Abstract
Background
Bronchoalveolar lavage (BAL) is a necessary procedure for diagnosis of various lung diseases. High-flow nasal cannula (HFNC) oxygen delivery was recently introduced. This study aimed to investigate the safety and effectiveness of HFNC oxygen supply during BAL procedure in patients with acute respiratory failure (ARF).
Methods
Patients who underwent BAL while using HFNC at a partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2/FiO2; PF) ratio of 300 or below among patients who had been admitted from March 2013 to May 2017 were retrospectively investigated.
Results
Thirty-three BAL procedures were confirmed. Their baseline PF ratio was 166.1±46.7. FiO2 values before, during, and after BAL were 0.45±0.12, 0.74±0.19, and 0.57±0.14, respectively. Flow (L/min) values before, during, and after BAL were 26.5±20.3, 49.0±7.2, and 40.8±14.2, respectively. Both FiO2 and flow during and after the procedure were significantly different from those before the procedure (both p<0.001). Oxygen saturation levels before, during, and after BAL measured by pulse oximeter were 94.8±2.9, 94.6±3.5, and 95.2±2.8%, respectively. There were no significant differences in oxygen saturation among the three groups. Complications of BAL procedure included transient hypoxemia, hypotension, and fever. However, there was no endotracheal intubation within 24 hours. Baseline PF ratio in “without HFNC” group was significantly higher than that in “with HFNC” group. There were no differences in complications between the two groups.
Conclusion
The use of HFNC during BAL procedure in ARF patients was effective and safe. However, there were no significant differences in oxygen saturation level and complications comparing “without HFNC” group in mild ARF. More studies are needed for moderate to severe ARF patients.
Keywords: Bronchoalveolar Lavage, Bronchoscopy, Nasal Cannula, Oxygen, Respiratory Failure
Introduction
Bronchoalveolar lavage (BAL) under bronchoscopy is an important procedure that is helpful for the diagnosis of lung disease and for the prediction of the treatment progress and prognosis1,2,3,4. Hypoxemia, however, may occur during BAL5, and serious complications like endotracheal intubation (ETI) may occur after the procedure. In the past, noninvasive positive-pressure ventilation (NPPV) was used to prevent hypoxemia when performing BAL6,7, but NPPV has been seldom used because it is a time-consuming and very demanding technique, requiring experienced personnel and sufficient adaptation time for application to patients.
High-flow nasal cannula (HFNC) oxygen therapy is easy to use and is well-tolerated and comfortable; thus, it is currently being used as a noninvasive method of supplying oxygen to hypoxemic patients8. Studies of acute respiratory failure (ARF) reported that the application of HFNC was proven to have had beneficial effects9, showing superior effects compared to the conventional oxygen delivery system or NPPV10.
Comparative studies using HFNC and NPPV during bronchoscopy on hypoxemic patients were recently reported11,12. A study where HFNC was used during BAL on patients with ARF in the intensive care unit (ICU) has also been reported13. As the number of patients reported was not big, however, it is not clear that the use of HFNC during BAL is reasonable. Moreover, the safety of using HFNC has not been clearly established. Therefore, this study was conducted to investigate the safety and effectiveness on oxygenation of HFNC oxygen supply during BAL in patients with ARF.
Materials and Methods
1. Study subjects
A retrospective, single-center study was performed, and data were collected by searching medical records. Of the patients who were admitted to the Department of Pulmonology, Daegu Catholic University Medical Center within the period from March 1, 2013, to May 31, 2017, the cases that met the following three inclusion criteria were targeted: (1) patients who underwent BAL during bronchoscopy; (2) patients with a partial pressure of oxygen in arterial blood/fraction of inspired oxygen (PaO2/FiO2; PF) ratio of 300 or less before bronchoscopy; and (3) patients for whom HFNC was used. Adopting Berlin's definition of acute respiratory distress syndrome (ARDS), a PF ratio of 300 or less was considered ARF14. The cases that met any of the following exclusion criteria were not included in the study: (1) patients for whom the use of HFNC was impossible; (2) patients who underwent ETI; and (3) patients for whom mechanical ventilation, including NPPV, was applied. Data of the patients who did not use HFNC were also collected if they met the criteria 1 and 2. This study had been approved by the Institutional Review Board of Daegu Catholic University Medical Center (IRB No. CR-17-089) and the requirement for written informed consent was waived due to retrospective study design.
2. Study protocol
The result of the arterial blood gas analysis performed within 48 hours before BAL was investigated, and the baseline PF ratio was calculated using this result. The severity of ARF was divided into three stages (mild, 200<PF ratio≤300; moderate, 100<PF ratio≤200; and severe, PF ratio≤100) based on Berlin's definition of ARDS. Before performing BAL, the oxygen delivery device, FiO2, supplied oxygen flow, and oxygen saturation measured by pulse oximeter (SpO2) were recorded. If the patient was receiving oxygen through a nasal cannula, the FiO2 value was calculated as 0.24 for 1 L/min, 0.28 for 2 L/min, 0.32 for 3 L/min, 0.36 for 4 L/min, and 0.4 for 5 L/min. If the patient wore a simple oxygen mask, the FiO2 value was calculated as 0.4 for 5–6 L/min. If the patient was receiving more than 10 L/min of oxygen with a mask with reservoir bag, the FiO2 value was calculated as 0.815. Additionally, the measured values of the FiO2, flow, and SpO2 were examined during and after the BAL procedure. During the procedure, the FiO2 and flow of HFNC were increased to maintain a SpO2 value 90% or above, and the maximum values were recorded. Also, for the SpO2 value during the procedure, the value maintained in the FiO2 and flow states adjusted to the maximum value was recorded.
The complications that occurred during and within 24 hours after the BAL procedure were investigated. Hypoxemia referred to the case where the SpO2 value dropped to below 90%, and transient hypoxemia referred to the case where it was maintained for less than 30 seconds during the BAL procedure. Hypotension was the case where the systolic blood pressure was less than 90 mm Hg or the diastolic blood pressure was less than 60 mm Hg16. Fever was a body temperature of 37.8℃ or higher measured at the tympanic membrane17. Even if fever was already present before the BAL procedure, it was considered a procedure-related fever if the patient had a fever of 37.8℃ or higher after the bronchoscopy. Several previous studies12,18,19,20 concluded that ETI that occurred within 8 hours before completing bronchoscopy is directly associated with bronchoscopy. In this study, however, the occurrence of ETI within 24 hours after the completion of bronchoscopy was checked by applying stricter criteria, as in the study of La Combe et al.13. And we followed cases for ETI until 2 weeks after the BAL procedure.
