Abstract
Background
Fasting during the month of Ramadan for Muslims is a unique metabolic model that includes abstinence from food and fluid intake during the period from dawn to sunset as well as a reduction in meal frequency and alterations in the sleep-wakefulness cycle. Leptin, neuropeptide-Y and insulin are thought to play an important role in long-term regulation of caloric intake and energy expenditure. However, the long-term changes and interactions between these factors during this pattern of fasting are not known.
Subjects and Methods
The study was conducted on 46 healthy female volunteers (age, 22±2 years; BMI, 25.3±0.7 kg/m2). Anthropometrical measurements, estimation of body fat and fasting serum levels of neuropeptide Y, leptin, insulin and glucose were estimated at baseline (day 1), days 14 and 28 of the month of Ramadan and 2 weeks after Ramadan.
Results
Baseline serum levels of leptin correlated positively with body fat (r=0.87, P=0.0002). Serum leptin levels exhibited a significant increase by approximately 41 % and neuropeptide-Y levels were decreased by 30.4% throughout the month of Ramadan. In addition, a significant correlation (r=0.63, P=0.0001) was found between changes in serum leptin and serum insulin. However, changes in serum neuropeptide-Y levels did not correlate with those of leptin or insulin
Conclusions
Long-term fasting with interrupted nocturnal eating is associated with significant elevations in serum leptin and insulin and reduction in serum neuropeptide-Y. The changes in serum leptin are likely mediated through insulin. However, changes in neuropeptide-Y appears to be mediated independently of leptin or insulin during this type of fasting
Keywords: Leptin, insulin, neuropeptide-Y, ramadan fasting
Both leptin and insulin have been identified as putative candidate adiposity signals that appear to play a key role in long-term regulation of body weight and energy homeostasis. Leptin conveys information to the brain about the size of energy stores and stimulates the hypothalamic centers responsible for regulation of energy intake and expenditure.1–3 The central effects of leptin are likely mediated by neuronal networks expressing a complex array of hypothalamic neurotransmitters, including neuropeptide-Y, agouti-related peptide (AGRP), pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART).1–3 However, the relationship between leptin and all these factors is complex and poorly understood. Many studies have focused on neuropeptide Y synthesis and release as a possible target and mediator of the actions of leptin.3 However, current evidence that neuropeptide Y mediates the effects of leptin under all conditions remains controversial.
Fasting triggers complex neural, metabolic, hormonal and behavioral adaptations with the goal of maintaining the energy substrates for use by the brain, protecting lean mass and promoting survival.3 During the month of Ramadan, Muslims abstain from food and fluid intake from dawn to sunset and eat during the whole night. These changes in the timing of food intake are also associated with a reduction in meal frequency and sleep duration.4 Food composition has also been reported to shift toward consuming more fat and less carbohydrates during Ramadan despite no change in total energy intake.5 Previous studies indicated that short-term total fasting or a chronic reduction in caloric intake6,7 results in reduction of leptin to 30% to 66% of basal levels. On the other hand, chronic overfeeding is associated with significantly elevated levels of leptin than would be expected with the increase in body mass index or percentage of body fat.7,8 The relationship between the changes in serum leptin, neuropeptide-Y and insulin associated with the unique type of metabolic changes during Ramadan fasting and a possible link to these metabolic changes, however, is unclear.
This study aimed to follow changes in serum levels of leptin, neuropeptide-Y and insulin during inversion in dietary and sleep habits associated with long-term diurnal fasting in Ramadan. We also aimed to test the correlation between changes in serum leptin, neuropeptide-Y and insulin with each other and with other factors such as anthropometrical variables, food intake and serum levels of glucose. All variables were measured during baseline (day 1), and days 14 and 28 of the month of Ramadan and two weeks after Ramadan fasting.
Subjects and Methods
Healthy female volunteers aged 18–45 years (mean, 22±2 years) were sought for participation in the study All participants were citizens of the GCC countries, mostly students and few employees of Arabian Gulf University. All subjects signed a written consent form before being involved and the experimental procedures included in the study were explained in the form. Subjects were specifically excluded if fasting blood glucose was > 6.3 mmol/L or blood pressure was > 140/90 mmHg, or if they were smokers. All Muslim subjects included in the study abstained from food and drink during the day from dawn to sunset and ate only during the night. The Research and Ethics Committee at the College of Medicine and Medical Sciences (CMMS), Arabian Gulf University, approved the experimental protocol.
