SUMMARY
Tuberculosis (TB) is the leading infectious cause of mortality worldwide, due in part to a limited understanding of its clinical pathogenic spectrum of infection and disease. Historically, scientific research, diagnostic testing, and drug treatment have focused on addressing one of two disease states: latent TB infection or active TB disease. Recent research has clearly demonstrated that human TB infection, from latent infection to active disease, exists within a continuous spectrum of metabolic bacterial activity and antagonistic immunological responses. This revised understanding leads us to propose two additional clinical states: incipient and subclinical TB. The recognition of incipient and subclinical TB, which helps divide latent and active TB along the clinical disease spectrum, provides opportunities for the development of diagnostic and therapeutic interventions to prevent progression to active TB disease and transmission of TB bacilli. In this report, we review the current understanding of the pathogenesis, immunology, clinical epidemiology, diagnosis, treatment, and prevention of both incipient and subclinical TB, two emerging clinical states of an ancient bacterium.
KEYWORDS: Mycobacterium tuberculosis, tuberculosis
INTRODUCTION
Mycobacterium tuberculosis is an ancient bacterium that was first presented as the cause of tuberculosis (TB) in 1882. Despite the global introduction of a vaccine and the discovery of an effective four-drug treatment regimen, M. tuberculosis still likely infects approximately one-quarter of the world's population and is the leading infectious cause of mortality worldwide (1, 2). The current TB pandemic is fueled not only by poverty and HIV/AIDS but also by an insufficient understanding of the spectrum of TB pathogenesis, which may be essential in developing new diagnostic tests and creating more-adaptable treatment regimens.
The “End TB Strategy” of the World Health Organization (WHO) seeks to reduce TB incidence to fewer than 10 cases per 105 people per year by 2035 (3). The primary approach for achieving this goal is to improve efforts to find and treat people with active TB disease, conduct universal screening of individuals at high risk, and provide preventive therapy for those at risk of progressing to active TB disease (3). While there are concentrated efforts to develop a new vaccine, this alone may not be enough to end the TB epidemic (4). A strong public health response will require new diagnostic tools and therapeutic options along with a better understanding of the microbiological and clinical spectrum of TB infection and disease.
The Spectrum of Tuberculosis Infection to Disease
A longstanding tenet of TB pathogenesis has been that M. tuberculosis exists in either a metabolically inactive latent state or a metabolically active disease state. In this framework, about 5% of people infected with TB progress rapidly to active disease, while the vast majority of people develop a latent infection and remain at risk for progression to active disease (“reactivation”) (5). Studies of pathogenesis in animal models and in humans suggest a more complex course, where, following initial contact between M. tuberculosis and a human host, the pathogen may progress to primary active TB disease or be completely eliminated through an innate and/or acquired immune response (4). For individuals with latent TB infection, the host maintains a dynamic relationship with M. tuberculosis through the regulation of available nutrients as well as the innate and acquired immune systems (6, 7).
This new paradigm for a dynamic continuum of M. tuberculosis infection suggests that additional categories of infection can be defined between latent infection and active TB disease (8). With a better understanding of the TB spectrum, we propose and describe two additional clinical states, called incipient and subclinical TB. Although the spectra of TB infection and disease remain continuous, categorizing M. tuberculosis into these discrete states may allow for better dialogue on TB pathogenesis and help foster the development of new diagnostic and therapeutic interventions to prevent progression to active TB disease and ongoing transmission of M. tuberculosis bacilli.
DEFINITIONS OF INCIPIENT AND SUBCLINICAL TUBERCULOSIS
We propose a new framework in which the pathophysiological spectrum is divided into five discrete categories for both conceptual and practical purposes. This five-state framework incorporates a recent understanding of clinical TB pathogenesis as well as advances in the diagnostic approaches to detecting viable TB and predicting the progression of disease activity. These definitions and categories are meant to build upon each other, as detailed in Table 1.
TABLE 1.
Defining criteria for the five categorical states of tuberculosis
| Categorical state of TB | Presence of criterion |
||||
|---|---|---|---|---|---|
| M. tuberculosis exposure | Person has viable M. tuberculosis pathogen | M. tuberculosis has metabolic activity to indicate ongoing or impending progression of infection | Radiographic abnormalities or microbiological evidence of active, viable M. tuberculosis | Person has symptoms suggestive of active M. tuberculosis disease | |
| Eliminated TB infection | ✕ | ||||
| Latent TB infection | ✕ | ✕ | |||
| Incipient TB infection | ✕ | ✕ | ✕ | ||
| Subclinical TB disease | ✕ | ✕ | ✕ | ✕ | |
| Active TB disease | ✕ | ✕ | ✕ | ✕ | ✕ |
Eliminated TB infection is an individual with prior exposure to M. tuberculosis who either has cleared the infection by innate and/or acquired immune responses or has been cured of the infection with anti-TB medications. This individual no longer has viable M. tuberculosis bacteria but may still have immunological evidence of prior infection.
Latent TB infection (LTBI) is infection with viable M. tuberculosis for which progression to TB disease is not expected to occur in the near future in the absence of any significant immunological compromise. This represents a more conceptual analogue to the current WHO definition, which considers LTBI “as having evidence of TB infection and no clinical, radiological or microbiological evidence of active TB disease” (9–11). Currently, there is no direct way of confirming LTBI or its microbiological load, as existing tests infer LTBI based on a T cell response to TB or TB-like antigens (12).
Incipient TB infection is an infection with viable M. tuberculosis bacteria that is likely to progress to active disease in the absence of further intervention but has not yet induced clinical symptoms, radiographic abnormalities, or microbiologic evidence consistent with active TB disease.
Subclinical TB disease is disease due to viable M. tuberculosis bacteria that does not cause clinical TB-related symptoms but causes other abnormalities that can be detected using existing radiologic or microbiologic assays.
Active TB disease is disease due to viable M. tuberculosis that causes clinical symptoms with radiographic abnormalities or microbiologic evidence consistent with active TB disease. This would remain consistent with the current WHO definition, which considers active TB disease as “symptomatic patients with radiological or microbiological evidence of M. tuberculosis” (13).
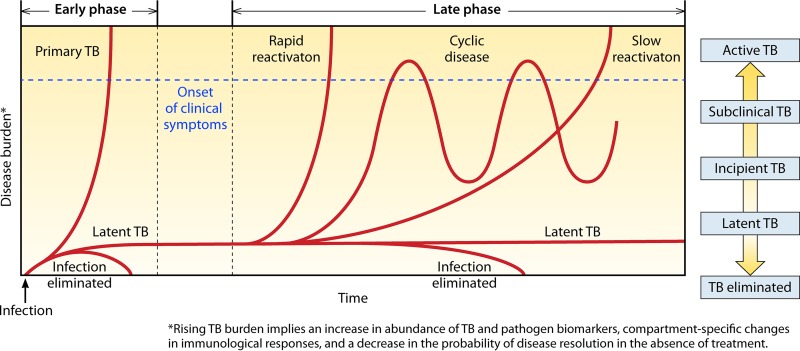
Following the establishment of latent infection, the pathways by which disease may naturally progress include (i) latency (the most common course, which encompasses persistent or eliminated disease burden), (ii) rapid or (iii) slow progression through incipient and subclinical disease to active TB, or (iv) a period of cycling through incipient and subclinical states that may precede the development of symptomatic disease or eventual disease resolution (Fig. 1). A larger disease burden may correlate with increasing host symptoms, a likelihood of transmission, a decreasing probability of spontaneous self-cure, an impaired or poor immunological response, and/or a larger abundance of TB bacilli and other pathogen biomarkers. While we have not depicted all possibilities for regression of disease burden, spontaneous recovery may occur in any of these clinical trajectories, including pathways that have progressed through active TB.
FIG 1.
Pathways of tuberculosis disease progression. After initial exposure, M. tuberculosis may be eliminated by the host immune response, persist as a latent infection, or progress to primary active disease. Following the establishment of latent infection, disease may persist in a latent form, naturally progress in a slow or rapid fashion to active tuberculosis, or cycle through incipient and subclinical states before developing into symptomatic disease or eventual disease resolution. Although not all possibilities for regression of disease burden are depicted, spontaneous recovery may occur in any of these clinical trajectories.
We acknowledge that there are limitations by applying discrete categorical definitions to a continuous process. First, this introduces challenges with categorical interpretation, particularly as people transition from one state to another. As such, the time spent within each disease state as well as routes of progression may be highly variable. Second, there may also be various levels of evidence for patients to fit these categorical definitions. For example, a culture with M. tuberculosis may be a higher level of diagnostic evidence for subclinical TB than chest radiography suggestive of upper lobe disease. Despite these limitations, we hope that applying new categories to the continuous spectrum of TB will help advance biomedical research and interventions to end the TB epidemic.
BACTERIAL PATHOGENESIS AND IMMUNE RESPONSE
Pathogenesis of Incipient and Subclinical Tuberculosis
M. tuberculosis exerts a wide spectrum of infectious pathophysiology. Evidence is mounting that genetic and phenotypic variation of the bacteria, along with the interaction between these bacteria and their individual hosts, can influence the progression of TB. To our knowledge, very few studies have focused specifically on the state of the pathogen during incipient TB or subclinical TB or progression through these states. Therefore, much of this section relies on making inferences from studies dichotomously conceptualizing latent TB infection and active TB disease.
At the population level, genetic heterogeneity between M. tuberculosis strains influences interactions with the host immune system and the consequent pathogenesis of TB infection. M. tuberculosis strains show substantial variation in their virulence and immunogenicity, which ultimately impact their propensity to induce or accelerate active disease (14–17). Based on whole-genome sequence analysis, human-adapted M. tuberculosis complex strains have been divided into seven phylogenetic lineages (15–20). Lineages 2 to 4, which harbor a deletion in the genomic region known as TbD1, are collectively referred to as “modern” because these strains diversified genetically more recently than the “ancient” lineages (lineages 1, 5, and 6) and “intermediate” lineage 7 (17, 18, 21). Numerous differences in host disease presentation have been ascribed to the different lineages, some of which are listed in Table 2. In addition, mechanistic studies have shown that different M. tuberculosis strains induce differential innate and adaptive immune responses via multiple effectors (16–18). Modern M. tuberculosis strains have been shown to elicit lower-level and delayed proinflammatory cytokine production from human peripheral blood mononuclear cells and replicate more rapidly in aerosol-infected mice and are more pathogenic to mice than more-ancient TB strains (17, 18, 20, 22–24). A well-characterized example is the HN878 strain of the lineage 2 Beijing family, which is highly virulent in mouse and rabbit models and has been implicated in multiple human TB outbreaks (25–27). HN878 produces a particular phenolic glycolipid in its cell wall (28, 29) and induces altered innate and adaptive immune responses (see Table 3 for examples). Together, these processes result in increased lung pathology and reduced survival in immunocompetent mice. In contrast, infections with ancient lineage 6 strains result in reduced bacterial burdens in marmosets and differential T cell responses in humans compared to modern-lineage strains and have been associated with reduced virulence and reduced progression to disease (30–32). As a result, it has been proposed that as human populations expanded rapidly, M. tuberculosis may have evolved traits of more-rapid disease progression and increased transmission (16–18). Given the influence of different M. tuberculosis strains on the host immune response and disease progression, the state of the pathogen is likely to play an important role in shaping the disease states for incipient and subclinical TB.
TABLE 2.
Summary of M. tuberculosis complex phylogenetic lineages, including some of the reported differences in disease presentation
| Lineage | Geographic distribution | Main lineage-specific genetic deletion(s) | Difference(s) in disease presentation | Reference(s) |
|---|---|---|---|---|
| 1 (Indo-Oceanic) | East Africa and Central, South, and Southeast Asia | RD239 | Elicits higher IFN-γ responses than lineage 2 and 3 strains; not associated with extrapulmonary disease | 237, 238 |
| 2 (East Asian [includes Beijing family]) | Central and East Asia, Eastern Europe, and South Africa | TbD1, RD105 | Associated with extrapulmonary disease; induces more-rapid wt loss than lineage 6; associated with relapse, treatment failure, and fever early during treatment | 30, 238–242 |
| 3 (East African-Indian) | East Africa and Central, South, and Southeast Asia | TbD1, RD750 | Associated with extrapulmonary disease; higher-level anti-inflammatory phenotype than with lineage 4 | 243 |
| 4 (Euro-American) | Asia, Europe, Africa, and the Americas | TbD1, pks15/1 | Associated with more α1-acid glycoprotein and C-reactive protein, higher neutrophil counts, and lower body mass index than with lineage 1; associated with more pulmonary than meningeal disease; lower mortality rates from meningeal TB; associated with lung consolidation in chest X ray | 32, 244, 245 |
| 5 (West Africa [Mycobacterium africanum]) | West Africa | RD9, RD711 | Lower rate of progression to disease than for M. tuberculosis | 32 |
| 6 (West Africa [M. africanum]) | West Africa | RD9, RD7, RD8, RD10, RD702 | Lower rate of progression to disease than for M. tuberculosis; more extrapulmonary spread | 30–32 |
| 7 (Ethiopia) | Ethiopia | RD3, RD11, 10 bp in mmpL9, 27 bp in lppH, 1.3 kb in lppO-sseB, 3.3 kb in Rv3467-rmlB2-mhpE | Delay in patients seeking treatment | 246 |
TABLE 3.
Examples of altered immune responses by M. tuberculosis strain HN878 relative to the CDC1551 or H37Rv strain
| Immune response | HN878-induced difference(s) vs H37Rv or CDC1551 | References |
|---|---|---|
| Innate | Altered interactions with pattern recognition receptors (e.g., Toll-like receptors) | 29, 247, 248 |
| Reduced activation of monocytes, macrophages, and dendritic cells | ||
| Adaptive | Raised levels of CD4+/CD25+/FoxP3+/CD223+/IL-10+ regulatory T cells | 27, 29, 249 |
| Cytokine | Induction of type I IFNs | 27, 29, 249 |
| Induction of TH2 cytokines IL-4 and IL-13 | ||
| Rapid repression of an initial spike in TH1 cytokines TNF-α, IL-6, IL-12, and IFN-γ |
Within the context of an individual patient, different infection sites within the lung create diverse microenvironments that induce bacterial phenotypic heterogeneity, which can in turn influence the immune response and progression of infection, from LTBI through the spectrum of incipient and subclinical TB and, ultimately, to active TB disease. Variations in the cellular compositions and activation levels of immune cells, epithelial cells, and the extracellular matrix that comprise granulomas expose the residing M. tuberculosis bacteria to differences in nutrient availability, reactive intermediates, and cytokine profiles as well as to differential drug penetration (5, 33–35). M. tuberculosis also reaches extrapulmonary sites even during asymptomatic infections, each with its own diverse milieu (36–39).
The types of lesions detectable in asymptomatic individuals correlate with the onset of subclinical TB disease and with progression toward active disease. One study surveying LTBI in individuals with asymptomatic HIV-1 infection with no clinical evidence of TB showed that over 70% of individuals harbored a broad range of abnormal lung lesions detected by [18F]fluoro-2-deoxy-d-glucose (FDG) positron emission tomography (PET) combined with computed tomography (PET/CT) imaging. Importantly, individuals with subclinical TB that harbored fibrotic scars or infiltrates or who were FDG active by PET/CT were more likely to develop active, symptomatic TB disease during a 6-month follow-up period (40, 41).
The mechanisms by which M. tuberculosis adapts to the diverse microenvironments created by these infection sites could contribute to differential disease progression (33, 34). Experiments in vitro and in mouse models have characterized molecular responses to some of the specific stresses encountered by M. tuberculosis during infection (e.g., oxygen and nutrient limitation) that promote a physiological shift toward reduced replication (33, 42, 43). Exposure of M. tuberculosis to either low oxygen or starvation in vitro triggers large-scale changes in gene and protein expression (44–47), which remodel key cellular processes, including energy metabolism (45, 47–49). Several mechanisms underlying the M. tuberculosis response to these stresses enact transcriptional, posttranslational, or allosteric control (50–60). How these responses integrate to influence patient-level outcomes for incipient or subclinical TB disease is currently unclear.
