Abstract
Objective
The progress of non-anticoagulated patients with atrial fibrillation (AF) undergoing hemodialysis has not been determined. Using data from the RAKUEN (Registry of Atrial fibrillation in chronic Kidney disease Under hEmodialysis from Niigata) study, we examined the clinical characteristics and outcomes among hemodialysis patients with AF who were not receiving a vitamin K antagonist (VKA).
Methods and Results
Forty-three of 423 patients undergoing hemodialysis (-10%) were prescribed a VKA. The remaining 380 patients (age 64.8±12.8 years, male 70%) were enrolled in the present study. During a mean observation period of 36 months, AF (n=55) was independently associated with all-cause death (hazard ratio, 1.82; 95% confidence interval, 1.12-2.94; p=0.014), but was not associated with ischemic stroke (hazard ratio, 1.91; 95% confidence interval, 0.74-4.92; p=0.177) and major bleeding (hazard ratio, 1.80; 95% confidence interval, 0.80-4.08; p=0.150). The crude incidence rates of all-cause death and ischemic stroke in the AF patients were 15.75 (2.5-fold higher compared to the non-AF patients) and 3.63 (1.7-fold higher compared to the non-AF patients) per 100 person-years, respectively.
Conclusion
A great impact on death, but not ischemic stroke, was observed in non-anticoagulated hemodialysis patients with AF in comparison to those without AF from the analysis of the RAKUEN study.
Keywords: anticoagulation, atrial fibrillation, hemodialysis, mortality, stroke
Introduction
Atrial fibrillation (AF) is a potent risk factor for stroke and death in the general population (1-3). Several studies have shown that AF is a common comorbidity in patients undergoing hemodialysis (4-6). However, the detailed clinical characteristics and outcomes of hemodialysis patients with AF have been unclear, and the appropriate management of such patients has not been established. Anticoagulation therapy with a vitamin K antagonist (VKA) is often indicated for the prevention of ischemic stroke in non-dialyzed AF patients (7); however, the Japanese Society for Dialysis Therapy (JSDT) guidelines suggest that a VKA should not be used in the treatment of AF without careful evaluation (8). As part of the RAKUEN (Registry of Atrial fibrillation in chronic Kidney disease Under hEmodialysis from Niigata) study, we recently reported that AF was independently associated with all-cause death and major bleeding, but not with an increased risk of ischemic stroke; notably, 33% of the study's AF patients were prescribed a VKA (6). There is an ongoing controversy as to whether AF is associated with the risk of stroke in hemodialysis patients. In the present study, we investigated the progress of AF/hemodialysis patients who were not treated with VKAs using the data from RAKUEN study.
Materials and Methods
Study population and data collection
The RAKUEN study was a single-center, retrospective observational study (UMIN-CTR No. UMIN 000026744), and the study protocol was reviewed and approved by the ethics committee of Shinrakuen Hospital (H26009). We obtained the medical records of the 423 patients who were on maintenance hemodialysis at Shinrakuen Hospital in August 2011 for baseline demographics, as described (6). We also collected the incidences of all-cause death and cardiovascular death, ischemic stroke, major bleeding (hemorrhagic stroke, gastrointestinal bleeding, and others), and heart failure among these patients from August 2011 to March 2015.
Electrocardiography was used to detect AF, and was performed every 6 months or any time that the patient had any symptoms that suggested the presence of arrhythmia, or if alterations in the rhythm were detected in the course of the dialysis session. We divided the cases of major bleeding (defined as bleeding from any site requiring hospitalization or blood transfusion) into hemorrhagic stroke, gastrointestinal bleeding, and ‘others.' Stroke was defined as the presence of a focal neurological deficit requiring hospitalization, and it was confirmed by computed tomography or magnetic resonance imaging. We calculated each patient's CHADS2 [Congestive heart failure, Hypertension, Age, Diabetes, and Stroke/transient ischemic attack (TIA)] score by assigning one point each for congestive heart failure, hypertension, age ≥75 years, and diabetes mellitus, and two points for history of stroke or TIA; the range of possible scores was thus 0 to 6 (7). We also calculated the HAS-BLED score for estimating the risk of major bleeding. The HAS-BLED score is calculated by assigning one point each for hypertension, abnormal renal function, abnormal liver function, history of stroke/TIA, history of bleeding, labile international normalized ratio, age ≥65 years, drug therapy [antiplatelet agent and/or nonsteroidal anti-inflammatory drugs (NSAIDs)], and alcohol intake; the range of possible scores is 1 to 8 (9).
