Abstract
A 50-year-old man who presented with a fever and epigastralgia was diagnosed to have esophageal carcinoma which was identified as poorly differentiated adenocarcinoma producing alpha-fetoprotein (AFP) with Barrett's esophagus. Computed tomography revealed multiple liver metastases and lymph node metastases surrounding the stomach. We first performed chemotherapy for the systemic lesions and proton beam therapy for the local control of lesions without complete remission and we were able to successfully control the frequently recurring lesions by proton beam therapy, cryotherapy and chemotherapy. A complete response has been maintained for 16 months and the overall survival time is 4 years and 2 months. Proton beam therapy for primary esophageal cancer and metastatic lesions was thus found to be an effective therapeutic option for such cases.
Keywords: alpha-fetoprotein, Barrett's cancer, proton beam therapy
Introduction
Esophageal cancer is a malignant tumor that is the sixth most common cause of cancer death worldwide, with occurrence rates varying greatly by geographic location (1). At diagnosis, approximately 50% of patients with esophageal cancer will have metastatic disease and thus will be candidates for palliative therapy (2). In particular, the prognoses of patients with liver metastases are extremely poor and it is difficult to successfully treat such cases.
Alpha-fetoprotein (AFP) is a glycoprotein normally produced by the fetal liver and yolk sac, beginning the sixth week of gestation. Although elevated at birth, the AFP level decreases to the normal adult range of 10-15 ng/mL over the first year of life. Tissues may regain the ability to produce AFP if they undergo malignant transformation AFP-producing gastric cancer represents 1-6 per cent of all gastric cancers (3), and it is associated with a poor prognosis because of high rates of liver and lymph node metastasis (4). AFP-producing esophageal adenocarcinoma is a rare occurrence (5). To our knowledge, our case is only the 17th case of AFP-producing esophageal adenocarcinoma to be reported in the literature. There have been few reports describing a long survival in patients with liver metastasis of AFP-producing esophago-gastric adenocarcinoma.
Proton beam therapy (PBT) is a promising modality for the management of thoracic malignancies, because protons have excellent dose localization according to the Bragg peak compared with photons, and are biologically equivalent to conventional X-ray treatment for malignancies. The use of protons to treat esophageal cancer has been reported as definitive treatment for locoregional esophageal squamous cell carcinoma (6, 7). However, there have been few reports regarding the treatment of liver metastasis from esophageal cancer (8).
Case Report
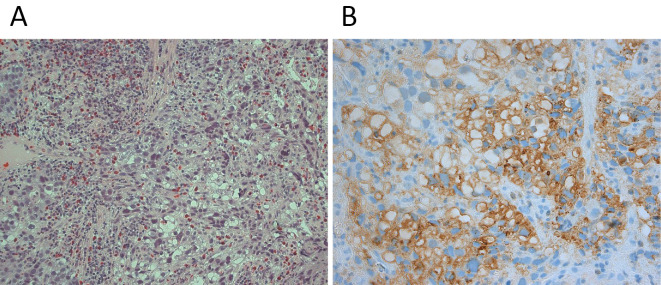
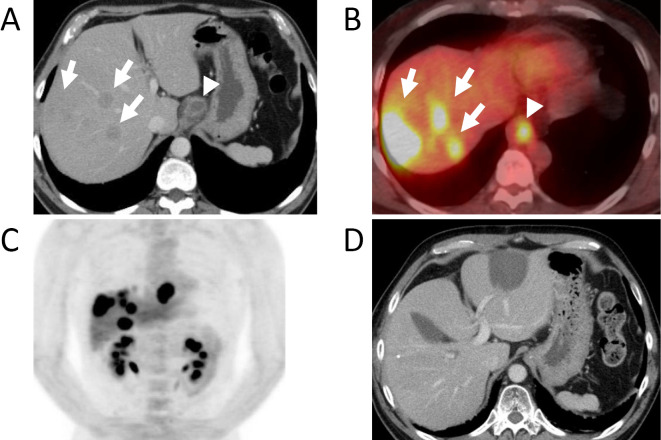
A 50-year-old man, with no cancer-related past history, was admitted to our hospital. He had been medicated for reflux esophagitis, hyperlipidemia, and diabetes mellitus. He had consumed a moderate amount of alcohol every day for 20 years and smoked approximately 15 cigarettes every day for 17 years. He had undergone regular check-ups by endoscopic examination for reflux esophagitis. Last year, he was diagnosed to have reflux esophagitis with Barrett's esophagus and an inflammatory polyp was observed. His chief complaints were fever and epigastralgia. He first presented with these