Primary Toxoplasma gondii infection is usually subclinical, but cervical lymphadenopathy or ocular disease can be present in some patients. Active infection is characterized by tachyzoites, while tissue cysts characterize latent disease.
KEYWORDS: T. gondii, Toxoplasma gondii, animal models, clindamycin, in vitro, in vivo, pyrimethamine, sulfadiazine, therapy, treatment
SUMMARY
Primary Toxoplasma gondii infection is usually subclinical, but cervical lymphadenopathy or ocular disease can be present in some patients. Active infection is characterized by tachyzoites, while tissue cysts characterize latent disease. Infection in the fetus and in immunocompromised patients can cause devastating disease. The combination of pyrimethamine and sulfadiazine (pyr-sulf), targeting the active stage of the infection, is the current gold standard for treating toxoplasmosis, but failure rates remain significant. Although other regimens are available, including pyrimethamine in combination with clindamycin, atovaquone, clarithromycin, or azithromycin or monotherapy with trimethoprim-sulfamethoxazole (TMP-SMX) or atovaquone, none have been found to be superior to pyr-sulf, and no regimen is active against the latent stage of the infection. Furthermore, the efficacy of these regimens against ocular disease remains uncertain. In multiple studies, systematic screening for Toxoplasma infection during gestation, followed by treatment with spiramycin for acute maternal infections and with pyr-sulf for those with established fetal infection, has been shown to be effective at preventing vertical transmission and minimizing the severity of congenital toxoplasmosis (CT). Despite significant progress in treating human disease, there is a strong impetus to develop novel therapeutics for both the acute and latent forms of the infection. Here we present an overview of toxoplasmosis treatment in humans and in animal models. Additional research is needed to identify novel drugs by use of innovative high-throughput screening technologies and to improve experimental models to reflect human disease. Such advances will pave the way for lead candidates to be tested in thoroughly designed clinical trials in defined patient populations.
INTRODUCTION
Toxoplasma gondii is an intracellular pathogen affecting approximately one-third of the human population. It exists in nature as oocysts, bradzyzoites (contained in latent tissue cysts), and replicating tachyzoites, with the last form being the hallmark of active disease (1). Human infection is acquired via ingesting food or water contaminated with sporulated oocysts or undercooked meat infected with latent cysts, by mother-to-child transmission, or via an infected allograft during organ transplantation. Acquisition via blood products or by accidental ingestion or inoculation of Toxoplasma in laboratories working with the parasite is rare. Acute infection is typically asymptomatic in immunocompetent individuals, but cervical lymphadenopathy or ocular disease can occur. Infection of immunocompetent individuals with more virulent strains of T. gondii, which are prevalent in Latin America, can result in severe pneumonia and disseminated disease, including death (2). In pregnant women, acute infection acquired during or shortly before gestation can lead to congenital toxoplasmosis (CT) even though the mother remains asymptomatic. Acute infection in the immunocompetent host is followed by asymptomatic latent infection, during which the parasite encysts in various organs, especially the cardiac and skeletal muscles, brain parenchyma, and retina. Latent infection can reactivate overtly in immunocompromised patients, with conversion of latent bradyzoites into rapidly replicating tachyzoites, causing severe, life-threatening disease with significant morbidity and 100% mortality if left untreated (1). Latent infection can also reactivate locally in the retinas of immunocompetent individuals, leading to significant loss of visual acuity and economic productivity.
Treatment of toxoplasmosis typically includes combinations of two antimicrobials, most often inhibitors of dihydrofolate reductase (DHFR) (pyrimethamine and trimethoprim) and dihydropteroate synthetase (sulfonamides, such as sulfadiazine, sulfamethoxazole, and sulfadoxine), which block folic acid synthesis. Pyrimethamine, a key DHFR inhibitor, appears to be the most effective drug against T. gondii and is the basis for effective regimens. These include pyrimethamine-sulfadiazine (pyr-sulf), the gold standard against which other regimens are measured, and pyrimethamine combined with clindamycin, atovaquone, clarithromycin, or azithromycin. However, in September 2015, the price of pyrimethamine, which has been in clinical use for more than 6 decades, was increased 5,000% overnight, from $13.50 to $750.00 per pill, by Turing Pharmaceuticals (now marketed by Vyera Pharmaceuticals), the sole manufacturer in the United States; this created confusion and prompted compound pharmacies to provide alternatives (3). In other regions of the world, the price of pyrimethamine is less than $1 per pill. This serves as an additional impetus to find new drugs and should encourage lawmakers in the United States to prohibit unethical and massive increases in prices of old and inexpensive drugs. Other regimens include trimethoprim, another DHFR inhibitor, in combination with sulfamethoxazole (TMP-SMX; also known as co-trimoxazole) and atovaquone alone or in combination with sulfadiazine. Unfortunately, all drugs used in clinical practice are solely active against the tachyzoite stage of the parasite and do not demonstrate activity against cysts containing bradyzoites, the latent stage of the parasite. Interestingly, resistance to the currently used drugs has not been described as a clinical problem.
Very few reviews have been published on the treatment of T. gondii infection in humans and in vivo models (4–6). Here we discuss studies on anti-T. gondii drugs performed in humans and in animal models. For studies performed using in vitro models and screening approaches for new drugs, the reader is referred to recent expert reviews by Alday and Doggett (7), McFarland et al. (8), Jin et al. (9), Kortagere (10), Montazeri et al. (11), and Sharif et al. (12).
EARLY DAYS OF RESEARCH ON ANTI-TOXOPLASMA THERAPIES
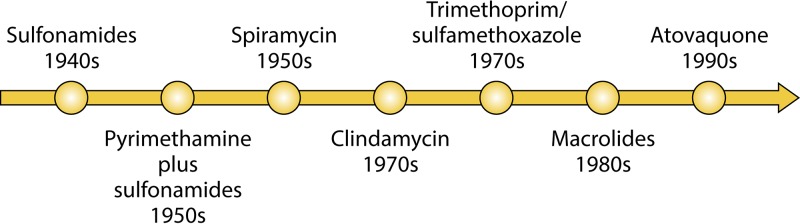
Early research on the efficacy of anti-Toxoplasma drugs started in the 1940s. Since then, many in vitro and in vivo models of T. gondii infection have been used to investigate antiparasitic therapies in humans. In 1942, Sabin and Warren (13) reported the effectiveness of sulfonamides against murine toxoplasmosis. Several sulfone compounds were soon found to be effective (5, 14, 15). In the early 1950s, Eyles and Coleman (16) observed the synergistic effect of combination therapy with pyr-sulf against experimental toxoplasmosis in mice. To this day, more than 60 years later, combination therapy with pyr-sulf remains the gold standard for the treatment of toxoplasmosis in humans. Beverley and Fry (17) conducted experiments on the treatment of toxoplasmosis by use of monotherapy and combination therapy with sulfadimidine, dapsone, and pyrimethamine in 1957. Concurrently, Garin and Eyles (18) found spiramycin to have antitoxoplasmic activity in mice. Since spiramycin is nontoxic and does not cross the placenta, it is still used prophylactically in pregnant women to prevent materno-fetal transmission of the parasite (19). Other drugs studied early on were, among others, tetracyclines (20), rifampin (21), and lincomycin (22).
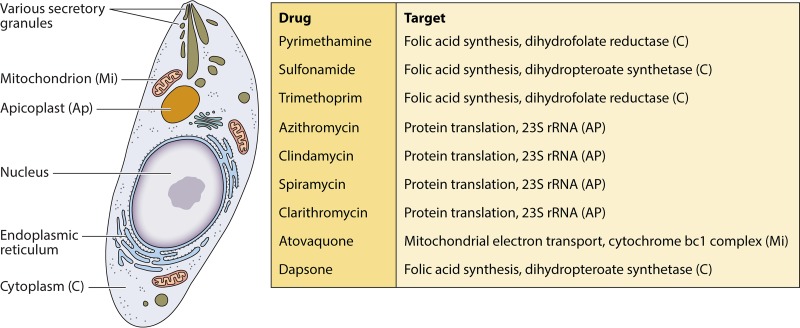
Unfortunately, only a few anti-Toxoplasma drugs have been approved for use in humans. Figure 1 depicts the timeline of introduction of important anti-T. gondii drugs to the market. Most of the studies on Toxoplasma treatment were geared toward developing inhibitors of enzymes involved in Toxoplasma metabolism, such as dihydrofolate reductase, NADH dehydrogenases, cysteine proteases, and dihydropteroate synthetase (elegantly reviewed by Kortagere [10]) (Fig. 2).
FIG 1.
Developmental timeline of anti-T. gondii drugs in clinical use.
FIG 2.
Parasite pathways targeted by anti-T. gondii drugs in clinical use.
TREATMENT OF TOXOPLASMOSIS IN HUMANS
Nonpregnant Immunocompetent Individuals
About 11% of the U.S. population is infected with T. gondii; acquisition is usually due to ingestion of contaminated food and water (23). Every year an estimated 85,000 foodborne toxoplasmosis cases occur in the United States (24). Acute Toxoplasma infection in healthy North American and European adults is generally benign and presents asymptomatically. Some patients may experience lymphadenopathy, fever, fatigue, chorioretinitis, myocarditis, myositis, and hepatosplenomegaly. Since most patients experience mild self-limited symptoms, acute toxoplasmosis in immunocompetent patients generally does not require treatment. However, treatment should be considered if a patient continues to have persistent and discomforting symptoms or displays severe symptoms. This may be particularly relevant to areas of the world, such as South America, where virulent strains of T. gondii have been reported to cause a severe illness with multivisceral involvement, sometimes with a fatal outcome (2, 25, 26). There are few data on the choice of therapy. Table 1 provides an overview of drug regimens used for the treatment of toxoplasmosis in immunocompetent patients. In one randomized placebo-controlled trial of 46 immunocompetent patients in Iran, treatment with TMP-SMX (8 mg of TMP component/kg of body weight/day) for 1 month was found to be effective at resolving lymphadenopathy (27). The pyr-sulf combination may be effective as well, but large randomized trials have not been performed (25, 26). Based on the efficacy in the treatment of Toxoplasma encephalitis (TE) in the HIV population, pyr-sulf plus folinic acid, pyrimethamine-clindamycin plus folinic acid, or TMP-SMX can be considered for severe or debilitating primary infection in immunocompetent patients. Although it is rare, laboratory-acquired Toxoplasma infection via accidental ingestion or cutaneous inoculation of laboratory specimens can occur. Case reports of such acute infections in apparently immunocompetent patients from the 1950s described severe systemic symptoms and even fatality (28–31). Hence, individuals with such infections should always be offered treatment. While the duration of treatment is not established, a 4- to 6-week course is reasonable, depending on the clinical course and the response to treatment if symptoms have developed.
TABLE 1.
Treatment of toxoplasmosis in immunocompetent patientsa
| Regimenb | Comments |
|---|---|
| Pyrimethamine (100 mg daily for 1 or 2 days and then 25–50 mg daily) plus sulfadiazine (1g every 6 h [q6h]) plus folinic acid (10–20 mg daily) | Blood counts, creatinine, and liver function should be monitored regularly |
| Adequate hydration should be ensured to prevent renal damage from crystalluria | |
| Pyrimethamine plus folinic acid (dosing as described above) plus clindamycin (300 mg q6h) | Blood counts should be monitored regularly |
| Clindamycin may cause diarrhea, including Clostridium difficile infection | |
| TMP-SMX (5/25–10/50 mg/kg/day in divided doses) | Blood counts, creatinine, and liver function should be monitored regularly |
| Adequate hydration should be ensured to prevent renal damage from crystalluria | |
| Atovaquone (1,500 mg twice daily) ± pyrimethamine plus folinic acid (dosing as described above) | Blood counts and liver function should be monitored regularly |
| Atovaquone should be taken with a high-fat diet | |
| Pyrimethamine plus folinic acid (dosing as described above) plus azithromycin (250–500 mg dailyc) | Blood counts should be monitored regularly |
| Azithromycin may cause hearing problems and a prolonged QT interval | |
| Intravitreal clindamycin (1 mg) plus dexamethasone (400 μg) | Only for ocular toxoplasmosis; may need to be repeated 1 or 2 times if response is suboptimal |
Treatment should be considered for immunocompetent patients with severe or persistent symptoms, ocular involvement, or laboratory-acquired infection. For ocular infection, concomitant steroids (prednisone at 0.5 to 1 mg/kg/day) with gradual tapering can be used; this decision is best made by the ophthalmologist.
Folinic acid is different from folic acid.
A higher dose of azithromycin (1,000 mg) should be considered for severe nonocular systemic disease.
Acute Toxoplasma infection in immunocompetent people may also present solely as ocular symptoms; its management in such cases is discussed below.
Following an acute episode of toxoplasmosis, the actively replicating tachyzoites encyst as bradyzoites in various tissues (tissue cysts), especially in the brain, cardiac and skeletal muscles, and retina. These cysts remain dormant in immunocompetent patients, with the exception of those in the retina; frequent reactivation of these bradyzoites can lead to recurrent chorioretinitis. However, the lifelong persistence of these cysts and long-term immunity against reinfection in the latently infected host were recently questioned (32). Unfortunately, there is no effective treatment for the eradication of bradyzoites in tissue cysts, as the available drugs are active only against tachyzoites. These cysts can reactivate when the host's immunity is compromised; intermittent subclinical reactivation of these cysts may also occur. A growing number of studies of animals and epidemiological studies of humans suggest a possible link between latent Toxoplasma infection and changes in behavior via altered or subverted host neuronal activity (33, 34). A “loss of fear” phenotype has been described for rodents (35), providing strong support for the behavioral manipulation hypothesis. In the wild, increased rodent predation caused by the loss of fear facilitates the spread of the parasite to members of the Felidae, the definitive hosts of T. gondii (35). However, the molecular mechanisms underlying the loss-of-fear phenotype are poorly delineated. Sutterland et al. (36) performed a meta-analysis of studies reporting the prevalence of T. gondii infection in patients with any psychiatric disorder compared to that in healthy controls; among 50 studies, significant associations with the presence of Toxoplasma-specific IgG antibodies were found for schizophrenia, bipolar disorder, obsessive-compulsive disorder, and addiction, but not major depression. However, four randomized controlled trials evaluating antiparasitic drugs in patients with schizophrenia did not demonstrate changes in psychopathology with adjunctive treatment (37). While these trials, using azithromycin, trimethoprim, artemisinin, and artemether, had severe limitations, there are currently no ongoing trials of anti-Toxoplasma therapy to determine the benefits of antiparasitic treatment for neuropsychiatric disorders.
Immunocompromised Patients
HIV-infected patients.
Toxoplasmosis commonly manifests as Toxoplasma encephalitis (TE) in HIV-infected patients with CD4 counts of <100/mm3. Other manifestations (pulmonary, gastrointestinal [GI], ocular, and disseminated) are less common, but the treatment modality is similar to that for TE. TE usually occurs via reactivation of a previously acquired latent infection and presents with either solitary or multiple (usually ring enhancing) parenchymal lesions on brain imaging (38, 39). Diffuse encephalitis without space-occupying lesions on magnetic resonance imaging (MRI) can also occur. Common clinical manifestations include headache, seizure, altered mentation, and focal neurologic signs. Fever may or may not be present and should not be relied on to exclude the diagnosis of TE (38, 40). The clinical course of TE is universally fatal if left untreated. Antimicrobial therapy should not be delayed to confirm the diagnosis by tissue biopsy for Toxoplasma-seropositive patients with advanced HIV presenting with clinical and radiologic features consistent with Toxoplasma reactivation. Pursuing histopathologic confirmation can delay the implementation of appropriate therapy and consequently lead to a poor outcome. In one study, although limited by a small sample size, the overall clinical response and survival for patients with biopsy-proven TE were found to be similar to those for patients who were treated empirically (41). In fact, some of the nonresponders in the biopsy-proven cohort had their treatment delayed due to biopsy and were already comatose when treatment was initiated. Since the differential diagnosis of ring-enhancing brain lesions in patients with advanced HIV includes lymphoma and, occasionally, other central nervous system (CNS) infections (e.g., bacterial abscess, nocardia infection, cryptococcus infection, etc.), these empirically treated patients should be monitored closely for treatment failure. AIDS patients with multiple ring-enhancing lesions, positive Toxoplasma IgG results, and CD4 counts of <100/mm3 and who are not receiving Toxoplasma prophylaxis have a high likelihood of having TE and should be treated empirically (41). The more that AIDS patients deviate from this profile, the higher the likelihood of non-TE lesions; these patients should be considered candidates for brain biopsy. About 85% of those who ultimately respond to treatment do so within the first week, and 90% do so by 2 weeks (38, 42). Hence, failure to respond within the first 1 to 2 weeks should raise a suspicion of another underlying etiology, and a brain biopsy should be considered. Late presentation with a decreased sensorium does not carry a favorable prognosis despite optimal treatment (40, 43, 44). It is important to note that radiologic improvement lags behind clinical improvement and does not necessarily signify unsuccessful therapy (44–48). Interestingly, drug resistance (either inherent or emerging during therapy) is not an issue in the treatment of toxoplasmosis, and failure to respond to appropriate therapy is related to a delay in diagnosis and to host factors (e.g., poor immune response).
