one of the main risk factors for the development of cardiovascular disease (CVD) is hypertension. According to the American Heart Association, CVD is the leading cause of death in the United States, with almost one in three deaths attributable to it. Remodeling of the vasculature is one of the first detectable changes that occurs early on during the development/progression of hypertension and is a risk factor for subsequent cardiovascular events (19, 23). In fact, recent reports have suggested that vascular remodeling, in particular vascular stiffening, precedes the development of hypertension and is itself a primary risk factor for CVD (2, 25). Vascular remodeling is primarily characterized by the presence of structural changes in the vascular wall that modify the passive lumenal diameter of blood vessels in the absence or presence of changes to the amount of wall material (17). We and others have demonstrated that both inward (the structural reduction in passive luminal diameter of blood vessels) and outward remodeling (the structural increase in passive luminal diameter) involves the production and activation of matrix metalloproteinases (MMPs), primarily MMP-2 and MMP-9 (6, 10, 18). MMPs are a family of enzymes that degrade components of the extracellular matrix that comprise the vessel wall to which vascular cells attach. The activity of these enzymes during vascular remodeling promotes multiple processes that facilitate the structural modification of the vascular wall including the detachment, repositioning, and migration of vascular smooth muscle cells as well as the formation of new extracellular matrix proteins and the production of inflammatory cytokines by different cells of the vascular wall.
A prominent physiological process that involves one of the most rapid and greatest changes in vascular remodeling is pregnancy. In both pregnant humans and rodent animal models, vascular remodeling allows for uterine blood flow to increase significantly to provide the nutrients and environment for the proper development of the growing embryo and fetus. This occurs in association with a blood volume increase over the course of pregnancy. Concomitantly, vascular resistance is reduced via the outward remodeling of spiral arteries and the reduction in vascular tone that takes place as the placenta is being formed. In particular, cytotrophoblast invasion into the decidualized endometrium and myometrium is posited to play an important role in the outward remodeling of spiral arteries within the uterine vasculature by transforming them into conduit arteries of low resistance (15). In normal pregnancies, increased MMP-9 activity is implicated in trophoblast invasion (1), and both MMP-2 and MMP-9 are implicated in endometrial tissue and vascular remodeling. During this time, maternal blood pressure remains at prepregnancy levels or is marginally decreased (20).
A common pregnancy-related disorder that endangers the proper development of the embryo/fetus as well as the health of the mother is preeclampsia, a pathological condition in which vascular resistance is aberrantly increased and leads to maternal hypertension. Preeclampsia is associated with restricted fetal development and blunted uterine growth/expansion, and although the underlying causal mechanisms leading to preeclampsia have not been fully elucidated, it is postulated that reduced uteroplacental perfusion pressure (RUPP) and the subsequent placental ischemia/hypoxia play a role in increasing maternal vascular resistance. In a recent article published in the American Journal of Physiology-Heart and Circulatory Physiology, Li et al. (13a) tested the hypothesis that placental ischemia could modulate the expression and activity of MMPs through an inflammatory cytokine-mediated mechanism. They demonstrated that the presence of MMP-1 and MMP-7 is increased not only in the uterus and placenta of a RUPP animal model of preeclampsia but also in the aortas of the dams. Furthermore, they demonstrated that the presence of these MMPs in the uterus, placenta, and maternal vasculature is associated with increased production of collagen type I in those tissues. This suggests that an increased activity of multiple MMPs in the maternal circulation may be responsible for inducing maternal vascular remodeling processes that prompt the development of preeclampsia. Moreover, as Li et al. discovered that exposure of tissues from RUPP rats to a TNF-α antagonist reduced their content of MMP-1 and MMP-7, their results further suggest that increased circulating levels of that inflammatory cytokine may be responsible for inducing the remodeling of maternal blood vessels and for prompting the development of preeclampsia.