Recent in vitro studies have shown that host stresses encountered during infection will also influence the cell-to-cell variability in the growth and metabolism of M. tuberculosis, creating a spectrum of phenotypically heterogeneous subpopulations even under uniform conditions (61, 62). One subpopulation with potentially high clinical relevance is a subset of cells that are metabolically viable but do not replicate, even after being returned to growth-permissive culture conditions. Phenotypically similar subpopulations of “differentially culturable tubercle bacilli” (DCTB), which could not form CFU when cultured on standard solid media but could be grown after exposure to liquid media supplemented with fresh M. tuberculosis culture filtrates, have been found in sputum samples of TB patients and sputum culture-negative, smear-negative individuals (63). The DCTB subpopulation appears to be phenotypically heterogeneous, as different subsets of DCTB replicate differentially when exposed to medium supplementations of resuscitation-promoting factors. The correlation between relative abundance levels of DCTB and HIV status in TB patients suggests an interaction between these bacilli and the immune response (64). However, additional studies are required to clarify the molecular mechanisms that generate DCTB and the consequences of this population in patients with a clinical phenotype of incipient or subclinical TB.
Bacterial heterogeneity also influences immune control. Primate and human experiments have shown diverse trajectories for individual granulomas (5, 35). In one study, the numbers of viable M. tuberculosis bacilli within individual granulomas varied by several orders of magnitude in both asymptomatic and symptomatic individuals, the histopathology of lesions correlated with the bacterial load, and individuals in both groups harbored granulomas that were completely clear of M. tuberculosis bacilli (65). Individuals who progressed to active disease differed from those with sustained asymptomatic infection in three key ways: (i) they had a subpopulation of lesions that exhibit a minimal bactericidal effect after the onset of adaptive immunity, (ii) they exhibited evidence of bacterial dissemination to form new granulomas at later time points, and (iii) they had increased inflammation in their lesions (5, 65). Thus, various host-directed therapies that could contain metabolically active and dividing M. tuberculosis bacilli within the existing granulomas and prevent escape may help stop disease progression (66).
Immune Correlates of Incipient and Subclinical Tuberculosis
A common feature of immune correlate studies has been the upregulation of interferon (IFN) signaling, decreased B and T cell signaling, and increased myeloid cell signaling, including phagocytosis. When analyzed kinetically, IFN signaling preceded all other transcriptional modules, suggesting the promise of an IFN-driven signature for the progression of incipient TB (67). Cell-specific validation experiments suggested that immune signatures may be attributed to neutrophils. To address the specificity of host transcriptional signatures for TB, these preliminary signatures have also been evaluated in sarcoidosis, pneumonia, systemic lupus erythematosus, and lung cancer. While some overlap was observed, four studies were able to develop a refined signature driven by IFN signaling that could possibly delineate TB from other inflammatory conditions that contained IFN-stimulated genes (68–71). However, these signatures remain to be externally validated by others.
Humoral immunity may provide protective immunity to M. tuberculosis (72–74). Circulating antibodies to immunodominant antigens of M. tuberculosis may serve as biomarkers of subclinical TB (75, 76). For example, in an antibody screening study, distinctive Fc effector functions and glycosylation patterns were strongly associated with active TB, compared to LTBI (74). Notably, pooled IgG antibodies from latently infected individuals, but not those with active TB, were able to contain M. tuberculosis in an in vitro macrophage culture assay. Low levels of M. tuberculosis-targeting IgG may foreshadow a transition through incipient and subclinical TB. Since humoral immunity is involved in the response to M. tuberculosis infection, quantification of families of antibodies may provide an approach to identifying subclinical TB.
Interferon gamma release assays (IGRAs), which measure M. tuberculosis-specific CD4+ T cell immunity, have value in discerning exposure to M. tuberculosis but fail to identify infection or to distinguish the disease stage. However, one study reported that an IGRA conversion value of >4 IU/ml in infants was associated with a 42-fold-elevated risk of progression to TB (77). The presence of interleukin-2 (IL-2) cytokine-secreting CD4+ T cells has been shown to correlate with bacterial burden and clinical disease (78, 79). People with active TB disease also have a more activated and proapoptotic phenotype of antigen-specific CD4+ T cells than people with LTBI (80, 81). Th17-type CD4+ T cell responses may be associated with a lower risk of progression to TB (67). Individuals with a lower risk for TB progression, as measured by an RNA host signature, had higher levels of Th17-associated transcripts, including IL-17F (67). Measuring T cell immune responses using both CD4+ and CD8+ cells may be capable of differentiating LTBI from subclinical or active TB, if the frequency of antigen-specific CD8+ T cells can serve as a reflection of the antigen load and disease burden. Two independent studies found CD8+ T cells at a higher frequency in smear-positive than in smear-negative active TB (79, 82). Furthermore, frequencies of TB-specific CD8+ T cells are increased in disease progression in nonhuman primates (83) and decreased with anti-TB treatment (79, 84). IFN-γ production from CD8+ T cells can provide a signature of TB in young children (85). Most studies have not delineated T cell immune responses by different TB disease states, incipient and subclinical TB.
As M. tuberculosis changes its gene expression in response to host immune pressure, the antigen repertoire may also change (86). A number of antigens, DosR-regulated proteins, and enduring-hypoxic-response proteins have been described as being selective for LTBI, but data from confirmatory studies have been inconsistent (87). At present, relatively little is known regarding antigens that are selectively expressed during early TB as well as progression of TB infection (88–90). Ag85B is highly expressed during early M. tuberculosis infection and appears to be repressed during chronic M. tuberculosis infection (91–93). Lung-specific antigens could also be of value, but only a few reports have defined these antigens (94, 95). Nevertheless, an additional understanding of antigen recognition by the human cellular immune system may be helpful for generating diagnostic tools for incipient and subclinical TB.
Nonclassically restricted T cells use invariant restriction molecules and can present peptidic and nonpeptidic antigens, including glycolipids (96) and TB-derived products (97, 98). HLA-E-restricted CD8+ T cells can recognize TB-derived peptides (98–100) and proliferate during active TB, compared to healthy controls (101). Frequencies of CD1b-c-restricted T cells, which recognize lipid antigens, are similarly increased in during LTBI, compared to TB-uninfected individuals (102, 103). In contrast, frequencies of MR1-restricted T cells, which recognize riboflavin biosynthetic products, are reduced during active TB, compared to LTBI (104–106). Frequencies of natural killer T (NKT) cells, restricted by CD1d and recognizing glycolipid antigens, are similarly reduced from the circulation of individuals with TB compared to healthy controls (107). In a prospective household contact study, NKT cells were significantly less prevalent in people who progressed to active TB than in people who did not progress to active TB (108). Cumulatively, circulating frequencies of nonclassically restricted T cells change in the context of the progression from LTBI to active TB disease and could be useful as biomarkers to discern bacterial burdens for incipient and subclinical TB.
Proteomic, metabolomic, and epigenetic data may also be used to define signatures of TB progression (109–114). A longitudinal analysis of soluble biomarkers of an adolescent cohort monitored for progression to TB suggested that the complement cascade was the earliest upregulated group of proteins and correlated with an upregulated IFN-driven host RNA signature (67). While few of these signatures have been prospectively validated, multiple approaches suggest that measurement of the host response to mycobacterial infection can serve to identify those with subclinical and incipient TB. Future technological advances will likely define specific immune subsets associated with both incipient and subclinical TB, but validation of these approaches will need to rely upon prospective clinical trials.
EPIDEMIOLOGY OF INCIPIENT AND SUBCLINICAL TUBERCULOSIS
Our limited understanding of incipient and subclinical TB at the population level reflects the dearth of available diagnostic tools. Tests that have been utilized to understand the epidemiology of incipient and subclinical TB include national prevalence surveys, active case-finding studies, and systematic screening of high-risk groups, including preventive-therapy studies and TB vaccine trials (115–138). Studies recording both patient symptom histories and diagnostic test results, as well as postmortem studies, have also helped to characterize the burden of subclinical TB in specific populations (139, 140).
Given that incipient TB is not detectable on the basis of symptoms, radiography, or microbiological testing, our understanding of the epidemiology of incipient TB is very limited at present. We are, however, able to make some inferences about this disease state. Assuming that all cases of active TB progress through a period of meeting the definition of incipient TB, the incidence must be at least that of active TB (i.e., an estimated 10.4 million cases in 2016 [141]). To the extent that some individuals with incipient TB do not progress to detectable active disease, the incidence of incipient TB would be expected to be even higher than that of active TB disease.
The reported prevalence of subclinical TB varies widely across epidemiological settings, populations, and screening tools used. The prevalence of subclinical TB is generally high in studies performing active case finding among high-risk groups, during which all participants are screened with high-sensitivity tests (119, 123), compared to broader prevalence surveys, during which sputum culture might be performed only for persons with chest radiography suggestive of TB (142). A review of 12 national prevalence surveys in Asia found that the percentages of all bacteriologically confirmed TB cases who did not report TB symptoms (often requiring cough with a duration of 2 to 3 weeks) ranged from 40% in Pakistan to 79% in Myanmar. In three countries with repeat survey data on symptoms (Cambodia, Republic of Korea, and China), the percentages of confirmed prevalent cases who did not report symptoms rose between 18 and 21% over a period of 5 to 10 years (115, 143–145). This suggested that traditional TB control measures may reduce the prevalence of symptomatic TB but not that of subclinical TB, which may increase the proportion of subclinical TB cases among all culture-positive TB cases.
Groups at high risk for subclinical TB are likely to be similar to those for active TB and include residence in high-incidence settings; persons with a history of TB (119, 123, 139); HIV-positive individuals, particularly those not treated with antiretroviral therapy (118–123, 125–134, 146–154); pregnant and postpartum women (135, 136, 148, 155); people with noncommunicable disease comorbidity, including renal disease and diabetes (139); and high-exposure-risk groups, including household or recent TB contacts (119), miners (124, 134), and prisoners (137). Certain known TB risk factors have not yet been studied in association with subclinical TB; these factors include immunosuppressive medications (e.g., tumor necrosis factor alpha [TNF-α] inhibitors or glucocorticoids) and residential or occupational exposure (e.g., health care workers). Further studies of these groups would presumably identify an elevated risk for subclinical TB. As better biomarker-based assays become available, researchers can prospectively monitor high-risk individuals to better describe the epidemiology of incipient and subclinical TB.
CLINICAL COURSE AND TRANSMISSIBILITY OF INCIPIENT AND SUBCLINICAL TB
Clinical Course
The clinical course of incipient and subclinical TB disease is driven by a complex interaction between pathogen and host defenses. As a result, the duration and trajectory of these disease states can differ substantially across patients (Fig. 1). It is possible that the highest-risk individuals (e.g., those with advanced HIV) progress rapidly from subclinical TB to active TB, whereas those at a more moderate risk remain in a protracted or cyclic disease state. Immunosuppressive conditions are important risk factors for progression and include less-prevalent but higher-risk conditions, such as HIV infection and TNF-α blockade, and more-common but lower-risk conditions, including malnutrition and diabetes mellitus (156, 157). The time since initial exposure is a risk factor for progression; for instance, reactivation of TB is highest in the first 2 to 5 years following infection (158). Importantly, while progression from latent to incipient, subclinical, and symptomatic TB disease is sometimes a unidirectional process, regression of disease may also occur (159).
Currently, little is known about the duration of incipient or subclinical TB disease states. Given this variable natural history, the time from initial infection to the development of incipient or subclinical TB may range from weeks to a lifetime. The duration of each disease phase is similarly heterogeneous, and a window period for incipient or subclinical TB disease may last from months to years. National prevalence surveys suggest that the duration of subclinical TB, as estimated by the prevalence-incidence ratio, is at least as long as that of symptomatic disease (115). However, observational studies in settings where HIV is endemic suggest that people with subclinical TB can quickly progress to active TB disease within weeks to a few months (119–122, 124).
The relationship between clinical symptoms and underlying disease burden may not be entirely proportional. To the extent that smear positivity measures bacillary burden in the lungs, subclinical TB is generally more likely to be smear negative than symptomatic TB, as suggested by national prevalence surveys (115) and observational studies in HIV-positive cohorts (118, 119, 126). However, since subclinical TB is a dynamic state, transitions from smear-negative to smear-positive disease or the development of new radiographic abnormalities as individuals progress from subclinical to symptomatic TB disease may occur (123, 124). Although the burden of M. tuberculosis in the lungs may correlate loosely with the presence of symptoms, extrapulmonary or disseminated TB in immunocompromised individuals is often asymptomatic, rapidly progressive, and fatal. Postmortem studies highlight the frequency of extrapulmonary disease in high-HIV-prevalence settings (140, 160, 161). Thus, heterogeneity in clinical presentation and diagnostic findings must be interpreted within the context of differing patterns of progression through incipient and subclinical TB as well as differing levels of host immune status.
Transmissibility
One of the key concerns is that persons who persistently or intermittently carry lower bacillary burdens in their lungs, as might occur with subclinical TB, may be important sources of transmission in the community (162). Transmission of TB can be considered to be a function of the degree of infectiousness, the duration of infectiousness, and the availability of susceptible contacts (163). Most models of TB transmission have not included a subclinical TB stage, which may constitute half of the average total duration of active TB prior to treatment. However, people with subclinical TB may likely be missed by current TB control efforts, especially in settings where TB culture is not included in intensified case-finding efforts. In a recent mathematical model where subclinical disease was included, active detection of 5% of prevalent TB cases had a substantially greater impact than a 20% increase in rates of passive case detection on diagnosis and mortality at 10 years in most epidemiological settings (163). More clinical studies are needed to fully characterize the contribution of subclinical TB disease to M. tuberculosis transmission.
SCREENING AND DIAGNOSTIC APPROACH
Until recently, screening and diagnostic tools have not been prioritized for either incipient or subclinical TB. Currently, there are no approved diagnostic tests for incipient TB, although several novel biomarkers are under investigation (108, 114). For diagnosing subclinical TB, conventional tools, including chest radiography, sputum smear microscopy, culture, and Xpert MTB/RIF (Cepheid Inc.), may be used with various utilities (4). Overall, better diagnostics for incipient and subclinical TB (2, 163–165), which could allow treatment of individuals before they become symptomatic and infectious, may be required to make significant progress for the WHO's “End TB Strategy” (166).
Current Approaches and Incremental Improvements for Incipient Tuberculosis
Tuberculin skin testing (TST) and IGRAs have be used to identify individuals with a persistent immune response to TB antigens. While these tests have well-described limitations (167–170), their biggest problem is that they poorly predict who is at greatest risk for disease progression (171, 172). In countries with a high burden of TB, a large proportion of the general population may be infected with M. tuberculosis, but few adults will progress to active TB disease within their lifetime. The TST and IGRAs are not specific for disease progression, and providing universal LTBI treatment is often impractical for national TB programs (173–176). Several newly developed tests for latent infection represent incremental improvements but appear to suffer from the same major shortcomings of not accurately predicting disease progression (177–181). One new assay, QuantiFERON TB Gold Plus (Qiagen), includes additional peptides designed to stimulate both CD4+ and CD8+ T cells. Early studies have demonstrated improved sensitivity over the traditional QuantiFERON-TB Gold in-tube assay and the potential to serve as a proxy for recent infection (178, 182–184). The WHO recently articulated the minimal and optimal characteristics for a target product profile of a test to predict progression from incipient TB infection to active TB disease (185).
Since incipient TB exists on a spectrum of TB progression, defining immunologically distinct characteristics has been challenging. Recent research on host transcriptional changes has relied on the measurement of immune markers and responses as a surrogate for TB disease burden. An early microarray identified innate antimicrobial genes that differentiated TB patients from LTBI patients (186). Independent blood-based genome-wide transcriptional profiles for patients in high-burden settings have revealed additional gene signatures, few of which have been mined for the enrichment of molecular pathways (69, 71, 187–189). In a whole-transcriptome approach, a 393-gene signature was derived by using a “molecular distance to health” composite score that incorporated the severity of radiographical disease (68). The more recent Adolescent Cohort Study, performed in South African adolescents with LTBI, highlighted a 16-gene signature that could identify subjects as early as 18 months prior to the development of active TB (67, 114). The CORTIS study, a prospective validation trial to identify patients with incipient TB and guide preventive therapy using the same 16-gene signature, is under way (190, 191). Further improvements in simplifying the host RNA signature to a 6-gene set may also be possible (Thomas Scriba, personal communication), but the practicality of implementing this approach may remain challenging.