Table 2 provides an overview of drug regimens used for the treatment of toxoplasmosis in immunocompromised patients. Early studies during the HIV epidemic demonstrated efficacy of pyr-sulf in the treatment of TE. The response rate is about 80% during the induction phase of therapy (38, 49–51). However, the therapeutic potency is marred by severe adverse reactions, namely, hematologic toxicity (38, 40, 41, 44, 49–51). Adverse reactions associated with pyr-sulf were noted in 60% of TE patients, leading to discontinuation of therapy in 45% of patients. Leukopenia, thrombocytopenia, cutaneous rash, and fever were reported for 40%, 12%, 19%, and 10% of patients, respectively (40). Leukopenia can occur anytime during the treatment course (median, 26 days) (38). Folinic acid is generally recommended to minimize the hematologic toxicity associated with pyrimethamine. It should be noted that folinic acid is different from folic acid.
TABLE 2.
Treatment of toxoplasmosis in immunocompromised patients
| Induction therapy | Maintenance therapy | Comments |
|---|---|---|
| For those with body wt of ≥60 kg, pyrimethamine (200 mg once and then 75 mg daily) plus sulfadiazine (1.5 g q6h) plus folinic acid (10–50 mg daily) | Pyrimethamine (50 mg daily) plus sulfadiazine (1 g q6h) plus folinic acid (10–25 mg daily) | Blood counts, creatinine, and liver function should be monitored regularly |
| Adequate hydration should be ensured to prevent renal damage from crystalluria | ||
| For those with body wt of <60 kg, pyrimethamine (200 mg once and then 50 mg daily) plus sulfadiazine (1 g q6h) plus folinic acid (10–50 mg daily) | Pyrimethamine (25 mg daily) plus sulfadiazine (0.5 g q6h) plus folinic acid (10–25 mg daily) | Blood counts, creatinine, and liver function should be monitored regularly |
| Adequate hydration should be ensured to prevent renal damage from crystalluria | ||
| Pyrimethamine plus folinic acid (dosing as described above) plus clindamycin (600 mg q6h) | Pyrimethamine plus folinic acid (dosing as described above) plus clindamycin (600 mg q8h) | Blood counts should be monitored regularly |
| Clindamycin may cause diarrhea, including Clostridium difficile infection | ||
| TMP-SMX (10/50 mg/kg/daya in divided doses) | TMP-SMX (5/25 mg/kg/day in divided doses) | Blood counts, creatinine, and liver function should be monitored regularly |
| Adequate hydration should be ensured to prevent renal damage from crystalluria | ||
| Atovaquone (1,500 mg twice daily) ± pyrimethamine plus folinic acid (dosing as described above) | Atovaquone (750–1,500 mg twice daily) ± pyrimethamine plus folinic acid (dosing as described above) | Blood counts and liver function should be monitored regularly |
| Atovaquone suspension should be taken with a high-fat diet to optimize bioavailability | ||
| Atovaquone plus sulfadiazine (dosing as described above) | Atovaquone plus sulfadiazine (dosing as described above) | Blood counts, creatinine, and liver function should be monitored regularly |
| Atovaquone suspension should be taken with a high-fat diet to optimize bioavailability | ||
| Pyrimethamine plus folinic acid (dosing as described above) plus azithromycinb (1,000 mg daily) | Pyrimethamine plus azithromycin not recommended due to a high relapse rate; one of the above regimens should be used instead | Blood counts should be monitored regularly |
| Azithromycin may cause hearing problems and a prolonged QT interval |
Higher doses of 15/75 or 20/100 mg/kg/day can be used.
Clarithromycin can be used instead of azithromycin but is associated with more GI intolerance and drug interactions. If used, a lower dose of 500 mg twice a day is preferred, especially in HIV-infected patients; the efficacy of this dosing regimen is not clear (see the text for details).
The rate of unacceptable adverse reactions, as well as the high pill burden associated with pyr-sulf, led to a quest for other therapeutic options. Initial, nonrandomized studies showed efficacy of clindamycin-based regimens, but the relapse rate was high for clindamycin-only maintenance therapy (42, 52). Two randomized trials subsequently established an equivalent efficacy of a pyrimethamine-clindamycin regimen for the treatment of TE (44, 51). A phase II randomized trial of pyr-sulf versus pyrimethamine-clindamycin involved 59 patients with TE. After 6 weeks of treatment, 70% and 65% of patients in the pyr-sulf and pyrimethamine-clindamycin arms, respectively, showed a partial or complete clinical response. In the multivariate analysis, survival and clinical response tended to be higher in the pyr-sulf arm, but most deaths were not directly related to TE. The radiological response appeared to be better in the pyrimethamine-clindamycin arm but was not statistically significant. Both groups had similar adverse reactions. Based on these findings, it was concluded that pyrimethamine-clindamycin is as effective as pyr-sulf and can be used as an alternative regimen for the treatment of TE (44). In a European multicenter prospective study, 299 patients with TE were randomized to pyr-sulf and pyrimethamine-clindamycin arms. No difference was observed in the complete or partial clinical response in the pyr-sulf and pyrimethamine-clindamycin groups (76 versus 68%) after induction therapy. However, relapse on maintenance therapy was found to be significantly higher in the pyrimethamine-clindamycin arm. Additionally, there was no difference in survival between the two groups. While the adverse reactions were similar, diarrhea was more common in the pyrimethamine-clindamycin group, while skin reactions and fever were more common in the pyr-sulf group. Although there was no significant difference in the proportion of patients who crossed over to the other treatment arm during the induction phase of treatment, 98% of switches from the pyr-sulf arm were due to medication-related adverse reactions, while 54% of switches from the pyrimethamine-clindamycin arm were due to a suboptimal response (51). Taken together, the data indicate that pyrimethamine-clindamycin appears to be more tolerable than pyr-sulf and is equally efficacious in the resolution of acute TE but may be less effective at preventing relapse during the maintenance phase of treatment.
TMP-SMX in various doses (from 6.6 to 20 mg/kg/day of TMP component) has also been found in small studies to be effective for TE treatment in the HIV population and is well tolerated (43, 53–56). In a retrospective study of 71 patients with AIDS-related TE, 87% of patients had a complete or partial resolution of symptoms after 4 weeks of TMP-SMX therapy (10 mg/kg/day of TMP component). Thirty-one percent of patients had adverse cutaneous reactions, while 7% had a relapse while on a maintenance regimen (48). Another prospective study of 83 patients with TE also showed a similar efficacy of TMP-SMX, with relapse seen mainly among noncompliant patients (57). In a multicenter prospective pilot study, 77 patients with TE were randomized to a pyr-sulf arm versus a TMP-SMX (10 mg/kg/day of TMP component) arm (48). After 30 days of treatment, 86% versus 84% of patients had a complete or partial response to treatment. Maintenance therapy was continued at half the dose of the induction regimen for 3 months, during which time only 1 patient in the TMP-SMX arm relapsed. While there was no difference in survival, adverse events were more frequent in the pyr-sulf arm. Surprisingly, a small randomized trial of pyr-sulf versus TMP-SMX found a higher mortality in the TMP-SMX arm (58). However, the results are largely uninterpretable, since the trial was prematurely terminated and had a very small sample size, with only 10 patients in the TMP-SMX arm. Moreover, the failure rates (composites of death rates and serious adverse event rates) were similar between the two arms.
A 2006 Cochrane meta-analysis found only three randomized trials that examined the efficacy of different treatment regimens for TE in HIV-infected patients; no significant difference in efficacy was found between pyr-sulf, pyrimethamine-clindamycin, and TMP-SMX (59). Recent systemic reviews and meta-analyses have also reached similar conclusions (60–62). One meta-analysis which used less stringent criteria and included randomized controlled trials as well as intention-to-treat analysis demonstrated a slightly better clinical response with pyr-sulf than that with pyrimethamine-clindamycin, albeit at the cost of higher drug toxicity (63). There was, however, no significant difference in clinical response between pyr-sulf and TMP-SMX.
The clinical experience with other treatment regimens is limited. Older studies of atovaquone showed that it was a somewhat useful agent for salvage therapy in patients who either could not tolerate or did not respond to standard TE therapy. In a study of 93 patients, atovaquone tablets (750 mg 4 times a day) were used as salvage treatment, with some efficacy. At week 6, 52% of patients showed some clinical response, but by week 18, only 26% of patients had a clinical response (64). Another study in which atovaquone, either alone (75% of patients) or with pyrimethamine or clindamycin, was used as maintenance therapy in patients who could not tolerate the standard regimen demonstrated that atovaquone was well tolerated, and only 26% of patients relapsed over a 1-year period (51). It should be noted that these studies used the tablet formulation of atovaquone, which has a lower bioavailability than that of the currently available suspension; this may have undermined its efficacy. Also, atovaquone monotherapy may not be very effective. In a noncomparative randomized trial of atovaquone suspension with either pyrimethamine or sulfadiazine, 75 and 82% of patients, respectively, responded to induction therapy. Only 1 of 20 patients developed a relapse. Notably, 28% of patients discontinued the therapy, mainly because of GI intolerance (65). Hence, atovaquone should be used in combination with pyrimethamine or sulfadiazine for both induction and maintenance therapy, as there may be a high risk of failure if it is used as a monotherapy.
In one small study of pyrimethamine plus clarithromycin, 11 of 13 patients clinically responded at 3 weeks, but only 8 patients could complete the 6-week treatment course. Adverse events occurred in 38% of patients and included gastrointestinal intolerance, skin rash, hematologic abnormality, and hearing loss (45). In that study, a high dose of clarithromycin (2 g per day) was used. Such a high dose is associated with gastrointestinal intolerance, hearing problems, and a prolonged QT interval and can potentially increase mortality in HIV-infected patients with concomitant Mycobacterium avium complex infection (66). A lower dose of 500 mg twice daily seems reasonable, but its efficacy is not clear. In a study of pyrimethamine plus azithromycin, 67% of 30 evaluable patients responded clinically, but almost 50% relapsed during maintenance therapy. Interestingly, there was no improvement of efficacy at doses higher than 900 mg of azithromycin per day. In fact, adverse effects were apparent when azithromycin was used at 1,500 mg per day (67). Pyrimethamine plus azithromycin at a lower azithromycin dose (500 mg/day) may also be effective during induction therapy, but the interpretation of the study is limited by a very small sample size (68).
In general, induction therapy should be continued for at least 6 weeks. Since the currently available anti-T. gondii therapy cannot eradicate the protozoan, induction therapy should be followed by maintenance therapy as long as the patient remains significantly immunosuppressed. The risk of relapse is more than 50% in the absence of maintenance therapy (38, 40, 49, 69). Moreover, long-term maintenance therapy may be able to completely resolve neurologic deficits in patients who only partially responded to induction therapy (49). Maintenance therapy can safely be stopped once the CD4 count is maintained above 200/mm3 for at least 6 months on antiretroviral therapy (ART), provided that the patient has responded well (70–73). In general, a daily maintenance regimen should be continued at half the induction dose. Although some small studies showed good efficacy of twice-weekly pyr-sulf or pyrimethamine-sulfadoxine therapy, a randomized open multicenter trial demonstrated worse outcomes, with higher relapse and mortality rates, for the twice-weekly regimen than for the daily regimen (69, 74, 75). A thrice-weekly regimen of pyr-sulf, however, seems to be comparable to the daily regimen (76), but a less-than-daily maintenance regimen is generally not recommended.
It is worth emphasizing that combination therapy with two antimicrobials should be used for the treatment of TE. While pyr-sulf combination therapy is the current gold standard for the treatment of acute TE in HIV patients, pyrimethamine-clindamycin and TMP-SMX also seem to be similarly effective; however, no regimen has been found to be superior to pyr-sulf. Pyrimethamine-clindamycin seemed to be somewhat less effective than pyr-sulf in preventing relapse during maintenance therapy in the European multicenter prospective study (51), although other, nonrandomized studies have shown both combination therapies to be equally effective at preventing relapses (38, 41). There is less experience with TMP-SMX, but the available literature suggests an efficacy comparable to that of pyr-sulf. Unfortunately, the pyr-sulf regimen is fraught with serious adverse effects. Pyrimethamine-clindamycin and TMP-SMX also have significant side effects but seem to be better tolerated. The choice of therapy should be guided by drug availability, tolerability, and the ability of patients to take medications enterally. For example, intravenous TMP-SMX may be more appropriate for patients with severe oral mucositis or compromised intestinal absorption, whereas patients with recent Clostridium difficile infection should avoid clindamycin-based therapy if possible. The cost of the drugs should also be taken into account when choosing the right therapy. This is especially true of pyrimethamine, as the price in the United States has skyrocketed 5,000% in recent years; the annual cost is now estimated to be around $645,000, thus rendering it unaffordable to many patients in the United States (3). TMP-SMX, on the other hand, is much less expensive and has been used successfully in many developing countries. Folinic acid should be used to supplement pyrimethamine-based regimens to minimize hematologic toxicity. The clinical experience with other regimens (atovaquone monotherapy, atovaquone with pyrimethamine or sulfadiazine, and pyrimethamine with azithromycin or clarithromycin) is limited, and these should be used only in the absence of alternatives. In particular, maintenance therapy with pyrimethamine plus azithromycin should be avoided due to a high relapse rate.
Other considerations for HIV-infected patients include the following.
(i) Role of ART.
The survival of HIV-infected patients with opportunistic infections has increased dramatically in the ART era; TE patients should thus start ART without delay (77). Immune reconstitution inflammatory syndrome (IRIS) is uncommon following initiation of ART for TE (78). Prior to ART, overall survival was poor despite optimal antimicrobial therapy (40). In the ART era, both mortality and relapse of TE have decreased (57, 76, 79–82). Surprisingly, a recent systemic review showed a higher relapse rate for TE in patients on TMP-SMX maintenance therapy in the ART era than in the pre-ART era (19.2% versus 14.9%) (83). This should, however, be interpreted cautiously due to the significant heterogeneity and small sample size of the patients studied. Other factors, such as poor compliance with medications, may also have accounted for this finding.
(ii) Role of steroids.
Though the beneficial effects of steroids are not clear, they can be considered if there is significant brain edema (40, 42, 84). Injudicious use of steroids, however, should be avoided, as this can be harmful (56).
(iii) PJP prophylaxis.
The treatment and prophylaxis of infection with Toxoplasma and Pneumocystis jirovecii overlap considerably. Pyr-sulf, TMP-SMX, and atovaquone-based regimens used in the treatment of Toxoplasma provide adequate coverage for P. jirovecii pneumonia (PJP), and separate PJP prophylaxis is not required (85, 86). Other regimens used in Toxoplasma prophylaxis, except for pyrimethamine plus dapsone, do not have reliable activity against PJP. Similarly, some regimens used for P. jirovecii prophylaxis or treatment (aerosolized pentamidine, clindamycin plus primaquine, and dapsone without pyrimethamine) do not confer adequate protection against toxoplasmosis.
HCT recipients.
Allogeneic hematopoietic cell transplant (allo-HCT) recipients account for more than 90% of toxoplasmosis cases in the HCT population and occur predominantly via reactivation of latent infection (87). Almost 90% of Toxoplasma disease in allogeneic HCT presents as CNS or disseminated disease and occurs within the first 6 months after HCT (87). Toxoplasma prophylaxis is thus recommended for pre-HCT-seropositive (pre-HCTSP) allo-HCT recipients during this high-risk period. Toxoplasma disease in allo-HCT is almost always fatal if not treated. Unfortunately, it can present with other concomitant infections, and the diagnosis can be delayed or missed, leading to a poor outcome (88). In fact, almost one-third of Toxoplasma cases in pre-HCTSP allo-HCT patients are diagnosed postmortem (87). Treatment should thus be started empirically in suspicious cases (e.g., pre-HCTSP patients presenting with typical brain lesions or diffuse ground-glass opacities on chest computed tomography, without an alternative explanation). Unlike that with HIV, the likelihood of diseases besides Toxoplasma (e.g., Aspergillus, Fusarium, Mucor, or Scedosporium infection or diseases with noninfectious etiologies, including malignancy) in HCT patients presenting with space-occupying lesions is reasonably higher. As treatments for these entities and their prognoses vary widely, immediate tissue diagnosis is preferred at the same time that empirical treatment for various pathogens (e.g., Toxoplasma, molds, and Nocardia) is instituted. If possible, peripheral blood should be obtained for Toxoplasma PCR prior to therapy: a positive test should trigger treatment initiation and may circumvent the need for more invasive tests. In fact, regular peripheral blood Toxoplasma PCR monitoring during the first 6 months posttransplant in pre-HCTSP allogeneic recipients can lead to early detection of Toxoplasma reactivation, before progression into end organ disease, and has been demonstrated to lower Toxoplasma-related mortality (87, 89, 90). A recent French study also showed a higher overall survival of allo-HCT recipients in centers that performed routine peripheral blood Toxoplasma PCR monitoring (91). This preemptive approach is particularly useful for patients who cannot tolerate Toxoplasma prophylaxis. Furthermore, compliance with prophylaxis cannot be guaranteed, and implementation of routine PCR monitoring in conjunction with prophylaxis can avert fatal toxoplasmosis (92).