3. Definitions of pre-BAL and post-BAL diagnosis
In this study, pre-BAL diagnosis was defined as symptoms, laboratory results and radiologic findings. Pre-BAL diagnosis included bacterial pneumonia, atypical pneumonia, interstitial lung disease, and alveolar hemorrhage.
Post-BAL diagnosis was defined based on the following criteria. Bacterial pneumonia was defined as a case (1) where clinical manifestation of typical pneumonia (fever, increased cough with sputum production, a change in the shadow on the chest X-ray, increased inflammatory marker) was shown, and the presence of bacteria was proven in the BAL procedure; or (2) where clinical manifestation of typical pneumonia was shown but bacteria were not detected, neutrophil predominance appeared in the result of BAL, and the clinical manifestation was improved by antibiotics. Atypical pneumonia was defined as a case (1) where the presence of atypical microorganisms such as Pneumocystis jiroveci pneumonia, viral pneumonia, and fungal pneumonia was proven; (2) where clinical manifestation of pneumonia and evidence of infection were shown, but it was not typical, and causative organisms were not proven; or (3) where aspiration pneumonia was present21. Organizing pneumonia was defined as a case (1) where it was diagnosed through lung biopsy; or (2) where there were pneumonia-like chest X-ray findings, there was no evidence of infection, lymphocyte predominance was shown in the result of BAL, and the clinical manifestation improved after the use of steroids22. Interstitial lung disease was defined as a case (1) where the lesions of interstitial pneumonia previously diagnosed were aggravated; or (2) where the patient had a connective tissue disease showing aggravation of the lesions of interstitial pneumonia in the chest X-ray findings, and there was no evidence of infectious lung disease. Alveolar hemorrhage was diagnosed after confirming it in the BAL fluid, and acute eosinophilic pneumonia was diagnosed in a case showing eosinophilia (>25%) in the BAL procedure. If differential diagnosis was difficult, the most likely clinical diagnosis was used as the post-BAL diagnosis.
Treatment changes were defined as changes in treatment occurring as a result of fluid examination obtained by the BAL procedure.
4. HFNC oxygen delivery
For HFNC, Optiflow (Fisher & Paykel, Auckland, New Zealand) or AIRVO2 (Fisher & Paykel) was used. HFNC was applied just before bronchoscopy, during the BAL procedure, and until the end of the bronchoscopy. If the SpO2 value was predicted to drop below 90% during the BAL procedure, the FiO2 and flow of HFNC were adjusted, and the values were maintained so that the SpO2 value would be 90% or above.
5. Fiberoptic bronchoscopy and BAL
All the bronchoscopy procedures were performed by three experienced (more than 10 years' experience) respiratory specialists. Before the procedure, atropine and pethidine were used to inhibit secretion and reduce the discomfort. For local anesthesia, lidocaine was nebulized into the nasal cavity and pharyngeal mucosa. First, the bronchoscope was inserted in the nasal cavity while oxygen was being supplied through the nose with HFNC. If it was difficult to insert the nasal cannula and bronchoscope at the same time, however, as the internal diameter of the nasal cavity was small, oxygen was supplied by inserting the nasal cannula in the oral cavity. After assessing the patient's condition, sedation was induced using midazolam, if necessary. The bronchoscope was inserted in the trachea, and then lidocaine was sprayed into the carina. After that, the bronchi were examined, and the bronchoscope was wedged into the appropriate segmental bronchus. For BAL, normal saline was used, and after instilling 30 mL aliquots, gentle suction was done, which was repeated 5 times, with 1 time added or subtracted depending on the patient's condition. The bronchoscopy time was measured from the time of insertion of the bronchoscope to the time of its removal. After the procedure, flumazenil was used to reverse the effect of midazolam.
6. Statistical analysis
For the test results, if the absolute values, percentages, and continuous values met the normality assumption, the mean and standard deviation were presented, and if they did not, the median, maximum value, and minimum value were presented. For the FiO2, flow, SpO2, and hemodynamic changes over time before, during, and after the BAL procedure under bronchoscopy, repeated-measure one-factor analysis was used. The differences in the implementation of ETI and in the immunosuppression status of the survivor and non-survivor groups were analyzed through Fisher exact test. Various quantitative factors were compared through the Mann-Whitney U test, and the difference in the post-BAL diagnosis was analyzed through the chi-square test. The statistical significance level was set to p<0.05. For the statistical analysis, SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) was used.
Results
1. Baseline characteristics of the patients
Of the patients who were admitted to Daegu Catholic University Medical Center within the period from March 1, 2013, to May 31, 2017, a total of 32 patients met the inclusion criteria. Among them, one patient underwent BAL 2 times; thus, the total number of BAL procedures was 33. The mean age was 63.9±15.0 years, and the male-to-female ratio was 18:15. The mean baseline PF ratio was 166.1±46.7, and the median was 159.4 (range, 89.1–269.5). Before the BAL procedure, 19 cases of HFNC and 14 cases of nasal cannula were used as oxygen delivery devices. Eight cases (24.2%) were classified as mild ARF; 24 cases (72.7%) as moderate ARF; and one case (3%) as severe ARF. For the comorbidities, 11 cases (33.3%) had connective tissue disease, followed by 10 cases (30.3%) of hypertension and seven cases (21.2%) of chronic lung disease, including chronic obstructive pulmonary disease, posttuberculous destroyed lung, and bronchiectasis. Eight patients (24.2%) were under the immunosuppression state, in which immunosuppressants or steroids needed to be administered for a long time, such as patients with connective tissue disease, interstitial lung disease, or acquired immune deficiency syndrome. Radiologic findings showed bilateral alveolar infiltration in 18 cases (54.5%), bilateral interstitial infiltration in seven cases (21.2%) and bilateral alveolar and interstitial infiltration in eight cases (24.2%). For the pre-BAL diagnosis, bacterial pneumonia was the most common, on the other hand, for the post-BAL diagnosis, atypical pneumonia was the most common. Organizing pneumonia and acute eosinophilic pneumonia were newly diagnosed in the post-BAL diagnosis (Table 1). Atypical pneumonia included P. jiroveci pneumonia, viral pneumonia, fungal pneumonia, and unknown-cause pneumonia.
