Abstract
Background
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a standard procedure to evaluate suspicious lymph node involvement of lung cancer because computed tomography (CT) and 18F-fluorodeoxyglucose positron emission tomography-CT (PET-CT) have limitations in their sensitivity and specificity. There are a number of benign causes of false positive lymph node such as anthracosis or anthracofibrosis, pneumoconiosis, old or active tuberculosis, interstitial lung disease, and other infectious conditions including pneumonia. The purpose of this study was to evaluate possible causes of false positive lymph node detected in chest CT or PET-CT.
Methods
Two hundred forty-seven patients who were initially diagnosed with lung cancer between May 2009 and December 2012, and underwent EBUS-TBNA to confirm suspicious lymph node involvement by chest CT or PET-CT were analyzed for the study.
Results
Of 247 cases, EBUS-TBNA confirmed malignancy in at least one lymph node in 189. The remaining 58 patients whose EBUS-TBNA results were negative were analyzed. Age ≥65, squamous cell carcinoma as the histologic type, and pneumoconiosis were related with false-positive lymph node involvement on imaging studies such as chest CT and PET-CT.
Conclusion
These findings suggest that lung cancer staging should be done more carefully when a patient has clinically benign lymph node characteristics including older age, squamous cell carcinoma, and benign lung conditions.
Keywords: Lymph Node; Chest; Tomography, X-Ray Computed; Positron Emission Tomography Computed Tomography; Lung Neoplasms
Introduction
Lung cancer is the leading cause of cancer deaths worldwide for both men and women1. To determine optimal therapeutic strategy for lung cancer patients, accurate staging is essential.
Mediastinoscopy is often considered as gold standard procedure for the mediastinal staging of lung cancer2. However, a prospective cohort study with 190 lung cancer patients found that diagnostic performance of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was comparable with that of mediastinoscopy3. A meta-analysis of 11 observational studies which compared EBUS-TBNA with mediastinoscopy also showed similar sensitivity and specificity4.
Computed tomography (CT) and 18F-fluorodeoxyglucose (FDG) positron emission tomography-CT (PET-CT) are standard imaging modalities for staging of lung cancer. However, chest CT or PET-CT have limited reliability for the sensitivity or specificity of lymph node staging5,6,7,8,9. Previous studies reported that the sensitivity and specificity of chest CT in identifying lymph node metastases were 55% and 81%, respectively10. In comparison to CT imaging, PET-CT had shown to have better sensitivity and specificity, 77% and 86% respectively10. However, in patients with granulomatous disease such as sarcoidosis, concurrent infectious and inflammatory disease such as tuberculosis, pneumonia and interstitial lung disease (ILD), PET-CT has been shown to increase the rates of false-positive malignancy in mediastinal lymph nodes11,12.
Therefore, in the present study, we investigated various benign causes of false-positive lymph node including anthracosis or anthracofibrosis, pneumoconiosis, old or active tuberculosis, ILD and other infectious conditions including pneumonia on chest CT or PET-CT using EBUS-TBNA.
Materials and methods
1. Patients
Data were collected from lung cancer patients who underwent EBUS-TBNA for suspicious lymph node involvement by chest CT or PET-CT from May 2009 to December 2012 in Seoul St. Mary's Hospital. The diagnosis of lymph node involvement was determined by EBUS-TBNA. True-positive was defined as a diagnosis of lymph node metastasis from lung cancer. True-negative was defined as a diagnosis of benign lymphoid tissue. We also defined false-positive group as patients with negative result of malignancy from EBUS-TBNA although chest CT or PET-CT were positive.
Anthracosis or anthracofibrosis were defined by bronchoscopic findings demonstrating black-brown pigment lining the endobronchial mucosa with or without fibrotic distortion/narrowing of the bronchus. Diagnosis of pneumoconiosis includes chest imaging consistent with pneumoconiosis plus occupational inhalation exposure history of sufficient amount and latency to coal or silica dust. Previous history of tuberculosis was defined as old tuberculosis. If lung cancer was concomitantly diagnosed with tuberculosis, it was defined as active tuberculosis. ILD was defined by typical radiological findings with symptoms and signs of ILD.