There are no randomized trials of Toxoplasma treatment in HCT recipients. While a retrospective analysis of toxoplasmosis in HCT showed a marginally better survival with pyr-sulf combination therapy, that study did not factor in other variables (duration and timing of therapy, underlying disease, etc.) affecting mortality (93). Most of the reported cases were treated with pyr-sulf or pyrimethamine-clindamycin; some were treated with TMP-SMX (our unpublished data). In the absence of data for HCT patients, it is reasonable to treat toxoplasmosis in HCT with the same regimens as those used for HIV-infected patients (see above). Acute (primary) toxoplasmosis in allo-HCT patients comprises only 10% of cases and should be treated the same as reactivated disease. Maintenance therapy should be continued as long as the patient remains immunosuppressed.
SOT recipients.
Toxoplasmosis in solid organ transplant (SOT) recipients occurs via either primary infection from a Toxoplasma-seropositive donor or reactivation of latent infection in the recipient. Reactivation is less common in SOT recipients than in HCT patients, likely because of the lower immune suppression used in SOT recipients. Primary infection via ingestion of contaminated food and water can also occur. The group at highest risk is the group of donor-seropositive and recipient-seronegative (D+ R−) heart or heart-lung transplant patients (94–96), as Toxoplasma cysts have a predilection for cardiac muscle. Toxoplasmosis in SOT recipients usually occurs as disseminated or localized (CNS, pulmonary, or ocular) disease within the first few months after transplant, but late-onset disease months to years posttransplant, presumably from contaminated food and water (non-donor-derived disease), is not uncommon (97–99). The presenting signs and symptoms are generally nonspecific and include fever, pneumonitis, and neurologic symptoms (95, 96). Treatment should be started promptly, as delay in instituting effective therapy can result in a worse outcome (96). The treatment of toxoplasmosis (primary or reactivation) has not been standardized for this patient population, but treatment regimens should be similar to those used in AIDS-related TE. If feasible, immunosuppressive medications should be deescalated cautiously.
Nontransplant, non-HIV-infected immunocompromised patients.
Toxoplasmosis has been reported in patients with malignancy and other T-cell and B-cell immune deficiencies and likely occurs via reactivation of latent infection (91, 100–102). A recent restrospective multicenter French study of 180 PCR-positive toxoplasmosis cases in immunocompromised patients found that 14% of cases occurred in the nontransplant, non-HIV-infected group, especially in patients with hematologic malignancy and connective tissue disease on immunosuppressive medications (91). Like HCT and SOT recipients, this group should be treated similarly to HIV-infected patients with TE. The Study Group for Infections in Compromised Hosts of the European Society of Clinical Microbiology and Infectious Diseases recently published an elegant consensus document on the safety of targeted and biological therapies from an infectious disease perspective (103), including a review of “opportunistic infection” in patients under treatment with therapies that include proinflammatory cytokines, interleukins, immunoglobulins, and other soluble immune mediators, cell surface receptors and associated signaling pathways, intracellular signaling pathways, and lymphoma and leukemia cell surface antigens.
Ocular Toxoplasmosis
Immunocompetent nonpregnant patients.
Unlike TE, ocular toxoplasmosis (OT) can occur in immunocompetent hosts and commonly presents as posterior uveitis. Although many cases are a result of congenital toxoplasmosis (CT), the ocular manifestation may not be apparent until adolescence or early adulthood (104, 105). Postnatally acquired OT, on the other hand, usually manifests after age 40; similar to CT, chorioretinitis may not develop until months to years after initial infection (106, 107). Regardless of the mode of acquisition, OT is complicated by frequent recurrences in more than 50% of affected patients (106, 108, 109). These recurrences can occur despite treatment of acute episodes and can be vision threatening (110).
Ryan and colleagues first reported favorable outcomes for 25 of 29 patients with presumptive active OT treated with pyr-sulf; visual acuity improved in 58% of patients (111). Subsequent studies also showed beneficial effects of pyr-sulf, including earlier healing of lesions with or without steroids (112, 113). A prospective multicenter study evaluating the efficacy of different OT treatment regimens showed that only pyr-sulf was associated with a significant decrease in lesion size compared to that of the untreated group. This suggests a potential role in treating lesions near the optic disc or the fovea, although there was no difference in visual acuity or inflammation duration (114). In a double-blind randomized trial of pyrimethamine monotherapy versus placebo for the treatment of presumed OT, improvement was observed in 77% of the treatment arm patients versus 50% of the placebo arm patients (98 patients in total; P < 0.02) (115). However, “improvement” was not well defined, and it was not clear whether visual acuity actually improved. Moreover, the difference was less apparent when only the acute OT cases were analyzed. Additionally, lesion size and severity were not described, thus making it difficult to judge the true efficacy of pyrimethamine monotherapy. In a double-blind randomized therapeutic trial of pyrimethamine-trisulfapyridine plus steroid versus steroid, all 20 patients with active recurrent OT improved, without any difference in the time to lesion resolution. One patient in each group had a recurrence over a 2-year period (116). While these findings suggest a lack of efficacy of antimicrobial treatment, it should be noted that this small study did not take into account important variables, such as disease severity, anatomical site, and lesion duration; the control group also did not have a true placebo (117). Both of these double-blind randomized trials suffer from methodological flaws and thus cannot prove or refute the efficacy of treatment.
Little is known about the therapeutic role of systemic clindamycin therapy (118, 119). In a prospective study that compared the therapeutic efficacies of pyr-sulf plus steroids, clindamycin-sulfadiazine plus steroids, and TMP-SMX plus steroids versus an untreated control group, no regimen impacted visual acuity, the duration of inflammation, or lesion recurrence (114). Intravitreal clindamycin with steroids, on the other hand, seems to hold a promising role in OT treatment (120–123). Two randomized trials demonstrated similar efficacies of intravitreal clindamycin plus steroids and pyr-sulf plus steroids in terms of improvement in visual acuity and reductions of lesion size and vitreal inflammation at specified intervals (124, 125). Overall, 83% of patients had an improvement in visual acuity, and there was no significant difference in rates of recurrence in both arms over a 2-year period. Most patients in the intravitreal arm required only one injection; no major complications were reported for intravitreal therapy, other than subconjunctival hemorrhage in 9% of cases (124). The results of these randomized trials, along with other reported cases, support the safety and efficacy of intravitreal clindamycin with steroids as an alternative treatment regimen. However, there was diagnostic uncertainty in one of the randomized trials, as only 50% patients had Toxoplasma IgG antibodies, whereas the other half had only Toxoplasma IgM antibodies (125). This is important because the presence of Toxoplasma IgM antibodies alone, without the presence of IgG antibodies, may represent a false-positive result (126). Additionally, the lack of difference in outcome for patients with Toxoplasma IgG and IgM antibodies casts doubt about the true efficacy of therapy.
In a randomized trial of steroids plus either TMP-SMX or pyr-sulf involving 59 patients with OT, all patients improved within 6 weeks. No difference in improvement of visual acuity, time to best visual acuity, lesion size decrease, vitreous inflammation reduction, recurrence rate, time to recurrence, or adverse effects was observed between treatment arms (127). In a prior comparative study of various treatment regimens, there were no beneficial effects of TMP-SMX, perhaps due to a lower TMP-SMX dose and duration (114). There are anecdotal reports of successful treatment with intravitreal TMP-SMX and intravitreal steroids as well, but more data regarding safety and efficacy are required (128–130).
There are very few data on the clinical utility of atovaquone in the treatment of OT. All 17 patients in a small study had a favorable response within 3 weeks of treatment; atovaquone efficacy could not be determined, however, as there was no placebo arm (131). It is better tolerated than pyr-sulf and seems to prolong the time to reactivation (109).
Azithromycin at 250 to 500 mg per day seems to be better tolerated in OT treatment. However, the time to lesion resolution appears to be longer at doses of 500 mg/day than that with pyr-sulf (132, 133). Combination therapy with pyrimethamine seems to increase efficacy even at a low dose of 250 mg azithromycin per day. In a prospective randomized open-label study of pyr-sulf plus steroids versus pyrimethamine plus azithromycin plus steroids in 46 patients with sight-threatening OT, visual acuity improved in nearly all patients after about 4 weeks of treatment (108). No difference in visual acuity improvement, inflammation duration, or change in lesion size was noted between the two groups. The 1-year recurrence rate was 33% for the pyrimethamine-plus-azithromycin group versus 56% for the pyr-sulf group, but the difference was not statistically significant. Adverse effects were, however, more commonly reported in the pyr-sulf group. Similarly, a recent small, randomized trial of 27 patients showed equivalent efficacies of oral azithromycin (500 mg followed by 250 mg daily) plus steroids and oral TMP-SMX (160/800 mg twice daily) plus steroids in decreasing retinal lesion size, clearing vitreous inflammation, and improving visual acuity after 6 to 12 weeks of treatment (134).
Despite the apparent beneficial effects of treatment seen in the above-mentioned studies, there is no definite proof that treatment improves the outcome. Since OT can resolve spontaneously, longitudinal observational or retrospective studies cannot prove treatment efficacy without a well-designed placebo control arm. Even randomized trials comparing two different therapeutic regimens cannot establish efficacy if the regimens appear to be equally efficacious. Unfortunately, the only two randomized double-blind trials that compared antimicrobial treatment to either a placebo or steroids for the treatment of acute OT, conducted more than half a century ago, suffered from methodological flaws and could not establish treatment efficacy (135). Hence, there is no consensus among experts on the beneficial effects of treatment and on the choice of antimicrobials (136). A 2011 report by the American Academy of Ophthalmology concluded that there is no level I evidence to support the routine use of antimicrobials in acute OT treatment in immunocompetent patients (137). A recent Cochrane review of randomized trials of antimicrobial treatment versus placebo or no treatment also did not find evidence supporting visual acuity improvement with treatment (138). Nonetheless, many uveitis experts treat lesions that are vision threatening or have significant inflammation because of fear of vision loss (136). If a decision is made to treat, pyr-sulf plus folinic acid or TMP-SMX or intravitreal clindamycin should be chosen based on their efficacy in several studies (60). There is less experience with other regimens (atovaquone or azithromycin, with or without pyrimethamine), and these should be used as alternatives.
On the other hand, suppressive therapy following standard treatment of an acute or recurrent chorioretinitis episode has proven to be effective at preventing recurrences. In a retrospective study of 352 patients who underwent secondary prophylaxis with a biweekly regimen of pyrimethamine and sulfadoxine for 6 months after an intensive 21-day treatment for active chorioretinitis, the probability of a 3-year recurrence-free period (long after prophylaxis was stopped) was 90% (139). In an open-label randomized study by Silveira et al., long-term intermittent suppressive treatment with TMP-SMX (160/800 mg every 3 days for up to 20 months) was found to decrease the recurrence rate compared to that in the no-treatment arm (7 versus 24%) (140). A recent randomized trial of this intermittent suppressive strategy with TMP-SMX for 12 months also demonstrated its efficacy in reducing recurrences compared to that of placebo. The recurrence risk over 12 months was 0% in the TMP-SMX arm versus 13% in the placebo arm (P = 0.03) (141). A larger follow-up study conducted by the same group randomized 141 patients after treatment for an acute episode to receive TMP-SMX every 2 days versus placebo for 1 year; this confirmed the efficacy of preventive treatment for up to 2 years after the treatment was stopped (recurrence rate at 3 years of 0 versus 20.3%) (142). The exact duration of protection after stopping preventive therapy is not known, since a long-term follow-up of a previous study by Silveira et al. showed loss of protection after 10 years (107). Nonetheless, the 1-year preventive strategy is justified for patients presenting with sight-threatening chorioretinal lesions near the macula or optic disc or patients with frequent severe recurrences (143).
Immunocompromised patients.
OT in the immunocompromised patient should always be treated, ideally with systemic therapy (pyr-sulf or pyrimethamine-clindamycin with folinic acid or TMP-SMX), and disseminated infection should be ruled out.
Role of steroids.
Steroids (systemic or intravitreal) are used in the treatment of OT, although their role is not clear (137, 144). Steriods were initially used to treat OT, as it was once thought to be a hypersensitivity reaction (145). It later became clear that steroids alone, without concomitant antimicrobials, can have detrimental effects on vision, including development of endophthalmitis (146, 147). Monotherapy with systemic steroids is also associated with a higher risk of recurrence (110, 148). Judicious use of steroids along with antimicrobial therapy for severe inflammation or when the lesions are near the fovea or optic disc may suppress the inflammation and can be beneficial, but excessive doses can yield a suboptimal response. While the optimal dose is not established, most experts use 0.5 to 1 mg/kg/day. Steroids are generally started a few days after initiation of antimicrobial therapy and continued for about 1 month, with gradual tapering (109, 136). It should be acknowledged that the evidence for adjuvant steroids is not based on high-quality data (149), and steroids must not be used without concomitant antimicrobial therapy, as this can lead to a complete loss of vision.
Congenital Toxoplasmosis
Treatment of toxoplasmosis during pregnancy.
Pregnant women with previously acquired latent Toxoplasma infection and no immunocompromising conditions do not experience systemic or CNS reactivation of Toxoplasma and hence do not transmit infection to the offspring (150). Isolated ocular reactivation can occur, but materno-fetal transmission in the absence of an underlying immunocompromised condition is unlikely even without anti-Toxoplasma therapy (151–153). On the other hand, primary infection during pregnancy or around the time of conception can lead to fetal infection (154). The severity of congenital toxoplasmosis (CT) ranges from asymptomatic infection to severe neurologic diseases and even fetal demise. Several studies have documented an increased risk of materno-fetal transmission with increasing gestational age at the time of maternal infection, but clinical severity of CT is less pronounced when the infection is acquired later in pregnancy (150, 155–163). In a study of 603 confirmed primary Toxoplasma infections during pregnancy, the overall risk of materno-fetal transmission was found to be 29%; the risk increased from 6% at 13 gestational weeks to 72% at 36 gestational weeks. The rate of clinical signs of infection before 3 years of age among patients with CT decreased from 61% for infections acquired at 13 weeks of gestation to 9% for infections acquired at 36 weeks of gestation. Because of the opposing effects of gestational age on risk of transmission and CT severity, the overall risk of having symptomatic CT is therefore highest when maternal infection occurs between 24 and 30 weeks of gestation and is estimated to be around 10% (156).
Because of the risk of developing CT after a primary maternal infection, antenatal treatment is generally offered to prevent vertical transmission and/or to decrease the severity of infection in case the fetus is infected. Table 3 provides an overview of drug regimens used for the treatment of toxoplasmosis in pregnant women. An early study demonstrated efficacy of antenatal treatment with spiramycin in reducing CT, with 22% of patients in the spiramycin-treated group developing definite CT versus 45% of those in the group that did not receive treatment (150). Other studies have also demonstrated a favorable outcome with antenatal treatment, with some studies especially supporting early initiation of therapy (164–166).
TABLE 3.
Treatment of acute toxoplasmosis in pregnant women and newborns
| Infection stage | Regimen | Comments |
|---|---|---|
| Maternal infection at <14 weeks of gestation, no fetal infection | Spiramycin (1 g [3 million units] every 8 h until delivery) | Spiramycin is not effective for treating established fetal infection and hence should be used only for prevention of vertical transmission |
| Amniocentesis and fetal ultrasound should be performed when feasible to rule out fetal infection | ||
| Maternal infection at >14 weeks of gestationa | Pyrimethamine (100 mg daily for 2 days and then 50 mg daily) plus sulfadiazine (1 g q8h [body wt of <80 kg] or 1 g q6h [body wt of ≥80 kg]) plus folinic acid (10–20 mg daily pending fetal USG and amniocentesis) | Pyrimethamine is teratogenic and should not be used in early pregnancy |
| If fetus is confirmed to be infected (abnormal USG and/or positive amniotic fluid PCR), continue pyrimethamine plus sulfadiazine plus folinic acid until delivery | Serial fetal USG and amniotic fluid PCR should be performed at 18 weeks of gestation | |
| If fetus is not infected (e.g., negative USG and amniotic fluid PCR), pyrimethamine plus sulfadiazine plus folinic acid may be switched to spiramycin | ||
| Alternatively, pyrimethamine plus sulfadiazine plus folinic acid can be continued until delivery or alternated with spiramycin on a monthly basis | ||
| Congenital infection in newborns | Pyrimethamine (1 mg/kg q12h for 2 days and then 1 mg/kg/day for 2–6 mo and then 1 mg/kg/day 3 times a week) plus sulfadiazine (50 mg/kg q12h) plus folinic acid (10 mg 3 times a week) | Treatment should be started as soon as feasible after birth and continued for at least 1 year |
The 14-week cutoff period for starting pyrimethamine and sulfadiazine in pregnant women may vary in different countries.