Table 1. Baseline characteristics of the patients.
| Characteristic | With HFNC (n=32) | Without HFNC (n=23) |
|---|---|---|
| Age, yr | 63.9±15.0 | 65.3±13.1 |
| Male sex | 18 (54.5) | 10 (43.5) |
| Baseline PaO2/FiO2 (mm Hg)* | 166.1±46.7 | 233.0±36.5 |
| Baseline PaO2 (mm Hg) | 69.3±16.2 | 65.0±9.9 |
| Baseline FiO2* | 0.44±0.11 | 0.29±0.06 |
| Severity of acute respiratory failure† | ||
| Mild (200<PaO2/FiO2 ≤300) | 8 (24.2) | 20 (87.0) |
| Moderate (100<PaO2/FiO2 ≤200) | 24 (72.7) | 3 (13.0) |
| Severe (PaO2/FiO2 ≤100) | 1 (3.0) | 0 (0) |
| Comorbid illness | ||
| Connective tissue disease | 11 (33.3) | 4 (17.4) |
| Hypertension | 10 (30.3) | 11 (47.8) |
| Chronic lung disease | 7 (21.2) | 1 (4.3) |
| Diabetes mellitus | 6 (18.2) | 3 (13.0) |
| Vascular disease | 4 (12.1) | 7 (30.4) |
| Interstitial lung disease | 3 (9.0) | 2 (8.7) |
| Malignancy | 2 (6.0) | 2 (8.7) |
| AIDS | 1 (3.0) | 0 (0) |
| Chronic kidney disease | 1 (3.0) | 0 (0) |
| Others‡ | 10 (30.3) | 5 (21.7) |
| Use of vasopressor | 1 (3.0) | 0 (0) |
| Immune suppression | 8 (24.2) | 5 (21.7) |
| Radiologic finding | ||
| Bilateral alveolar infiltration | 18 (54.5) | 9 (39.1) |
| Bilateral interstitial infiltration | 7 (21.2) | 5 (21.7) |
| Bilateral alveolar and interstitial infiltration | 8 (24.2) | 9 (39.1) |
| Pre-BAL diagnosis | ||
| Bacterial pneumonia | 18 (54.5) | 10 (43.5) |
| Atypical pneumonia | 12 (36.4) | 8 (34.8) |
| Interstitial lung disease | 2 (6.1) | 5 (21.7) |
| Alveolar hemorrhage | 1 (3.0) | 0 (0) |
| Post-BAL diagnosis | ||
| Atypical pneumonia | 14 (42.4) | 5 (21.7) |
| Bacterial pneumonia | 10 (30.3) | 6 (26.1) |
| Organizing pneumonia | 4 (12.1) | 5 (21.7) |
| Interstitial lung disease | 2 (6.1) | 4 (17.4) |
| Alveolar hemorrhage | 2 (6.1) | 0 (0) |
| Acute eosinophilic pneumonia | 1 (3.0) | 1 (4.3) |
| Pulmonary tuberculosis | 0 (0) | 2 (8.7) |
Values are presented as mean±standard deviation or number (%).
*p<0.001, Mann-Whitney U test between “with HFNC” and “without HFNC” groups. †p<0.001, through the chi-square test between “with HFNC” and “without HFNC” groups. The severity of acute respiratory failure was divided into three stages (mild, moderate and severe) based on Berlin's definition of acute respiratory distress syndrome14. ‡Others included atrial fibrillation, chronic liver disease, chronic pancreatitis, congestive heart failure, epilepsy, femur fracture, herniated nucleus pulposus, hypothyroidism, schizophrenia and spinal stenosis.
HFNC: high-flow nasal cannula; PaO2: partial pressure of oxygen in arterial blood; FiO2: fraction of inspired oxygen; AIDS: acquired immune deficiency syndrome; BAL: bronchoalveolar lavage.
2. BAL procedure
BAL was successfully performed in all the patients. The time spent performing BAL was 14.4±7.2 minutes. The amount of normal saline infused during the BAL procedure was 140.9±27.5 mL, the amount of specimens obtained by BAL was 75.1±25.5 mL, and the recovery rate compared to the instilled volume was 53.1±14.5%. In terms of the BAL site in the bronchus, the right upper lobe had six cases, the right middle lobe had nine cases, the right lower lobe had 13 cases, the left upper lobe had one case, and the left lower lobe had four cases. Midazolam was injected during bronchoscopy in 26 cases, and the amount of injection was 2.67±1.0 mg. In 17 cases, flumazenil was administrated immediately after bronchoscopy, and the amount injected was 0.31±0.11 mg. The total cell counts of BAL fluid were 290/µL (range, 40–25,000/µL) and the percentage of each cell was as follows: macrophages 28% (range, 0%–76%), neutrophils 25% (range, 0%–89%), lymphocytes 17% (range, 1%–71%), and eosinophils 1% (range, 0%–53%). Infectious agents were identified in 16 cases as follows; bacteria in six cases, cytomegalovirus in four cases, fungus in three cases, P. jiroveci in three cases, and respiratory virus in three cases. After BAL procedure, the treatment plan was changed in 19 cases (57.6%) (Table 2).
Table 2. Clinical status in 33 cases of acute respiratory failures received bronchoalveolar lavage (BAL) using high-flow nasal cannula oxygen delivery.
