, although on day 1, a trend was present for increased cleaved caspase 3. To appraise nonapoptotic cell death, we measured necroptosome-inducing proteins receptor-interacting serine/threonine-protein kinase 1 (RIPK1) and phospho-mixed lineage kinase domain-like protein (MLKL) in the alveolar region (Fig. 7, C and D). Both proteins increased at day 1 and subsided over 2 days. RIPK1 specifically transduces inflammatory and cell-death signals, activation of pathogen recognition receptors, and DNA damage. The kinase associates with RIPK3 to allow homotrimerization of phospho-MLKL pseudokinase, forming a necroptosis-inducing complex that promotes cell death. Necroptosis is a common feature of TNF receptor-1 ligation by TNF-α family cytokines, which is blocked by normal mitophagy and accelerated by failed mitophagy (20).
Fig. 7.
TdT-mediated dUTP nick-end labeling (TUNEL) and lung AT2 cell death markers after S. aureus inoculation. A: scatterplots showing an increase in alveolar cell death by TUNEL staining after S. aureus inoculation. Cell death increased by 7- to 8-fold at days 1 and 2 before falling to near control levels by day 3. B: cleaved to native caspase-3 ratio follows a similar pattern as the TUNEL staining. Peak apoptosis occurs on day 2 and subsides by day 3. C: scatterplots showing increased levels of receptor-interacting serine/threonine-protein kinase 1 (RIPK1) on day 1 followed by a decline on day 2. D: scatterplots for mixed lineage kinase domain-like protein phosphoprotein (pMLKL) show a dramatic increase on day 1 followed by normalization on days 2 and 3 postinoculation. C and D: necroptosis on day 1 consistent with failing mitophagy after inoculation. E: photomicrograph of immunochemical stains for sufactant protein-C (S-C; green) and TUNEL (red) on formalin-fixed control lung section. Nuclei are stained with DAPI (blue). Control lung shows little or no evidence of alveolar cell death. F: the same 3 stains (SP-C, TUNEL, and DAPI) on formalin-fixed lung sections obtained at day 1 indicate dying AT2 cells (long arrows). Extensive neutrophil and macrophage influx is noted, and the occasional macrophage has collected SP-C, perhaps from surfactant clearance (short arrow). G: the 3 stains applied to day 3 lung indicate neutrophil clearance and near resolution of AT2 cell death. Some flat TUNEL-positive cell nuclei are consistent with dying alveolar type I (AT1) cells (arrows). Scatterplot bars are means ± SE for n = 6 per time point; *P < 0.05 vs. time 0 by one-way ANOVA.
Finally, TUNEL-positive nuclei were colocalized within AT2 cells (Fig. 7, E–G). TUNEL and SP-C costaining was used to identify dying AT2 cells. Interspersed were small numbers of dying inflammatory cells distinguished by round TUNEL-stained nuclei without the presence of SP-C, while AT1 cells were identified by TUNEL-stained broad, flat nuclei (Fig. 7, F–G). In Fig. 7E, no dying cells were apparent in large areas of control lungs (day 0), but at day 1 postinoculation, a large number of dying AT2 cells were visible along with dying inflammatory cells, mostly macrophages. (Fig. 7G). AT1 cells rarely showed TUNEL-positive nuclei at day 1, but more were stained on day 2, probably because many AT1 cells had been lysed on day 1 by direct contact with α-hemolysin, the main toxin of this S. aureus strain. By day 2, only a few dying AT2 cells were found, while cell death was abundant in AT1 cells and mononuclear inflammatory cells (Fig. 7G).
