Abstract
Type B insulin resistance syndrome is a rare autoimmune disease and no effective therapy has yet been established. On the other hand, it is known that Saibokuto, one type of Japanese Kampo medicine, may have beneficial effects on various symptoms associated with this disease and it is therefore occasionally prescribed for various immune disorders. We herein describe a case of type B insulin resistance syndrome in which anti-insulin receptor antibody disappeared and the patient's glycemic control markedly improved after the administration of Saibokuto. At first, we administered various anti-oral diabetic drugs and insulin therapy, but the patient's glycemic control became further aggravated. In addition, Helicobacter pylori eradication therapy was not effective, although its benefit has been reported. Interestingly, after the patient started taking Saibokuto, her glycemic control markedly improved. In addition, the patient's plasma insulin levels markedly decreased and anti-insulin receptor antibody became negative after taking Saibokuto. Taken together, there is a possibility that Saibokuto may one of the options for type B insulin resistance syndrome therapy.
Keywords: type B insulin resistance syndrome, Saibokuto, anti-insulin receptor antibody
Introduction
Insulin resistance is a common problem in subjects with type 2 diabetes mellitus (T2DM). Some patients with T2DM gradually become less sensitive to the effects of insulin, and insulin which is secreted from pancreatic β-cells helps to regulate the blood glucose levels. There are actually two types of insulin resistance, insulin resistance syndrome type A and type B. With insulin resistance syndrome type A, insulin cannot bind to its receptor because the insulin receptors are either absent or non-functional. This is because there is a mutation in the genes that code for the insulin receptors so that the receptors become defective and cannot bind to insulin. Type B insulin resistance syndrome is a rare autoimmune disease triggered by auto-antibodies to the insulin receptor and it is associated with other autoimmune diseases (1-3). There is no established effective therapy for it (4). Therefore, it is very difficult to manage glycemic control, because both hyperglycemia and hypoglycemia occur in subjects with this disease. At present, type B insulin resistance syndrome is mainly treated with immunosuppressive therapy (5).
On the other hand, Saibokuto, a Japanese Kampo medicine, is known to be one of the most popular and best studied anti-asthmatic Kampo medicines and is commonly used both in Japan and China (6, 7). It has beneficial effects on various symptoms, such as appetite loss, sore throat and cough. In addition, it is thought that Saibokuto is, at least in part, involved in the immune response. Indeed, it has been occasionally prescribed for various immune disorders such as asthma and collagen disease (8, 9).
We experienced a case of type B insulin resistance syndrome in which anti-insulin receptor antibody disappeared and glycemic control was improved after the administration of Saibokuto. We think that this case may thus be very important and informative from a clinical point of view, because there is a possibility that Saibokuto may be one of the options for type B insulin resistance syndrome therapy.
Case Report
A 69-year-old woman who had been regularly taking 20 mg of olmesartan for hypertension developed hyperglycemia. At that time her plasma glucose level was 197 mg/dL and hemoglobin A1c was 6.9%. She was diagnosed to have diabetes mellitus, and she thus started diet therapy of 1,600 kcal/day (about 29 kcal/ ideal body weight kg). At 5 months after the diagnosis (HbA1c 8.3%), we started to administer 25 mg/day of alogliptin, and then at 9 months after the diagnosis (HbA1c 9.5%), we added 1 mg/day of glimepiride. However, her glycemic control became further aggravated and her weight significantly decreased (10.0 kg decrease in 4 months). Therefore, she was hospitalized at our institution in order to improve her glycemic control.
