The pathogenic entomophthoralean fungi cause infection in insects and mammalian hosts. Basidiobolus and Conidiobolus species can be found in soil and insect, reptile, and amphibian droppings in tropical and subtropical areas.
KEYWORDS: Entomophthorales, Entomophthoramycota, Basidiobolus, Conidiobolus, basidiobolomycosis, conidiobolomycosis, rhinoconidiobolomycosis, entomophthoramycosis, rhinoentomophthoramycosis
SUMMARY
The pathogenic entomophthoralean fungi cause infection in insects and mammalian hosts. Basidiobolus and Conidiobolus species can be found in soil and insect, reptile, and amphibian droppings in tropical and subtropical areas. The life cycles of these fungi occur in these environments where infecting sticky conidia are developed. The infection is acquired by insect bite or contact with contaminated environments through open skin. Conidiobolus coronatus typically causes chronic rhinofacial disease in immunocompetent hosts, whereas some Conidiobolus species can be found in immunocompromised patients. Basidiobolus ranarum infection is restricted to subcutaneous tissues but may be involved in intestinal and disseminated infections. Its early diagnosis remains challenging due to clinical similarities to other intestinal diseases. Infected tissues characteristically display eosinophilic granulomas with the Splendore-Höeppli phenomenon. However, in immunocompromised patients, the above-mentioned inflammatory reaction is absent. Laboratory diagnosis includes wet mount, culture serological assays, and molecular methodologies. The management of entomophthoralean fungi relies on traditional antifungal therapies, such as potassium iodide (KI), amphotericin B, itraconazole, and ketoconazole, and surgery. These species are intrinsically resistant to some antifungals, prompting physicians to experiment with combinations of therapies. Research is needed to investigate the immunology of entomophthoralean fungi in infected hosts. The absence of an animal model and lack of funding severely limit research on these fungi.
INTRODUCTION
Most of what we know about the described species comprising the Entomophthorales comes from studies done in the last 150 years (1–10). These studies initially focused on the biology of some species known for their ability to multiply their propagules inside insects, resulting in the death of the affected species (5–9). Originally, this was an object of interest because of its putative application in industry for the biological control of insects (4, 6). A good example of this was Entomophthora musca (10), causing true epizootics in Musca domestica (common housefly). Moreover, previously studied entomophthoralean fungi were found to affect a variety of economically important insect species (11, 12), including species which consume a great variety of vegetables during their caterpillar life cycles (11, 13). Good examples are aphids, cabbage (white) butterfly caterpillars, caterpillars of the Noctuidae, orthopterous insects, gnats, and others (11).
These studies also found several Entomophthorales species colonizing soils in tropical and subtropical areas rich in organic matter, especially in areas covered with leaves and other organic materials (3–7). Of importance was also the finding of entomophthoralean species (Basidiobolus ranarum) in the intestinal tract of some amphibians and reptile species without invasion or disease (8, 11). Thus, early in the history of the entomophthoraleans, it was obvious that these species can be found multiplying inside insects or as saprotrophic fungi in ecological niches rich in organic matter and in the intestines of amphibian and reptile species (7, 12). In these ecological niches and during infection in insects, these fungi developed broad hyaline coenocytic hyphae, sometimes with a few septa and the production of forcibly discharged mono- and multinucleated asexual conidia. Under special conditions, they could also form spherical thick-walled sexual zygospores after the mating of two adjacent hyphal segments (see Biology, below).
Prior to 1950, few studies addressed the classification of Entomophthorales species found in nature or those that complete part of their life cycle inside insects (11). Although Van Overeem (14) found for the first time Basidiobolus species causing disease in a horse, it was 31 years later when Basidiobolus and Conidiobolus species were again incriminated as pathogenic etiologies of humans and lower animals (15–19). At this point, the need to address the taxonomics of the Entomophthorales was postulated (11, 20). Until this time, those working with the so-called “lower fungi,” including saprotrophic and pathogenic species, applied morphological features only for the classification of these unique species (10, 21–23). In the early 1950s and 1960s, the inclusion of physiological, cytological, genetic, as well as pathological studies in insects provided a better perspective for a more comprehensive classification of the Entomophthorales (24–26). This classic taxonomic approach was recently challenged (27–30). Using molecular phylogenetic approaches, a new proposal organized the entomophthoraleans into the classification currently in place (30) (Table 1) (see Taxonomy and Phylogeny, below).
TABLE 1.
Classification of the phylum Entomophthoramycota using multiple gene phylogeniesa
| Classification of the phylum Entomophthoramycota |
|---|
| Basidiobolomycetes |
| Basidiobolales |
| Basidiobolaceae |
| Basidiobolus |
| Entomophthoromycetes |
| Entomophthorales |
| Ancylistaceae |
| Ancylistes |
| Conidiobolus |
| Completoriaceae |
| Completoria |
| Entomophthoraceae |
| Entomophaga |
| Entomophthora |
| Pandora |
| Meristcraceae |
| Meristacrum |
| Tabanomyces |
| Neozygitomycetes |
| Neozygitales |
| Neozygitaceae |
| Apterivorax |
| Neozygites |
See reference 30. In this review, we adopted the spelling “Entomophthoramycota” for the phylum. The order in which the classifications are shown is as follows: class, order, family, and genus. The phylum Mucormycota is not included.
Human-Infecting Entomophthorales: Historical Perspectives
The etymology of the terms Entomophthoramycota and Entomophthorales derives from the original genus type Entomophthora (from the Greek entomo, for insect, and phthora, for destruction) introduced by early investigators studying unusual fungal insect pathogens (9, 10). Since the first report of infections caused by entomophthoralean fungi in mammalian hosts (14), only two genera, Basidiobolus and Conidiobolus, have been documented as human pathogens. The terms basidiobolomycosis and conidiobolomycosis are derived from these genera, and the general term entomophthoramycosis comes from the high-rank name Entomophthoramycota (12). Infections due to entomophthoralean fungi rarely disseminate from the initial lesion to other areas of the body; instead, the pathogens spread near the originally infected areas. They are characterized by the development of painless slow-growing subcutaneous granulomas, sometime causing deformity of the affected anatomical areas. Lesions may remain indolent for years, but some cases of spontaneous cure have also been mentioned (3, 31). Typically, Conidiobolus species have been frequently found affecting the face and the nostril areas. Basidiobolus affects the thorax, trunk, limbs, and intestinal tract, and in unusual cases, it causes systemic infections (31–35).
The first report of Entomophthorales fungi infecting humans occurred in Indonesia (15), in a boy with subcutaneous swelling from whom B. ranarum was recovered in pure culture. Those authors mentioned that in 1925, Van Overeem (14), also in Indonesia, described a horse with chronic suppurating granulomas with discharging sinus tracts, and a fungus identified as B. ranarum was recovered. They also mentioned that in 1931, Casagrandi found similar broad hyphae within an intestinal ulcer of a man by histopathology, but cultures were not obtained. This claim, however, needs confirmation. Four years later, four additional cases, also in children 4 to 8 years old from Indonesia, from which B. ranarum isolates were recovered in culture were recorded (36). At least one of these isolates (the isolate from the first case) was later studied by Lie Kian Joe et al. (15) and confirmed to be B. ranarum. In 1960 in London, Symmers (37) diagnosed another case of Basidiobolus infection, this time in a Dutch girl who had visited Indonesia. It was believed that the girl had contracted the infection while traveling in this area of endemicity. The diagnosis was based on the location of the lesion (back) and the eosinophilic reaction by histopathology, as cultures were not available. A case of a Cameroonian man with swelling of the upper lip with a similar eosinophilic reaction but without culture was reported 1 year later (2). Those authors believe that Basidiobolus was the etiologic agent, but based on the anatomical location of the lesion, it was probably due to Conidiobolus sp., an understandable mistake since Conidiobolus infection in humans was reported 4 years later (3). In addition, Lynch and Husband (38), based on histopathological findings (hyphae with the Splendore-Höeppli phenomenon), reported a similar case of a 5-year-old boy in Sudan with painless progressive swelling in the upper right forearm (15). Despite the lack of cultures, this case could be attributed to B. ranarum based on histopathology and the anatomical location of the lesion.
Gatti et al. (39) reported the first case from Africa and gave a detailed history of the reported cases involving entomophthoralean fungi until 1968. Since then, numerous cases have been reported from tropical and subtropical areas of the world, including the United States (31–35, 40–42). Although earlier controversies on the taxonomic Basidiobolus species affecting humans suggested the presence of two other species, B. haptosporus (8) and B. meristosporus (9), it was later found that B. ranarum was the only species affecting humans and lower animals such as horses, dogs, and others (14, 26). The finding of B. meristosporus causing intestinal disease in an HIV-positive Cameroonian woman by the use of molecular procedures without culture was recently reported (43). However, taxonomic and comprehensive molecular and phylogenetic data are necessary to validate this finding.
Conidiobolus infection was provably first described in humans in 1961 (2), but the diagnosis was based on histopathological analysis without culture. Those researchers believed that the hyphae in the infected tissues were Basidiobolus sp. hyphae, but the anatomical location (upper lip) pointed to Conidiobolus as the most likely etiology (see above). In 1961, Emmons and Bridges (17) reported the first cases of conidiobolomycosis in Texan horses. The first well-documented case of human Conidiobolus coronatus infection, in an 11-year-old Jamaican boy from the Grand Caiman Island, came from Bras et al. (3). The lesion was limited to the nose and paranasal sinuses, and C. coronatus was obtained in pure culture. Two years later, reports of several human cases of infection caused by C. coronatus were reported in the same journal (44–46). Two of these cases were the first reports of the infection in South America, in Brazil (44) and Colombia (46), plus 11 new cases from Nigeria, all involving the nasal passages (45).
Systemic conidiobolomycosis in a U.S. infant was reported by Gilbert et al. (47), and their isolate was later characterized as Conidiobolus incongruus by King and Jong (48). Another systemic case involving C. incongruus was reported 7 years later (49). More recently, Conidiobolus lamprauges was also reported, first in several Brazilian sheep (19) and later in a human with systemic infection in Japan (50). In the following years, numerous cases involving B. ranarum and Conidiobolus species were reported, including numerous reviews (1, 31–34, 51–53).
TAXONOMY AND PHYLOGENY
Entomophthorales: Traditional and Current Phylogenetic and Genomic Analyses
The taxonomic placement of the Entomophthorales in their own group was traditionally based on the presence of coenocytic (or sparsely septate) hyphae, the production of forcibly ejected multinucleated asexual conidia, and the development of sexual diploid zygospores in nature and culture (20, 54, 55). As a result, the entomophthoraleans were properly separated from mucoralean fungi, and both were included within the now obsolete phylum Zygomycota. The entomophthoralean fungi were at that time further divided into six families (29, 30). Their taxonomic characteristics have been thoroughly reviewed numerous times (1, 27–29, 56, 57). This characterization was widely accepted until phylogenetic analysis indicated that the “Zygomycota” were a polyphyletic group, with Basidiobolus sharing phylogenetic features with monoflagellated chytrid fungi (30, 58–60). Of interest for its current classification was a report that molecular-based approaches, using amino acid sequences (including those exons used for DNA phylogeny), had placed the genus Basidiobolus away from the Entomophthorales and closely related to fungal chytrids (58, 61, 62). These findings led to a proposal to remove the entire Basidiobolus genus from the Entomophthorales. However, many researchers remained skeptical on the actual interpretation of such results (30, 63, 64).
Investigators called attention to Basidiobolus because of its unusually huge nucleus (65) and its large genome size (66) and because it develops a 9-by-3 microtubular arrangement in centrioles and kinetosomes (67). This is an unusual feature among eukaryotic phyla without flagella but is consistently found on fungal microbes displaying flagella, such as the chytrids (43, 67). Despite these controversies, the placement of the genus Basidiobolus with the entomophthoralean fungi is still supported by many (30, 58, 63, 64).
In a recent comprehensive phylogenetic study, Gryganskyi et al. (63) included more genes from a broader range of entomophthoralean species. This study confirmed previous analyses showing the Entomophthorales as a monophyletic group, basal to the mucoralean fungi and directly related to the chytrids (58, 62, 68, 69). As highlighted by Humber (30), both traditional taxonomic data and current phylogenetic analyses agree that entomophthoralean fungi possess features distinct from those of other fungi occupying a position basal to nonflagellate fungi (70). Based on these data, Humber (30) proposed the phylum Entomophthoramycota, which comprises 3 new classes (Basidiobolomycetes, Neozygitomycetes, and Entomophthoromycetes) and one new order (Neozygitales). Table 1 illustrates the nomenclatural features of the proposed phylum Entomophthoramycota (30).
A genome-bar phylogenetic analysis of zygomycete species, on the other hand (64), recently introduced a new view on the nomenclatural classification of coenocytic fungi. Based on genome data for 25 “zygomycetes” and 192 proteins, those researchers proposed the creation of 2 phyla (Mucormycota and Zoopagomycota), 6 subphyla, 4 classes, and 16 orders (Table 2). As those authors pointed out, “the interpretation of bootstrap support for branches in genome-bar phylogenies is still poorly understood given that some genes within a particular genome may have different evolutionary histories” (64). Thus, adopting and interpreting such data need the support of further studies to validate the proposed changes. Moreover, the use of these data does not exclude the possibility of genome duplication, even with the use of conservative orthologs, and issues related to ancient lineage sorting events (71). As DNA sequence technologies advance, new changes in the higher classification of these fungi are expected.
TABLE 2.
Classification of “zygomycetes” using genome-scale dataa
| Classification of “zygomycetes” |
|---|
| Mucormycota |
| Glomeromycotina |
| Glomeromycetes |
| Archaeosporales |
| Diversisporales |
| Glomerales |
| Paraglomerales |
| Mortierellomycotina |
| Mortieralles |
| Mucoromycotina |
| Endogonales |
| Mucorales |
| Mucor |
| Rhizopus |
| Umbelopsidales |
| Zoopagomycota |
| Entomophthoromycotina |
| Basidiobolomycetes |
| Basidiobolales |
| Basidiobolus |
| Entomophthoromycetes |
| Entomophthorales |
| Conidiobolus |
| Neozygitomycetes |
| Neozygitales |
| Kickxellomycotina |
| Asellariales |
| Dimargaritales |
| Harpellales |
| Kickxellales |
| Zoopagomycotina |
| Zoopagales |
See reference 64. The order in which the classifications are shown is as follows: phylum, subphylum, class, order, and genus.
The Terms Zygomycetes and Zygomycosis
The abolishment of the disease name “zygomycosis” (32, 72) had created an inherent problem for those involved in clinical cases of “zygomycosis” and for those with appointments in the teaching of medically important fungi. The suggestion of the terms mucormycosis and entomophthoramycosis for the infections caused by filamentous coenocytic pathogens in mammals did not address the need for a single term linking both groups of coenocytic pathogens (32, 72). Although the term zygomycosis has been incorrectly used for the infections caused by mucoralean fungi, it is wise to maintain the original intent of the term zygomycosis to name the infections caused by both groups of coenocytic pathogens: the Mucormycota (30), (Mucoromycotina according to Spatafora et al. [64]) and the Entomophthoramycota (30) (Zoopagomycota according to Spatafora et al. [64]). Following the use of the epithet “zygomycetes” by Spatafora et al. (64), we use this general term to address the microscopic features of coenocytic fungi in infected tissues and in culture. Thus, the terms zygomycetes and zygomycosis are used throughout this review with the understanding that they represent coenocytic fungal species developing sexual zygospores in nature and in culture.
BIOLOGY
Entomophthoraleans are fast-growing fungi forming spores by developing abundant forcibly discharged sticky conidia (8, 11). They are considered primary or secondary mesotrophic colonizers of difficult-to-degrade substrates, such as cellulose, suberin, chitin, and others (11). Their physiological characteristics and biochemical properties (e.g., the production of chitinolytic and saccharolytic enzymes) make them successful competitors among fungi and common inhabitants of soil and organic debris (73). Some Conidiobolus species are able to develop within living insects and mites (C. coronatus) (74). Basidiobolus species had been found in the gastrointestinal tract of amphibians and reptiles and are readily recovered in their feces (11, 75). These species are characterized by the development of complex conidium systems to facilitate their spread in nature (54). Entomophthoralean species are also parasites of nematodes, arthropods, and insects (8, 9, 11).
The Entomophthoramycota comprise around 280 species (63, 71). Their detection in nature requires special methods; thus, their occurrence in these environments was seldom noted in early mycological studies (75). In nature, these fungi develop short-lived forcibly ejected multinucleated (some species) conidia (6, 76). When a conidium lands on a potential host, it germinates and develops a germ tube. If the tip of the germ tube detects the presence of open skin, it could develop an appressorium for infection (76) (see Pathophysiology, below). Likewise, if the ejected conidia land on a place other than the target host, they are able to develop secondary small conidia that are also forcibly ejected, allowing the pathogen another chance to target a new host (11, 77).
Entomophthoramycota species develop wide coenocytic hyphae and usually place a septum to separate the active cytoplasm at the tip of the structure. Robinow (78) extensively studied the developmental stages of B. ranarum hyphae. He reports that under his culture conditions, B. ranarum hyphae increased in size “at a rate of about 4.4 μm per minute,” suggesting that, under the right conditions, these fungi develop very quickly. He describes that the B. ranarum nucleus, “close to 25 microns in length, lies at a variable, but always considerable, distance from the tip of the cell” (78). As the tip of the hypha increases in length, a septum is formed to separate the active cytoplasm from the rest of the empty hyphae (67, 78). In cases when branches are formed, the division of the nucleus is first observed, and a septum is then formed, separating the divided nuclei. The hyphal branch usually develops below the newly developed septum, and the process continues (67, 78–80).
Nuclear division in B. ranarum was brilliantly described first by Robinow (78) and later by Sun (65). The details are so impressive, we have directly transcribed Robinow's observations (78):
Mitosis in Basidiobolus seems rigidly linked to cell division. Not one of a large number of cells in which I have watched the course of mitosis failed to divide, and I have rarely seen a cell with two mature nuclei in the same cytoplasm. At the end of telophase the distal daughter nucleus moves, or is moved, rapidly toward the tip of the cell. The proximal nucleus moves for a much shorter distance in the opposite direction only to reverse its migration shortly after, when a sharply defined transverse septum begins to grow inward from the cell wall at a level close to the plane in which the metaphase plate had first become visible.
Conidiobolus Species Life Cycle
Entomophthoralean fungi develop conidia under appropriate conditions in nature and during their life cycle in insects, amphibians, and lizards (6, 8, 11). Based on the type of conidia and the way in which they are released, Brefeld (4) introduced the genus Conidiobolus in 1884. Close to 35 Conidiobolus species of saprophytic insect pathogens and mammalian pathogens (C. coronatus, C. incongruus, and C. lamprauges) have been described so far (19, 32, 35, 50, 74, 81–83). Vilela et al. (19) gave a detailed description of the major morphological features of pathogenic species from clinical cultures.
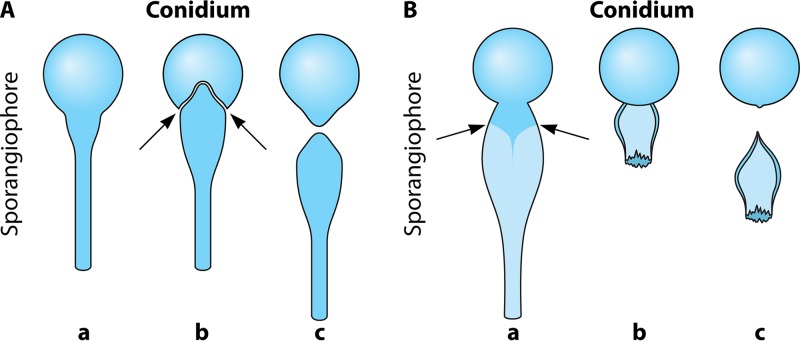
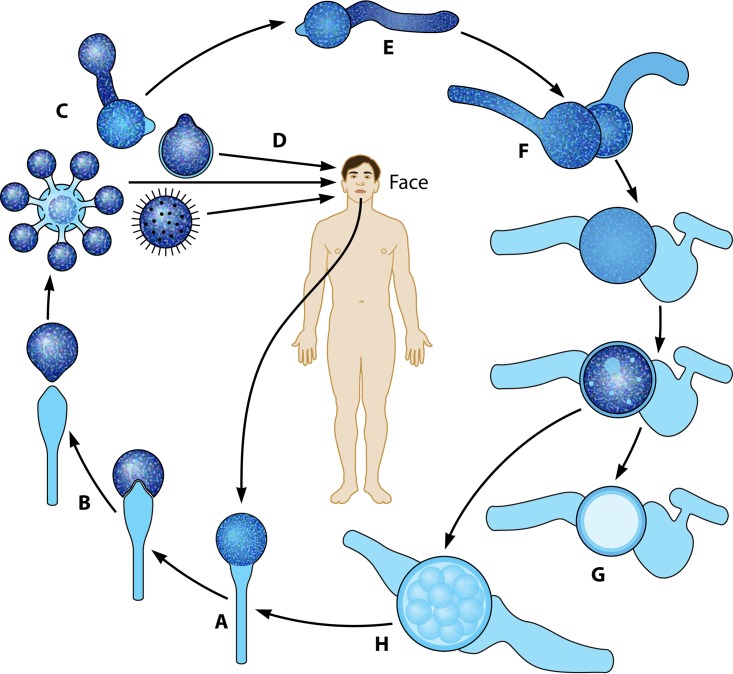
For the genus Conidiobolus, the life cycle begins with the formation of uni- or multinucleated conidia, which are produced at the top of single unbranched sporangiophores (Fig. 1A). First, the cytoplasm migrates from the coenocytic hypha, forming single or multinucleated conidia (Fig. 1Aa). At the base of the conidium, an invagination is formed, exerting pressure at the base of the single conidiophore (Fig. 1Ab, arrows) (8). Eventually, the invagination suddenly everts, propelling the sticky conidium away from the sporangiophore (Fig. 1Ac). In the genus Conidiobolus, at least four types of asexual conidia have been reported in nature and in culture (Fig. 2 to 4) (5, 7, 10). The presence of sexual structures (zygospores) has been detected in the majority of the members of this genus (Fig. 3B and C and 4B and C) but not in C. coronatus. However, under specific laboratory conditions (water agar plates), this species develops villose conidia, a unique taxonomic characteristic used for its identification in clinical isolates (Fig. 2K and L) (26). C. incongruus and C. lamprauges develop sexual spores by the projections from two terminally enlarged hyphal bodies (positive and negative). These apical unequally sized swellings come into contact and conjugate as the intervening cell wall connects through an opening. The contents of the two cells (including their nuclei) merge, and a thick-walled zygospore is formed (Fig. 3 to 6) (18, 19, 65, 78).
FIG 1.
(Aa) Formation of a sporangiophore of the genus Conidiobolus developing a sticky conidium on top of the structure. (b) As the conidium matures, an invagination is formed at the base of the conidium (arrows), exerting pressure on the base. (c) The invagination suddenly everts, propelling the conidium away from the sporangiophore. (Ba) Basidiobolus sporangiophore developing a conidium at the top of the structure. The subconidial portion of the sporangiophore accumulates liquid that becomes turgid (arrows). (b) The continuing accumulation of liquid exerts extreme pressure at the base, forming a subconidial vesicle, and the pressure results in rupture and the abrupt release of the conidium. (c) The subconidial structure remains attached, but it detaches upon landing.