However, studies published between 1999 and 2006 cast doubt over the efficacy of antenatal treatment in reducing the incidence and severity of CT (167, 168). Nevertheless, studies published since 2007 have consistently supported the efficacy of antenatal treatment. The main two factors leading to the misleading conclusions in the 1999-2006 studies have to do with excluding severe cases and fetal demises and the fact that spiramycin is likely to paradoxically increase the number of infected babies that are born alive with mild infection who would have otherwise been born with severe disease or dead. Furthermore, heterogeneous populations were studied in the 1999-2006 studies, and the interpretations were biased due to a poor knowledge of CT pathogenesis.
(i) Studies from 1999 to 2006.
In a study of 144 pregnant women with maternal infection, antenatal treatment did not appear to impact transmission, but neurologic sequelae were less common among those treated with either spiramycin or pyr-sulf (169). In a large European CT study involving 11 centers, antenatal spiramycin or pyr-sulf treatment did not prevent vertical transmission irrespective of the timing of antimicrobial initiation (157). In another European study involving 13 centers, prenatal treatment within 4 weeks of seroconversion reduced clinical signs, but there was no difference between pyr-sulf and spiramycin (170).
In another European multicenter study of primary infection in pregnancy between 1987 and 1995, neither materno-fetal transmission nor clinical manifestations in children with CT were consistently lower in centers with more intensive antenatal treatment protocols (158). In a separate analysis of 554 pregnant women with primary infection from a French cohort, early initiation (within 4 weeks of seroconversion) or the choice of antimicrobial therapy (spiramycin or pyr-sulf) also did not impact the transmission risk (158). When the analysis was restricted to 181 live-born children with CT, the study failed to demonstrate a beneficial effect of antenatal pyr-sulf or spiramycin treatment on clinical manifestations, irrespective of treatment timing (171). Unfortunately, the data from these studies were erroneously interpreted as evidence for a lack of efficacy of antenatal treatment. Since 2007, several studies from different cohorts and countries have consistently shown that antenatal treatment is associated with lower transmission rates and severity (159–163, 172).
(ii) Studies from 2007 to the present.
In a 2007 meta-analysis of European studies, earlier antenatal treatment seemed to decrease vertical transmission but had no effect on clinical manifestations in those born with CT; this analysis excluded severe cases and fetal demises (163). A retrospective study of 300 newborns with CT showed that a delay in antenatal treatment increased the risk of OT before 2 years of age, thus indirectly supporting the efficacy of antenatal treatment (172). Hotop and colleagues also demonstrated late treatment (more than 8 weeks after maternal seroconversion) as a risk factor for symptomatic CT (159). A follow-up of a 14-center European CT study that included severe CT cases (unlike previous studies, in which severe CT cases were excluded) showed that antenatal treatment significantly decreased the risk of severe neurologic sequelae or death after adjusting for gestational age; the highest efficacy was seen in the first trimester. Pyr-sulf was not found to be more effective than spiramycin, perhaps because of a limited power to detect the difference (161). In an updated report of the findings on the impact of antenatal treatment from 1985 through 2008 from France, the authors demonstrated a decline in vertical transmission of Toxoplasma infection after mandatory monthly Toxoplasma serological screening was introduced in 1992 (160). This likely resulted from earlier initiation of anti-Toxoplasma therapy, since maternal infection was also diagnosed earlier. Moreover, among newborns with CT that could not be prevented, there were improved clinical outcomes after implementation of PCR for screening of fetal infection in 1995. This may have led to earlier diagnosis of fetal infection and initiation of anti-Toxoplasma therapy (160). This landmark study demonstrated the usefulness of maternal screening for preventing CT and improving CT outcomes (among infected newborns) by routine antenatal diagnosis via amniotic fluid PCR testing. An Austrian study also demonstrated a 6-fold decrease in CT among babies born to mothers who received appropriate antenatal treatment versus no treatment (162). A recent randomized, open-label trial in 36 French centers, comparing pyr-sulf plus folinic acid to spiramycin following seroconversion in pregnant women, revealed a lower rate of placental transmission with pyr-sulf than that with spiramycin; the study showed a trend toward lower transmission rates but failed to show statistical significance, since only 143 women were enrolled between November 2010 and January 2014 (173). The difference was strongest when treatment was started within 3 weeks after seroconversion, and the incidence of abnormal ultrasound findings was significantly lower for patients receiving pyr-sulf than for those receiving spiramycin (173).
Thus, despite conflicting results observed in earlier studies, recent long-term data favor antimicrobial therapy to prevent vertical transmission and to decrease the occurrence of neurologic sequelae in Toxoplasma-infected fetuses (174). If maternal infection occurs before 14 weeks of gestation, spiramycin should be started to prevent fetal infection. It should be pointed out that this 14-week limitation may be slightly different in other countries. Amniocentesis for PCR analysis of amniotic fluid and ultrasonography (USG) should be done as soon as feasible to determine whether the fetus has been infected. If fetal infection is confirmed or suspected, spiramycin should be switched to pyr-sulf: although pyrimethamine is teratogenic and should be avoided in early pregnancy, spiramycin can only prevent fetal infection and is ineffective at treating established infection. If maternal infection occurs after 14 weeks of gestation, pyr-sulf with folinic acid should be started empirically pending fetal USG and amniocentesis because of the higher likelihood of fetal infection with increasing gestational age at the time of maternal infection. Pyr-sulf with folinic acid should be continued until delivery for cases of confirmed or suspected fetal infection. If fetal infection is ruled out, pyr-sulf may be switched to spiramycin or continued throughout pregnancy. The role of other anti-Toxoplasma therapies (atovaquone, pyrimethamine-clindamycin, pyrimethamine-azithromycin, etc.) in pregnancy is not established, and these should not be used. TMP-SMX is not validated for use in pregnancy but seems to be well tolerated and may be effective at preventing CT (175). In one study, the combination of spiramycin and TMP-SMX was found to decrease vertical transmission compared to that with spiramycin alone, although there was no significant difference compared to that with pyr-sulf (176). TMP-SMX can also cause kernicterus in the newborn, especially when used during late pregnancy. At present, more data on its safety and efficacy are required before TMP-SMX is routinely used during pregnancy.
Treatment of congenital toxoplasmosis in newborns.
The severity of CT varies from asymptomatic presentation at birth to severe neurologic disease and even neonatal death. In some countries, such as France, where pregnant women are universally screened for primary Toxoplasma infection (with monthly Toxoplasma serology) and offered antenatal treatment upon evidence of primary infection, most CT cases present without any apparent signs or symptoms and generally have a good prognosis if anti-Toxoplasma therapy is started after birth (177, 178). Clinical manifestations are likely to be prominent in those whose mothers were not diagnosed or treated antenatally for primary toxoplasmosis. Screening for congenital infection by serology, cranial imaging, and ophthalmic examination should be done for all newborns born to mothers with primary maternal infection to detect asymptomatic cases. This is important because even newborns with no signs or symptoms of clinical disease can later develop clinical manifestations, including significant sequelae, especially in the absence of treatment (104, 105, 179). Postnatal treatment has also been found to decrease both the development of new signs and symptoms and the clinical worsening of existing signs and symptoms (179–181). In a large CT outcome study between 1981 and 2004, 120 newborns with CT were treated with pyr-sulf for 1 year and monitored prospectively for clinical outcomes. Even among children with severe presentations at birth, 80% had normal motor function, 73% had an IQ of >70, 64% did not develop new eye lesions, and none developed sensorineural hearing loss. The neurologic outcomes for treated children were found to be significantly better than those for historical controls with no or suboptimal treatment (182).
Since most children with CT appear to have a good overall outcome with normal neurologic development with treatment (even among infants presenting with severe CNS involvement, such as microcephaly and hydrocephalus), treatment should be started as soon as feasible after birth in neonates with CT, including asymptomatic patients (183). Short courses of therapy and delay in initiating therapy can lead to severe disabilities. The latter scenario is particular relevant when presenting signs of CT are overlooked or when the infant with CT is asymptomatic at birth but develops signs and symptoms a few weeks later. The treatment of choice is pyr-sulf plus folinic acid. Pyrimethamine-sulfadoxine plus folinic acid is also used in some places and seems to be well tolerated (184). Spiramycin does not treat established infection and should not be used. The role of other medications is not clear. Treatment should be continued for at least 1 year, with patients monitored clinically and serologically for treatment failure. Since some clinical manifestations, especially eye lesions, may not manifest until later in life, long-term follow-up is required.
PREVENTION OF TOXOPLASMOSIS IN HUMANS
Prevention of Primary Infection in High-Risk Populations
Everyone, including immunocompetent patients, should be educated about toxoplasmosis risk factors and ways to minimize the risks. Various intervention strategies to reduce the burden of T. gondii infection were recently reviewed, with a focus on CT and protection of the general population (185). In particular, ingestion of raw meat, oysters, clams, and mussels should be avoided, and vegetables should be washed properly before consumption. Cat feces may contain Toxoplasma oocysts, and high-risk individuals (e.g., seronegative pregnant women and transplant recipients) should not handle cat litter boxes, if possible. As oocysts take 1 to 2 days to become infectious, cat feces should be disposed of daily. Protective gloves should be worn, followed by handwashing, while disposing of cat feces and gardening. In addition, prophylaxis should be employed in pretransplant Toxoplasma serology-negative SOT recipients who receive grafts from seropositive donors (D+ R−) for at least 6 months after the transplant (94, 96, 186).
Special considerations in pregnancy.
Since at least 50% of pregnant women with acute toxoplasmosis do not have risk factors or illness consistent with acute toxoplasmosis, counseling women to avoid risk factors cannot completely prevent acquisition of disease (187). A recent paper from Austria also demonstrated that only 28% of infections acquired during pregnancy could be avoided by strict adherence to preventive strategies, such as avoiding ingestion of raw meat. This suggests that there are other, unknown means by which acute infection can occur (188). Systematic monthly antenatal screening of Toxoplasma-seronegative pregnant women can identify recent infections and allow prompt therapy to prevent CT and, in those infected, severe sequelae and death. Such an antenatal screening program has been implemented in France, where CT is less severe than that in the United States, where such a screening program does not exist (178). A mathematical model found the implementation of universal maternal screening in the United States to be cost-effective, but its impact diminished when the incidence of CT also decreased unless the cost of serological testing was also adjusted (189). A recent European paper also demonstrates the cost-effectiveness of universal maternal screening (190).
Prevention of Reactivation in Immunocompromised Patients
Latent infection can reactivate in patients with HIV and in allo-HCT recipients. In Toxoplasma-seropositive HIV-infected patients, prophylaxis is started when the CD4 count drops below 100/mm3 and stopped when the CD4 count remains above 200/mm3 for at least 6 months on ART (70) (discussed in detail above). In pre-HCTSP allo-HCT recipients, prophylaxis is recommended for at least 6 months after transplant or while the patient remains significantly immunosuppressed (87). Preemptive treatment with serial monitoring for Toxoplasma DNA in blood, in lieu of prophylaxis (in those who cannot tolerate prophylaxis) or as an adjunct to prophylaxis, is also an option. Secondary prophylaxis (maintenance treatment) should be continued as long as the patient remains immunosuppressed (for HIV, see the above discussion of TE) (87). While reactivation is less common in pretransplant Toxoplasma-seropositive SOT recipients, they should nonetheless be considered for prophylaxis or preemptive therapy following treatment for an acute allograft rejection.
TREATMENT OF EXPERIMENTAL TOXOPLASMOSIS IN ANIMAL MODELS
Animal models have frequently been used to investigate the therapeutic effects and safety of drugs against T. gondii infection. In most cases, these animal models have been employed to address efficacy and safety during the different stages (acute, latent, and reactivation) of T. gondii infection based on results obtained in prior in vitro studies. Many of these studies have been instrumental in establishing currently available therapies.
To our knowledge, reviews of antiparasitic drugs for the treatment of T. gondii infection in animal models are scarce and were published 2 decades ago (4, 191). Although in most cases animal studies are not directly applicable to humans, most show marked similarities between mammals and humans with regard to drug potency. Here we review studies in animal models with a focus on antiparasitic drugs that are in clinical practice.
Laboratory animals, including mice, rats, rabbits, pigs, and nonhuman primates (192–194), are most often used to study the efficacy of drugs against T. gondii infection. However, the type of laboratory animal plays a decisive role in the outcome of infection; the reader is referred to recent review articles for more detailed perspectives (195, 196). Mice are most commonly used to study the efficiency of antiparasitic drugs. While murine models have many advantages, extrapolating results to other species must be done with care (197). Depending on the strain, mice are greatly susceptible to T. gondii infection, and their use in furthering our understanding may not be ideal for all clinical presentations. For instance, fetal infection can occur in successive murine pregnancies. This is not the case in rats or sheep, which are more resistant to the disease and may therefore be more relevant to studying treatment of human CT (197–199). Furthermore, the mouse strain (195, 196), parasite strain (virulence and lethality versus nonlethality [200]), parasite inoculum, and route of infection (oral versus intraperitoneal [201]) determine the course of infection (195, 196). Coinfections with other microorganisms have also been studied in animal models to mimic this scenario in immunocompromised hosts (202).
The natural course of human infection is characterized by oral infection with oocysts or tissue cysts; these convert into the rapidly replicating tachyzoites during acute infection. Importantly, the vast majority of currently used antiparasitic drugs demonstrate efficacy only against the tachyzoite stage, not against the latent stage, which is characterized by the presence of bradyzoites and tissue cysts. Therefore, most antiparasitic drugs investigated allow control of the acute phase of infection but are not able to clear parasites contained in tissue cysts from the host in the latent stage to establish sterile immunity. Successful treatment and prevention of reactivation of latent infection therefore continue to be unmet medical needs in immunocompromised patients.
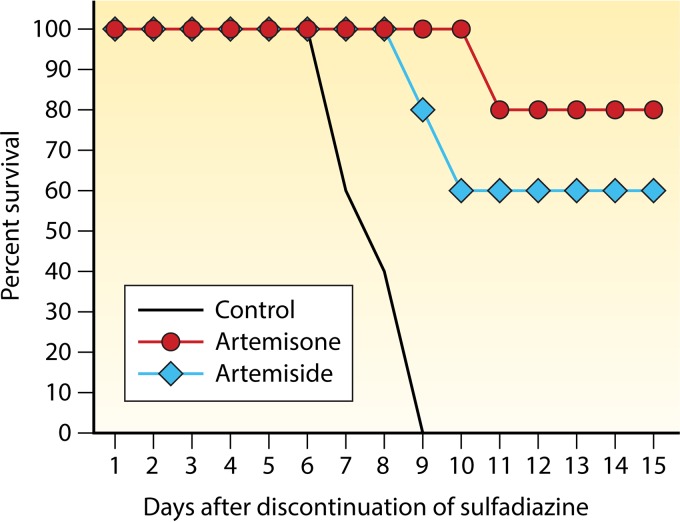
Animal models of infection allow determination of the efficacy of antiparasitic drugs by applying diverse readouts; these include survival of infected animals (Fig. 3), histopathological changes in affected organs, and/or tachyzoite or cyst loads in various organs, determined using staining techniques, PCR, or subinoculation of tissue into naive mice or cell cultures (203).
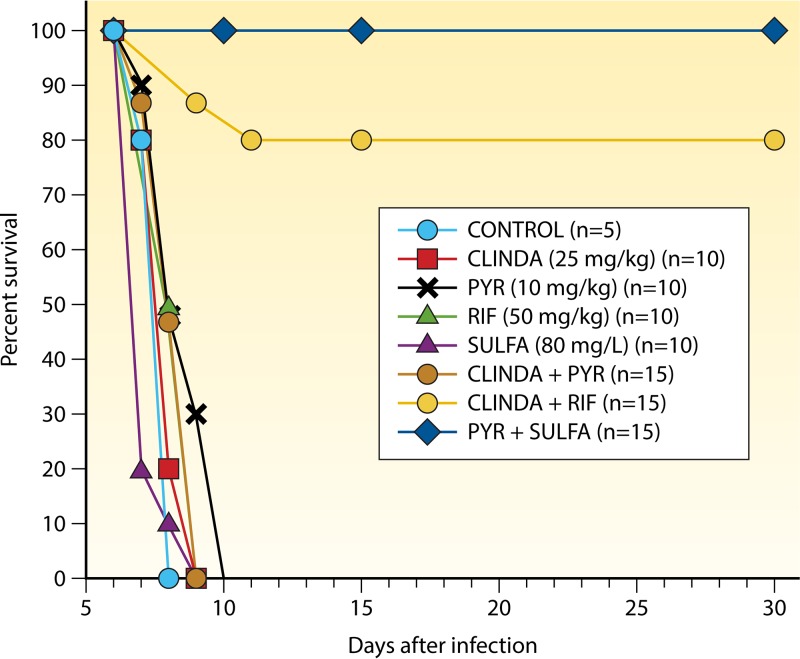
FIG 3.
Survival of T. gondii-infected mice treated with drug combinations. The graph shows the survival of infected mice treated with the combination of pyrimethamine plus sulfadiazine compared to that of mice treated with monotherapy with either drug alone or with other treatment regimens (4). CLINDA, clindamycin; PYR, pyrimethamine; RIF, rifampin; SULFA, sulfadiazine.