All subjects provided written informed consent for the procedure, and the study protocol was approved by the Institutional Review Board of Seoul St. Mary's Hospital, The Catholic University of Korea (IRB approval number: KC16RISI0733).
2. EBUS-TBNA
The EBUS-TBNA was done by two experts using a flexible linear (convex) ultrasound bronchoscopy system (Olympus, Tokyo, Japan). The procedure was performed with the patient under conscious sedation by midazolam, and local anesthesia (lidocaine) was applied. After scanning the mediastinal lymph node, each target lymph node was aspirated at least 3 times with a 22-gauge needle. Lymph node location was classified according to the International Association for the Study of Lung Cancer (IASLC) lymph node map13. The aspirated tissue was fixed promptly with 95% alcohol, and stained using Papanicolaou. An expert lung pathologist (K.Y. Lee) performed the cytopathological examinations.
3. Chest CT
Multi-detector computed tomography (SOMATOM Definition; Siemens, Erlangen, Germany and Discovery CT750 HD; GE Healthcare, Waukesha, WI, USA) was performed using a slice thickness of 1 mm reconstructed at 0.75-mm intervals with contrast injection by a mechanical injector at a rate of 3 mL/sec, for a total dose of 100 mL. Axial, sagittal and coronal images were reconstructed at 3-mm interval and were transferred to a PACS workstation (Maroview; Marotech, Seoul, Korea). All abnormal findings on chest CT were reviewed in lymph node stations according to the guidelines of the IASLC13. On chest CT, positive lymph node was defined as short diameter longer than 1 cm14.
4. PET-CT
Patients were fasted for at least 6 hours before PET-CT scanning. Scanning was done 60 minutes later after FDG injection (5.5–7.4 MBq/kg). None of the patients had a blood glucose level higher than 130 mg/dL before injection. No intravenous contrast agent was administered. Studies were acquired on combined PET-CT in-line systems, either Biograph Duo or Biograph Truepoint (Siemens Medical Solutions, Knoxville, TN, USA). The maximum standardized uptake value (SUV) was used to define positive lymph node on PET-CT, and the SUV was obtained by locating a region of interest on a lesion. A maximum SUV >2.5 on a lymph node was interpreted as positive15.
5. Statistical analysis
The descriptive statistics were calculated and are presented as number (percentage) for categorical variables, and the mean±SD and median (range) for continuous variables. Differences between the false-positive and true-positive group were compared using the Wilcoxon rank-sum test for continuous variables and the chi-square or Fisher exact test for categorical variables. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy of chest CT and PET-CT were measured for the detection of mediastinal lymph node metastases. The receiver operating characteristic (ROC) curve and the Youden index identified an optimal age cut-off value for mediastinal lymph node metastases. Univariate and multivariable logistic regressions analyses were performed to determine odds ratios (ORs) for identify independent predictors of false-positive. Analyses were conducted using the SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC, USA). The significance level for statistical tests were set at α=0.05 (two-tailed).
Results
1. Patients' characteristics
Among the 247 patients who underwent EBUS-TBNA, male patients were 199 (80.6%), and mean and median age was 64.9 and 66.0, respectively (Table 1). Predominant histologic type was adenocarcinoma (116 cases, 47.0%). The number of nodes sampled per patients was one in 123 (49.8%), two in 107 (43.3%) and three in 17 (6.9%). Staging workup indicated that 95 patients (49.0%) were classified as stage IV in non-small cell lung cancer, and 39 (73.6%) were extensive disease in small cell lung cancer. Mediastinal lymph node metastases were confirmed by EBUS-TBNA in 258 nodal stations from total 388 lymph nodes.