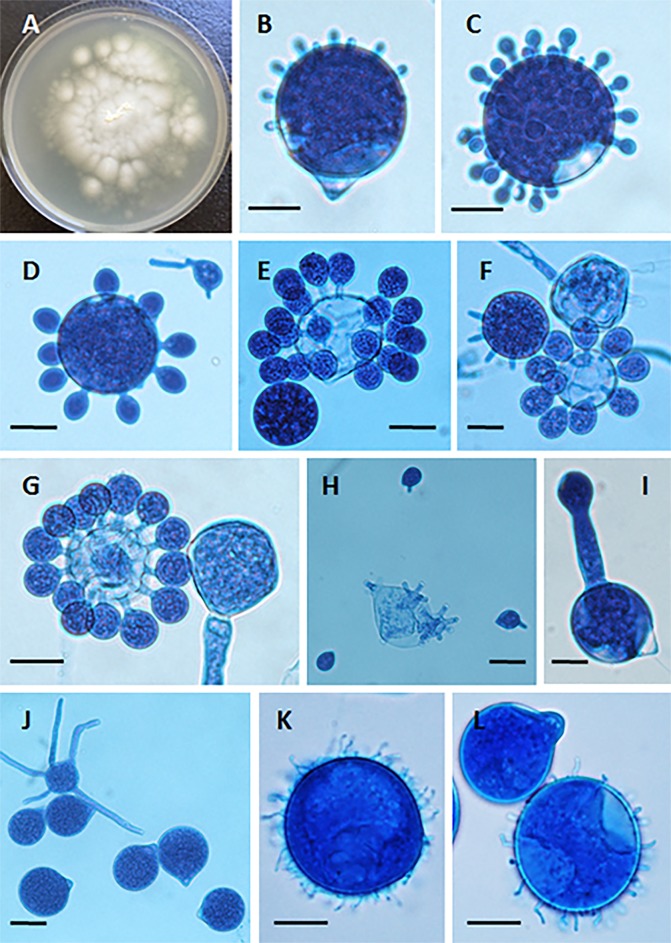
FIG 2.
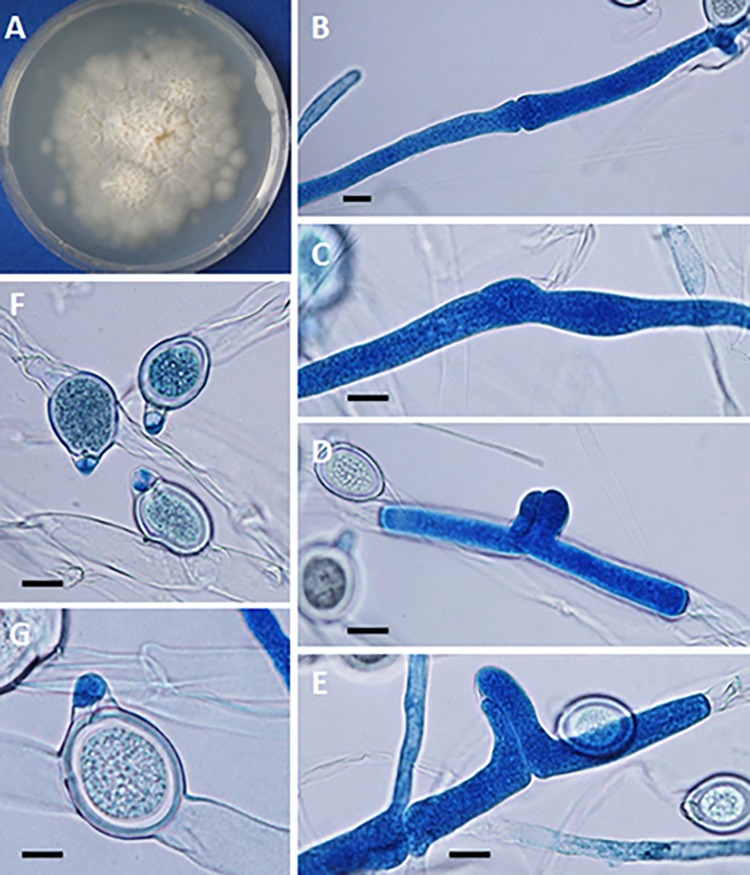
(A) Yellow-white colony of Conidiobolus coronatus on 2% Sabouraud dextrose agar (SDA) from a primary culture after 48 h. The presence of a few aerial hyphae and satellite colonies due to the ejection of conidia from sporangiophores, detected on the lid of the SDA plate (data not shown), is also observed. (B to G) Formation of secondary conidia from a primary multinucleate conidium of C. coronatus in lactophenol blue. Bars, 20 μm (B, D, and G), 22 μm (C and E), and 21 μm (F). Note the different steps of development into fully formed corona secondary conidia in panels F and G. (H) Ejected secondary conidia and also several empty sporangiophores and three small ejected secondary conidia, some of which are developing germ tubes. Bar, 22 μm. (I and J) Single conidiophore with a secondary sporangiophore and conidium (I) and several primary C. coronatus conidia, one with several coenocytic hyphae (J). Bars, 20.0 μm (I) and 22 μm (J). (K and L) Presence of C. coronatus villose conidia in water agar cultures. Bars, 18 μm (K) and 19 μm (L).
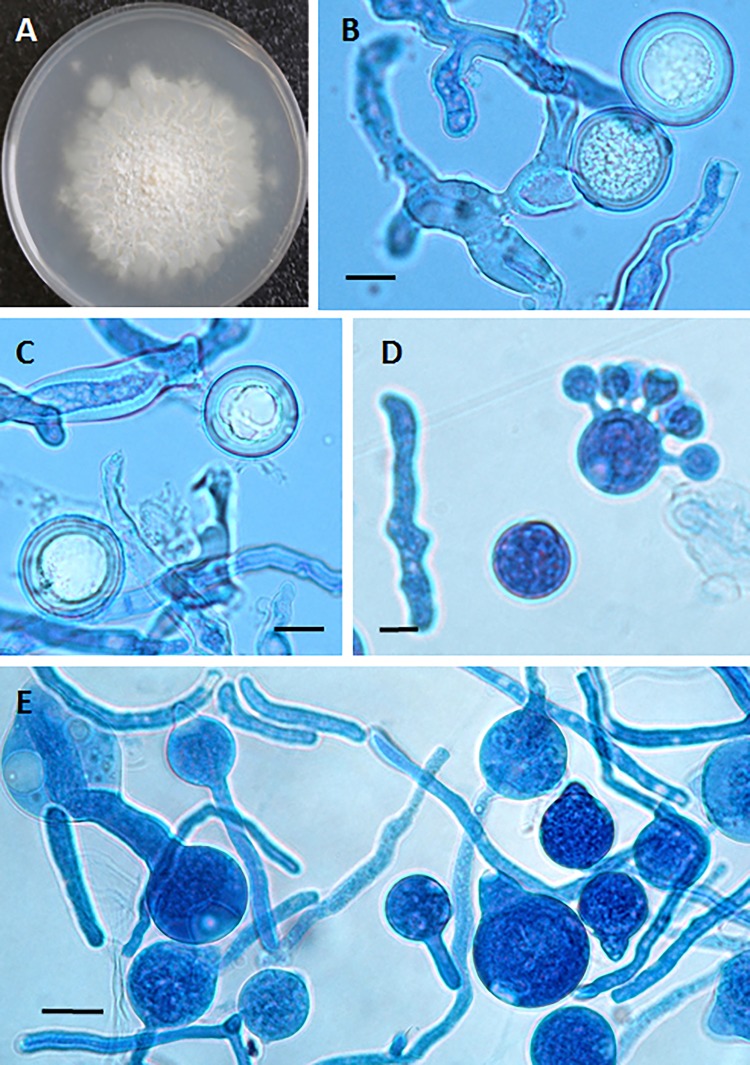
FIG 4.
(A) Creamy powdery colony of Conidiobolus lamprauges with radiating folds from the center of the colony at 72 h. (B and C) C. lamprauges smooth spherical sexual zygospores with thick cell walls in lactophenol blue. Bars, 20 μm (B) and 11 μm (C). (D and E) Asexual conidia found in culture plates of C. lamprauges (lactophenol blue). Note the smooth papilla projection of the conidia contrasting with that in C. incongruus (Fig. 3). Bars, 10 μm. (F) Secondary replicative conidium in lactophenol blue. Bar, 10 μm.
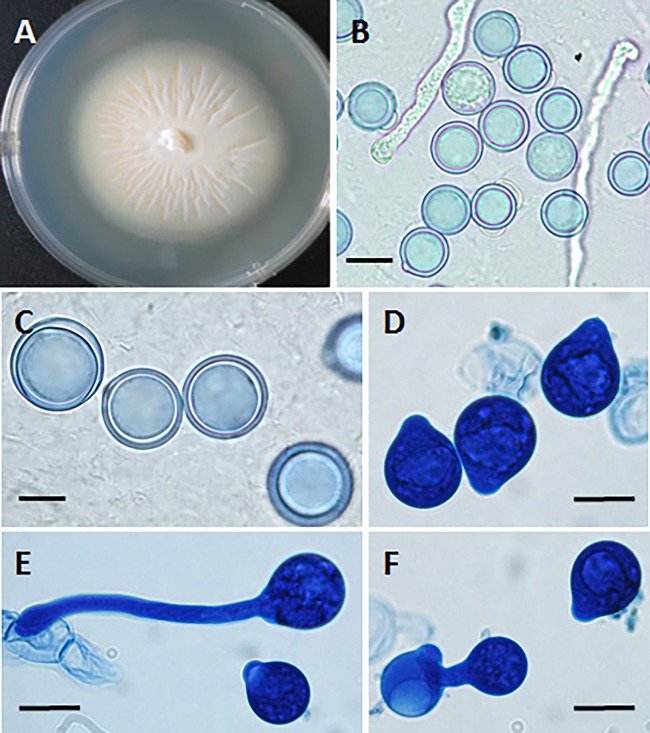
FIG 3.
(A) Powdery creamy colony of Conidiobolus incongruus at 72 h with a few aerial hyphae and some satellite colonies. (B and C) Sexual zygospores typical of C. incongruus in culture in lactophenol blue. Bars, 10 μm. (D) Multireplicative conidium bearing several secondary small conidia and a hyphal fragment (lactophenol blue). Bar, 8.0 μm. (E) Numerous primary conidia with sharp-pointed papillae. Some of the conidia are in the process of developing germ tubes, and at least one has a secondary replicative conidium. Bar, 15 μm.
FIG 6.
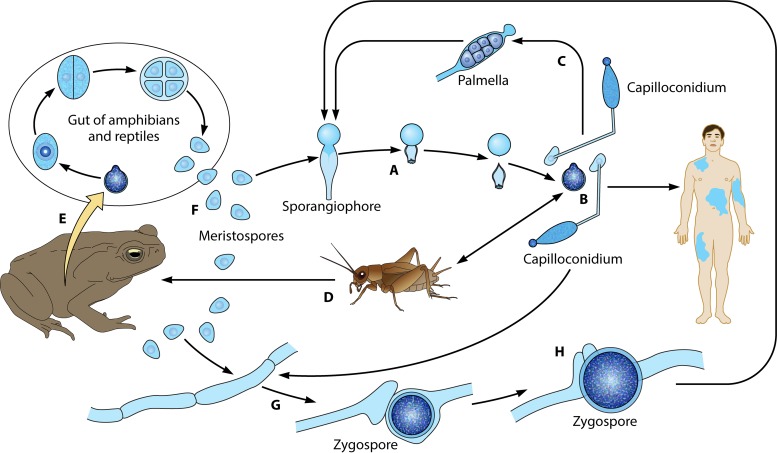
The life cycle of Basidiobolus ranarum. (A) The life cycle starts when sticky conidia are forcibly ejected from sporangiophores. (B) The sticky primary conidium could attach to a passing host (humans or insects) or develop an elongated adhesive conidium (capilloconidium), which also can attach to passing hosts. (C) The latter secondary elongated structure could develop to contain a sticky beak haptor that divides to form numerous “Palmella” endospores, some of which are released outside the broken capilloconidium cell wall, giving rise to new hyphae and single sporangiophores (A). (D and E) The target insects (D) can be ingested by reptiles or amphibians (E), initiating a new cycle inside the intestinal tract of these animals. (F) In this new environment, hundreds of resistant meristospores are produced and then secreted in feces. When environmental conditions are right, coenocytic hyphae are developed (G). (H) If two opposite-sex hyphae contact each other, their exchange of genetic material leads to the formation of sexual zygospores (see also Fig. 7). Zygospores can develop into sporangiophores (long arrow).
It is believed that infections by the entomophthoralean fungi in humans and other animals are acquired after contact with conidia through small skin injuries (31, 32). Sticky conidia attach to injured tissue and develop a germ tube that eventually penetrates the open skin, causing conidiobolomycosis (Fig. 5) (see Pathophysiology, below). As mentioned above, the anatomical areas commonly affected by Conidiobolus species include the face (nostrils) and, less frequently, other body areas, including systemic infections (1, 32, 35) (Fig. 5).
FIG 5.
(A and B) The life cycle of Conidiobolus species starting with the development of a sporangiophore from hyphae (A) and the ejection of multireplicative primary conidia at the top of sporangiophores (B) (Fig. 2). (C and D) The primary conidia could replicate into secondary conidia that could also attach to passing hosts (C) or directly attach to the skin of humans (including the villose conidia of C. coronatus) (D). Clinical samples can be cultured, leading to the development of hyphae and sporangiophores (long arrow). (E to H) In nature and in culture, the primary conidia could form coenocytic filaments, which, after the interchange of genetic material, could lead to the formation of sexual zygospores, and the cycle starts all over again.
Basidiobolus ranarum Life Cycle
The genus Basidiobolus was introduced by Eidam in 1886 (84). It is not considered a traditional entomopathogenic fungus, although it is commonly isolated from droppings and/or intestinal contents of amphibians and reptiles (85). Thus, members of this genus may infect some insects, or perhaps these animals come into contact with the sticky propagules of Basidiobolus becoming host carriers (83, 86). The development of advancing hyphae triggers the expression of abundant quantities of chitin at the distal section of the hyphal structure (9, 87). Although both Conidiobolus and Basidiobolus have developed ballistic conidia as a means of dissemination, the mechanisms by which these genera release their conidia are quite different. In contrast to the mechanism employed by Conidiobolus (discussed above), in Basidiobolus, a single uninucleate conidium on top a single sporangiophore is formed before discharge (Fig. 1B) (86). However, in Basidiobolus, the subconidial portion of the sporangiophore becomes turgid due to the accumulation of liquid below the formed conidium. The continuing accumulation of liquid exerts extreme pressure at the base (below the conidium base) (Fig. 1Ba, arrows), forming a subconidial vesicle (88). This pressure results in the rupture of this structure and the abrupt release of the conidium far away from the sporangiophore (Fig. 1Bb). The subconidial structure remains attached to the released conidium, but it detaches soon after landing (Fig. 1Bb and c) (9, 85, 86, 88). In turn, the released conidia could attach to passing insects, such as mites and beetles, or to mammals, causing basidiobolomycosis in the latter hosts (Fig. 6A and B) (11, 83). In turn, the targeted insects can be ingested by amphibians and reptiles, initiating a new cycle in the intestinal tract of these hosts (Fig. 6D and E) (53, 85, 86). In the intestinal tract of amphibians and reptiles, the conidium divides by fission several times until numerous meristospores are formed, which are eventually released in the droppings of the hosts (Fig. 6E and F). The meristospores are rugged structures that can survive severe environmental conditions for months. These structures develop into mycelia and sporangiophores (Fig. 6A and B), from which new ballistic conidia can form. The germination of a primary ballistosporic conidium on enriched agar will form a structure called the “Palmella” stage (Fig. 6C). This stage is characterized by the germination of a secondary capilloconidium containing a sticky beak haptor (Fig. 6B and C). Eventually, the capilloconidium divides to form numerous Palmella endospores (Fig. 6C); some of these are released outside the broken capilloconidium cell wall, giving rise to new hyphae and single sporangiophores (Fig. 6A).
In nature and culture, B. ranarum developed sexual structures termed zygospores. The zygospores are formed when the tips of two parents' hyphae from the same isolate (homothallic) encounter each other (Fig. 6G and H and see Fig. 7B to E). The process is initiated when a septum is placed at the point where the two parents' hyphae met (Fig. 7B and C). A small protuberance is formed at that particular point, with the development of parallel beaks that had initiated nuclear division on each side of the hypha (84) (Fig. 7D and E). One of the two replicated nuclei, in each hypha, will be digested, and one of the remaining nuclei migrates through a pore into the adjacent hypha. The fusion of the two nuclei occurs, giving rise to a zygospore with prominent undulate and/or smooth cell walls (Fig. 7D, F, and G) (9, 15, 53). The studies of Hutchinson et al. (25) showed that some features used in the past, such as odor, growth at different temperatures, and the presence of undulated zygospores, to separate B. ranarum into different species were not valid.
FIG 7.
(A) Creamy rugose colony of Basidiobolus ranarum on 2% Sabouraud dextrose agar. (B to E) Encounter of opposite-sex hyphae before the formation of lateral beaks. Bars, 12 μm (B to D) and 10 μm (E). (F and G) After the exchange of genetic material, zygospores develop with their characteristic beak. Bars, 25 μm (F) and 15 μm (G).
Basidiobolomycosis occurs when the sticky propagules of B. ranarum come into contact with skin or injured mucosa through which the propagules of this zygomycete can penetrate a mammalian host (Fig. 6B) (see Pathophysiology, below). B. ranarum infections are commonly diagnosed in humans, affecting the limbs, chest, back, and intestinal tract and less frequently causing systemic infection (32, 89). Similar anatomically preferred infection sites have been observed in lower animals (17, 19, 90, 91).
PHYSIOLOGICAL ASPECTS OF ENTOMOPHTHORALEAN FUNGI
Few studies related to the cell wall composition of entomophthoralean fungi are available (73, 92–95). In 1939, Couch (5) conducted the first study on the cell wall composition of these fungi. Based on this study, he was the first to separate Basidiobolus ranarum from the other entomophthoralean fungi based on its cellulose content, data confirmed later by others (75, 79, 93). The presence of chitin in the cell wall of Basidiobolus and Conidiobolus was also determined in early studies (22, 96). Farkaš (97) mentioned that in general, the cell wall composition of zygomycetes might include glucuronomannoproteins, polyglucuronic acid, chitosan, and chitin.
Early studies on the cell wall composition of Entomophthora and Conidiobolus species suggested that in vitro, entomophthoralean fungi display similar cell wall components, mainly β(1-3)-glucans associated with chitin (98). Farkaš (97) reported that in some members of the Mucorales, chitosan, a linear polymer of β(1-4)-d-glucosamine, is one of the main cell wall components of these fungi. Although studies concerning the presence of this polymer in entomophthoralean fungi are scarce, there is no evidence that this polysaccharide is involved in the deacetylation of nascent chitin (94, 98). According to these studies, glucose, mannose, chitin, and β(1-3)-glucan are the main cell wall components of most entomophthoralean fungi. The absence of chitosan in entomophthoralean species was used in early studies to separate this group of coenocytic fungi from the Mucorales (73).
In nature, these fungi cannot compete with microbes that utilize rich nutritional compounds, such as the Mucorales (29, 65). However, entomophthoralean fungi are well known for their abilities to release chemical compounds to degrade substances, such as cellulose, that most fungi cannot utilize (65, 78). Thus, they are frequently found in environments with high temperatures and high humidity, such as tropical environments with high vegetation (33). In these environments and in cultures, the lower section of the advancing hypha places a septum, to separate the advancing cytoplasm. As the tip of the hypha advances, a new septum is formed, leaving behind a long chain of empty cells (11). Eventually, if the surrounding conditions are appropriate, the formation of sporangiophores and zygospores could take place (11).
Nutritional Requirements and Secreted Metabolites
Physiological studies of entomophthoralean fungi indicate that these species are able to synthetize most vitamins and growth factors by absorbing amino acids and inorganic nitrogen from external sources (11, 65). To retrieve these compounds from nature, these fungi release powerful enzymes, ensuring survival in poor nutritional environments (65, 78, 79). Probably during evolution, they adapted these weapons to invade the tissues of animals, including arthropods, insects, and, later, mammals (23). For instance, the release of some metabolites allows insect pathogenic entomophthoralean species to secrete several chitinases, successfully penetrating the exoskeleton of their prey (see below) (11, 23, 76, 99–101). The release of powerful enzymes in culture and during infection has been reported for Basidiobolus and Conidiobolus species (73, 99, 102).
The expressions of extracellular cellulases, lipases, and proteases in these fungi have been widely reported (73, 99, 102–105). Likely, Basidiobolus and Conidiobolus took advantage of this ancestral trait to survive in nature and adapted their life cycles to growth inside the environment provided by infected host species (23). Interestingly, both Basidiobolus ranarum and Conidiobolus species trigger similar inflammatory responses in infected mammalian species, suggesting the in vivo expression of analogous virulence factors (see below). The expressions of secondary toxic metabolites such as azoxybenzene dicarboxylic acid and hydroxymethyl azoxybenzene in C. coronatus during culture growth were reported (106). However, their release during infection in insects and mammals has yet to be confirmed (23, 106, 107).
GENETICS AND EVOLUTION
Exactly how the mammalian pathogenic Basidiobolus and Conidiobolus species evolved to infect lower animals and mammals is unclear. Some insect fungal pathogens, included in the entomophthoralean fungi, had evolved mechanisms to infect animals (insects and arthropods) several times in their evolutionary history with different degrees of specificity and virulence (108). It is then quite possible that both Basidiobolus and Conidiobolus share with these fungi similar evolutionary traits to become effective pathogenic species, first to lower animals and then to mammalian hosts (12, 23, 54).
The genomes of members of the Entomophthoramycota are among of the largest genomes ever sequenced (8,000 Mb) (77). This is the case for B. ranarum, which has a large haploid genome of around 350 Mb (66). This huge size is striking compared to those of other fungal species genomes, which are around only 40 Mb (71). Earlier studies based on morphological appearance only predicted the presence of hundreds of chromosomes in B. ranarum, but this is not the case for other entomophthoralean species, with an average of 8 to 32 chromosomes (12, 23, 65, 77). The entomophthoraleans are considered haploid fungi. It is not clear if this feature has something to do with gene duplication in B. ranarum or the development of a large genome (66). Large nuclei with numerous chromosomes, as is the case for B. ranarum, have also been found in other Entomophthoramycota fungi, suggesting that this may be a trait in these species (12, 23, 66, 77). In contrast, the genome size of C. coronatus has been calculated to be around 39.9 Mb, which is similar to the genome size of traditional fungi (∼40 Mb) (77).