complaints in February 2013. He saw his primary care doctor and underwent ultrasonography (US) the next day. US showed multiple low echoic lesions in his liver. He was referred to our hospital for a detailed examination. Serum tumor marker studies showed a clear elevation in AFP to 1,728 ng/mL, an elevated carcinoembryonic antigen (CEA) value of 20.0 ng/mL and normal carbohydrate-19-9 antigen levels of 13 U/mL, respectively. An endoscopic examination revealed a 9 cm long lesion with a type 2 esophageal cancer with a long segment Barrett's esophagus with a 7 cm long Barrett's epithelium (Fig. 1). On biopsy, the lesion was diagnosed to be poorly differentiated adenocarcinoma (Fig. 2A) and the background epithelium was identified as demonstrating a specialized columnar epithelium. Immunohistochemical staining for AFP showed positive granules in the cytoplasm, mainly in the medullary growing lesions (Fig. 2B). Immunohistochemical staining for human epidermal growth factor receptor-related 2 (HER2) was negative. Computed tomography (CT) and 18-Fluorodeoxyglucose-Positron emission tomography (FDG-PET) revealed a mass in the lower esophagus, multiple liver metastases and lymph node metastases surrounding the stomach (Fig. 3). We diagnosed Barrett's esophageal cancer Siewert type 1, cT3N1M1(Liver) cStage IV.
Figure 1.
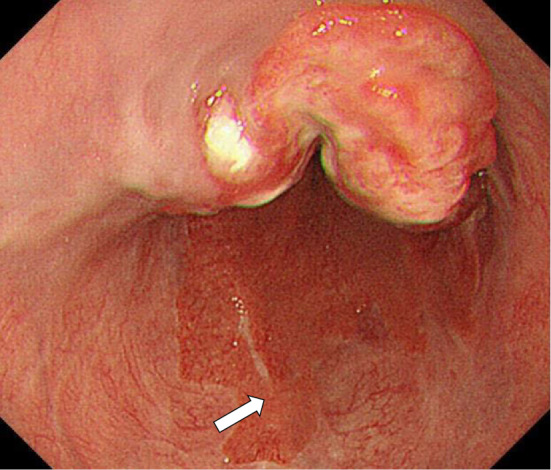
Upper endoscopy revealing a type 2 tumor in the lower third of the esophagus at a site 30-39 cm from the incisors. The white arrow indicates Barrett epithelium.
Figure 2.
A: A forceps biopsy of the esophageal tumor revealing poorly differentiated adenocarcinoma. B: Granules in the cytoplasm, mainly in the medullary growing lesions stained positive for alfa fetoprotein by immunohistochemistry.
Figure 3.
A: Computed tomography (CT) of the abdomen revealing multiple masses (white arrows) throughout the liver consistent with metastatic disease and abnormal thickening in the esophagus (white arrow head). B: 18F-2-fluoro-2-deoxy-d-glucose positron emission tomography CT (FDG-PET CT) revealing a high FDG uptake in the liver masses (white arrows) and abdominal esophagus (white arrow head). C: Coronal view FDG-PET revealing a high FDG uptake in the liver masses and abdominal esophagus. D: CT of abdomen revealing remission of multiple liver metastases after chemotherapy, proton beam therapy and transcatheter arterial chemoembolization + cryoablation.
The patient was given two courses of chemotherapy with S-1 120 mg/day for 21 days and cisplatin (CDDP) 150 mg on the 8th day. The serum AFP levels responded well to chemotherapy. His primary lesion and liver metastatic nests both decreased in size and a partial response was identified according to the Response Evaluation Criteria In Solid Tumors (RECIST) ver1.0 at 2 courses of the regimen. Unfortunately, after 4 courses of treatment the lesions of liver metastasis increased in size. As a result, we held discussions in order to select the next treatment strategy. The patient next underwent chemotherapy using irinotecan (CPT-11), but the response was poor. Then, he underwent PBT with concurrent chemotherapy using paclitaxel (PTX). Radiation was then administered for the primary lesion, cardiac lymph node metastasis and three liver metastases in the right lobe. The dose of PBT was 60 Gy (RBE)/30fractions/49days for the primary lesion and cardiac lymph node metastasis and 72.6 Gy(RBE)/22fractions/35days for the liver metastases.