Table 1. Baseline characteristics of the 247 lung cancer patients.
| Variable | No. (%) (n=247) |
|---|---|
| Sex | |
| Male | 199 (80.6) |
| Female | 48 (19.4) |
| Age, yr | |
| Mean±SD | 64.9±10.0 |
| Median (range) | 66.0 (33.0–87.0) |
| Histology | |
| Adenocarcinoma | 116 (47.0) |
| Squamous cell carcinoma | 67 (27.1) |
| Large cell carcinoma | 5 (2.0) |
| Small cell carcinoma | 53 (21.5) |
| Others | 6 (2.4) |
| Stage | |
| NSCLC (n=194) | |
| IA | 6 (3.1) |
| IB | 3 (1.5) |
| IIA | 9 (4.6) |
| IIB | 2 (1.0) |
| IIIA | 47 (24.2) |
| IIIB | 32 (16.5) |
| IV | 95 (49.0) |
| SCLC (n=53) | |
| Limited disease | 14 (26.4) |
| Extensive disease | 39 (73.6) |
NSCLC: non-small cell lung cancer; SCLC: small cell lung cancer.
2. Diagnostic performance of chest CT and/or PET-CT according to lymph node
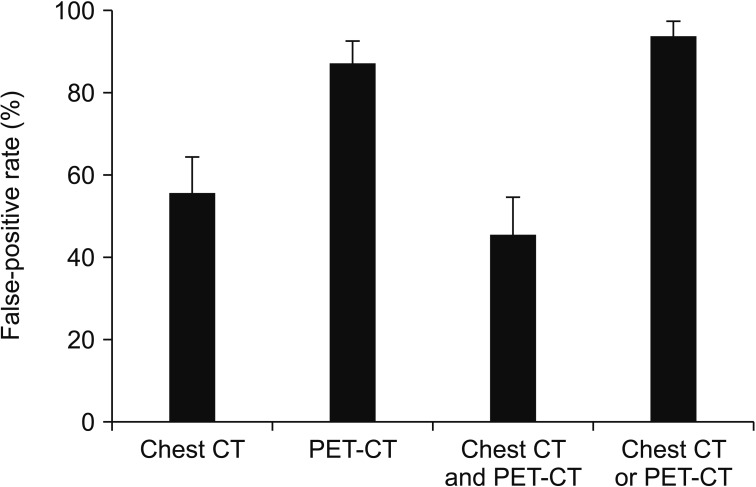
Table 2 presents sensitivity, specificity, PPV, NPV, accuracy, and area under the curve (AUC) of chest CT and/or PET-CT. Sensitivity was the highest in chest CT or PET-CT group, but this group presented the lowest specificity. Chest CT and PET-CT group showed the highest specificity, but the specificity was only 54.6%. The false-positive rate according to lymph nodes was 55.4% in chest CT, 87% in PET-CT, 45.4% in chest CT and PET-CT, and 93.5% in chest CT or PET-CT group (Figure 1).
Table 2. Diagnostic performance of chest CT, PET-CT, chest CT and/or PET-CT according to lymph node positivity by EBUS-TBNA.
| Sensitivity (95% CI, %) | Specificity (95% CI, %) | PPV (95% CI, %) | NPV (95% CI, %) | Accuracy (95% CI, %) | AUC (95% CI) | |
|---|---|---|---|---|---|---|
| Chest CT | 88.8 (84.3–92.3) | 44.6 (35.9–53.6) | 76.1 (70.9–80.8) | 66.7 (55.8–76.4) | 74.0 (69.3–78.3) | 0.667 (0.618–0.714) |
| PET-CT | 99.2 (97.1–99.9) | 13.0 (7.6–20.3) | 69.9 (64.8–74.6) | 88.9 (65.3–98.6) | 70.8 (65.9–75.3) | 0.561 (0.509–0.612) |
| Chest CT and PET-CT | 85.7 (80.8–89.7) | 54.6 (45.7–63.4) | 78.9 (73.7–83.6) | 65.7 (56.0–74.6) | 75.3 (70.7–79.5) | 0.701 (0.653–0.747) |
| Chest CT or PET-CT | 100.0 (98.5–100.0) | 6.5 (2.8–12.4) | 68.5 (63.5–73.2) | 100.0 (63.1–100.0) | 69.2 (64.2–73.8) | 0.533 (0.480–0.584) |
CT: computed tomography; PET-CT: positron emission tomography-CT; EBUS-TBNA: endobronchial ultrasound-guided transbronchial needle aspiration; CI: confidence interval; PPV: positive predictive value; NPV: negative predictive value; AUC: area under the curve.