Subjects were weighed bare-footed, wearing light clothes and standing on a standard pre-calibrated balance. Measurements were approximated to the nearest 1 kg and height was simultaneously measured by using a graded bar included with the scale. Waist circumference was measured with a standard tape at a level midway between the xiphisternum and the symphysis pubis. Hip circumference was measured at the widest point in the hip region. The measurements of the height and waist and hip were approximated to the nearest 0.1 cm. Body fat was assessed by “Bioelectrical Impedance Analysis” (OMRON BF 302, Matsusaka co, Japan). This method analyzes the electrical resistance of the body tissues by sending an extremely weak electrical current through the body. Since fat has little or no electric conductivity, it is possible to determine the fat tissue compared to other tissues. Percent fat was calculated from the body weight (kg) and amount of fat (kg).
During each of the experimental days, subjects were given a form where they reported the type and exact quantities of all the food they had eaten during the night. Nutrient intakes were estimated utilizing a computer program (Diet Expert for Windows, version 2.1) and from food composition tables published by the Nutrition Unit at the Ministry of Health in Bahrain.9
In addition to the anthropometrical and blood pressure measurements, fasting venous samples were taken at 1:00–2:00 PM for measurement of serum levels of leptin, neuropeptide Y, insulin,, glucose and lipids. Serum leptin levels were measured by radioimmunoassay (Linco, St. Charles, MO) as previously described.10 The leptin assay detects leptin sensitivity of 0.05 μg/L. The intra- and inter-assay coefficients were 7.5% and 8.9%, respectively. Neuropeptide Y was assayed by competitive radioimmunoassay by direct assay without extraction using reagents (Euro-Diagnostica AB, Sweden). The method has sensitivity to detect the lowest concentration of 6 pmol/L and recovery of 84%. The method has shown intra-assay variation coefficient of variation (CV) of 4% and interassay variation CV of 12%. The human insulin specific radioimmunoassay detects insulin with a sensitivity of 2μU/mL. The intra- and inter-assay coefficients were less than 4.4% and 6.0%, respectively. Blood glucose was measured by the hexokinase method (Roche Diagnostics, Mannheim, Germany) in plasma from a fluoride oxalate tube. Serum cholesterol was measured by the cholesterol oxidase method (Roche Diagnostics, Mannheim, Germany) and serum triacylglycerol was measured by the enzymatic method using glycerol kinase and peroxidase (Roche Diagnostics, Mannheim, Germany). All the samples of collected sera were kept at −20°C until analysis and all the hormonal assays were run in the same assay in duplicate.
Data are expressed as mean ± SEM. Data were analyzed using SSPS program (version 11) by analysis of variance (ANOVA) for repeated measures. A P value <0.05 was considered statistically significant. Pearson’s correlation coefficient was used to determine possible linear correlations between the different variables.
Results
Despite the tendency for body weight, BMI, body fat and waist circumference to decrease at the end of Ramadan, changes in anthropometrical variables in women (n=46) did not reach statistical significance (Table 1). Neither were significant changes observed across the study period. However, energy intake was significantly increased (P<0.05) compared with baseline levels in women.
Table 1.
Anthropometrical variables and energy intake during the study (mean ± SEM).
| Time in relation to Ramadan | ||||
|---|---|---|---|---|
| Early | Mid | End | Post | |
| Weight (kg) | 80.8 ± 1.4 | 79.9 ± 2.1 | 80.1 ± 2.0 | 80.5 ± 1.8 |
|
| ||||
| Body mass index (kg/m2) | 25.3 ± 0.7 | 25.3 ± 0.8 | 24.2 ± 0.9 | 25.4 ± 1.0 |
|
| ||||
| Fat (kg) | 23.9 ± 1.8 | 24.0 ± 1.8 | 22.4 ± 1.7 | 23.7 ± 1.9 |
|
| ||||
| Fat (%) | 32.8 ± 1.3 | 32.3 ± 1.3 | 32.5 ± 1.4 | 32.9 ± 1.7 |
|
| ||||
| Waist circumference (cm) | 77.7 ± 1.6 | 77.8 ± 75.8 | 75.8 ± 1.5 | 77.2 ± 1.9 |
|
| ||||
| Waist/hip ratio | 0.76 ± 0.01 | 0.76 ± 0.01 | 0.75 ± 0.01 | 0.78 ± 0.01 |
|
| ||||
| Energy intake (Kcal/day) | 1660 ± 311 | 1751 ± 344* | 1671 ± 278 | - |
P<0.05 compared with early Ramadan
Serum leptin levels were significantly increased (P<0.05) during the month of Ramadan (Table 2). This increase in serum leptin was associated with comparable and significant (P<0.05) increases in serum insulin and decreases in serum neuropeptide Y (P<0.05). Although serum triglycerides were increased during the month of Ramadan, these changes were not significant (P=0.06) No changes in serum glucose were observed during the study.
