Abstract
We describe a rare case of Propionibacterium acnes native-valve endocarditis that silently progressed in a 67-year-old man with hybrid dialysis. The patient was scheduled for kidney transplantation, and pre-operative investigation incidentally detected a vegetative structure at his native mitral valve that had increased in size. He underwent cardiac surgery and P. acnes was detected in cultures of a resected cardiac valve specimen and blood. This case highlights that P. acnes can silently cause infective endocarditis in a native-valve, and that physicians should consider the possibility of infection when P. acnes is isolated in blood cultures.
Keywords: anaerobe, hemodialysis, infective endocarditis, native valve, Propionibacterium acnes
Introduction
Propionibacterium acnes is a facultative anaerobic, non-spore-forming, slow-growing Gram-positive rod bacterium that is a main constituent of the normal flora of the human skin (1). The isolation of P. acnes in blood cultures is considered to be clinically insignificant and is disregarded as a skin flora contaminant in most cases (2). According to a previous large single-center study, only 18 of 522 (3.5%) cases of P. acnes bacteremia were found to be true infections (3). However, it has the potential to cause fatal infection involving the central nervous system, spine, and surgical sites (4).
Several studies focusing on the clinical significance of this organism have described its relationship with infective endocarditis (IE) (5-8). In a case series of 58 patients with Propionibacterium endocarditis, 79% of the patients had prosthetic cardiac devices (5). One of two recent single-centered retrospective studies reported that all 13 cases of P. acnes endocarditis occurred in patients after valve replacement surgery (6); the other reported that 23 of 24 cases (95.8%) involved either a prosthetic valve or annuloplasty ring (7). In addition, the most recent multi-center study in Spain reported that P. acnes accounted for only 14 of approximately 2,500 (0.56%) IE cases, of which 12 (85.7%) cases occurred in patients with prosthetic devices (8). Native valve involvement of the pathogen is thus rare and its clinical characteristics remain unknown. We herein describe a rare case of P. acnes endocarditis that progressed completely silently at the native mitral valve of a patient who was receiving hybrid dialysis.
Case Report
A 67-year-old man with a past history of chronic kidney disease was referred to our hospital for living donor kidney transplantation. He had received hybrid dialysis, a combination of peritoneal dialysis and hemodialysis, for the two previous years due to chronic kidney disease. The patient had never had abdominal symptoms or turbid peritoneal fluid suggesting peritonitis. He was not taking any immunosuppressive medicines. He had undergone echocardiography three years previously, which revealed moderate mitral regurgitation. Echocardiography, which was performed as a routine examination before transplantation surgery incidentally revealed a movable vegetative structure on his mitral valve (3×8 mm), and the patient referred to a cardiac surgeon for further investigation.
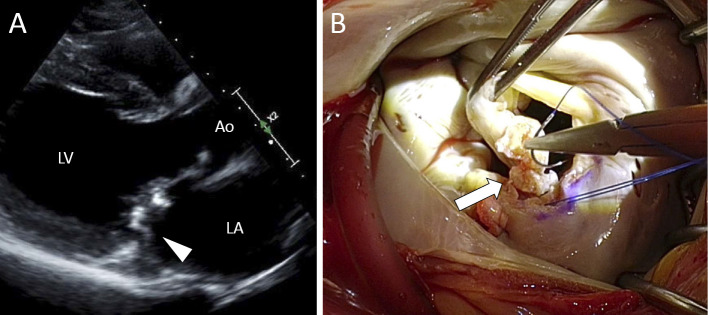
The patient was afebrile and did not complain of any symptoms. A physical examination ruled out any findings suggestive of peripheral embolism or heart failure, but auscultation revealed a cardiac murmur at the right lower parasternal border. The laboratory data revealed that inflammatory markers such as the peripheral white blood cell count (5,420 /μL) and the serum C-reactive protein level (CRP, 0.67 mg/dL) were within the normal ranges. A chest X-ray revealed no evidence of cardiomegaly or pulmonary edema. Echocardiography revealed left ventricular dilatation (diastolic/systolic dimensions; 62/49 mm) and moderately impaired cardiac contraction (ejection fraction, 41%). Movable vegetation was found attached to the A3 scallop, and moderate mitral regurgitation was detected (Figure A). No abnormalities were detected in the coronary artery system on cardiac computed tomography. Two sets of blood cultures remained negative after 2 weeks of cultivation (BacT/ALERT system; bioMérieux, Marcy l'Etoile, France), and the patient's preoperative clinical condition did not fulfill the Duke criteria for the diagnosis of IE (1 major criterion and 1 minor criterion). However, one month later, the size of the