Current Approaches and Incremental Improvements for Subclinical Tuberculosis
In principle, tools used to diagnose active TB can also detect subclinical TB, although their sensitivity may be lower (192, 193). Since persons with subclinical TB do not have a symptomatic cough, they may be unable to produce quality sputum specimens, so novel assays may be necessary for use on non-sputum-based specimen types (194). Traditional diagnostic tests to confirm active TB disease include sputum smear microscopy, which has low diagnostic sensitivity (195), and mycobacterial culture, which has major infrastructure requirements and takes weeks to yield results (196). Xpert MTB/RIF, a nucleic acid amplification assay, has higher sensitivity than smear microscopy and important advantages over culture but has limited sensitivity to detect early or paucibacillary disease (197, 198). The next-generation Xpert MTB/RIF Ultra assay has improved sensitivity (199), but its ability to detect subclinical TB is unknown and still requires a sputum sample for the diagnosis of pulmonary TB. Several non-sputum-based approaches are currently under development for diagnosing active TB in both adults and children, which include M. tuberculosis nucleic acid detection in oral mucosa and stool (200, 201) as well the as detection of M. tuberculosis proteins and metabolites in urine (202). The most developed non-sputum-based assay detects urinary lipoarabinomannan (LAM) (203) and is indicated for use in severely immunocompromised HIV-infected hospitalized patients (204). However, the test is only moderately sensitive (∼60%) among HIV positive adults with CD4 counts of <100 cells/mm3 and has lower sensitivity in patients with CD4 counts of >100 cells/mm3. Urinary LAM has been shown to detect subclinical TB in 25% (7/28) of HIV-infected ambulatory patients in South Africa (205). Despite these limitations, there is the potential to improve diagnostic sensitivity in next-generation urinary LAM assays (206–209).
Transformational Approaches and Remaining Challenges for Diagnostics
Transformational and novel approaches may be necessary to overcome limitations in diagnosing incipient or subclinical TB. In particular for incipient TB, a high-sensitivity test may be achieved by measuring the host immune response, as opposed to direct pathogen detection (210, 211). Several systems biology “omics”-based diagnostic approaches appear promising (212, 213). The most advanced approach has been peripheral blood host RNA-based signatures, which show the potential to differentiate the entire spectrum of TB (67) and may be suitable for the detection of incipient and subclinical TB. Multiple RNA signatures have been reported (69, 71, 214–217), only some of which have focused on incipient or subclinical TB. Recently, a meta-analysis of RNA data sets identified a 3-gene signature for differentiating TB from other respiratory diseases (218) and may be useful for detecting incipient TB (Purvesh Khatri, personal communication). Other novel approaches investigated for incipient TB, including cell activation and differentiation markers, cytokine levels in blood, and antigen-specific T cells, have also been described (219, 220).
While a more comprehensive approach may be promising, the eventual development, validation, and implementation of a complicated assay would be challenging. Translating a host RNA signature into a simple, affordable, and globally applicable test poses formidable technical challenges (221). Host-based signatures typically do not require simply differentiating “signal from noise” (i.e., a qualitative/binary assessment of the presence versus the absence of a pathogen marker) but instead require a precise quantification of multiple biomarkers. Furthermore, validating possible diagnostic tests will pose unique challenges for tests targeting subclinical or incipient TB due to the lack of a good reference standard as well as requirements for sample size and longitudinal follow-up (185). Ensuring broadly generalizable accuracy estimates will also be difficult since host response signatures will likely vary depending on a multitude of factors, including age, ethnicity, medical comorbidities, the local environment, and various exposures to pathogens and immunological stressors (222, 223). Validation across a wide range of populations will be critical, but high levels of participant dropout can occur in the cascade of care for active TB (224, 225). Finally, many practical implementation questions will also need to be resolved, and additional work is needed on evaluating combinatorial approaches and complex algorithms.
TREATMENT AND PREVENTION OF INCIPIENT AND SUBCLINICAL TUBERCULOSIS
The basic principles underpinning treatment of TB infection and disease also apply to both incipient and subclinical TB (12). These include the microbiological burden (inferred from tests of bacterial load); disease extent (inferred from radiological studies); testing of susceptibility to major first- and second-line drugs; the site of disease for drug penetration; the risk of adverse events and medication side effects, including hepatotoxicity; and prognostic features and comorbidities, such as diabetes and HIV coinfection, with an increased risk of drug-drug interactions. Several of these factors may influence the drug-specific selection, dose, or duration of therapy for incipient and subclinical TB. All patients with suspected incipient or subclinical TB should have a comprehensive clinical evaluation for other comorbidities, including HIV.
Incipient Tuberculosis
Although pathophysiologically characterized, incipient TB has not previously had a clearly defined diagnostic definition, which has precluded the evaluation of a specific treatment regimen for a defined subgroup. However, incipient TB remains a critically important entity to diagnose, as preventing progression to active TB will optimize the use of health care resources. Although speculative, the conventional regimens for LTBI, which include 6 to 12 months of isoniazid, 3 months of a combination of rifamycin and isoniazid, or 3 to 4 months of a rifamycin alone, may be effective for incipient TB, assuming a relatively low burden of disease. However, newer, existing, and repurposed drugs (bedaquiline, delamanid, linezolid, likely sutezolid in the future, and fluoroquinolones) are now being used in clinical practice to treat drug-resistant TB (226, 227). Once issues of toxicity, teratogenicity, and effectiveness are clarified, newer and repurposed drugs offer the prospect of simplifying and shortening regimens for incipient TB of any susceptibility profile in the future. Another treatment option is providing a high dose of rifamycins, which have the potential to shorten the duration of conventional treatment for active TB and are well tolerated (228). Clinical trials are already in progress to evaluate the utility of different drugs, including delamanid and fluoroquinolones, for the treatment of multidrug-resistant LTBI (229). If successful, then they may also be appropriate for treating incipient TB. Future, alternative approaches for the treatment of incipient TB might include therapeutic vaccines, host-directed therapies, or combining immunosuppressive agents and anti-TB drugs (230). Future clinical trials will also need to address the issue of duration of therapy for incipient TB. In countries where TB is endemic, the WHO recommends treating presumed LTBI in HIV-infected persons with isoniazid for 36 months (231), which may be a starting point for incipient TB.
Subclinical Tuberculosis
Treatment of subclinical TB should include concurrent HIV testing and, where possible, obtaining a biological sample for drug susceptibility testing. The presence of HIV coinfection will impact the choice of antiretroviral therapy and raise considerations related to immune reconstitution inflammatory syndrome, while regimens for the treatment of drug-resistant TB will depend on the susceptibility profile of the M. tuberculosis isolate. However, in general, treatment for subclinical TB should be identical to that of conventional active TB disease while taking into account comorbidities, potential drug-drug interactions, and other considerations described above. Once results from clinical studies that are evaluating newer and repurposed drugs become available, additional trials may need to be initiated to evaluate the possibility for shortening the duration of treatment for drug-sensitive subclinical TB, based on disease extent and bacterial burden. One clinical trial is currently evaluating the utility of a short 4-month treatment course, compared to a standard 6-month regimen, based on bacterial burden and the radiological extent of disease (232). While ongoing studies are directed at patients with active TB disease, future studies will need to validate or improve treatment options for patients with subclinical TB.
Isoniazid Preventive Therapy
Persons with incipient or subclinical TB are sometimes identified as having LTBI and may be started on isoniazid preventive therapy (IPT). While this practice raises concerns for the emergence of isoniazid resistance and the potential for ongoing disease transmission (233, 234), IPT-driven drug resistance, despite decades of use, has not been confirmed at the population level (235). In one study, treatment was switched to a four-drug regimen upon the recognition of subclinical TB, and participants had a good response (123). Furthermore, a comparison of recently IPT-treated and -untreated individuals with subclinical disease did not show differences in the proportions of individuals who subsequently developed active TB (119). Although evidence is limited, observational studies have not suggested a benefit of IPT on the clinical progression or outcomes of subclinical TB disease (155). Despite a lack of evidence, it will be essential to first exclude subclinical TB when considering treatment for LTBI. In contrast, preventive regimens are appropriate in the setting of incipient TB.
Treatment Outcomes
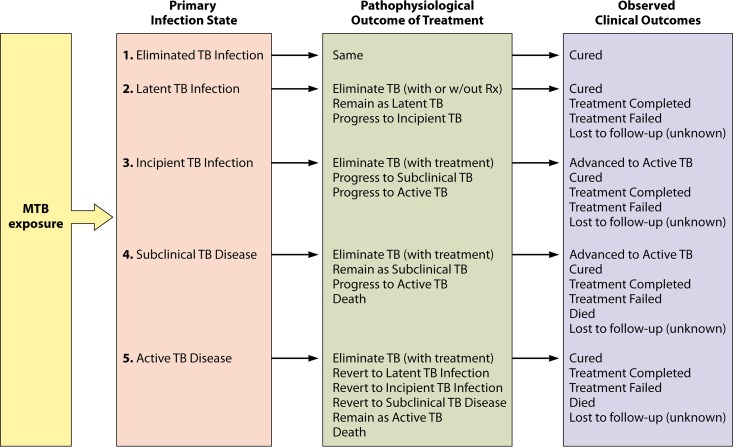
Responses to treatment for each stage of disease are variable (Fig. 2). Clinical outcomes are rarely recorded for the treatment of LTBI, and the appropriate treatment course for incipient or subclinical TB remains uncertain. Currently, treatment outcomes for active TB are often reported simply as treatment success (i.e., completion of a full course of treatment and resolution of symptoms) versus failure (persistent disease), loss to follow-up, or death (13). Cure is sometimes confirmed microbiologically, but even microbiological cure does not necessarily equate to pathophysiological cure (i.e., the absence of viable bacilli in the host). One study that profiled patients from multiple cohorts infected with drug-sensitive M. tuberculosis after 6 months of anti-TB therapy found that a substantial number of people still showed ongoing inflammation in the lung by FDG PET/CT and that some developed new or intensified lesions during the course of therapy (41, 236). Thus, “treatment success” as recorded clinically may correspond with regression to an earlier stage in the pathophysiological spectrum of disease.
FIG 2.
Primary and secondary disease states for the five categorical states of TB. Clinical outcomes following treatment are variable and depend on the respective pathophysiological outcomes. MTB, M. tuberculosis.
RESEARCH NEEDS AND PRIORITIES
Earlier diagnosis and prompt therapy for incipient or subclinical TB may be critical to preventing TB disease progression and transmission of M. tuberculosis bacilli. Since both incipient TB and subclinical TB are relatively new concepts, many clinical, translational, and basic science research questions remain. In Table 4, we list some of the key research needs and priorities for each of the domains presented in this review. This list is not meant to be comprehensive but rather is meant to present some prior research questions for the current field.
TABLE 4.
Priority research questions for incipient and subclinical tuberculosis
| Question |
|---|
| Pathogenesis and immune response |
| What triggers/prevents progression from incipient or subclinical to active TB disease? |
| How do different strains of M. tuberculosis alter proliferation and progression to active TB? |
| Can the immune system be harnessed to stop M. tuberculosis in the incipient or subclinical stage? |
| Epidemiology |
| How does the prevalence of incipient and subclinical TB vary across populations? |
| Are there host genetic factors that may increase the risk of incipient or subclinical TB? |
| Clinical course and transmissibility |
| How common is a waxing/waning picture of adults with subclinical and active TB? |
| What are the risk factors for progression of disease through incipient and subclinical TB? |
| How transmissible is TB in the incipient or subclinical disease stage? |
| Should contacts of a patient with incipient or subclinical disease be evaluated for TB? |
| Screening and diagnostic approach |
| What are the optimal screening and diagnostics tests for incipient TB? |
| What are the optimal screening and diagnostics tests for subclinical TB? |
| What is the recommended screening frequency for either stage in high-TB-burden regions? |
| Treatment and prevention |
| Should incipient TB be treated with isoniazid monotherapy or another regimen, and for how long? |
| Should subclinical TB be treated with monotherapy, 4-drug therapy, or a new drug combination? |
Several basic science questions pertain to the complex host-pathogen interactions that may either promote or halt the progression of infection from an incipient or subclinical TB stage to active TB disease. Additional research is needed to determine whether the powers of the host immune system may be harnessed to existing or novel host-directed therapies to stop M. tuberculosis in the incipient or subclinical stage. To improve the understanding of TB epidemiology, more research is needed on the underlying prevalence and incidence of both incipient and subclinical TB across various high- and low-risk populations. Many research questions also exist for the clinical course and transmissibility of incipient and subclinical TB. Some of these questions include the general pattern of disease progression and the associated risk factors, while the apparent transmissibility of subclinical TB is largely unknown but clearly important for adequate infection control and public health responses. Finally, the optimal diagnostic modalities and treatment of incipient and subclinical TB have been largely underexplored but are quickly becoming important priority topics.
CONCLUSIONS
The recognition of incipient and subclinical TB as distinct states between latent and active TB along the clinical disease spectrum provides opportunities for an improved understanding of disease dynamics as well as the development of diagnostic and therapeutic interventions to prevent progression to active TB disease and transmission of active TB bacilli. However, this revised understanding of TB may not currently change the therapy directed to individual patients, mainly because of the major limitations with the available diagnostic methods and tools. In this review, we have highlighted the current knowledge of incipient and subclinical TB and offered opportunities and priorities for further exploration.
In 2018, the United Nations General Assembly will hold its first high-level meeting on M. tuberculosis and the global TB epidemic, roughly 136 years after the initial discovery of a causative agent for TB. The meeting will likely highlight the need for more innovative solutions to an ancient disease, which should include priority research questions around incipient and subclinical TB.
Through the development of a better understanding of the clinical pathogenic spectrum of TB infection and disease, we may be able to devise more advanced and specific solutions to the TB epidemic. By synthesizing the expanding knowledge and addressing existing research gaps for both incipient and subclinical TB, the research and public health communities may move forward together on developing sustainable solutions to end the TB epidemic.
Biographies

Paul K. Drain, M.D., M.P.H., is an Assistant Professor of Global Health, Medicine, and Epidemiology at the University of Washington and a practicing Infectious Disease physician in Seattle. His research group focuses on development and implementation of diagnostic testing and clinic-based screening, including novel point-of-care technologies, to improve clinical care and patient-centered outcomes for tuberculosis and HIV in resource-limited settings. He is Associate Director of the Tuberculosis Research and Training Center at the University of Washington. His research has been supported by the National Institutes of Health, the Infectious Diseases Society of America, the Bill and Melinda Gates Foundation, the Harvard Global Health Institute, both the University of Washington and Harvard Centers for AIDS Research, and the AIDS Healthcare Foundation. He completed infectious disease training at Massachusetts General Hospital and Brigham and Women's Hospital at Harvard Medical School in Boston, MA.

Kristina L. Bajema, M.D., M.Sc., is a senior infectious disease fellow at the University of Washington. She received her M.D. and M.Sc. from the University of Washington and completed her residency at Providence Portland Medical Center in Oregon. Her primary research interest is in the intersection of infectious and noncommunicable diseases in low- and middle-income countries, with a specific focus on HIV-related tuberculosis and diabetes.

David Dowdy, M.D., Ph.D., is an Associate Professor of Epidemiology at the Johns Hopkins Bloomberg School of Public Health and a practicing general internist. He received his M.D. and Ph.D. from Johns Hopkins University and completed his residency in Internal Medicine at the University of California, San Francisco, before returning to the faculty at Johns Hopkins, where he has served since 2011. His primary research interests lie at the nexus of epidemiology, modeling, health economics, and decision-making in the global fight against tuberculosis. He has worked in this field for 12 years.

Keertan Dheda, M.B.B.Ch., Ph.D., graduated in 1992 from the University of the Witwatersrand in Johannesburg, specialized in respiratory medicine in London, and obtained a Ph.D. in mycobacterial immunity from UCL in 2004. He is head of the Division of Pulmonology at Groote Schuur Hospital and the University of Cape Town. His research interests include the diagnosis and management of pulmonary infections, including multidrug-resistant tuberculosis.

Kogieleum Naidoo, M.B.Ch.B., Ph.D., is head of the Treatment Research Program at the Centre of the AIDS Programme of Research in South Africa (CAPRISA). She received her M.B.Ch.B. and Ph.D. from the University of KwaZulu-Natal. Her research on addressing the challenges of tuberculosis-HIV integration has been influential in defining treatment strategies for clinical complications of tuberculosis-HIV integration. Her research has been supported by the U.S. Centers for Disease Control, the Newton Fund, the South African Medical Research Council, the National Institutes of Health, the Bill and Melinda Gates Foundation, the Howard Hughes Medical Institute, and the Presidents Emergency Fund for AIDS Relief. She has authored more than 60 peer-reviewed publications and coauthored chapters in several global health books. She was awarded the 2013 Union Scientific Prize awarded by the International Union against Tuberculosis and Lung Disease.