Based on the huge genome of B. ranarum, some had speculated the possibility of a relationship between this genomic feature and the fact that B. ranarum developed genetic adaptation to interact with amphibians, reptiles, insects, and mammals (77). It has been observed that host specialization in other pathogenic fungi is related to gene expansion and whole-gene duplication (109). Thus, it is quite possible that this is the case for B. ranarum. However, the average size of the C. coronatus genome and its ability to interact with insects, nematodes, and mammals suggests that more genomes have to be sequenced to determine if large genomes are the norm for the Entomophthoramycota and if pathogenicity to animals is linked to gene duplication and genome expansion. Genome information on this type of fungi will soon be available with the addition of data on several entomophthoralean pathogens, including the complete genome of C. coronatus (NRRL 28632) and the ongoing projects of C. incongruus (B7586), B. meristosporus (B9252 and CBS 931.73), B. heterosporus (B8920), and other important entomophthoralean fungi (77).
ECOLOGY AND EPIDEMIOLOGY
Distribution in Nature and Contact with Human Hosts
As mentioned above, entomophthoralean fungi can be found in association with organic material in nature and as part of the intestinal microbiota of animals such as lizards and amphibians (see Biology, above) (11, 12, 20, 33, 53, 110). The sticky forcibly ejected propagules produced by these fungi during their life cycle in nature (Fig. 6 and 7) come into contact with animal hosts, including humans, and eventually penetrate the hosts through small cuts in the skin to develop infection (32, 85) (see below). It is important that the clinical picture for mucoralean fungi differs from those for the Entomophthorales, in that Mucorales infections typically occur in immunocompromised hosts, whereas entomophthoralean fungi infect mostly apparently healthy hosts (12, 31, 33, 35). However, the true immunological predisposing factors leading to entomophthoramycosis in humans remain unknown (32, 35). Moreover, infections caused by the Entomophthorales lead to the development of swelling of the infected tissue (usually subcutaneous and intestinal) and have some predilection for vascular tissues (mainly in immunocompromised hosts) but not as strong as is the case for the Mucorales. In addition, the entomophthoralean fungi trigger a distinctive eosinophilic inflammatory response, with the formation of eosinophilic material around the invasive coenocytic hyphae of Basidiobolus and Conidiobolus species (Splendore-Höeppli phenomenon [see below]). This typical eosinophilic inflammatory response with the Splendore-Höeppli phenomenon is absent in infections caused by the Mucorales. Interestingly, eosinophilic granulomas have also been found in infections caused by parasites and by the invading sparsely septate hypha-like structures of the Oomycota (Pythium insidiosum, Lagenidium species, and Paralagenidium species) causing infections in humans and other mammals (32, 111–113). These eukaryotic pathogens, in addition to triggering a typical eosinophilic response, all display the Splendore-Höeppli phenomenon (see below) around the invading propagules in the hosts' infected tissues (32, 114, 115). Interestingly, unusual cases caused by C. incongruus and C. lamprauges, in immunocompromised hosts, do not display this phenomenon (35, 49, 50, 116).
Natural Habitat of Basidiobolus ranarum
Basidiobolus ranarum is a saprotrophic fungus found in soil, decaying vegetation, and the intestinal tract and excreta of amphibians and reptiles (117). B. ranarum has been isolated as a saprotrophic fungus from the above-mentioned sources around the world, especially in tropical and subtropical areas (20, 53). It is believed that amphibians and lizards ingest propagules of Basidiobolus spp. by capturing and ingesting insects carrying their spores or those that are already infected (11). The Basidiobolus spores in the intestinal tract of amphibians and reptiles multiply by fission (meristospores) and return to the environment in the excreta of the host (Fig. 6F). If the conditions of moisture and soil organic matter are appropriate, the spores develop a coenocytic germ tube, and the cycle in nature is reinitiated (see above). There is at least one report of B. ranarum causing mortality in Canadian toads (Bufo hemiophrys) due to extensive dermatitis (118). Humans can be exposed to Basidiobolus propagules through open skin and contact with amphibian and lizard droppings, organic matter, insect bites, contaminated food, and soil containing the propagules of these fungi (Fig. 6B) (31, 119). Of interest was a report of six cases from Arizona (101), suggesting that this pathogen may be inhabiting dry areas of the southwestern United States where B. ranarum may be cycling in nature with desert reptiles, a finding supported by similar cases in the dry regions of the Middle East (120–126).
Natural Habitat of Conidiobolus spp.
Conidiobolus spp. are also found in soil and decaying vegetation rich in organic matter, from which they have been isolated as saprotrophic fungi (8, 117, 127). Conidiobolus species have also been recovered from insects (13, 74, 128). It is likely that human hosts can be exposed to Conidiobolus species when injured skin is exposed to contaminated insects, organic matter, or soil (32). Through laboratory experiments, it was demonstrated that maximum germination properties of Conidiobolus species are achieved at high humidity levels (98 to 100%) and at temperatures of between 16°C and 30°C. These findings suggest that these species thrive better in tropical and subtropical environments (8, 85, 117, 129). Incidentally, these data support the exact geographical distribution of Basidiobolus and Conidiobolus infections in humans (25, 129).
Geographic Distribution
The cases reported in the literature in 1956 (15), caused by B. ranarum in Indonesia, and in 1965, caused by C. coronatus in Jamaica (3), clearly showed that infections caused by the Entomophthoramycota occurred in areas of high humidity and high temperatures typical of tropical and subtropical environments. These areas include numerous countries in Africa (Cameroon, Congo, Ghana, Kenya, Nigeria, Ivory Coast, Senegal, Sudan, Zaire, and others), America (Brazil, Colombia, Costa Rica, Mexico, Venezuela, Dominican Republic, Jamaica, Puerto Rico, and the United States), and Asia (Burma [Myanmar], China, Indonesia, India, Philippines, Thailand, Taiwan, Vietnam, Australia, New Zealand, New Guinea, and others) (25, 119, 129).
Age and Occupational Distributions of Human Entomophthoramycosis Cases
Infections caused by the Entomophthoramycota involve children and young adults (31, 32, 129). The disease occurs more frequently in males than in females. In some areas of Africa, the male-to-female ratio is close to 3:1, whereas all cases of the world together showed a ratio of 8:1, very close to data from previous reports (129, 130). This trend of high infection rates in males may be related to occupational activities. In most of the areas where the disease is endemic, males are more in contact with contaminated environments than females because males are usually working in fields, whereas females tend to remain at home taking care of the family. In the same way, children are infected with both Basidiobolus and Conidiobolus because they are constantly playing in the field, more often becoming in contact with their environments in areas of endemicity (119, 129).
IMMUNOLOGY OF THE FUNGI CAUSING ENTOMOPHTHORAMYCOSIS
Few studies leading to an understanding of the molecular immunological events triggered by Basidiobolus and Conidiobolus virulence factors expressed during infection have been reported. Most of what we know about the host immunological responses to coenocytic hypha invasion came from histopathological and serological analyses of infected tissues or immunoglobulin detected during infection (31, 32, 131–133). The lack of information on the virulence factors expressed by the entomophthoralean fungi in infected hosts, and how the host responds to this assault, in part has delayed the way in which we approach the diagnosis and management of infections by these pathogens. Thus, this issue needs to be addressed soon.
The main problem for investigators has been the facts that these pathogens affect countries with poor populations and that the infections are sporadic rather than epidemic (31, 32, 134). Thus, most funding agencies have neglected this group of fungi, and the lack of funding for research into these pathogens has resulted in fewer investigators interested in this subject (31, 32, 134). To make matters more difficult, there is no animal model to study their virulence factors and immunological capabilities. Only recently have de Gody et al. (135) introduced a rodent model (gerbils) for C. lamprauges, a good first step to study the pathogenic capabilities of the entomophthoraleans and host immunological defenses.
Fortunately, another group of pathogens, the tapeworm eukaryotic parasites, has shown similar inflammatory responses in infected hosts (111–113); thus, we could use their experimental data to extrapolate these findings to the Entomophthorales. For instance, tapeworms in humans trigger Th2 eosinophilic granulomas with the Splendore-Höeppli phenomenon around the parasites, in the same manner in which the entomophthoralean fungi develop eosinophilic granulomas in infected humans (31, 32, 119). It is likely that the immunological events triggered by these parasites mimic the cellular and molecular responses activated by entomophthoralean fungi during human infection. Thus, we could take advantage of the parasite data to develop hypothetical immunological models.
T Helper Subsets during Infection
During entomophthoramycosis, the invading hyphae trigger the migration of numerous inflammatory cells, including high numbers of eosinophils, cytotoxic lymphocytes, giant cells, mast cells, mononuclear macrophages, natural killer cells, and plasma cells and a few neutrophils, to the infection site (53, 90, 136–139). Furthermore, Khan et al. (120) reported high levels of Th2 interleukins in human patients with basidiobolomycosis, a finding that supports the tenet that during Basidiobolus and Conidiobolus infections, a Th2 response is likely activated by these fungi. Incidentally, an increase in the blood counts of circulating eosinophils has also been encountered in patients infected with these fungi (121, 140–142).
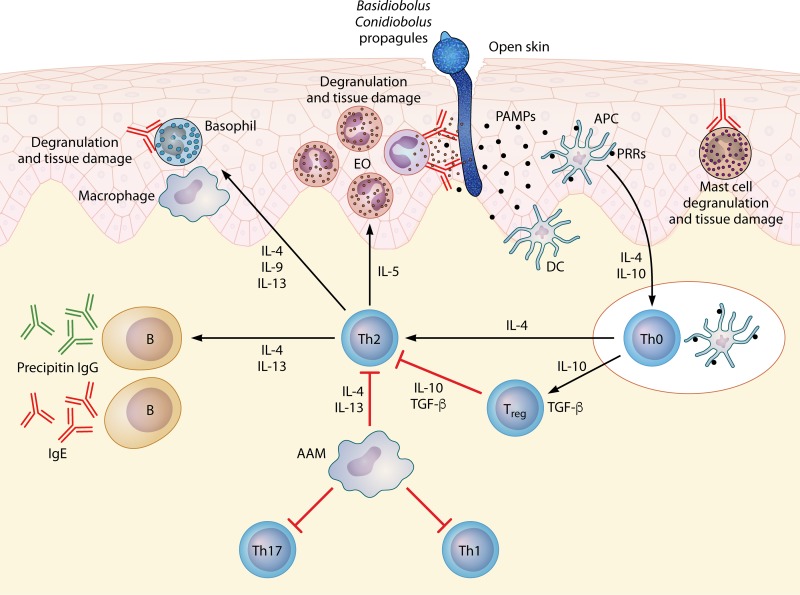
It is well known that the detection of microbial pathogens is mediated by Toll-like pattern recognition receptors (PRRs) that recognize molecules known as pathogen-associated molecular patterns (PAMPs) (136, 137). Upon PAMP recognition, Toll-like receptors initiate programmed signals essential for the first line of host defenses against microbes (Fig. 8). As occurs in the parasites (137), PRR signaling could also stimulate the maturation of dendritic cells to become antigen-presenting cells (APCs), a step that stimulates the host's adaptive immunity, which eventually controls the infection and eliminates the pathogen (Fig. 9). A likely sequence of events after penetration by Basidiobolus or Conidiobolus hyphae is the putative release of PAMPs and their contact with local dendritic cells. These stimulated APCs seem to trigger the production of interleukin-4 (IL-4) and migrate to nearby lymph nodes to present these antigens to Th0 naive cells. These cells in turn initiate the release of more IL-4, locking the immune response in a Th2 mode (Fig. 9).
FIG 8.
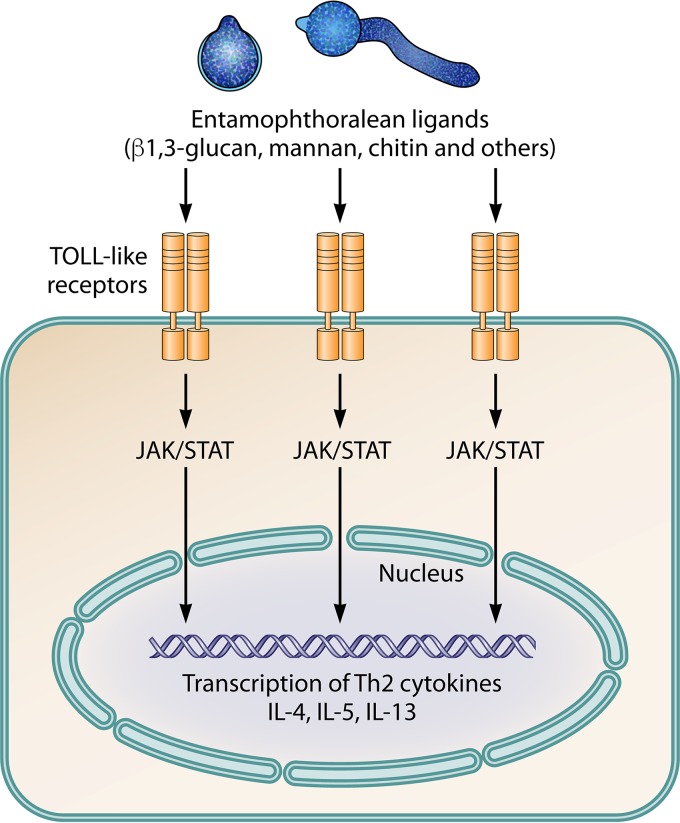
Presentation of key antigenic molecules from invading entomophthoralean hyphae, based on the development of key Th2 cytokines and the typical eosinophilic reactions hypothetically triggered by entomophthoralean fungi during infection. The pathogen-associated molecular pattern (probably related to β-1,3-glucan, mannan, chitin, and others) could stimulate Toll-like cell receptors through signaling via Janus kinase (JAK) and signal transducer and activator of transcription (STAT). These molecules signal the nucleus to activate a cascade of events leading to the production of Th2-related cytokines (IL-4, IL-5, and IL-13), locking the host's immune system in a Th2 mode.
FIG 9.
Putative events based on histopathological and immunological findings during entomophthoramycosis and during parasitic infections (112, 133, 137). Under this scenario, through open skin, a conidium attaches to the host and produces a germ tube penetrating the host. The invading hyphae then release secretory immunogens (pathogen-associated molecular patterns [PAMPs]). Dendritic cells (DC), through pattern recognition receptors (PRRs), contact the antigen and are activated, becoming antigen-presenting cells (APCs). The activated APCs process the antigens and release IL-4 during migration to nearby lymph nodes to present the antigen to Th0 naive cells. The Th0 naive cells in turn release IL-4 and IL-10 and become a powerful Th2 subset. The Th2 subset releases more IL-4, IL-5, IL-13, and IL-10, resulting in the downregulation of Th17 and Th1 subsets. These interleukins activate the differentiation of alternative activated macrophages (AAM) that could inhibit the proliferation of cells such as Th1, Th2, and Th17 cells. However, the exacerbated production of IL-4 and IL-5 by the Th2 subset drives the immune response into a strong Th2 subset. In turn, IL-4 and IL-13 stimulate B cells to produce precipitin IgG (detected by serological assays in cases of entomophthoramycosis) and IgE as well as the activation of effector cells such as mast cells, eosinophils (EO), and basophils. The released IgE will also specifically bind to the invading hyphae, and the eosinophils will in turn attach to the Fc region of IgE, triggering the degranulation of the eosinophils around the invading hyphae. A similar outcome occurs after IgE binding to mast cells and basophils, causing fibrosis and tissue damage, consistent with the clinical features of entomophthoramycosis. TGF-β, transforming growth factor β.
Activation of Basophils, Eosinophils, and Mast Cells during Infection
The presence of granulocytic cells, including mononuclear macrophages and giant cells, in the infected tissue has been consistently reported for Basidiobolus and Conidiobolus infections and parasitic infections (52, 53, 90, 120, 121, 140–143). The degranulation of these cells at the site of infection has been largely blamed for the tissue damage typically observed in histopathological preparations (52, 120, 137, 138). According to the data shown in Fig. 9, the release of Th2 cytokines (IL-4, IL-5, and IL-13) could activate a cascade of events that trigger the proliferation of inflammatory cells related to a Th2 event: eosinophils, mast cells, basophils, and others (52, 144). The release of IL-4 and IL-13 could also stimulate the production of IgE and IgG antibodies by circulating B cells, as previously reported by some investigators (131–133, 138). IgG antibodies are readily detected by serological methods in cases of human entomophthoramycosis (131, 132, 138). The IgG antibodies released during infection are considered precipitin antibodies that do not protect the host against the invader. Circulating IgE antibodies are probably some of the most important players in tissue damage during Basidiobolus and Conidiobolus infections (120, 138, 139). The release of IL-5 attracts eosinophils to the IgE Fc region bound to the cell wall of the invading hyphae (Fig. 9). This results in the rupture of eosinophil cell walls and their degranulation around the hyphae, forming the Splendore-Höeppli phenomenon and tissue damage (31, 32, 120, 137–139) (see below).
Upregulation of Th2 Cytokines and Downregulation of Th1 during Infection
It has been found that pathogens such as parasites (111, 112, 137), Oomycota (145, 146), and Entomophthoramycota (31–34, 53, 120, 143, 145) can survive in mammalian tissues only if a Th2 cellular subset is in place. It is likely that these pathogens ensure their survival in infected tissues by stimulating immunological processes that downregulate other T helper subsets (92, 111, 120, 143). These eukaryotic pathogens achieve this objective by the expression of a specific set of immunogens that, when processed by antigen-presenting cells through pattern recognition receptors, results in the upregulation of Th0 naive cells into a Th2 subset (Fig. 9). The expression of key Th2 cytokines (IL-4, IL-10, and IL-13) by the stimulated Th2 subset results in the downregulation of the Th1, Th17, and other subsets, ensuring pathogen survival (52, 92, 137, 138, 143). This is exemplified by the elevated titers of IL-4, IL-10, and alpha interferon (IFN-α) in a 41-year-old man with intestinal basidiobolomycosis, implying that a Th2 response was in place in this patient (120). The tissue damage observed in these cases could be the result of the release of proteases and other enzymes from the pathogen or by the release of cytotoxic compounds from granulocytic cells, as has been reported for other systems (147). Thus, the release of immunogens from Basidiobolus and Conidiobolus species in infected tissues triggers a continuing Th2 response, which contributes to the formation of the typical indolent subcutaneous lesions in mammalian hosts (120, 143).
The presence of numerous entomophthoralean hyphal elements in internal organs and the lack of an eosinophilic reaction and the Splendore-Höeppli phenomenon in immunocompromised hosts have consistently been reported (35, 49, 110). These patients share in common underlying conditions, drug addiction (110), organ transplantation (35), and concomitant diseases, such as lymphoma and others (49). Some of the patients also displayed neutropenia and, in some cases (49), thrombocytopenia. It is well known that first cell line of defense during infections is neutrophils (49, 112, 137). Thus, the low counts of these key inflammatory cells in immunocompromised hosts infected with entomophthoralean fungi could facilitate the spread of hyphal elements without control (49). Immunocompromised individuals with defective immune responses may produce few to no eosinophils, mast cells, and other inflammatory cells (35, 49, 110). This in part could explain the lack of both eosinophils and the Splendore-Höeppli phenomenon in this type of patient (35, 49, 110). Furthermore, the low cell counts could also play a role in the low levels of key circulating interleukins (anergic stage), a factor that could help in their propagation in internal organs.
Anti-Basidiobolus/Conidiobolus IgG and IgM Antibodies in Infected Hosts
Studies from the late 1980s (148, 149) suggested that entomophthoralean immunogens expressed during experimental infection could stimulate antibodies when injected into animals. Kaufman et al. (132) reported the presence of anti-Basidiobolus and anti-Conidiobolus IgG and IgM antibodies in the sera of mammalian hosts infected with these pathogenic species by using immunodiffusion (ID) and exoantigens extracted from Basidiobolus and Conidiobolus species. In that study, at least 10 human patients with subcutaneous and/or abdominal infections due to B. ranarum and 5 human patients with chronic nasofacial lesions caused by C. coronatus were evaluated. Additionally, test data from horses with C. coronatus infections were presented. This report indicated that the sensitivity of immunodiffusion was low, but its specificity for culture-positive patients was 100%. Six years later, Imwidthaya and Srimuang (131) used this approach to test two patients with subcutaneous basidiobolomycosis. In this case, the immunodiffusion test detected specific precipitin antibodies with high sensitivity and specificity. Khan et al. (133) also used immunodiffusion to confirm a case of intestinal basidiobolomycosis in a 41-year-old Indian man. At the same time, they developed and tested an enzyme-linked immunosorbent assay (ELISA). They reported that the ELISA detected the presence of IgG1, IgG3, and IgM anti-B. ranarum antibodies, suggesting that this sensitive test could be of value for the diagnosis of this disease in humans (120, 133). The IgE levels in infected hosts with entomophthoramycosis have yet to be investigated.
The Splendore-Höeppli Phenomenon: an Immunological Perspective
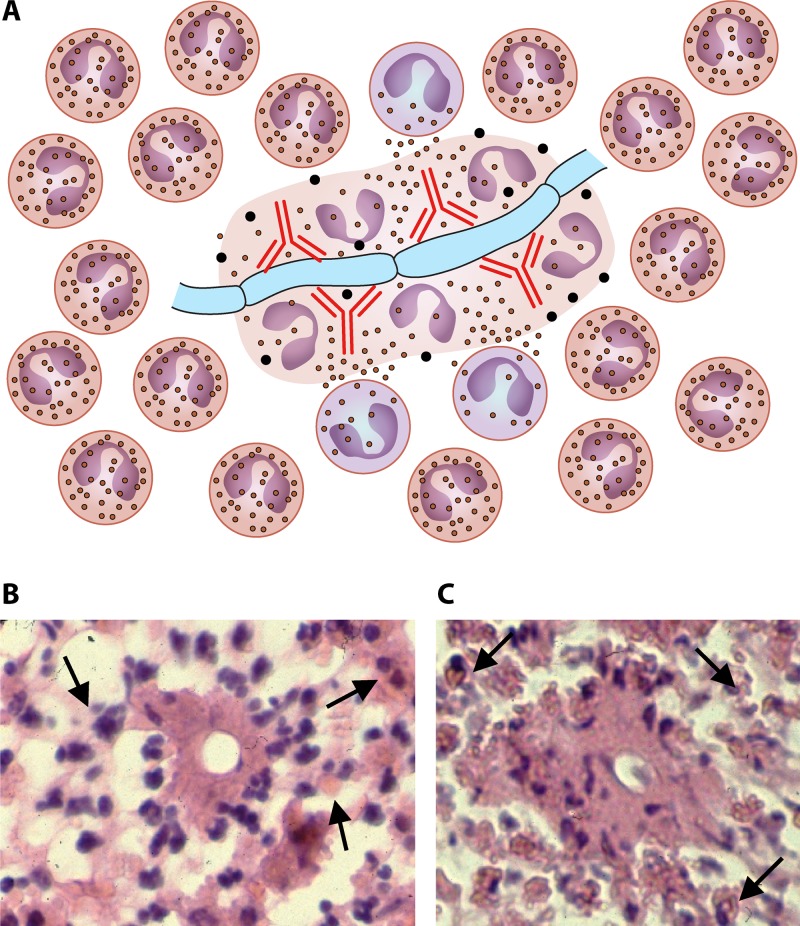
One of the main characteristics of entomophthoralean fungi in healthy mammalian hosts is the formation of the Splendore-Höeppli phenomenon, an eosinophilic reaction around the invading hyphae (Fig. 10) (31, 115, 145, 150). As mentioned above, this phenomenon is not restricted to Basidiobolus or Conidiobolus species but has also been found in Oomycota pathogens, such as P. insidiosum (114, 139), Lagenidium species (146), and Paralagenidium species (146), and in other fungi (139). This phenomenon has also been reported with invading parasites (111, 112, 119) and bacteria (139, 151) and on inert bodies such as silk sutures (152).