movable vegetation was found to have increased in size (6×10 mm) and cardiac surgery was planned before kidney transplantation. During the operation, caseous vegetation was found on A3 and P3, and the anterior leaflet was found to be prolapsed due to torn chordae (Figure B). Considering the planned kidney transplantation, ring annuloplasty was performed instead of valve replacement. A histopathologic examination of the resected vegetation showed an acute inflammatory change which was consistent with active IE. Although Gram-staining was negative, P. acnes was isolated from the resected vegetation after surgery. The organism was identified by an API Rapid ID 32A test (bioMérieux) and matrix assisted laser desorption ionization-time of flight mass spectrometry (Bruker Daltonics, Bremen, Germany). In addition, P. acnes was identified in 1 set of blood cultures after an incubation period of 10 days from blood samples that were drawn before surgery. Based on the results that were obtained after surgery, the pathological criteria of Duke's criteria were met and we made a definitive diagnosis of P. acnes-associated IE involving the native mitral valve. It was not possible to reliably assess the antimicrobial susceptibility of the pathogen because no appropriate methods were available at our laboratory.
Figure.
Infective vegetation caused by Propionibacterium acnes. Echocardiography (A: arrowhead) revealed as well as the intraoperative findings (B: arrow) revealed hyperplasia of the mitral valve. Caseous and fibrotic changes were observed at the resection plane of the hypertrophic valve. LA: left atrium, LV: left ventricle, Ao: ascending aorta
The patient's post-operative course was complicated by nosocomial pneumonia, and the patient was treated with piperacillin/tazobactam and vancomycin for 2 weeks. The antibiotic therapy was followed by 4 weeks of ceftriaxone, and the patient recovered well without recurrence.
Discussion
We reported a rare case of P. acnes endocarditis involving the native mitral valve that was incidentally diagnosed during a systemic investigation prior to renal transplantation. The clinical course of the patient suggested two important possibilities: P. acnes potentially causes native valve endocarditis, and such cases may progress silently without apparent clinical signs or symptoms.
The occurrence of native valve endocarditis with P. acnes is comparatively rare. Propionibacterium species form biofilm and thus tend to cause infections at the site of foreign materials (9). Three previous reviews on Propionibacterium endocarditis noted that the disease involved prosthetic cardiac devices in 79% (5), 57.6% (8), and 85.7% (10) of the cases; in particular, prosthetic valves were involved in 67% (5), 42.4% (10), and 78.6% of the cases (8). In these reports, 8 of 12 patients (66.7%) (5) and 10 of 14 cases patients (71.4%) (10) without prosthetic cardiac valves had a history of heart valve abnormality before the development of endocarditis, similarly to our case.
Hybrid dialysis might have been a rare trigger of infection in our case. P. acnes is part of the normal skin flora, and the organism may have entered the blood stream through the site at which the dialysis catheter was inserted. In fact, a recent prospective study of 16 consecutive cases of P. acnes-associated prosthetic valve endocarditis (PVE) indicated that the disease may frequently be acquired secondary to bacteremia of mucocutaneous origin (11).
The silent course of the patient was the highlight of the present case. Patients with Propionibacterium endocarditis tend to present only minimal clinical signs or symptoms. However, the majority of such patients had systemic symptoms such as fever, malaise, fatigue, night sweat, or shortness of breath (5, 10). The laboratory findings of patients with IE usually show inflammatory marker elevation. The median CRP level of patients with Propionibacterium endocarditis was 5.1 mg/dL (10). It is noteworthy, however, that our patient was completely asymptomatic and had no laboratory abnormalities at presentation. To the best of our knowledge, no other cases of “clinically silent” Propionibacterium endocarditis have been reported, even though the organism is not so highly pathogenic. A delayed diagnosis can result in valvular or perivalvular destruction, and even extracardiac involvement (11); and an early diagnosis is essential for achieving a better prognosis.