Samuel G. Schumacher, Ph.D., M.Sc., is a Senior Scientific Officer at the Foundation for Innovative New Diagnostics (FIND) in Geneva, Switzerland. He received his M.Sc. in Molecular Biotechnology from the Technical University of Munich and his Ph.D. in Epidemiology from McGill University. He has a keen interest in the development and evaluation of technology to address global health problems and in applying rigorous research methodology. His main research focus is on tuberculosis diagnostics, with more than 10 years of experience in a broad range of countries and settings. At FIND, his main role is in the planning and conduct of clinical trials, definition of standards for diagnostics and study design, evaluation of the patient- and population-level impacts of diagnostics, and in supporting the WHO's policy development process.

Shuyi Ma, Ph.D., is a postdoctoral scientist at the Center for Infectious Disease Research and the University of Washington Pathobiology Program. She did her graduate training at the University of Illinois at Urbana-Champaign and the Institute for Systems Biology. She has studied Mycobacterium tuberculosis since 2012, by combining computational and experimental systems biology approaches. Her work has included modeling genome-scale metabolism and transcriptional regulation in M. tuberculosis and integrating these models with omics-based experimental strategies to reveal how M. tuberculosis responds to drugs and stresses.

Erin Meermeier, Ph.D., is a postdoctoral fellow at Oregon Health and Science University in the Department of Pulmonary and Critical Care Medicine. She received her Ph.D. from Oregon Health and Science University in the Department of Molecular Microbiol and Immunology. Her research interests focus on using human genomics and cellular immunology to reveal mechanisms that support vaccine design for infectious diseases, with a current focus on tuberculosis. She has been working in the field of tuberculosis immunology for six years.

David M. Lewinsohn, M.D., Ph.D., received his undergraduate degree at Haverford College and his M.D. and Ph.D. at Stanford University. He received his training in Internal Medicine at UCSF and Pulmonary and Critical Care training at the University of Washington. He was a Senior Fellow/Acting Instructor at the University of Washington and Investigator at the Infectious Disease Research Institute in Seattle. He is currently a Professor of Pulmonary and Critical Care Medicine at Oregon Health and Science University and holds adjunct appointments in Molecular Microbiology and Immunology as well as the Vaccine and Gene Therapy Institute. He is also the director of the Oregon Health and Science University Center for Global Child Health Research. Dr. Lewinsohn's primary research interest is in understanding the mechanisms by which the human immune system recognizes the M. tuberculosis-infected cell. This interest began during his postdoctoral training at the University of Washington.

David R. Sherman, Ph.D., is Associate Director and Professor at the Center for Infectious Disease Research and Affiliate Professor in the Department of Global Health at the University of Washington in Seattle. He earned his B.A. from UC Berkeley and Ph.D. in Biochemistry from Vanderbilt University and performed postgraduate work at the Rockefeller University and at Washington University in St. Louis, MO. He began work on M. tuberculosis while at PathoGenesis Corp., where he played a lead role in the discovery and early development of the antituberculosis agent pretomanid that is now in clinical trials. The Sherman laboratory is widely recognized for integrating approaches of systems biology into tuberculosis research.
REFERENCES
- 1.World Health Organization. 2016. Global tuberculosis report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Houben RM, Dodd PJ. 2016. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 13:e1002152. doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. 2014. The end TB strategy. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, Ginsberg A, Swaminathan S, Spigelman M, Getahun H, Menzies D, Raviglione M. 2016. Tuberculosis. Nat Rev Dis Primers 2:16076. doi: 10.1038/nrdp.2016.76. [DOI] [PubMed] [Google Scholar]
- 5.Cadena AM, Fortune SM, Flynn JL. 2017. Heterogeneity in tuberculosis. Nat Rev Immunol 17:691–702. doi: 10.1038/nri.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barry CE III, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. 2009. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwander S, Dheda K. 2011. Human lung immunity against Mycobacterium tuberculosis: insights into pathogenesis and protection. Am J Respir Crit Care Med 183:696–707. doi: 10.1164/rccm.201006-0963PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Achkar JM, Jenny-Avital ER. 2011. Incipient and subclinical tuberculosis: defining early disease states in the context of host immune response. J Infect Dis 204(Suppl 4):S1179–S1186. doi: 10.1093/infdis/jir451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. 2017. Consensus meeting report: development of a target product profile (TPP) and a framework for evaluation for a test for predicting progression from tuberculosis infection to active disease. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 10.World Health Organization. 2015. Guidelines on the management of latent tuberculosis infection. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 11.World Health Organization. 2018. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 12.Dheda K, Barry CE III, Maartens G. 2016. Tuberculosis. Lancet 387:1211–1226. doi: 10.1016/S0140-6736(15)00151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. 2010. Treatment of tuberculosis guidelines, 4th ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 14.Lopez B, Aguilar D, Orozco H, Burger M, Espitia C, Ritacco V, Barrera L, Kremer K, Hernandez-Pando R, Huygen K, van Soolingen D. 2003. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin Exp Immunol 133:30–37. doi: 10.1046/j.1365-2249.2003.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, Narayanan S, Nicol M, Niemann S, Kremer K, Gutierrez MC, Hilty M, Hopewell PC, Small PM. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 103:2869–2873. doi: 10.1073/pnas.0511240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coscolla M, Gagneux S. 2010. Does M. tuberculosis genomic diversity explain disease diversity? Drug Discov Today Dis Mech 7:e43–e59. doi: 10.1016/j.ddmec.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tientcheu LD, Koch A, Ndengane M, Andoseh G, Kampmann B, Wilkinson RJ. 2017. Immunological consequences of strain variation within the Mycobacterium tuberculosis complex. Eur J Immunol 47:432–445. doi: 10.1002/eji.201646562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coscolla M, Gagneux S. 2014. Consequences of genomic diversity in Mycobacterium tuberculosis. Semin Immunol 26:431–444. doi: 10.1016/j.smim.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nebenzahl-Guimaraes H, Yimer SA, Holm-Hansen C, de Beer J, Brosch R, van Soolingen D. 2016. Genomic characterization of Mycobacterium tuberculosis lineage 7 and a proposed name: ‘Aethiops vetus’. Microb Genom 2:e000063. doi: 10.1099/mgen.0.000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagneux S. 2018. Ecology and evolution of Mycobacterium tuberculosis. Nat Rev Microbiol 16:202–213. doi: 10.1038/nrmicro.2018.8. [DOI] [PubMed] [Google Scholar]
- 21.Firdessa R, Berg S, Hailu E, Schelling E, Gumi B, Erenso G, Gadisa E, Kiros T, Habtamu M, Hussein J, Zinsstag J, Robertson BD, Ameni G, Lohan AJ, Loftus B, Comas I, Gagneux S, Tschopp R, Yamuah L, Hewinson G, Gordon SV, Young DB, Aseffa A. 2013. Mycobacterial lineages causing pulmonary and extrapulmonary tuberculosis, Ethiopia. Emerg Infect Dis 19:460–463. doi: 10.3201/eid1903.120256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro SC, Gomes LL, Amaral EP, Andrade MR, Almeida FM, Rezende AL, Lanes VR, Carvalho EC, Suffys PN, Mokrousov I, Lasunskaia EB. 2014. Mycobacterium tuberculosis strains of the modern sublineage of the Beijing family are more likely to display increased virulence than strains of the ancient sublineage. J Clin Microbiol 52:2615–2624. doi: 10.1128/JCM.00498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portevin D, Gagneux S, Comas I, Young D. 2011. Human macrophage responses to clinical isolates from the Mycobacterium tuberculosis complex discriminate between ancient and modern lineages. PLoS Pathog 7:e1001307. doi: 10.1371/journal.ppat.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiling N, Homolka S, Walter K, Brandenburg J, Niwinski L, Ernst M, Herzmann C, Lange C, Diel R, Ehlers S, Niemann S. 2013. Clade-specific virulence patterns of Mycobacterium tuberculosis complex strains in human primary macrophages and aerogenically infected mice. mBio 4:e00250-13. doi: 10.1128/mBio.00250-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsenova L, Ellison E, Harbacheuski R, Moreira AL, Kurepina N, Reed MB, Mathema B, Barry CE III, Kaplan G. 2005. Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J Infect Dis 192:98–106. doi: 10.1086/430614. [DOI] [PubMed] [Google Scholar]
- 26.Manca C, Tsenova L, Barry CE III, Bergtold A, Freeman S, Haslett PA, Musser JM, Freedman VH, Kaplan G. 1999. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J Immunol 162:6740–6746. [PubMed] [Google Scholar]
- 27.Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, Barry CE III, Freedman VH, Kaplan G. 2001. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha/beta. Proc Natl Acad Sci U S A 98:5752–5757. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed MB, Domenech P, Manca C, Su H, Barczak AK, Kreiswirth BN, Kaplan G, Barry CE III. 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431:84–87. doi: 10.1038/nature02837. [DOI] [PubMed] [Google Scholar]
- 29.Manca C, Reed MB, Freeman S, Mathema B, Kreiswirth B, Barry CE III, Kaplan G. 2004. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect Immun 72:5511–5514. doi: 10.1128/IAI.72.9.5511-5514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Via LE, Weiner DM, Schimel D, Lin PL, Dayao E, Tankersley SL, Cai Y, Coleman MT, Tomko J, Paripati P, Orandle M, Kastenmayer RJ, Tartakovsky M, Rosenthal A, Portevin D, Eum SY, Lahouar S, Gagneux S, Young DB, Flynn JL, Barry CE III. 2013. Differential virulence and disease progression following Mycobacterium tuberculosis complex infection of the common marmoset (Callithrix jacchus). Infect Immun 81:2909–2919. doi: 10.1128/IAI.00632-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tientcheu LD, Sutherland JS, de Jong BC, Kampmann B, Jafali J, Adetifa IM, Antonio M, Dockrell HM, Ota MO. 2014. Differences in T-cell responses between Mycobacterium tuberculosis and Mycobacterium africanum-infected patients. Eur J Immunol 44:1387–1398. doi: 10.1002/eji.201343956. [DOI] [PubMed] [Google Scholar]
- 32.de Jong BC, Hill PC, Aiken A, Awine T, Antonio M, Adetifa IM, Jackson-Sillah DJ, Fox A, Deriemer K, Gagneux S, Borgdorff MW, McAdam KP, Corrah T, Small PM, Adegbola RA. 2008. Progression to active tuberculosis, but not transmission, varies by Mycobacterium tuberculosis lineage in The Gambia. J Infect Dis 198:1037–1043. doi: 10.1086/591504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutta NK, Karakousis PC. 2014. Latent tuberculosis infection: myths, models, and molecular mechanisms. Microbiol Mol Biol Rev 78:343–371. doi: 10.1128/MMBR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhar N, McKinney J, Manina G. 2016. Phenotypic heterogeneity in Mycobacterium tuberculosis. Microbiol Spectr 4:TBTB2–0021-2016. doi: 10.1128/microbiolspec.TBTB2-0021-2016. [DOI] [PubMed] [Google Scholar]
- 35.Lenaerts A, Barry CE III, Dartois V. 2015. Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol Rev 264:288–307. doi: 10.1111/imr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C. 1916. An experimental study of latent tuberculosis. Lancet 188:417–419. doi: 10.1016/S0140-6736(00)58936-3. [DOI] [Google Scholar]
- 37.Loomis H. 1890. Some facts in the etiology of tuberculosis, evidenced by thirty autopsies and experiments upon animals. Med Rec (1866-1922) 38:689. [Google Scholar]
- 38.Opie E. 1927. Tubercle bacilli in latent tuberculous lesions and in the lung tissue without tuberculous lesions. Arch Pathol Lab Med 4:1–21. [Google Scholar]
- 39.Neyrolles O, Hernandez-Pando R, Pietri-Rouxel F, Fornes P, Tailleux L, Barrios Payan JA, Pivert E, Bordat Y, Aguilar D, Prevost MC, Petit C, Gicquel B. 2006. Is adipose tissue a place for Mycobacterium tuberculosis persistence? PLoS One 1:e43. doi: 10.1371/journal.pone.0000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esmail H, Lai RP, Lesosky M, Wilkinson KA, Graham CM, Coussens AK, Oni T, Warwick JM, Said-Hartley Q, Koegelenberg CF, Walzl G, Flynn JL, Young DB, Barry CE III, O'Garra A, Wilkinson RJ. 2016. Characterization of progressive HIV-associated tuberculosis using 2-deoxy-2-[(18)F]fluoro-d-glucose positron emission and computed tomography. Nat Med 22:1090–1093. doi: 10.1038/nm.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnappinger D, Ehrt S. 2016. A broader spectrum of tuberculosis. Nat Med 22:1076–1077. doi: 10.1038/nm.4186. [DOI] [PubMed] [Google Scholar]
- 42.Gengenbacher M, Kaufmann SH. 2012. Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol Rev 36:514–532. doi: 10.1111/j.1574-6976.2012.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lipworth S, Hammond RJ, Baron VO, Hu Y, Coates A, Gillespie SH. 2016. Defining dormancy in mycobacterial disease. Tuberculosis (Edinb) 99:131–142. doi: 10.1016/j.tube.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Albrethsen J, Agner J, Piersma SR, Hojrup P, Pham TV, Weldingh K, Jimenez CR, Andersen P, Rosenkrands I. 2013. Proteomic profiling of Mycobacterium tuberculosis identifies nutrient-starvation-responsive toxin-antitoxin systems. Mol Cell Proteomics 12:1180–1191. doi: 10.1074/mcp.M112.018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol 43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 46.Schubert OT, Ludwig C, Kogadeeva M, Zimmermann M, Rosenberger G, Gengenbacher M, Gillet LC, Collins BC, Rost HL, Kaufmann SH, Sauer U, Aebersold R. 2015. Absolute proteome composition and dynamics during dormancy and resuscitation of Mycobacterium tuberculosis. Cell Host Microbe 18:96–108. doi: 10.1016/j.chom.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Rustad TR, Harrell MI, Liao R, Sherman DR. 2008. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS One 3:e1502. doi: 10.1371/journal.pone.0001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boshoff HI, Barry CE III. 2005. Tuberculosis—metabolism and respiration in the absence of growth. Nat Rev Microbiol 3:70–80. doi: 10.1038/nrmicro1065. [DOI] [PubMed] [Google Scholar]