FIG 10.
As highlighted in Fig. 9, the Th2 subset will trigger the release of IL-4, IL-5, and IL-13, and B cells will express IgE (red immunoglobulins). (A) The eosinophilic reaction around an invading hypha encases basidiobolomycosis or conidiobolomycosis. Note the presence of IgE, the degranulation of eosinophils around the hyphae, and several pyknotic nuclei within degranulate eosinophilic material. (B and C) Histopathological sections from a human case of intestinal basidiobolomycosis. Note the presence of numerous eosinophils surrounding the cross sections of hyphae that appear as spherical or oval structures in the center of the Splendore-Höeppli phenomenon. Under this perspective, the released IgE (red immunoglobulins) will bind to cell wall antigens that attract eosinophils to the site of infection. Panels A and B display the binding and degranulation of the eosinophils on the hyphae triggered by IgE (panel A, red immunoglobulins). The eosinophil nuclei at this stage appear at the periphery of the eosinophilic precipitate (A and B). As the lesion becomes old (chronic stages), other eosinophils will bind the complex, and after degranulation, their nuclei are also incorporated into the eosinophilic material and become pyknotic, giving rise to the Splendore-Höeppli phenomenon (C). In some instances, only the eosinophilic material will be expressed around the invading microbe (115, 137).
The first mention of this phenomenon appeared in a 1907 report of rats infected with Sporothrix schenckii (153) and was mentioned again by Splendore in 1908 (154). In the latter publication, Splendore proposed the novel species “Sporotrichum asteroides,” after the finding of the same “asteroid bodies” in the studied animals, but the species name was not accepted. Several years later, Höeppli (155) observed a similar eosinophilic reaction, this time around Schistosoma species in experimentally infect animals. Soon after, several investigators reported this phenomenon in different eukaryotic and prokaryotic pathogenic species and named its appearance either the Splendore or Höeppli phenomenon (139, 156–158). Later, the combination Splendore-Höeppli, giving priority to the first report by Splendore, was adopted (115, 139, 159).
Numerous hypotheses have been proposed to explain the morphogenesis and the elements present within this eosinophilic material around invading pathogens, but the exact mechanism of its formation has yet to be elucidated (150, 151, 160). The main hypothesis suggested that the Splendore-Höeppli phenomenon was an antigen-antibody reaction forming complexes from debris of dead inflammatory cells (eosinophils and others) (158). Glycoproteins, lipidic fractions, complement, immunoglobulins, and antigenic fractions have been detected in these complexes (115, 161). Inflammatory events triggered by parasites and fungi (Fig. 9) offer a plausible mechanism for the Splendore-Höeppli phenomenon during Basidiobolus and Conidiobolus infections.
Under this scenario, after the formation of a germ tube by the entomophthoralean fungi, the release of antigenic fractions (virulence factors) from the invading hyphae, as shown in Fig. 9, would contribute to the establishment of the typical Th2 response observed in these fungi (32). This includes the release of IL-4, IL-5, and IL-13 by proinflammatory cells, resulting in the downregulation of Th1 and Th17 (52, 92, 111, 139, 144). The upregulated Th2 response triggers the release of very large quantities of IgE and the migration of mast cells, basophils, eosinophils, and other Th2-related inflammatory cells to the site of infection. Once released, IgE binds to the advancing hyphae, which in turn attract eosinophils. The bound IgE's Fc region contacts the eosinophil receptors and induces the degranulation of the eosinophils around the invading hyphae (Fig. 10B and C). The content of the degranulated cells (pyknotic nuclei and cytoplasmic and lipid membranes) remains in close contact with the hyphae, forming an eosinophilic blanket (Fig. 10A to C).
The hyphae of the invading pathogen continue to produce antigens, which diffuse through the eosinophilic material. In turn, new eosinophils arrive, and the process is repeated (Fig. 10). Thus, the formation of the Splendore-Höeppli phenomenon in entomophthoralean fungi likely requires a Th2 response in place; the presence of IgE, eosinophils, and other inflammatory cells; and their degranulation around the invading hyphae. The proposed mechanism for the formation of the Splendore-Höeppli phenomenon in part agrees with data from previous studies regarding the elements present within the eosinophilic material (115, 139, 157–161). It is quite possible that this phenomenon is an evolutionary strategy of some pathogens to hide important antigens from the host immune response and, thus, is a key factor for their survival in infected hosts (145).
PATHOPHYSIOLOGY
As is the case for other fungal pathogens, it is likely that entomophthoralean fungi cannot penetrate intact skin of a potential host (1, 12, 33, 146). They need a traumatic lesion on the host's skin to attach their conidia and for the formation of a germ tube to penetrate the infected tissue using lipolytic and proteolytic enzymes. In fact, the expression of these types of enzymes in cultures of entomophthoralean species has been reported (12, 102, 106, 107). Most of these enzymes have been found to play important roles in infected insects (11, 76, 99, 107). In insects and other species, the infection starts with contact with ballistic sticky conidia landing on the insect's cuticle (11, 13, 83, 162). Once in contact with the host body, the entomophthoralean fungi secrete chitinases, which break the strong insect cuticle, and the fungus' germ tube penetrates the host (11, 22, 107, 128). At this stage, the fungus actively secretes powerful lipases and proteases that practically digest the body content of the infected insect (11, 76, 81, 163).
The finding that Basidiobolus and Conidiobolus express numerous proteases, glucanases, and lipases in culture is of importance and may shed light on the pathophysiology of Basidiobolus and Conidiobolus species in mammalian hosts. For instance, Fromentin and Ravisse (164) reported the expression of lipases and proteases in cultures of both C. coronatus and B. ranarum and speculated on their role as putative virulence factors during human infection. In addition, the trypsin-like serine protease-coding gene was recently PCR amplified from C. coronatus, C. lamprauges, and B. ranarum (163). Since this finding, numerous authors have suggested that the secreted enzymes in these species might play a role in the pathophysiology of the infection, acting, as is the case in insects, as virulence factors (31, 102–105). Thus, the mammalian pathogenic entomophthoralean fungi expressed, in culture, a cocktail of lipases and proteases, such as elastase, esterase, collagenase, and several other enzymes, that can be successfully used to penetrate and invade human tissues (31, 79, 102, 104–106).
Putative Mechanisms of Disease and the Host-Pathogen Relationship
It is possible that Basidiobolus and Conidiobolus species use the same approach to invade and cause disease in other hosts, as has been reported for infected insects (76, 81). Pathogens of insects and nematodes are able to penetrate the intact chitinous cuticle; in contrast, the propagules of Basidiobolus and Conidiobolus require skin injury to attach to the exposed wound (12, 102, 107). Cases of entomophthoramycosis after insect bites and exposure through open skin in contaminated environments have been the norm (12, 32, 119). Moreover, in reported cases of intestinal or systemic infections, the inhalation or ingestion of these fungal propagules has also been proposed to be the initiator of the infection (32, 35, 119, 133, 138). Although there have been some reports pointing out that abdominal basidiobolomycosis arose from a cutaneous focus, the majority of cases seem to have an intestinal origin (165). The pathogenesis of Conidiobolus pericarditis is believed to be similar to that of Aspergillus pericarditis (49). It had been suggested that during Conidiobolus pulmonary infection, the pericardium might be directly invaded from the pulmonary focus or by transmural passage and myocardial invasion (49). The ability of entomophthorales to separate dead hyphae with a septum from those containing viable cytoplasma could give rise to small hyphal elements, such as that shown in Fig. 3D. These propagules can easily travel through blood vessels, allowing the pathogen to reach distant anatomical sites in immunocompromised individuals.
Probably, once the sticky conidia of entomophthoralean fungi land on an open wound (skin or mucosa) or inside lung tissue, they could become attached to its surface and stimulated by the host's temperature (thermophilic fungi), and the development a germ tube may occur. The tip of the germ tube, possibly directed by genetically controlled genes, will orient the tip toward the open wound and actively penetrate, assisted by the release of elastase, esterase, collagenase, and lipases (31, 79, 102, 104). Once in the host's tissues, by the release of the above-mentioned enzymes and almost certainly by the production of other immunogens, the invading fungi will encounter the host's immune system, initiating the typical Th2 immune response to the attack (see above). However, the host's Th2 response is not able to control or kill the invading hyphae, ensuring the pathogen's survival as it becomes enclosed by the Splendore-Höeppli phenomenon (see above). In cases of subcutaneous infection, the host's immune response to the pathogen's immunogens seems to restrain the invading hyphae to the subcutaneous tissues, from which it advances very slowly (31, 32, 166–170).
Chronic localized fibrosing leukocytoclastic vasculitis (CLFLCV) was recently suggested as a factor in cases of entomophthoramycosis of the face (171). Those authors stated that this phenomenon in infected areas could explain the tumofactive response to entomophthoralean fungi in these hosts. They stated that the detection of fibrosing vasculitis by histopathology is probably due to the persistence of immune complexes that could lead to tissue vasculitis-granulation, resulting in the accumulation of layered fibrous tissue (171). More importantly, fibrotic tissue could decrease lymphatic drainage, which could contribute to the accumulation of antigen and immune complexes in the infected areas. Choon et al. proposed that “CLFLCV is the driving process for inflammatory pseudotumor formation and lymphedema found in conidiobolomycosis” (171).
In addition, Choon et al. (171) suggested that chronic lymphedema could also play a role in the facial deformity observed in extreme chronic cases of rhinoconidiobolomycosis (118, 130). According to the CLFLCV hypothesis, long-standing lymphostasis could induce permanent tissue overgrowth, as in elephantiasis cases (130). Choon et al. (171) indicated that in chronic cases, the immune response does not control fungal growth. This fact could lead to inadequate lymphatic clearance of circulating fungal antigens, exacerbating localized lymphedema and favoring tissue overgrowth.
In cases of gastrointestinal basidiobolomycosis, exposure to entomophthoralean fungi could be caused by the ingestion of food contaminated with propagules of this fungus. Basidiobolus ranarum seems to survive gastric acidity, as per the numerous cases of intestinal basidiobolomycosis (171). It is likely that, after ingestion, B. ranarum propagules first survive the stomach acidic environment and then come into contact with the mucous membranes of the intestines. If the propagules encounter small intestinal abrasions (caused by sharp vegetable bodies, disrupted polyps, bacterial infection, or conditions of a disrupted epithelium), the propagules would attach to these sites, as described above, and initiate an infection (79, 102, 104) (Fig. 9 and 10). Interestingly, in immunocompromised hosts infected with C. incongruus, the typical eosinophilic Th2 response (eosinophils, Splendore-Höeppli phenomenon, and others) is missing (35, 49, 172). These hosts frequently develop dissemination of the pathogen to different organs, including lungs (49).
In summary, the ancestors of the entomophthoralean fungi developed an enzymatic approach early in their evolutionary history, probably to digest hardy organic matter usually not available to other microbes (11, 104). It is likely that they later transformed this enzymatic approach into a weapon to infect insects and eventually adapted this mechanism to cause disease in mammals (11, 102, 108). Usually, the entomophthoralean fungi remain in subcutaneous tissues as chronic granulomas that can persist for years in infected hosts (31, 32, 108).
CLINICAL FEATURES OF ENTOMOPHTHORAMYCOSIS
Conidiobolomycosis
Of all described Conidiobolus species, only three have been incriminated in human conidiobolomycosis: C. coronatus, C. incongruus, and C. lamprauges (35, 44, 50, 166, 170, 173). One of the main clinical features of infections caused by these species in humans is their preference for the subcutaneous tissues of the face (32, 119). Depending on the species involved, other anatomical sites and, more rarely, dissemination to internal organs are expected (35, 42, 50, 164). For an accurate diagnosis, the clinical aspects of the disease and a comprehensive history of the health status of the host, the epidemiology of the pathogen, the extended time of the infection, and its anatomical location are of importance (31, 32, 35). Once the clinical presentations of both conidiobolomycosis and basidiobolomycosis are documented, the use of computed tomography (CT) and/or magnetic resonance imaging (MRI) could be of help to support the clinical findings (see Clinical and Laboratory Diagnosis, below) (31, 138).
Conidiobolus coronatus.
Infections with C. coronatus commonly affect the subcutaneous tissues of the face (3, 31, 32, 164, 167). Good examples of this anatomical preference came from early studies of patients with rhinofacial swelling, and it was soon evident that this particular species has a strong tropism for subcutaneous tissues of the face (2, 45, 118, 167, 169). Rhinoconidiobolomycosis is typically caused by C. coronatus and starts with painless swelling around the soft tissues of the nose, slowly (weeks) extending to include adjacent tissue (44, 45, 174). It is usually found bilaterally, but some unilateral cases have also been mentioned (169). If not treated, the infection becomes chronic (several months to even years in some extreme cases) and progresses to indolent facial deformity (130). In early and chronic conidiobolomycosis cases, the terms atypical, early, intermediate, and late disease have been suggested (130). Under this proposal, (i) “atypical disease” is defined as ulcerations of the skin and/or invasion of the orbit, central nervous system, visceral organs, bones, muscles, and/or lymph nodes within 11 months after the onset of disease. (ii) “Early disease” includes cases in which disease is 1 or more weeks old and is diagnosed before the occurrence of the characteristic nodule at the nostril. This stage includes rhinitis, intermittent episodes of epistaxis, sinus pain, and mild swelling of the infected areas (Fig. 11A) (118, 130, 167, 169). (iii) “Intermediate disease” involves cases with 1 or more months of infection and coryza, epistaxis, nasal obstruction, noticeable reddishness, and swelling of the nose and nearby tissue, including enlarged lymph nodes (130). (iv) “Late disease” consists of chronic nontreated cases (several months and sometimes years) with the development of extreme facial deformity, and facial elephantiasis is the main feature of this stage, which may also involve regional lymph nodes (Fig. 11B and C) (130, 173, 174). The diagnosis of conidiobolomycosis at a particular stage is of importance because it seems to be an indication of the expected response to treatment. For instance, cases of early and intermediate disease responded very well to combination therapies (see below), whereas cases of both late and atypical disease are resilient to treatment (130).
FIG 11.
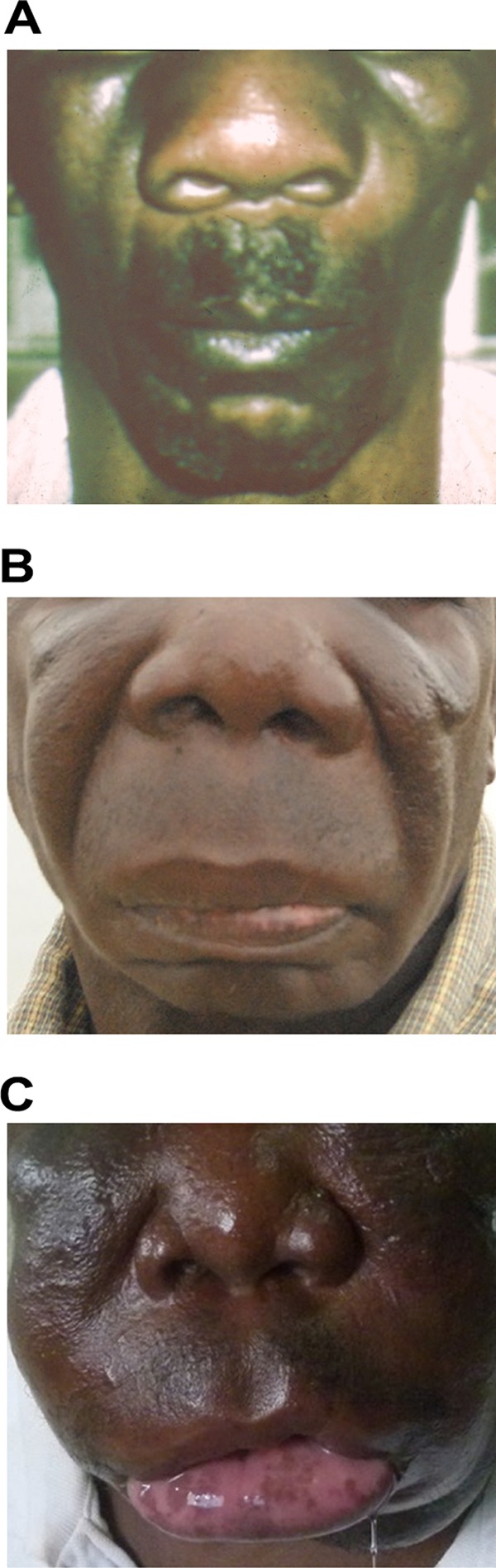
(A) An early case of conidiobolomycosis. (B and C) Two examples of late conidiobolomycosis infection. Extreme facial deformity of the patient is observed in panel B, whereas panel C depicts a case of facial elephantiasis caused by C. coronatus in a chronic nontreated case. (Panels B and C are reprinted from reference 130.)
Systemic cases involving C. coronatus in immunocompetent individuals, immunocompromised individuals, and drug users have been mentioned in many instances, without culture data (32, 35, 40, 119, 175). Good examples are the unusual finding of villous conidia in blood samples of infected patients using electron microscopy (176, 177) and a report of Conidiobolus hyphae in blood by Hoogendijk et al. (177). The presence of putative villous conidia in blood samples (176, 177) attributed to C. coronatus is not consistent with the large size of the fungus villous conidia (25 to 45 μm) versus the red blood cell size (6 to 8 μm) shown in their preparation. Likewise, the entomophthoralean ribbon-type hyphae, 9 to 18 μm in diameter, found near red blood cells (6 to 8 μm) cannot be related to entomophthoralean fungi (177). Although those researchers confirmed the presence of Conidiobolus by PCR, details on the specificity of the molecular test are missing.
Walker et al. (42) reported a systemic infection by C. coronatus, but the structures displayed in Fig. 7 are comparable to the zygospores developed by C. incongruus in culture. Similarly, Coelho-Filho (178) and Marwaha et al. (179) also reported systemic cases caused by putative Conidiobolus species involving the superior vena cava without culture. However, entomophthoralean fungi rarely invade blood vessels, and the pathological features of the observed slender hyphae are not typical of these fungi, so the possibility of infection caused by P. insidiosum has to be taken into consideration (114). Similar cases involving P. insidiosum within the lumen of large blood vessels have been reported in Thailand (114, 180, 181). Thus, the presence of C. coronatus in blood vessels is possible, but it is probably a rare event (32). Differential diagnoses include diseases such as actinomycosis, pythiosis, rhinosporidiosis, sporotrichosis, squamous cell carcinoma, subcutaneous mucormycosis, and others (31, 32, 35). Table 3 shows prominent clinical findings in cases involving Conidiobolus species.
TABLE 3.
Common clinical findings in individuals with subcutaneous, intestinal, or systemic basidiobolomycosis/conidiobolomycosis
| Species | Description (reference[s]) |
|||
|---|---|---|---|---|
| Infection of the face | Systemic infection | Subcutaneous infection other than the face | Intestinal infection | |
| Conidiobolus coronatus | Reported in apparently healthy individuals; clinical signs of early infection include serosanguinous nasal discharge and single or multiple nasal granulomas leading to nasal passage blockage; slowly growing granulomas involving adjacent tissue of the face including lips, mouth, sinus, nasopharynx, and others (164, 167, 210) | Some cases in immunocompromised and organ transplant patients (42); fever, anorexia, pleuritic and chest pain, fever, severe wt loss with lung involvement, and dissemination to blood vessels and brain are the main findings (165, 169, 171) | Unusual cases involving subcutaneous tissues in anatomical areas other than the face have been reported (45, 209) | There is a rare report without culture suggesting disseminating infection due to C. coronatus from the face to liver, kidney, and small intestine (210) |
| Conidiobolus incongruus | Cases in humans are rare; patients with this infection showed refractory fever, cellulitis of the forehead with sinusitis, obstruction of the nares, periorbital edema, and orbital inflammation (82, 172, 174, 180) | Systemic infections are common in immunocompromised hosts (35, 47); involvement of the lungs was a common feature in these patients; anorexia, persistent cough with or without hemoptysis, fever, and wt loss; invasion of internal organs, including liver and lymph nodes (49) | A case affecting the right foot in a diabetic female was diagnosed (179); the clinical description lacks details, but pain of the right foot with violaceous swelling and ulcers was noted | An unusual case involving the small intestine (jejunum) was reported (49); the infection started as a subcutaneous infection and spread to other organs, including intestine |
| Conidiobolus lamprauges | No human cases involving the face have been reported; however, in sheep, the main affected areas are the face and nasal passages with eosinophilia and the Splendore-Höeppli reaction (19) | Only one report of a 61-yr-old lymphoma patient has been documented (50); the patient developed severe pulmonary infection and expired soon after diagnosis; at autopsy, C. lamprauges was found in bladder, blood vessels, heart, kidney, lungs, spleen, thyroid gland, and urethra | Yet to be reported | Yet to be reported |
| Basidiobolus ranarum | A 5-yr-old Brazilian boy was diagnosed with a large swelling involving the right side of the face; the granuloma extended to the submandibular and retroauricular areas (189); an usual case involving the eye causing endophthalmitis was also recorded in India (190) | Rare infection among cases of intestinal basidiobolomycosis; usually, patients with intestinal infection with dissemination to liver, small bowel, gallbladder, pancreas, kidney, or retroperitoneum have been reported (142, 185, 186, 197, 227); pulmonary infection spread from a cutaneous lesion was also recorded (191) | Reported to occur in apparently healthy hosts; lesions are painless and found around the neck, trunk, limbs, buttocks, and, less frequently, other sites (51, 53, 183); usually, edematous extensive single granulomatous lesions are observed; in the infected areas, there is moderate to severe pruritus around nodular lesions with eroded to ulcerative granulomas (184, 188) | Reported to occur in apparently healthy individuals; common clinical signs include abdominal pain, abdominal distention, constipation, fever, vomiting, wt lost, rectal bleeding, hepatomegaly, perianal swelling, and palpable abdominal masses (142); these masses can be visualized in colon and rectum using CTa (101, 120, 193) |
See Table 4.
Mucormycosis restricted to subcutaneous tissues has been diagnosed in humans with severe skin trauma (122, 182, 183). The clinical features of subcutaneous mucormycosis share some characteristics with those of infections caused by entomophthoralean fungi. However, the inflammatory response in the infected tissues is remarkably different. Subcutaneous zygomycosis caused by mucoralean species does not trigger an eosinophilic response in the infected area, and the invading hypha lacks the Splendore-Höeppli phenomenon typical of entomophthoralean pathogens. Thus, the histopathological features of both diseases are key for an accurate differential diagnosis (32, 119, 122, 182).