Statistical analysis
Values were expressed as the mean±standard deviation (SD). Student's t-test was used for the descriptive analysis of the data. Proportions were compared using the chi-squared test. A survival analysis was conducted by comparing Kaplan-Meier curves using the log-rank test. A Cox proportional hazards regression model analysis was conducted to determine the prognostic significance of certain factors. Hazard ratios (HRs) and 95% confidence interval (CI) were also calculated. p values of <0.05 were considered to indicate statistical significance. All statistical analyses were performed using the SPSS software program [ver. 22 (for Windows), SPSS, Chicago, USA].
Results
VKAs were prescribed to 43 of the 423 patients undergoing hemodialysis (-10%). We examined the cases of the remaining 380 patients [age 64.8±12.8 years; male, n=266 (70%); female, n=114 (30%); mean duration of hemodialysis, 134±121 months].
The baseline clinical features of the patients
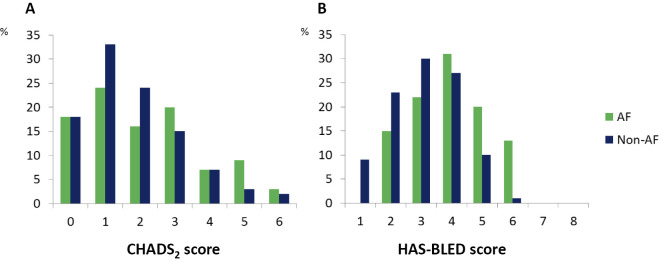
We divided the patients into an AF group (n=55) and a non-AF group (n=325). The groups' baseline characteristics are summarized in Table 1. In comparison to the non-AF group, the AF patients were significantly older (p<0.01) and had a significantly longer duration of hemodialysis (p<0.01). The AF group showed a significantly higher prevalence of ischemic stroke (22% vs. 11%, p=0.02). A tendency to have had other cardiovascular comorbidities was also observed in the AF patients, who also showed a significantly higher prevalence of structured abnormalities on echocardiography. The rate of aspirin use in the AF patients was significantly higher than that in the non-AF patients (p=0.03). Fig. 1 illustrates the distributions of the CHADS2 and HAS-BLED scores at baseline. The CHADS2 scores of the AF patients at baseline were significantly higher than those of the non-AF patients (p=0.01). The AF patients also showed significantly higher HAS-BLED scores (p<0.01); however, the rates of hemorrhagic stroke and gastrointestinal bleeding rates were similar between the two groups.
Table 1.
Baseline Characteristics of the 380 Non-anticoagulated Hemodialysis Patients in August 2011.
| Variable | AF (n=55) | Non-AF (n=325) | p | |||
|---|---|---|---|---|---|---|
| Age, yrs | 71.9±10.1 | 63.7±12.8 | <0.01 | |||
| Male | 39 | (71%) | 227 | (70%) | 0.87 | |
| BMI, kg/m2 | 21.7±4.1 | 20.6±3.7 | 0.05 | |||
| Duration of hemodialysis, mo | 187±157 | 126±113 | <0.01 | |||
| History of: | ||||||
| Coronary artery disease | 12 | (22%) | 43 | (13%) | 0.09 | |
| Congestive heart failure | 15 | (27%) | 58 | (18%) | 0.10 | |
| PCI | 9 | (16%) | 22 | (6%) | 0.01 | |
| CABG | 1 | (2%) | 5 | (1%) | 0.87 | |