Figure 1. Comparison of false-positive rate (%) of lymph node involvement according to different diagnostic modalities. CT: computed tomography; PET-CT: positron emission tomography-CT.
3. Factors associated with false-positive lymph node according to patients
False-positive lymph nodes depending on the lymph node location was shown according to IASLC (Table 3) and the demographic differences between the false-positive group and the true-positive group are presented in Table 4. The false-positive patients had significantly higher mean and median age than the true-positive patients (mean age, 68.2±9.5 vs. 63.9±10.0, p=0.004; median age, 69.5 [45.0–87.0] vs. 65.0 [33.0–85.0], p=0.004). According to the histologic type, false-positive rate was the highest in patients with squamous cell carcinoma, and the lowest in small cell carcinoma. Except old or active tuberculosis, other benign conditions such as anthracosis or anthracofibrosis, ILD and pneumonia showed a higher tendency of false-positive rate, but were not statistically significant. Patients with pneumoconiosis had higher false-positive rate than those without pneumoconiosis.
Table 3. False-positive lymph nodes depending on the lymph node location according to IASLC.
| Lymph node location | No. of lymph nodes studied | No. of false-positive lymph nodes | False-positive rate (%) |
|---|---|---|---|
| Right lower cervical, supraclavicular and sternal notch node (1R) | 2 | 2 | 100.0 |
| Right upper paratracheal node (2R) | 6 | 2 | 33.3 |
| Right lower paratracheal node (4R) | 141 | 44 | 31.2 |
| Right hilar node (10R) | 12 | 4 | 33.3 |
| Right interlobar node (11R) | 10 | 1 | 10.0 |
| Right lobar lymph node (12R) | 2 | 1 | 50.0 |
| Subcarinal node (7) | 157 | 61 | 38.9 |
| Left lower cervical, supraclavicular, and sternal notch node (1L) | 1 | 0 | 0.0 |
| Left upper paratracheal node (2L) | 2 | 0 | 0.0 |
| Left lower paratracheal node (4L) | 48 | 13 | 27.1 |
| Left hilar node (10L) | 4 | 2 | 50.0 |
| Left interlobar node (11L) | 2 | 0 | 0.0 |
| Retrotracheal lymph node (3P) | 1 | 0 | 0.0 |
| Total No. of lymph nodes | 388 | 130 | 33.5 |
IASLC: International Association for the Study of Lung Cancer.
Table 4. Factors associated with false-positive patients of chest CT and PET-CT.
| Variable | False-positive (n=58) | True-positive (n=189) | p-value |
|---|---|---|---|
| Sex | |||
| Male | 49 (84.5) | 150 (79.4) | 0.389 |
| Female | 9 (15.5) | 39 (20.6) | |
| Age, yr | |||
| Mean±SD | 68.2±9.5 | 63.9±10.0 | 0.004 |
| Median (range) | 69.5 (45.0–87.0) | 65.0 (33.0–85.0) | 0.004 |
| <65 | 16 (27.6) | 92 (48.7) | 0.005 |
| ≥65 | 42 (72.4) | 97 (51.3) | |
| Histology | |||
| Adenocarcinoma | 25 (43.1) | 91 (48.1) | <0.001 |
| Squamous cell carcinoma | 26 (44.8) | 41 (21.7) | |
| Large cell carcinoma | 1 (1.7) | 4 (2.1) | |
| Small cell carcinoma | 4 (6.9) | 49 (25.9) | |
| Others | 2 (3.4) | 4 (2.1) | |
| Anthracosis or anthracofibrosis | |||
| Absence | 52 (89.7) | 177 (93.7) | 0.262 |
| Presence | 6 (10.3) | 12 (6.3) | |
| Pneumoconiosis | |||
| Absence | 53 (91.4) | 186 (98.4) | 0.019 |
| Presence | 5 (8.6) | 3 (1.6) | |
| Old or active tuberculosis | |||
| Absence | 47 (81.0) | 147 (77.8) | 0.597 |
| Presence | 11 (19.0) | 42 (22.2) | |
| ILD | |||
| Absence | 52 (89.7) | 173 (91.5) | 0.660 |
| Presence | 6 (10.3) | 16 (8.5) | |
| Pneumonia | |||
| Absence | 51 (87.9) | 167 (88.4) | 0.929 |
| Presence | 7 (12.1) | 22 (11.6) |
Values are presented as number (%) or mean±SD.