After proton beam therapy, we performed chemotherapy as a systemic therapy. We selected the FLEP regimen (5-fluorouracil; 5-FU, leucovorin; LV, etoposide; ETP and CDDP) then. New lesion of liver metastasis and lymph node metastasis on the anterior surface of the pancreatic head appeared while the patient received chemotherapy. The patient underwent transcatheter arterial chemoembolization (TACE) and percutaneous cryoablation for the new lesion of liver metastasis. In addition, he also underwent PBT for new lesion of lymph node metastasis. The dose of PBT was 52 Gy (RBE)/26fractions/42days. We continued the FLEP therapy after treatment. After 5 courses of FLEP, he underwent chemotherapy using FLEP without CDDP due to a decline in his renal function. We finally stopped chemotherapy, because we evaluated complete response (CR) at 18 months after beginning the first treatment. However, left supraclavicular lymph node metastasis appeared on FDG-PET 2 months later, he underwent PBT again for left supraclavicular lymph node metastasis with ETP only. The dose of PBT was 60 Gy (RBE)/30fractions/51days. After PBT, the patient received adjuvant chemotherapy (ETP) in our hospital. Right lung metastasis in S3 and right tracheobronchial lymph node metastasis were detected by FDG-PET 32 months after beginning the first treatment. Then, he underwent PBT to treat lung metastasis and right tracheobronchial lymph node metastasis with capecitabine (2,000 mg/m2/day days1-14) and oxaliplatin (130 mg/m2 day 1). The dose of PBT was 57.5 Gy (RBE)/25fractions/42days to lung metastasis and 50 Gy (RBE)/25fractions/42days to right tracheobronchial lymph node metastasis. After PBT, we continued to administer capecitabine and oxaliplatin therapy (CapeOx) for 9 courses. The CR has been maintained for 16 months and the overall survival time is 4 years and 2 months. The patient is currently alive without cancer or any treatment related complications. His AFP level is checked every month and CT or FDG-PET is performed once every three months.
Discussion
The incidence of adenocarcinoma of the esophagus, including the esophagogastric junction, has been increasing rapidly since 1960s in the United States as well as in several Western European countries (9). The risk factors for esophageal adenocarcinoma including Barrett's esophageal cancer have been reported as follows: gastroesophageal reflux disease, obesity and cigarette smoking (10, 11). Whereas infection with Helicobacter pylori and use of non-steroidal anti-inflammatory drugs are associated with a reduced risk, low intakes of fruit, vegetables, and cereal fibers seem to increase the risk of esophageal adenocarcinoma (10). Our case had also a smoking habit, reflux esophagitis, obesity and no infection of Helicobacter pylori.
AFP producing gastric cancer has been reported to have a poor prognosis and high liver metastatic potential in many studies. To our knowledge, there have only been 17 reports about AFP producing esophageal cancer (5, 12-28). Most cases described in these reports were advanced cases with liver metastasis. The optimal treatment strategy of AFP producing esophageal cancer remains controversial. We first selected chemotherapy as systemic therapy. The selected chemotherapy regimen was combination therapy of S-1 and CDDP which is generally acknowledged to be the standard therapy in Japan (29). The treatment effect was high and we planned to perform surgery at a later for local control. However, both liver metastasis and lymph node metastasis grew rapidly during the one-month period that we evaluated the patient and determined the treatment plan.
We therefore abandoned surgery since our case was no longer indicated for surgery, and we instead selected proton beam therapy to treat the primary lesion, lymph node metastasis and liver metastasis. After proton beam therapy, we continued to administer chemotherapy as systemic therapy. We selected FLEP chemotherapy, because this regimen has been reported to achieve a better effect in patients with AFP producing gastric cancer than in those with non-AFP-producing gastric cancer (30). Kochi reported the response rate, conversion rate, disease free and overall survival to all be superior in patients with AFP producing stage IV inoperable gastric cancer than in those with AFP non-producing cancer (30). Mochizuki et al. also reported the overall response rate to be 40.8% (20/49 patients) and the median survival time was 12.6 months in a phase II study for patients with advanced gastric cancer that was considered to be incurable (31).