On admission (April, 2012), her height, body weight and BMI were 160.0 cm, 49.0 kg and 19.38 kg/m2, respectively. Her physical examination was unremarkable. She did not have Acanthosis nigricans, which is often associated with type B insulin resistance syndrome. She had no family history of diabetes. The laboratory data were as follows: white blood cell count, 2,970/μL (neutrophil 34.0%); red blood cell count, 346×104/μL; hemoglobin, 11.9 g/dL; platelet, 11.9/μL. The diabetes-associated data were as follows: fasting plasma glucose, 77 mg/dL; plasma insulin, 324.6 μU/mL; C-peptide, 3.7 ng/mL; glycoalbumin 37.6%. Anti-glutamic acid decarboxylase (GAD) antibody and anti-IA-2 antibody were negative. Anti-insulin antibody was positive (450.4 nU/mL), but the binding rate was as low as 1.5%. Anti-insulin receptor antibody was positive (45%) (radio immunoassay, SRL, Tokyo, Japan). Scatchard analysis plots of insulin autoantibodies (SRL) were not detected, because the binding rate of anti-insulin antibody was very low. The plasma adiponectin level was 58.2 μg/mL. She did not develop hypoglycemia and also showed no symptoms of hypoglycemia. Other endocrine hormone levels were within the normal range; adrenocorticotropic hormone (ACTH), 45.2 pg/mL; cortisol, 10.1 μg/dL; dehydroepiandrosterone sulfate (DHEA-S), 26 μg/dL; thyroid stimulating hormone (TSH), 3.906 μU/L; FT4, 1.19 ng/mL; FT3, 2.27 pg/mL. Lipid-related factor levels were also normal; low density lipoprotein (LDL) cholesterol, 116 mg/dL; high density lipoprotein (HDL) cholesterol, 79 mg/dL; triglyceride, 49 mg/dL. Renal and liver function was also within normal range: creatinine, 0.72 mg/dL; BUN, 16 mg/dL; aspartate aminotransferase (AST), 22 U/L; alanine aminotransferase (ALT), 10 U/L; γ-glutamyltranspeptidase (GTP), 19 U/L. The serum IgG, IgA and IgM levels were 1,820 mg/dL, 386 mg/dL and 73 mg/dL, respectively. In addition, since it is known that insulin resistance syndrome is often accompanied by various kinds of collagen diseases, we measured various collagen disease-related antibodies. Antinuclear antibody was positive (67.0) and rheumatoid factor was 22 U/L. Other collagen disease-related antibodies (anti-ds-DNA antibody, anti-Sm antibody, anti-SS-A antibody, anti-SS-B antibody, anti-Scl-70 antibody, anti-Jo-1 antibody, anti-TPO antibody, anti-TR antibody) were all negative (Table). Based on these data, it seemed that she did not have any overt autoimmune disease and she was not treated with steroids. As a result, this case was not a typical case of insulin resistance syndrome. However, insulin resistance syndrome is not necessarily accompanied by collagen diseases in all cases, and we think that anti-insulin receptor antibody may have in fact been truly positive in this case, although we could not totally rule out the potential for false positive anti-insulin receptor antibody.
Table.
Laboratory Data on Admission in This Subject.
| Variable | Result | Reference Range | Variable | Result | Reference Range | |||
|---|---|---|---|---|---|---|---|---|
| Peripheral blood | Diabetes marker | |||||||
| White blood cells (/μL) | 2,970 | 4,000-9,000 | FPG (mg/dL) | 77 | 70-110 | |||
| Neutrophil (%) | 34.0 | 28.0-78.0 | Hemoglobin A1c (%) | 9.8 | 4.6-6.2 | |||
| Red blood cells (×104/μL) | 346 | 427-570 | Glycoalbumin (%) | 37.6 | 12.4-16.3 | |||
| Hemoglobin (g/dL) | 11.9 | 14.0-18.0 | Insulin (μU/mL) | 324.6 | 1.84-12.2 | |||
| Platelets (×104/μL) | 11.9 | 15.0-35.0 | C-peptide (ng/mL) | 3.7 | 0.61-2.09 | |||
| Blood biochemistry | GAD Ab. (U/mL) | <1.3 | 0-4.9 | |||||
| Total protein (g/dL) | 7.8 | 6.7-8.3 | IA-2 Ab. (U/mL) | <0.4 | 0-0.3 | |||