Conidiobolus incongruus.
Infections caused by C. incongruus have been diagnosed in both apparently healthy and immunocompromised hosts (32, 49, 172, 174, 184). In contrast to C. coronatus, C. incongruus has been recovered in culture more often from cases of systemic infections than from cases of localized subcutaneous disease (31, 35, 40, 47–49). Thus, according to Blumentrath et al. (130), most infections involving C. incongruus are of the atypical form. This disease has also been reported to occur in other mammalian species (116, 185). Gilbert et al. (47) first reported an infant with a systemic infection caused by a Conidiobolus species. Eckert et al. (40) later evaluated this case, and the original fungal isolate was correctly identified as C. incongruus by King and Jong (48).
The above-described systemic cases start with mild swelling at the initially infected area (49, 167, 184). Depending on the initial anatomical site, the disease can spread to nearby tissues. In cases of orbital disease, the rhino-orbitocerebral area could be severely compromised (176). Symptoms and clinical signs in these areas include periorbital edema, pyroptosis, pruritus, visual impairment, and others (47, 174). Cases of dissemination from the orbital region to the cranial cavity have been observed (49, 172, 174, 184).
Conidiobolus lamprauges.
Infections caused by C. lamprauges were first diagnosed in lower animals (18, 19). Soon after, the only known human case involving this unusual species was reported, involving a Japanese patient with clinical symptoms of a systemic infection (50). The patient was a 61-year-old male undergoing treatment for relapsed lymphoma. During treatment, the patient developed fever and was later treated with antibiotics, without a response. A chest X ray showed bilateral lung infiltration, and laboratory testing found levels of β(1→3)-d-glucan consistent with a putative case of fungal infection. Despite treatment with several antifungal drugs, the patient expired.
By histopathology, the presence of a filamentous fungus invading blood vessels was found in the bladder, heart, kidney, lungs, spleen, thyroid gland, and urethra (50). Culture of the infected tissues revealed the presence of broad hyphae with numerous spherical thick-cell-wall zygospores similar to those seen with C. lamprauges. Molecular-based methodologies confirmed that C. lamprauges was the etiologic agent of this fatal case of disseminated infection. Of interest was the finding that this particular isolate has tropism for blood vessels and does not display the Splendore-Höeppli phenomenon in the infected areas. However, C. lamprauges in animals has a different clinical presentation than that seen in the described human case (50). In horses and sheep, infections caused by C. lamprauges are localized around the nasopharyngeal area and display an eosinophilic reaction with the Splendore-Höeppli phenomenon. In lower animals, dissemination to other organs is rare (19).
As discussed above, C. coronatus is commonly found to affect the face. Walker et al. (42) reported an unusual case of pulmonary disseminated infection caused by C. coronatus in an immunosuppressed individual. However, a closer look at the microscopic features of the isolate described by Walker et al. (42) showed the presence of sexual zygospores (named chlamydoconidia by those authors) very similar to the sexual structures found in C. incongruus. Thus, their isolate likely was a C. incongruus isolate causing disseminated infection, a plausible scenario supported by similar cases (see above) (35, 47, 49). In fact, lung infections involving C. incongruus and C. lamprauges have been sporadically reported (35, 49, 50). Clinical signs in patients with Conidiobolus lung infection include cough and lower lobe rales, in some cases with high fever or without fever. Some patients complained of pleuritic chest pain with low lobular intensive infiltrates, as also shown by chest radiography (35, 42). Most patients with lung involvement did not survive their infections because of dissemination of the pathogen to other anatomical areas (31). Table 3 shows prominent clinical findings in cases involving Conidiobolus species.
Basidiobolomycosis: Basidiobolus ranarum
Cutaneous and subcutaneous forms.
Basidiobolus ranarum was the first entomophthoralean fungus isolated from a horse (14) and later from a human with cutaneous basidiobolomycosis; both reports came from Indonesia (15). Basidiobolus ranarum is the most common species affecting mammals. As mentioned above, there is at least one report, confirmed by molecular methodologies, of B. meristosporus infection in humans (43). However, this report needs confirmation. In humans, the disease is characterized by the development of swelling erythematous nodular lesions with a “bathing suit” distribution around the buttocks, thighs, and limbs (Fig. 12A) (31, 186–190). The general conditions of infected hosts are remarkably good, and unless lesions become contaminated with bacteria, they are usually painless and evolve slowly for weeks and in some cases for years without fever. Characteristically, the affected skin areas do not indent after applied pressure, and lesions could ulcerate after nodular masses expand, increasing pressure over the subcutaneous affected areas (Fig. 12A) (191–193) (Table 3). Cases with an involvement of regional lymph nodes could resemble Burkitt's lymphoma cases (123, 124, 194, 195). In addition, an unusual case of endophthalmitis caused by B. ranarum was recently reported in a 59-year-old Indian man (125). Differential diagnoses comprise actinomycosis due to Actinomyces or Nocardia, chromoblastomycosis, cutaneous pythiosis, cutaneous tuberculosis caused by atypical Mycobacterium species, primary subcutaneous mucormycosis, and others.
FIG 12.
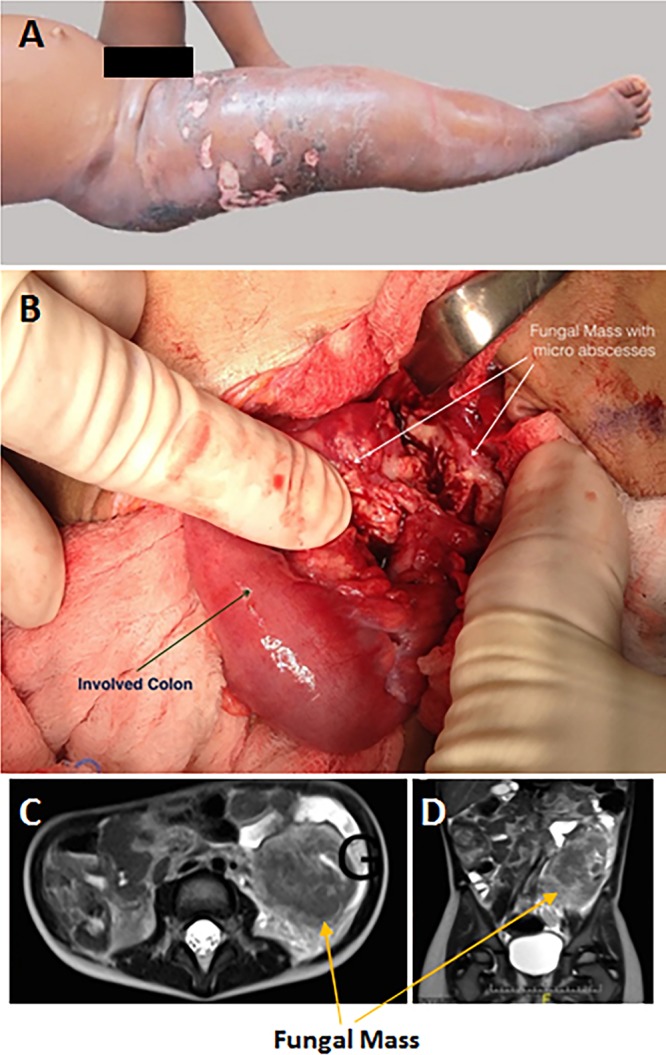
(A) A 3-year-old girl with extensive swelling on the upper section of her right lower limb affecting the inguinal area and buttocks. The presence of multiple ulcers and skin discoloration is also evident. (Reprinted from reference 187 with permission of the publisher.) (B) Exploratory intestinal laparotomy showing an edematous colon (arrow) surrounded by granulomatous masses displaying several microabscesses (arrows) in a case of human intestinal basidiobolomycosis. (C and D) Prior to surgery, MRI had shown a large predominantly hypointense retrocolic mass (arrows). (Panels B through D are reprinted from reference 200.)
Intestinal basidiobolomycosis.
In contrast to subcutaneous disease, the clinical symptoms of intestinal basidiobolomycosis are more difficult to detect, and thus, its diagnosis challenging (32, 164, 196). Most cases of intestinal basidiobolomycosis have been diagnosed by histopathology of biopsied tissues in the absence of culture (32). The presence of the Splendore-Höeppli phenomenon in the infected areas has been used to implicate B. ranarum as the etiologic agent (31, 186, 196–198). In fact, the first culture-proven case involving B. ranarum was reported in the United States in 1986 (89). Common clinical symptoms related to intestinal basidiobolomycosis include (i) bloody, mucous diarrhea; (ii) epigastric abdominal pain; (iii) intermittent low fever; (iv) intestinal bleeding; (v) vomiting; and (vi) the development of tumor-like masses involving the stomach and intestinal tissues (Fig. 12B to D) (199–202) (Table 3). Magnetic resonance imaging (Table 4) has been used to visualize tumor-like masses around the intestinal areas before surgical intervention (200). Spread of the invading hyphae to lymph nodes, liver, and pancreas has also been reported (170, 203, 204). Unusual cases of systemic infection involving lungs, maxillary sinus and palate, and muscle have also been described (41, 133, 191). Differential diagnoses comprises colon carcinoma, inflammatory bowel disease, intestinal pythiosis, intestinal parasitic infections, intestinal mucormycosis, and others (31, 32, 183, 199, 204). Table 3 shows prominent clinical findings in cases involving B. ranarum.
TABLE 4.
Computed tomography, magnetic resonance imaging, X ray, and ultrasound as diagnostic aids for putative cases of entomophthoramycosisa
| Procedure | Description (reference[s]) |
||
|---|---|---|---|
| Conidiobolomycosis |
Intestinal basidiobolomycosis | ||
| Face | Disseminated | ||
| CT | Evidence of tumor-like masses in left or right (or both) nasal cavity, with involvement of ethmoidal and/or sphenoidal sinuses (207); soft tissue thickening (126) | Frontal hypodensity suggesting intracerebral abscesses, pansinusitis, periorbital edema, orbital inflammation (172) | Thickening of the intestines with single or multiple tumor-like masses of the colon or rectum; in cases of disseminated infection, similar masses are found in liver, small bowel, gallbladder, pancreas, kidney, and/or peritoneum (192, 203, 210) |
| MRI | Increase in T2 signals of the nose and soft tissues of the upper lips; involvement of the dorsal cavity and infiltration of maxillary sinuses and nasal concha (167) | A study of a case of pericarditis caused by C. incongruus found large pericardial effusion, causing pericardial tamponade; irregular echogenic densities were also found near the left ventricle (35) | Circumferential thickening of cecum and rectum with significant luminal narrowing; retroperitoneal lymphoadenopathies (211) |
| Traditional X ray | Presence of soft tissue and opacities due to nasal and facial swelling, with obstruction of the airway passages of left or right antrum; frontal and maxillary sinusitis; no signs of bone involvement (208) | Generalized cardiac enlargement, bilateral pulmonary infiltration, posterior mediastinal tumor-like masses (40); progressive low infiltration of the lungs that soon evolves to adjacent sections of the affected lungs (35, 42, 50) | Abnormal collection of gas in the descending colon region; upper gastrointestinal thickened folds in the fundus and body of the stomach and the proximal duodenum (212) |
| Ultrasound | Recorded only for the right foot, attributed to C. incongruus (184) | ND | Detection of tumor-like masses in abdomen and pelvis and thickening of the colon and rectum (120); heterogeneous mass around the abdominal aorta (203) |
Shown are the most common findings with each of the selected procedures. ND, not determined (no reports).
PATHOLOGY
By histopathology, the infection seems confined to subcutaneous tissues and is rarely observed to invade nearby anatomical sites (164). The histopathological features of infections caused by the entomophthoralean fungi are comparable (Fig. 13A to D). In hematoxylin and eosin (H&E)-stained sections, infected tissues show extensive fibrosis and an inflammatory response, in cases of both conidiobolomycosis and basidiobolomycosis, containing numerous eosinophils and the formation of a reddish blanket (Splendore-Höeppli phenomenon) surrounding the invading hyphae (Fig. 13A to D). Characteristically, the hyphae appear unbranched (8 to 12 μm), forming small rings (transversally sectioned hypha) or short ribbon-type filaments with the presence of an occasional septum (Fig. 13A, C, and F). The inflammatory response could change depending on the stage of the disease (32, 137, 205). For instance, in acute cases, neutrophils and eosinophils can be found around the hyphae. In chronic cases, the presence of histiocytes, lymphocytes, and occasional giant cells along with some eosinophils are observed. It is important to mention that both entomophthoralean and mucoralean fungi display broad hyphae in infected tissues, and the presence of eosinophils and the Splendore-Höeppli phenomenon becomes a key element to separate these two groups of pathogens (31, 32, 139, 205, 206). However, cases in immunocompromised hosts infected with entomophthoralean species lacked the presence of eosinophils and the Splendore-Höeppli phenomenon. In these cases, the finding of coenocytic hyphae in infected tissues has to be interpreted with caution (31, 32, 35).
FIG 13.
(A) H&E-stained cross sections of Basidiobolus ranarum hyphae (arrows) from a case of intestinal basidiobolomycosis. Note the numerous eosinophils and the Splendore-Höeppli phenomenon. Bar, 20 μm. (B) Longitudinal B. ranarum hypha surrounded by numerous eosinophils and the Splendore-Höeppli phenomenon from a case of subcutaneous infection. Bar, 50 μm. (C and D) H&E-stained cross section (arrows) (bar, 10 μm) (C) and longitudinal hyphae of Conidiobolus coronatus surrounded by numerous eosinophils and the Splendore-Höeppli phenomenon (D) (bar, 12 μm) from a biopsy specimen of a conidiobolomycosis case involving the nostrils. (E) C. coronatus longitudinal hypha stained with periodic acid-Schiff stain. Bar, 10 μm. The content of the hypha is poorly stained, but the Splendore-Höeppli phenomenon is enhanced with the periodic acid-Schiff stain, indicating the presence of glycoproteins and polysaccharides. (F) C. coronatus stained with Grocott's methenamine silver. Bar, 8 μm. The presence of poorly stained coenocytic transverse and longitudinal filaments is evident.
Periodic acid-Schiff stain poorly stains the hyphal elements of both Conidiobolus and Basidiobolus (Fig. 13E and F). This stain reacts very well with the blanket around the hypha (Splendore-Höeppli phenomenon), indicating the presence of polysaccharides, such as glycoproteins and glycolipids, derived from the degranulation of eosinophils (Fig. 13E) (see above). Silver stain can also be used. Coenocytic hyphae appeared as dark hyphal elements with this stain (Fig. 13F).
CLINICAL AND LABORATORY DIAGNOSIS
Computed Tomography, Magnetic Resonance Imaging, X Ray, and Ultrasound
Noninvasive methodologies such as computed tomography (CT), magnetic resonance imaging (MRI), traditional X ray, and ultrasound have been widely used to aid in the clinical diagnosis of human entomophthoramycosis (Table 4). However, the presence of tissue thickening or infiltrates or the finding of tumor-like masses using the above-mentioned methodologies always needs confirmation by histopathological and/or laboratory procedures (49, 172). Using any of the above-mentioned methodologies, it was possible to visualize putative cases of entomophthoramycosis of the face (35, 42, 126, 189, 192). For instance, the finding in the nasal cavity of infiltration of the maxillary sinus, tumor-like masses, and opacities due to facial swelling was of importance not just to support the diagnosis of rhinoconidiobolomycosis but also for management (see below) (126, 167, 207, 208). Likewise, these tools were instrumental in determining the extent of damage in cases of disseminated conidiobolomycosis involving the brain (172) and the lungs (32, 35, 42, 50, 209) (Table 4). These methodologies have also been extensively used for cases of intestinal and disseminated basidiobolomycosis (120, 140, 192, 203, 210–212). Thickening of the intestine and the presence of tumor-like masses in the colon, rectum and, in cases of disseminated infection, liver, gallbladder, pancreas, and kidney were typical findings. In addition, these tools could be of special importance for physicians to determine the precise site for specimen collection.
Culture and Wet Mount Procedures
In addition to serum samples for serological analysis (see below), in suspected cases of entomophthoramycosis, a valuable specimen for the laboratory is biopsied tissue (206). The sample has to be collected in the advancing areas of the skin or from intestinal tumor-like masses. The biopsy specimen must be transported to the laboratory at room temperature in closed containers to avoid contamination. The use of cool temperatures (4°C) for transportation was found to inhibit B. ranarum growth in culture media (52, 197, 213–215). In the laboratory, the biopsy specimen is cut into 2-mm by 2-mm blocks, and it must be pushed into 2% Sabouraud dextrose agar (SDA) or blood agar plates before incubation. Plates are incubated at 37°C and at room temperature for 5 days (32, 52, 197). Colonies develop very rapidly at 37°C (24 to 48 h) and slightly more slowly (2 to 4 days) at room temperature for both Conidiobolus species and B. ranarum (Fig. 3 to 5 and 8). The colonies are identified as C. coronatus, C. incongruus, C. lamprauges, or B. ranarum after microscopic evaluation of their conidia (Fig. 2 to 4 and 7) (see above). The remaining 2-mm blocks are placed onto glass slides with 1 to 2 drops of 10% KOH (10 g KOH, 10 ml glycerol, 80 ml distilled water). The samples are gently warmed, and after 30 min of incubation in a wet chamber, they are microscopically inspected for the presence of broad hyphae. In positive samples, the hyphae appear hyaline and are surrounded by an opaque sheet of debris.
Serology
Although standardized serological tests for entomophthoramycosis have yet to be developed, several assays have been used to test sera from well-documented human and animal cases (131, 132, 138, 213). Typical cases of conidiobolomycosis of the face are traditionally diagnosed by histopathology and wet mounts (32, 137, 205). Although Kaufman et al. (132) found circulating anti-Conidiobolus precipitins in patients with conidiobolomycosis, the use of a serological approach for the diagnosis of Conidiobolus infection of the face is rare. Serological diagnosis of intestinal Basidiobolus infection, on the other hand, is a key element to support the finding of intestinal masses detected by CT, MRI, and X ray, which is confirmed by the presence of hyphae with eosinophils by histopathology (see above) (Table 4).
The first serological assays targeting entomophthoralean fungi were developed in the mid-1980s (132). Those investigators used immunodiffusion (ID) and exoantigens extracted from B. ranarum and C. coronatus to detect the presence of antibodies specific for entomophthoralean fungi in the sera of patients with entomophthoramycosis. Similar results were achieved by others using similar approaches (101, 202, 204, 206). ID was found to be 100% specific and 85 to 95% sensitive, although it has yet to be tested on the sera of immunocompromised hosts (131, 132).
An in-house ELISA was successfully used by Arya et al. (138) as an aid in the diagnosis of a case of basidiobolomycosis. In addition, a C. coronatus ELISA was also successfully tested in horses with conidiobolomycosis (Robert Glass, personal communication). However, the lack of standardization is a barrier to evaluate its true sensitivity and specificity features. The finding of a high-molecular-weight C. coronatus antigen that cross-reacted with sera containing anti-P. insidiosum antibodies in a Western blot suggested that the results of in-house ELISAs should be carefully monitored (213). Of interest was a report that the detection of β(1-3)-d-glucan antigen in an ELISA helped investigators suspect a fungal infection in a patient with a systemic infection, which was later identified as being caused by C. lamprauges (50). However, Wüppenhorst et al. (172) failed to detect this compound in the serum of a C. coronatus-infected patient. This report suggests that the detection of β(1-3)-d-glucan antigen in putative cases of entomophthoramycosis should be evaluated cautiously.
Molecular Tools
Few PCR primers have been tested to identify entomophthoralean fungi in infected tissues of putative cases of conidiobolomycosis or basidiobolomycosis (198, 199, 214, 216–218). However, as is the case with serology, the introduced PCR primers targeting C. coronatus and B. ranarum need to be standardized to validate their usefulness. Despite this drawback, Voigt et al. (218) designed two sets of primers, Cc1-Cc2 for C. coronatus and Ba1-Ba2 for B. haptosporus (B. ranarum), that specifically amplify 491 bp and 651 bp, respectively, of their 28S large-subunit ribosomal DNAs (rDNAs). Likewise, Lyon et al. (219), El-Shabrawi et al. (199), and Rothhardt et al. (220) used the above-described primers and specifically detected the presence of B. ranarum in the infected tissues of their patients. Kimura et al. (50) used universal primers for the internal transcribed spacer (ITS) region and sequencing analysis to confirm the presence of C. lamprauges in the infected tissues of an unusual case of systemic entomophthoramycosis. Preliminary steps for PCR standardization specifically targeting B. ranarum were done by Gómez-Muñoz et al. (217). They used two sets of primers from the 18S small-subunit rDNA (BasF611 and BasR1340) and tested them against several microbial DNAs, including those of fungi, parasites, and bacteria. Their results showed that the designed primers were highly specific. Overall, molecular methodologies are powerful tools that can be used to identify the presence of entomophthoralean fungi in infected tissues following appropriate standardization of primers and methodologies (217).
MANAGEMENT
Ever since the first report of entomophthoramycosis, iodine has been used with some success (130, 221–223). In vitro intrinsic resistance to amphotericin B was documented for Basidiobolus and Conidiobolus isolates (50, 138, 223, 224). However, this antifungal showed some success alone or in parallel with azoles (47, 53, 130). So far, there has been no consensus on the optimal antifungal drug to treat these infections (32, 50, 171, 221). The lack of standardized protocols and effective antifungal drugs to treat entomophthoramycosis, however, forced physicians to investigate new drugs and tools. Recent studies recognized that in the past 10 years, the most widely used therapeutic approaches were surgery (n = 37; 84% efficacy) and itraconazole (n = 27; 73% efficacy), followed by amphotericin B (n = 8; 22%) (142). Those investigators reported that among six patients (n = 37) treated only by surgery, four of them relapsed and later died of disseminated intra-abdominal basidiobolomycosis. The other two had no documented follow-up. Their results are consistent with the available data for the use of surgery alone (53, 138, 188, 225, 226). For instance, a recent retrospective review of 16 cases of subcutaneous basidiobolomycosis in children by Raveenthiran et al. (227) found that 8 surgically treated cases relapsed and eventually switched to KI and/or antifungals. Eleven children (including eight cases of recurrence after surgery) treated with KI alone were cured without relapses, three did not continue treatment, two had to be switched to itraconazole or amphotericin B, and one died, with perineal involvement. This study suggests that the use of KI is still a reliable option in low-income countries. Interestingly, in vitro studies showed that entomophthoralean fungi are resistant to KI (222). The precise in vivo mechanism of the KI killing effect on entomophthoralean fungi is unknown (223). Sterling et al. (228), working with this compound, suggested that the killing may be related to in vivo fungal cell wall degeneration and the activation of key immunological mechanisms. However, the finding that monocyte or neutrophil killing of fungi was not increased after treatment with KI does not support the hypothesis of in vivo immunological enhancement (228).