CT: computed tomography; PET-CT: positron emission tomography-CT; ILD: interstitial lung disease.
4. Univariate and multivariate analyses of false-positive patients
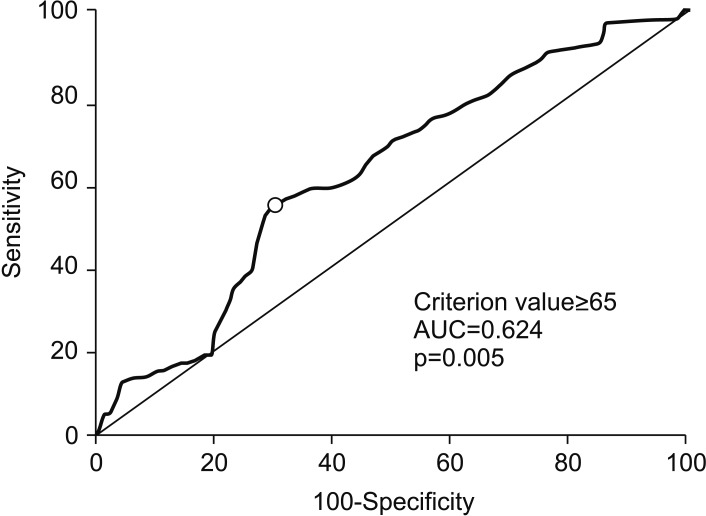
Logistic regression analysis of clinicopathological parameters for the prediction of false-positive patients with lung cancer is shown in Table 5. As mentioned above, older age was associated with increased risk of having a false-positive result on chest CT or PET-CT. We determined the optimal discriminator value for age using ROC curve analysis (Figure 2). AUC was the highest (0.624) when patients were divided into two groups according to age 65. Logistic regression analysis revealed that false-positive rate was significantly higher (OR, 2.21; 95% confidence interval [CI], 1.12–4.36; p=0.022) in the older age group (Table 5).
Table 5. Logistic regression analysis of clinicopathological parameters for the prediction of false-positive patients with lung cancer.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age, yr | ||||
| <65 | 1.00 | 0.005 | 1.00 | 0.022 |
| ≥65 | 2.49 (1.31–4.73) | 2.21 (1.12–4.36) | ||
| Histology | ||||
| Adenocarcinoma vs. squamous cell carcinoma, large cell carcinoma, small cell carcinoma, and others | 0.82 (0.45–1.48) | 0.501 | - | - |
| Squamous cell carcinoma vs. adenocarcinoma, large cell carcinoma, small cell carcinoma, and others | 2.93 (1.57–5.47) | 0.001 | 2.84 (1.48–5.43) | 0.002 |
| Large cell carcinoma vs. adenocarcinoma, squamous cell carcinoma, small cell carcinoma, and others | 0.81 (0.09–7.41) | 0.853 | - | - |
| Small cell carcinoma vs. adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and others | 0.21 (0.07–0.62) | 0.004 | 0.18 (0.06–0.55) | 0.003 |
| Others vs. adenocarcinoma, squamous cell carcinoma, large cell carcinoma, small cell carcinoma | 1.65 (0.30–9.26) | 0.568 | - | - |
| Anthracosis or anthracofibrosis | ||||
| Absence | 1.00 | 0.310 | - | - |
| Presence | 1.70 (0.61–4.76) | - | ||
| Pneumoconiosis | ||||
| Absence | 1.00 | 0.018 | 1.00 | 0.020 |
| Presence | 5.85 (1.35–25.27) | 6.33 (1.34–29.96) | ||
| Old and active tuberculosis | ||||
| Absence | 1.00 | 0.598 | - | - |
| Presence | 0.82 (0.39–1.72) | - | ||
| ILD | ||||
| Absence | 1.00 | 0.661 | - | - |
| Presence | 1.25 (0.46–3.35) | - | ||
| Pneumonia | - | |||
| Absence | 1.00 | 0.929 | - | - |
| Presence | 1.04 (0.42–2.58) | - | ||
OR: odds ratio; CI: confidence interval; ILD: interstitial lung disease.