A diagnosis of Propionibacterium endocarditis is challenging to make for two reasons. First, the sensitivity of blood culture examinations is low. One study reported that blood cultures were negative in one third of cases of Propionibacterium endocarditis (5). Similarly, in two recent studies, the organism was detected by blood culturing in only 75% (7) and 71.4% (8) of the cases, respectively. Even in positive cases, a relatively long incubation period is required because of its slow growth (8, 12); the median time required for positive results was 7 days (ranging 3-14 days) (7, 10, 13). Additionally, bacterial culturing of resected valve specimens provided a positive result in only 12 of 22 cases 55%) in the case series (7). Thus, the incidence of P. acnes endocarditis is likely to be under-recognized when conventional diagnostic approaches are applied (14). A PCR-based diagnosis targeting 16s rDNA was reported to show a very high rate of positivity (20/21 cases, 95%) (7); however, this method cannot be routinely applied. Notably, approximately half of the cases of P. acnes endocarditis in the series could not be appropriately diagnosed without sequencing of resected valve (7). Second, the organisms grown by blood culturing are often considered to be skin contaminants. When P. acnes is isolatedin blood cultures, it is usually considered to be a contaminant (2). In previous studies, all 48 cases (15) and 46 cases (16) of P. acnes identified in blood cultures were considered to be contaminants. Third, Propionibacterium species may also contaminate valve cultures (17, 18). In our case, when P. acnes was isolated from the valve culture, we initially attributed it to contamination. However, the histopathological examination of the resected valve indicated the case to be an active infection and the fact that cultures of both blood and the resected valve specimens were positive for P. acnes led us to the final diagnosis of P. acnes endocarditis. The modified Duke criteria is generally applied when diagnosing IE (19); however, approximately one-fifth of the previous cases of Propionibacterium endocarditis were not categorized as definite cases based on these criteria (10). Our patient did fulfill the required criteria and was considered to be a possible case before surgery.
Antibiotic therapy and surgical debridement are essential for the treatment of P. acnes endocarditis. Basically, P. acnes is widely susceptible to various antimicrobial agents (20). A recent Japanese study demonstrated that the organism usually shows in vitro susceptibility to beta-lactams, carbapenems, and tetracycline, while it is frequently resistant to macrolides and clindamycin (21). Because of the procedure is complicated, we do not routinely perform antimicrobial susceptibility testing for P. acnes, which is recommended by authorities such as the Clinical and Laboratory Standards Institute. Due to the limited number of cases, an appropriate antibiotic therapy has yet established. However, most previous cases of P. acnes PVE have been treated with penicillin in combination with rifampicin or gentamicin (6, 22, 23). Penicillin-resistant Propionibacterium isolates are extremely rare; however, one such IE case has been described (24). The administration of rifampicin is preferred, especially in cases of prosthetic valve endocarditis, based on its high propensity for biofilm-penetration (5). Daptomycin combined with rifampicin is also a treatment of choice for biofilm-associated infection (23). Six weeks of treatment appears to be sufficient for P. acnes PVE (5, 6, 13). Surgical resection is required in most cases of P. acnes PVE; however, conservative treatment was shown to be successful in a few cases (25). In a recent case series, all of 22 patients who underwent surgery for P. acnes endocarditis survived for more than 1 year (7). The mortality rates in patients who received surgical and non-surgical treatment were 90.9% (10/11 cases) and 66.7% (2/3 cases), respectively (8). Despite the limited number of reported cases, these results indicate the effectiveness of surgery in the treatment of P. acnes endocarditis.