| No | Age (yr)/ Sex | PF ratio | Before BAL | After BAL | During BAL | Procedure time (min) | Intubation* (time to intubation, hr) | Immune Suppression | 6-Month survival | Complications | Pre-BAL diagnosis | Infectious agent | Post-BAL diagnosis | Treatment change | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FiO2 | Flow (L/min) | SpO2 (%) | FiO2 | Flow (L/min) | SpO2 (%) | FiO2 | Flow (L/min) | SpO2 (%) | ||||||||||||
| 1 | 80/F | 109.6 | 0.7 | 40 | 93 | 0.9 | 40 | 98 | 0.8 | 40 | 90 | 20 | + (26) | - | Expired | THX, fever | PN | Acinetobacter baumanii | PN | - |
| 2 | 56/M | 165.6 | 0.6 | 50 | 90 | 0.9 | 50 | 96 | 0.6 | 50 | 90 | 5 | + (76) | - | Expired | THX, fever | ILD | - | ILD | - |
| 3 | 43/F | 133.3 | 0.4 | 5 | 96 | 0.7 | 60 | 98 | 0.7 | 60 | 98 | 20 | - | - | Expired | Fever | PN | - | AH | + |
| 4† | 42/F | 165.3 | 0.4 | 5 | 97 | 0.9 | 50 | 96 | 0.4 | 5 | 95 | 30 | - | - | Expired | Fever | PN | PCP | APN | + |
| 5† | 42/F | 173.8 | 0.4 | 5 | 93 | 0.94 | 50 | 94 | 0.65 | 50 | 98 | 30 | - | - | Expired | THX, fever | APN | PCP | APN | - |
| 6 | 71/M | 159.3 | 0.4 | 5 | 98 | 0.5 | 50 | 98 | 0.5 | 50 | 96 | 20 | - | - | Survived | Fever | PN | - | PN | - |
| 7 | 83/M | 138.9 | 0.45 | 47 | 98 | 0.7 | 47 | 96 | 0.5 | 47 | 97 | 20 | - | - | Survived | - | PN | - | OP | + |
| 8 | 73/F | 115.8 | 0.5 | 50 | 90 | 0.9 | 50 | 95 | 0.6 | 50 | 98 | 15 | - | - | Survived | - | PN | - | OP | + |
| 9 | 56/M | 200.0 | 0.4 | 30 | 98 | 0.56 | 50 | 91 | 0.5 | 40 | 99 | 15 | - | - | Expired | THX | PN | Staphylococcusaureus | PN | + |
| 10 | 68/F | 212.3 | 0.4 | 5 | 96 | 0.6 | 50 | 97 | 0.4 | 5 | 96 | 15 | - | - | Survived | Hypotension | PN | A. baumanii,S. aureus | PN | + |
| 11 | 82/F | 213.9 | 0.4 | 5 | 99 | 0.6 | 45 | 99 | 0.4 | 5 | 96 | 9 | - | + | Survived | - | APN | - | APN | - |
| 12 | 53/M | 269.5 | 0.4 | 40 | 97 | 0.4 | 40 | 90 | 0.4 | 40 | 99 | 25 | + (32) | - | Expired | THX | APN | RSV | APN | + |
| 13 | 23/M | 203.8 | 0.3 | 3 | 96 | 1.0 | 55 | 86 | 0.6 | 40 | 94 | 7 | - | - | Survived | THX | APN | - | AEP | + |
| 14 | 70/M | 149.5 | 0.4 | 30 | 96 | 0.6 | 50 | 90 | 0.4 | 30 | 95 | 9 | - | - | Survived | - | PN | CMV | APN | + |
| 15 | 73/F | 106.3 | 0.6 | 50 | 94 | 0.8 | 55 | 92 | 0.7 | 50 | 93 | 10 | + (70) | - | Expired | THX, fever | APN | - | APN | - |
| 16 | 58/M | 178.2 | 0.6 | 50 | 91 | 1.0 | 50 | 88 | 0.8 | 50 | 91 | 21 | - | - | Survived | THX | PN | RSV | OP | + |
| 17 | 64/M | 120.8 | 0.4 | 40 | 96 | 0.8 | 50 | 90 | 0.4 | 40 | 96 | 7 | - | - | Survived | - | PN | - | PN | - |
| 18 | 69/M | 128.0 | 0.4 | 35 | 98 | 0.4 | 35 | 97 | 0.4 | 35 | 97 | 9 | - | - | Expired | - | PN | - | PN | - |
| 19 | 64/F | 248.0 | 0.4 | 40 | 100 | 0.8 | 40 | 97 | 0.4 | 40 | 98 | 7 | - | - | Survived | - | APN | - | APN | - |
| 20 | 76/F | 123.1 | 0.65 | 45 | 98 | 1.0 | 60 | 94 | 0.65 | 45 | 95 | 20 | + (78) | + | Expired | THX | APN | - | APN | - |
| 21 | 70/M | 244.6 | 0.28 | 2 | 92 | 0.6 | 40 | 95 | 0.8 | 15 | 96 | 8 | + (144) | - | Expired | THX, fever | ILD | Candida albicans | APN | + |
| 22 | 56/F | 189.4 | 0.32 | 3 | 91 | 0.6 | 40 | 96 | 0.6 | 40 | 98 | 25 | - | + | Expired | THX | APN | CMV | APN | + |
| 23 | 72/M | 159.4 | 0.4 | 5 | 92 | 0.7 | 40 | 95 | 0.6 | 50 | 98 | 17 | - | + | Expired | THX | APN | CMV | APN | + |
| 24 | 64/M | 184.7 | 0.32 | 3 | 90 | 0.7 | 50 | 96 | 0.4 | 40 | 92 | 17 | + (120) | - | Expired | THX, fever | APN | - | APN | - |
| 25 | 36/F | 136.8 | 0.7 | 50 | 95 | 1.0 | 50 | 88 | 0.8 | 50 | 90 | 5 | - | - | Survived | THX | PN | - | OP | + |
| 26 | 81/M | 128.8 | 0.5 | 40 | 94 | 0.9 | 60 | 94 | 0.5 | 40 | 93 | 5 | - | - | Survived | THX | PN | C. albicans | PN | - |
| 27 | 68/F | 156.0 | 0.5 | 40 | 96 | 0.7 | 50 | 94 | 0.6 | 50 | 93 | 10 | + (72) | + | Expired | THX | AH | - | AH | - |
| 28 | 43/M | 89.1 | 0.7 | 60 | 91 | 0.8 | 60 | 93 | 0.8 | 60 | 95 | 7 | + (274) | + | Expired | THX | APN | PCP, CMV, Rhinovirus | APN | + |
| 29 | 71/M | 208.9 | 0.28 | 2 | 91 | 0.5 | 40 | 98 | 0.5 | 40 | 97 | 15 | - | - | Expired | - | PN | - | PN | + |
| 30 | 81/F | 245.8 | 0.4 | 40 | 95 | 0.8 | 50 | 97 | 0.6 | 50 | 100 | 12 | - | + | Expired | - | PN | C. albicans | ILD | + |
| 31 | 80/F | 141.0 | 0.4 | 5 | 98 | 1.0 | 60 | 100 | 0.6 | 50 | 93 | 14 | - | + | Expired | - | APN | - | APN | - |
| 32 | 64/M | 102.6 | 0.5 | 40 | 95 | 0.8 | 60 | 95 | 0.7 | 50 | 92 | 8 | - | - | Survived | - | PN | S. aureus | PN | + |
| 33 | 75/M | 179.2 | 0.4 | 5 | 95 | 0.4 | 40 | 99 | 0.4 | 40 | 95 | 18 | - | - | Survived | - | PN | Pseudomonas aeruginosa | PN | + |
*Cases for endotracheal intubation were followed until 2 weeks after the BAL procedure. †One patient received bronchoscopy twice for BAL.