Figure 2. Receiver operating characteristic curve analysis showing the maximum sensitivity and specificity of age (65 years) of cut-off value for discrimination of false-positive patients with lung cancer. AUC: area under the curve.
Histology of squamous cell carcinoma indicated significantly high false-positive rate (OR, 2.84; 95% CI, 1.48–5.43; p=0.002), whereas small cell carcinoma showed significantly low false-positive rate (OR, 0.18; 95% CI, 0.06–0.55; p=0.003). Patients with pneumoconiosis also showed significantly high false-positive rate (OR, 6.33; 95% CI, 1.34–29.96; p=0.020).
Discussion
The aim of the present study was to examine the possible causes of false-positive lymph node detected in chest CT or PET-CT in patients with lung cancer by using EBUS-TBNA. We found that older age, histologic type of squamous cell carcinoma and benign condition such as pneumoconiosis were related with false-positive lymph node.
In the present study, older age was identified as a risk factor for false-positive lymph node. Possible explanations include many age-associated changes due to less robust immune response in the respiratory system. Many complex changes in both the innate and adaptive immune systems might contribute to increase susceptibility to infections16. Cumulative episodes of previous infectious or inflammatory lung conditions might also be the causes of increasing tendency of false-positive lymph node in elderly patients.
Previous studies indicated that patients with adenocarcinoma had low FDG uptake compared to those with squamous cell carcinoma17,18,19. Hwangbo et al.20 reported that higher rate of lymph node metastasis was detected in patients with adenocarcinoma compared to those with squamous cell carcinoma, although PET-CT scan findings were negative. According to a study by Lu et al.21, PET-CT showed higher false positivity in squamous cell carcinoma group than in adenocarcinoma group. These previous results correspond well with the present study that patients with squamous cell carcinoma had more false-positive lymph node on chest CT and PET-CT than those with adenocarcinoma. A possible explanation for the differences between squamous cell carcinoma and adenocarcinoma is that most of squamous cell carcinomas are central bronchogenic carcinoma and are usually accompanied with obstructive pneumonia and atelectasis, and this may activate macrophages and inflammatory cells which could cause reactive hyperplasia of lymph nodes21.
If histologic findings of specimens from EBUS-TBNA are not related to tumors, benign findings include reactive lymphadenitis, anthracotic pigmentations with or without fibrosis, and granulomatous inflammation.
Interestingly, univariate and multivariate analyses indicated that small cell carcinoma had significantly low false-positive rate compared with adenocarcinoma and squamous cell carcinoma. Moreover, squamous cell carcinoma showed significantly high false-positive rate compared with adenocarcinoma and small cell carcinoma. Considering these findings, one might think that small cell carcinoma and adenocarcinoma could metastasize although chest CT and PET-CT could not detect lymph node metastasis20.
Metser and Even-Sapir22 have reported that inflammatory lesions in the tracheobronchial tree (tracheitis and infected bronchiectasis), lung (viral, bacterial, fungal infection, abscess, ILD, cryptogenic organizing pneumonia, lipoid pneumonia, Langerhans cell histiocytosis, vasculitis, sarcoidosis, Wegner's granulomatosis, and inhalational lung disease), and pleura (empyema) induces false-positive findings due to increased uptake of FDG in the chest. Radiologic findings of bronchial obstructive lesion and mediastinal lymph nodes in bronchial anthracofibrosis could mimic cancer23. In the present study, patients with pneumoconiosis had higher false-positive rate than those without pneumoconiosis. However, patients with anthracosis or anthracofibrosis, ILD and pneumonia showed statistically insignificant. The present study investigated old or active tuberculosis which includes pulmonary and extrapulmonary tuberculosis. The incidence of extrapulmonary tuberculosis showed 11%–17% of total tuberculosis in Korea24. Tuberculous lymphadenopathy is one of the extrapulmonary tuberculosis manifestation. Therefore, limited number of patients as well as total tuberculosis cases studied in the present study can explain the reason that tuberculosis was not related with false-positive lymph node in this study.