| Valve surgery | 0 | 0 | ||||
| Pacemaker/ICD | 5 | (9%) | 2 | (1%) | <0.01 | |
| Ischemic stroke | 12 | (22%) | 36 | (11%) | 0.02 | |
| Peripheral artery disease | 15 | (27%) | 54 | (17%) | 0.06 | |
| Hypertension | 34 | (62%) | 231 | (71%) | 0.16 | |
| Dyslipidemia | 9 | (16%) | 80 | (25%) | 0.18 | |
| Diabetes | 14 | (26%) | 88 | (27%) | 0.80 | |
| Hemorrhagic stroke | 6 | (11%) | 21 | (7%) | 0.23 | |
| Gastrointestinal bleeding | 10 | (18%) | 35 | (11%) | 0.23 | |
| Cause of ESRD: | ||||||
| Diabetes | 14 | (26%) | 82 | (25%) | ||
| Glomerulonephritis | 21 | (38%) | 150 | (46%) | ||
| Hypertension | 4 | (7%) | 27 | (8%) | 0.64 | |
| Echocardiographic findings: | ||||||
| LVEF, % | 61±12 | 65±9 | <0.01 | |||
| LA, mm | 39±10 | 38±6 | 0.25 | |||
| LVH | 26 | (48%) | 120 | (37%) | 0.12 | |
| Laboratory data (pre-dialysis): | ||||||
| Creatinine, mg/dL | 8.6±2.9 | 11.2±4.3 | <0.01 | |||
| Hematocrit, % | 30.8±4.1 | 30.8±3.7 | 0.98 | |||
| Antithrombotic therapy | ||||||
| Aspirin | 17 | (31%) | 60 | (19%) | 0.03 | |
| Thienopyridine | 9 | (16%) | 35 | (11%) | 0.23 | |
| CHADS2score | 2.2±1.7 | 1.7±1.3 | 0.01 | |||
| HAS-BLED score | 4.0±1.2 | 3.1±1.1 | <0.01 | |||
AF: atrial fibrillation, BMI: body mass index, CABG: coronary artery bypass grafting, ESRD: end stage renal disease, ICD: implantable cardioverter defibrillator, LVDd: left ventricular diastolic dimension, LVEF: left ventricular ejection fraction, LVH: left ventricular hypertrophy, PCI: percutaneous coronary intervention
Figure 1.
The distribution of (A) CHADS2 scores and (B) HAS-BLED scores in the AF (n=55) and non-AF (n=325) hemodialysis patient groups. AF: atrial fibrillation
Mortality, stroke and major bleeding events during the study period
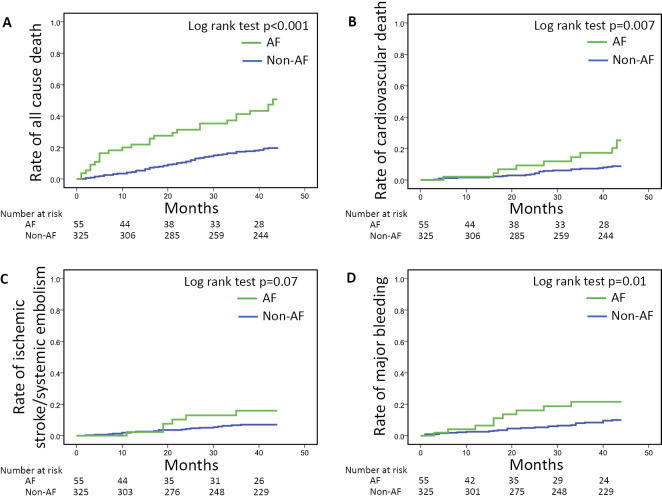
During the 36-month observation period, the Cox proportional model with adjustment for age, duration of hemodialysis, aspirin use, thienopyridine use, and left ventricular ejection fraction revealed that AF was independently associated with all-cause death (HR 1.69, 95%CI 1.04-2.74, p=0.034), but not associated with ischemic stroke or major bleeding events (Table 2). The results of the Kaplan-Meier analysis revealed that the rates of all-cause death, cardiovascular death, and major bleeding events were significantly higher in the AF patients (p<0.001, p=0.007 and p=0.01, respectively), whereas the rates of ischemic stroke in the AF and non-AF groups did not differ to a statistically significant extent (Fig. 2). The crude incidence rates of all-cause death and ischemic stroke in the AF patients were 15.75 (2.5-fold higher in comparison to non-AF patients) and 3.63 (1.7-fold higher in comparison to the non-AF patients) per 100 person-years (Table 3).
Table 2.