The results from Li et al. provide new insights on our limited understanding of the mechanisms that cause preeclampsia and introduce a novel link between the role that RUPP may have on the regulation of MMP-1 and MMP-7 expression in maternal tissues during pregnancy and how the inflammatory cytokine TNF-α may regulate that expression and the development of vascular remodeling/fibrosis and preeclampsia. Although novel and exciting, the study of Li et al. has a number of limitations that allow for the development of important questions that remain unanswered and should be addressed with additional research. For instance, it remains to be determined how TNF-α exerts its influence on the expression of MMP-1 and MMP-7 and whether that effect is limited to those MMPs only. It also remains to be determined whether the increased presence of MMP-1 and MMP-7 is indeed associated with an increased tissular activity of those enzymes. Moreover, it must be found if an increased activity of MMP-1 and MMP-7 is truly responsible for inducing maternal vascular remodeling/stiffening, increased vascular resistance, and hypertension during the development of preeclampsia. Further research should also determine if the dysregulation of MMP-1 and MMP-7 presence/activity in preeclampsia affects the outward remodeling of the uterine vasculature directly or if the effect is indirect. For example, the activity of multiple MMPs is implicated in the cytotrophoblast switch to an invasive phenotype; thus, dysregulation of MMPs may lead to reduced infiltration of cytrophoblasts and a subsequent reduction in spiral artery transformation. Inadequate spiral artery transformation is a common feature of preeclampsia (26). There are reports showing that periarterial trophoblast cell expression of MMP-7 is reduced in early onset preeclamptic patients compared with healthy women (21) and that MMP-1 and MMP-7 gene expression in decidua basalis tissue from preeclamptic patients is downregulated and MMP-1 protein levels are reduced in trophoblasts from the same tissue (14). These reports demonstrating a reduction in MMP-1 and MMP-7 in trophoblasts suggest that the elevated MMP-1 and MMP-7 levels in tissue from RUPP rats observed by Li et al. (13a) may be exerting effects independently of modulating the trophoblast invasion phenotype in preeclamptic pregnancies.
As mentioned above, the results by Li et al. demonstrate that the inflammatory cytokine TNF-α plays a role in the upregulation of MMP-1 and MMP-7 expression, but the mechanisms by which TNF-α may upregulate MMP-1 and MMP-7 in preeclampsia are not known. Serum TNF-α levels have been reported to be elevated during pregnancy in humans (13), although it would appear that the levels of TNF-α change over the course of pregnancy. In a large longitudinal study, maternal plasma TNF-α was reported to progressively decrease from the first, second, and third trimesters in healthy pregnancies (9). In preeclamptic patients, plasma TNF-α levels have been reported to be higher than those in normotensive women in the third trimester of pregnancy (11, 12), suggesting that failure to attenuate TNF-α signaling may play a role in the development of preeclampsia, through the aberrant overexpression of MMPs.
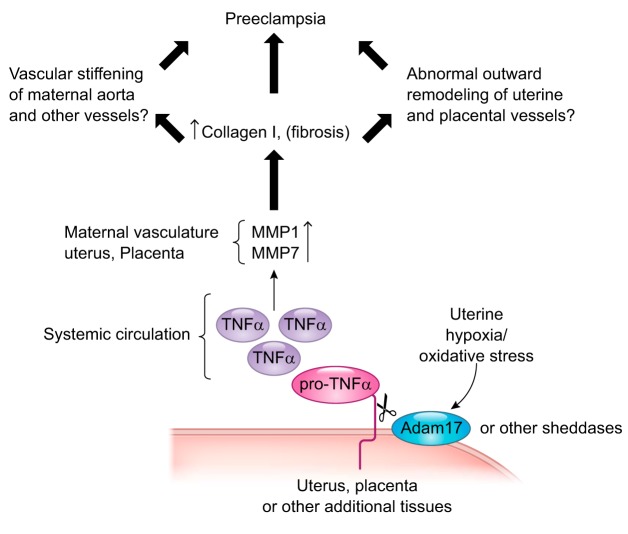
TNF-α is a cell surface molecule activated by ectodomain shedding. Pro-TNF-α is tethered to the membrane via a transmembrane domain that is cleaved by a number of MMPs to generate the soluble, active form of the cytokine. The main sheddase implicated in the release of soluble TNF-α into the circulatory system is a disintegrin and metalloproteinase-17 (ADAM17), also referred to as TNF-α-converting enzyme (TACE) (4). One possible source for increased TNF-α in preeclamptic pregnancies could be the placenta, which is subjected to hypoxia/oxidative stress as a consequence of reduced uteroplacental perfusion pressure (8). Oxidative stress is a known activator of ADAM17 (5). Lipid peroxides (24), a byproduct of oxidative stress, are increased in preeclamptic placentas, as is ADAM17 expression (7, 16). It therefore follows that TNF-α levels are also elevated in placentas from preeclamptic pregnancies (22, 24). It would appear that hypoxia/oxidative stress plays a role in this increase, as exposure of placental tissue explants, harvested from normal pregnancies, to low oxygen or cobalt chloride to mimic the hypoxic response significantly increases the release of TNF-α (3). Thus, a plausible model emerges in which hypoxia/oxidative stress in the placenta activates ADAM17, leading to the shedding and activation of TNF-α and the subsequent increased expression activity of MMP-1 and MMP-7 and likely other MMPs that impairs vascular remodeling and promotes preeclampsia (Fig. 1).