In conclusion, we documented a clinically rare case of native valve endocarditis caused by P. acnes. The patient was free of any clinical symptoms and the infection progressed silently and latently until it was found incidentally. The results suggest physicians should consider the possibility of infection when P. acnes is isolated in blood cultures.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol 9: 244-253, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eady EA, Ingham E. Propionibacterium acnes-friend or foe? Rev Med Microbiol 5: 163-173, 1994. [Google Scholar]
- 3.Park HJ, Na S, Park SY, et al. . Clinical significance of Propionibacterium acnes recovered from blood cultures: analysis of 524 episodes. J Clin Microbiol 49: 1598-1601, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy O, Iyer S, Atoun E, et al. . Propionibacterium acnes: an underestimated etiology in the pathogenesis of osteoarthritis? J Shoulder Elbow Surg 22: 505-511, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Sohail MR, Gray A, Baddour L, Tleyjeh I, Virk A. Infective endocarditis due to Propionibacterium species. Clin Microbiol Infect 15: 387-394, 2009. [DOI] [PubMed] [Google Scholar]
- 6.van Valen R, van Wijngaarden RAdL, Verkaik NJ, Mokhles MM, Bogers AJ. Prosthetic valve endocarditis due to Propionibacterium acnes. Interact Cardiovasc Thorac Surg 23: 150-155, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banzon JM, Rehm SJ, Gordon SM, Hussain ST, Pettersson GB, Shrestha NK. Propionibacterium acnes endocarditis: a case series. Clin Microbiol Infect 23: 396-399, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Kestler M, Munoz P, Marin M, et al. . Endocarditis caused by anaerobic bacteria. Anaerobe 47: 33-38, 2017. [DOI] [PubMed] [Google Scholar]
- 9.Portillo ME, Corvec S, Borens O, Trampuz A. Propionibacterium acnes: an underestimated pathogen in implant-associated infections. Biomed Res Int 2013: 804391, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clayton JJ, Baig W, Reynolds GW, Sandoe JA. Endocarditis caused by Propionibacterium species: a report of three cases and a review of clinical features and diagnostic difficulties. J Med Microbiol 55: 981-987, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Guío L, Sarriá C, de las Cuevas C, Gamallo C, Duarte J. Chronic prosthetic valve endocarditis due to Propionibacterium acnes: an unexpected cause of prosthetic valve dysfunction. Rev Esp Cardiol (Engl Ed) 62: 167-177, 2009. [DOI] [PubMed] [Google Scholar]
- 12.O'Neill TM, Hone R, Blake S. Prosthetic valve endocarditis caused by Propionibacterium acnes. Br Med J (Clin Res Ed) 296: 1444, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lalani T, Person AK, Hedayati SS, et al. . Propionibacterium endocarditis: a case series from the international collaboration on endocarditis merged database and prospective cohort study. Scand J Infect Dis 39: 840-848, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Tattevin P, Watt G, Revest M, Arvieux C, Fournier P-E. Update on blood culture-negative endocarditis. Med Mal Infect 45: 1-8, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein MP, Towns ML, Quartey SM, et al. . The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis 24: 584-602, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Muttaiyah S, Paviour S, Buckwell L, Roberts SA. Anaerobic bacteraemia in patients admitted to Auckland City Hospital: its clinical significance. N Z Med J 120: U2809, 2007. [PubMed] [Google Scholar]
- 17.Campbell WN, Tsai W, Mispireta LA. Evaluation of the practice of routine culturing of native valves during valve replacement surgery. Ann Thorac Surg 69: 548-550, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Giladi M, Szold O, Elami A, Bruckner D, Johnson B. Microbiological cultures of heart valves and valve tags are not valuable for patients without infective endocarditis who are undergoing valve replacement. Clin Infect Dis 24: 884-888, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Li JS, Sexton DJ, Mick N, et al. . Proposed modifications to the duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30: 633-638, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Achermann Y, Goldstein EJ, Coenye T, Shirtliff ME. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev 27: 419-440, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakase K, Nakaminami H, Takenaka Y, Hayashi N, Kawashima M, Noguchi N. Propionibacterium acnes is developing gradual increase in resistance to oral tetracyclines. J Med Microbiol 66: 8-12, 2017. [DOI] [PubMed] [Google Scholar]
- 22.Aubin G, Portillo M, Trampuz A, Corvec S. Propionibacterium acnes, an emerging pathogen: from acne to implant-infections, from phylotype to resistance. Med Mal Infect 44: 241-250, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Tafin UF, Corvec S, Betrisey B, Zimmerli W, Trampuz A. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother 56: 1885-1891, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis JF, Abramson JH. Endocarditis due to Propionibacterium acnes. Am J Clin Pathol 74: 690-692, 1980. [DOI] [PubMed] [Google Scholar]
- 25.Kurz M, Kaufmann BA, Baddour LM, Widmer AF. Propionibacterium acnes prosthetic valve endocarditis with abscess formation: a case report. BMC Infect Dis 14: 1, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]