Outcomes Associated with AF in the 380 Non- anticoagulated Hemodialysis Patients.
| HR | 95%CI | p | |
|---|---|---|---|
| All-cause death | 1.69 | 1.04-2.74 | 0.034 |
| Cardiovascular death | 1.42 | 0.62-3.21 | 0.400 |
| Ischemic stroke/systemic embolism | 1.68 | 0.65-4.34 | 0.280 |
| Major bleeding | 2.01 | 0.86-4.69 | 0.105 |
Age, duration of hemodialysis, aspirin use, thienopyridine use, and left ventricular ejection fraction were adjusted in the Cox proportional model.
AF: atrial fibrillation, HR: hazard ratio, CI: confidence interval
Figure 2.
Kaplan-Meier curves for (A) all-cause death, (B) cardiovascular death, (C) ischemic stroke/systemic embolism, and (D) major bleeding for the AF (n=55, green) and non-AF (n=325, blue) hemodialysis patients. The rates of all-cause death, cardiovascular death, and major bleeding were significantly higher in the AF patients, whereas the rates of ischemic stroke/systemic embolism in AF and non-AF patients did not differ to a statistically significant extent. AF: atrial fibrillation
Table 3.
Crude Incident Rates for the Studied Events during 36 Months Observation.
| AF (n=55) No. of events |
Incident rate per 100 person-yrs |
Non-AF (n=325) No. of events |
Incident rate per 100 person-yrs |
|
|---|---|---|---|---|
| All-cause death | 26 | 15.75 | 62 | 6.35 |
| Cardiovascular death | 9 | 5.45 | 25 | 2.56 |
| Ischemic stroke/systemic embolism | 6 | 3.63 | 20 | 2.05 |
| Major bleeding | 9 | 5.45 | 28 | 2.87 |
| Gastrointestinal bleeding | 4 | 2.42 | 21 | 2.15 |
| Hemorrhagic stroke | 1 | 0.60 | 8 | 0.82 |
| Heart failure | 6 | 3.63 | 23 | 2.35 |
AF: atrial fibrillation
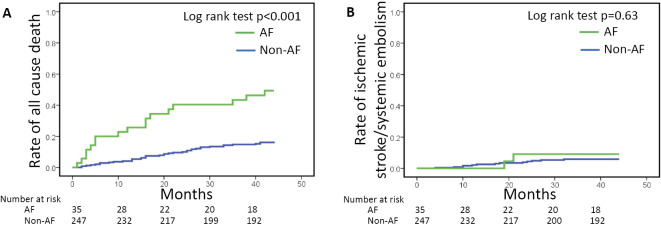
We also examined the patients who were treated without antithrombotic therapy (VKA, aspirin, and thienopyridine). AF (n=35) remained associated with all-cause death but not with an increased risk of ischemic stroke (Table 4 and Fig. 3).
Table 4.
Baseline Characteristics of the 282 Hemodialysis Patients without Antithrombotic Therapy in August 2011.
| Variable | AF (n=35) | Non-AF (n=247) | p | ||
|---|---|---|---|---|---|
| Age, yrs | 73.4±9.4 | 63.2±13.0 | <0.01 | ||
| Male | 23 | (65%) | 167 | (67%) | 0.82 |
| BMI, kg/m2 | 22.5±4.3 | 20.8±3.7 | 0.01 | ||
| Duration of hemodialysis, mo | 177±149 | 125±106 | 0.05 | ||
| History of: | |||||
| Coronary artery disease | 2 | (5%) | 8 | (3%) | 0.45 |
| Congestive heart failure | 9 | (25%) | 34 | (13%) | 0.06 |
| Ischemic stroke | 3 | (8%) | 19 | (7%) | 0.85 |
| Peripheral artery disease | 6 | (17%) | 28 | (11%) | 0.32 |
| Hypertension | 20 | (57%) | 176 | (71%) | 0.09 |
| Dyslipidemia | 6 | (17%) | 46 | (18%) | 0.83 |
| Diabetes | 8 | (22%) | 50 | (20%) | 0.72 |
| Hemorrhagic stroke | 3 | (8%) | 18 | (7%) | 0.78 |
| Gastrointestinal bleeding | 5 | (14%) | 26 | (10%) | 0.50 |
| Cause of ESRD: | |||||
| Diabetes | 7 | (20%) | 49 | (19%) | |
| Glomerulonephritis | 13 | (37%) | 126 | (51%) | |
| Hypertension | 3 | (8%) | 19 | (7%) | 0.60 |
| Echocardiographic findings: | |||||
| LVEF, % | 60±12 | 65±8 | <0.01 | ||
| LA, mm | 39±11 | 37±5 | 0.21 | ||
| LVH | 15 | (42%) | 87 | (35%) | 0.38 |
| Laboratory data (pre-dialysis): | |||||
| Creatinine, mg/dL | 8.6±2.9 | 11.4±4.6 | <0.01 | ||
| Hematocrit, % | 30.3±4.1 | 30.5±3.7 | 0.71 | ||
AF: atrial fibrillation, BMI: body mass index, ESRD: end stage renal disease, LVEF: left ventricular ejection fraction, LVH: left ventricular hypertrophy
Figure 3.