Fig. 1.
Plausible model in which hypoxia/oxidative stress in the placenta activates a disintegrin and metalloproteinase-17 (ADAM17), leading to the shedding and activation of TNF-α and the subsequent increased expression/activity of matrix metalloproteinase (MMP)-1 and MMP-7 and likely other MMPs that impair vascular remodeling and promote preeclampsia.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-088105 (to L. A. Martinez-Lemus).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.A.F. and L.A.M.-L. prepared figures; C.A.F. drafted manuscript; C.A.F. and L.A.M.-L. approved final version of manuscript; L.A.M.-L. conceived and designed research; L.A.M.-L. edited and revised manuscript.
REFERENCES
- 1.Anacker J, Segerer SE, Hagemann C, Feix S, Kapp M, Bausch R, Kämmerer U. Human decidua and invasive trophoblasts are rich sources of nearly all human matrix metalloproteinases. Mol Hum Reprod : 637–652, 2011. doi: 10.1093/molehr/gar033. [DOI] [PubMed] [Google Scholar]
- 2.Bender SB, Castorena-Gonzalez JA, Garro M, Reyes-Aldasoro CC, Sowers JR, DeMarco VG, Martinez-Lemus LA. Regional variation in arterial stiffening and dysfunction in Western diet-induced obesity. Am J Physiol Heart Circ Physiol : H574–H582, 2015. doi: 10.1152/ajpheart.00155.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab : 1582–1588, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature : 729–733, 1997. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 5.Brill A, Chauhan AK, Canault M, Walsh MT, Bergmeier W, Wagner DD. Oxidative stress activates ADAM17/TACE and induces its target receptor shedding in platelets in a p38-dependent fashion. Cardiovasc Res : 137–144, 2009. doi: 10.1093/cvr/cvp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro MM, Rizzi E, Ceron CS, Guimaraes DA, Rodrigues GJ, Bendhack LM, Gerlach RF, Tanus-Santos JE. Doxycycline ameliorates 2K-1C hypertension-induced vascular dysfunction in rats by attenuating oxidative stress and improving nitric oxide bioavailability. Nitric Oxide : 162–168, 2012. doi: 10.1016/j.niox.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol : 240–249, 1997. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 8.Cornelius DC, Amaral LM, Wallace K, Campbell N, Thomas AJ, Scott J, Herse F, Wallukat G, Dechend R, LaMarca B. Reduced uterine perfusion pressure T-helper 17 cells cause pathophysiology associated with preeclampsia during pregnancy. Am J Physiol Regul Integr Comp Physiol : R1192–R1199, 2016. doi: 10.1152/ajpregu.00117.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denney JM, Nelson EL, Wadhwa PD, Waters TP, Mathew L, Chung EK, Goldenberg RL, Culhane JF. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine : 170–177, 2011. doi: 10.1016/j.cyto.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumont O, Loufrani L, Henrion D. Key role of the NO-pathway and matrix metalloprotease-9 in high blood flow-induced remodeling of rat resistance arteries. Arterioscler Thromb Vasc Biol : 317–324, 2007. doi: 10.1161/01.ATV.0000254684.80662.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi M, Ueda Y, Yamaguchi T, Sohma R, Shibazaki M, Ohkura T, Inaba N. Tumor necrosis factor-alpha in the placenta is not elevated in pre-eclamptic patients despite its elevation in peripheral blood. Am J Reprod Immunol : 113–119, 2005. doi: 10.1111/j.1600-0897.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 12.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol : 1752–1759, 1994. [PubMed] [Google Scholar]