Table 2.
Serum levels of biochemical variables during the study (mean ± SEM).
| Time in relation to Ramadan | ||||
|---|---|---|---|---|
| Early | Mid | End | Post | |
| Glucose (mmol/L) | 5.41 ± 0.14 | 5.14 ± 0.17 | 5.27 ± 0.20 | 5.83 ± 0.16 |
|
| ||||
| Triglycerides (mmol/L) | 0.79 ± 0.06 | 0.77 ± 0.05 | 0.84 ± 0.06 | 0.84 ± 0.10 |
|
| ||||
| Cholesterol (mmol/L) | 4.41 ± 0.10 | 4.44 ± 0.10 | 4.58 ± 0.10 | 4.36 ± 0.25 |
|
| ||||
| Insulin (μU/mL) | 7.37 ± 1.57 | 8.34 ± 1.32 | 8.83 ± 1.20 | 9.73 ± 1.77 |
|
| ||||
| Leptin (μg/mL) | 10.57 ± 0.82 | 13.59 ± 1.01 | 14.83 ± 1.01 | 13.54 ± 1.11 |
|
| ||||
| Neuropeptide Y (pmol/L) | 77.53 ± 6.79 | 53.93 ± 5.43 | 66.41 ± 7.26 | - |
P<0.05 compared with early Ramadan
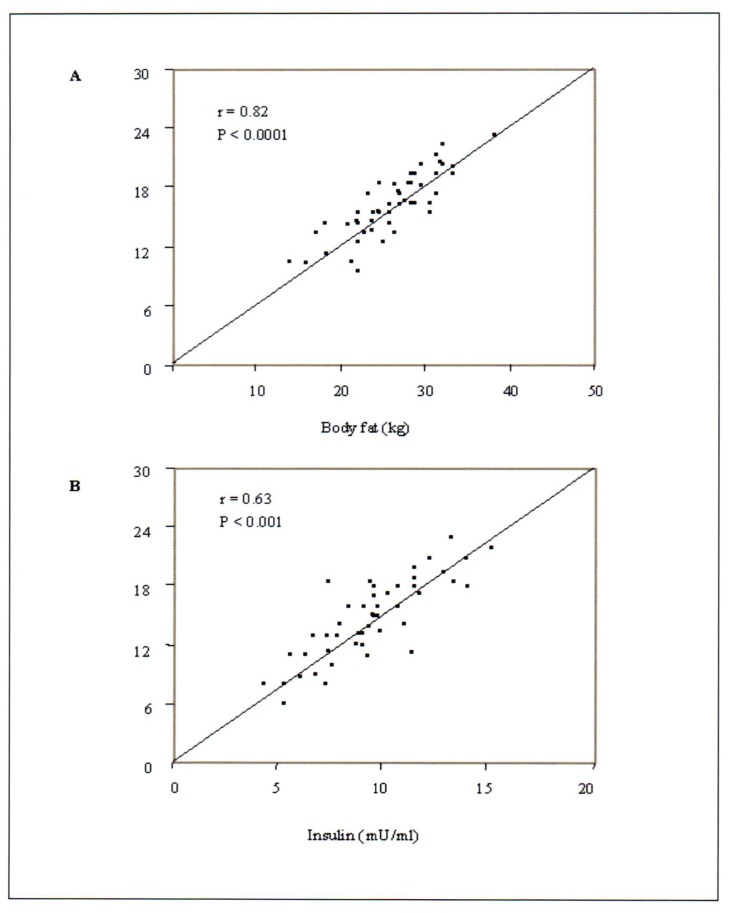
Significant correlations were found between serum leptin and body fat (r=0.82, P<0.001) and also between leptin and serum insulin (r=0.63, P<0.001) (Figure 1, panel A and B).
Figure 1.
Correlations between serum leptin (μg/L) and both body fat (kg) [A] and serum insulin (μU/mL) [B]. The data indicate the correlations during day 14 (mid-Ramadan) of fasting. Significant correlations were also observed during other periods of the study.
Discussion
Follow-up of serum levels of leptin, neuropeptide-Y and insulin during the unique type of fasting in Ramadan indicate that as Muslims progress during the month, there is a relative hyperinsulinemia and hyperleptinemia associated with reduction in neuropeptide-Y. The changes in serum leptin and insulin correlated positively with each other during the study. However, changes in neuropeptide-Y levels did not correlate with either leptin or insulin.