- 49.Shi L, Sohaskey CD, Kana BD, Dawes S, North RJ, Mizrahi V, Gennaro ML. 2005. Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc Natl Acad Sci U S A 102:15629–15634. doi: 10.1073/pnas.0507850102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voskuil MI, Schnappinger D, Visconti KC, Harrell MI, Dolganov GM, Sherman DR, Schoolnik GK. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med 198:705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherman DR, Voskuil M, Schnappinger D, Liao R, Harrell MI, Schoolnik GK. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc Natl Acad Sci U S A 98:7534–7539. doi: 10.1073/pnas.121172498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galagan JE, Minch K, Peterson M, Lyubetskaya A, Azizi E, Sweet L, Gomes A, Rustad T, Dolganov G, Glotova I, Abeel T, Mahwinney C, Kennedy AD, Allard R, Brabant W, Krueger A, Jaini S, Honda B, Yu WH, Hickey MJ, Zucker J, Garay C, Weiner B, Sisk P, Stolte C, Winkler JK, Van de Peer Y, Iazzetti P, Camacho D, Dreyfuss J, Liu Y, Dorhoi A, Mollenkopf HJ, Drogaris P, Lamontagne J, Zhou Y, Piquenot J, Park ST, Raman S, Kaufmann SH, Mohney RP, Chelsky D, Moody DB, Sherman DR, Schoolnik GK. 2013. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature 499:178–183. doi: 10.1038/nature12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chauhan R, Ravi J, Datta P, Chen T, Schnappinger D, Bassler KE, Balazsi G, Gennaro ML. 2016. Reconstruction and topological characterization of the sigma factor regulatory network of Mycobacterium tuberculosis. Nat Commun 7:11062. doi: 10.1038/ncomms11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanyal S, Banerjee SK, Banerjee R, Mukhopadhyay J, Kundu M. 2013. Polyphosphate kinase 1, a central node in the stress response network of Mycobacterium tuberculosis, connects the two-component systems MprAB and SenX3-RegX3 and the extracytoplasmic function sigma factor, sigma E. Microbiology 159:2074–2086. doi: 10.1099/mic.0.068452-0. [DOI] [PubMed] [Google Scholar]
- 55.Dutta NK, Mehra S, Martinez AN, Alvarez X, Renner NA, Morici LA, Pahar B, Maclean AG, Lackner AA, Kaushal D. 2012. The stress-response factor SigH modulates the interaction between Mycobacterium tuberculosis and host phagocytes. PLoS One 7:e28958. doi: 10.1371/journal.pone.0028958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fontan PA, Voskuil MI, Gomez M, Tan D, Pardini M, Manganelli R, Fattorini L, Schoolnik GK, Smith I. 2009. The Mycobacterium tuberculosis sigma factor sigmaB is required for full response to cell envelope stress and hypoxia in vitro, but it is dispensable for in vivo growth. J Bacteriol 191:5628–5633. doi: 10.1128/JB.00510-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ortega C, Liao R, Anderson LN, Rustad T, Ollodart AR, Wright AT, Sherman DR, Grundner C. 2014. Mycobacterium tuberculosis Ser/Thr protein kinase B mediates an oxygen-dependent replication switch. PLoS Biol 12:e1001746. doi: 10.1371/journal.pbio.1001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sureka K, Dey S, Datta P, Singh AK, Dasgupta A, Rodrigue S, Basu J, Kundu M. 2007. Polyphosphate kinase is involved in stress-induced mprAB-sigE-rel signalling in mycobacteria. Mol Microbiol 65:261–276. doi: 10.1111/j.1365-2958.2007.05814.x. [DOI] [PubMed] [Google Scholar]
- 59.Avarbock D, Avarbock A, Rubin H. 2000. Differential regulation of opposing RelMtb activities by the aminoacylation state of a tRNA·ribosome·mRNA·RelMtb complex. Biochemistry 39:11640–11648. doi: 10.1021/bi001256k. [DOI] [PubMed] [Google Scholar]
- 60.Sajish M, Tiwari D, Rananaware D, Nandicoori VK, Prakash B. 2007. A charge reversal differentiates (p)ppGpp synthesis by monofunctional and bifunctional Rel proteins. J Biol Chem 282:34977–34983. doi: 10.1074/jbc.M704828200. [DOI] [PubMed] [Google Scholar]
- 61.Manina G, Dhar N, McKinney JD. 2015. Stress and host immunity amplify Mycobacterium tuberculosis phenotypic heterogeneity and induce nongrowing metabolically active forms. Cell Host Microbe 17:32–46. doi: 10.1016/j.chom.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 62.Mouton JM, Helaine S, Holden DW, Sampson SL. 2016. Elucidating population-wide mycobacterial replication dynamics at the single-cell level. Microbiology 162:966–978. doi: 10.1099/mic.0.000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dartois V, Saito K, Warrier T, Nathan C. 2016. New evidence for the complexity of the population structure of Mycobacterium tuberculosis increases the diagnostic and biologic challenges. Am J Respir Crit Care Med 194:1448–1451. doi: 10.1164/rccm.201607-1431ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chengalroyen MD, Beukes GM, Gordhan BG, Streicher EM, Churchyard G, Hafner R, Warren R, Otwombe K, Martinson N, Kana BD. 2016. Detection and quantification of differentially culturable tubercle bacteria in sputum from patients with tuberculosis. Am J Respir Crit Care Med 194:1532–1540. doi: 10.1164/rccm.201604-0769OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin PL, Ford CB, Coleman MT, Myers AJ, Gawande R, Ioerger T, Sacchettini J, Fortune SM, Flynn JL. 2014. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat Med 20:75–79. doi: 10.1038/nm.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hawn TR, Shah JA, Kalman D. 2015. New tricks for old dogs: countering antibiotic resistance in tuberculosis with host-directed therapeutics. Immunol Rev 264:344–362. doi: 10.1111/imr.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scriba TJ, Penn-Nicholson A, Shankar S, Hraha T, Thompson EG, Sterling D, Nemes E, Darboe F, Suliman S, Amon LM, Mahomed H, Erasmus M, Whatney W, Johnson JL, Boom WH, Hatherill M, Valvo J, De Groote MA, Ochsner UA, Aderem A, Hanekom WA, Zak DE. 2017. Sequential inflammatory processes define human progression from M. tuberculosis infection to tuberculosis disease. PLoS Pathog 13:e1006687. doi: 10.1371/journal.ppat.1006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O'Garra A. 2010. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaforou M, Wright VJ, Oni T, French N, Anderson ST, Bangani N, Banwell CM, Brent AJ, Crampin AC, Dockrell HM, Eley B, Heyderman RS, Hibberd ML, Kern F, Langford PR, Ling L, Mendelson M, Ottenhoff TH, Zgambo F, Wilkinson RJ, Coin LJ, Levin M. 2013. Detection of tuberculosis in HIV-infected and -uninfected African adults using whole blood RNA expression signatures: a case-control study. PLoS Med 10:e1001538. doi: 10.1371/journal.pmed.1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bloom CI, Graham CM, Berry MP, Rozakeas F, Redford PS, Wang Y, Xu Z, Wilkinson KA, Wilkinson RJ, Kendrick Y, Devouassoux G, Ferry T, Miyara M, Bouvry D, Valeyre D, Gorochov G, Blankenship D, Saadatian M, Vanhems P, Beynon H, Vancheeswaran R, Wickremasinghe M, Chaussabel D, Banchereau J, Pascual V, Ho LP, Lipman M, O'Garra A. 2013. Transcriptional blood signatures distinguish pulmonary tuberculosis, pulmonary sarcoidosis, pneumonias and lung cancers. PLoS One 8:e70630. doi: 10.1371/journal.pone.0070630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson ST, Kaforou M, Brent AJ, Wright VJ, Banwell CM, Chagaluka G, Crampin AC, Dockrell HM, French N, Hamilton MS, Hibberd ML, Kern F, Langford PR, Ling L, Mlotha R, Ottenhoff THM, Pienaar S, Pillay V, Scott JAG, Twahir H, Wilkinson RJ, Coin LJ, Heyderman RS, Levin M, Eley B. 2014. Diagnosis of childhood tuberculosis and host RNA expression in Africa. N Engl J Med 370:1712–1723. doi: 10.1056/NEJMoa1303657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Phuah JY, Mattila JT, Lin PL, Flynn JL. 2012. Activated B cells in the granulomas of nonhuman primates infected with Mycobacterium tuberculosis. Am J Pathol 181:508–514. doi: 10.1016/j.ajpath.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Torrado E, Fountain JJ, Robinson RT, Martino CA, Pearl JE, Rangel-Moreno J, Tighe M, Dunn R, Cooper AM. 2013. Differential and site specific impact of B cells in the protective immune response to Mycobacterium tuberculosis in the mouse. PLoS One 8:e61681. doi: 10.1371/journal.pone.0061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, Schoen MK, Tafesse F, Martin C, Leung V, Mahan AE, Sips M, Kumar MP, Tedesco J, Robinson H, Tkachenko E, Draghi M, Freedberg KJ, Streeck H, Suscovich TJ, Lauffenburger DA, Restrepo BI, Day C, Fortune SM, Alter G. 2016. A functional role for antibodies in tuberculosis. Cell 167:433.e14–443.e14. doi: 10.1016/j.cell.2016.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wanchu A, Dong Y, Sethi S, Myneedu VP, Nadas A, Liu Z, Belisle J, Laal S. 2008. Biomarkers for clinical and incipient tuberculosis: performance in a TB-endemic country. PLoS One 3:e2071. doi: 10.1371/journal.pone.0002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bulut Y, Michelsen KS, Hayrapetian L, Naiki Y, Spallek R, Singh M, Arditi M. 2005. Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate pro-inflammatory signals. J Biol Chem 280:20961–20967. doi: 10.1074/jbc.M411379200. [DOI] [PubMed] [Google Scholar]
- 77.Andrews JR, Nemes E, Tameris M, Landry BS, Mahomed H, McClain JB, Fletcher HA, Hanekom WA, Wood R, McShane H, Scriba TJ, Hatherill M. 2017. Serial QuantiFERON testing and tuberculosis disease risk among young children: an observational cohort study. Lancet Respir Med 5:282–290. doi: 10.1016/S2213-2600(17)30060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Millington KA, Innes JA, Hackforth S, Hinks TS, Deeks JJ, Dosanjh DP, Guyot-Revol V, Gunatheesan R, Klenerman P, Lalvani A. 2007. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J Immunol 178:5217–5226. doi: 10.4049/jimmunol.178.8.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O'Rie T, Pienaar B, de Kock M, Kaplan G, Mahomed H, Dheda K, Hanekom WA. 2011. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol 187:2222–2232. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Day CL, Moshi ND, Abrahams DA, van Rooyen M, O'Rie T, de Kock M, Hanekom WA. 2014. Patients with tuberculosis disease have Mycobacterium tuberculosis-specific CD8 T cells with a pro-apoptotic phenotype and impaired proliferative capacity, which is not restored following treatment. PLoS One 9:e94949. doi: 10.1371/journal.pone.0094949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adekambi T, Ibegbu CC, Cagle S, Kalokhe AS, Wang YF, Hu Y, Day CL, Ray SM, Rengarajan J. 2015. Biomarkers on patient T cells diagnose active tuberculosis and monitor treatment response. J Clin Invest 125:3723. doi: 10.1172/JCI83279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rozot V, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, Perreau M, Lazor-Blanchet C, Petruccioli E, Hanekom W, Goletti D, Bart PA, Nicod L, Pantaleo G, Harari A. 2013. Mycobacterium tuberculosis-specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. Eur J Immunol 43:1568–1577. doi: 10.1002/eji.201243262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lewinsohn DM, Tydeman IS, Frieder M, Grotzke JE, Lines RA, Ahmed S, Prongay KD, Primack SL, Colgin LM, Lewis AD, Lewinsohn DA. 2006. High resolution radiographic and fine immunologic definition of TB disease progression in the rhesus macaque. Microbes Infect 8:2587–2598. doi: 10.1016/j.micinf.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 84.Nyendak MR, Park B, Null MD, Baseke J, Swarbrick G, Mayanja-Kizza H, Nsereko M, Johnson DF, Gitta P, Okwera A, Goldberg S, Bozeman L, Johnson JL, Boom WH, Lewinsohn DA, Lewinsohn DM, Tuberculosis Research Unit and the Tuberculosis Trials Consortium. 2013. Mycobacterium tuberculosis specific CD8(+) T cells rapidly decline with antituberculosis treatment. PLoS One 8:e81564. doi: 10.1371/journal.pone.0081564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lancioni C, Nyendak M, Kiguli S, Zalwango S, Mori T, Mayanja-Kizza H, Balyejusa S, Null M, Baseke J, Mulindwa D, Byrd L, Swarbrick G, Scott C, Johnson DF, Malone L, Mudido-Musoke P, Boom WH, Lewinsohn DM, Lewinsohn DA. 2012. CD8+ T cells provide an immunologic signature of tuberculosis in young children. Am J Respir Crit Care Med 185:206–212. doi: 10.1164/rccm.201107-1355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ernst JD. 2012. The immunological life cycle of tuberculosis. Nat Rev Immunol 12:581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 87.Lindestam Arlehamn CS, Lewinsohn D, Sette A, Lewinsohn D. 2014. Antigens for CD4 and CD8 T cells in tuberculosis. Cold Spring Harb Perspect Med 4:a018465. doi: 10.1101/cshperspect.a018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Geluk A, van Meijgaarden KE, Joosten SA, Commandeur S, Ottenhoff TH. 2014. Innovative strategies to identify M. tuberculosis antigens and epitopes using genome-wide analyses. Front Immunol 5:256. doi: 10.3389/fimmu.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chegou NN, Essone PN, Loxton AG, Stanley K, Black GF, van der Spuy GD, van Helden PD, Franken KL, Parida SK, Klein MR, Kaufmann SH, Ottenhoff TH, Walzl G. 2012. Potential of host markers produced by infection phase-dependent antigen-stimulated cells for the diagnosis of tuberculosis in a highly endemic area. PLoS One 7:e38501. doi: 10.1371/journal.pone.0038501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Demissie A, Leyten EM, Abebe M, Wassie L, Aseffa A, Abate G, Fletcher H, Owiafe P, Hill PC, Brookes R, Rook G, Zumla A, Arend SM, Klein M, Ottenhoff TH, Andersen P, Doherty TM, VACSEL Study Group. 2006. Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin Vaccine Immunol 13:179–186. doi: 10.1128/CVI.13.2.179-186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moguche AO, Musvosvi M, Penn-Nicholson A, Plumlee CR, Mearns H, Geldenhuys H, Smit E, Abrahams D, Rozot V, Dintwe O, Hoff ST, Kromann I, Ruhwald M, Bang P, Larson RP, Shafiani S, Ma S, Sherman DR, Sette A, Lindestam Arlehamn CS, McKinney DM, Maecker H, Hanekom WA, Hatherill M, Andersen P, Scriba TJ, Urdahl KB. 2017. Antigen availability shapes T cell differentiation and function during tuberculosis. Cell Host Microbe 21:695.e5–706.e5. doi: 10.1016/j.chom.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi L, North R, Gennaro ML. 2004. Effect of growth state on transcription levels of genes encoding major secreted antigens of Mycobacterium tuberculosis in the mouse lung. Infect Immun 72:2420–2424. doi: 10.1128/IAI.72.4.2420-2424.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rogerson BJ, Jung YJ, LaCourse R, Ryan L, Enright N, North RJ. 2006. Expression levels of Mycobacterium tuberculosis antigen-encoding genes versus production levels of antigen-specific T cells during stationary level lung infection in mice. Immunology 118:195–201. doi: 10.1111/j.1365-2567.2006.02355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mustafa T, Leversen NA, Sviland L, Wiker HG. 2014. Differential in vivo expression of mycobacterial antigens in Mycobacterium tuberculosis infected lungs and lymph node tissues. BMC Infect Dis 14:535. doi: 10.1186/1471-2334-14-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coppola M, van Meijgaarden KE, Franken KL, Commandeur S, Dolganov G, Kramnik I, Schoolnik GK, Comas I, Lund O, Prins C, van den Eeden SJ, Korsvold GE, Oftung F, Geluk A, Ottenhoff TH. 2016. New genome-wide algorithm identifies novel in-vivo expressed Mycobacterium tuberculosis antigens inducing human T-cell responses with classical and unconventional cytokine profiles. Sci Rep 6:37793. doi: 10.1038/srep37793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. 2015. The burgeoning family of unconventional T cells. Nat Immunol 16:1114–1123. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 97.De Libero G, Singhal A, Lepore M, Mori L. 2014. Nonclassical T cells and their antigens in tuberculosis. Cold Spring Harb Perspect Med 4:a018473. doi: 10.1101/cshperspect.a018473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Meijgaarden KE, Haks MC, Caccamo N, Dieli F, Ottenhoff TH, Joosten SA. 2015. Human CD8+ T-cells recognizing peptides from Mycobacterium tuberculosis (Mtb) presented by HLA-E have an unorthodox Th2-like, multifunctional, Mtb inhibitory phenotype and represent a novel human T-cell subset. PLoS Pathog 11:e1004671. doi: 10.1371/journal.ppat.1004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heinzel AS, Grotzke JE, Lines RA, Lewinsohn DA, McNabb AL, Streblow DN, Braud VM, Grieser HJ, Belisle JT, Lewinsohn DM. 2002. HLA-E-dependent presentation of Mtb-derived antigen to human CD8+ T cells. J Exp Med 196:1473–1481. doi: 10.1084/jem.20020609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Joosten SA, van Meijgaarden KE, van Weeren PC, Kazi F, Geluk A, Savage ND, Drijfhout JW, Flower DR, Hanekom WA, Klein MR, Ottenhoff TH. 2010. Mycobacterium tuberculosis peptides presented by HLA-E molecules are targets for human CD8 T-cells with cytotoxic as well as regulatory activity. PLoS Pathog 6:e1000782. doi: 10.1371/journal.ppat.1000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caccamo N, Pietra G, Sullivan LC, Brooks AG, Prezzemolo T, La Manna MP, Di Liberto D, Joosten SA, van Meijgaarden KE, Di Carlo P, Titone L, Moretta L, Mingari MC, Ottenhoff TH, Dieli F. 2015. Human CD8 T lymphocytes recognize Mycobacterium tuberculosis antigens presented by HLA-E during active tuberculosis and express type 2 cytokines. Eur J Immunol 45:1069–1081. doi: 10.1002/eji.201445193. [DOI] [PubMed] [Google Scholar]