Drugs for Treatment of Acute T. gondii Infection in Mammals
Pyrimethamine and sulfadiazine.
Eyles and Coleman (204) showed in 1952 that the antimalarial drug pyrimethamine, which inhibits the folate synthetic pathway, protected mice against toxoplasmosis. Following reports of successful treatment in humans (205, 206), the efficacy of pyrimethamine monotherapy was also demonstrated in mice, rats, and rabbits (207–209). Combination therapy using pyrimethamine plus sulfonamides was also shown to be successful by Beverley and Fry (17), and subsequently by Piketty et al. (203). Folinic acid was added to the combination of pyr-sulf to mitigate the hematological side effects of pyrimethamine (210, 211). In the 1970s, the potencies of multiple sulfonamide-containing drug combinations were compared during both stages of infection in mice (212). The parasitic cure rate (indicated by the failure of infected brain from surviving mice to initiate infection when injected into naive mice) was higher for mice treated with pyrimethamine plus sulfamethoxypyridazine (92%) than for those receiving clindamycin plus sulfamethoxypyridazine (75%). Khan and Araujo (4) later demonstrated the superior efficacy of the combination of pyr-sulf over those of other treatment regimens in mice (Fig. 3). These studies performed decades ago provided the basis for the current recommendation for the combination of pyr-sulf plus folinic acid as first-line treatment of toxoplasmosis in humans.
Pyrimethamine was also studied in combination with other drugs. The therapeutic effect of dapsone alone or combined with pyrimethamine was highlighted in a murine acute toxoplasmosis model (213). Dapsone plus pyrimethamine, but not dapsone alone, cleared parasites from blood and organs. Starting treatment early, right after infection, resulted in survival of 100% of mice, without relapse, suggesting a great efficacy of the drug combination in preventing infection.
Trimethoprim-sulfamethoxazole.
The efficacy of sulfonamides against toxoplasmosis was demonstrated in animal models as early as 1942 (13, 214). While treatment with trimethoprim alone was unsuccessful in mice infected with the virulent type I RH strain via intraperitoneal inoculation, sulfamethoxazole, sulfadiazine, and pyrimethamine demonstrated marked therapeutic effects. The therapeutic effect of sulfamethoxazole was increased when it was used in combination with trimethoprim. Treatment with TMP-SMX was found to reduce the level of cysts and to inhibit anti-T. gondii-antibody production (215, 216). However, the TMP-SMX combination was inferior to the pyr-sulf combination (217).
Spiramycin.
Spiramycin has been studied extensively in animal models due to its ability to prevent human CT. Unlike pyrimethamine and sulfonamides, it has a favorable safety profile, achieving a significant placental concentration. However, spiramycin was less efficacious than the combination of pyrimethamine and sulfonamides in reducing mortality or parasite loads with the RH strain (215, 218). Elsewhere, spiramycin demonstrated marked antiparasitic activity in a murine model of peroral infection (219): treatment was characterized by elevated protection and a reduced brain cyst burden months after infection. More recently, the coadministration of spiramycin with metronidazole was also found to significantly reduce the number of intracerebral T. gondii cysts due to increased spiramycin brain penetration (220).
Clindamycin.
In contrast to the lack of in vitro efficacy of clindamycin demonstrated in early reports, lincomycin potency was demonstrated in mice acutely infected with T. gondii (22, 221, 222). The efficacy of prolonged clindamycin treatment during chronic infection was shown by parasite clearance from the blood, spleen, and liver (223), and clindamycin prevented death from TE in intracerebrally infected mice (224). Histological characteristics of cerebral infection, such as large numbers of cysts and inflammatory infiltrates with tissue necrosis, were not present in the CNS of clindamycin-treated mice. Combination therapy with clindamycin plus atovaquone during acute toxoplasmosis in mice appears to be superior to monotherapy with either drug, based on increased survival and elimination of parasites in some mice (225).
Atovaquone.
Atovaquone, formerly known as 566C80, is a hydroxynaphthoquinone derivative and has in vivo activity against T. gondii during both infection stages (226, 227). Romand et al. (228) reported that the combination of atovaquone with clarithromycin, pyrimethamine, or sulfadiazine compared to monotherapy with any of these drugs resulted in a higher survival rate. However, the parasite burdens in blood and various organs were not significantly decreased in the combination groups. A marked improvement in efficacy was demonstrated by combining atovaquone with either sulfadiazine or pyrimethamine in murine models of intraperitoneal infection with rapidly multiplying tachyzoites or oral infection with cysts (229). The combination of atovaquone plus clindamycin also showed antiparasitic potency in models of acute and chronic murine infection, as demonstrated by significantly decreased cyst burdens compared to those with monotherapy with either drug (230).
Drugs with activity against T. gondii cysts.
The development of anti-T. gondii drugs with activity against cysts during the latent stage of infection is highly desirable to lower the cyst burden in the host, and possibly even eradicate the parasite from the host to establish sterile immunity. Since the vast majority of drugs currently used to treat toxoplasmosis in humans do not demonstrate such activity, eradication of the parasite from the host has not been achieved. Several groups have reported a reduction of cyst numbers in mouse models of acute or latent infection by use of, among others, the anticoccidial drug ponazuril (231, 232), artemisinin derivates (233), and 2-hydroxy-3-(1-propen-3-phenyl)-1,4-naphthoquinone (PHNQ6) alone or combined with sulfadiazine (234). In an elegant series of in vitro and in vivo experiments, the endochin-like quinolone ELQ-316 demonstrated a favorable median effective dose (ED50) compared to that of atovaquone when administered orally to acutely infected mice; furthermore, ELQ-316 at 25 mg/kg reduced the cyst burden significantly compared to that with atovaquone (88% versus 44% reduction) after 16 days of treatment, without showing overt toxicity in mice (235). Guanabenz, an FDA-approved drug for the treatment of hypertension, protected mice against lethal acute infection and also significantly reduced the number of brain cysts in chronically infected mice due to its excellent passage through the blood-brain barrier (BBB) (236). Guanabenz may potentially be repurposed in humans as an effective antiparasitic drug with a unique ability to reduce tissue cysts in the brain. Similarly, the maximum concentration (Cmax) of spiramycin in the brain increased more than 70% when spiramycin was coadministered with metronidazole (which lacks efficacy against toxoplasmosis); this regimen resulted in a 10-fold reduction in brain cysts during latent infection compared to the level with spiramycin treatment alone (220). Recently, inhibitors of the calcium-dependent protein kinase 1 were found to penetrate the CNS and significantly reduced the number of brain cysts in acute and latent mouse models of T. gondii infection (237). Most importantly, treatment of gamma interferon (IFN-γ) receptor 1-deficient mice, which are normally extremely susceptible to infection, not only prolonged survival after reactivation but also cured some of the animals (238). It was hypothesized that interfering with tachyzoite invasion and egress contributed to the results, but a blockage of bradyzoite invasion in vitro suggested a direct effect on cyst formation and turnover (238).
Immunotherapy.
There are several reports on immunotherapy in the field of toxoplasmosis. While immunotherapy nowadays often denotes blockage of checkpoint inhibitors, including CTLA-4 as well as PD-1 in cancer and sepsis (239), its use in the form of serum and immune modulators to boost the efficacy of anti-T. gondii drugs has previously been evaluated. As early as 1977, Werner et al. (240) reported a greater reduction of cysts in the CNS following treatment with anti-Toxoplasma serum combined with sulfonamide plus pyrimethamine than after treatment with sulfonamide plus pyrimethamine alone. In another study, more than 40% of mice receiving combination therapy with azithromycin plus IFN-γ survived, whereas azithromycin monotherapy protected less than 10% of mice and IFN-γ alone did not protect against a lethal dose of the parasite (241). Similarly, administration of interleukin 12 (IL-12) beginning 24 h before infection, followed by therapy with atovaquone or clindamycin, significantly increased the survival of mice compared to that of controls treated with either drug or IL-12 alone (242). These data support the efficacy of immunotherapy in combination with anti-Toxoplasma drugs.
Animal Models of Coinfection
Coinfections with opportunistic pathogens, including Pneumocystis jirovecii, T. gondii, and members of the Mycobacterium avium complex, were frequently observed in AIDS patients before the introduction of ART (243). To investigate the efficacy of primary and secondary prophylaxis for opportunistic infections, coinfection models were developed in rats (202, 244). Following corticosteroid-induced immunosuppression, treatment with TMP-SMX or with pyrimethamine plus dapsone simultaneously cleared T. gondii and prevented P. jirovecii pneumonia. Roxithromycin monotherapy protected against T. gondii but not P. jirovecii infection, and atovaquone showed partial efficacy against pneumocystosis and toxoplasmosis (202, 244). In separate studies, three folate reductase inhibitors, PS-15, epiroprim, and pyrimethamine, were also tested alone or in combination with dapsone. Monotherapy with PS-15 showed efficacy against P. jirovecii but not against T. gondii, while combination therapy with PS-15 plus dapsone was highly effective against T. gondii and P. jirovecii (202). The combination of dapsone with epiroprim and pyrimethamine also prevented pneumocystosis and toxoplasmosis (202). Combination therapies of clarithromycin plus sulfamethoxazole or atovaquone, roxithromycin plus sulfamethoxazole or dapsone, and rifabutin plus atovaquone all demonstrated efficacy against T. gondii, P. jirovecii, and M. avium in coinfected rats, whereas PS-15 plus dapsone or TMP-SMX was active only against T. gondii and P. jirovecii and not against M. avium (245).
Ocular Infection
While OT is an important cause of human retinochoroiditis (246), the efficacy of currently available antiparasitic therapies is unclear. Moreover, the available drugs are ineffective against tissue cysts, which are the likely source of clinical reactivation (114). Evaluation of anti-Toxoplasma drugs for OT requires retinal biopsy to study the effect on active infection as well as the persistence of cysts. For ethical reasons, these studies cannot be carried out on human volunteers. Therefore, animal models have been used to study the efficacy of anti-Toxoplasma therapy in ocular infection (195, 196). Gormley et al. (247) investigated the efficacy of atovaquone compared to that of conventional therapies for OT treatment in Syrian golden hamsters. None of these treatments changed the development of acute infection, based on clinical examination. However, atovaquone, but not the conventional therapies, significantly reduced the number of cerebral cysts after acute infection and during chronic disease. Retinal cysts were only rarely detected in control and treated hamsters. Tabbara et al. (248) investigated the efficacy of clindamycin in rabbits. Clinical improvement was observed in 28% of control rabbits and more than 70% of clindamycin-treated rabbits. While parasites were found in 78% of eyes of untreated rabbits, they were not detected in those of treated rabbits. The retinal architecture was preserved and the alteration of inflammation was mild to moderate in the majority of treated rabbits. In contrast, the vast majority of eyes of untreated rabbits demonstrated severe retinochoroiditis, characterized by disruption of the normal retinal architecture and significant tissue destruction, supporting findings for humans. The efficacy of orally administered clindamycin was also investigated in cats, but the morphometric severity of ocular posterior segment lesions did not differ between control and treated animals. Paradoxically, clindamycin administration was associated with disseminated toxoplasmosis, manifested as hepatitis and interstitial pneumonia (249). Reasons for the lack of efficacy of clindamycin may be, among others, the suboptimal dosage, delay in treatment initiation, and shorter duration of treatment.
Other drugs have also demonstrated some efficacy in the treatment or prevention of OT in animal studies. Minocycline, a semisynthetic tetracycline, ameliorated clinical signs of TE, sterilized ocular tissues from parasites, and prevented death in 75% of animals (250). The potency of azithromycin in diminishing congenital transmission of the parasite was shown in the vesper mouse, Calomys callosus (251). After peroral infection, azithromycin treatment reduced the number of cysts, and parasites were absent from fetal eyes.
Congenital Infection
As previously discussed for OT, animal studies on CT treatment are complicated by the complexity of the experimental model and a lack of similarity with the human course of disease. The ability of clindamycin to prevent CT in animal models during acute infection was evaluated by Araujo and Remington (252). Pregnant mice infected intraperitoneally with tachyzoites were given clindamycin in their diet immediately after infection. Subinoculation studies revealed that almost all infected control mice, but none of the infected mice treated with clindamycin, bred infected offspring. Similarly, Schoondermark-van de Ven et al. (253) demonstrated the efficacy of pyr-sulf plus folinic acid in rhesus monkeys with congenital infection during the second trimester. Initiation of combination treatment after establishment of fetal infection cleared parasites from amniotic fluid samples and from the neonates at birth. The same authors also investigated the efficacy of spiramycin (254). In monkeys that completed several weeks of treatment with spiramycin, the placenta, amniotic fluid, and neonatal tissues were free of parasites, suggesting that initial treatment with spiramycin is able to prevent fetal transmission of the parasite.
The large vesper mouse model was used to demonstrate the capability of azithromycin to prevent congenital T. gondii transmission (251). Following peroral infection, tachyzoites were detected in the eyes of control fetuses and fetuses treated with pyr-sulf plus folinic acid but not in those of fetuses treated with azithromycin. The authors concluded that azithromycin might be an effective alternative treatment for toxoplasmosis during pregnancy.
Reactivated Toxoplasmosis
Although reactivation of the latent phase of infection usually manifests as TE, the immunopathogenesis of TE is not fully understood. The efficacy of antiparasitic drugs for the treatment of TE has been evaluated in animal models of acute and reactivated infections. While models of reactivated infection more accurately reflect reactivation in humans, we are not aware of studies directly comparing antiparasitic drugs in either model. Therefore, it remains to be shown whether animal models of reactivated disease, as described below, are better suited to predict the efficacy of antiparasitic drugs in humans.
Direct inoculation of tachyzoites into brain tissue of experimental animals can induce focal encephalitis, but these models are difficult to use in large-scale studies (224). Cellular immunity is key for containing reactivation of T. gondii, and immunosuppressive treatments, including administration of antibodies against CD8+ and CD4+ T lymphocytes (255), IFN-γ (256, 257), or immunosuppressive drugs targeting a broad range of immune pathways (258–260), induce focal reactivation in the brain in animal models of infection. A cyclophosphamide-induced murine model of reactivated toxoplasmosis was used to show the prophylactic efficacy of the synthetic dipeptide pidotimod, a potent immunostimulating drug. Treatment resulted in reduced parasite loads and amelioration of histopathology in the brain through enhanced Th1-mediated cellular immunity (261). Djurkovic-Djakovic et al. (230) introduced an alternative immunosuppression model in which dexamethasone-sodium phosphate and hydrocortisone-21-acetate induced immunosuppression in latently infected mice. In this model, atovaquone in combination with clindamycin significantly increased survival and markedly decreased the cyst burden. The latter effect was long lasting: the cyst burden in treated mice continued to decrease up to 3 months later, whereas it increased in the untreated mice.
Genetically modified mice that are depleted of cellular or other components of the protective (T) cell response develop disseminated but not focal infection (262–264). We and others have established new mouse models to mimic the development of reactivated infection observed in humans. Based on earlier studies in infected IFN-γ-deficient mice (265), we used mice deficient in the interferon-regulated-factor 8 (IRF-8) gene, which do not produce IL-12p40 (266). When these animals were treated with anti-Toxoplasma drugs shortly after infection, they developed latent Toxoplasma infection, which reactivated upon withdrawal of the anti-Toxoplasma drugs (Fig. 4A). Thus, this murine model of reactivated disease allows investigation of the efficacy of acute therapy. The model also lends itself to assessment of the efficacy of maintenance therapy (Fig. 4B). Following oral parasite challenge, sulfadiazine is administered for 4 weeks. The treatment prevents acute symptomatic disease but allows development of latent infection with the development of cysts in the CNS. Discontinuation of sulfadiazine treatment leads to cyst reactivation into active replicating tachyzoites, resulting in development of TE. Using our models, we studied the efficacy of new atovaquone formulations. An atovaquone nanosuspension coated with the surfactant polysorbate 80 (Tween 80) demonstrated elevated bioavailability and better penetration through the blood-brain barrier (BBB) than that of conventional atovaquone, and it prevented TE in animals challenged with T. gondii (266). Similarly, sodium dodecyl sulfate (SDS) coating of atovaquone nanosuspensions resulted in higher oral bioavailability and improved CNS uptake, resulting in less brain damage and reduced parasite numbers in brains of treated mice (267).
FIG 4.
(A) Murine model of reactivated toxoplasmosis to investigate the efficacy of drugs for acute therapy. Following induction of latent infection in immunodeficient (IRF-8−/−) mice by use of sulfadiazine, treatment is withdrawn to allow reactivation. The specific treatment of interest, e.g., atovaquone nanosuspensions, is then administered. Histological changes in brain tissue, including parasite counts, and survival of mice can be determined to evaluate the efficacy of treatment for reactivated infection. (Modified from reference 266 with permission.) (B) Murine model of reactivated infection to investigate the efficacy of drugs for maintenance therapy. Following induction of latent infection in immunodeficient (IRF-8−/−) mice by use of sulfadiazine, treatment is withdrawn to allow reactivation. Acute therapy against reactivation is then administered, and maintenance treatment (secondary prophylaxis) with the drug of interest is initiated. Histological changes in brain tissue, including parasite counts, and survival of mice can be evaluated to determine the potency of maintenance treatment. (Modified from reference 268 with permission.) ANS, atovaquone nanosuspensions; i.v., intravenous.