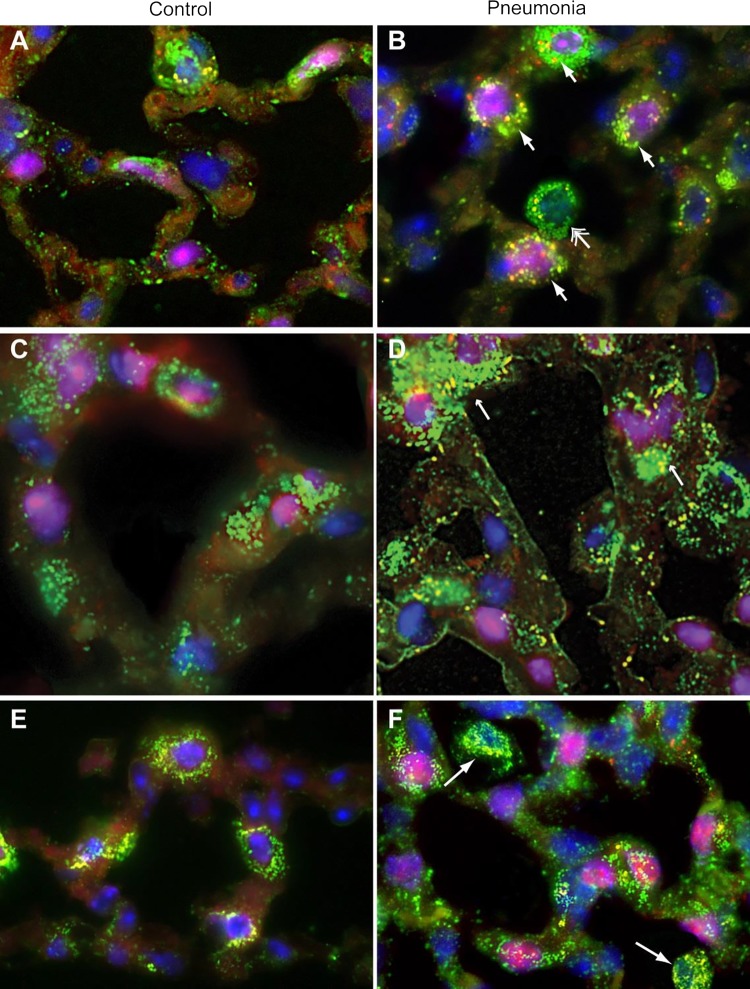
These molecular studies have established that mitochondrial QC responds to S. aureus pneumonia and that mitophagy is an important factor in the cellular response to infectious inflammation in the alveolar region. Because of the influx of inflammatory cells and the many normal cell types in the parenchyma, mitochondrial QC localization studies using immunofluorescence markers and confocal microscopy were performed on formalin-fixed lung sections to compare the responses of different cell types to the infection (Fig. 8). These studies were designed to identify and localize autophagy/mitophagy in the cytoplasm of various alveolar cells, but out of interest and for technical reasons, this was most successfully accomplished in the AT2 cell using a unique nuclear localization marker protein, thyroid transcription factor-1 (TTF1). Nuclear TTF1 was compared with nuclear NRF-1 localization, a marker of activation of mitochondrial biogenesis. Mitochondrial density was tracked using immunofluorescent antibody labeling of CS (TCA cycle enzyme), which we also colocalized with the mitophagy proteins Pink1 and Park2. The pre- and postpneumonia comparisons indicated the extensive activation of both mitochondrial QC processes in the alveolar region as shown in marked AT2 cells.
Fig. 8.
Confocal immunofluorescence micrographs of the alveolar region pre-/post-S. aureus inoculation. A and B: confocal micrograph of lung alveolar sections stained for LC3II (red), citrate synthase (green), and TTF1 (magenta, AT2 cells), nuclei are counterstained with DAPI. Arrows denotes overlay of LC3 and citrate synthase (yellow/orange florescence). A: control lung section showing a macrophage with few LC3 puncta. B: section from day 2 lung showing increased citrate synthase expression and LC3 puncta in AT2 cells and macrophages. C and D: confocal micrographs of lung sections stained for Pink1 (red), citrate synthase (green), and TTF1 (magenta, AT2 cells), and nuclei are counterstained with DAPI. Arrows denote overlay of Pink1 and citrate synthase (yellow/orange florescence). C: control lung section showing few Pink1 puncta colocalized with mitochondria. D: pneumonia section shows increased citrate synthase on day 2 colocalized with Pink1 (yellow/orange florescence) in AT1, AT2, and mononuclear cells. Arrows denotes overlay of Pink1 and citrate synthase implying that S. aureus pneumonia stimulates Pink1 colocalization to mitochondria. E and F: confocal micrograph of lung alveolar sections stained for Parkin (Park2) (red), citrate synthase (green), and NRF-1 (magenta, nuclear translocation in both AT2 and macrophage cells), nuclei are counterstained with DAPI. E: control lung section shows little Parkin colocalized with mitochondria. F: lung section on day 2 shows increased citrate synthase colocalized with Parkin (yellow/orange fluorescence). Postinoculation, nuclear NRF-1 is seen in AT2 cells (pink nuclei) but not in macrophages (arrows) consistent with mitochondrial biogenesis. Localization of Parkin with citrate synthase is seen both in AT2 cells (displaying nuclear NRF-1) and macrophages (blue nuclei) showing mitochondrial QC induction by pneumonia. Magnification = ×1,000. Data are representative of 3 sets of mice.