It is unlikely that this increase in serum leptin and insulin is mediated by changes in body adiposity since body fat and other indices of obesity did not change significantly during the study. Potential non-adiposity factors that could be responsible for elevated serum leptin and insulin could be those related to diet such as the amount of energy intake or food composition. Leptin secretion decreases in response to fasting7,11 and increases in response to positive energy balance induced by overfeeding.6 Food composition also alters the secretion of leptin, although the data on this issue has been controversial.12–14 Since energy intake was increased during Ramadan, it is possible that elevated levels of leptin and insulin may reflect a state of positive energy balance due to a compensatory increase in food intake during the night. These observations agree with previous studies indicating that energy intake is increased,15 but contradict those that indicate that energy intake is reduced16 during Ramadan. However, our study confirms that with prolonged diurnal fasting, leptin and insulin become more sensitive to other signals such as caloric intake.
Plasma leptin is secreted in a pulsatile fashion with peak nocturnal levels and a nadir at noon.17 Although the underlying mechanisms responsible for the circadian leptin rhythm are unclear, there is evidence that the diurnal rhythm of leptin secretion is entrained to the meal pattern and that shifting meal timing causes a comparable shift in plasma leptin rhythm.18 Since Ramadan fasting is associated with forward shifting of lunchtime by approximately 6 hours, another possible cause of increased levels of leptin in our study could be attributed to a shift in the circadian pattern of leptin with progressive proximity towards the peak nocturnal levels. Additional studies that take frequent samples over 24 hours will be required to examine the circadian variations in serum leptin during Ramadan fasting.
Evidence supporting an interaction between leptin and neuropeptide Y has come from studies demonstrating that the expression of neuropeptide Y mRNA in the arcuate nucleus is increased in situations where functional leptin levels are low, such as in ob/ob mice and after fasting.19,20 Furthermore, in fasted animals, intra-cerebroventricular injection of leptin reduces the concentration of neuropeptide Y protein in the paraventricular nucleus as well as in the dorsomedial hypothalamus and the arcuate nucleus.21 Ramadan fasting involves not only abstinence from food and water, but also an associated unique spiritual pattern that makes it difficult to compare with other types of fasting. However, this study confirms previous findings that increased serum leptin during Ramadan is associated with reduced levels of neuropeptide Y. But the lack of correlation between the changes in leptin and that of neuropeptide Y indicate that the latter is unlikely to play an important role in long-term regulation of leptin secretion, especially during prolonged diurnal fasting.
A recent study has also indicated that fasting insulin and leptin levels were closely correlated in weight-reduced obese women, suggesting that insulin could contribute to the long-term regulation of plasma leptin.22 These observations are supported by others, which found that insulin and glucose might regulate leptin secretion, especially in response to alterations in energy status.23 Energy restriction studies have indicated that serum insulin and leptin levels are decreased with fasting, and the decrease in leptin levels correlates closely with the reduction in plasma glucose.7,8 Furthermore, leptin levels remain unchanged when serum insulin was prevented from decline by intravenous infusion of small amounts of glucose.11 Therefore, our data confirm and support a role for insulin in the long-term regulation of leptin secretion under conditions of chronic diurnal fasting followed by nocturnal feeding.
In conclusion, we found that circulating leptin and insulin levels progressively increase and neuropeptide Y levels decrease throughout the month of Ramadan. Elevations in serum leptin correlate with comparable increases in serum insulin. However, the reduction in serum neuropeptide Y did not correlate with those of insulin or leptin. These data provide an insight into the important role of insulin and leptin in long-term regulation of energy balance during conditions of chronic fasting and re-feeding during Ramadan. However, neuropeptide Y does not appear to be involved in this process.
Acknowledgment
The authors also express their sincere thanks to Mr. Hani Badr El-Sheikh for excellent technical assistance. The authors would like also to thank Mr. Mohammad Hussein from the Clinical Biochemistry Section in Salmaniya Medical Complex for his vital role in the chemical assay of serum leptin and insulin.