- 102.Gilleron M, Stenger S, Mazorra Z, Wittke F, Mariotti S, Bohmer G, Prandi J, Mori L, Puzo G, De Libero G. 2004. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J Exp Med 199:649–659. doi: 10.1084/jem.20031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moody DB, Ulrichs T, Muhlecker W, Young DC, Gurcha SS, Grant E, Rosat JP, Brenner MB, Costello CE, Besra GS, Porcelli SA. 2000. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature 404:884–888. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]
- 104.Kwon YS, Cho YN, Kim MJ, Jin HM, Jung HJ, Kang JH, Park KJ, Kim TJ, Kee HJ, Kim N, Kee SJ, Park YW. 2015. Mucosal-associated invariant T cells are numerically and functionally deficient in patients with mycobacterial infection and reflect disease activity. Tuberculosis (Edinb) 95:267–274. doi: 10.1016/j.tube.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 105.Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, Winata E, Swarbrick GM, Chua W-J, Yu YYL, Lantz O, Cook MS, Null MD, Jacoby DB, Harriff MJ, Lewinsohn DA, Hansen TH, Lewinsohn DM. 2010. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol 8:e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, Levy E, Dusseaux M, Meyssonnier V, Premel V, Ngo C, Riteau B, Duban L, Robert D, Huang S, Rottman M, Soudais C, Lantz O. 2010. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol 11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 107.Snyder-Cappione JE, Nixon DF, Loo CP, Chapman JM, Meiklejohn DA, Melo FF, Costa PR, Sandberg JK, Rodrigues DS, Kallas EG. 2007. Individuals with pulmonary tuberculosis have lower levels of circulating CD1d-restricted NKT cells. J Infect Dis 195:1361–1364. doi: 10.1086/513567. [DOI] [PubMed] [Google Scholar]
- 108.Sutherland JS, Hill PC, Adetifa IM, de Jong BC, Donkor S, Joosten SA, Opmeer L, Haks MC, Ottenhoff TH, Adegbola RA, Ota MO. 2011. Identification of probable early-onset biomarkers for tuberculosis disease progression. PLoS One 6:e25230. doi: 10.1371/journal.pone.0025230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sigal GB, Segal MR, Mathew A, Jarlsberg L, Wang M, Barbero S, Small N, Haynesworth K, Davis JL, Weiner M, Whitworth WC, Jacobs J, Schorey J, Lewinsohn DM, Nahid P. 2017. Biomarkers of tuberculosis severity and treatment effect: a directed screen of 70 host markers in a randomized clinical trial. EBioMedicine 25:112–121. doi: 10.1016/j.ebiom.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bark CM, Manceur AM, Malone LL, Nsereko M, Okware B, Mayanja HK, Joloba ML, Rajotte I, Mentinova M, Kay P, Lo S, Tremblay P, Stein CM, Boom WH, Paramithiotis E. 2017. Identification of host proteins predictive of early stage Mycobacterium tuberculosis infection. EBioMedicine 21:150–157. doi: 10.1016/j.ebiom.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Groote MA, Nahid P, Jarlsberg L, Johnson JL, Weiner M, Muzanyi G, Janjic N, Sterling DG, Ochsner UA. 2013. Elucidating novel serum biomarkers associated with pulmonary tuberculosis treatment. PLoS One 8:e61002. doi: 10.1371/journal.pone.0061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Esterhuyse MM, Weiner J III, Caron E, Loxton AG, Iannaccone M, Wagman C, Saikali P, Stanley K, Wolski WE, Mollenkopf HJ, Schick M, Aebersold R, Linhart H, Walzl G, Kaufmann SH. 2015. Epigenetics and proteomics join transcriptomics in the quest for tuberculosis biomarkers. mBio 6:e01187-15. doi: 10.1128/mBio.01187-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weiner J III, Parida SK, Maertzdorf J, Black GF, Repsilber D, Telaar A, Mohney RP, Arndt-Sullivan C, Ganoza CA, Fae KC, Walzl G, Kaufmann SH. 2012. Biomarkers of inflammation, immunosuppression and stress with active disease are revealed by metabolomic profiling of tuberculosis patients. PLoS One 7:e40221. doi: 10.1371/journal.pone.0040221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zak DE, Penn-Nicholson A, Scriba TJ, Thompson E, Suliman S, Amon LM, Mahomed H, Erasmus M, Whatney W, Hussey GD, Abrahams D, Kafaar F, Hawkridge T, Verver S, Hughes EJ, Ota M, Sutherland J, Howe R, Dockrell HM, Boom WH, Thiel B, Ottenhoff THM, Mayanja-Kizza H, Crampin AC, Downing K, Hatherill M, Valvo J, Shankar S, Parida SK, Kaufmann SHE, Walzl G, Aderem A, Hanekom WA. 2016. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet 387:2312–2322. doi: 10.1016/S0140-6736(15)01316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Onozaki I, Law I, Sismanidis C, Zignol M, Glaziou P, Floyd K. 2015. National tuberculosis prevalence surveys in Asia, 1990-2012: an overview of results and lessons learned. Trop Med Int Health 20:1128–1145. doi: 10.1111/tmi.12534. [DOI] [PubMed] [Google Scholar]
- 116.Mao TE, Okada K, Yamada N, Peou S, Ota M, Saint S, Kouet P, Chea M, Keo S, Pheng SH, Tieng S, Khun KE, Sugamoto T, Matsumoto H, Yoshiyama T, Ito K, Onozaki I. 2014. Cross-sectional studies of tuberculosis prevalence in Cambodia between 2002 and 2011. Bull World Health Organ 92:573–581. doi: 10.2471/BLT.13.131581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guwatudde D, Nakakeeto M, Jones-Lopez EC, Maganda A, Chiunda A, Mugerwa RD, Ellner JJ, Bukenya G, Whalen CC. 2003. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol 158:887–898. doi: 10.1093/aje/kwg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van't Hoog AH, Laserson KF, Githui WA, Meme HK, Agaya JA, Odeny LO, Muchiri BG, Marston BJ, DeCock KM, Borgdorff MW. 2011. High prevalence of pulmonary tuberculosis and inadequate case finding in rural western Kenya. Am J Respir Crit Care Med 183:1245–1253. doi: 10.1164/rccm.201008-1269OC. [DOI] [PubMed] [Google Scholar]
- 119.Oni T, Burke R, Tsekela R, Bangani N, Seldon R, Gideon HP, Wood K, Wilkinson KA, Ottenhoff TH, Wilkinson RJ. 2011. High prevalence of subclinical tuberculosis in HIV-1-infected persons without advanced immunodeficiency: implications for TB screening. Thorax 66:669–673. doi: 10.1136/thx.2011.160168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lawn SD, Brooks SV, Kranzer K, Nicol MP, Whitelaw A, Vogt M, Bekker LG, Wood R. 2011. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med 8:e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lawn SD, Kerkhoff AD, Wood R. 2011. Progression of subclinical culture-positive tuberculosis to symptomatic disease in HIV-infected individuals. AIDS 25:2190–2191. doi: 10.1097/QAD.0b013e32834cda4e. [DOI] [PubMed] [Google Scholar]
- 122.Corbett EL, Bandason T, Cheung YB, Munyati S, Godfrey-Faussett P, Hayes R, Churchyard G, Butterworth A, Mason P. 2007. Epidemiology of tuberculosis in a high HIV prevalence population provided with enhanced diagnosis of symptomatic disease. PLoS Med 4:e22. doi: 10.1371/journal.pmed.0040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mtei L, Matee M, Herfort O, Bakari M, Horsburgh CR, Waddell R, Cole BF, Vuola JM, Tvaroha S, Kreiswirth B, Pallangyo K, von Reyn CF. 2005. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis 40:1500–1507. doi: 10.1086/429825. [DOI] [PubMed] [Google Scholar]
- 124.Lewis JJ, Charalambous S, Day JH, Fielding KL, Grant AD, Hayes RJ, Corbett EL, Churchyard GJ. 2009. HIV infection does not affect active case finding of tuberculosis in South African gold miners. Am J Respir Crit Care Med 180:1271–1278. doi: 10.1164/rccm.200806-846OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Swindells S, Komarow L, Tripathy S, Cain KP, MacGregor RR, Achkar JM, Gupta A, Veloso VG, Asmelash A, Omoz-Oarhe AE, Gengiah S, Lalloo U, Allen R, Shiboski C, Andersen J, Qasba SS, Katzenstein DK. 2013. Screening for pulmonary tuberculosis in HIV-infected individuals: AIDS Clinical Trials Group protocol A5253. Int J Tuberc Lung Dis 17:532–539. doi: 10.5588/ijtld.12.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Swaminathan S, Paramasivan CN, Kumar SR, Mohan V, Venkatesan P. 2004. Unrecognised tuberculosis in HIV-infected patients: sputum culture is a useful tool. Int J Tuberc Lung Dis 8:896–898. [PubMed] [Google Scholar]
- 127.Bassett IV, Wang B, Chetty S, Giddy J, Losina E, Mazibuko M, Bearnot B, Allen J, Walensky RP, Freedberg KA. 2010. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis 51:823–829. doi: 10.1086/656282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Henostroza G, Harris JB, Chitambi R, Siyambango M, Turnbull ER, Maggard KR, Kruuner A, Kapata N, Reid SE. 2016. High prevalence of tuberculosis in newly enrolled HIV patients in Zambia: need for enhanced screening approach. Int J Tuberc Lung Dis 20:1033–1039. doi: 10.5588/ijtld.15.0651. [DOI] [PubMed] [Google Scholar]
- 129.Kimerling ME, Schuchter J, Chanthol E, Kunthy T, Stuer F, Glaziou P, Ee O. 2002. Prevalence of pulmonary tuberculosis among HIV-infected persons in a home care program in Phnom Penh, Cambodia. Int J Tuberc Lung Dis 6:988–994. [PubMed] [Google Scholar]
- 130.Kufa T, Mngomezulu V, Charalambous S, Hanifa Y, Fielding K, Grant AD, Wada N, Chaisson RE, Churchyard GJ, Gounder CR. 2012. Undiagnosed tuberculosis among HIV clinic attendees: association with antiretroviral therapy and implications for intensified case finding, isoniazid preventive therapy, and infection control. J Acquir Immune Defic Syndr 60:e22–e28. doi: 10.1097/QAI.0b013e318251ae0b. [DOI] [PubMed] [Google Scholar]
- 131.Shah S, Demissie M, Lambert L, Ahmed J, Leulseged S, Kebede T, Melaku Z, Mengistu Y, Lemma E, Wells CD, Wuhib T, Nelson LJ. 2009. Intensified tuberculosis case finding among HIV-infected persons from a voluntary counseling and testing center in Addis Ababa, Ethiopia. J Acquir Immune Defic Syndr 50:537–545. doi: 10.1097/QAI.0b013e318196761c. [DOI] [PubMed] [Google Scholar]
- 132.Chheng P, Tamhane A, Natpratan C, Tan V, Lay V, Sar B, Kimerling ME. 2008. Pulmonary tuberculosis among patients visiting a voluntary confidential counseling and testing center, Cambodia. Int J Tuberc Lung Dis 12:54–62. [PubMed] [Google Scholar]
- 133.Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, Kaplan G, Huebner R, McIntyre J, Bekker LG. 2007. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med 175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Day JH, Charalambous S, Fielding KL, Hayes RJ, Churchyard GJ, Grant AD. 2006. Screening for tuberculosis prior to isoniazid preventive therapy among HIV-infected gold miners in South Africa. Int J Tuberc Lung Dis 10:523–529. [PubMed] [Google Scholar]
- 135.Kancheya N, Luhanga D, Harris JB, Morse J, Kapata N, Bweupe M, Henostroza G, Reid SE. 2014. Integrating active tuberculosis case finding in antenatal services in Zambia. Int J Tuberc Lung Dis 18:1466–1472. doi: 10.5588/ijtld.14.0920. [DOI] [PubMed] [Google Scholar]
- 136.LaCourse SM, Cranmer LM, Matemo D, Kinuthia J, Richardson BA, John-Stewart G, Horne DJ. 2016. Tuberculosis case finding in HIV-infected pregnant women in Kenya reveals poor performance of symptom screening and rapid diagnostic tests. J Acquir Immune Defic Syndr 71:219–227. doi: 10.1097/QAI.0000000000000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Telisinghe L, Fielding KL, Malden JL, Hanifa Y, Churchyard GJ, Grant AD, Charalambous S. 2014. High tuberculosis prevalence in a South African prison: the need for routine tuberculosis screening. PLoS One 9:e87262. doi: 10.1371/journal.pone.0087262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Agizew TB, Arwady MA, Yoon JC, Nyirenda S, Mosimaneotsile B, Tedla Z, Motsamai O, Kilmarx PH, Wells CD, Samandari T. 2010. Tuberculosis in asymptomatic HIV-infected adults with abnormal chest radiographs screened for tuberculosis prevention. Int J Tuberc Lung Dis 14:45–51. [PubMed] [Google Scholar]
- 139.Bates M, O'Grady J, Mwaba P, Chilukutu L, Mzyece J, Cheelo B, Chilufya M, Mukonda L, Mumba M, Tembo J, Chomba M, Kapata N, Rachow A, Clowes P, Maeurer M, Hoelscher M, Zumla A. 2012. Evaluation of the burden of unsuspected pulmonary tuberculosis and comorbidity with non-communicable diseases in sputum producing adult inpatients. PLoS One 7:e40774. doi: 10.1371/journal.pone.0040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cohen T, Murray M, Wallengren K, Alvarez GG, Samuel EY, Wilson D. 2010. The prevalence and drug sensitivity of tuberculosis among patients dying in hospital in KwaZulu-Natal, South Africa: a postmortem study. PLoS Med 7:e1000296. doi: 10.1371/journal.pmed.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.World Health Organization. 2017. Global tuberculosis report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 142.Hoa NB, Sy DN, Nhung NV, Tiemersma EW, Borgdorff MW, Cobelens FG. 2010. National survey of tuberculosis prevalence in Viet Nam. Bull World Health Organ 88:273–280. doi: 10.2471/BLT.09.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.National Tuberculosis Control Program. 2012. Report: second national tuberculosis prevalence survey Cambodia, 2011. Ministry of Health, Phnom Penh, Cambodia. [Google Scholar]
- 144.Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. 1998. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis 2:27–36. [PubMed] [Google Scholar]
- 145.Wang L, Zhang H, Ruan Y, Chin DP, Xia Y, Cheng S, Chen M, Zhao Y, Jiang S, Du X, He G, Li J, Wang S, Chen W, Xu C, Huang F, Liu X, Wang Y. 2014. Tuberculosis prevalence in China, 1990-2010; a longitudinal analysis of national survey data. Lancet 383:2057–2064. doi: 10.1016/S0140-6736(13)62639-2. [DOI] [PubMed] [Google Scholar]