PF ratio: baseline PaO2/FiO2; FiO2: fraction of inspired oxygen; Flow: gas flow rate; SpO2: oxygen saturation recorded by pulse oximetry; F: female; THX: transient hypoxemia; PN: bacterial pneumonia; M: male; ILD: interstitial lung disease; AH: alveolar hemorrhage; PCP: Pneumocystis jiroveci pneumonia; APN: atypical pneumonia; OP: organizing pneumonia; RSV: respiratory syncytial virus; AEP: acute eosinophilic pneumonia; CMV: cytomegalovirus.
3. SpO2, FiO2, and flow
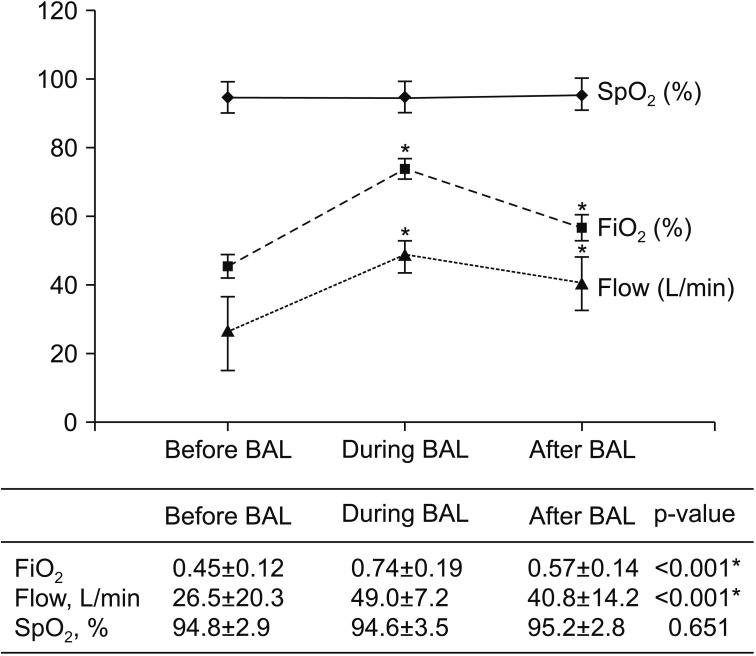
Before the BAL procedure, the FiO2 was 0.45±0.12, the flow was 26.5±20.3 L/min, and the SpO2 was 94.8±2.9%. During the BAL procedure, the FiO2 was 0.74±0.19, the flow was 49.0±7.2 L/min, and the SpO2 was 94.6±3.5%. After the BAL procedure, the FiO2 was 0.57±0.14, the flow was 40.8±14.2 L/min, and the SpO2 was 95.2±2.8%. There were no differences in SpO2 among the three groups (p=0.651), but there were statistically significant differences in FiO2 and flow during and after the BAL procedure compared to before the BAL procedure (p<0.001) (Figure 1).
Figure 1. Changes in saturation measured by pulse oximeter (SpO2), fraction of inspired oxygen (FiO2), and gas flow rate before, during and after the bronchoalveolar lavage (BAL) using high-flow nasal cannula oxygen delivery. The FiO2 values in the table are shown as percent (%) in the graph. *p<0.001, Multiple comparison result by contrast between before BAL and during BAL, before BAL and after BAL, respectively.
4. Hemodynamic changes
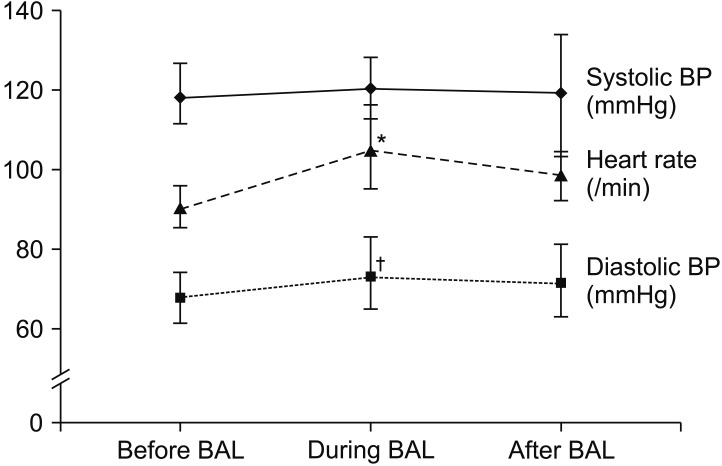
The systolic blood pressure was 114.9±15.8 mm Hg before the BAL procedure, 120.3±22.5 mm Hg during the BAL procedure, and 119.2±19.0 mm Hg after the BAL procedure (p=0.201). As for the diastolic blood pressure, it was 68.0±10.0 mm Hg before the BAL procedure, 73.4±12.6 during the BAL procedure, and 71.6±11.5 mm Hg after the procedure (p=0.025). Before the procedure, there was no difference in the systolic blood pressure, but the diastolic blood pressure significantly increased during and after the BAL procedure compared to before the BAL procedure. The heart rate per minute was 90.5±13.5 times before the procedure, 104.7±17.1 times during the procedure, and 98.7±13.5 times after the procedure, showing statistically significant differences (p<0.001) (Figure 2).
Figure 2. Hemodynamic changes before, during and after the bronchoalveolar lavage (BAL) using high-flow nasal cannula oxygen delivery. *p<0.001, †p=0.025, repeated measure one factor analysis between before BAL and during BAL, before BAL and after BAL, respectively. BP: blood pressure.
5. Complications of the BAL procedure
During the BAL procedure, transient hypoxemia appeared in 17 cases (51.5%), SpO2 was maintained 90% or above by increasing the FiO2 and flow. There was no hypoxemia, however, that lasted for more than 30 seconds. Hypotension occurred in one case (3%), but the patient recovered after 30 minutes through fluid hydration and the use of inotropics. This case was associated with sepsis caused by bacterial pneumonia. Deterioration of the clinical status due to hypotension was not observed. After the BAL procedure, fever was developed in nine cases (27.3%). In five cases, fever that was not present before the bronchoscopy occurred after the bronchoscopy, but the fever was less than 39℃ and was alleviated without additional antibiotics within 1 day in most cases. There was no ETI within 24 hours after the BAL procedure (Table 3), but ETI was performed 26 hours after the procedure in one case. The 6-month survival rate was 42.4%, and the 30-day mortality rate after bronchoscopy was 45.5%.
Table 3. Complications of the bronchoalveolar lavage.
| Complications | With HFNC | Without HFNC |
|---|---|---|
| During bronchoalveolar lavage | ||
| Transient hypoxemia* | 17 (51.5) | 9 (39.1) |
| Hypotension† | 1 (3.0) | 0 (0) |
| After bronchoalveolar lavage | ||
| Fever‡ | 9 (27.3) | 3 (13.0) |
| ETI within 24 hours | 0 (0) | 0 (0) |
Values are presented as number (%).