Kaplan-Meier curves for (A) all-cause death, and (B) ischemic stroke/systemic embolism for 282 hemodialysis patients who did not receive antithrombotic therapy. The rate of all-cause death was significantly higher in the AF patients (n=35, green), whereas the rate of ischemic stroke/systemic embolism in the AF and non-AF patients (n=247, blue) did not differ to a statistically significant extent. AF: atrial fibrillation
Discussion
AF is one of the most important causes of ischemic stroke in the general population (1). In the Framingham Heart Study, AF patients were found to have a higher risk of stroke in comparison to non-AF patients (2). The data obtained in several clinical trials supported the use of VKAs and direct oral anticoagulants to prevent stroke in AF patients (1, 10). Anticoagulation therapy based on a patient's CHADS2 score is widely accepted for the prevention of stroke in the majority of AF patients (7); however, the JSDT guidelines suggest that a VKA should not be used in the treatment of AF without careful evaluation (8). To date, there are two unsolved problems regarding the management of AF in hemodialysis patients: [1] whether AF is associated with the risk of stroke, and [2] whether VKA treatment can effectively prevent ischemic stroke. We investigated problem [1] using the data from the RAKUEN study. Our analysis revealed that AF was independently associated with all-cause death but not with an increased risk of ischemic stroke in non-anticoagulated hemodialysis patients. In Japan, where racial differences in mortality and morbidity have been observed in comparison to Western countries (10), there is a lack of data regarding AF in hemodialysis patients without VKA treatment; this situation makes our present findings especially helpful.
In a systematic review, the incidence of ischemic stroke in AF patients undergoing hemodialysis was 5.2 per 100 person-years (11). Our present study showed that the incidence of ischemic stroke/systemic embolism in the non-anticoagulated AF patients was 3.63 per 100 person-years. A large population-based Canadian retrospective cohort study revealed similar results in non-anticoagulated AF patients (12). In that study, the crude incidence rate of stroke in non-anticoagulated AF patients on dialysis was 2.91 per 100 person-years. Our AF patients had a 1.7-fold higher risk of ischemic stroke in comparison to the non-AF patients; this difference became nonsignificant in the Cox proportional hazards regression model analysis. The risk ratio for stroke in non-dialysis AF patients ranged from 4 to 8 (10). Hemodialysis patients may be protected from AF-related ischemic stroke by routine heparin infusion during dialysis sessions.
On the other hand, our present study also revealed a higher risk of death in non-anticoagulated AF patients (15.75 per 100 person-years, 2.5-fold higher in comparison to non-AF patients). It has been reported that the incidence of death in non-dialysis patients with AF was approximately 1.0% per year in Japan and 5.3% per year in Europe (10, 13). Previous reports showed that the mortality rates in the first and second year of follow-up were 38% and 53% in an AF group and 14% and 31% in a sinus rhythm group, respectively (14). Cardiovascular death was the leading cause of death in the present study. Several other cardiovascular comorbidities were observed in our AF patients undergoing hemodialysis (6), and these comorbidities may have contributed to their higher mortality rate.