- 146.Cain KP, McCarthy KD, Heilig CM, Monkongdee P, Tasaneeyapan T, Kanara N, Kimerling ME, Chheng P, Thai S, Sar B, Phanuphak P, Teeratakulpisarn N, Phanuphak N, Nguyen HD, Hoang TQ, Le HT, Varma JK. 2010. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med 362:707–716. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- 147.Ahmad Khan F, Verkuijl S, Parrish A, Chikwava F, Ntumy R, El-Sadr W, Howard AA. 2014. Performance of symptom-based tuberculosis screening among people living with HIV: not as great as hoped. AIDS 28:1463–1472. doi: 10.1097/QAD.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Modi S, Cavanaugh JS, Shiraishi RW, Alexander HL, McCarthy KD, Burmen B, Muttai H, Heilig CM, Nakashima AK, Cain KP. 2016. Performance of clinical screening algorithms for tuberculosis intensified case finding among people living with HIV in Western Kenya. PLoS One 11:e0167685. doi: 10.1371/journal.pone.0167685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rangaka MX, Wilkinson RJ, Glynn JR, Boulle A, van Cutsem G, Goliath R, Mathee S, Maartens G. 2012. Effect of antiretroviral therapy on the diagnostic accuracy of symptom screening for intensified tuberculosis case finding in a South African HIV clinic. Clin Infect Dis 55:1698–1706. doi: 10.1093/cid/cis775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ayles H, Schaap A, Nota A, Sismanidis C, Tembwe R, De Haas P, Muyoyeta M, Beyers N. 2009. Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: implications for tuberculosis control in the era of HIV. PLoS One 4:e5602. doi: 10.1371/journal.pone.0005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Corbett EL, Zezai A, Cheung YB, Bandason T, Dauya E, Munyati SS, Butterworth AE, Rusikaniko S, Churchyard GJ, Mungofa S, Hayes RJ, Mason PR. 2010. Provider-initiated symptom screening for tuberculosis in Zimbabwe: diagnostic value and the effect of HIV status. Bull World Health Organ 88:13–21. doi: 10.2471/BLT.08.055467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Corbett EL, Bandason T, Cheung YB, Makamure B, Dauya E, Munyati SS, Churchyard GJ, Williams BG, Butterworth AE, Mungofa S, Hayes RJ, Mason PR. 2009. Prevalent infectious tuberculosis in Harare, Zimbabwe: burden, risk factors and implications for control. Int J Tuberc Lung Dis 13:1231–1237. [PMC free article] [PubMed] [Google Scholar]
- 153.Kerkhoff AD, Wood R, Lowe DM, Vogt M, Lawn SD. 2013. Blood neutrophil counts in HIV-infected patients with pulmonary tuberculosis: association with sputum mycobacterial load. PLoS One 8:e67956. doi: 10.1371/journal.pone.0067956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lawn SD, Kranzer K, Edwards DJ, McNally M, Bekker LG, Wood R. 2010. Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS 24:1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hoffmann CJ, Variava E, Rakgokong M, Masonoke K, van der Watt M, Chaisson RE, Martinson NA. 2013. High prevalence of pulmonary tuberculosis but low sensitivity of symptom screening among HIV-infected pregnant women in South Africa. PLoS One 8:e62211. doi: 10.1371/journal.pone.0062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ai JW, Ruan QL, Liu QH, Zhang WH. 2016. Updates on the risk factors for latent tuberculosis reactivation and their managements. Emerg Microbes Infect 5:e10. doi: 10.1038/emi.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Flynn JL, Chan J. 2001. Tuberculosis: latency and reactivation. Infect Immun 69:4195–4201. doi: 10.1128/IAI.69.7.4195-4201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Comstock GW. 1982. Epidemiology of tuberculosis. Am Rev Respir Dis 125:8–15. [DOI] [PubMed] [Google Scholar]
- 159.Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJ. 2011. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS One 6:e17601. doi: 10.1371/journal.pone.0017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bates M, Mudenda V, Shibemba A, Kaluwaji J, Tembo J, Kabwe M, Chimoga C, Chilukutu L, Chilufya M, Kapata N, Hoelscher M, Maeurer M, Mwaba P, Zumla A. 2015. Burden of tuberculosis at post mortem in inpatients at a tertiary referral centre in sub-Saharan Africa: a prospective descriptive autopsy study. Lancet Infect Dis 15:544–551. doi: 10.1016/S1473-3099(15)70058-7. [DOI] [PubMed] [Google Scholar]
- 161.Gupta RK, Lucas SB, Fielding KL, Lawn SD. 2015. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS 29:1987–2002. doi: 10.1097/QAD.0000000000000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Calligaro GL, Zijenah LS, Peter JG, Theron G, Buser V, McNerney R, Bara W, Bandason T, Govender U, Tomasicchio M, Smith L, Mayosi BM, Dheda K. 2017. Effect of new tuberculosis diagnostic technologies on community-based intensified case finding: a multicentre randomised controlled trial. Lancet Infect Dis 17:441–450. doi: 10.1016/S1473-3099(16)30384-X. [DOI] [PubMed] [Google Scholar]
- 163.Dowdy DW, Basu S, Andrews JR. 2013. Is passive diagnosis enough? The impact of subclinical disease on diagnostic strategies for tuberculosis. Am J Respir Crit Care Med 187:543–551. doi: 10.1164/rccm.201207-1217OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reid A, Grant AD, White RG, Dye C, Vynnycky E, Fielding K, Churchyard G, Pillay Y. 2015. Accelerating progress towards tuberculosis elimination: the need for combination treatment and prevention. Int J Tuberc Lung Dis 19:5–9. doi: 10.5588/ijtld.14.0078. [DOI] [PubMed] [Google Scholar]
- 165.Houben R, Menzies NA, Sumner T, Huynh GH, Arinaminpathy N, Goldhaber-Fiebert JD, Lin HH, Wu CY, Mandal S, Pandey S, Suen SC, Bendavid E, Azman AS, Dowdy DW, Bacaer N, Rhines AS, Feldman MW, Handel A, Whalen CC, Chang ST, Wagner BG, Eckhoff PA, Trauer JM, Denholm JT, McBryde ES, Cohen T, Salomon JA, Pretorius C, Lalli M, Eaton JW, Boccia D, Hosseini M, Gomez GB, Sahu S, Daniels C, Ditiu L, Chin DP, Wang L, Chadha VK, Rade K, Dewan P, Hippner P, Charalambous S, Grant AD, Churchyard G, Pillay Y, Mametja LD, Kimerling ME, Vassall A, White RG. 2016. Feasibility of achieving the 2025 WHO global tuberculosis targets in South Africa, China, and India: a combined analysis of 11 mathematical models. Lancet Glob Health 4:e806–e815. doi: 10.1016/S2214-109X(16)30199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Dias HM, Falzon D, Floyd K, Gargioni G, Getahun H, Gilpin C, Glaziou P, Grzemska M, Mirzayev F, Nakatani H, Raviglione M. 2015. WHO's new end TB strategy. Lancet 385:1799–1801. doi: 10.1016/S0140-6736(15)60570-0. [DOI] [PubMed] [Google Scholar]
- 167.Wang L, Turner MO, Elwood RK, Schulzer M, FitzGerald JM. 2002. A meta-analysis of the effect of bacille Calmette Guerin vaccination on tuberculin skin test measurements. Thorax 57:804–809. doi: 10.1136/thorax.57.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Farhat M, Greenaway C, Pai M, Menzies D. 2006. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis 10:1192–1204. [PubMed] [Google Scholar]
- 169.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, Metcalfe JZ, Cattamanchi A, Dowdy DW, Dheda K, Banaei N. 2014. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tagmouti S, Slater M, Benedetti A, Kik SV, Banaei N, Cattamanchi A, Metcalfe J, Dowdy D, van Zyl Smit R, Dendukuri N, Pai M, Denkinger C. 2014. Reproducibility of interferon gamma (IFN-gamma) release assays. A systematic review. Ann Am Thorac Soc 11:1267–1276. doi: 10.1513/AnnalsATS.201405-188OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rangaka MX, Wilkinson KA, Glynn JR, Ling D, Menzies D, Mwansa-Kambafwile J, Fielding K, Wilkinson RJ, Pai M. 2012. Predictive value of interferon-gamma release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 12:45–55. doi: 10.1016/S1473-3099(11)70210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Diel R, Loddenkemper R, Nienhaus A. 2012. Predictive value of interferon-gamma release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest 142:63–75. doi: 10.1378/chest.11-3157. [DOI] [PubMed] [Google Scholar]
- 173.Dye C. 2011. Practical preventive therapy for tuberculosis? N Engl J Med 365:2230–2231. doi: 10.1056/NEJMe1111859. [DOI] [PubMed] [Google Scholar]
- 174.Kasprowicz VO, Churchyard G, Lawn SD, Squire SB, Lalvani A. 2011. Diagnosing latent tuberculosis in high-risk individuals: rising to the challenge in high-burden areas. J Infect Dis 204(Suppl 4):S1168–S1178. doi: 10.1093/infdis/jir449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Robertson BD, Altmann D, Barry C, Bishai B, Cole S, Dick T, Duncan K, Dye C, Ehrt S, Esmail H, Flynn J, Hafner R, Handley G, Hanekom W, van Helden P, Kaplan G, Kaufmann SH, Kim P, Lienhardt C, Mizrahi V, Rubin E, Schnappinger D, Sherman D, Thole J, Vandal O, Walzl G, Warner D, Wilkinson R, Young D. 2012. Detection and treatment of subclinical tuberculosis. Tuberculosis (Edinb) 92:447–452. doi: 10.1016/j.tube.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 176.Esmail H, Barry CE III, Young DB, Wilkinson RJ. 2014. The ongoing challenge of latent tuberculosis. Philos Trans R Soc Lond B Biol Sci 369:20130437. doi: 10.1098/rstb.2013.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hoff ST, Peter JG, Theron G, Pascoe M, Tingskov PN, Aggerbeck H, Kolbus D, Ruhwald M, Andersen P, Dheda K. 2016. Sensitivity of C-Tb: a novel RD-1-specific skin test for the diagnosis of tuberculosis infection. Eur Respir J 47:919–928. doi: 10.1183/13993003.01464-2015. [DOI] [PubMed] [Google Scholar]
- 178.Barcellini L, Borroni E, Brown J, Brunetti E, Codecasa L, Cugnata F, Dal Monte P, Di Serio C, Goletti D, Lombardi G, Lipman M, Rancoita PM, Tadolini M, Cirillo DM. 2016. First independent evaluation of QuantiFERON-TB Plus performance. Eur Respir J 47:1587–1590. doi: 10.1183/13993003.02033-2015. [DOI] [PubMed] [Google Scholar]
- 179.Li F, Xu M, Qin C, Xia L, Xiong Y, Xi X, Fan X, Gu J, Pu J, Wu Q, Lu S, Wang G. 2016. Recombinant fusion ESAT6-CFP10 immunogen as a skin test reagent for tuberculosis diagnosis: an open-label, randomized, two-centre phase 2a clinical trial. Clin Microbiol Infect 22:889.e9–889.e16. doi: 10.1016/j.cmi.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 180.Ruhwald M, Aggerbeck H, Gallardo RV, Hoff ST, Villate JI, Borregaard B, Martinez JA, Kromann I, Penas A, Anibarro LL, de Souza-Galvao ML, Sanchez F, Rodrigo-Pendas JA, Noguera-Julian A, Martinez-Lacasa X, Tunez MV, Fernandez VL, Millet JP, Moreno A, Cobos N, Miro JM, Roldan L, Orcau A, Andersen P, Cayla JA. 2017. Safety and efficacy of the C-Tb skin test to diagnose Mycobacterium tuberculosis infection, compared with an interferon gamma release assay and the tuberculin skin test: a phase 3, double-blind, randomised, controlled trial. Lancet Respir Med 5:259–268. doi: 10.1016/S2213-2600(16)30436-2. [DOI] [PubMed] [Google Scholar]
- 181.Aksenova V. 2011. DIASKINTEST as a screening metod [sic] at the mass child health examination for tuberculosis in Russia. Eur Respir J 38:295.21233272 [Google Scholar]
- 182.Yi L, Sasaki Y, Nagai H, Ishikawa S, Takamori M, Sakashita K, Saito T, Fukushima K, Igarashi Y, Aono A, Chikamatsu K, Yamada H, Takaki A, Mori T, Mitarai S. 2016. Evaluation of QuantiFERON-TB Gold Plus for detection of Mycobacterium tuberculosis infection in Japan. Sci Rep 6:30617. doi: 10.1038/srep30617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hoffmann H, Avsar K, Gores R, Mavi SC, Hofmann-Thiel S. 2016. Equal sensitivity of the new generation QuantiFERON-TB Gold plus in direct comparison with the previous test version QuantiFERON-TB Gold IT. Clin Microbiol Infect 22:701–703. doi: 10.1016/j.cmi.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 184.Nikolova M, Markova R, Drenska R, Muhtarova M, Todorova Y, Dimitrov V, Taskov H, Saltini C, Amicosante M. 2013. Antigen-specific CD4- and CD8-positive signatures in different phases of Mycobacterium tuberculosis infection. Diagn Microbiol Infect Dis 75:277–281. doi: 10.1016/j.diagmicrobio.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 185.World Health Organization. 2017. Consensus meeting report: development of a target product profile (TPP) and a framework for evaluation for a test for predicting progression from tuberculosis infection to active disease. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 186.Jacobsen M, Repsilber D, Gutschmidt A, Neher A, Feldmann K, Mollenkopf HJ, Ziegler A, Kaufmann SH. 2007. Candidate biomarkers for discrimination between infection and disease caused by Mycobacterium tuberculosis. J Mol Med (Berl) 85:613–621. doi: 10.1007/s00109-007-0157-6. [DOI] [PubMed] [Google Scholar]
- 187.Cliff JM, Lee JS, Constantinou N, Cho JE, Clark TG, Ronacher K, King EC, Lukey PT, Duncan K, Van Helden PD, Walzl G, Dockrell HM. 2013. Distinct phases of blood gene expression pattern through tuberculosis treatment reflect modulation of the humoral immune response. J Infect Dis 207:18–29. doi: 10.1093/infdis/jis499. [DOI] [PubMed] [Google Scholar]
- 188.Maertzdorf J, Repsilber D, Parida SK, Stanley K, Roberts T, Black G, Walzl G, Kaufmann SH. 2011. Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes Immun 12:15–22. doi: 10.1038/gene.2010.51. [DOI] [PubMed] [Google Scholar]
- 189.Ottenhoff TH, Verreck FA, Hoeve MA, van de Vosse E, Ottenhoff THM, Verreck FAW, Hoeve MA, van de Vosse E. 2005. Control of human host immunity to mycobacteria. Tuberculosis 85:53–64. doi: 10.1016/j.tube.2004.09.011 (Erratum, 86:74–75, 2006.) [DOI] [PubMed] [Google Scholar]
- 190.Petruccioli E, Chiacchio T, Pepponi I, Vanini V, Urso R, Cuzzi G, Barcellini L, Cirillo DM, Palmieri F, Ippolito G, Goletti D. 2016. First characterization of the CD4 and CD8 T-cell responses to QuantiFERON-TB Plus. J Infect 73:588–597. doi: 10.1016/j.jinf.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 191.Fiore-Gartland A, Carpp L, Naidoo K, Thompson E, Zak D, Self S, Churchyard G, Walzl G, Penn-Nicholson A, Scriba T, Hatherill M. 2018. Considerations for biomarker-targeted intervention strategies for tuberculosis disease prevention. Tuberculosis (Edinb) 109:61–68. doi: 10.1016/j.tube.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Manabe YC, Breen R, Perti T, Girardi E, Sterling TR. 2009. Unmasked tuberculosis and tuberculosis immune reconstitution inflammatory disease: a disease spectrum after initiation of antiretroviral therapy. J Infect Dis 199:437–444. doi: 10.1086/595985. [DOI] [PubMed] [Google Scholar]
- 193.Sohn H, Aero AD, Menzies D, Behr M, Schwartzman K, Alvarez GG, Dan A, McIntosh F, Pai M, Denkinger CM. 2014. Xpert MTB/RIF testing in a low tuberculosis incidence, high-resource setting: limitations in accuracy and clinical impact. Clin Infect Dis 58:970–976. doi: 10.1093/cid/ciu022. [DOI] [PubMed] [Google Scholar]
- 194.World Health Organization. 2014. High-priority target product profiles for new tuberculosis diagnostics. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 195.Steingart KR, Henry M, Ng V, Hopewell PC, Ramsay A, Cunningham J, Urbanczik R, Perkins M, Aziz MA, Pai M. 2006. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis 6:570–581. doi: 10.1016/S1473-3099(06)70578-3. [DOI] [PubMed] [Google Scholar]
- 196.Parsons LM, Somoskovi A, Gutierrez C, Lee E, Paramasivan CN, Abimiku A, Spector S, Roscigno G, Nkengasong J. 2011. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev 24:314–350. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, Gler MT, Blakemore R, Worodria W, Gray C, Huang L, Caceres T, Mehdiyev R, Raymond L, Whitelaw A, Sagadevan K, Alexander H, Albert H, Cobelens F, Cox H, Alland D, Perkins MD. 2011. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, Hall SL, Chakravorty S, Cirillo DM, Tukvadze N, Bablishvili N, Stevens W, Scott L, Rodrigues C, Kazi MI, Joloba M, Nakiyingi L, Nicol MP, Ghebrekristos Y, Anyango I, Murithi W, Dietze R, Lyrio Peres R, Skrahina A, Auchynka V, Chopra KK, Hanif M, Liu X, Yuan X, Boehme CC, Ellner JJ, Denkinger CM. 2018. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 18:76–84. doi: 10.1016/S1473-3099(17)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wood RC, Luabeya AK, Weigel KM, Wilbur AK, Jones-Engel L, Hatherill M, Cangelosi GA. 2015. Detection of Mycobacterium tuberculosis DNA on the oral mucosa of tuberculosis patients. Sci Rep 5:8668. doi: 10.1038/srep08668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.LaCourse SM, Pavlinac PB, Cranmer LM, Njuguna IN, Mugo C, Gatimu J, Stern J, Walson JL, Maleche-Obimbo E, Oyugi J, Wamalwa D, John-Stewart G. 2018. Stool Xpert MTB/RIF and urine lipoarabinomannan for the diagnosis of tuberculosis in hospitalized HIV-infected children. AIDS 32:69–78. doi: 10.1097/QAD.0000000000001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Young BL, Mlamla Z, Gqamana PP, Smit S, Roberts T, Peter J, Theron G, Govender U, Dheda K, Blackburn J. 2014. The identification of tuberculosis biomarkers in human urine samples. Eur Respir J 43:1719–1729. doi: 10.1183/09031936.00175113. [DOI] [PubMed] [Google Scholar]