In another study, an atovaquone nanosuspension used as oral maintenance therapy was found to be superior to standard therapy with pyr-sulf in preserving brain pathology and reducing parasite numbers (268). To further enhance the capacity of the atovaquone nanosuspension to cross the BBB, several novel surface modifications were developed (269). The modified drugs have enhanced in vivo CNS uptake, as measured by mass spectrometry, and lead to atovaquone accumulation on endothelial cells (269, 270).
Other drugs, such as artemisone and artemiside, have also been tested as potential treatments in IFN-γ-deficient mice (271). Both artemiside and artemisone increased survival of animals with reactivated toxoplasmosis, although artemiside demonstrated superior therapeutic efficacy (Fig. 5). These drugs were able to control the early phases of reactivation, but all mice died within 25 days of discontinuing treatment, indicating a failure to eradicate the parasite. These results likely indicate a lack of efficacy against the latent stage of infection, which is characterized by the presence of bradyzoite-containing cysts.
FIG 5.
Survival of mice following treatment of reactivated toxoplasmosis with artemisinin derivatives. (Republished from reference 271 with permission.)
CONCLUSIONS
Toxoplasmosis continues to be a health threat for diverse patient populations, including immunocompromised patients, pregnant women, and patients with ocular disease. Since currently available treatments are insufficiently effective, require prolonged courses ranging from weeks to more than a year, or demonstrate significant toxicity, new therapeutic options for the treatment of toxoplasmosis are warranted. While development of therapeutics against T. gondii in the past was driven by the use of existing drugs with potent activity against other pathogens, future drug development should focus on new target molecules that lead to highly potent drugs without toxicity or on improved formulations for targeted delivery of established drugs. These approaches must also consider the efficacy of drugs against the bradyzoite stage of T. gondii, since curing patients from the infection is the most desirable strategy for preventing reactivation.
These development efforts will require appropriate screening technologies, followed by characterization and testing in validated in vitro models, before moving on to studies with validated animal models. Lastly, controlled clinical trials should be conducted on patient populations with the greatest need, including pregnant women and patients with ocular disease.
Research efforts in different fields, including chemistry, parasitology, pharmaceutical sciences, clinical microbiology, and infectious diseases, must come together to successfully address the vast number of remaining challenges.
ACKNOWLEDGMENTS
We thank Anis Khan for expert assistance. We are grateful to Jack Remington for his continuous support of us and his lifelong contributions to the field of Toxoplasma research.
We have no conflicts of interest.
Biographies

Ildiko Rita Dunay, Ph.D., is an Associate Professor at the Institute of Inflammation and Neurodegeneration at the Otto-von-Guericke University, Medical Faculty, in Germany. She obtained her M.Sc. and Dr. Pharm. degrees at Semmelweis Medical University in Budapest, Hungary. Her Ph.D. thesis on medical microbiology, completed at the Free University of Berlin and at the Department of Medical Microbiology of the Charité Medical School, Berlin, Germany, referred to novel treatment strategies for Toxoplasma gondii infection. She performed postdoctoral training at the Washington University School of Medicine in St. Louis, MO, in the Department of Molecular Microbiology, supported by the German Research Foundation. Her lab is interested in characterizing immune cell subsets and novel mechanisms that regulate host-pathogen interactions in chronic infections and the interaction of immune cells with the nervous system.

Kiran Gajurel, M.D., is a Clinical Assistant Professor of Medicine in the Division of Infectious Diseases at the University of Iowa. A native of Nepal, he graduated from Calcutta Medical College, India, and completed an internal medicine residency at the Nassau University Medical Center, NY. This was followed by an infectious disease fellowship at the William Beaumont Hospital, MI, and an immunocompromised host infectious disease fellowship at Stanford University, CA. Dr. Gajurel's research interests include toxoplasmosis and infections in hematopoietic cell and solid organ transplant recipients.

Reshika Dhakal, M.D., M.P.H., is a Research Scientist in the Vaccine and Treatment Evaluation Unit (VTEU) at the Carver College of Medicine, University of Iowa. She is a graduate of Rangpur Medical College, Bangladesh. After coming to the United States from her native Nepal, she worked under the guidance of Jose G. Montoya at the National Reference Center for the Study and Diagnosis of Toxoplasmosis in Palo Alto, CA. She later joined the University of Iowa. Dr. Dhakal's research focuses on toxoplasmosis and clinical vaccine trials.

Oliver Liesenfeld holds M.D. and Dr. Med. degrees from the Free University of Berlin, Germany. He completed his residency in medical microbiology and infection epidemiology in the Department of Medical Microbiology at the Charité Medical School, Berlin, Germany, followed by a postdoctoral fellowship in the Division of Infectious Diseases and Geographic Medicine at Stanford University. He was an Associate Professor of Medical Microbiology and Infection Immunology at the Charité Medical School from 2001 to 2008, where his laboratory performed studies on the immune response, diagnosis, and treatment of infections, with a focus on toxoplasmosis. From 2008 to 2012, he was Director of the Department of Medical and Scientific Affairs at Roche Molecular Diagnostics, and he headed the Department as Chief Medical Officer from 2012 to 2017. In 2018, he became Chief Medical Officer and Head of Clinical Affairs at Inflammatix Inc., a molecular diagnostics company developing rapid molecular tests that read the immune system to diagnose and risk stratify acute bacterial and viral infections and sepsis.

Jose G. Montoya, M.D., is originally from Cali, Colombia, and completed his medical degree with honors at the Universidad del Valle. He trained in internal medicine at Tulane University in New Orleans. Following his residency, he completed a fellowship in infectious diseases at Stanford University, Palo Alto, CA, under the mentorship of Jack S. Remington. He is currently a Professor in the Department of Medicine and Division of Infectious Diseases and Geographic Medicine at the Stanford University School of Medicine (https://med.stanford.edu/profiles/Jose_Montoya). He is also the Director of the National Reference Laboratory for the Diagnosis and Management of Toxoplasmosis in the United States at the Palo Alto Medical Foundation in Palo Alto, CA (http://www.pamf.org/Serology/), and is the founder of the Immunocompromised Host Service (Infectious Diseases) at the Stanford University Medical Center. He was elected a Fellow of the American College of Physicians (FACP), in recognition of his commitment to the internal medicine community, and a Fellow of the Infectious Diseases Society of America (FIDSA) for having achieved professional excellence in the field of infectious diseases. His research interests include toxoplasmosis, infectious complications in immunocompromised hosts, and chronic unexplained illnesses likely triggered or aggravated by infection.
REFERENCES
- 1.Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Demar M, Ajzenberg D, Maubon D, Djossou F, Panchoe D, Punwasi W, Valery N, Peneau C, Daigre JL, Aznar C, Cottrelle B, Terzan L, Darde ML, Carme B. 2007. Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clin Infect Dis 45:e88–e95. doi: 10.1086/521246. [DOI] [PubMed] [Google Scholar]
- 3.Gallant J. 2015. Get rich quick with old generic drugs! The pyrimethamine pricing scandal. Open Forum Infect Dis 2:ofv177. doi: 10.1093/ofid/ofv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan AA, Araujo FG. 1996. Recent developments in the search for therapeutic interventions against Toxoplasma gondii infection, p 65–77. In Recent research developments in antimicrobial agents and chemotherapy, vol 1 Research Signpost, Thiruvananthapuram, Kerala, India. [Google Scholar]
- 5.Eyles DE. 1953. The present status of the chemotherapy of toxoplasmosis. Am J Trop Med Hyg 2:429–444. doi: 10.4269/ajtmh.1953.2.429. [DOI] [PubMed] [Google Scholar]
- 6.Eyles DE. 1956. Newer knowledge of the chemotherapy of toxoplasmosis. Ann N Y Acad Sci 64:252–267. doi: 10.1111/j.1749-6632.1956.tb36617.x. [DOI] [Google Scholar]
- 7.Alday PH, Doggett JS. 2017. Drugs in development for toxoplasmosis: advances, challenges, and current status. Drug Des Devel Ther 11:273–293. doi: 10.2147/DDDT.S60973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFarland MM, Zach SJ, Wang X, Potluri LP, Neville AJ, Vennerstrom JL, Davis PH. 2016. Review of experimental compounds demonstrating anti-Toxoplasma activity. Antimicrob Agents Chemother 60:7017–7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin C, Jung SY, Kim SY, Song HO, Park H. 2012. Simple and efficient model systems of screening anti-Toxoplasma drugs in vitro. Expert Opin Drug Discov 7:195–205. doi: 10.1517/17460441.2012.660479. [DOI] [PubMed] [Google Scholar]
- 10.Kortagere S. 2012. Screening for small molecule inhibitors of Toxoplasma gondii. Expert Opin Drug Discov 7:1193–1206. doi: 10.1517/17460441.2012.729036. [DOI] [PubMed] [Google Scholar]
- 11.Montazeri M, Sharif M, Sarvi S, Mehrzadi S, Ahmadpour E, Daryani A. 2017. A systematic review of in vitro and in vivo activities of anti-Toxoplasma drugs and compounds (2006–2016). Front Microbiol 8:25. doi: 10.3389/fmicb.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharif M, Sarvi S, Pagheh AS, Asfaram S, Rahimi MT, Mehrzadi S, Ahmadpour E, Gholami S, Daryani A. 2016. The efficacy of herbal medicines against Toxoplasma gondii during the last 3 decades: a systematic review. Can J Physiol Pharmacol 94:1237–1248. doi: 10.1139/cjpp-2016-0039. [DOI] [PubMed] [Google Scholar]
- 13.Sabin AB, Warren J. 1942. Therapeutic effectiveness of certain sulfonamides on infection by an intracellular protozoon (Toxoplasma). Proc Soc Exp Biol Med 51:19–23. doi: 10.3181/00379727-51-13809. [DOI] [Google Scholar]
- 14.Oshima S, Inami Y, Tanaka H. 1970. Prophylactic effect of 2-sulfamoyl-4,4′-diaminodiphenylsulfone (SDDS) on experimental infection with Toxoplasma in pigs. Am J Trop Med Hyg 19:422–426. doi: 10.4269/ajtmh.1970.19.422. [DOI] [PubMed] [Google Scholar]
- 15.Summers WA. 1949. The effects of oral administration of aureomycin, sulfathiazole, sulfamerazine and 4,4′-diamino diphenyl sulfone on toxoplasmosis in mice. Am J Trop Med Hyg 29:889–893. [DOI] [PubMed] [Google Scholar]
- 16.Eyles DE, Coleman N. 1953. Synergistic effect of sulfadiazine and daraprim against experimental toxoplasmosis in the mouse. Antibiot Chemother (Northfield) 3:483–490. [PubMed] [Google Scholar]
- 17.Beverley JK, Fry BA. 1957. Sulphadimidine, pyrimethamine and dapsone in the treatment of toxoplasmosis in mice. Br J Pharmacol Chemother 12:189–193. doi: 10.1111/j.1476-5381.1957.tb00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garin JP, Eyles DE. 1958. Spiramycin therapy of experimental toxoplasmosis in mice. Presse Med 66:957–958. [PubMed] [Google Scholar]
- 19.Montoya JG, Remington JS. 2008. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis 47:554–566. doi: 10.1086/590149. [DOI] [PubMed] [Google Scholar]
- 20.Eyles DE, Coleman N. 1954. Notes on the treatment of acute experimental toxoplasmosis of the mouse with chlortetracycline and tetracycline. Antibiot Chemother (Northfield) 4:988–991. [PubMed] [Google Scholar]
- 21.Remington JS, Yagura T, Robinson WS. 1970. The effect of rifampin on Toxoplasma gondii. Proc Soc Exp Biol Med 135:167–172. doi: 10.3181/00379727-135-35011. [DOI] [PubMed] [Google Scholar]
- 22.McMaster PR, Powers KG, Finerty JF, Lunde MN. 1973. The effect of two chlorinated lincomycin analogues against acute toxoplasmosis in mice. Am J Trop Med Hyg 22:14–17. doi: 10.4269/ajtmh.1973.22.14. [DOI] [PubMed] [Google Scholar]
- 23.Jones JL, Kruszon-Moran D, Elder S, Rivera HN, Press C, Montoya JG, McQuillan GM. 2018. Toxoplasma gondii infection in the United States, 2011–2014. Am J Trop Med Hyg 98:551–557. doi: 10.4269/ajtmh.17-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carme B, Bissuel F, Ajzenberg D, Bouyne R, Aznar C, Demar M, Bichat S, Louvel D, Bourbigot AM, Peneau C, Neron P, Darde ML. 2002. Severe acquired toxoplasmosis in immunocompetent adult patients in French Guiana. J Clin Microbiol 40:4037–4044. doi: 10.1128/JCM.40.11.4037-4044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demar M, Hommel D, Djossou F, Peneau C, Boukhari R, Louvel D, Bourbigot AM, Nasser V, Ajzenberg D, Darde ML, Carme B. 2012. Acute toxoplasmoses in immunocompetent patients hospitalized in an intensive care unit in French Guiana. Clin Microbiol Infect 18:E221–E231. doi: 10.1111/j.1469-0691.2011.03648.x. [DOI] [PubMed] [Google Scholar]
- 27.Alavi SM, Alavi L. 2010. Treatment of toxoplasmic lymphadenitis with co-trimoxazole: double-blind, randomized clinical trial. Int J Infect Dis 14(Suppl 3):e67–e69. doi: 10.1016/j.ijid.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Strom J. 1951. Toxoplasmosis due to laboratory infection in two adults. Acta Med Scand 139:244–252. doi: 10.1111/j.0954-6820.1951.tb17165.x. [DOI] [PubMed] [Google Scholar]
- 29.Wettingfeld RF, Rowe J, Eyles DE. 1956. Treatment of toxoplasmosis with pyrimethamine (daraprim) and triple sulfonamide. Ann Intern Med 44:557–564. doi: 10.7326/0003-4819-44-3-557. [DOI] [PubMed] [Google Scholar]
- 30.Sexton RC Jr, Eyles DE, Dillman RE. 1953. Adult toxoplasmosis. Am J Med 14:366–377. doi: 10.1016/0002-9343(53)90047-3. [DOI] [PubMed] [Google Scholar]
- 31.Kayhoe DE, Jacobs L, Beye HK, McCullough NB. 1957. Acquired toxoplasmosis. Observations on two parasitologically proved cases treated with pyrimethamine and triple sulfonamides. N Engl J Med 257:1247–1254. [DOI] [PubMed] [Google Scholar]
- 32.Rougier S, Montoya JG, Peyron F. 2017. Lifelong persistence of Toxoplasma cysts: a questionable dogma? Trends Parasitol 33:93–101. doi: 10.1016/j.pt.2016.10.007 (Erratum, 33:414, doi:.) [DOI] [PubMed] [Google Scholar]
- 33.Tedford E, McConkey G. 2017. Neurophysiological changes induced by chronic Toxoplasma gondii infection. Pathogens 6:E19. doi: 10.3390/pathogens6020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurley RA, Taber KH. 2012. Latent toxoplasmosis gondii: emerging evidence for influences on neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci 24:376–383. doi: 10.1176/appi.neuropsych.12100234. [DOI] [PubMed] [Google Scholar]