| Albumin (g/dL) | 4.2 | 3.8-5.2 | ICA Ab. (U) | <1.25 | <1.25 | |||
| Total bilirubin (mg/dL) | 0.80 | 0.00-1.00 | Insulin Ab. (nU/mL) | 450.4 | 0-125.0 | |||
| AST (U/L) | 22 | 8-35 | binding rate | 1.5 | 0-0.4 | |||
| ALT (U/L) | 10 | 5-43 | Anti-insulin receptor Ab. (%) | 45.0 | ||||
| LDH (U/L) | 153 | 106-211 | Plasma adiponectin (μg/mL) | 58.2 | ||||
| ALP (U/L) | 220 | 104-338 | HLA-DNA typing | HLA-DRB1 12:01:01 | ||||
| γ-GTP (U/L) | 19 | 2-72 | Endocrine marker | |||||
| BUN (mg/dL) | 16.0 | 8.0-20.0 | ACTH (pg/mL) | 45.2 | 7.2-63.3 | |||
| Creatinine (mg/dL) | 0.72 | 0.3-1.1 | Cortisol (μg/dL) | 10.1 | 6.24-18.0 | |||
| Cholinesterase (U/L) | 171 | 170-430 | DHEA-S (μg/dL) | 26 | 76-386 | |||
| Creatine Kinase (U/L) | 78 | 38-213 | TSH (μU/mL) | 3.90 | 0.35-4.94 | |||
| CRP (mg/dL) | 0.07 | 0.00-0.50 | Free thyroxine (ng/dL) | 1.19 | 0.70-1.48 | |||
| Sodium (mEq/L) | 136 | 135-148 | Aldosterone (pg/mL) | 126 | 35.7-240 | |||
| Potassium (mEq/L) | 3.8 | 3.3-5.0 | Renin activity (ng/mL/hr) | 2.7 | 0.3-2.9 | |||
| Chloride (mEq/L) | 100 | 98-109 | Collagen disease-related antibodies | |||||
| Amylase (U/L) | 113 | 40-134 | Anti-nuclear Ab. | 67.0 | 0-39 | |||
| Urea breath test (%o) | 14.8 | 0.0-2.4 | rheumatoid factor (U/L) | 22 | <20 | |||
| Urinary test | anti-ds-DNA Ab. (IU/mL) | <10 | <12.0 | |||||
| Urinary pH | 5.5 | anti-Sm Ab. (IU/mL) | ≤7.0 | <10.0 | ||||
| Urinary protein | ± | anti-SS-A Ab. (IU/mL) | ≤7.0 | <10.0 | ||||
| Urinary sugar | 3+ | anti-SS-B Ab. (IU/mL) | ≤7.0 | <10.0 | ||||
| Urinary ketone body | - | anti-Scl-70 Ab. (IU/mL) | ≤7.0 | <10.0 | ||||
| anti-Jo-1 Ab. (IU/mL) | ≤7.0 | <10.0 | ||||||
| PAIgG (ng/107cells) | 180 | 0-46 | ||||||
AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyltranspeptidase, BUN: blood urea nitrogen, CRP: C-reactive protein, P-amylase: pancreatic amylase, P-phospholipase A2: pancreatic phospholipase A2, FPG: fasting plasma glucose, GAD: anti-glutamic acid decarboxylase, Ab.: antibody, IA-2: anti-insulinoma-associated tyrosine phosphatase-like protein-2, ICA: anti-islet cell antigen, DHEA-S: dehydroepiandrosterone sulfate, TSH: thyroid stimulating hormone, PAIgG: platelet-associated immunoglobulin
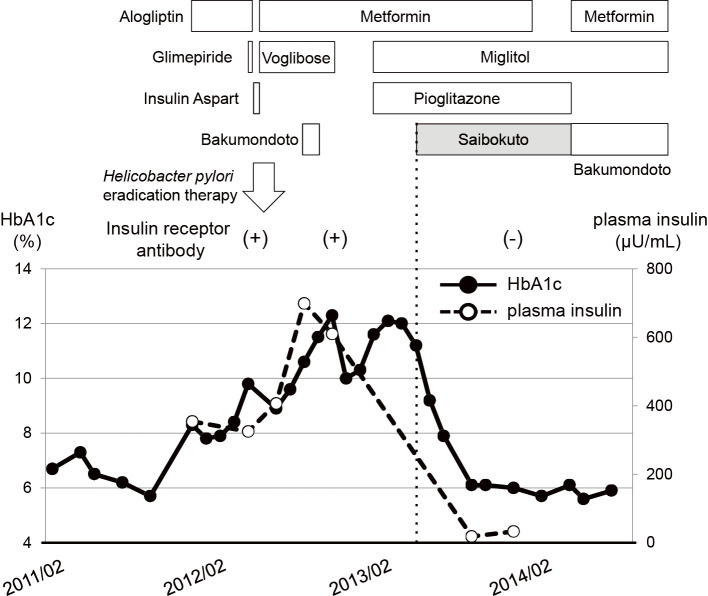
At first we thought she had type 2 diabetes and thus administered bolus insulin (4 units of aspart before each meal) for 2 weeks, but after obtaining the findings that her anti-insulin receptor antibody was positive (45%), we diagnosed her to have type B insulin resistance syndrome, and thereby stopped the insulin (maximum dose: 14 U/day of aspart) and started the patient on anti-oral diabetic drugs (metformin and voglibose). As shown in Figure, we tried various kinds of anti-oral diabetic drugs, such as alogliptin, glimepiride, metformin, voglibose, miglitol and pioglitazone, but all of them failed to sufficiently improve her glycemic control, although some drug showed a transient effect. In addition, urea breath test was positive (14.8‰), indicating that she was infected with Helicobacter pylori. While it is known that idiopathic thrombocytopenia is often accompanied by Helicobacter pylori infection (10), platelet-associated immunoglobulin G was also as high as 180 ng/107cells in this subject. Therefore, we tried Helicobacter pylori eradication therapy. Furthermore, Helicobacter pylori eradication therapy has been reported to be effective for type B insulin resistance syndrome (11). Helicobacter pylori was successfully eradicated [urea breath test was negative 5 weeks after eradication therapy (0.8‰)], however, her glycemic control was further aggravated.