- 203.Shah M, Hanrahan C, Wang ZY, Dendukuri N, Lawn SD, Denkinger CM, Steingart KR. 2016. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database Syst Rev 2016:Cd011420. doi: 10.1002/14651858.CD011420.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.World Health Organization. 2015. The use of lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis and screening of active tuberculosis in people living with HIV. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 205.Bajema K, Losina E, Coleman S, Giddy J, Ross D, Freedberg K, Bassett I, Drain P. 2017. Subclinical tuberculosis among HIV positive adults in South Africa: a cohort study, abstr SOA-395-13. 48th Union World Conf Lung Health, Guadalajara, Mexico, 11 to 14 October 2017 http://www.abstractserver.com/TheUnion2017/TheUnion2017_Abstracts_Web.pdf. [Google Scholar]
- 206.Crawford AC, Laurentius LB, Mulvihill TS, Granger JH, Spencer JS, Chatterjee D, Hanson KE, Porter MD. 2016. Detection of the tuberculosis antigenic marker mannose-capped lipoarabinomannan in pretreated serum by surface-enhanced Raman scattering. Analyst 142:186–196. doi: 10.1039/C6AN02110G. [DOI] [PubMed] [Google Scholar]
- 207.Hamasur B, Bruchfeld J, van Helden P, Kallenius G, Svenson S. 2015. A sensitive urinary lipoarabinomannan test for tuberculosis. PLoS One 10:e0123457. doi: 10.1371/journal.pone.0123457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Laurentius LB, Crawford AC, Mulvihill TS, Granger JH, Robinson R, Spencer JS, Chatterjee D, Hanson KE, Porter MD. 2016. Importance of specimen pretreatment for the low-level detection of mycobacterial lipoarabinomannan in human serum. Analyst 142:177–185. doi: 10.1039/C6AN02109C. [DOI] [PubMed] [Google Scholar]
- 209.Liu C, Zhao Z, Fan J, Lyon CJ, Wu HJ, Nedelkov D, Zelazny AM, Olivier KN, Cazares LH, Holland SM, Graviss EA, Hu Y. 2017. Quantification of circulating Mycobacterium tuberculosis antigen peptides allows rapid diagnosis of active disease and treatment monitoring. Proc Natl Acad Sci U S A 114:3969–3974. doi: 10.1073/pnas.1621360114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ko ER, Yang WE, McClain MT, Woods CW, Ginsburg GS, Tsalik EL. 2015. What was old is new again: using the host response to diagnose infectious disease. Expert Rev Mol Diagn 15:1143–1158. doi: 10.1586/14737159.2015.1059278. [DOI] [PubMed] [Google Scholar]
- 211.Holcomb ZE, Tsalik EL, Woods CW, McClain MT. 2017. Host-based peripheral blood gene expression analysis for diagnosis of infectious diseases. J Clin Microbiol 55:360–368. doi: 10.1128/JCM.01057-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Walzl G, Haks MC, Joosten SA, Kleynhans L, Ronacher K, Ottenhoff TH. 2014. Clinical immunology and multiplex biomarkers of human tuberculosis. Cold Spring Harb Perspect Med 5:a018515. doi: 10.1101/cshperspect.a018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Haas CT, Roe JK, Pollara G, Mehta M, Noursadeghi M. 2016. Diagnostic ‘omics’ for active tuberculosis. BMC Med 14:37. doi: 10.1186/s12916-016-0583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bloom CI, Graham CM, Berry MP, Wilkinson KA, Oni T, Rozakeas F, Xu Z, Rossello-Urgell J, Chaussabel D, Banchereau J, Pascual V, Lipman M, Wilkinson RJ, O'Garra A. 2012. Detectable changes in the blood transcriptome are present after two weeks of antituberculosis therapy. PLoS One 7:e46191. doi: 10.1371/journal.pone.0046191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Latorre I, Leidinger P, Backes C, Dominguez J, de Souza-Galvao ML, Maldonado J, Prat C, Ruiz-Manzano J, Sanchez F, Casas I, Keller A, von Briesen H, Knobel H, Meese E, Meyerhans A. 2015. A novel whole-blood miRNA signature for a rapid diagnosis of pulmonary tuberculosis. Eur Respir J 45:1173–1176. doi: 10.1183/09031936.00221514. [DOI] [PubMed] [Google Scholar]
- 216.Kaforou M, Wright VJ, Levin M. 2014. Host RNA signatures for diagnostics: an example from paediatric tuberculosis in Africa. J Infect 69(Suppl 1):S28–S31. doi: 10.1016/j.jinf.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 217.Satproedprai N, Wichukchinda N, Suphankong S, Inunchot W, Kuntima T, Kumpeerasart S, Wattanapokayakit S, Nedsuwan S, Yanai H, Higuchi K, Harada N, Mahasirimongkol S. 2015. Diagnostic value of blood gene expression signatures in active tuberculosis in Thais: a pilot study. Genes Immun 16:253–260. doi: 10.1038/gene.2015.4. [DOI] [PubMed] [Google Scholar]
- 218.Sweeney TE, Braviak L, Tato CM, Khatri P. 2016. Genome-wide expression for diagnosis of pulmonary tuberculosis: a multicohort analysis. Lancet Respir Med 4:213–224. doi: 10.1016/S2213-2600(16)00048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Petruccioli E, Scriba TJ, Petrone L, Hatherill M, Cirillo DM, Joosten SA, Ottenhoff TH, Denkinger CM, Goletti D. 2016. Correlates of tuberculosis risk: predictive biomarkers for progression to active tuberculosis. Eur Respir J 48:1751–1763. doi: 10.1183/13993003.01012-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Djoba Siawaya JF, Ruhwald M, Eugen-Olsen J, Walzl G. 2007. Correlates for disease progression and prognosis during concurrent HIV/TB infection. Int J Infect Dis 11:289–299. doi: 10.1016/j.ijid.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 221.Gliddon HD, Herberg JA, Levin M, Kaforou M. 2018. Genome-wide host RNA signatures of infectious diseases: discovery and clinical translation. Immunology 153:171–178. doi: 10.1111/imm.12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gibson G. 2008. The environmental contribution to gene expression profiles. Nat Rev Genet 9:575–581. doi: 10.1038/nrg2383. [DOI] [PubMed] [Google Scholar]
- 223.Joosten SA, Fletcher HA, Ottenhoff TH. 2013. A helicopter perspective on TB biomarkers: pathway and process based analysis of gene expression data provides new insight into TB pathogenesis. PLoS One 8:e73230. doi: 10.1371/journal.pone.0073230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sandgren A, Vonk Noordegraaf-Schouten M, van Kessel F, Stuurman A, Oordt-Speets A, van der Werf MJ. 2016. Initiation and completion rates for latent tuberculosis infection treatment: a systematic review. BMC Infect Dis 16:204. doi: 10.1186/s12879-016-1550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. 2016. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis 16:1269–1278. doi: 10.1016/S1473-3099(16)30216-X. [DOI] [PubMed] [Google Scholar]
- 226.Dheda K, Chang KC, Guglielmetti L, Furin J, Schaaf HS, Chesov D, Esmail A, Lange C. 2017. Clinical management of adults and children with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Microbiol Infect 23:131–140. doi: 10.1016/j.cmi.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 227.Dheda K, Gumbo T, Gandhi NR, Murray M, Theron G, Udwadia Z, Migliori G, Warren R. 2014. Global control of tuberculosis: from extensively drug-resistant to untreatable tuberculosis. Lancet Respir Med 2:321–338. doi: 10.1016/S2213-2600(14)70031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Boeree MJ, Heinrich N, Aarnoutse R, Diacon AH, Dawson R, Rehal S, Kibiki GS, Churchyard G, Sanne I, Ntinginya NE, Minja LT, Hunt RD, Charalambous S, Hanekom M, Semvua HH, Mpagama SG, Manyama C, Mtafya B, Reither K, Wallis RS, Venter A, Narunsky K, Mekota A, Henne S, Colbers A, van Balen GP, Gillespie SH, Phillips PPJ, Hoelscher M, PanACEA Consortium. 2017. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 17:39–49. doi: 10.1016/S1473-3099(16)30274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dheda K, Gumbo T, Maartens G, Dooley KE, McNerney R, Murray M, Furin J, Nardell EA, London L, Lessem E, Theron G, van Helden P, Niemann S, Merker M, Dowdy D, Van Rie A, Siu GK, Pasipanodya JG, Rodrigues C, Clark TG, Sirgel FA, Esmail A, Lin HH, Atre SR, Schaaf HS, Chang KC, Lange C, Nahid P, Udwadia ZF, Horsburgh CR Jr, Churchyard GJ, Menzies D, Hesseling AC, Nuermberger E, McIlleron H, Fennelly KP, Goemaere E, Jaramillo E, Low M, Jara CM, Padayatchi N, Warren RM. 2017. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med 5:291–360. doi: 10.1016/S2213-2600(17)30079-6. [DOI] [PubMed] [Google Scholar]
- 230.Kaufmann SHE, Dorhoi A, Hotchkiss RS, Bartenschlager R. 2018. Host-directed therapies for bacterial and viral infections. Nat Rev Drug Discov 17:35–56. doi: 10.1038/nrd.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.World Health Organization. 2011. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 232.Anonymous. 2016. Using biomarkers to predict TB treatment duration. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02821832 (registration number NCT02821832) Accessed 17 January 2018.
- 233.Cohen T, Lipsitch M, Walensky RP, Murray M. 2006. Beneficial and perverse effects of isoniazid preventive therapy for latent tuberculosis infection in HIV-tuberculosis coinfected populations. Proc Natl Acad Sci U S A 103:7042–7047. doi: 10.1073/pnas.0600349103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kunkel A, Colijn C, Lipsitch M, Cohen T. 2015. How could preventive therapy affect the prevalence of drug resistance? Causes and consequences. Philos Trans R Soc Lond B Biol Sci 370:20140306. doi: 10.1098/rstb.2014.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Balcells ME, Thomas SL, Godfrey-Faussett P, Grant AD. 2006. Isoniazid preventive therapy and risk for resistant tuberculosis. Emerg Infect Dis 12:744–751. doi: 10.3201/eid1205.050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Malherbe ST, Shenai S, Ronacher K, Loxton AG, Dolganov G, Kriel M, Van T, Chen RY, Warwick J, Via LE, Song T, Lee M, Schoolnik G, Tromp G, Alland D, Barry CE III, Winter J, Walzl G, Lucas L, Spuy GV, Stanley K, Thiart L, Smith B, Du Plessis N, Beltran CG, Maasdorp E, Ellmann A, Choi H, Joh J, Dodd LE, Allwood B, Koegelenberg C, Vorster M, Griffith-Richards S. 2016. Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nat Med 22:1094–1100. doi: 10.1038/nm.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rakotosamimanana N, Raharimanga V, Andriamandimby SF, Soares JL, Doherty TM, Ratsitorahina M, Ramarokoto H, Zumla A, Huggett J, Rook G, Richard V, Gicquel B, Rasolofo-Razanamparany V. 2010. Variation in gamma interferon responses to different infecting strains of Mycobacterium tuberculosis in acid-fast bacillus smear-positive patients and household contacts in Antananarivo, Madagascar. Clin Vaccine Immunol 17:1094–1103. doi: 10.1128/CVI.00049-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kong Y, Cave MD, Zhang L, Foxman B, Marrs CF, Bates JH, Yang ZH. 2006. Population-based study of deletions in five different genomic regions of Mycobacterium tuberculosis and possible clinical relevance of the deletions. J Clin Microbiol 44:3940–3946. doi: 10.1128/JCM.01146-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lan NTN, Lien HTK, Tung LB, Borgdorff MW, Kremer K, van Soolingen D. 2003. Mycobacterium tuberculosis Beijing genotype and risk for treatment failure and relapse, Vietnam. Emerg Infect Dis 9:1633–1635. doi: 10.3201/eid0912.030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sun YJ, Lee AS, Wong SY, Paton NI. 2006. Association of Mycobacterium tuberculosis Beijing genotype with tuberculosis relapse in Singapore. Epidemiol Infect 134:329–332. doi: 10.1017/S095026880500525X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Parwati I, Alisjahbana B, Apriani L, Soetikno RD, Ottenhoff TH, van der Zanden AG, van der Meer J, van Soolingen D, van Crevel R. 2010. Mycobacterium tuberculosis Beijing genotype is an independent risk factor for tuberculosis treatment failure in Indonesia. J Infect Dis 201:553–557. doi: 10.1086/650311. [DOI] [PubMed] [Google Scholar]
- 242.van Crevel R, Nelwan RH, de Lenne W, Veeraragu Y, van der Zanden AG, Amin Z, van der Meer JW, van Soolingen D. 2001. Mycobacterium tuberculosis Beijing genotype strains associated with febrile response to treatment. Emerg Infect Dis 7:880–883. doi: 10.3201/eid0705.017518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lari N, Rindi L, Cristofani R, Rastogi N, Tortoli E, Garzelli C. 2009. Association of Mycobacterium tuberculosis complex isolates of BOVIS and Central Asian (CAS) genotypic lineages with extrapulmonary disease. Clin Microbiol Infect 15:538–543. doi: 10.1111/j.1469-0691.2009.02712.x. [DOI] [PubMed] [Google Scholar]
- 244.Caws M, Thwaites G, Dunstan S, Hawn TR, Lan NT, Thuong NT, Stepniewska K, Huyen MN, Bang ND, Loc TH, Gagneux S, van Soolingen D, Kremer K, van der Sande M, Small P, Anh PT, Chinh NT, Quy HT, Duyen NT, Tho DQ, Hieu NT, Torok E, Hien TT, Dung NH, Nhu NT, Duy PM, van Vinh Chau N, Farrar J. 2008. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog 4:e1000034. doi: 10.1371/journal.ppat.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Thwaites G, Caws M, Chau TT, D'Sa A, Lan NT, Huyen MN, Gagneux S, Anh PT, Tho DQ, Torok E, Nhu NT, Duyen NT, Duy PM, Richenberg J, Simmons C, Hien TT, Farrar J. 2008. Relationship between Mycobacterium tuberculosis genotype and the clinical phenotype of pulmonary and meningeal tuberculosis. J Clin Microbiol 46:1363–1368. doi: 10.1128/JCM.02180-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yimer SA, Norheim G, Namouchi A, Zegeye ED, Kinander W, Tonjum T, Bekele S, Mannsaker T, Bjune G, Aseffa A, Holm-Hansen C. 2015. Mycobacterium tuberculosis lineage 7 strains are associated with prolonged patient delay in seeking treatment for pulmonary tuberculosis in Amhara Region, Ethiopia. J Clin Microbiol 53:1301–1309. doi: 10.1128/JCM.03566-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Carmona J, Cruz A, Moreira-Teixeira L, Sousa C, Sousa J, Osorio NS, Saraiva AL, Svenson S, Kallenius G, Pedrosa J, Rodrigues F, Castro AG, Saraiva M. 2013. Mycobacterium tuberculosis strains are differentially recognized by TLRs with an impact on the immune response. PLoS One 8:e67277. doi: 10.1371/journal.pone.0067277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Reyes-Martinez JE, Nieto-Patlan E, Nieto-Patlan A, Gonzaga-Bernachi J, Santos-Mendoza T, Serafin-Lopez J, Chavez-Blanco A, Sandoval-Montes C, Flores-Romo L, Estrada-Parra S, Estrada-Garcia I, Chacon-Salinas R. 2014. Differential activation of dendritic cells by Mycobacterium tuberculosis Beijing genotype. Immunol Invest 43:436–446. doi: 10.3109/08820139.2014.880120. [DOI] [PubMed] [Google Scholar]
- 249.Ordway D, Henao-Tamayo M, Harton M, Palanisamy G, Troudt J, Shanley C, Basaraba RJ, Orme IM. 2007. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J Immunol 179:522–531. doi: 10.4049/jimmunol.179.1.522. [DOI] [PubMed] [Google Scholar]