A synergistic effect was reported when this antifungal was used in combination with azoles (53, 162, 222). An example of a putative synergistic effect was reported by Fischer et al. (167). They found by in vitro susceptibility testing that C. coronatus was resistant to both amphotericin B and itraconazole. Conversely, when both antifungals were used in a host with rhinoconidiobolomycosis, cure was achieved (167). Thus, the synergistic effect of amphotericin B and azoles, like itraconazole, is promising, and more synergy studies for putative cases of entomophthoramycosis need to be performed. Because studies of the management of both conidiobolomycosis and basidiobolomycosis with antifungal drugs, iodine, and surgery showed controversial results, a combination of strategies was recently proposed (31, 50, 82, 130).
A good example of the multiple-therapy approach is a report by Temple et al. (82). These investigators successfully treated C. incongruus orbital disease in a girl with a combination of antifungals (amphotericin B plus itraconazole), hyperbaric oxygen, and surgery. Kimura et al. (50) achieved partial improvement using a combination of hyperbaric oxygen, miconazole, trimethoprim-sulfamethoxazole, and surgical resection, although the patient later died of systemic infection. Recently, Shaikh et al. (31), after reviewing the available therapeutic choices, concluded that a combination of therapies is the best option in the majority of cases involving Basidiobolus and Conidiobolus species.
Because of the intrinsic resistance to amphotericin B and its severe side effects, it is not the ideal first choice of physicians; thus, other antifungals have been tested, with variable success (222). Itraconazole and ketoconazole are good examples (see above) (197, 223, 225, 228) (Table 5). Two other imidazoles have recently been used successfully in cases of intestinal basidiobolomycosis, posaconazole (165) and voriconazole (121, 140–142, 197). Posaconazole is effective as prophylaxis and treatment in patients with hematologic malignancies (229). This antifungal was successfully tested first in a systemic infection caused by the mucoralean fungus Rhizopus microsporus (229) and more recently in a case of a 67-year-old patient with gastrointestinal basidiobolomycosis (165). Although posaconazole has been used in only one case, there are potential advantages: (i) it does not require dose adjustment to avoid hepatic or renal side effects; (ii) it is well tolerated, with few side effects (loss of hair and dry mouth and skin); (iii) it is not susceptible to changes induced by other medications; and (iv) it can be taken orally. However, the cost, the lack of an intravenous formulation (intensive care patients), and its various absorption patterns have to be evaluated carefully. A case reported by Rose et al. (165) successfully responded to 1 year of oral posaconazole (200 mg/day). Voriconazole is a triazole with a broad spectrum of antifungal activity. This drug was recently used by Albaradi et al. (140) to treat an 11-year-old at doses of 150 mg twice a day; the boy responded to the therapy, without the need for surgical intervention. Similar outcomes were also reported for children with intestinal basidiobolomycosis (121, 141). The above-described cases treated successfully with these two new triazoles suggest that these antifungals can be recommended as monotherapies. However, more cases need to be evaluated to validate their antifungal efficacies against Conidiobolus and Basidiobolus species.
TABLE 5.
Common procedures for the management of basidiobolomycosis and conidiobolomycosisa
| Etiology | Treatment |
|||
|---|---|---|---|---|
| Antifungal(s) | KI | Surgery alone or with antifungals | Other | |
| Conidiobolus coronatus | AB (35%), F (19%), FL (9%), K (32%), ITC (19%), M (5%), T (2%)b | 63% | 32% | Hyperbaric O2, radiation, steroids (12%)c |
| Conidiobolus incongruus | AB alone or in combination with CL, FL, ITC, K, or Vc | Rarely used in systemic cases | Surgery used for at least 1 case of face infectionf | Hyperbaric O2 or radiation did not predict a favorable resultc |
| Conidiobolus lamprauges | AB alone and in combination with MF and V did not cure the only known case; the recovered isolate showed resistance to several antifungalsd | ND | ND | ND |
| Basidiobolus ranarum | AB (22%), ITC (73%), K (8%), V (5%)b; P was used successfully in 1 casee | Used successfullyg | 84% | ND |
n = 172 (171) and n = 44 (142). ND, not done; AB, amphotericin B; CL, clotrimazole; F, fluconazole; FC, flucytosine; ITC, itraconazole; K, ketoconazole; M, miconazole; MF, micafungin; P, posaconazole; T, terbinafine; V, voriconazole. Percentages are success rates.
See reference 142.
See reference 171.
See reference 50.
See reference 165.
See reference 82.
See reference 123.
Overall, the findings of the last 30 years have shown that chronic, persistent, or progressing cases of entomophthoramycosis responded poorly to the available monotherapies (31, 32, 130, 230–232). In comparison, good prognoses were the case for patients treated early with antifungals, iodine, hyperbaric oxygen, surgery, or a combination of the above-mentioned therapeutic choices (31, 130). Despite the above-described therapeutic approaches, the prognosis for immunocompromised patients with disseminated C. incongruus and C. lamprauges infections remains reserved (31, 49, 50). Table 5 shows the most common antifungal drugs used so far in the management of Basidiobolus and Conidiobolus infections.
The lack of both an animal model and research funds to investigate this neglected group of pathogens has an impact on the way in which we approach their management. We believe that the search for new drugs and methodologies could directly benefit patients in underdeveloped countries. The findings of new drugs could open the door for novel interventions, such as immunotherapy, that could also have potential applications for other mycoses.
CONCLUSION
The human pathogenic entomophthoralean fungi B. ranarum and Conidiobolus species have been identified as causing subcutaneous, intestinal, and systemic disease in humans inhabiting tropical and subtropical areas. The fact that the disease is frequently diagnosed in poor populations of the world and is often reported as single cases rather than as causing epidemics has played a role in the lack of interest from the scientific community, the pharmaceutical industry, and funding agencies. However, as the accuracy and availability of diagnostic tools have improved, the number of cases reported in areas of endemicity has increased. Research on these neglected pathogens could reveal novel features that could lead to an understanding of the management of other pathogens. For instance, the histopathological features seen in pathogenic entomophthoralean fungal infections are comparable to those engendered by other pathogenic microbes, such as parasites. The immunogens of P. insidiosum, another neglected pathogen with an identical inflammatory response, for example, displayed immunoregulatory features, a finding also replicated in parasitic infections. Thus, one can speculate that these pathogens express yet-to-be-investigated antigens that can be used to modulate the immune response for the management of these and other processes. We believe that new research strategies for the entomophthoralean fungi have to be explored to investigate the molecular interaction of these organisms with the immune systems of their hosts. The development of this type of data could convince funding agencies to include these pathogens in their priorities.
ACKNOWLEDGMENTS
We thank C. G. Blumentrath and F. Schaumburg (Fig. 11B and C) for the figures used for the clinical aspects of conidiobolomycosis and A. Sackey (Fig. 12A) and P. Mandhan (Fig. 12B to D) for the figures used for the clinical aspects of basidiobolomycosis.
Biographies

Raquel Vilela, M.Sc., M.D., Ph.D., graduated in Pharmacy and Biochemistry from the Federal University of Minas Gerais (UFMG), Brazil. Later, she concluded her master and doctorate studies on infectious diseases at the UFMG and Michigan State University (MSU) followed by two postdoctorals at MSU. Later in her career, she obtained her final degree in human medicine. Currently, she conducts research on infectious diseases of anomalous pathogens. She is also involved in projects dealing with Mechanical Engineering of 3D prostheses and biotechnology, where she developed topical therapeutic drugs for skin regeneration in patients undergoing radiotherapy. She is Adjunct Professor at the UFMG and holds an Adjunct Professorship at the Biomedical Laboratory Diagnostics, MSU. For the period from 2005 to 2008, she was adjunct professor at the Department of Dermatology, School of Human Medicine, MSU. She has published numerous scientific articles as well as book chapters. Current research is focused on molecular topics related to neglected microbes.

Leonel Mendoza, M.Sc., Ph.D., concluded his master degree at the University of Guelph, Canada, and his Ph.D. at the University of Texas at Austin. He currently is a Professor at Michigan State University. His research focus is on the infections caused by the stramenopilan oomycete pathogen Pythium insidiosum. Other research interests involve the study of the fungus-like organisms Rhinosporidium seeberi, Lagenidium giganteum, and Paralagenidium spp. Because R. seeberi is intractable to culture, it remained a taxonomic mystery. Using molecular approaches, he recently found that R. seeberi was a protistan organism closely related to fish pathogens. In collaboration with Dr. Vilela, he studied Lacazia loboi, another uncultivated pathogen of dolphins and humans. The dolphin pathogen was a variety of Paracoccidioides brasiliensis named by them P. brasiliensis var. ceti, and the pathogen causing human disease was a species in the genus Paracoccidioides, now P. loboi. Current research is focused on molecular topics related to these neglected microbes.
REFERENCES
- 1.Ader P, Dodd JK. 1979. Mucormycosis and entomophthoromycosis. Mycopathologia 68:67–99. doi: 10.1007/BF00441088. [DOI] [PubMed] [Google Scholar]
- 2.Blaché R, Destombes P, Nazimoff O. 1961. Mycoses sous-cutanées nouvelles an Sud-Cameroun. Bull Soc Path Exot 54:56–63. [Google Scholar]
- 3.Bras G, Gordon CC, Emmons CW, Prendegast KM, Sugar M. 1965. A case of phycomycosis observed in Jamaica; infection with Entomphthora coronata. Am J Trop Med Hyg 14:141–145. doi: 10.4269/ajtmh.1965.14.141. [DOI] [PubMed] [Google Scholar]
- 4.Brefeld O. 1884. Conidiobolus utricolosis und minor. Untersuchungen aus dem Gesamtgebiete der. Mykologie 4:75–78. [Google Scholar]
- 5.Couch JN. 1939. A new Conidiobolus with sexual reproduction. Am J Bot 26:119–130. doi: 10.1002/j.1537-2197.1939.tb12878.x. [DOI] [Google Scholar]
- 6.Callaghan AA. 1969. Light and spore discharge in Entomophthorales. Trans Br Mycol Soc 53:87–97. doi: 10.1016/S0007-1536(69)80010-0. [DOI] [Google Scholar]
- 7.Constatin M. 1897. Sur un Entomorphthoree nouvelle. Bull Soc Mycol France 13:38–43. [Google Scholar]
- 8.Drechsler C. 1956. Widespread distribution of Delacroixia coronata and other saprophytic Entomophthoraceae in plant detritus. Science 115:575–576. doi: 10.1126/science.115.2995.575. [DOI] [PubMed] [Google Scholar]
- 9.Drechsler C. 1958. Formation of sporangia from conidia and hyphal segments in an Indonesian Basidiobolus. Am J Bot 45:632–638. doi: 10.1002/j.1537-2197.1958.tb10595.x. [DOI] [Google Scholar]
- 10.Fresenius G. 1858. Über die Pilzgattung Entomophthora. Abh Seckenberg Naturforsch Ges 2:201–210. [Google Scholar]
- 11.Batko A. 1974. Phylogenesis and taxonomic structure of the Entomophthoraceae, p 209–305. In Nowinski C. (ed), Ewolucja biologiczna: szkice teoretyczne i metodologiczne. Polska Akademia Nauk, Instytyt Filozofii i Socjologii, Warsaw, Poland. [Google Scholar]
- 12.Humber RA. 2008. Evolution of entomopathogenicity in fungi. J Invertebr Pathol 98:262–266. doi: 10.1016/j.jip.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Hall IM, Dunn PH. 1957. Entomophthorous fungi parasitic on the spotted alfafa aphid. Hilgardia 27:159–167. doi: 10.3733/hilg.v27n04p159. [DOI] [Google Scholar]
- 14.Van Overeem C. 1925. Uber ain merkwurdiges Vorkommen von Basidiobolus ranarum Eidam. Bull Jardin Bot 7:423–431. [Google Scholar]
- 15.Joe LK, Njo-Injo TE, Pohan A, Van Der Maulen H. 1956. Basidiobolus ranarum as a cause of subcutaneous phycomycosis in Indonesia. Arch Dermatol Syphol 74:378–383. doi: 10.1001/archderm.1956.01550100046008. [DOI] [PubMed] [Google Scholar]
- 16.Connole MD. 1973. Equine phycomycosis. Aust Vet J 49:214–215. doi: 10.1111/j.1751-0813.1973.tb06796.x. [DOI] [PubMed] [Google Scholar]
- 17.Emmons CW, Bridges CH. 1961. Entomophthora coronata, the etiologic agent of a phycomycosis of horses. Mycologia 53:307–312. doi: 10.2307/3756277. [DOI] [Google Scholar]
- 18.Humber RA, Brown CC, Kornegay RW. 1989. Equine zygomycosis caused by Conidiobolus lamprauges. J Clin Microbiol 27:573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vilela R, Silva SM, Riet-Correa F, Dominguez E, Mendoza L. 2010. Morphologic and phylogenetic characterization of Conidiobolus lamprauges recovered from infected sheep. J Clin Microbiol 48:427–432. doi: 10.1128/JCM.01589-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greer DL, Friedman L. 1966. Studies on the genus Basidiobolus with reclassification of the species pathogenic for man. Sabouraudia 4:231–241. doi: 10.1080/00362176685190521. [DOI] [PubMed] [Google Scholar]
- 21.Lakon G. 1939. Entomophthoraceen-Studien V-VI. Z Angew Entomol 26:517–521. doi: 10.1111/j.1439-0418.1939.tb01576.x. [DOI] [Google Scholar]
- 22.Frey R. 1950. Chitin und zellulose in Pilzzellwanden. Ber Schweiz Bot Ges 60:199–230. [Google Scholar]
- 23.Humber RA. 1984. Foundations for an evolutionary classification of the Entomophthorales (Zygomycetes), p 166–183. In Wheeler Q, Blackwell M (ed), Fungus/insect relationships: perspectives in ecology and evolution. Columbia University Press, New York, NY. [Google Scholar]
- 24.Ainsworth GC. 1973. Introduction and keys to higher taxa, p 1–7. In Ainsworth GC, Sparrow FK, Sussman AS (ed), The Fungi. IVA. A taxonomic review with keys. Academic Press, New York, NY. [Google Scholar]
- 25.Hutchinson JA, King DS, Nickerson MA. 1972. Studies on temperature requirements, odor production and zygospore wall undulation of the genus Brasidiobolus. Micologia 64:467–474. doi: 10.2307/3757864. [DOI] [PubMed] [Google Scholar]
- 26.McGinnis MR. 1980. Recent taxonomic developments and changes in medical mycology. Annu Rev Microbiol 34:109–135. doi: 10.1146/annurev.mi.34.100180.000545. [DOI] [PubMed] [Google Scholar]
- 27.Humber RA. 1982. Strongwellsea vs. Erynia: the case for a phylogenetic classification of the Entomophthorales (Zygomycetes). Mycotaxon 15:167–184. [Google Scholar]
- 28.Humber RA. 1981. An alternative view of certain taxonomic criteria used in the Entomophthorales (Zygomycetes). Mycotaxon 13:191–240. [Google Scholar]
- 29.Humber RA. 1989. Synopsis of a revised classification for the Entomophthorales (Zygomycotina). Mycotaxon 34:441–460. [Google Scholar]
- 30.Humber RA. 2012. Entomophthoromycota: a new phylum and reclassification for entomophthoroid fungi. Mycotaxon 120:477–492. doi: 10.5248/120.477. [DOI] [Google Scholar]
- 31.Shaikh N, Hussain KA, Petraitiene R, Schuetz AN, Walsh TJ. 2016. Entomophthoramycosis: a neglected tropical mycosis. Clin Microbiol Infect 22:688–694. doi: 10.1016/j.cmi.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Mendoza L, Vilela R, Voelz K, Ibrahim AS, Lee SC. 2015. Human fungal pathogenic of Mucorales and Entomophthorales. Cold Spring Harb Perspect Med 5:a019562. doi: 10.1101/cshperspect.a019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson M. 2009. The ecology of the zygomycetes and its impact on environmental exposure. Clin Microbiol Infect 15(Suppl 5):2–9. doi: 10.1111/j.1469-0691.2009.02972.x. [DOI] [PubMed] [Google Scholar]
- 34.Ribes JA, Vanover-Sams CL, Baker DJ. 2000. Zygomycosis in human disease. Clin Microbiol Rev 13:236–301. doi: 10.1128/CMR.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh TJ, Renshaw G, Andrews J, Kwon-Chung J, Cunnion RC, Pass HI, Taubenberger J, Wilson W, Pizzo PA. 1994. Invasive zygomycosis due to Conidiobolus incongruus. Clin Infect Dis 19:423–430. doi: 10.1093/clinids/19.3.423. [DOI] [PubMed] [Google Scholar]
- 36.Joe LK, Harsono T, Rukmono, Eng N-IT. 1962. Subcutaneous phycomycosis in man in Indonesia: description of three new cases. Trop Med Hyg 65:38–42. [Google Scholar]
- 37.Symmers WSC. 1960. Mucormycotic granuloma possibly due to Basidiobolus ranarum. Br Med J i:1331–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynch JB, Husband AD. 1962. Subcutaneous phycomycetosis. J Clin Pathol 15:126–132. doi: 10.1136/jcp.15.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gatti F, Renoirtte P, Nootens J, Seynhaeve V. 1968. Premier cas de phycomycose sous-cutanée due à Basidiobolus meristosporus observe en République Démocratique du Congo. Ann Soc Belge Med Trop 48:455–462. [PubMed] [Google Scholar]
- 40.Eckert HL, Khoury GH, Pore RS, Gilbert EF, Gaskell JR. 1972. Deep Entomophthora phycomycotic infection reported for the first time in the United States. Chest 61:392–394. doi: 10.1378/chest.61.4.392. [DOI] [PubMed] [Google Scholar]
- 41.Dworzack DL, Pollock AS, Hodges GR, Barnes WG, Ajello L, Padhye A. 1978. Zygomycosis of the maxillary sinus and palate caused by Basidiobolus haptosporus. Arch Intern Med 138:1274–1276. doi: 10.1001/archinte.1978.03630330074022. [DOI] [PubMed] [Google Scholar]
- 42.Walker SD, Clark RV, King CT, Humphries JE, Lytle LS, Butkus DE. 1992. Fatal disseminated Conidiobolus coronatus infection in a renal transplant patient. Am J Clin Pathol 98:559–564. doi: 10.1093/ajcp/98.6.559. [DOI] [PubMed] [Google Scholar]
- 43.Sitterlé E, Rodriguez C, Mounier R, Calderaro J, Foulet F, Develoux M, Pawlotsky JM, Botterel F. 2017. Contribution of ultra deep sequencing in the clinical diagnosis of a new fungal pathogen species: Basidiobolus meristosporus. Front Microbiol 8:334. doi: 10.3389/fmicb.2017.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andrade ZA, Paula LA, Sherlock IA, Cheever AW. 1967. Nasal granuloma caused by Entomophthora coronata. Am J Trop Med Hyg 16:31–33. doi: 10.4269/ajtmh.1967.16.31. [DOI] [PubMed] [Google Scholar]
- 45.Martinson FD, Clark BM. 1967. Rhinophycomycosis entomophthorae in Nigeria. Am J Trop Med Hyg 16:40–47. doi: 10.4269/ajtmh.1967.16.40. [DOI] [PubMed] [Google Scholar]
- 46.Restrepo MA, Greer DL, Robledo VM, Diaz GC, Lopez MR, Bravo RC. 1967. Subcutaneous phycomycosis: report of the first case observed in Colombia, South America. Am J Trop Med Hyg 16:34–39. doi: 10.4269/ajtmh.1967.16.34. [DOI] [PubMed] [Google Scholar]
- 47.Gilbert EF, Khoury EM, Pore RS. 1970. Histopathological identification of Entomophthora phycomycosis. Deep mycotic infection in an infant. Arch Pathol 90:583–587. [PubMed] [Google Scholar]
- 48.King DS, Jong SC. 1976. Identity of the etiological agent of the first deep entomophthoraceous infection of man in the United States. Mycologia 68:181–183. doi: 10.2307/3758911. [DOI] [PubMed] [Google Scholar]
- 49.Busapakum R, Youngchaiyud U, Srimpai S, Segretain G, Frommentin H. 1983. Disseminated infection with Conidiobolus incongruus. Sabouraudia 21:323–330. doi: 10.1080/00362178385380471. [DOI] [PubMed] [Google Scholar]
- 50.Kimura M, Yaguchi T, Sutton DA, Fothergill AW, Thompson EH, Wickes BL. 2011. Disseminated human conidiobolomycosis due to Conidiobolus lamprauges. J Clin Microbiol 49:752–756. doi: 10.1128/JCM.01484-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bittencourt AL, Londero AT, Araujo MGS, Mendonça N, Bastos JLA. 1979. Occurrence of subcutaneous zygomycosis caused by Basidiobolus haptosporus in Brazil. Mycopathologia 68:101–104. doi: 10.1007/BF00441089. [DOI] [PubMed] [Google Scholar]
- 52.Geramizadeh B, Heidari M, Shekarkhar G. 2015. Gastrointestinal basidiobolomycosis, a rare and under-diagnosed fungal infection in immunocompetent hosts: a review article. Iran J Med Sci 4:90–97. [PMC free article] [PubMed] [Google Scholar]