In the Framingham Heart Study, AF was associated with an approximately 1.5- to 2.0-fold higher mortality risk and a 3- to 5-fold higher stroke risk in comparison to non-AF patients (2, 3). The risk of ischemic stroke is higher than that of death in non-dialyzed AF patients. In our study, however, AF had less impact on the stroke risk in hemodialysis patients in comparison to non-dialyzed patients. In another Danish population-based study, the death rate was over 5-fold higher than the stroke rate in hemodialysis patients with AF (15). A study using the records of the U.S. Renal Data System from 1995 to 2007 indicated that >60% of the AF patients on dialysis died before the occurrence of stroke (16). In the above-cited Taiwanese population-based study, the risk of stroke was only modestly higher in the hemodialysis patients with AF in comparison to those without AF. This difference became nonsignificant after considering in-hospital death as a competing risk (17). These findings are similar to the findings of our study of Japanese hemodialysis patients, and suggest that the risk of death surpasses the risk of ischemic stroke in AF patients undergoing hemodialysis.
Study limitations
The present study is associated with several limitations. Our patient series was relatively small, the study was performed in a single center, and the study design was retrospective. In addition, as in any observational study, the associations found may not be causal, and the possibility of bias due to unmeasured factors cannot be excluded. Moreover, the decision to use anticoagulation therapy is affected by each clinician's perceived risk of thromboembolism and bleeding. Further large prospective studies are needed to identify the risks of death and ischemic stroke in hemodialysis patients with AF.
Conclusion
In the present study, AF had less impact on the risk of ischemic stroke in non-anticoagulated patients undergoing hemodialysis over a mean observation period of 36 months. In addition, the risk of death was higher than that of ischemic stroke. We should be aware of the increased risks of not only stroke but also death in the management of non-anticoagulated AF patients.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Hart RG, Halperin JL. Atrial fibrillation and thromboembolism: a decade of progress in stroke prevention. Ann Intern Med 131: 688-695, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22: 983-988, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 98: 946-952, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Stack AG, Bloembergen WE. Prevalence and clinical correlates of coronary artery disease among new dialysis patients in the United States: a cross-sectional study. J Am Soc Nephrol 12: 1516-1523, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Winkelmayer WC, Patrick AR, Liu J, Brookhart MA, Setoguchi S. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol 22: 349-357, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitsuma W, Matsubara T, Hatada K, et al. . Clinical characteristics of hemodialysis patients with atrial fibrillation: The RAKUEN (Registry of atrial fibrillation in chronic kidney disease under hemodialysis from Niigata) study. J Cardiol 68: 148-155, 2016. [DOI] [PubMed] [Google Scholar]
- 7.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 137: 263-272, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Hirakata H, Nitta K, Inaba M, et al. . Japanese Society for Dialysis Therapy guidelines for management of cardiovascular diseases in patients on chronic hemodialysis. Ther Apher Dial 16: 387-435, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 138: 1093-1100, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita T. Recent mortality and morbidity rates of Japanese atrial fibrillation patients: racial differences and risk stratification. Circ J 77: 864-868, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman D, Sood MM, Rigatto C, Holden RM, Hiremath S, Clase CM. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol Dial Transplant 27: 3816-3822, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Shah M, Avgil Tsadok M, Jackevicius CA, et al. . Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation 129: 1196-1203, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Andersson T, Magnuson A, Bryngelsson IL, et al. . All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995-2008: a Swedish nationwide long-term case-control study. Eur Heart J 34: 1061-1067, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vázquez-Ruiz de Castroviejoa E, Sánchez-Perales C, Lozano-Cabezas C, et al. . Incidence of atrial fibrillation in hemodialysis patients. A prospective long-term follow-up study. Rev Esp Cardiol 59: 779-784, 2006. [PubMed] [Google Scholar]
- 15.Olesen JB, Lip GY, Kamper AL, et al. . Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 367: 625-635, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein BA, Arce CM, Hlatky MA, Turakhia M, Setoguchi S, Winkelmayer WC. Trends in the incidence of atrial fibrillation in older patients initiating dialysis in the United States. Circulation 126: 2293-2301, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih CJ, Ou SM, Chao PW, et al. . Risks of death and stroke in patients undergoing hemodialysis with new-onset atrial fibrillation: a competing-risk analysis of a nationwide cohort. Circulation 133: 265-272, 2016. [DOI] [PubMed] [Google Scholar]