Abstract
Background
Post-operative morbidity following tonsillectomy remains an important clinical problem despite advances in anesthetic and surgical techniques. This study investigated the effect of a one-day course of intravenous dexamethasone on recovery from tonsillectomy.
Patients and Methods
In a double-blind, randomized, placebo-controlled trial, 30 consecutive adult patients between 18 and 35 years of age, who had no previous or known contraindications to steroid therapy, were randomly assigned at the time of surgery to either a 24-hour course of dexamethasone (3 doses of 6 mg IV) or placebo with the first dose administered during surgery, and subsequent doses given after 8 and 16 hours. The same surgeon treated all patients. Postoperative signs and symptoms, including pain, nausea, vomiting, progress of healing and the degree of granulation, were evaluated for 2 weeks.
Results
Patients treated with dexamethasone showed significantly less pain, nausea and vomiting, better healing and less granulation. There were no side effects reported.
Conclusion
Application of 3 doses of dexamethasone within 24 hours during and after tonsillectomy is advisable because of the reduction of postoperative morbidity, especially pain and edema.
Keywords: Tonsillectomy, dexamethasone, post-operative morbidity, randomized controlled trial, Saudi Arabia
Although tonsillectomy is one of the most common surgical methods performed worldwide, postoperative morbidity still remains a significant clinical problem despite advances in anesthetic and surgical techniques. The most common reason for return to the hospital or clinic is dehydration. Because of the pain associated with tonsillectomy, many patients do not eat or drink sufficiently. Significant pain is to be expected for at least a week in children and two weeks in adults, and may last longer. Appropriate analgesia is important in allowing patients to eat adequately. The effect of systemic corticosteroids on the healing process has been investigated in numerous studies. Data in adult patients undergoing tonsillectomy are rare. The results of meta-analyses1 suggest a statistically significant reduction in postoperative morbidity with single-dose dexamethasone therapy given during pediatric tonsillectomy or adenotonsillectomy with a significant reduction in post-tonsillectomy emesis during the first 24 hours and an increase in the number of patients advancing to a soft or solid diet on postoperative day 1. However, data from prospective, randomized, double-blind trials from the last decade on the treatment of adult patients that refer to different doses and applications of corticosteroids in the pre- and postoperative phases of tonsillectomy, including the 7-day oral route,2 the single-dose intravenous (12 mg) and the peri-infiltrative (up to 12 mg dexamethasone) application. However, data are still controversial regarding pain.3,4,5,7 There are no studies on the dose-response relationship of dexamethasone in this patient group.
Dexamethasone, a powerful synthetic steroid, is well-known as an effective antiemetic in adult patients receiving emetogenic chemotherapy. Because of its antipholgistic and anti-edematous effects, the drug plays a primary role in the pain therapy of tumor patients.8 The mean dose for application in tumor patients lies between 8 and 32 mg/day. Dexamethasone is preferred because of its absolute glucocorticoid and long-term effect. Short-term application, even in high doses, is not associated with severe side-effects, and the overall costs are low.
The hypothesis that a triple intravenous application of dexamethasone with a cumulative dose of 18 mg within the first 24 perioperative hours may improve the post-surgical outcome of adult patients is based on clinical experience and the outcome of the aforementioned published randomized studies on pediatric and adult patients. The rationale for this investigation was to determine the postoperative benefits, if any, of an intravenous steroid (dexamethasone, 9a-fluor-l 6a-methyprednisolone) with the first dose administered at the onset of tonsillectomy and another two doses given within 24 hours postoperatively.