Table shows a summary of the AFP-producing esophageal adenocarcinoma cases. These cases show a high liver metastatic potential and a poor prognosis (Table). The majority of cases were treated with a combination of surgery and a chemotherapy regimen involving fluorouracil-related and platinum-based agents. Only four of the reported cases were still alive at the time of the last follow-up. Our case has the longest survival time among the reported patients with liver metastasis and is therefore regarded as being a successful case. About half of all cases had Barrett's esophageal cancer.
Table.
Summary of AFP-producing Esophageal Adenocarcinomas.
| Reference | Year | Age | Sex | Location | Pathology | Liver metastasis | Barrett’s Esophagus | Treatment | Protocol | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|
| 12) | 1993 | 80 | male | middle | AFP-producing | yes | no | paliative surgery+ chemotherapy | Tegafur+ Lentinan | 4mo. Died |
| 18) | 1995 | 80 | female | lower | hepatoid | yes | yes | chemotherapy | Bleomycin | 2mo. Died |
| 28) | 1996 | 53 | male | lower | hepatoid | yes | yes(LSBE) | surgery+ chemotherapy | S-1+CDDP | 36mo. Alive |
| 15) | 1999 | 59 | male | lower | AFP-producing | yes | yes | chemotherapy | 5-FU+CDDP | 2mo. Died |
| 19) | 2001 | 51 | male | lower | AFP-producing | no | no | surgery+ chemotherapy | 5-FU+CDDP | 67mo. Died |
| 20) | 2002 | 44 | female | lower | hepatoid | yes | yes | surgery+ chemotherapy | N/A | 6mo. Died |
| 14) | 2003 | 69 | male | lower | AFP-producing adenosqumous | no | N/A | surgery+ chemotherapy | 5-FU+CDDP | 6mo. Alive |
| 13) | 2005 | 47 | male | lower | hepatoid | yes | yes(SSBE) | surgery+ chemotherapy | TXL+CDDP | 14mo. Died |
| 21) | 2005 | 55 | male | lower | hepatoid | no | no | surgery+ chemotherapy | UFTE+CDDP | 9mo. |
| 22) | 2008 | 56 | male | lower | hepatoid | yes | no | N/A | N/A | N/A |
| 26) | 2009 | 49 | male | lower | AFP-producing | no | yes(SSBE) | surgery+ chemotherapy | 5-FU+CDDP+ MTX | 96mo. Alive |
| 23) | 2011 | 76 | male | lower | hepatoid | no | yes | surgery | - | 3mo. Alive |
| 24) | 2012 | 58 | male | lower | hepatoid | no | yes | surgery+ chemotherapy | S-1 | 22mo. Alive |
| 16) | 2013 | 45 | male | lower | AFP-producing | no | no | surgery+ chemotherapy | 5-FU+L-OHP | 19mo. Died |
| 27) | 2014 | 62 | male | lower | hepatoid | yes | no | surgery+ chemotherapy | S-1+CDDP | 25mo. Alive |
| 25) | 2015 | 83 | male | lower | hepatoid | yes | yes | none | - | 4mo. Died |
| 5) | 2017 | 51 | male | lower | AFP-producing | yes | no | paliative stent | FOLFOX/ Herceptin | 2mo. Died |
| Our case | 50 | male | lower | AFP-producing | yes | yes(SSBE) | PBT+ chemotherapy | S-1+CDDP, FLEP, CapeOx | 50mo. Alive |
SSBE: short segment Barrett’s esophagus, N/A: not available, AFP: alpha-fetoprotein, 5-FU: 5-fluorouracil, CDDP: cisplatin, UFT: Tegafur/Uracil, L-OHP: oxaliplatin, FOLFOX: 5-FU+Leucovorin+oxaliplatin, FLEP: 5-FU+Leucovorin+Etoposide, CapeOx: capecitabine+oxaliplatin
In general, we choose chemotherapy for systemic disease with distant metastases. However, we usually cannot control tumor growth by chemotherapy alone. This case is therefore considered to be a successful case of multimodality therapy since we administered PBT, chemotherapy and percutaneous cryoablation.