Figure.
Clinical time course in a subject with type B insulin resistance syndrome. Insulin receptor antibody was positive, and high HbA1c and plasma insulin levels were observed. After beginning to administer Saibokuto (7.5 mg/day), however, anti-insulin receptor antibody disappeared and her HbA1c and plasma insulin levels markedly decreased. HbA1c: hemoglobin A1c
Some Japanese Kampo medicines have been reported to be effective for taste disorder and glossitis (12), and she had both taste disorder and glossitis. Thus she occasionally took Bakumondoto, a Japanese Kampo medicine. In April 2013, she started taking Saibokuto (7.5 g/day), another Japanese Kampo medicine because Bakumondoto was no longer effective. Interestingly, her glycemic control thereafter markedly improved without changing any of the anti-diabetic drugs (15 mg/day of pioglitazone, 750 mg/day metformin, and 150 mg/day of miglitol), although many of the anti-diabetic drugs we had used were not effective for obtaining her glycemic control (Figure). The HbA1c level before and 1, 2 and 4 months after Saibokuto treatment was 11.2%, 9.2%, 7.9% and 6.1%, respectively, and her glycemic control was well controlled with pioglitazone, metformin and miglitol. The plasma insulin levels markedly decreased from 324.6-699.0 μU/mL to 18.5-32.8 μU/mL after starting Saibokuto. We stopped Saibokuto about 6 months later, because it was not effective for taste disorder. Since good glycemic control was maintained for several months with metformin and miglitol, we examined anti-insulin receptor antibody again several months after starting Saibokuto treatment. Interestingly, anti-insulin receptor antibody became negative. Anti-insulin antibody also became negative, although the anti-insulin antibody titer and binding rate were both slightly high. It is likely that Saibokuto treatment brought out negative conversion of anti-insulin receptor antibody. However antinuclear antibody was still positive (59.8-74.5) and the platelet-associated immunoglobulin G level had decreased, but it was still high (85 ng/107cells).
Discussion
We herein describe a case of type B insulin resistance syndrome in which anti-insulin receptor antibody disappeared and glycemic control markedly improved after the administration of Saibokuto. No effective therapy has yet been established for type B insulin resistance syndrome, although Helicobacter pylori eradication therapy has been reported to be effective (11). Therefore, type B insulin resistance syndrome tends to mainly be treated by immunosuppressive therapy (5). For this patient, we did not use steroid therapy, plasmapheresis or CD20 monoclonal therapy, all of which are thought to be possible therapeutic options for insulin resistance syndrome (13-17). On the other hand, Saibokuto has been occasionally prescribed for various immune disorders. It was reported that 11β-hydroxysteroid dehydrogenase was inhibited by Saibokuto and that Saibokuto treatment increased the prednisolone/prednisone ratio (18, 19). These data suggest that Saibokuto is, at least in part, involved in the immune response. Therefore, we assume that Saibokuto treatment caused the disappearance of anti-insulin receptor antibody through some modification of the immune response which finally stabilized glycemic control in this patient. Since the cost of Saibokuto is relatively low, Saibokuto is useful from an economic point of view. It is noted, however, that although Saibokuto is a relatively safe drug, interstitial pneumonia, pseudoaldosteronism, myopathy, hepatic dysfunction and jaundice are known to be side effects associated with this drug. Therefore, when such symptoms occur, the administration should be discontinued.
This study is associated with one limitation. Helicobacter pylori was successfully eradicated, but the patient's glycemic control did not improve after more than one year. It is therefore possible that Helicobacter pylori eradication therapy may require a long period of time before it becomes effective. In addition, the patient was taking various medications. Kawahito et al. reported that the peroxisome proliferator-activated receptor-gamma could function as an immune-inflammatory mediator (20). Since she was taking pioglitazone before her HbA1c levels decreased, there is a possibility that pioglitazone may have affected her symptoms. On the other hand, it was previously reported that anti-insulin receptor antibody decreased spontaneously in some patients (21, 22). Therefore, although we think that Saibokuto was involved in the disappearance of anti-insulin receptor antibody from the clinical course in this patient, we cannot rule out the possibility that anti-insulin receptor antibody may have spontaneously disappeared.