- 53.Gugnani HC. 1999. A review of zygomycosis due to Basidiobolus ranarum. Eur J Epidemiol 15:923–929. doi: 10.1023/A:1007656818038. [DOI] [PubMed] [Google Scholar]
- 54.Gryganskyi AP, Humber RA, Smith ME, Hodge K, Huang B, Voigt K, Vilgalys R. 2013. Phylogenetic lineages in Entomophthoromycota. Persoonia 30:94–105. doi: 10.3767/003158513X666330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waterhouse GM. 1973. Entomophthorales, p 219–229. In Ainsworth GC, Sparrow FK, Sussman AS (ed), The Fungi. IVB. Academic Press, New York, NY. [Google Scholar]
- 56.Srinivasan MC, Thirumalachar MJ. 1967. Evaluation of taxonomic characters in the genus Conidiobolus, with key to known species. Mycologia 59:689–713. doi: 10.2307/3757097.6042868 [DOI] [Google Scholar]
- 57.Tyrrell D, MacLeod D. 1972. A taxonomic proposal regarding Delacroixia coronata (Entomopgthoraceae). J Invertebr Pathol 20:11–13. doi: 10.1016/0022-2011(72)90074-2. [DOI] [Google Scholar]
- 58.James TY, Kauff F, Schoch C, Matheny PB, Hofstetter V, Cox CJ, Celio G, Gueidan C, Fraker E, Miadlikowska J, Lumbsch HT, Rauhut A, Reeb V, Arnold AE, Amtoft A, Stajich JE, Hosaka K, Sung GH, Johnson D, O'Rourke B, Crockett M, Binder M, Curtis JM, Slot JC, Wang Z, Wilson AW, Schüssler A, Longcore JE, O'Donnell K, Mozley-Standridge S, Porter D, Letcher PM, Powell MJ, Taylor JW, White MM, Griffith GW, Davies DR, Humber RA, Morton JB, Sugiyama J, Rossman AY, Rogers JD, Pfister DH, Hewitt D, Hansen K, Hambleton S, Shoemaker RA, Kohlmeyer J, Volkmann-Kohlmeyer B, Spotts RA, et al. . 2006. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443:818–822. doi: 10.1038/nature05110. [DOI] [PubMed] [Google Scholar]
- 59.Jensen AB, Gargas A, Eilenberg J, Rosendahl S. 1998. Relationship of the insect-pathogenic order Entomophthorales (Zygomycota, Fungi) based on phylogenetic analyses of nuclear small subunit ribosomal DNA sequences (SSU rDNA). Fungal Genet Biol 24:325–334. doi: 10.1006/fgbi.1998.1063. [DOI] [PubMed] [Google Scholar]
- 60.Nagahama T, Sato H, Shimazu M, Sugiyama J. 1995. Phylogenetic divergence of the entomophthoralean fungi: evidence from nuclear 18S ribosomal RNA gene sequences. Mycologia 87:203–209. doi: 10.2307/3760906. [DOI] [Google Scholar]
- 61.Liu X-Y, Voigt K. 2010. Molecular characters of zygomycetous fungi, p 93–105. In Gherbawy Y, Voigt K (ed), Molecular identification of fungi. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 62.Voigt K, Kirk PM. 2011. Recent developments in the taxonomic affiliation and phylogenetic positioning of fungi: impact in applied microbiology and environmental biotechnology. Appl Microbiol Biotechnol 90:41–57. doi: 10.1007/s00253-011-3143-4. [DOI] [PubMed] [Google Scholar]
- 63.Gryganskyi AP, Humber RA, Smith ME, Miadlikovska J, Wu S, Voigt K, Walther G, Anishchenko IM, Vilgalys R. 2012. Molecular phylogeny of the Entomophthoromycota. Mol Phylogenet Evol 65:682–694. doi: 10.1016/j.ympev.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 64.Spatafora JW, Chang Y, Benny GL, Lazarus K, Smith ME, Berbee ML, Bonito G, Corradi N, Grigoriev I, Gryganskyi A, James TY, O'Donnell K, Roberson RW, Taylor TN, Uehling J, Vilgalys R, White MM, Stajich JE. 2016. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108:1028–1046. doi: 10.3852/16-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun NC. 1970. The ultra-structure of the nuclear division of Basidiobolus ranarum Eidam. PhD dissertation. Iowa State University, Ames, IA. [Google Scholar]
- 66.Henk DA, Fisher MC. 2012. The gut fungus Basidiobolus ranarum has a large genome and different copy numbers of putatively functionally redundant elongation factor genes. PLoS One 7:e31268. doi: 10.1371/journal.pone.0031268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McKerracher LJ, Heath IB. 1985. The structure and cycle of the nucleus-associated organelle in two species of Basidiobolus. Mycologia 77:412–417. doi: 10.2307/3793197. [DOI] [Google Scholar]
- 68.Tanabe Y, Watanabe MM, Sugiyama J. 2005. Evolutionary relationships among basal fungi (Chytridiomycota and Zygomycota): insights from molecular phylogenetics. J Gen Appl Microbiol 51:267–276. doi: 10.2323/jgam.51.267. [DOI] [PubMed] [Google Scholar]
- 69.White MM, James TY, O'Donnell KO, Cafaro MJ, Tanabe Y, Sugiyama J. 2006. Phylogeny of the Zygomycota based on nuclear ribosomal sequence data. Mycologia 98:872–884. doi: 10.1080/15572536.2006.11832617. [DOI] [PubMed] [Google Scholar]
- 70.Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lücking R, Lumbsch HT, Lutzoni F, Matheny PB, McLaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai YC, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Kõljalg U, Kurtzman CP, Larsson KH, Lichtwardt R, Longcore J, Miadlikowska J, Miller A, Moncalvo JM, Mozley-Standridge S, Oberwinkler F, Parmasto E, Reeb V, et al. . 2007. A higher-level phylogenetic classification of the Fungi. Mycol Res 111:509–547. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Gryganskyi AP, Muszewska A. 2014. Whole genome sequencing and the Zygomycota. Fungal Genom Biol 4:e116. doi: 10.4172/2165-8056.1000e116. [DOI] [Google Scholar]
- 72.Kwon-Chung KJ. 2012. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clin Infect Dis 54(Suppl 1):S8–S15. doi: 10.1093/cid/cir864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoddinott J, Olsen OA. 1972. A study of the carbohydrates in the cell wall of some species of the Entomophthorales. Can J Bot 50:1675–1679. doi: 10.1139/b72-206. [DOI] [Google Scholar]
- 74.Lowe RE, Rumbauch RB, Patterson RS. 1968. Entomophthora coronata as a pathogen of mosquitoes. J Invertebr Pathol 11:506–507. doi: 10.1016/0022-2011(68)90201-2. [DOI] [PubMed] [Google Scholar]
- 75.Coremans-Pelseneer J. 1976. Biologie des champignos du genere Basidiobolus Eidman 1886: saprophytisme et puvoir pathogene. Acta Zool Pathol Antverp 60:1–143. [PubMed] [Google Scholar]
- 76.Roy HE, Steinkraus DC, Eilenberg J, Hajek AE, Pell JK. 2006. Bizarre interactions and endgames: entomopathogenic fungi and their arthropod hosts. Annu Rev Entomol 51:331–357. doi: 10.1146/annurev.ento.51.110104.150941. [DOI] [PubMed] [Google Scholar]
- 77.De Fine Licht HH, Hajek AE, Eilenberg J, Jensen AB. 2016. Utilizing genomics to study entomopathogenicity in the fungal phylum Entomophthoromycota: a review of current genetic resources. Adv Genet 94:41–65. doi: 10.1016/bs.adgen.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Robinow CF. 1963. Observations on cell growth, mitosis and division in the fungus Basidiobolus ranarum. J Cell Biol 17:123–152. doi: 10.1083/jcb.17.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garrison RG, Mariat F, Boyd KS, Tally JF. 1975. Ultrastructure and electron cytochemical studies of Entomophthora coronata. Ann Microbiol (Paris) 126:149–173. [PubMed] [Google Scholar]
- 80.Gull K, Trinci APJ. 1975. Septa ultrastructure in Basidiobolus ranarum. Sabouraudia 13:49–51. doi: 10.1080/00362177585190101. [DOI] [PubMed] [Google Scholar]
- 81.MacLeod DM, Müller-Kögler E. 1970. Insect pathogens: species described originally from their resting spores mostly as Tarichium species (Entomophthorales: Entomophthoraceae). Mycologia 62:33–66. doi: 10.2307/3757710. [DOI] [PubMed] [Google Scholar]
- 82.Temple ME, Brady MT, Koranyi KI, Nahata MC. 2001. Periorbital cellulitis secondary to Conidiobolus incongruus. Pharmacotherapy 21:351–354. doi: 10.1592/phco.21.3.351.34210. [DOI] [PubMed] [Google Scholar]
- 83.Werner S, Persoh D, Rambold G. 2012. Basidiobolus haptosporus is frequently associated with the gamasid mite Leptogamasus obesus. Fungal Biol 116:90–97. doi: 10.1016/j.funbio.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 84.Eidam E. 1886. Basidiobolus eineneue gattung der Entomophthoracean. Beitr Biol Pflanz 4:181–251. [Google Scholar]
- 85.Dykstra MJ. 1994. Ballistosporic conidia in Basidiobolus ranarum: the influence of light and nutrition on the production of conidia and endospores (sporangiospores). Mycologia 86:494–501. doi: 10.2307/3760741. [DOI] [Google Scholar]
- 86.Ingold CT. 1934. The spore discharge mechanism in Basidiobolus ranarum. Phytologist 33:274–277. doi: 10.1111/j.1469-8137.1934.tb06814.x. [DOI] [Google Scholar]
- 87.Kopecek P, Raclavský V. 1999. Comparison of chitin content in the apical and distal parts of fungal hyphae in Basidiobolus ranarum, Neurospora crassa and Coprinus sterquilinus. Folia Microbiol (Praha) 44:397–400. doi: 10.1007/BF02903712. [DOI] [PubMed] [Google Scholar]
- 88.Yafetto L, Carroll L, Cui Y, Davis DJ, Fischer MW, Henterly AC, Kessler JD, Kilroy HA, Shidler JB, Stolze-Rybczynski JL, Sugawara Z, Money NP. 2008. The fastest flights in nature: high-speed spore discharge mechanisms among fungi. PLoS One 3:e3237. doi: 10.1371/journal.pone.0003237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmidt JH, Howard RJ, Jane L, Chen JL, Pierson KK. 1986. First culture-proven gastrointestinal entophthoromycosis in the United States: a case report and review of the literature. Mycopathologia 95:101–104. doi: 10.1007/BF00437168. [DOI] [PubMed] [Google Scholar]
- 90.Mendoza L, Alfaro AA. 1985. Equine subcutaneous zygomycosis in Costa Rica. Mykosen 28:545–549. doi: 10.1111/j.1439-0507.1985.tb02084.x. [DOI] [PubMed] [Google Scholar]
- 91.Okada K, Amano S, Kawamura Y, Kagawa Y. 2015. Gastrointestinal basidiobolomycosis in a dog. J Vet Med Sci 77:1311–1313. doi: 10.1292/jvms.15-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Erwig LP, Gow NAR. 2016. Interaction of fungal pathogens with phagocytes. Nat Rev Microbiol 14:163–176. doi: 10.1038/nrmicro.2015.21. [DOI] [PubMed] [Google Scholar]
- 93.De Bievre C, Fromentin H, Mariat F. 1978. Etude de la composition en sucre de souches de Conidiobolus et l'animal. Mycopathologia 63:167–172. doi: 10.1007/BF00490932. [DOI] [PubMed] [Google Scholar]
- 94.Feofilova EP. 2009. The fungal cell wall: modern concepts of its composition and biological function. Microbiology 79:711–720. doi: 10.1134/S0026261710060019. [DOI] [PubMed] [Google Scholar]
- 95.Gow NAR, Latge JP, Munro CA. 2017. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr 5:FUNK-0035-2016. doi: 10.1128/microbiolspec.FUNK-0035-2016. [DOI] [PubMed] [Google Scholar]
- 96.Raclavský V, Kopecek P. 1994. Effect of the fluorescent brightener Rylux BSU on the cell wall chitin content in Basidiobolus ranarum. Acta Univ Palacki Olomuc Fac Med 138:19–20. [PubMed] [Google Scholar]
- 97.Farkaš V. 1990. Fungal cell walls: their structure, biosynthesis and biochemical aspects. Acta Biotechnol 10:225–238. doi: 10.1002/abio.370100303. [DOI] [Google Scholar]
- 98.Beauvais A, Latgé JP. 1989. Chitin and β(1-3) glucan synthases in the protoplasmic Entomophthorales. Arch Microbiol 152:229–236. doi: 10.1007/BF00409656. [DOI] [Google Scholar]
- 99.Freimoser FM, Screen S, Hu G, St Leger R. 2003. EST analysis of genes expressed by the zygomycete pathogen Conidiobolus coronatus during growth on insect cuticle. Microbiology 149:1893–1900. doi: 10.1099/mic.0.26252-0. [DOI] [PubMed] [Google Scholar]
- 100.Malagocka J, Grell MN, Lange L, Eilenberg J, Jensen AB. 2015. Transcriptome of an entomophthoralean fungus (Pandora formica) shows molecular machinery adjusted for successful host exploitation and transmission. J Invertebr Pathol 128:47–56. doi: 10.1016/j.jip.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 101.Yousef OM, Smilack JD, Kerr DM, Ramsey R, Rosati L, Colby TV. 1999. Gastrointestinal basidiobolomycosis: morphological findings in the cluster of six cases. Am J Clin Pathol 112:610–616. doi: 10.1093/ajcp/112.5.610. [DOI] [PubMed] [Google Scholar]
- 102.Echetebu KO, Ononogbu IC. 1982. Extracellular lipase and proteinase of Basidiobolus haptosporus: possible role in subcutaneous mycosis. Mycopathologia 80:171–177. doi: 10.1007/BF00437580. [DOI] [PubMed] [Google Scholar]
- 103.Okafor JI, Gugnani HC. 1990. Lipase activity of Basidiobolus and Conidiobolus species. Mycoses 33:81–85. doi: 10.1111/myc.1990.33.2.81. [DOI] [PubMed] [Google Scholar]
- 104.Okafor JI, Gugnani HC, TeStrake D, Yangoo BC. 1987. Extracellular enzyme activities by Basidiobolus and Conidiobolus isolates on solid media. Mykosen 30:404–407. doi: 10.1111/j.1439-0507.1987.tb03637.x. [DOI] [PubMed] [Google Scholar]
- 105.Okafor JI. 1994. Purification and characterization of protease enzymes of Basidiobolus and Conidiobolus species. Mycoses 37:265–269. doi: 10.1111/j.1439-0507.1994.tb00424.x. [DOI] [PubMed] [Google Scholar]
- 106.Claydon N. 1978. Insecticidal secondary metabolites from entomophagous fungi: Entomophthora virulenta. J Invertebr Pathol 32:319–324. doi: 10.1016/0022-2011(78)90195-7. [DOI] [Google Scholar]
- 107.Boguœ MI, Wieloch W, Ligęza-Żuber M. 2017. Coronatin-2 from the entomopathogenic fungus Conidiobolus coronatus kills Galleria mellonella larvae and incapacitates hemocytes. Bull Entomol Res 107:66–76. doi: 10.1017/S0007485316000638. [DOI] [PubMed] [Google Scholar]
- 108.Butt TM, Coates CJ, Dubovskiy IM, Ratcliffe NA. 2016. Entomopathogenic fungi: new insights into host-pathogen interactions. Adv Genet 94:307–364. doi: 10.1016/bs.adgen.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 109.Raffaele S, Kamoun S. 2012. Genome evolution in filamentous plant pathogens: why bigger can be better. Nat Rev Microbiol 10:417–430. doi: 10.1038/nrmicro2790. [DOI] [PubMed] [Google Scholar]
- 110.Lakon G. 1919. Die Insektenfeinde aus der Familie der Entomophthoraceen. Z Angew Entomol 5:161–216. doi: 10.1111/j.1439-0418.1919.tb01419.x. [DOI] [Google Scholar]
- 111.Ditgen D, Anandarajah EM, Meissner KA, Brattig N, Wrenger C, Liebau E. 2014. Harnessing the helminth secretome for therapeutic immunomodulators. Biomed Res Int 2014:964350. doi: 10.1155/2014/964350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang L, Appleton JD. 2016. Eosinophils in helminth infection: defenders and dupes. Trends Parasitol 32:798–807. doi: 10.1016/j.pt.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reyes-Hernadez JL, Leing G, McKay DM. 2013. Cestode regulation of inflammation and inflammatory diseases. Int J Parasitol 43:233–243. doi: 10.1016/j.ijpara.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 114.Gaastra W, Lipman LJA, DeCock AWAM, Exel TK, Pegge RB, Scheurwater J, Vilela R, Mendoza L. 2010. Pythium insidiosum: an overview. Vet Microbiol 146:1–16. doi: 10.1016/j.vetmic.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 115.Hussein MR. 2008. Mucocutaneous Splendore-Hoeppli phenomenon. J Cutan Pathol 35:979–988. doi: 10.1111/j.1600-0560.2008.01045.x. [DOI] [PubMed] [Google Scholar]
- 116.Madson DM, Loynachan AT, Kariyawasam S, Opriessnig T. 2009. Systemic Conidiobolus incongruus infection and hypertrophic osteopathy in a white-tailed deer (Odocoileus virginianus). J Vet Diagn Invest 21:167–170. doi: 10.1177/104063870902100131. [DOI] [PubMed] [Google Scholar]
- 117.Manning RJ, Waters SD, Callaghan AA. 2007. Saprotrophy of Conidiobolus and Basidiobolus in leaf litter. Mycol Res 111:1437–1449. doi: 10.1016/j.mycres.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 118.Taylor SK, Williams ES, Mills KW. 1999. Experimental exposure of Canadian toads to Basidiobolus ranarum. J Wildl Dis 35:58–63. doi: 10.7589/0090-3558-35.1.58. [DOI] [PubMed] [Google Scholar]
- 119.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Dimitrios P, Kontoyiannis DP, Walsh TJ. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 120.Khan ZU, Khoursheed M, Makar R, Al-Waheeb S, Al-Bader I, Al-Muzaine A, Chandy R, Mustafa AS. 2001. Basidiobolus ranarum as an etiologic agent of gastrointestinal zygomycosis. J Clin Microbiol 39:2360–2363. doi: 10.1128/JCM.39.6.2360-2363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ageel HI, Arishi HM, Kamli AA, Hussein AM, Bhavanarushi S. 2017. Unusual presentation of gastrointestinal basidiobolomycosis in a 7-year-old child—case report. Am J Med Rep 5:131–134. [Google Scholar]
- 122.Al-Hedaithy M. 1998. Cutaneous zygomycosis due to Saksenaea vasiformis: case report and literature review. Ann Saudi Med 18:428–431. doi: 10.5144/0256-4947.1998.428. [DOI] [PubMed] [Google Scholar]
- 123.Bittencourt AL, Serra G, Sadigursky M, Araujo MG, Campos MC, Sampaio LC. 1982. Subcutaneous zygomycosis caused by Basidiobolus haptosporus: presentation of a case mimicking Burkitt's lymphoma. Am J Trop Med Hyg 31:370–373. doi: 10.4269/ajtmh.1982.31.370. [DOI] [PubMed] [Google Scholar]
- 124.Kumar Verma R, Shivaprakash MR, Shanker A, Panda NK. 2012. Subcutaneous zygomycosis of the cervicotemporal region: due to Basidiobolus ranarum. Med Mycol Case Rep 1:59–62. doi: 10.1016/j.mmcr.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Annamalai R, Kindo AJ, Muthayya M. 2017. Postoperative fungal endophthalmitis due to Basidiobolus ranarum: report of a rare case. J Clin Ophthalmol Res 4:89–91. doi: 10.4103/2320-3897.183719. [DOI] [Google Scholar]
- 126.Cao C, Khader JA. 2018. Rhinofacial entomophthoromycosis. N Engl J Med 378:e13 doi: 10.1056/NEJMicm1709956. [DOI] [PubMed] [Google Scholar]
- 127.Gustafsson M. 1965. On the species of the genus Entomophthora Fries in Sweden. I. Classification and distribution. Lantbruk Shogsk 31:103–114. [Google Scholar]
- 128.Yendol WG, Paschke JD. 1965. Pathology of an Entomophthora infection in the eastern subterranean termite Reticulitermes flavipes (Kollar). J Invertebr Pathol 7:434–443. doi: 10.1016/0022-2011(65)90115-1. [DOI] [Google Scholar]
- 129.Clark BM. 1975. The epidemiology of entomophthoromycoses, p 178–196. In Doory YA. (ed), The epidemiology of human mycotic diseases. Charles C Thomas, Springfield, IL. [Google Scholar]
- 130.Blumentrath CG, Grobusch MP, Matsiégui PB, Pahlke F, Zoleko-Manego R, Nzenze-Aféne S, Mabicka B, Sanguinetti M, Kremsner PG, Schaumburg F. 2015. Classification of rhinoentomophthoromycosis into atypical, early, intermediate, and late disease: a proposal. PLoS Negl Trop Dis 9:e0003984. doi: 10.1371/journal.pntd.0003984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Imwidthaya P, Srimuang S. 1992. Immunodiffusion test for diagnosing basidiobolomycosis. Mycopathologia 118:127–131. doi: 10.1007/BF00437144. [DOI] [PubMed] [Google Scholar]
- 132.Kaufman L, Mendoza L, Standard PG. 1990. Immunodiffusion test for serodiagnosing subcutaneous zygomycosis. J Clin Microbiol 28:1887–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Khan ZU, Parkash B, Kapoor M, Medda JP, Chandy R. 1998. Basidiobolomycosis of the rectum masquerading as Crohn's disease. Clin Infect Dis 26:521–523. doi: 10.1086/517107. [DOI] [PubMed] [Google Scholar]
- 134.Köhler JR, Hube B, Puccia R, Casadaval A, Perfect JR. 2017. Fungi that infect humans. Microbiol Spectr 5:FUNK-0014-2016. doi: 10.1128/microbiolspec.FUNK-0014-2016. [DOI] [PubMed] [Google Scholar]
- 135.de Gody I, de Campos CG, Pecador CA, Galceran JVA, Cândido SL, Dutra V, Nakazato L. 2017. Experimental infection in gerbils by Conidiobolus lamprauges. Microb Pathog 105:251–254. doi: 10.1016/j.micpath.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 136.Kawai T, Akira S. 2011. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immun Rev 34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 137.McSorley HJ, Hewitson JP, Maizels RM. 2013. Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol 43:301–310. doi: 10.1016/j.ijpara.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 138.Arya AA, Naik C, Desmukh S, Babanagare SV, Muntode P. 2011. Mastoid infection caused by Entomophthorales: a rare fungal disease. J Laryngol Otol 125:630–632. doi: 10.1017/S0022215111000661. [DOI] [PubMed] [Google Scholar]
- 139.Chandler FW, Kaplan W, Ajello L. Color atlas and text of the histopathology of mycotic diseases, p 122–127, 298–300 Year Book Medical Publisher Inc, Chicago, IL. [Google Scholar]
- 140.Albaradi BA, Bibiker AMI, Al-Qahtani H. 2014. Successful treatment of gastrointestinal basidiobolomycosis with voriconazole without intervention. J Trop Pediatr 60:476–479. doi: 10.1093/tropej/fmu047. [DOI] [PubMed] [Google Scholar]