Nagai et al. reported a case of AFP-producing esophagogastric junction cancer with liver metastases, which demonstrated a long term survival for about 2 years after surgery after being treated by both surgery and chemotherapy (17). The treatment strategy for high-grade AFP-producing esophageal cancer needs both systematic chemotherapy and local control treatment, such as surgery or radiation.
Concurrent chemoradiotherapy is considered to be beneficial regarding the prognosis, mortality and quality of life after treatment (32, 33). A reduction of the irradiation doses to the organ at risk is a simple and effective method to reduce the rate of adverse events. In this context, PBT may provide therapeutic advantages over conformal X-ray therapy for patients with esophageal cancer (6, 7, 34). PBT achieves a better distribution of the biologically effective dose for many sites than is feasible by high technology X-ray therapy. Charged particles, such as protons and carbon-ions, offer advantageous physical properties in comparison to radiation therapy for the treatment of various cancers when compared with photons, because they exhibit a spread-out Bragg peak, and may be utilized to achieve a desirable dose distribution to the target volume by using specified beam modulation (35). Because this treatment is effective for localized target lesions, it thus causes less damage to normal tissue around lesion. In addition, PBT is an attractive radiation therapy option, especially for locally advanced deep lesions, such as for esophageal cancer and solitary liver metastasis. Muroi et al. reported the first case with liver metastasis from esophageal carcinoma that underwent PBT (8). Their case achieved a CR owing to the administration of PBT and chemotherapy. In our case, the patient underwent chemotherapy, PBT and concurrent chemotherapy and cryotherapy for metastatic lesions. PBT is one of the best treatment tools to control local lesions. The treatment strategy using multiple procedures thus made it possible to control both local lesions and systemic disease.
We performed CT guided percutaneous cryoablation for one site of solitary liver metastasis. Cryoablation has been reported to be effective for the treatment of the liver, adrenal, lung, bone and soft tissue (36, 37). Compared with radiofrequency ablation, cryoablation has the following advantages:
A frozen area of cryoablation appears as a well-defined low-density area on CT images, which enables real-time monitoring of the treated area; it is less painful, and the procedure can be performed with local anesthesia; and multiple needles can be used simultaneously, which enables the creation of a larger frozen area by cryoablation (38). One disadvantage of this combination therapy is its high cost (37). We selected this procedure for these reasons and to also prevent the occurrence of collateral organ damage.
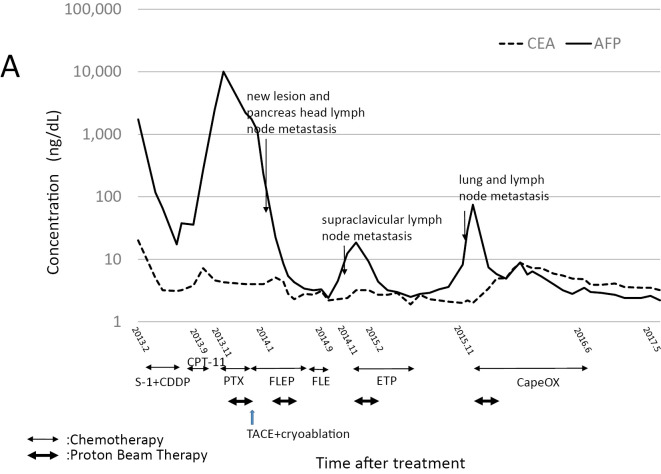
Serial measurement of the serum AFP levels in AFP-producing esophageal adenocarcinoma may be useful for monitoring the tumor status and the response to treatment. In our case, the serum AFP levels reflected tumor growth, the occurrence of new metastases and the response to treatment (Fig. 4). Some other reports have reported a relationship to exist between the serum AFP level and tumor progression or the treatment effect (5, 13, 19).
Figure 4.
Changes in the serum concentrations of AFP, CEA and the treatment course. AFP: alpha-fetoprotein, CEA: carcinoembryonic antigen, PBT: proton beam therapy, CDDP: cisplatin, PTX: paclitaxel, CPT-11: irinotecan, FLEP: 5-fluorouracil + leucovorin + etoposide + CDDP, EP: etoposide, CapeOx: capecitabine and oxaliplatin therapy, TACE: transcatheter arterial chemoembolization
In conclusion, the serial measurement of the serum AFP level may be useful for monitoring the clinical status and treatment response in AFP producing esophageal adenocarcinoma. PBT for primary esophageal cancer and metastatic lesions is therefore considered to be an effective therapeutic option.