- 35.Vyas A, Kim S-K, Giacomini N, Boothroyd JC, Sapolsky RM. 2007. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci U S A 104:6442–6447. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutterland AL, Fond G, Kuin A, Koeter MW, Lutter R, van Gool T, Yolken R, Szoke A, Leboyer M, de Haan L. 2015. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta-analysis. Acta Psychiatr Scand 132:161–179. doi: 10.1111/acps.12423. [DOI] [PubMed] [Google Scholar]
- 37.Chorlton SD. 2017. Toxoplasma gondii and schizophrenia: a review of published RCTs. Parasitol Res 116:1793–1799. doi: 10.1007/s00436-017-5478-y. [DOI] [PubMed] [Google Scholar]
- 38.Porter SB, Sande MA. 1992. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med 327:1643–1648. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 39.Luft BJ, Brooks RG, Conley FK, McCabe RE, Remington JS. 1984. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA 252:913–917. [PubMed] [Google Scholar]
- 40.Haverkos HW. 1987. Assessment of therapy for toxoplasma encephalitis. The TE Study Group Am J Med 82:907–914. [DOI] [PubMed] [Google Scholar]
- 41.Cohn JA, McMeeking A, Cohen W, Jacobs J, Holzman RS. 1989. Evaluation of the policy of empiric treatment of suspected Toxoplasma encephalitis in patients with the acquired immunodeficiency syndrome. Am J Med 86:521–527. doi: 10.1016/0002-9343(89)90378-1. [DOI] [PubMed] [Google Scholar]
- 42.Luft BJ, Hafner R, Korzun AH, Leport C, Antoniskis D, Bosler EM, Bourland DD III, Uttamchandani R, Fuhrer J, Jacobson J, Morlat P, Vilde J-L, Remington JS, ACTG 077p/ANRS 009 Study Team. 1993. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. Members of the ACTG 077p/ANRS 009 Study Team. N Engl J Med 329:995–1000. [DOI] [PubMed] [Google Scholar]
- 43.Canessa A, Del Bono V, De Leo P, Piersantelli N, Terragna A. 1992. Cotrimoxazole therapy of Toxoplasma gondii encephalitis in AIDS patients. Eur J Clin Microbiol Infect Dis 11:125–130. doi: 10.1007/BF01967063. [DOI] [PubMed] [Google Scholar]
- 44.Dannemann B, McCutchan JA, Israelski D, Antoniskis D, Leport C, Luft B, Nussbaum J, Clumeck N, Morlat P, Chiu J, Vilde J-L, Orellana M, Feigal D, Bartok A, Heseltine P, Leedom J, Remington J. 1992. Treatment of toxoplasmic encephalitis in patients with AIDS. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. The California Collaborative Treatment Group. Ann Intern Med 116:33–43. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez-Martin J, Leport C, Morlat P, Meyohas MC, Chauvin JP, Vilde JL. 1991. Pyrimethamine-clarithromycin combination for therapy of acute Toxoplasma encephalitis in patients with AIDS. Antimicrob Agents Chemother 35:2049–2052. doi: 10.1128/AAC.35.10.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foppa CU, Bini T, Gregis G, Lazzarin A, Esposito R, Moroni M. 1991. A retrospective study of primary and maintenance therapy of toxoplasmic encephalitis with oral clindamycin and pyrimethamine. Eur J Clin Microbiol Infect Dis 10:187–189. doi: 10.1007/BF01964458. [DOI] [PubMed] [Google Scholar]
- 47.Remington JS, Vilde JL. 1991. Clindamycin for toxoplasma encephalitis in AIDS. Lancet 338:1142–1143. [DOI] [PubMed] [Google Scholar]
- 48.Torre D, Casari S, Speranza F, Donisi A, Gregis G, Poggio A, Ranieri S, Orani A, Angarano G, Chiodo F, Fiori G, Carosi G. 1998. Randomized trial of trimethoprim-sulfamethoxazole versus pyrimethamine-sulfadiazine for therapy of toxoplasmic encephalitis in patients with AIDS. Italian Collaborative Study Group. Antimicrob Agents Chemother 42:1346–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leport C, Raffi F, Matheron S, Katlama C, Regnier B, Saimot AG, Marche C, Vedrenne C, Vilde JL. 1988. Treatment of central nervous system toxoplasmosis with pyrimethamine/sulfadiazine combination in 35 patients with the acquired immunodeficiency syndrome. Efficacy of long-term continuous therapy. Am J Med 84:94–100. [DOI] [PubMed] [Google Scholar]
- 50.Antinori A, Ammassari A, Maiuro G, Camilli G, Damiano F, Federico G, Pizzigallo E, Tamburrini E. 1992. Comparison of two medications in central nervous system toxoplasmosis in patients with AIDS. Ital J Neurol Sci 13:475–479. doi: 10.1007/BF02230867. [DOI] [PubMed] [Google Scholar]
- 51.Katlama C, De Wit S, O'Doherty E, Van Glabeke M, Clumeck N. 1996. Pyrimethamine-clindamycin vs. pyrimethamine-sulfadiazine as acute and long-term therapy for toxoplasmic encephalitis in patients with AIDS. Clin Infect Dis 22:268–275. doi: 10.1093/clinids/22.2.268. [DOI] [PubMed] [Google Scholar]
- 52.Dannemann BR, Israelski DM, Remington JS. 1988. Treatment of toxoplasmic encephalitis with intravenous clindamycin. Arch Intern Med 148:2477–2482. [PubMed] [Google Scholar]
- 53.Solbreux P, Sonnet J, Zech F. 1990. A retrospective study about the use of cotrimoxazole as diagnostic support and treatment of suspected cerebral toxoplasmosis in AIDS. Acta Clin Belg 45:85–96. doi: 10.1080/17843286.1990.11718072. [DOI] [PubMed] [Google Scholar]
- 54.Duval X, Pajot O, Le Moing V, Longuet P, Ecobichon JL, Mentre F, Leport C, Vilde JL. 2004. Maintenance therapy with cotrimoxazole for toxoplasmic encephalitis in the era of highly active antiretroviral therapy. AIDS 18:1342–1344. doi: 10.1097/00002030-200406180-00016. [DOI] [PubMed] [Google Scholar]
- 55.Francis P, Patel VB, Bill PL, Bhigjee AI. 2004. Oral trimethoprim-sulfamethoxazole in the treatment of cerebral toxoplasmosis in AIDS patients—a prospective study. S Afr Med J 94:51–53. [PubMed] [Google Scholar]
- 56.Arens J, Barnes K, Crowley N, Maartens G. 2007. Treating AIDS-associated cerebral toxoplasmosis-pyrimethamine plus sulfadiazine compared with cotrimoxazole, and outcome with adjunctive glucocorticoids. S Afr Med J 97:956–958. [PubMed] [Google Scholar]
- 57.Beraud G, Pierre-Francois S, Foltzer A, Abel S, Liautaud B, Smadja D, Cabie A. 2009. Cotrimoxazole for treatment of cerebral toxoplasmosis: an observational cohort study during 1994–2006. Am J Trop Med Hyg 80:583–587. [PubMed] [Google Scholar]
- 58.Kongsaengdao S, Samintarapanya K, Oranratnachai K, Prapakarn W, Apichartpiyakul C. 2008. Randomized controlled trial of pyrimethamine plus sulfadiazine versus trimethoprim plus sulfamethoxazole for treatment of toxoplasmic encephalitis in AIDS patients. J Int Assoc Physicians AIDS Care (Chic) 7:11–16. doi: 10.1177/1545109707301244. [DOI] [PubMed] [Google Scholar]
- 59.Dedicoat M, Livesley N. 2006. Management of toxoplasmic encephalitis in HIV-infected adults (with an emphasis on resource-poor settings). Cochrane Database Syst Rev 2006:CD005420. [DOI] [PubMed] [Google Scholar]
- 60.Rajapakse S, Chrishan Shivanthan M, Samaranayake N, Rodrigo C, Deepika Fernando S. 2013. Antibiotics for human toxoplasmosis: a systematic review of randomized trials. Pathog Glob Health 107:162–169. doi: 10.1179/2047773213Y.0000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hernandez AV, Thota P, Pellegrino D, Pasupuleti V, Benites-Zapata VA, Deshpande A, Penalva de Oliveira AC, Vidal JE. 2017. A systematic review and meta-analysis of the relative efficacy and safety of treatment regimens for HIV-associated cerebral toxoplasmosis: is trimethoprim-sulfamethoxazole a real option? HIV Med 18:115–124. doi: 10.1111/hiv.12402. [DOI] [PubMed] [Google Scholar]
- 62.Wei HX, Wei SS, Lindsay DS, Peng HJ. 2015. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS One 10:e0138204. doi: 10.1371/journal.pone.0138204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan J, Huang B, Liu G, Wu B, Huang S, Zheng H, Shen J, Lun ZR, Wang Y, Lu F. 2013. Meta-analysis of prevention and treatment of toxoplasmic encephalitis in HIV-infected patients. Acta Trop 127:236–244. doi: 10.1016/j.actatropica.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Torres RA, Weinberg W, Stansell J, Leoung G, Kovacs J, Rogers M, Scott J. 1997. Atovaquone for salvage treatment and suppression of toxoplasmic encephalitis in patients with AIDS. Atovaquone/Toxoplasmic Encephalitis Study Group. Clin Infect Dis 24:422–429. doi: 10.1093/clinids/24.3.422. [DOI] [PubMed] [Google Scholar]
- 65.Chirgwin K, Hafner R, Leport C, Remington J, Andersen J, Bosler EM, Roque C, Rajicic N, McAuliffe V, Morlat P, Jayaweera DT, Vilde JL, Luft BJ. 2002. Randomized phase II trial of atovaquone with pyrimethamine or sulfadiazine for treatment of toxoplasmic encephalitis in patients with acquired immunodeficiency syndrome: ACTG 237/ANRS 039 Study. AIDS Clinical Trials Group 237/Agence Nationale de Recherche sur le SIDA, Essai 039. Clin Infect Dis 34:1243–1250. [DOI] [PubMed] [Google Scholar]
- 66.Cohn DL, Fisher EJ, Peng GT, Hodges JS, Chesnut J, Child CC, Franchino B, Gibert CL, El-Sadr W, Hafner R, Korvick J, Ropka M, Heifets L, Clotfelter J, Munroe D, Horsburgh CR Jr. 1999. A prospective randomized trial of four three-drug regimens in the treatment of disseminated Mycobacterium avium complex disease in AIDS patients: excess mortality associated with high-dose clarithromycin. Terry Beirn Community Programs for Clinical Research on AIDS. Clin Infect Dis 29:125–133. [DOI] [PubMed] [Google Scholar]
- 67.Jacobson JM, Hafner R, Remington J, Farthing C, Holden-Wiltse J, Bosler EM, Harris C, Jayaweera DT, Roque C, Luft BJ, ACTG 156 Study Team. 2001. Dose-escalation, phase I/II study of azithromycin and pyrimethamine for the treatment of toxoplasmic encephalitis in AIDS. AIDS 15:583–589. doi: 10.1097/00002030-200103300-00007. [DOI] [PubMed] [Google Scholar]
- 68.Saba J, Morlat P, Raffi F, Hazebroucq V, Joly V, Leport C, Vilde JL. 1993. Pyrimethamine plus azithromycin for treatment of acute toxoplasmic encephalitis in patients with AIDS. Eur J Clin Microbiol Infect Dis 12:853–856. doi: 10.1007/BF02000407. [DOI] [PubMed] [Google Scholar]
- 69.Pedrol E, Gonzalez-Clemente JM, Gatell JM, Mallolas J, Miro JM, Graus F, Alvarez R, Mercader JM, Berenguer J, Jimenez de Anta MT, Valls ME, Soriano E. 1990. Central nervous system toxoplasmosis in AIDS patients: efficacy of an intermittent maintenance therapy. AIDS 4:511–517. doi: 10.1097/00002030-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Miro JM, Lopez JC, Podzamczer D, Pena JM, Alberdi JC, Martinez E, Domingo P, Cosin J, Claramonte X, Arribas JR, Santin M, Ribera E. 2006. Discontinuation of primary and secondary Toxoplasma gondii prophylaxis is safe in HIV-infected patients after immunological restoration with highly active antiretroviral therapy: results of an open, randomized, multicenter clinical trial. Clin Infect Dis 43:79–89. doi: 10.1086/504872. [DOI] [PubMed] [Google Scholar]
- 71.Bertschy S, Opravil M, Cavassini M, Bernasconi E, Schiffer V, Schmid P, Flepp M, Chave JP, Christen A, Furrer H. 2006. Discontinuation of maintenance therapy against toxoplasma encephalitis in AIDS patients with sustained response to anti-retroviral therapy. Clin Microbiol Infect 12:666–671. doi: 10.1111/j.1469-0691.2006.01459.x. [DOI] [PubMed] [Google Scholar]
- 72.Kirk O, Reiss P, Uberti-Foppa C, Bickel M, Gerstoft J, Pradier C, Wit FW, Ledergerber B, Lundgren JD, Furrer H. 2002. Safe interruption of maintenance therapy against previous infection with four common HIV-associated opportunistic pathogens during potent antiretroviral therapy. Ann Intern Med 137:239–250. doi: 10.7326/0003-4819-137-4-200208200-00008. [DOI] [PubMed] [Google Scholar]
- 73.Zeller V, Truffot C, Agher R, Bossi P, Tubiana R, Caumes E, Jouan M, Bricaire F, Katlama C. 2002. Discontinuation of secondary prophylaxis against disseminated Mycobacterium avium complex infection and toxoplasmic encephalitis. Clin Infect Dis 34:662–667. doi: 10.1086/338816. [DOI] [PubMed] [Google Scholar]
- 74.Ruf B, Schurmann D, Bergmann F, Schuler-Maue W, Grunewald T, Gottschalk HJ, Witt H, Pohle HD. 1993. Efficacy of pyrimethamine/sulfadoxine in the prevention of toxoplasmic encephalitis relapses and Pneumocystis carinii pneumonia in HIV-infected patients. Eur J Clin Microbiol Infect Dis 12:325–329. doi: 10.1007/BF01964427. [DOI] [PubMed] [Google Scholar]
- 75.Podzamczer D, Miro JM, Bolao F, Gatell JM, Cosin J, Sirera G, Domingo P, Laguna F, Santamaria J, Verdejo J. 1995. Twice-weekly maintenance therapy with sulfadiazine-pyrimethamine to prevent recurrent toxoplasmic encephalitis in patients with AIDS. Spanish Toxoplasmosis Study Group. Ann Intern Med 123:175–180. [DOI] [PubMed] [Google Scholar]
- 76.Podzamczer D, Miro JM, Ferrer E, Gatell JM, Ramon JM, Ribera E, Sirera G, Cruceta A, Knobel H, Domingo P, Polo R, Leyes M, Cosin J, Farinas MC, Arrizabalaga J, Martinez-Lacasa J, Gudiol F. 2000. Thrice-weekly sulfadiazine-pyrimethamine for maintenance therapy of toxoplasmic encephalitis in HIV-infected patients. Spanish Toxoplasmosis Study Group. Eur J Clin Microbiol Infect Dis 19:89–95. doi: 10.1007/s100960050436. [DOI] [PubMed] [Google Scholar]
- 77.Djawe K, Buchacz K, Hsu L, Chen MJ, Selik RM, Rose C, Williams T, Brooks JT, Schwarcz S. 2015. Mortality risk after AIDS—defining opportunistic illness among HIV-infected persons—San Francisco, 1981–2012. J Infect Dis 212:1366–1375. doi: 10.1093/infdis/jiv235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martin-Blondel G, Alvarez M, Delobel P, Uro-Coste E, Cuzin L, Cuvinciuc V, Fillaux J, Massip P, Marchou B. 2011. Toxoplasmic encephalitis IRIS in HIV-infected patients: a case series and review of the literature. J Neurol Neurosurg Psychiatry 82:691–693. doi: 10.1136/jnnp.2009.199919. [DOI] [PubMed] [Google Scholar]
- 79.Vidal JE, Hernandez AV, de Oliveira AC, Dauar RF, Barbosa SP Jr, Focaccia R. 2005. Cerebral toxoplasmosis in HIV-positive patients in Brazil: clinical features and predictors of treatment response in the HAART era. AIDS Patient Care STDS 19:626–634. doi: 10.1089/apc.2005.19.626. [DOI] [PubMed] [Google Scholar]
- 80.Connolly MP, Goodwin E, Schey C, Zummo J. 2017. Toxoplasmic encephalitis relapse rates with pyrimethamine-based therapy: systematic review and meta-analysis. Pathog Glob Health 111:31–44. doi: 10.1080/20477724.2016.1273597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mayor AM, Fernandez Santos DM, Dworkin MS, Rios-Olivares E, Hunter-Mellado RF. 2011. Toxoplasmic encephalitis in an AIDS cohort at Puerto Rico before and after highly active antiretroviral therapy (HAART). Am J Trop Med Hyg 84:838–841. doi: 10.4269/ajtmh.2011.10-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martin-Iguacel R, Ahlstrom MG, Touma M, Engsig FN, Staerke NB, Staerkind M, Obel N, Rasmussen LD. 2017. Incidence, presentation and outcome of toxoplasmosis in HIV infected in the combination antiretroviral therapy era. J Infect 75:263–273. doi: 10.1016/j.jinf.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 83.Connolly MP, Haitsma G, Hernandez AV, Vidal JE. 2017. Systematic review and meta-analysis of secondary prophylaxis for prevention of HIV-related toxoplasmic encephalitis relapse using trimethoprim-sulfamethoxazole. Pathog Glob Health 111:327–331. doi: 10.1080/20477724.2017.1377974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rothova A, Buitenhuis HJ, Meenken C, Baarsma GS, Boen-Tan TN, de Jong PT, Schweitzer CM, Timmerman Z, de Vries J, Zaal MJ, Kijlstra A. 1989. Therapy of ocular toxoplasmosis. Int Ophthalmol 13:415–419. doi: 10.1007/BF02306491. [DOI] [PubMed] [Google Scholar]