*Transient hypoxemia referred to the case where the SpO2 value dropped to below 90% was maintained for less than 30 seconds during the bronchoalveolar lavage procedure. †Hypotension was the case where the systolic blood pressure was less than 90 mm Hg or the diastolic blood pressure was less than 60 mm Hg16. ‡Fever was a body temperature of 37.8℃ or higher measured at the tympanic membrane17.
HFNC: high-flow nasal cannula; ETI: endotracheal intubation; SpO2: oxygen saturation recorded by pulse oximetry.
6. Comparison of the survivor and non-survivor groups
After the patients' division into the survivor and non-survivor groups, ETI was performed only in the non-survivor group. The median of the time spent until ETI after the BAL procedure was 76 hours (range, 26–274 hours). There were no differences between the survivor and non-survivor groups in the age, PF ratio, immunosuppression, FiO2, and flow used before, during, and after the procedure, as well as in the SpO2, diastolic blood pressure, and heart rate. There was a difference, however, in the systolic blood pressure before the procedure between the two groups (p=0.030). The mean systolic blood pressure of the survivor group was 110.8±16.6 mm Hg, which was slightly lower than that of the non-survivor group (118.7±14.7 mm Hg). Additionally, there was a difference between the two groups in terms of the diagnosis. In the survivor group, some were diagnosed with organizing pneumonia or acute eosinophilic pneumonia, both of which show good prognosis. In the non-survivor group, on the other hand, there were more patients with atypical pneumonia (whose treatment is difficult) compared to the survivor group. Additionally, the patients with interstitial pneumonia and pulmonary hemorrhage all died (Table 4). Multivariate logistic regression analysis showed no significant predictors of mortality.
Table 4. Comparisons between survivor and non-survivor groups in patients using high-flow nasal cannula oxygen delivery.
| Characteristic | Survivor (n=14) | Non-survivor (n=19) | p-value |
|---|---|---|---|
| Age, yr | 65.1±17.0 | 62.9±13.7 | 0.465 |
| Baseline PaO2/FiO2, mm Hg | 163.4±43.6 | 168.1±50.0 | 0.855 |
| Immune suppression | 1 (7.1) | 7 (35.8) | 0.098 |
| Before BAL | |||
| SpO2, % | 95.6±2.8 | 94.2±3.0 | 0.240 |
| FiO2 | 0.46±0.10 | 0.45±0.13 | 0.577 |
| Flow, L/min | 29.3±19.8 | 24.5±21.0 | 0.377 |
| During BAL | |||
| SpO2, % | 93.7±4.5 | 95.3±2.6 | 0.506 |
| FiO2 | 0.75±0.20 | 0.73±0.19 | 0.706 |
| Flow, L/min | 49.8±5.9 | 48.4±8.2 | 0.706 |
| After BAL | |||
| SpO2, % | 94.8±2.5 | 95.6±3.0 | 0.377 |
| FiO2 | 0.53±0.15 | 0.59±0.13 | 0.174 |
| Flow, L/min | 38.4±15.4 | 42.6±13.5 | 0.418 |
| Volume, recovered, mL | 75.9±6.4 | 74.4±6.2 | 0.471 |
| Procedure time, min | 11.9±6.0 | 16.3±7.5 | 0.092 |
| Intubation | 0 (0) | 9 (47.4) | 0.004* |
| Time to intubation, median (range), hr | 0 (0) | 76.0 (26-274) | 0.003† |
| Post-BAL diagnosis | 0.019‡ | ||
| Atypical pneumonia | 3 (21.4) | 11 (57.9) | |
| Bacterial pneumonia | 6 (42.9) | 4 (21.1) | |
| Organizing pneumonia | 4 (28.6) | 0 (0) | |
| Interstitial lung disease | 0 (0) | 2 (10.5) | |
| Alveolar hemorrhage | 0 (0) | 2 (10.5) | |
| Acute eosinophilic pneumonia | 1 (7.1) | 0 (0) |
Values are presented as mean±standard deviation or number (%) unless otherwise indicated.
*p<0.05, Fisher exact test between survivor and non-survivor groups. †p<0.05, Mann-Whitney U test between survivor and non-survivor groups. ‡p<0.05, chi-square test between survivor and non-survivor groups.
PaO2: partial pressure of oxygen in arterial blood; FiO2: fraction of inspired oxygen; BAL: bronchoalveolar lavage; SpO2: saturation measured by pulse oximeter.
7. Comparison between “without HFNC” and “with HFNC” groups
We compared the characteristics, safety and complications of the BAL procedure “without HFNC” to “with HFNC” in patients with PF ratio of 300 or less during the same investigation period. There were 23 cases in “without HFNC” group. In “without HFNC” group, the mean baseline PF ratio was 233±36.5, that was significantly higher than the “with HFNC” group (p<0.001). Twenty cases (87.0%) were classified as mild ARF; three cases (13%) as moderate ARF (Table 1). In “without HFNC” group, the number of cases for mild ARF is higher than in “with HFNC” group (p<0.001). There were no differences between “without HFNC” and “with HFNC” groups in the age, the male-to-female ratio and patients under the immunosuppression state. In “without HFNC” group, transient hypoxemia occurred in nine cases (39.1%) that did not show significant difference compared with the “with HFNC” group (p=0.289). There were no significant differences in fever and hypotension (p=0.322 and p=0.400, respectively). There was also no ETI within 24 hours after the BAL procedure in “without HFNC” group (Table 3).
Discussion
When conducting bronchoscopy in patients with ARF, the PaO2 can be decreased to up to 10–20 mm Hg23, and BAL may cause severe hypoxemia24. The American Thoracic Society recommended in 1990 that BAL not be performed if it is impossible to correct the PaO2 to at least 75 mm Hg, or the SaO2 to 90%, by supplying oxygen25. Later, NPPV was introduced to prevent the occurrence of hypoxemia during the BAL procedure. NPPV well maintains oxygenation compared to the use of a face mask, and has less ETI complications6. There were limitations in the use of NPPV, however, because of the following disadvantages: it takes time to properly adjust it, and experienced medical personnel are required. In particular, when applying NPPV to an alert patient, the straps should be tightened for mask fitting; the agitation that may be caused by this will lower the patient's oxygen saturation, thereby making BAL difficult to perform26. As an alternative to NPPV, a laryngeal mask was once used to prevent BAL-associated hypoxemia19,26. This method, however, is rarely used at present because it has the burden of general anesthesia.