In conclusion, Saibokuto is a possible therapy for type B insulin resistance syndrome, although further evaluation with a substantial number of subjects with type B insulin resistance syndrome would be necessary in order to strengthen this hypothesis.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Gehi A, Webb A, Nolte M, Davis J Jr. Treatment of systemic lupus erythematosus-associated type B insulin resistance syndrome with cyclophosphamide and mycophenolate mofetil. Arthritis Rheum 48: 1067-1070, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Yang GQ, Li YJ, Dou JT, Wang BA, Lu JM, Mu YM. Type B insulin resistance syndrome with Scleroderma successfully treated with multiple immune suppressants after eradication of Helicobacter pylori infection: a case report. BMC Endocr Disord 16: 20, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang H, Zhao J, Li Y, Lv F, Zhang S, Li Y. Successful treatment of type B insulin resistance with mixed connective tissue disease by pulse glucocorticoids and cyclophosphamide. J Diabetes Investig 8: 626-628, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn CR, Flier JS, Bar RS, et al. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med 294: 739-745, 1976. [DOI] [PubMed] [Google Scholar]
- 5.Sims RE, Rushford FE, Huston DP, Cunningham GR. Successful immunosuppressive therapy in a patient with autoantibodies to insulin receptors and immune complex glomerulonephritis. South Med J 80: 903-906, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Bielory L, Lupoli K. Herbal interventions in asthma and allergy. J Asthma 36: 1-65, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Naito A, Satoh H, Sekizawa K. Asthma as well as anxiety improved by the Kampo extract Saiboku-to. Eur J Intern Med 16: 621, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Huntley A, Ernst E. Herbal medicines for asthma: a systematic review. Thorax 55: 925-929, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shergis JL, Wu L, Zhang AL, Guo X, Lu C, Xue CC. Herbal medicine for adults with asthma: a systematic review. J Asthma 53: 650-659, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Gasbarrini A, Franceschi F, Tartaglione R, Landolfi R, Pola P, Gasbarrini G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet 352: 878, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Imai J, Yamada T, Saito T, et al. Eradication of insulin resistance. Lancet 374: 264, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Ara T, Hattori T, Imamura Y, Wang PL. Development of novel therapy for oral diseases using kampo medicines. J Oral Biosci 52: 100-106, 2010. [Google Scholar]
- 13.Kawanishi K, Kawamura K, Nishina Y, Goto A, Okada S. Successful immunosuppressive therapy in insulin resistant diabetes caused by anti-insulin receptor autoantibodies. J Clin Endocrinol Metab 44: 15-21, 1977. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson JW, Bremell T, Eliasson B, Fowelin J, Fredriksson L, Yu ZW. Successful treatment with plasmapheresis, cyclophosphamide, and cyclosporin A in type B syndrome of insulin resistance. Case report. Diabetes Care 21: 1217-1220, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Coll AP, Thomas S, Mufti GJ. Rituximab therapy for the type B syndrome of severe insulin resistance. N Engl J Med 350: 310-311, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Takei M, Ishii H, Kawai Y, et al. Efficacy of oral glucocorticoid and cyclosporine in a case of rituximab-refractory type B insulin resistance syndrome. J Diabetes Investig 6: 734-738, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto Y, Yamada H, Funazaki S, Suzuki D, Kakei M, Hara K. Effect of liraglutide on Type B insulin resistance syndrome and insulin allergy in type 2 diabetes: a case report. Diabetes Ther 8: 1191-1194, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Homma M, Oka K, Niitsuma T, Itoh H. A novel 11 beta-hydroxysteroid dehydrogenase inhibitor contained in saiboku-to, a herbal remedy for steroid-dependent bronchial asthma. J Pharm Pharmacol 46: 305-309, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Homma M, Oka K, Ikeshima K, et al. Different effects of traditional Chinese medicines containing similar herbal constituents on prednisolone pharmacokinetics. J Pharm Pharmacol 47: 687-692, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Kawahito Y, Kondo M, Tsubouchi Y, et al. 15-deoxy-delta(12,14)-PGJ(2) induces synoviocyte apoptosis and suppresses adjuvant-induced arthritis in rats. J Clin Invest 106: 189-197, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flier JS, Bar RS, Muggeo M, Kahn CR, Roth J, Gorden P. The evolving clinical course of patients with insulin receptor autoantibodies: spontaneous remission or receptor proliferation with hypoglycemia. J Clin Endocrinol Metab 47: 985-995, 1978. [DOI] [PubMed] [Google Scholar]
- 22.Kato T, Itoh M, Hanashita J, et al. Severe hypoglycaemia in a person with insulin autoimmune syndrome accompanied by insulin receptor anomaly type B. Diabet Med 24: 1279-1281, 2007. [DOI] [PubMed] [Google Scholar]