Throughout many micrographs, both mitophagy and mitochondrial biogenesis were detected simultaneously in the same cell (Fig. 8). Moreover, some alveolar macrophages displayed evidence of mitophagy, and a few also showed increases in mitochondrial density. However, the latter in most cases was unusual because it occurred with no increase in nuclear NRF-1 expression. This may indicate that alveolar macrophages employ other transcription factors for mitochondrial biogenesis or that they scavenge mitochondria expelled into the air spaces by damaged alveolar cells. In some cases, tiny free mitochondria were seen in the air spaces (postpneumonia panel; Fig. 8, middle), leaving arriving macrophages free to phagocytose them like bacteria.
DISCUSSION
ALI is well understood physiologically, but our understanding at the cell and molecular levels remains challenged. Almost all ALI produces intense alveolar inflammation from the damaging agent and the host response. The upshot is epithelial cell death, loss of alveolar barrier function, edema, and hypoxemia (23). This alveolar damage can be resolved by replacement of dying epithelial cells, thereby allowing alveolar liquid clearance to be restored (29). Short of cell death, damage mechanisms are not as well defined, so the extent of mitochondrial injury and mitochondrial replacement was evaluated in the alveolar region in a validated S. aureus mouse pneumonia model (1). We compared the induction of mitochondrial biogenesis and mitophagy with the extent of cell death in alveolar epithelial cells.
We had information that mitochondrial QC processes promote the ability of lung cells to eliminate and replace damaged mitochondria and thereby support cell survival (26). This has also been well-documented in other tissues and in sepsis but not during pneumonia. There were signs of nucleic acid damage to mitochondrial genomes from the decrease in mtDNA copy number. There were also increases in tissue mRNA levels for mitochondrial antioxidant enzymes (SOD2, Prdx3, and GPX4) and the HO-1/CO system at 1 day postinoculation corresponding to increases in lung inflammatory cell infiltration, TLR2 activation, NLRP3-dependent cytokine induction. HO-1 is required for mitochondrial heme clearance and produces endogenous CO, a counterinflammatory agent (21), while SOD2 protects mitochondria against excessive superoxide production and from the initiation of intrinsic apoptosis by allowing H2O2 to be released across the outer mitochondrial membrane (7). Prdx 3 is a reductase that scavenges alkyl hydroperoxides (19), and Gpx4, the mitochondrial phospholipid-hydroperoxide glutathione peroxidase, protects the organelle from lipid peroxidation and the cell from apoptosis (10). The inducible HO-1/CO system is known to drive early mitochondrial biogenesis by induction of NRF-1 and PGC-1α genes and their nuclear accumulation (27). This pathway for mitochondrial biogenesis was observed in AT2 cells within 24 h of inoculation.
The induction of mitochondrial biogenesis was followed closely by the production of mitophagy proteins, which are required to eliminate damaged mitochondria. This response was detected in the lung on day 1 by elevated Sqstm1 (p62) protein levels, an autophagy receptor that interacts directly with both the cargo to become degraded and an autophagy modifier of the MAP1LC3 family. Then, increases in the ratio of LC3II/LC3I, an ubiquitin-like protein modifier cleaved in autophagosome formation, were consistent with mitophagy (40). LC3 was found by coimmunoprecipitation to bind the mtDNA transcription factor Tfam throughout the entire postinoculation study period. Moreover, Pink1 and Park2 upregulation was apparent on day 1 and peaked on day 2. Pink1 is involved in the clearance of damaged mitochondria by mediating activation and translocation of Parkin (Park2) (11).
By lung microscopy, the selected mitochondrial regulatory proteins all appeared to be concentrated in the AT2 cell population. These cells not only produce and recycle surfactant but act as progenitors for AT1 cells, which are necessary for the alveolar barrier function and edema clearance (22). The loss of AT2 cells would also impair surfactant production and delay alveolar repair and restoration of alveolar liquid clearance. Many of these cells were found to be TUNEL positive on day 1 postinoculation, but AT2 cell death had largely resolved by day 2 when the alveolar region mitochondrial QC mechanisms had been activated. Although AT1 cells also contain mitochondria, their very large surface area and low mitochondrial volume density makes it difficult to assess them in situ, and we cannot conclude anything meaningful about their mitochondrial QC responses. However, due to the nature of the infection, the amount of alveolar protein leak and edema, loss of compliance, and persistence of RAGE, we surmised that high levels of AT1 cell death had also been present.