Patients and Methods
Patients undergoing tonsillectomy were serially admitted into the study one day before surgery. All cases were admitted to the same hospital and treated by the same surgeon. Patients were randomly assigned to either a 24-hour course of dexamethasone (Decadron), 3 doses of 6 mg IV, or placebo (normal saline), according to a double-blind randomization. The first dose was administered during surgery and the subsequent doses were given at 8 and 16 hours post-surgery. Surgical procedures were done under general anaesthesia by using cautery in the case of patients undergoing tonsillectomy only. All patients underwent clinical examination and evaluation of medical history prior to surgery. Patients were excluded from the study if there were any contraindications for the application of steroids, such as gastrointestinal ulcer, psychiatric history, acute herpes zoster or glaucoma. The dexamethasone group (15 patients) was matched for age and sex with the control group. The male:female ratio was 8:7. All patients consented to participation in the trial and the ethical committee approved the study.
The post-operative signs and symptoms of pain, nausea, vomiting, progress of healing, and degree of granulation were evaluated for two weeks. Patients were evaluated in two phases of the recovery period: initially during their hospital stay, and later through their recovery period at home. In hospital, each patient was monitored for length of hospital stay, the duration and amount of intravenous fluid given, the total dose of postoperative analgesics, oral intake, temperature and any complications of surgery. At home, patients were asked to keep daily written records for 10 days on fluid intake, food intake, temperature, severity of pain, and medication taken for pain. The course of pain was scored on a visual analog scale (VAS) with a vertical drawer pulled along a ruler. The ruler was scaled from 0 to 10, with 0 defined as “no pain” and 10 defined as “worst pain”. Pain measurement began immediately after surgery and continued each morning before using analgesics. The data were collected in the third postoperative week. All patients were followed up by a clinic visit and daily telephone calls in the 2 weeks following surgery.
The statistical analysis was based on all patients enrolled in this study. The prescription rate and dosage of each drug was documented during the study. Continuous variables were described by mean value, standard deviation, median minimum and maximum, and a summary analysis was carried out. Chi-square and student’s t test were used as tests of significance at the 5% level.
Results
Thirty adult patients, aged between 18 and 35 years, took part in this study, with 15 patients in each study group. There was no difference between the two groups in either age or sex, or the duration of in-patient therapy. All patients left the hospital one day after surgery. None of the patients treated with dexamethasone complained of nausea and vomiting, but there were two cases of vomiting within the first 24 hours requiring antiemetics in the placebo group.
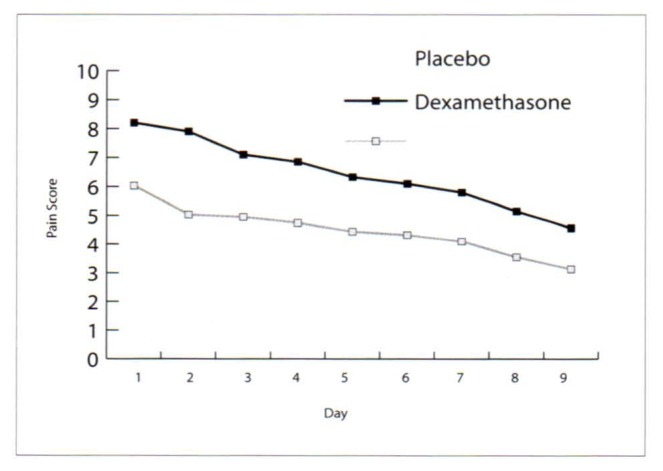
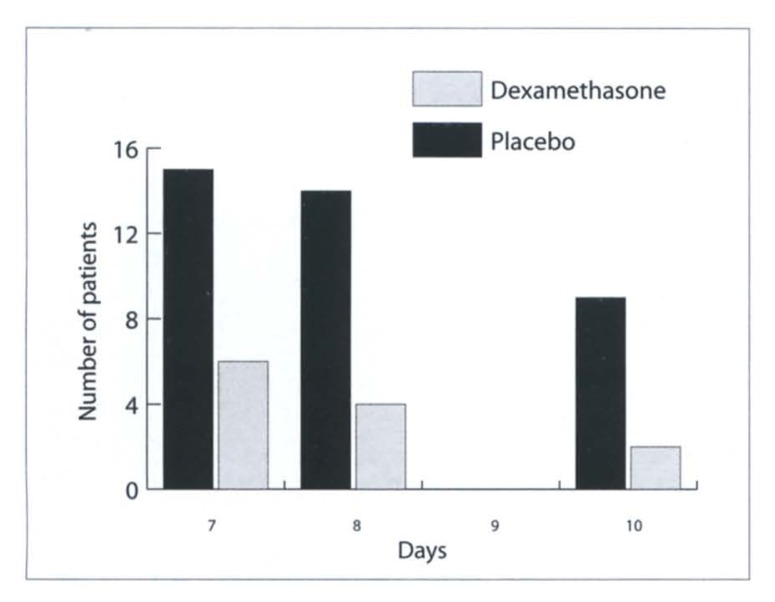
The dosage of postoperative analgesics during in-patient treatment and during the out-patient period were the same in all patients, consisting of two doses of 1000 mg paracetamol per day. None of the patients took any additional medication. The evaluated pain scores showed clearly reduced pain in the dexamethasone group in the postoperative period, with a mean value of 8.2±0.84 for the placebo group and a mean value of 6.03±0.95 for the dexamethasone group on day 1. On day 8, the placebo group showed a mean value of 4.57±1.18 on the pain score, while the dexamethasone group had a mean value of 3.13±0.81. The differences were statistically significant (P<0.05; Figure 1). Consistent with the reduced pain scores, the patients in the steroid group reduced their intake of pain medication earlier than the placebo group (Figure 2).