Recently, HFNC oxygen therapy started to be used as a replacement method for NPPV when performing BAL in patients with ARF27. HFNC can supply humidified and heated oxygen through the nose up to 100%, flow up to 60 L/min, and FiO2 exactly. HFNC washes out the pharyngeal dead spaces, reduces the nasopharyngeal resistance, provides a low degree of continuous positive airway pressure8,28, and is partially involved in alveolar recruitment8,29. Through these various mechanisms, HFNC allows BAL to be performed without severe hypoxemia.
HFNC has excellent advantages, such as that it is well tolerable compared to NPPV and can be easily started and discontinued. That is, HFNC can be used more easily in patients requiring oxygen before undergoing BAL30. If HFNC can be applied easily, it will help prevent a delay in the performance of the BAL procedure, and will allow more rapid diagnosis. If the patient is intolerable to NPPV, the application of NPPV may lead to ETI as it will not be able to improve oxygenation31. Since Miyagi et al.30 first reported the success of BAL with HFNC in five patients with ARF in 2014, the relevant study of La Combe et al.13 was published. Based on this, it was expected that HFNC could be more easily applied to patients than NPPV when performing BAL.
La Combe et al.13 used HFNC to perform BAL in 30 patients with ARF who were admitted to the ICU, and the median PF ratio of the patients was 169 (range, 145–196). Out study included all ICU and general ward patients, and the median PF ratio was 159.4 (range, 89.1–269.5). As opposed to the result of the study of La Combe et al.13, there was no difference in the median PF ratio, but our study included mild ARF of eight cases (24.2%). The important difference between the two studies' results was in the number of cases of NPPV or ETI occurring within 24 hours after BAL. La Combe et al.13 reported four cases of NPPV and one case of ETI while there were no such cases in the present study. The reasons for such difference between the two studies are as follows: the present study included eight cases (24.2%) with a PF ratio of over 200 (mild grade) in patients with ARF, and one case (3%) used inotropics among the subjects of the BAL procedure. This seems to be due to the fact that the procedure was performed in the state where hemodynamic stability had been obtained. Study investigators may have different criteria for ETI, and as delayed ETI can increase the mortality rate32, in this study, ETI was first conducted for the patients with serious general conditions due to ARF. Therefore, the cases where ETI was performed before applying HFNC were excluded from the analysis in this study. This implies that the BAL result can be safely checked, without ETI occurrence within 24 hours after the procedure, if the BAL procedure can be performed by applying HFNC with proper timing when hemodynamic stability is obtained in patients with ARF.
There was no difference in the systolic blood pressure during and after the BAL procedure compared to before the procedure. The diastolic blood pressure increased statistically during and after the procedure compared to before the procedure, showing a borderline statistical significance of p=0.025. Also, the heart rate significantly increased during and after the procedure compared to before the procedure (p<0.001), which seems to be due to secondary sympathetic hyperactivity for stress situations such as agitation and transient dyspnea.
In the present study, nine cases underwent ETI after BAL, and the median time to ETI after BAL was 76 hours (approximately 3 days) after BAL. These results support the conclusion that BAL in patients with ARF does not cause ETI due to the complications of BAL itself. When the patients were divided into the survivor and non-survivor groups, there were no differences in age, PF ratio, FiO2, flow, and SpO2 between the two groups. On the other hand, the cases with ETI were all in the non-survivor group. In particular, the frequency of the presence of diseases whose treatment is difficult was higher in the non-survivor group than in the survivor group. This means that the cause of ETI is not related to the BAL procedure or the use of HFNC but to the underlying disease itself. In other words, it suggests that the underlying disease present before BAL is an important factor in determining the prognosis of patients. The systolic blood pressure before the procedure was significantly higher in the non-survivor group, which seems to be the result of sympathetic hyperactivity caused by dyspnea associated with the patient's underlying disease.
There were no statistical differences in the safety and complication between “without HFNC” and “with HFNC” group in patients with PF ratio 300 or less. However, the number of patients with mild ARF was significantly higher in “without HFNC” group than “with HFNC” group. Because the BAL procedure was performed for mild cases in “without HFNC” group, there seems to be no differences in the safety and complication, compared to “with HFNC” group.
The limitation of this study was that it was a retrospective single-center study. The number of patients was 33, which is insufficient for proper analysis. When the bronchoscope and HFNC were inserted into the nasal cavity simultaneously, there was a possibility that the FiO2 could not supplied accurately. In this study, SpO2, a noninvasive test method, was used as a BAL-associated parameter, which may be inaccurate compared to the invasive measure of SaO2. As SpO2, however, is a method that can be clinically used most easily and conveniently, the actual clinical significance will not be different from that of SaO2.
Footnotes
Authors' Contributions: Conceptualization: Kim KC. Methodology: Kim EJ, Kim KC. Formal analysis: Kim EJ, Kim KC. Data curation: Kim EJ. Software: Kim EJ. Validation: Jung CY, Kim KC. Investigation: Kim EJ, Kim KC. Writing - original draft preparation: Kim EJ. Writing - review and editing: all authors. Approval of final manuscript: all authors.