- 13.Kupferminc MJ, Peaceman AM, Wigton TR, Tamura RK, Rehnberg KA, Socol ML. Immunoreactive tumor necrosis factor-alpha is elevated in maternal plasma but undetected in amniotic fluid in the second trimester. Am J Obstet Gynecol : 976–979, 1994. doi: 10.1016/0002-9378(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 13a.Li W, Cui N, Mazzuca MQ, Mata KM, Khalil RA. Increased vascular and uteroplacental matrix metalloproteinase-1 and -7 levels and collagen i deposition in hypertension-in-pregnancy. Role of TNF-α. Am J Physiol Heart Circ Physiol; doi: 10.1152/ajpheart.00207.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lian IA, Toft JH, Olsen GD, Langaas M, Bjørge L, Eide IP, Børdahl PE, Austgulen R. Matrix metalloproteinase 1 in pre-eclampsia and fetal growth restriction: reduced gene expression in decidual tissue and protein expression in extravillous trophoblasts. Placenta : 615–620, 2010. doi: 10.1016/j.placenta.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Lyall F, Bulmer JN, Duffie E, Cousins F, Theriault A, Robson SC. Human trophoblast invasion and spiral artery transformation: the role of PECAM-1 in normal pregnancy, preeclampsia, and fetal growth restriction. Am J Pathol : 1713–1721, 2001. doi: 10.1016/S0002-9440(10)64127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma R, Gu Y, Groome LJ, Wang Y. ADAM17 regulates TNFα production by placental trophoblasts. Placenta : 975–980, 2011. doi: 10.1016/j.placenta.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) : 45–57, 2009. doi: 10.1152/physiol.00029.2008. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Lemus LA, Zhao G, Galiñanes EL, Boone M. Inward remodeling of resistance arteries requires reactive oxygen species-dependent activation of matrix metalloproteinases. Am J Physiol Heart Circ Physiol : H2005–H2015, 2011. doi: 10.1152/ajpheart.01066.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathiassen ON, Buus NH, Larsen ML, Mulvany MJ, Christensen KL. Small artery structure adapts to vasodilatation rather than to blood pressure during antihypertensive treatment. J Hypertens : 1027–1034, 2007. doi: 10.1097/HJH.0b013e3280acac75. [DOI] [PubMed] [Google Scholar]
- 20.Osol G, Moore LG. Maternal uterine vascular remodeling during pregnancy. Microcirculation : 38–47, 2014. doi: 10.1111/micc.12080. [DOI] [PubMed] [Google Scholar]
- 21.Reister F, Kingdom JC, Ruck P, Marzusch K, Heyl W, Pauer U, Kaufmann P, Rath W, Huppertz B. Altered protease expression by periarterial trophoblast cells in severe early-onset preeclampsia with IUGR. J Perinat Med : 272–279, 2006. doi: 10.1515/JPM.2006.052. [DOI] [PubMed] [Google Scholar]
- 22.Rinehart BK, Terrone DA, Lagoo-Deenadayalan S, Barber WH, Hale EA, Martin JN Jr, Bennett WA. Expression of the placental cytokines tumor necrosis factor alpha, interleukin 1beta, and interleukin 10 is increased in preeclampsia. Am J Obstet Gynecol : 915–920, 1999. doi: 10.1016/S0002-9378(99)70325-X. [DOI] [PubMed] [Google Scholar]
- 23.Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation : 2230–2235, 2003. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Walsh SW. TNF alpha concentrations and mRNA expression are increased in preeclamptic placentas. J Reprod Immunol : 157–169, 1996. doi: 10.1016/S0165-0378(96)00998-9. [DOI] [PubMed] [Google Scholar]
- 25.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension : 1105–1110, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitley GS, Cartwright JE. Trophoblast-mediated spiral artery remodelling: a role for apoptosis. J Anat : 21–26, 2009. doi: 10.1111/j.1469-7580.2008.01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]