This report was approved by the institutional review board of our hospital, patient provided written informed consent before report.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported in part by the following grants and foundations: Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS); grant numbers 16K11339, 16K10450, 15H04932, 16K15606, 17K10534 0006, 17K16529 0007 and 17K14982 0001
Acknowledgement
We thank Ms. Tomoko Yano, Ms. Sayaka Okada, Ms. Tomoko Ubukata, Ms. Yuka Matsui, and Ms. Yukie Saito for their excellent assistance.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 61: 69-90, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Enzinger PC, Ilson DH, Kelsen DP. Chemotherapy in esophageal cancer. Semin Oncol 26: 12-20, 1999. [PubMed] [Google Scholar]
- 3.Okita K, Noda K, Kodama T, et al. Carcino-fetal proteins and gastric cancer: the site of alpha-fetoprotein synthesis in gastric cancer. Gastroenterol Jpn 12: 400-406, 1977. [PubMed] [Google Scholar]
- 4.Kono K, Amemiya H, Sekikawa T, et al. Clinicopathologic features of gastric cancers producing alpha-fetoprotein. Dig Surg 19: 359-365; discussion 365-, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Liu W, Parikh K, Post AB. Alpha-fetoprotein-producing esophageal adenocarcinoma: a mimicker of hepatocellular carcinoma. Clin J Gastroenterol 10: 7-12, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa H, Hashimoto T, Moriwaki T, et al. Proton beam therapy combined with concurrent chemotherapy for esophageal cancer. Anticancer Res 35: 1757-1762, 2015. [PubMed] [Google Scholar]
- 7.Lin SH, Komaki R, Liao Z, et al. Proton beam therapy and concurrent chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 83: e345-e351, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muroi H, Nakajima M, Satomura H, et al. Effectiveness of proton beam therapy on liver metastases of esophageal cancer: report of a case. Int Surg 100: 180-184, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown LM, Silverman DT, Pottern LM, et al. Adenocarcinoma of the esophagus and esophagogastric junction in white men in the United States: alcohol, tobacco, and socioeconomic factors. Cancer Causes Control 5: 333-340, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Pera M, Manterola C, Vidal O, Grande L. Epidemiology of esophageal adenocarcinoma. J Surg Oncol 92: 151-159, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki T, Kitagawa Y, Kuwano H, et al. Decreased risk of esophageal cancer owing to cigarette and alcohol cessation in smokers and drinkers: a systematic review and meta-analysis. Esophagus 14: 290-302, 2017. [Google Scholar]
- 12.Sawada H, Watanabe A, Yamada Y, Yano T, Nakano H, Konishi Y. Alpha-fetoprotein-producing esophageal adenocarcinoma: report of a case. Surg Today 23: 1103-1107, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Chiba N, Yoshioka T, Sakayori M, et al. AFP-producing hepatoid adenocarcinoma in association with Barrett's esophagus with multiple liver metastasis responding to paclitaxel/CDDP: a case report. Anticancer Res 25: 2965-2968, 2005. [PubMed] [Google Scholar]
- 14.Kawai H, Sekine S, Sanada T, Andoh T, Takechi Y, Okada S. Alpha-fetoprotein-producing esophageal carcinoma: a case report. Anticancer Res 23: 3837-3840, 2003. [PubMed] [Google Scholar]
- 15.Shimakawa T, Ogawa K, Naritaka Y, et al. Alpha-fetoprotein producing Barrett's esophageal adenocarcinoma: a case report. Anticancer Res 19: 4369-4373, 1999. [PubMed] [Google Scholar]