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Artigas A, Castella X. Bronchoalveolar lavage (BAL) in adult respiratory distress syndrome (ARDS) In: Vincent JL, editor. Update in intensive care and emergency medicine. Vol. 14. Berline: Springer; 1991. pp. 192–197. update 1991. [Google Scholar]
- 2.Crystal RG, Reynolds HY, Kalica AR. Bronchoalveolar lavage: the report of an international conference. Chest. 1986;90:122–131. doi: 10.1378/chest.90.1.122. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds HY. Bronchoalveolar lavage. Am Rev Respir Dis. 1987;135:250–263. doi: 10.1164/arrd.1987.135.1.250. [DOI] [PubMed] [Google Scholar]
- 4.The BAL Cooperative Group Steering Committee. Bronchoalveolar lavage constituents in healthy individuals, idiopathic pulmonary fibrosis, and selected comparison groups. Am Rev Respir Dis. 1990;141(5 Pt 2):S169–S202. doi: 10.1164/ajrccm/141.5_Pt_2.S169. [DOI] [PubMed] [Google Scholar]
- 5.Albertini RE, Harrell JH, 2nd, Kurihara N, Moser KM. Arterial hypoxemia induced by fiberoptic bronchoscopy. JAMA. 1974;230:1666–1667. [PubMed] [Google Scholar]
- 6.Antonelli M, Conti G, Riccioni L, Meduri GU. Noninvasive positive-pressure ventilation via face mask during bronchoscopy with BAL in high-risk hypoxemic patients. Chest. 1996;110:724–728. doi: 10.1378/chest.110.3.724. [DOI] [PubMed] [Google Scholar]
- 7.Song JU, Kim SA, Choi ER, SM Kim, Choi HJ, Lim SY, et al. Prediction of intubation after bronchoscopy with non-invasive positive pressure ventilation support in patients with acute hypoxemic respiratory failure. Tuberc Respir Dis. 2009;67:21–26. [Google Scholar]
- 8.Ricard JD. High flow nasal oxygen in acute respiratory failure. Minerva Anestesiol. 2012;78:836–841. [PubMed] [Google Scholar]
- 9.Sztrymf B, Messika J, Mayot T, Lenglet H, Dreyfuss D, Ricard JD. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure: a prospective observational study. J Crit Care. 2012;27:324.e9–324.e13. doi: 10.1016/j.jcrc.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 10.Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 11.Lucangelo U, Vassallo FG, Marras E, Ferluga M, Beziza E, Comuzzi L, et al. High-flow nasal interface improves oxygenation in patients undergoing bronchoscopy. Crit Care Res Pract. 2012;2012:506382. doi: 10.1155/2012/506382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon M, Braune S, Frings D, Wiontzek AK, Klose H, Kluge S. High-flow nasal cannula oxygen versus non-invasive ventilation in patients with acute hypoxaemic respiratory failure undergoing flexible bronchoscopy: a prospective randomised trial. Crit Care. 2014;18:712. doi: 10.1186/s13054-014-0712-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Combe B, Messika J, Labbe V, Razazi K, Maitre B, Sztrymf B, et al. High-flow nasal oxygen for bronchoalveolar lavage in acute respiratory failure patients. Eur Respir J. 2016;47:1283–1286. doi: 10.1183/13993003.01883-2015. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 15.Cairo JM. Administering medical gases: regulators, flowmeters, and controlling device. In: Cairo JM, Pilbeam SP, editors. Mosby's respiratory care equipment. 8th ed. St. Louis: Mosby; 2010. pp. 59–87. [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 17.Sund-Levander M, Forsberg C, Wahren LK. Normal oral, rectal, tympanic and axillary body temperature in adult men and women: a systematic literature review. Scand J Caring Sci. 2002;16:122–128. doi: 10.1046/j.1471-6712.2002.00069.x. [DOI] [PubMed] [Google Scholar]
- 18.Maitre B, Jaber S, Maggiore SM, Bergot E, Richard JC, Bakthiari H, et al. Continuous positive airway pressure during fiberoptic bronchoscopy in hypoxemic patients: a randomized double-blind study using a new device. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1063–1067. doi: 10.1164/ajrccm.162.3.9910117. [DOI] [PubMed] [Google Scholar]
- 19.Hilbert G, Gruson D, Vargas F, Valentino R, Favier JC, Portel L, et al. Bronchoscopy with bronchoalveolar lavage via the laryngeal mask airway in high-risk hypoxemic immunosuppressed patients. Crit Care Med. 2001;29:249–255. doi: 10.1097/00003246-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Baumann HJ, Klose H, Simon M, Ghadban T, Braune SA, Hennigs JK, et al. Fiber optic bronchoscopy in patients with acute hypoxemic respiratory failure requiring noninvasive ventilation: a feasibility study. Crit Care. 2011;15:R179. doi: 10.1186/cc10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishman JA. Approach to the patient with pulmonary infection. In: Grippi MA, Elias JA, Fishman JA, Kotloff RM, Pack AI, Senior RM, et al., editors. Fishman's pulmonary diseases and disorders. 5th ed. New York: McGraw-Hill Co, Inc.; 2015. pp. 1853–1879. [Google Scholar]
- 22.Cordier JF. Cryptogenic organising pneumonia. Eur Respir J. 2006;28:422–446. doi: 10.1183/09031936.06.00013505. [DOI] [PubMed] [Google Scholar]
- 23.Payne CB, Jr, Goyal PC, Gupta SC. Effects of transoral and transnasal fiberoptic bronchoscopy on oxygenation and cardiac rhythm. Endoscopy. 1986;18:1–3. doi: 10.1055/s-2007-1018309. [DOI] [PubMed] [Google Scholar]
- 24.Katz AS, Michelson EL, Stawicki J, Holford FD. Cardiac arrhythmias: frequency during fiberoptic bronchoscopy and correlation with hypoxemia. Arch Intern Med. 1981;141:603–606. [PubMed] [Google Scholar]
- 25.Goldstein RA, Rohatgi PK, Bergofsky EH, Block ER, Daniele RP, Dantzker DR, et al. Clinical role of bronchoalveolar lavage in adults with pulmonary disease. Am Rev Respir Dis. 1990;142:481–486. doi: 10.1164/ajrccm/142.2.481. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto T, Sato Y, Fukuda S, Katayama S, Miyazaki Y, Ozaki M, et al. Safety and efficacy of bronchoalveolar lavage using a laryngeal mask airway in cases of acute hypoxaemic respiratory failure with diffuse lung infiltrates. Intern Med. 2015;54:731–735. doi: 10.2169/internalmedicine.54.2686. [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Rehder KJ, Williford L, Cheifetz IM, Turner DA. Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature. Intensive Care Med. 2013;39:247–257. doi: 10.1007/s00134-012-2743-5. [DOI] [PubMed] [Google Scholar]
- 28.Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth. 2009;103:886–890. doi: 10.1093/bja/aep280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gotera C, Diaz Lobato S, Pinto T, Winck JC. Clinical evidence on high flow oxygen therapy and active humidification in adults. Rev Port Pneumol. 2013;19:217–227. doi: 10.1016/j.rppneu.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Miyagi K, Haranaga S, Higa F, Tateyama M, Fujita J. Implementation of bronchoalveolar lavage using a high-flow nasal cannula in five cases of acute respiratory failure. Respir Investig. 2014;52:310–314. doi: 10.1016/j.resinv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Delclaux C, L'Her E, Alberti C, Mancebo J, Abroug F, Conti G, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA. 2000;284:2352–2360. doi: 10.1001/jama.284.18.2352. [DOI] [PubMed] [Google Scholar]
- 32.Demoule A, Girou E, Richard JC, Taille S, Brochard L. Increased use of noninvasive ventilation in French intensive care units. Intensive Care Med. 2006;32:1747–1755. doi: 10.1007/s00134-006-0229-z. [DOI] [PubMed] [Google Scholar]