- 141.Almoosa Z, Alsuhaibani M, AlDandan S, Alsharani D. 2017. Pediatric gastrointestinal basidiobolomycosis mimicking malignancy. Med Mycol Case Rep 18:31–33. doi: 10.1016/j.mmcr.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vikram HR, Smilack JD, Leighton JA, Crowell MD, De Petris G. 2012. Emergence of gastrointestinal basidiobolomycosis in the United States, with a review of worldwide cases. Clin Infect Dis 54:1685–1691. doi: 10.1093/cid/cis250. [DOI] [PubMed] [Google Scholar]
- 143.Tadano T, Paim NP, Hueb M, Fontes CJF. 2005. Entomophthoromycosis (zygomycosis) caused by Conidiobolus coronatus in Mato Grosso (Brazil): case report. Rev Soc Bras Med Trop 38:188–190. doi: 10.1590/S0037-86822005000200013. [DOI] [PubMed] [Google Scholar]
- 144.Rane S, Jayaraman A, Puranik S, Deshmukh S, Bapat V. 2002. Entomopthoromycosis—report of four cases. Indian J Dermatol Venereol Leprol 68:296–297. [PubMed] [Google Scholar]
- 145.Mendoza L, Newton JC. 2005. Immunology and immunotherapy of the infections caused by Pythium insidiosum. Med Mycol 43:477–486. doi: 10.1080/13693780500279882. [DOI] [PubMed] [Google Scholar]
- 146.Mendoza L, Vilela R. 2013. The mammalian pathogenic oomycetes. Curr Fungal Infect Rep 7:198–208. doi: 10.1007/s12281-013-0144-z. [DOI] [Google Scholar]
- 147.Lauvau G, Loke P, Hohl TM. 2015. Monocyte-mediated defense against bacteria, fungi, and parasites. Semin Immunol 27:397–409. doi: 10.1016/j.smim.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Toriello C, Zerón E, Latgé JP, Mier T. 1989. Immunological separation of Entomophthorales genera. J Invertebr Pathol 53:358–360. doi: 10.1016/0022-2011(89)90100-6. [DOI] [PubMed] [Google Scholar]
- 149.Yango BG, Nettlow A, Okafor JI, Park J, Strake DT. 1986. Comparative antigenic studies of species of Basidiobolus and other medically important fungi. J Clin Microbiol 23:679–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Williams AO, Von Lichtenberg F, Smith JH, Martinson FD. 1969. Ultrastructure of phycomycosis due to Entomophthora, Basidiobolus, and associated “Splendore-Hoeppli” phenomenon. Arch Pathol 87:459–468. [PubMed] [Google Scholar]
- 151.Rodig SJ, Dorfman DM. 2001. Splendore-Hoeppli phenomenon. Arch Pathol Lab Med 125:1515–1516. [DOI] [PubMed] [Google Scholar]
- 152.Liber AF, Choi HS. 1973. Splendore-Hoeppli phenomenon about silk sutures in tissue. Arch Pathol 5:217–220. [PubMed] [Google Scholar]
- 153.Lutz A, Splendore A. 1907. Uber eine bei menschen und ratten beobachtete mykosen. Ein beitrag zur kenntnis der sogenanntes sporotrichosen. Zentralbl Bakteriol I 45:631–638. [Google Scholar]
- 154.Splendore A. 1908. Sobre a cultura de uma nova especie de cogumelo pathogénica do homen (Sporotrichum splendore) ou “Sporotrichum asteroides” n. sp. Rev Soc Cient Sau Paulo 3:62–62. [Google Scholar]
- 155.Hoeppli R. 1932. Histological observations in experimental schistosomiasis Japonica. Chin Med J 46:1179–1186. [Google Scholar]
- 156.Drexler H, Marlchinger H, Müller J. 1979. Asteroid bodies bei akuter, generaliserter aspergillosis in organen einer leukämine-pateintin. Schwerpunkt Med 2:42–52. [Google Scholar]
- 157.Moore M, Ackerman LV. 1946. Sporotrichosis with radiate formation in tissue, report of a case. Arch Dermatol Syph 53:253–264. doi: 10.1001/archderm.1946.01510320043007. [DOI] [PubMed] [Google Scholar]
- 158.Smith JH, Von Lichtenberg F. 1967. The Hoeppli phenomenon in schistosomiasis. II. Histochemistry. Am J Pathol 50:993–1008. [PMC free article] [PubMed] [Google Scholar]
- 159.Müller J. 2000. Asteroid bodies: host-pathogen reactions in mycoses. Mycoses 43(Suppl 1):29–35. doi: 10.1046/j.1439-0507.2000.00547.x. [DOI] [PubMed] [Google Scholar]
- 160.Hiruma M, Kawada A, Ishibashi A. 1991. Ultrastructure of asteroid bodies in sporotrichosis. Mycoses 34:103–107. doi: 10.1111/j.1439-0507.1991.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 161.Read RW, Zhang J, Albini T, Evans M, Rao NA. 2005. Splendore-Hoeppli phenomenon in the conjunctiva: immunohistochemical analysis. Am J Ophthalmol 140:262–266. doi: 10.1016/j.ajo.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 162.Schweizer G. 1947. Über die Kultur von Empusa muscae Dohn und anderer Entomophthoraceen auf Kaltsterilisierter Nährböden. Planta 35:132–176. doi: 10.1007/BF01989176. [DOI] [Google Scholar]
- 163.Lu HL, St Leger RJ. 2016. Insect immunity to entomopathogenic fungi. Adv Genet 94:251–285. doi: 10.1016/bs.adgen.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 164.Fromentin H, Ravisse P. 1977. Les entomophthoromycoses tropicales. Acta Trop 34:375–394. [PubMed] [Google Scholar]
- 165.Rose SR, Lindsley MD, Hurst SF, Paddack CD, Damodaran T, Bennett J. 2013. Gastrointestinal basidiobolomycosis treated with posaconazole. Med Mycol Case Rep 2:11–14. doi: 10.1016/j.mmcr.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maiti PK, Bose R, Bandyopadhyay S, Bhattacharya S, Dey JB, Ray A. 2004. Entomophthoromycosis in South Bengal (Eastern India): a 9 years study. Indian J Pathol Microbiol 47:295–297. [PubMed] [Google Scholar]
- 167.Fischer N, Reuf C, Ebnother C, Bachli EB. 2008. Rhinofacial Conidiobolus coronatus infection presenting with nasal enlargement. Infection 36:594–596. doi: 10.1007/s15010-008-8056-5. [DOI] [PubMed] [Google Scholar]
- 168.Anaparthy UR, Deepika G. 2014. A case of subcutaneous zygomycosis. Indian Dermatol Online J 5:51–54. doi: 10.4103/2229-5178.126033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Isa-Isa R, Arenas R, Fernández RF, Isa M. 2012. Rhinofacial conidiobolomycosis (entomophthoramycosis). Clin Dermatol 30:409–412. doi: 10.1016/j.clindermatol.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 170.Onuigbo WIB, Gugnani HC. 1976. Deep mycoses prevalent in the Igbos of Nigeria. Int J Dermatol 15:432–437. doi: 10.1111/j.1365-4362.1976.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 171.Choon S-E, Kang J, Neafie RC, Ragsdale B, Klassen-Fischer M, Carlson JA. 2012. Conidiobolomycosis in a young Malaysian woman showing chronic localized fibrosing leukocytoclastic vasculitis: a case report and meta-analysis focusing on clinicopathologic and therapeutic correlations with outcome. Am J Dermatopathol 34:511–522. doi: 10.1097/DAD.0b013e31823db5c1. [DOI] [PubMed] [Google Scholar]
- 172.Wüppenhorst N, Lee MK, Rappold E, Kayser G, Beckervordersandforth J, de With K, Serr A. 2010. Rhino-orbitocerebral zygomycosis caused by Conidiobolus incongruus in an immunocompromised patient in Germany. J Clin Microbiol 48:4322–4325. doi: 10.1128/JCM.01188-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gugnani HC. 1992. Entomophthoromycosis due to Conidiobolus. Eur J Epidemiol 8:391–396. doi: 10.1007/BF00158574. [DOI] [PubMed] [Google Scholar]
- 174.Al-Hajjar S, Perfect J, Hashem F, Tufenkeji H, Kayes S. 1996. Orbitofascial conidiobolomycosis in a child. Pediatr Infect Dis J 15:1130–1132. doi: 10.1097/00006454-199612000-00018. [DOI] [PubMed] [Google Scholar]
- 175.Jaffey PB, Haque AK, el-Zaatari M, Pasarell L, McGinnis MR. 1990. Disseminated Conidiobolus infection with endocarditis in a cocaine abuser. Arch Pathol Lab Med 114:1276–1278. [PubMed] [Google Scholar]
- 176.Hoogendijk CF, van Heerden WF, Pretorius E, Vismer HF, Jacobs JF. 2006. Rhino-orbitocerebral entomophthoramycosis. Int J Oral Maxillofac Surg 35:277–280. doi: 10.1016/j.ijom.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 177.Hoogendijk CF, Pretorius E, Marx J, Van Heerden WE, Imhof A, Schneemann M. 2006. Detection of villous conidia of Conidiobolus coronatus in a blood sample by scanning electron microscopy investigation. Ultrastruct Pathol 30:53–58. doi: 10.1080/01913120500482013. [DOI] [PubMed] [Google Scholar]
- 178.Coelho-Filho JC, Pereira J, Rabello A Jr. 1989. Mediastinal and pulmonary entomophthoromycosis with superior vena cava syndrome: case report. Rev Inst Med Trop Sao Paulo 31:430–433. doi: 10.1590/S0036-46651989000600010. [DOI] [PubMed] [Google Scholar]
- 179.Marwaha RK, Banerjee AK, Thapa BR, Agrawal SM. 1985. Mediastinal zygomycosis. Postgrad Med J 61:733–735. doi: 10.1136/pgmj.61.718.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Thianprasit M. 1990. Human pythiosis. Trop Dermatol 4:1–4. [Google Scholar]
- 181.Wanachiwanawin W, Thianprasit M, Fucharoen S, Chaiprasert A, Sudasna N, Ayudhya N, Sirithanaratkul N, Piankijagum A. 1993. Fatal infection due to Pythium insidiosum infection in patients with thalassaemia. Trans R Soc Trop Med Hyg 87:296–298. doi: 10.1016/0035-9203(93)90135-D. [DOI] [PubMed] [Google Scholar]
- 182.Padhye AA, Koshi G, Anandi V, Ponniah J, Sitaram V, Jacob M, Mathai R, Ajello L, Chandler FW. 1988. First case of subcutaneous zygomycosis caused by Saksenaea vasiformis in India. Diagn Microbiol Infect Dis 9:69–77. doi: 10.1016/0732-8893(88)90099-5. [DOI] [PubMed] [Google Scholar]
- 183.Pritchard RC, Muir DB, Archer KH, Beith JM. 1986. Subcutaneous zygomycosis due to Saksenaea vasiformis in an infant. Med J Aust 145:630–631. [PubMed] [Google Scholar]
- 184.Hernandez MJ, Landaeta W, Salazar BN, Vargas J, Rodriguez-Morales AJ. 2007. Subcutaneous zygomycosis due to Conidiobolus incongruus. Int J Infect Dis 11:468–470. doi: 10.1016/j.ijid.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 185.Ketterer PJ, Kelly MA, Connole MD, Ajello A. 1992. Rhinocerebral and nasal zygomycosis in sheep caused by Conidiobolus incongruus. Aust Vet J 69:85–87. doi: 10.1111/j.1751-0813.1992.tb15556.x. [DOI] [PubMed] [Google Scholar]
- 186.Al-Jarie A, Al-Azraki T, Al-Mohsen I, Al-Jumaah S, Almutawa A, Fahim YM, Al-Sheri M, Dayah AA, Ibrahim A, Shabana MM, Hussein MRA. 2011. Basidiobolomycosis: case series. J Mycol Med 21:37–45. doi: 10.1016/j.mycmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 187.Sackey A, Ghatey N, Gyasi R. 2017. Subcutaneous basidiobolomycosis: a case report. Ghana Med J 51:43–46. doi: 10.4314/gmj.v51i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Atadokpédé F, Gnossikè J, Adégbidi H, Dégboé B, Sissinto-Savi de Tovè Y, Adéyé A, Koudoukpo C, Chauty A, Chabasse D, Saint-André JP, Dieng MT, Koeppel MC, Yedomon HG, do-Ango-Padonou F. 2017. Cutaneous basidiobolomycosis: seven cases in southern Benin. Ann Dermatol Venereol 144:250–254. doi: 10.1016/j.annder.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 189.Sujatha S, Sheeladevi C, Khyriem AB, Parija SC, Thappa DM. 2003. Subcutaneous zygomycosis caused by Basidiobolus ranarum—a case report. Indian J Med Microbiol 21:205–206. [PubMed] [Google Scholar]
- 190.Bittencourt AL, Melo CR, Jalil OM, Andrade ZA. 1977. Basidiobolomicose. Apresentação de um caso. Rev Inst Med Trop Sao Paulo 19:208–212. [PubMed] [Google Scholar]
- 191.Bittencourt AL, Araujo MG, Fontoura MS. 1980. Occurrence of subcutaneous zygomycosis (entomophthoromycosis basidiobolae) caused by Basidiobolus haptosporus with pulmonary involvement. Mycopathologia 71:155–158. doi: 10.1007/BF00473063. [DOI] [PubMed] [Google Scholar]
- 192.Bigliazzi C, Poletti V, Dell'Amore D, Saragoni L, Cilby TV. 2004. Disseminated basidiobolomycosis in an immunocompetent woman. J Clin Microbiol 42:1367–1369. doi: 10.1128/JCM.42.3.1367-1369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Emmons CW, Lie-Kian-Joe, Eng N-IT, Pohan A, Kertopati S, Van der Mulen H. 1957. Basidiobolus and Cercospora from human infections. Mycologia 49:1–10. [Google Scholar]
- 194.Kamalan A, Thambiah AS. 1982. Muscle invasion by Basidiobolus haptosporus. Sabouraudia 22:273–277. doi: 10.1080/00362178485380471. [DOI] [PubMed] [Google Scholar]
- 195.Michael RC, Michael JS, Mathews MS, Rupa V. 2009. Unusual presentation of entomophthoromycosis. Indian J Med Microbiol 27:156–165. doi: 10.4103/0255-0857.49432. [DOI] [PubMed] [Google Scholar]
- 196.Bittencourt AL, Ayala MAR, Ramos EAG. 1979. A new form of abdominal zygomycosis different from mucormycosis: report of two cases and review of the literature. Am J Trop Med Hyg 28:564–569. doi: 10.4269/ajtmh.1979.28.564. [DOI] [PubMed] [Google Scholar]
- 197.Al-Naemi AQ, Khan LA, Al-Naemi I, Amin K, Athlawy YA, Awad LA, Sun Z. 2015. A case report of gastrointestinal basidiobolomycosis treated with voriconazole. Medicine 94:e1430. doi: 10.1097/MD.0000000000001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.El-Shabrawi MH, Kamal NM, Jouini R, Al-Harbi A, Voigt K, Al-Malki T. 2011. Gastrointestinal basidiobolomycosis: an emerging fungal infection causing bowel perforation in a child. J Med Microbiol 60:1395–1402. doi: 10.1099/jmm.0.028613-0. [DOI] [PubMed] [Google Scholar]
- 199.El-Shabrawi MH, Kamal NM, Kaerger K, Voigt K. 2014. Diagnosis of gastrointestinal basidiobolomycosis: a mini-review. Mycoses 57(Suppl 3):S138–S143. doi: 10.1111/myc.12231. [DOI] [PubMed] [Google Scholar]
- 200.Mandhan P, Hassan KO, Samaan SM, Ali MJ. 2015. Visceral basidiobolomycosis: an overlooked infection in immunocompetent children. Afr J Paediatr Surg 12:193–196. doi: 10.4103/0189-6725.170218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Saadah OI, Farouq MF, Daajani NA, Kamal JS, Ghanem AT. 2012. Gastrointestinal basidiobolomycosis in a child; an unusual fungal infection mimicking fistulising Crohn's disease. J Crohns Colitis 6:368–372. doi: 10.1016/j.crohns.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 202.Smilack JD. 1998. Gastrointestinal basidiobolomycosis. Clin Infect Dis 27:663–664. doi: 10.1086/517154. [DOI] [PubMed] [Google Scholar]
- 203.van den Berk GE, Noorduyn LA, van Ketel RJ, van Leeuwen J, Bemelman WA, Prins JM. 2006. A fatal pseudo-tumor: disseminated basidiobolomycosis. BMC Infect Dis 6:140–144. doi: 10.1186/1471-2334-6-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pasha TM, Leighton JA, Smilack JD, Heppell J, Colby TV, Kaufman L. 1997. Basidiobolomycosis: an unusual fungal infection mimicking inflammatory bowel disease. Gastroenterology 112:250–254. doi: 10.1016/S0016-5085(97)70242-7. [DOI] [PubMed] [Google Scholar]
- 205.Hussein MR, Musalam AO, Assiry MH, Eid RA, El Motawa AM, Gamel AM. 2007. Histological and ultrastructural features of gastrointestinal basidiobolomycosis. Mycol Res 111:926–930. doi: 10.1016/j.mycres.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 206.Zavasky DM, Samovitz W, Loftus T, Segal H, Carroll K. 1999. Gastrointestinal zygomycotic infection caused by Basidiobolus ranarum: case report and review. Clin Infect Dis 28:1244–1248. doi: 10.1086/514781. [DOI] [PubMed] [Google Scholar]
- 207.Nayak DR, Pillai S, Rao L. 2004. Rhinofacial zygomycosis caused by Conidiobolus coronatus. Indian J Otolaryngol Head Neck Surg 56:225–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cockshott WP, Clark BM, Martinson FD. 1968. Upper respiratory infection due to Entomophthora coronata. Radiology 90:1016–1019. doi: 10.1148/90.5.1016. [DOI] [PubMed] [Google Scholar]
- 209.Tan DCL, Hsu LY, Koh LP, Goh YT, Koh M. 2005. Severe conidiobolomycosis complicating induction chemotherapy in a patient with acute lymphoblastic leukaemia. Br J Haematol 129:447. doi: 10.1111/j.1365-2141.2005.05429.x. [DOI] [PubMed] [Google Scholar]
- 210.Saeed MA, Al Khuwaitir TS, Attia TH. 2014. Gastrointestinal basidiobolomycosis with hepatic dissemination: a case report. JMM Case Rep 1:e003269. doi: 10.1099/jmmcr.0.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ilyas MIM, Jordan SA, Nfonsam V. 2015. Fungal inflammatory masses masquerading as colorectal cancer: a case report. BMC Res Notes 8:32. doi: 10.1186/s13104-014-0962-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Flicek KT, Vikram HR, De Petris GD, Johnson CD. 2015. Abdominal imaging findings in gastrointestinal basidiobolomycosis. Abdom Imaging 40:246–250. doi: 10.1007/s00261-014-0212-z. [DOI] [PubMed] [Google Scholar]
- 213.Mendoza L, Nicholson V, Prescott JF. 1992. Immunoblot analysis of the humoral immune response to Pythium insidiosum in horses with pythiosis. J Clin Microbiol 30:2980–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.El-Shabrawi MHF, Arnaout H, Madkour L, Kamal NM. 2014. Entomophthoromycosis: a challenging emerging disease. Mycoses 57:132–137. doi: 10.1111/myc.12248. [DOI] [PubMed] [Google Scholar]
- 215.Davis SR, Ellis DH, Goldwater P, Dimitriou S, Byard R. 1994. First human culture-proven Australian case of entomophthoromycosis caused by Basidiobolus ranarum. J Med Vet Mycol 32:225–230. doi: 10.1080/02681219480000291. [DOI] [PubMed] [Google Scholar]
- 216.Dannaoui E. 2009. Molecular tools for the identification of Zygomycetes and the diagnosis of zygomycosis. Clin Microbiol Infect 15(Suppl 5):66–70. doi: 10.1111/j.1469-0691.2009.02983.x. [DOI] [PubMed] [Google Scholar]
- 217.Gómez-Muñoz MT, Fernández-Barredo S, Martínez-Díaz RA, Pérez-Gracia MT, Ponce-Gordo F. 2012. Development of a specific polymerase chain reaction assay for the detection of Basidiobolus. Mycologia 104:585–591. doi: 10.3852/10-271. [DOI] [PubMed] [Google Scholar]
- 218.Voigt K, Cigelnik E, O'Donnell K. 1999. Phylogeny and PCR identification of clinically important zygomycetes based on nuclear ribosomal-DNA sequence data. J Clin Microbiol 37:3957–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lyon GM, Smilack JD, Komatsu KK, Leighton JA, Guarner J, Colby TV, Lindsley MD, Phelan M, Warnock DW, Hajjeh RA. 2001. Gastrointestinal basidiobolomycosis in Arizona: clinical and epidemiological characteristics and review of the literature. Clin Infect Dis 32:1448–1455. doi: 10.1086/320161. [DOI] [PubMed] [Google Scholar]
- 220.Rothhardt JE, Schwartze VU, Voigt K. 2011. Entomophthorales, p 709–720. In Liu D. (ed), Molecular detection of human fungal pathogens. Taylor & Francis, New York, NY. [Google Scholar]
- 221.Mathew R, Kumaravel S, Kuruvilla S, Varghese RG, Shashikala, Srinivasan S, Mani MZ. 2005. Successful treatment of extensive basidiobolomycosis with oral itraconazole in a child. Int J Dermatol 44:572–575. doi: 10.1111/j.1365-4632.2004.02419.x. [DOI] [PubMed] [Google Scholar]
- 222.Guarro J, Aguilar C, Pujol I. 1999. In-vitro antifungal susceptibilities of Basidiobolus and Conidiobolus spp. strains. J Antimicrob Chemother 44:557–560. doi: 10.1093/jac/44.4.557. [DOI] [PubMed] [Google Scholar]
- 223.Kamalan A, Thambiah AS. 1979. Entomophthoromycosis basidiobolae successfully treated with KI. Mykosen 22:82–84. doi: 10.1111/j.1439-0507.1979.tb01718.x. [DOI] [PubMed] [Google Scholar]
- 224.Taylor GD, Sekhon AS, Tyrrell DLJ, Goldsand G. 1987. Rhinofacial zygomycosis by Conidiobolus coronatus: a case report including in vitro sensitivity to antimycotic agents. Am J Trop Med Hyg 36:398–401. doi: 10.4269/ajtmh.1987.36.398. [DOI] [PubMed] [Google Scholar]
- 225.Towersey L, Wanke B, Estrella RR, Londero AT, Mendonca AMN, Neves RG. 1988. Conidiobolus coronatus infection treated with ketoconazole. Arch Dermatol 124:1392–1396. doi: 10.1001/archderm.1988.01670090048010. [DOI] [PubMed] [Google Scholar]
- 226.Al-Shanafey S, AlRobean F, Bin Hussain I. 2012. Surgical management of gastrointestinal basidiobolomycosis in pediatric patients. J Pediatr Surg 47:949–951. doi: 10.1016/j.jpedsurg.2012.01.053. [DOI] [PubMed] [Google Scholar]
- 227.Raveenthiran V, Mangayarkarasin V, Kousalya M, Viswanathan P, Dhanalakshmi M, Anandi V. 2015. Subcutaneous entomophthoromycosis mimicking soft-tissue sarcoma in children. J Pediatr Surg 50:1150–1155. doi: 10.1016/j.jpedsurg.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 228.Sterling JB, Heymann WR. 2000. Potassium iodine in dermatology: a 19th century drug for the 21st century—uses, pharmacology, adverse effects, and contraindications. J Am Acad Dermatol 43:691–697. doi: 10.1067/mjd.2000.107247. [DOI] [PubMed] [Google Scholar]
- 229.Peel T, Daffy J, Thursky K, Stanley P, Buising K. 2008. Posaconazole as first line treatment for disseminated zygomycosis. Mycoses 51:542–545. doi: 10.1111/j.1439-0507.2008.01499.x. [DOI] [PubMed] [Google Scholar]
- 230.Avendaño MV, Contreras SA, Dhoble CY, Camacho AR, Medina M, Ameri KR. 2015. A rare case of subcutaneous zygomycosis on the neck of an immunocompetent patient. Case Rep Int Med 2:576–562. [Google Scholar]
- 231.Ridley DS, Wise M. 1965. Unusual disseminated infection with a phycomycete. J Pathol Bacteriol 90:675–679. doi: 10.1002/path.1700900242. [DOI] [PubMed] [Google Scholar]
- 232.Foss NT, Rocha MR, Lima VT, Velludo MA, Roselino AM. 1996. Entomophthoramycosis: therapeutic success by using amphotericin B and terbinafine. Dermatology 193:258–260. doi: 10.1159/000246260. [DOI] [PubMed] [Google Scholar]