The present study has several limitations. First, this is a retrospective study with a relatively small number of patients. Second, surgical confirmation for mediastinal lymph node was limited in the present study and only 28 patients underwent surgery. Among them, two patients' 4R lymph nodes were upstaged from N0 to N2. Therefore, there can be another false-negative results of EBUS-TBNA. Third, specificity of imaging modalities from the present study was lower than previous reports. Previous studies have reported that specificity of chest CT and PET-CT is 66%–90% and 67%–94%, respectively2,10. This discrepancy may be related to different selection criteria. The present study defined positive lymph node involvement as short diameter longer than 10 mm on axial image of CT scan, and maximum FDG uptake above 2.5 on PET-CT. Different studies have performed using different criteria for the definition of lymph node positivity on chest CT and PET-CT. In addition, the present study performed EBUS-TBNA when there was positive lymph node finding on either chest CT or PET-CT.
In conclusion, the present study suggests that lung cancer staging should be performed more carefully when a patient has clinically benign lymph node characteristics including older age, histologic type of squamous cell carcinoma and benign lung conditions.
Acknowledgments
The statistical consultation was supported by Catholic Research Coordinating Center of the Korea Health 21 R&D Project (A070001), Ministry of Health & Welfare, Republic of Korea. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2014R1A2A1A11052422).
Footnotes
Authors' Contributions: Conceptualization: Kim SJ. Methodology: Lee J. Formal analysis: Kim YK. Data curation: Seo YY, Choi EK, Lee DS. Software: Kim HB, Park MS, Yim HW. Validation: Kim YS, Hong SH, Kang JH, Lee KY, Park JK, Sung SW. Investigation: Lee J, Kim SJ. Writing - original draft preparation: Lee J, Kim YK, Kim SJ. Writing - review and editing: Seo YY, Choi EK, Lee DS, Kim HB, Park MS, Yim HW, Kim YS, Hong SH, Kang JH, Lee KY, Park JK, Sung SW. Approval of final manuscript: all authors.
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Kramer H, Groen HJ. Current concepts in the mediastinal lymph node staging of nonsmall cell lung cancer. Ann Surg. 2003;238:180–188. doi: 10.1097/01.SLA.0000081086.37779.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasufuku K, Pierre A, Darling G, de Perrot M, Waddell T, Johnston M, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg. 2011;142:1393–1400.e1. doi: 10.1016/j.jtcvs.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 4.Gu P, Zhao YZ, Jiang LY, Zhang W, Xin Y, Han BH. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer. 2009;45:1389–1396. doi: 10.1016/j.ejca.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 5.Dwamena BA, Sonnad SS, Angobaldo JO, Wahl RL. Metastases from non-small cell lung cancer: mediastinal staging in the 1990s: meta-analytic comparison of PET and CT. Radiology. 1999;213:530–536. doi: 10.1148/radiology.213.2.r99nv46530. [DOI] [PubMed] [Google Scholar]
- 6.Gould MK, Kuschner WG, Rydzak CE, Maclean CC, Demas AN, Shigemitsu H, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a metaanalysis. Ann Intern Med. 2003;139:879–892. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 7.Konishi J, Yamazaki K, Tsukamoto E, Tamaki N, Onodera Y, Otake T, et al. Mediastinal lymph node staging by FDG-PET in patients with non-small cell lung cancer: analysis of false-positive FDG-PET findings. Respiration. 2003;70:500–506. doi: 10.1159/000074207. [DOI] [PubMed] [Google Scholar]
- 8.Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest. 2003;123(1 Suppl):137S–146S. doi: 10.1378/chest.123.1_suppl.137s. [DOI] [PubMed] [Google Scholar]