- 16.Chen YY, Hsu WH, Hu HM, Wu DC, Lin WY. A case of alpha-fetoprotein-producing esophageal adenocarcinoma. Kaohsiung J Med Sci 29: 106-110, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Nagai Y, Kato T, Harano M, et al. [A case of AFP-producing esophagogastric junction cancer with liver metastases with a good response to chemotherapy]. Gan to kagaku ryoho 41: 2349-2351, 2014(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 18.Motoyama T, Higuchi M, Taguchi J. Combined choriocarcinoma, hepatoid adenocarcinoma, small cell carcinoma and tubular adenocarcinoma in the oesophagus. Virchows Archiv 427: 451-454, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi N, Ohbu M, Kuroyama S, et al. Alpha-Fetoprotein-producing esophageal adenocarcinoma: report of a case. Surg today 31: 915-919, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Tanigawa H, Kida Y, Kuwao S, et al. Hepatoid adenocarcinoma in Barrett's esophagus associated with achalasia: first case report. Pathol Int 52: 141-146, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Fukuzawa J, Terashima H, Nakano Y, et al. Alpha-fetoprotein producing adenocarcinoma of esophagogastric junction: a case report. Jpn J Gastroenterol Surg 38: 401-405, 2005(in Japanese, Abstract in English). [Google Scholar]
- 22.Atiq M, Husain M, Dang S, Olden KW, Malik AH, Brown DK. Hepatoid esophageal carcinoma: a rare cause of elevated alpha fetoprotein. J Gastrointest Cancer 39: 58-60, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Kuroda N, Onishi K, Lee GH. Combined tubular adenocarcinoma and hepatoid adenocarcinoma arising in Barrett esophagus. Annals of diagnostic pathology 15: 450-453, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Takeyama D, Miyata G, Onodera K, et al. A case of alphafetoprotein-producing barrett esophageal carcinoma with different histological patterns. Jpn J Gastroenterol Surg 45: 834-841, 2012(in Japanese). [Google Scholar]
- 25.Kashani A, Ellis JC, Kahn M, Jamil LH. Liver metastasis from hepatoid adenocarcinoma of the esophagus mimicking hepatocellular carcinoma. Gastroenterology report 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akashi Y, Izumi Y, Kato T, Miura A. A long-term survival case of alpha-fetoprotein producing barrett esophageal adenocarcinoma. Jpn J Gastroenterol Surg 42: 355-361, 2009(in Japanese, Abstract in English). [Google Scholar]
- 27.Nagai Y, Kato T, Harano M, et al. A case of AFP-producing esophagogastric junction cancer with liver metastases with a good response to chemotherapy. Gan To Kagaku Ryoho 41: 2349-2351, 2014(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 28.Inoue K, Unemura Y, Doi N, Takemura T. A case of α-fetoprotein producing Barrett Adenocarcinoma of the esophagus. Journal of Japanese Society for Clinical Surgery 57: 1622-1625, 1996(in Japanese, Abstract in English). [Google Scholar]
- 29.Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9: 215-221, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Kochi M, Fujii M, Kaiga T, et al. FLEP chemotherapy for alpha-fetoprotein-producing gastric cancer. Oncology 66: 445-449, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Mochizuki F, Fujii M, Kasakura Y, et al. Combination chemotherapy comprising 5-fluorouracil, leucovorin, etoposide, and cis-diamminedichloroplatinum for the treatment of advanced gastric cancer. J Cancer Res Clin Oncol 128: 493-496, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 281: 1623-1627, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 326: 1593-1598, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Makishima H, Ishikawa H, Terunuma T, et al. Comparison of adverse effects of proton and X-ray chemoradiotherapy for esophageal cancer using an adaptive dose-volume histogram analysis. J Radiat Res 56: 568-576, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakurai H, Ishikawa H, Okumura T. Proton beam therapy in Japan: current and future status. Jpn J Clin Oncol 46: 885-892, 2016. [DOI] [PubMed] [Google Scholar]
- 36.Shafir M, Shapiro R, Sung M, Warner R, Sicular A, Klipfel A. Cryoablation of unresectable malignant liver tumors. Am J Surg 171: 27-31, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki M, Iguchi T, Takaki H, et al. Ablation protocols and ancillary procedures in tumor ablation therapy: consensus from Japanese experts. Jpn J Radiol 34: 647-656, 2016. [DOI] [PubMed] [Google Scholar]
- 38.Kumasaka S, Miyazaki M, Tsushima Y. CT-guided percutaneous cryoablation of an aggressive osteoblastoma: a case report. J Vasc Interv Radiol 26: 1746-1748, 2015. [DOI] [PubMed] [Google Scholar]