Figure 1.
Pain scores on a 10-degree visual analog scale recorded in the morning before intake of analgesics in the dexamethasone and placebo groups (day 1: day of surgery) (documentation of days 10–14 incomplete)(P<0.05 dexamethasone vs. placebo groups).
Figure 2.
Number of patients taking pain medication per day (paracetamol 1000 mg times 2 doses per day) in the second week following tonsillectomy.
Both groups received intravenous fluids until patients were fully conscious, and started on oral fluid 6 hours after surgery. Both groups also changed to normal diet on the first post-surgical day. Patients with steroid treatment, however, tolerated fluid and diet better because of less pain and edema. Patients in the dexamethasone group showed fewer granulations and faster healing than those of the placebo group. The difference was statistically significant (P<0.05).
Discussion
The effects of systemic corticosteroids on postoperative morbidity after tonsillectomy have been studied for decades, as this remains a significant clinical problem for the patient as well as physician.9 In this prospective, randomized, double-blind study, we determined the postoperative effects of steroids in tonsillectomy Though numerous studies support the antiemetic effect of corticosteroids, the mechanism of that effect in dexamethasone is still unknown. Despite this, dexamethasone is commonly used as an adjuvant with emetogenic chemotherapy, and its antiemetic effect is widely accepted.8 In a meta-analysis, dexamethasone dosages varied among studies in both dosing (0.15–1.0 mg/kg) and maximum dose (8–25 mg), given as single-dose for pediatric patients. The authors found a marginal statistical significance for an improved antiemetic effect with increasing dexamethasone dose, which is consistent with the results of this study. Palme et al4 reported less nausea and vomiting on days 4 to 7 after a 7-day course of oral prednisolone given as a dose of 0.5 mg/kg in patients aged 12 years and older. Compared to the results of the present study, the oral route with higher dosages of corticosteroids over a longer period did not have better results in preventing emesis than short-term application.
The ability to return to a semi-full diet or a full diet was easier for the patients treated with dexamethasone than for those of the control group. Early return to a regular diet may be due to pain reduction, an antiemetic effect, reduced edema10 and/or stimulation of appetite as a result of steroid therapy. Further reading of studies on single-dose steroid therapy in children showed a statistically significant increase in the number of patients returning to a soft or solid diet during the first 24 hours;2,3,10,11 the delayed post-surgical period showed an improved, but not significant benefit from steroid therapy.12
Patients treated with steroids in the present study reported much less pain, the results being statistically significant (P<0.05). This positive outcome might be due to the route of administration and the higher cumulative dosage and frequency of the applied steroid as compared to most studies. Studies on single-dose intravenous or peritonsillar application of dexamethasone in children did not support the hypothesis of pain reduction,2,3,6 whereas Carr et al7 reported only slightly reduced pain over the first 10 days after surgery in non-pediatric patients undergoing electrocautery tonsillectomy, given an intravenous single dose of 20-mg of dexamethasone intraoperatively. Because of the small study groups there should be further investigations to affirm whether a cumulative 24-hour low-dose application of dexamethasone should be favored in comparison with a supraphysiological single-dose bolus.