Mitochondrial damage recovery is often overlooked in the lung, but aerobic energy provision supports most pulmonary physiological processes (26) Each function requires lung cell homeostasis, and each can be disrupted by inflammation and release of cytokines, nitric oxide, and other mediators. Thus damage to lung cell mitochondria may occur without or preceding cell death. In ALI, alveolar barrier failure occurs mainly through the loss of AT1 epithelial cell coverage, through cell retraction, cell loss, or both. If surface denudation is extensive, there is also protein-rich edema and flooding of alveolar spaces. This causes widespread surfactant inactivation, ventilation-perfusion (Va/Q) mismatch, and shunt, resulting in profound hypoxemia (23).
Mitochondrial damage promotes epithelial barrier dysfunction through energy failure, calcium dysregulation, loss of heme homeostasis, and activation of apoptosis (31). Moreover, the expected damage to AT1 cells is extensive because of their large, delicate surface area and sensitivity to lysis by α-hemolysin and cell damage by the host response. By comparison, the cuboid AT2 cell is generally more resistant to inflammation and oxidative stress (4). The surviving AT2 cells impart a stem function; they proliferate and transdifferentiate into AT1 epithelial cells to help restore alveolar barrier function (3). However, AT2 cells can implement this function only by first repairing cell damage, eliminating irreversibly damaged mitochondria, and replacing them with healthy ones. This mitochondrial QC process is fundamental to all aerobic cells (37) and requires bigenomic cooperation between the nuclear and mitochondrial genomes to maintain appropriate rates of mitochondrial turnover.
The present findings thus support the presence of substantial mitochondrial damage preceding alveolar cell death in murine S. aureus pneumonia. This mechanism is distinct from direct cell contact with the microbe and immediate cell death. The pathogenic isolate (Seattle 45) used here secretes largely α-hemolysin, a pore-forming toxin that kills epithelial cells by interacting with its receptor, the zinc-dependent metalloprotease ADAM10 on plasma membranes (14). This effect is projected to involve primarily AT1 cells both from a surface area effect and from ADAM10 distribution largely on epithelial and endothelial cells.
The induction of mitochondrial biogenesis and mitophagy was sometimes observed in the same cell, and both events were detected within the first 24 to 48 h. Thus ongoing mitochondrial repopulation in lung cells containing damaged mitochondria is important for both cell survival and return to function. Our findings raise new questions about the signals that lead to the induction of mitochondrial QC responses in the distal lung. In other living systems, energy failure is a fundamental driver mitochondrial QC genetic responses; but that mechanism risks cell death before the cell has reestablished an effective mitochondrial population. The host response can also stimulate mitochondrial biogenesis through the induction of Nfe2l2, NRF-1, CREB1 (38), and other rapidly inducible transcription factors. Equally importantly, mitochondrial oxidants such as H2O2 provide direct redox signals for the induction of mitochondrial biogenesis and mitophagy. Accordingly, damaged lung AT2 cells show nuclear accumulation of NRF-1 and PGC-1α nuclear binding partners, which regulate nuclear-encoded genes for mitochondrial proteins (30). These factors transactivate genes for mitochondrial proteins, including certain mitophagy proteins, such as Pink1 and Park2 (36). Future studies of distinct alveolar cell populations will be necessary to determine the extent to which mitochondrial QC responses are called upon by the range of cell types in ALI.
GRANTS
This study was funded by National Institute of Allergy and Infectious Diseases Grant R01-AI-095424 (to C. A. Piantadosi, Principal Investigator).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.B.S. and C.A.P. conceived and designed research; H.B.S. performed experiments; H.B.S., B.D.K., R.R.B., L.C., and K.E.W.-W. analyzed data; H.B.S., B.D.K., R.R.B., L.C., K.E.W.-W., and C.A.P. interpreted results of experiments; H.B.S. prepared figures; H.B.S. and C.A.P. drafted manuscript; H.B.S., B.D.K., R.R.B., L.C., K.E.W.-W., and C.A.P. edited and revised manuscript; H.B.S., B.D.K., R.R.B., L.C., K.E.W.-W., and C.A.P. approved final version of manuscript.