- 9.Silvestri GA, Gould MK, Margolis ML, Tanoue LT, McCrory D, Toloza E, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):178S–201S. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 10.Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e211S–e250S. doi: 10.1378/chest.12-2355. [DOI] [PubMed] [Google Scholar]
- 11.Birim O, Kappetein AP, Stijnen T, Bogers AJ. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg. 2005;79:375–382. doi: 10.1016/j.athoracsur.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 12.Deppen S, Putnam JB, Jr, Andrade G, Speroff T, Nesbitt JC, Lambright ES, et al. Accuracy of FDG-PET to diagnose lung cancer in a region of endemic granulomatous disease. Ann Thorac Surg. 2011;92:428–432. doi: 10.1016/j.athoracsur.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–577. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 14.Glazer GM, Orringer MB, Gross BH, Quint LE. The mediastinum in non-small cell lung cancer: CT-surgical correlation. AJR Am J Roentgenol. 1984;142:1101–1105. doi: 10.2214/ajr.142.6.1101. [DOI] [PubMed] [Google Scholar]
- 15.Hellwig D, Graeter TP, Ukena D, Groeschel A, Sybrecht GW, Schaefers HJ, et al. 18F-FDG PET for mediastinal staging of lung cancer: which SUV threshold makes sense? J Nucl Med. 2007;48:1761–1766. doi: 10.2967/jnumed.107.044362. [DOI] [PubMed] [Google Scholar]
- 16.Lowery EM, Brubaker AL, Kuhlmann E, Kovacs EJ. The aging lung. Clin Interv Aging. 2013;8:1489–1496. doi: 10.2147/CIA.S51152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vesselle H, Salskov A, Turcotte E, Wiens L, Schmidt R, Jordan CD, et al. Relationship between non-small cell lung cancer FDG uptake at PET, tumor histology, and Ki-67 proliferation index. J Thorac Oncol. 2008;3:971–978. doi: 10.1097/JTO.0b013e31818307a7. [DOI] [PubMed] [Google Scholar]
- 18.Downey RJ, Akhurst T, Gonen M, Vincent A, Bains MS, Larson S, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol. 2004;22:3255–3260. doi: 10.1200/JCO.2004.11.109. [DOI] [PubMed] [Google Scholar]
- 19.Jeong HJ, Min JJ, Park JM, Chung JK, Kim BT, Jeong JM, et al. Determination of the prognostic value of [(18)F]fluorodeoxyglucose uptake by using positron emission tomography in patients with non-small cell lung cancer. Nucl Med Commun. 2002;23:865–870. doi: 10.1097/00006231-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Hwangbo B, Kim SK, Lee HS, Lee HS, Kim MS, Lee JM, et al. Application of endobronchial ultrasound-guided transbronchial needle aspiration following integrated PET/CT in mediastinal staging of potentially operable non-small cell lung cancer. Chest. 2009;135:1280–1287. doi: 10.1378/chest.08-2019. [DOI] [PubMed] [Google Scholar]
- 21.Lu P, Sun Y, Sun Y, Yu L. The role of (18)F-FDG PET/CT for evaluation of metastatic mediastinal lymph nodes in patients with lung squamous-cell carcinoma or adenocarcinoma. Lung Cancer. 2014;85:53–58. doi: 10.1016/j.lungcan.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Metser U, Even-Sapir E. Increased (18)F-fluorodeoxyglucose uptake in benign, nonphysiologic lesions found on wholebody positron emission tomography/computed tomography (PET/CT): accumulated data from four years of experience with PET/CT. Semin Nucl Med. 2007;37:206–222. doi: 10.1053/j.semnuclmed.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Wynn GJ, Turkington PM, O'Driscoll BR. Anthracofibrosis, bronchial stenosis with overlying anthracotic mucosa: possibly a new occupational lung disorder: a series of seven cases From one UK hospital. Chest. 2008;134:1069–1073. doi: 10.1378/chest.08-0814. [DOI] [PubMed] [Google Scholar]
- 24.Joint Committee for the Revision of Korean Guidelines for Tuberculosis. Korean guidelines for tuberculosis. 3rd ed. Cheongju: Korea Centers for Disease Control and Prevention; 2007. [Google Scholar]