Short-term applications of steroids even in higher doses are routinely used and believed to be safe when applied to otherwise healthy patients in case of surgery in the head and neck region, to reduce edema and protect function. The present study showed that the intravenous application of 3 doses of dexamethasone, cumulating up to 18 mg within 24 hours in and after tonsillectomy is advisable because of reduction of postoperative morbidity, especially pain and edema.
References
- 1.Steward DL, Welge JA, Myer CM. Do steroids reduce morbidity of tonsillectomy? Meta-analysis of randomized trials. Laryngoscope. 2001;111:1712–1718. doi: 10.1097/00005537-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Palme CE, Tomasevic P, Pohl DV. Evaluating the effects of oral prednisolone on recovery after tonsillectomy: a prospective, double-blind, randomized trial. The Laryngoscope. 2000;10(12):2000–2004. doi: 10.1097/00005537-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Vosdiganis F, Baines DB. The effect of single dose intravenous dexamethasone in tonsillectomy in children. Anaesth intensive care. 1999;27(5):489–492. doi: 10.1177/0310057X9902700509. [DOI] [PubMed] [Google Scholar]
- 4.Ohlms LA, Wilder RT, Weston B. Use intraoperative corticosteroids in pediatric tonsillectomy. Arch Otolaryngol Head Neck Surg. 1995;121(7):737–742. doi: 10.1001/archotol.1995.01890070023006. [DOI] [PubMed] [Google Scholar]
- 5.Williams PM, Strome M, Eliachar I, Lavertu P, Wood BG, Vito KJ. Impact of steroids on recovery after uvulopalatopharyngoplasty. The Laryngoscope. 1999;109(12):1941–1946. doi: 10.1097/00005537-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Egeli E, Akkaya S. The effect of peritonsillar corticosteriod infiltration in tonsillectomy. Auris Nasus Larynx. 1997;24(2):179–183. doi: 10.1016/S0385-8146(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 7.Carr MM, Williams JG, Carmichael L, Nasser JG. Effect of steroids on posttonsillectomy pain in adults. Arch Otolaryngol Head Neck Surg. 1999;125(12):1361–1364. doi: 10.1001/archotol.125.12.1361. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Cancer Pain Relief. Geneva: WHO; 1986. [Google Scholar]
- 9.Randall D, Hoffer M. Complications of tonsillectomy and adenoidectomy. Otolaryngol Head Neck Surg. 1998;118:61–68. doi: 10.1016/S0194-5998(98)70376-6. [DOI] [PubMed] [Google Scholar]
- 10.Tom LW, Templeton JJ, Thompson ME, Marsh RR. Dexamethasone in adenotonsillectomy. Int J Ped Otorhinolaryngol. 1996;37(2):115–120. doi: 10.1016/0165-5876(96)01388-2. [DOI] [PubMed] [Google Scholar]
- 11.April MM, Callan ND, Nowak DM, Hausdroff MA. The effect of intravenous dexamethasone in pediatric adenotonsillectomy. Arch Otolaryngol Head Neck Surg. 1996;122(2):117–120. doi: 10.1001/archotol.1996.01890140007003. [DOI] [PubMed] [Google Scholar]
- 12.Goldman AC, Govindaraj A, Rosenfeld RM. A meta-analysis of dexamethasone use with tonsillectomy. Arch Otolaryngol Head Neck Surg. 2000;123(6):682–686. doi: 10.1067/mhn.2000.111354. [DOI] [PubMed] [Google Scholar]