References
- 1.Havel PJ. Leptin production and action: relevance to energy balance in humans. Am J Clin Nutr. 1998;67:355–356. doi: 10.1093/ajcn/67.3.355. [DOI] [PubMed] [Google Scholar]
- 2.Woods SC, Seeley RJ, Porte DJ, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 3.Ahima RS, Flier JS. Leptin. Ann Rev Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 4.Angel JF, Schwartz NE. Metabolic changes resulting from decreased meal frequency in adult male Muslims during the Ramadan Fast. Nutr Rev. 1975;11:29–39. [Google Scholar]
- 5.El Ati J, Beji C, Danguir J. Increased fat oxidation during Ramadan fasting in healthy women: An adaptive mechanism for body weight maintenance. Am J Clin Nutr. 1995;62:302–307. doi: 10.1093/ajcn/62.2.302. [DOI] [PubMed] [Google Scholar]
- 6.Kolaczynski JW, Considine RV, Ohanessian J, et al. Responses of leptin to short-term fasting and refeeding in humans. Diabetes. 1996;45:1511–1515. doi: 10.2337/diab.45.11.1511. [DOI] [PubMed] [Google Scholar]
- 7.Dubuc GR, Phinney SD, Stern JS, Havel PJ. Changes of serum leptin and endocrine and metabolic parameters after 7 days of energy restriction in men and women. Metabolism. 1998;47:429–434. doi: 10.1016/s0026-0495(98)90055-5. [DOI] [PubMed] [Google Scholar]
- 8.Wisse BE, Campfield LA, Marliss EB, Morais JA, Tenenbaum R, Gougeon R. Effect of prolonged moderate and severe energy restriction and refeeding on plasma leptin concentration in obese women. Am J Clin Nutr. 1999;70:321–330. doi: 10.1093/ajcn/70.3.321. [DOI] [PubMed] [Google Scholar]
- 9.Musaiger AA, AlDallal ZS. Food composition tables for use in Bahrain. 1st edition. Bahrain: Ministry of Health; 1985. [Google Scholar]
- 10.Horn R, Geldszus R, Potter E, von zur Muhlen A, Brabant G. Radioimmunoassay for the detection of leptin in human serum. Exp Clin Endocrinol Diabetes. 1996;104:454–458. doi: 10.1055/s-0029-1211484. [DOI] [PubMed] [Google Scholar]
- 11.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Endocrinol Clin Metab. 1996;81:3419–3423. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- 12.Havel PJ, Townsend R, Chaump L, Teff K. High fat meals reduce 24 h circulating leptin concentration in women. Diabetes. 1999;48:334–341. doi: 10.2337/diabetes.48.2.334. [DOI] [PubMed] [Google Scholar]
- 13.Ahren B, Mansson S, Gingerich RL, Havel PJ. Regulation of plasma leptin in mice: influence of age, high fat diet and fasting. Am J Physiol. 1997;273:R113–R120. doi: 10.1152/ajpregu.1997.273.1.R113. [DOI] [PubMed] [Google Scholar]
- 14.Shrauwen P, van Marken Lichtenbelt WD, Westerterp KR, Saris WH. Effect of diet composition on leptin concentration in lean subjects. Metabolism. 1997;96:420–424. doi: 10.1016/s0026-0495(97)90059-7. [DOI] [PubMed] [Google Scholar]
- 15.Frost G, Pirani S. Meal frequency and nutritional intake during Ramadan: A pilot study. Hum Nutr Appl Nutr. 1987;41:47–50. [PubMed] [Google Scholar]
- 16.Husain R, Duncan MT, Cheah SH, Ching SL. Effects of fasting in Ramadan on tropical Asiatic Moslems. Br J Nutr. 1978;58:41–48. doi: 10.1079/bjn19870067. [DOI] [PubMed] [Google Scholar]
- 17.Sinha MK, Ohannesian JP, Heiman ML, Criauciunas A, Stephens TW, Magosin S, Marco C, Caro JF. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoeller DA, Cella LK, Caro JF. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest. 1997;100:1882–1887. doi: 10.1172/JCI119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz MW, Seely RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in the rat hypothalamus. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz MW, Erickson JC, Baskin DG, Palmiter RD. Effects of fasting and leptin deficiency on hypothalamic neuropeptide Y gene transcription in vivo revealed by expression of lacZ reporter gene. Endocrinology. 1998;139:2623–2635. doi: 10.1210/endo.139.5.6000. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Bing C, Al Barazanji K, Mossakowaska DE, Wang XM, McBay DI. Interactions between leptin and hypothalamic neuropeptide Y neurons in the control of food intake and energy homeostasis. Diabetes. 1997;46:335–341. doi: 10.2337/diab.46.3.335. [DOI] [PubMed] [Google Scholar]
- 22.Guven S, El-Bershawi A, Sonnenberg GE, et al. Plasma leptin and insulin levels in weight-reduced obese women with normal body mass index: relationships with body composition and insulin. Diabetes. 1999;48:347–358. doi: 10.2337/diabetes.48.2.347. [DOI] [PubMed] [Google Scholar]
- 23.Dagogo-Jack S, Fanelli C, Parmore D, Brothers J, Landt M. Plasma leptin and insulin relationships in obese and non-obese humans. Diabetes. 1996;45:695–698. doi: 10.2337/diab.45.5.695. [DOI] [PubMed] [Google Scholar]