REFERENCES
- 1.Athale J, Ulrich A, MacGarvey NC, Bartz RR, Welty-Wolf KE, Suliman HB, Piantadosi CA. Nrf2 promotes alveolar mitochondrial biogenesis and resolution of lung injury in Staphylococcus aureus pneumonia in mice. Free Radic Biol Med : 1584–1594, 2012. doi: 10.1016/j.freeradbiomed.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest : 3025–3036, 2013. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol : 593–615, 2013. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 5.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol : 629–641, 2009. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang AL, Ulrich A, Suliman HB, Piantadosi CA. Redox regulation of mitophagy in the lung during murine Staphylococcus aureus sepsis. Free Radic Biol Med : 179–189, 2015. doi: 10.1016/j.freeradbiomed.2014.10.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dasgupta J, Subbaram S, Connor KM, Rodriguez AM, Tirosh O, Beckman JS, Jourd’Heuil D, Melendez JA. Manganese superoxide dismutase protects from TNF-alpha-induced apoptosis by increasing the steady-state production of H2O2. Antioxid Redox Signal : 1295–1305, 2006. doi: 10.1089/ars.2006.8.1295. [DOI] [PubMed] [Google Scholar]
- 8.De Nardo D, De Nardo CM, Latz E. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. Am J Pathol : 42–54, 2014. doi: 10.1016/j.ajpath.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding WX, Yin XM. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem : 547–564, 2012. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garry MR, Kavanagh TJ, Faustman EM, Sidhu JS, Liao R, Ware C, Vliet PA, Deeb SS. Sensitivity of mouse lung fibroblasts heterozygous for GPx4 to oxidative stress. Free Radic Biol Med : 1075–1087, 2008. doi: 10.1016/j.freeradbiomed.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Gelmetti V, De Rosa P, Torosantucci L, Marini ES, Romagnoli A, Di Rienzo M, Arena G, Vignone D, Fimia GM, Valente EM. PINK1 and BECN1 relocalize at mitochondria-associated membranes during mitophagy and promote ER-mitochondria tethering and autophagosome formation. Autophagy : 654–669, 2017. doi: 10.1080/15548627.2016.1277309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenlee-Wacker M, DeLeo FR, Nauseef WM. How methicillin-resistant Staphylococcus aureus evade neutrophil killing. Curr Opin Hematol : 30–35, 2015. doi: 10.1097/MOH.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hüttemann M, Lee I, Gao X, Pecina P, Pecinova A, Liu J, Aras S, Sommer N, Sanderson TH, Tost M, Neff F, Aguilar-Pimentel JA, Becker L, Naton B, Rathkolb B, Rozman J, Favor J, Hans W, Prehn C, Puk O, Schrewe A, Sun M, Höfler H, Adamski J, Bekeredjian R, Graw J, Adler T, Busch DH, Klingenspor M, Klopstock T, Ollert M, Wolf E, Fuchs H, Gailus-Durner V, Hrabě de Angelis M, Weissmann N, Doan JW, Bassett DJ, Grossman LI. Cytochrome c oxidase subunit 4 isoform 2-knockout mice show reduced enzyme activity, airway hyporeactivity, and lung pathology. FASEB J : 3916–3930, 2012. doi: 10.1096/fj.11-203273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, Wang Y, Bubeck Wardenburg J. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med : 1310–1314, 2011. doi: 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, Eisenbarth SC, Nauseef WM, Cassel SL, Sutterwala FS. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity : 311–323, 2013. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jabaudon M, Blondonnet R, Roszyk L, Bouvier D, Audard J, Clairefond G, Fournier M, Marceau G, Déchelotte P, Pereira B, Sapin V, Constantin JM. Soluble Receptor for Advanced Glycation End-Products Predicts Impaired Alveolar Fluid Clearance in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med : 191–199, 2015. doi: 10.1164/rccm.201501-0020OC. [DOI] [PubMed] [Google Scholar]
- 17.Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, Jania C, Doerschuk CM, Tilley SL, Duncan JA. Staphylococcus aureus α-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis : 807–817, 2012. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, Wachtel S, Bueno S, Prince A. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog : e1004820, 2015. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Shoji W, Takano H, Nishimura N, Aoki Y, Takahashi R, Goto S, Kaifu T, Takai T, Obinata M. Increased susceptibility of MER5 (peroxiredoxin III) knockout mice to LPS-induced oxidative stress. Biochem Biophys Res Commun : 715–721, 2007. doi: 10.1016/j.bbrc.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Lu W, Sun J, Yoon JS, Zhang Y, Zheng L, Murphy E, Mattson MP, Lenardo MJ. Mitochondrial protein PGAM5 regulates mitophagic protection against cell necroptosis. PLoS One : e0147792, 2016. doi: 10.1371/journal.pone.0147792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maines MD. The heme oxygenase system: update 2005. Antioxid Redox Signal : 1761–1766, 2005. doi: 10.1089/ars.2005.7.1761. [DOI] [PubMed] [Google Scholar]
- 22.Matthay MA, Robriquet L, Fang X. Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thorac Soc : 206–213, 2005. doi: 10.1513/pats.200501-009AC. [DOI] [PubMed] [Google Scholar]
- 23.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol : 147–163, 2011. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci USA : 11282–11287, 2012. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol : 222–230, 2011. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piantadosi CA, Suliman HB. Mitochondrial dysfunction in lung pathogenesis. Annu Rev Physiol : 495–515, 2017. doi: 10.1146/annurev-physiol-022516-034322. [DOI] [PubMed] [Google Scholar]
- 27.Piantadosi CA, Withers CM, Bartz RR, MacGarvey NC, Fu P, Sweeney TE, Welty-Wolf KE, Suliman HB. Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J Biol Chem : 16374–16385, 2011. doi: 10.1074/jbc.M110.207738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron : 257–273, 2015. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rokkam D, Lafemina MJ, Lee JW, Matthay MA, Frank JA. Claudin-4 levels are associated with intact alveolar fluid clearance in human lungs. Am J Pathol : 1081–1087, 2011. doi: 10.1016/j.ajpath.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta : 1269–1278, 2011. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schumacker PT, Gillespie MN, Nakahira K, Choi AM, Crouser ED, Piantadosi CA, Bhattacharya J. Mitochondria in lung biology and pathology: more than just a powerhouse. Am J Physiol Lung Cell Mol Physiol : L962–L974, 2014. doi: 10.1152/ajplung.00073.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity : 401–414, 2012. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell : 348–361, 2013. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suliman HB, Carraway MS, Piantadosi CA. Postlipopolysaccharide oxidative damage of mitochondrial DNA. Am J Respir Crit Care Med : 570–579, 2003. doi: 10.1164/rccm.200206-518OC. [DOI] [PubMed] [Google Scholar]
- 35.Suliman HB, Carraway MS, Welty-Wolf KE, Whorton AR, Piantadosi CA. Lipopolysaccharide stimulates mitochondrial biogenesis via activation of nuclear respiratory factor-1. J Biol Chem : 41510–41518, 2003. doi: 10.1074/jbc.M304719200. [DOI] [PubMed] [Google Scholar]
- 36.Suliman HB, Keenan JE, Piantadosi CA. Mitochondrial quality-control dysregulation in conditional HO-1(-/-) mice. JCI Insight : e89676, 2017. doi: 10.1172/jci.insight.89676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suliman HB, Piantadosi CA. Mitochondrial quality control as a therapeutic target. Pharmacol Rev : 20–48, 2016. doi: 10.1124/pr.115.011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suliman HB, Sweeney TE, Withers CM, Piantadosi CA. Co-regulation of nuclear respiratory factor-1 by NFkappaB and CREB links LPS-induced inflammation to mitochondrial biogenesis. J Cell Sci : 2565–2575, 2010. doi: 10.1242/jcs.064089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C, Armstrong SM, Sugiyama MG, Tabuchi A, Krauszman A, Kuebler WM, Mullen B, Advani S, Advani A, Lee WL. Influenza-induced priming and leak of human lung microvascular endothelium upon exposure to Staphylococcus aureus. Am J Respir Cell Mol Biol : 459–470, 2015. doi: 10.1165/rcmb.2014-0373OC. [DOI] [PubMed] [Google Scholar]
- 40.Weinlich R, Oberst A, Beere HM, Green DR. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol : 127–136, 2017. doi: 10.1038/nrm.2016.149. [DOI] [PubMed] [Google Scholar]
- 41.Xu SX, Gilmore KJ, Szabo PA, Zeppa JJ, Baroja ML, Haeryfar SM, McCormick JK. Superantigens subvert the neutrophil response to promote abscess formation and enhance Staphylococcus aureus survival in vivo. Infect Immun : 3588–3598, 2014. doi: 10.1128/IAI.02110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome qactivation. Nature : 221–225, 2011. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]