- 85.Leport C, Tournerie C, Raguin G, Fernandez-Martin J, Niyongabo T, Vilde JL. 1991. Long-term follow-up of patients with AIDS on maintenance therapy for toxoplasmosis. Eur J Clin Microbiol Infect Dis 10:191–193. doi: 10.1007/BF01964460. [DOI] [PubMed] [Google Scholar]
- 86.Heald A, Flepp M, Chave JP, Malinverni R, Ruttimann S, Gabriel V, Renold C, Sugar A, Hirschel B. 1991. Treatment for cerebral toxoplasmosis protects against Pneumocystis carinii pneumonia in patients with AIDS. The Swiss HIV Cohort Study. Ann Intern Med 115:760–763. [DOI] [PubMed] [Google Scholar]
- 87.Gajurel K, Dhakal R, Montoya JG. 2015. Toxoplasma prophylaxis in haematopoietic cell transplant recipients: a review of the literature and recommendations. Curr Opin Infect Dis 28:283–292. doi: 10.1097/QCO.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 88.Sumi M, Aosai F, Norose K, Takeda W, Kirihara T, Sato K, Fujikawa Y, Shimizu I, Ueki T, Hirosima Y, Ueno M, Ichikawa N, Watanabe M, Kobayashi H. 2013. Acute exacerbation of Toxoplasma gondii infection after hematopoietic stem cell transplantation: five case reports among 279 recipients. Int J Hematol 98:214–222. doi: 10.1007/s12185-013-1379-8. [DOI] [PubMed] [Google Scholar]
- 89.Martino R, Bretagne S, Einsele H, Maertens J, Ullmann AJ, Parody R, Schumacher U, Pautas C, Theunissen K, Schindel C, Munoz C, Margall N, Cordonnier C, Infectious Disease Working Party of the European Group for Blood and Marrow Transplantation. 2005. Early detection of Toxoplasma infection by molecular monitoring of Toxoplasma gondii in peripheral blood samples after allogeneic stem cell transplantation. Clin Infect Dis 40:67–78. doi: 10.1086/426447. [DOI] [PubMed] [Google Scholar]
- 90.Meers S, Lagrou K, Theunissen K, Dierickx D, Delforge M, Devos T, Janssens A, Meersseman W, Verhoef G, Van Eldere J, Maertens J. 2010. Myeloablative conditioning predisposes patients for Toxoplasma gondii reactivation after allogeneic stem cell transplantation. Clin Infect Dis 50:1127–1134. doi: 10.1086/651266. [DOI] [PubMed] [Google Scholar]
- 91.Robert-Gangneux F, Sterkers Y, Yera H, Accoceberry I, Menotti J, Cassaing S, Brenier-Pinchart MP, Hennequin C, Delhaes L, Bonhomme J, Villena I, Scherer E, Dalle F, Touafek F, Filisetti D, Varlet-Marie E, Pelloux H, Bastien P. 2015. Molecular diagnosis of toxoplasmosis in immunocompromised patients: a 3-year multicenter retrospective study. J Clin Microbiol 53:1677–1684. doi: 10.1128/JCM.03282-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Isa F, Saito K, Huang YT, Schuetz A, Babady NE, Salvatore S, Pessin M, van Besien K, Perales MA, Giralt S, Sepkowitz K, Papanicolaou GA, Soave R, Kamboj M. 2016. Implementation of molecular surveillance after a cluster of fatal toxoplasmosis at 2 neighboring transplant centers. Clin Infect Dis 63:565–568. doi: 10.1093/cid/ciw330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mele A, Paterson PJ, Prentice HG, Leoni P, Kibbler CC. 2002. Toxoplasmosis in bone marrow transplantation: a report of two cases and systematic review of the literature. Bone Marrow Transplant 29:691–698. doi: 10.1038/sj.bmt.1703425. [DOI] [PubMed] [Google Scholar]
- 94.Derouin F, Pelloux H, ESCMID Study Group on Clinical Parasitology . 2008. Prevention of toxoplasmosis in transplant patients. Clin Microbiol Infect 14:1089–1101. doi: 10.1111/j.1469-0691.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 95.Fernandez-Sabe N, Cervera C, Farinas MC, Bodro M, Munoz P, Gurgui M, Torre-Cisneros J, Martin-Davila P, Noblejas A, Len O, Garcia-Reyne A, Del Pozo JL, Carratala J. 2012. Risk factors, clinical features, and outcomes of toxoplasmosis in solid-organ transplant recipients: a matched case-control study. Clin Infect Dis 54:355–361. doi: 10.1093/cid/cir806. [DOI] [PubMed] [Google Scholar]
- 96.Campbell AL, Goldberg CL, Magid MS, Gondolesi G, Rumbo C, Herold BC. 2006. First case of toxoplasmosis following small bowel transplantation and systematic review of tissue-invasive toxoplasmosis following noncardiac solid organ transplantation. Transplantation 81:408–417. doi: 10.1097/01.tp.0000188183.49025.d5. [DOI] [PubMed] [Google Scholar]
- 97.Morollon N, Rodriguez F, Duarte J, Sanchez R, Camacho FI, Campo E. 2017. Brain lesions in a long-term kidney transplant recipient: primary cerebral lymphoma or cerebral toxoplasmosis? Neurologia 32:268–270. doi: 10.1016/j.nrl.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 98.Lanfranco L, Olle Delahaye P, Dorr G, Del Bello A, Kamar N. 2016. Late isolated ocular toxoplasmosis in a belatacept-treated kidney transplant patient. Transpl Int 29:1352–1353. doi: 10.1111/tri.12853. [DOI] [PubMed] [Google Scholar]
- 99.de Joode AA, Riezebos-Brilman A, Manson WL, Homan van der Heide JJ. 2011. Tissue is the issue: a solitary cerebral lesion 15 years after kidney transplantation. NDT Plus 4:410–412. doi: 10.1093/ndtplus/sfr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Israelski DM, Remington JS. 1993. Toxoplasmosis in patients with cancer. Clin Infect Dis 17(Suppl 2):S423–S435. doi: 10.1093/clinids/17.Supplement_2.S423. [DOI] [PubMed] [Google Scholar]
- 101.Scerra S, Coignard-Biehler H, Lanternier F, Suarez F, Charlier-Woerther C, Bougnoux ME, Gilquin J, Lecuit M, Hermine O, Lortholary O. 2013. Disseminated toxoplasmosis in non-allografted patients with hematologic malignancies: report of two cases and literature review. Eur J Clin Microbiol Infect Dis 32:1259–1268. doi: 10.1007/s10096-013-1879-8. [DOI] [PubMed] [Google Scholar]
- 102.Morjaria S, Epstein DJ, Romero FA, Taur Y, Seo SK, Papanicolaou GA, Hatzoglou V, Rosenblum M, Perales MA, Scordo M, Kaltsas A. 2016. Toxoplasma encephalitis in atypical hosts at an academic cancer center. Open Forum Infect Dis 3:ofw070. doi: 10.1093/ofid/ofw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fernandez-Ruiz M, Meije Y, Manuel O, Akan H, Carratala J, Aguado JM, Delaloye J. 2018. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (introduction). Clin Microbiol Infect 24(Suppl 2):S2–S9. doi: 10.1016/j.cmi.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 104.Desmonts G, Couvreur J. 1974. Congenital toxoplasmosis. A prospective study of 378 pregnancies. N Engl J Med 290:1110–1116. [DOI] [PubMed] [Google Scholar]
- 105.Koppe JG, Loewer-Sieger DH, de Roever-Bonnet H. 1986. Results of 20-year follow-up of congenital toxoplasmosis. Lancet i:254–256. [DOI] [PubMed] [Google Scholar]
- 106.Arantes TE, Silveira C, Holland GN, Muccioli C, Yu F, Jones JL, Goldhardt R, Lewis KG, Belfort R Jr. 2015. Ocular involvement following postnatally acquired Toxoplasma gondii infection in Southern Brazil: a 28-year experience. Am J Ophthalmol 159:1002.e2–1012.e2. doi: 10.1016/j.ajo.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 107.Silveira C, Muccioli C, Holland GN, Jones JL, Yu F, de Paulo A, Belfort R Jr. 2015. Ocular involvement following an epidemic of Toxoplasma gondii infection in Santa Isabel do Ivai, Brazil. Am J Ophthalmol 159:1013.e3–1021.e3. doi: 10.1016/j.ajo.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 108.Bosch-Driessen LE, Berendschot TT, Ongkosuwito JV, Rothova A. 2002. Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology 109:869–878. doi: 10.1016/S0161-6420(02)00990-9. [DOI] [PubMed] [Google Scholar]
- 109.Winterhalter S, Severing K, Stammen J, Maier AK, Godehardt E, Joussen AM. 2010. Does atovaquone prolong the disease-free interval of toxoplasmic retinochoroiditis? Graefes Arch Clin Exp Ophthalmol 248:1187–1192. doi: 10.1007/s00417-010-1379-9. [DOI] [PubMed] [Google Scholar]
- 110.Reich M, Becker MD, Mackensen F. 2016. Influence of drug therapy on the risk of recurrence of ocular toxoplasmosis. Br J Ophthalmol 100:195–199. doi: 10.1136/bjophthalmol-2015-306650. [DOI] [PubMed] [Google Scholar]
- 111.Culligan JJ, Gunkel RD, Jacobs L, Cook MK. 1954. Diagnosis and treatment of toxoplasmic uveitis. Trans Am Acad Ophthalmol Otolaryngol 58:867–884. [PubMed] [Google Scholar]
- 112.Beuerman VA, Burnham CJ. 1956. Toxoplasmic uveitis; treatment with pyrimethamine and sulfadiazine. Am J Ophthalmol 42:217–227. doi: 10.1016/0002-9394(56)90925-4. [DOI] [PubMed] [Google Scholar]
- 113.Fajardo RV, Furgiuele FP, Leopold IH. 1962. Treatment of toxoplasmosis uveitis. Arch Ophthalmol 67:712–720. doi: 10.1001/archopht.1962.00960020712004. [DOI] [PubMed] [Google Scholar]
- 114.Rothova A, Meenken C, Buitenhuis HJ, Brinkman CJ, Baarsma GS, Boen-Tan TN, de Jong PT, Klaassen-Broekema N, Schweitzer CM, Timmerman Z, de Vries J, Zaal MJW, Kijlstra A. 1993. Therapy for ocular toxoplasmosis. Am J Ophthalmol 115:517–523. doi: 10.1016/S0002-9394(14)74456-3. [DOI] [PubMed] [Google Scholar]
- 115.Perkins ES, Schofield PB, Smith CH. 1956. Treatment of uveitis with pyrimethamine (daraprim). Br J Ophthalmol 40:577–586. doi: 10.1136/bjo.40.10.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Acers TE. 1964. Toxoplasmic retinochoroiditis: a double blind therapeutic study. Arch Ophthalmol 71:58–62. doi: 10.1001/archopht.1964.00970010074010. [DOI] [PubMed] [Google Scholar]
- 117.O'Connor GR, Remington JS. 1964. Therapy of toxoplasmic retinochoroiditis. Arch Ophthalmol 71:883–885. doi: 10.1001/archopht.1964.00970010899023. [DOI] [PubMed] [Google Scholar]
- 118.Tabbara KF, O'Connor GR. 1980. Treatment of ocular toxoplasmosis with clindamycin and sulfadiazine. Ophthalmology 87:129–134. doi: 10.1016/S0161-6420(80)35268-8. [DOI] [PubMed] [Google Scholar]
- 119.Lakhanpal V, Schocket SS, Nirankari VS. 1983. Clindamycin in the treatment of toxoplasmic retinochoroiditis. Am J Ophthalmol 95:605–613. doi: 10.1016/0002-9394(83)90378-1. [DOI] [PubMed] [Google Scholar]
- 120.Lasave AF, Diaz-Llopis M, Muccioli C, Belfort R Jr, Arevalo JF. 2010. Intravitreal clindamycin and dexamethasone for zone 1 toxoplasmic retinochoroiditis at twenty-four months. Ophthalmology 117:1831–1838. doi: 10.1016/j.ophtha.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 121.Kishore K, Conway MD, Peyman GA. 2001. Intravitreal clindamycin and dexamethasone for toxoplasmic retinochoroiditis. Ophthalmic Surg Lasers 32:183–192. [PubMed] [Google Scholar]
- 122.Sobrin L, Kump LI, Foster CS. 2007. Intravitreal clindamycin for toxoplasmic retinochoroiditis. Retina 27:952–957. doi: 10.1097/IAE.0b013e31804b3f0d. [DOI] [PubMed] [Google Scholar]
- 123.Martinez CE, Zhang D, Conway MD, Peyman GA. 1998. Successful management of ocular toxoplasmosis during pregnancy using combined intraocular clindamycin and dexamethasone with systemic sulfadiazine. Int Ophthalmol 22:85–88. doi: 10.1023/A:1006129422690. [DOI] [PubMed] [Google Scholar]
- 124.Soheilian M, Ramezani A, Azimzadeh A, Sadoughi MM, Dehghan MH, Shahghadami R, Yaseri M, Peyman GA. 2011. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology 118:134–141. doi: 10.1016/j.ophtha.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 125.Baharivand N, Mahdavifard A, Fouladi RF. 2013. Intravitreal clindamycin plus dexamethasone versus classic oral therapy in toxoplasmic retinochoroiditis: a prospective randomized clinical trial. Int Ophthalmol 33:39–46. doi: 10.1007/s10792-012-9634-1. [DOI] [PubMed] [Google Scholar]
- 126.Dhakal R, Gajurel K, Pomares C, Talucod J, Press CJ, Montoya JG. 2015. Significance of a positive Toxoplasma immunoglobulin M test result in the United States. J Clin Microbiol 53:3601–3605. doi: 10.1128/JCM.01663-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Soheilian M, Sadoughi MM, Ghajarnia M, Dehghan MH, Yazdani S, Behboudi H, Anisian A, Peyman GA. 2005. Prospective randomized trial of trimethoprim/sulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology 112:1876–1882. doi: 10.1016/j.ophtha.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 128.Tripathy K. 2017. Comment on: “Intravitreal injection of sulfamethoxazole and trimethoprim associated with dexamethasone as an alternative therapy for ocular toxoplasmosis.” Ocul Immunol Inflamm 2017:1359307. doi: 10.1080/09273948.2017.1359307. [DOI] [PubMed] [Google Scholar]
- 129.Souza CE, Nascimento H, Lima A, Muccioli C, Belfort R Jr. 2017. Intravitreal injection of sulfamethoxazole and trimethoprim associated with dexamethasone as an alternative therapy for ocular toxoplasmosis. Ocul Immunol Inflamm 2017:1307420. doi: 10.1080/09273948.2017.1307420. [DOI] [PubMed] [Google Scholar]
- 130.Choudhury H, Jindal A, Pathengay A, Bawdekar A, Albini T, Flynn HW Jr. 2015. The role of intravitreal trimethoprim/sulfamethoxazole in the treatment of toxoplasma retinochoroiditis. Ophthalmic Surg Lasers Imaging Retina 46:137–140. doi: 10.3928/23258160-20150101-27. [DOI] [PubMed] [Google Scholar]
- 131.Pearson PA, Piracha AR, Sen HA, Jaffe GJ. 1999. Atovaquone for the treatment of toxoplasma retinochoroiditis in immunocompetent patients. Ophthalmology 106:148–153. doi: 10.1016/S0161-6420(99)90021-0. [DOI] [PubMed] [Google Scholar]
- 132.Balaskas K, Vaudaux J, Boillat-Blanco N, Guex-Crosier Y. 2012. Azithromycin versus sulfadiazine and pyrimethamine for non-vision-threatening toxoplasmic retinochoroiditis: a pilot study. Med Sci Monit 18:CR296–CR302. doi: 10.12659/MSM.882735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rothova A, Bosch-Driessen LE, van Loon NH, Treffers WF. 1998. Azithromycin for ocular toxoplasmosis. Br J Ophthalmol 82:1306–1308. doi: 10.1136/bjo.82.11.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lashay A, Mirshahi A, Parandin N, Riazi Esfahani H, Mazloumi M, Reza Lashay M, Johari MK, Ashrafi E. 2017. A prospective randomized trial of azithromycin versus trimethoprim/sulfamethoxazole in treatment of toxoplasmic retinochoroiditis. J Curr Ophthalmol 29:120–125. doi: 10.1016/j.joco.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stanford MR, See SE, Jones LV, Gilbert RE. 2003. Antibiotics for toxoplasmic retinochoroiditis: an evidence-based systematic review. Ophthalmology 110:926–931. doi: 10.1016/S0161-6420(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 136.Holland GN, Lewis KG. 2002. An update on current practices in the management of ocular toxoplasmosis. Am J Ophthalmol 134:102–114. doi: 10.1016/S0002-9394(02)01526-X. [DOI] [PubMed] [Google Scholar]
- 137.Kim SJ, Scott IU, Brown GC, Brown MM, Ho AC, Ip MS, Recchia FM. 2013. Interventions for toxoplasma retinochoroiditis: a report by the American Academy of Ophthalmology. Ophthalmology 120:371–378. doi: 10.1016/j.ophtha.2012.07.061. [DOI] [PubMed] [Google Scholar]
- 138.Pradhan E, Bhandari S, Gilbert RE, Stanford M. 2016. Antibiotics versus no treatment for toxoplasma retinochoroiditis. Cochrane Database Syst Rev 2016:CD002218. doi: 10.1002/14651858.CD002218.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Borkowski PK, Brydak-Godowska J, Basiak W, Switaj K, Zarnowska-Prymek H, Olszynska-Krowicka M, Kajfasz P, Rabczenko D. 2016. The impact of short-term, intensive antifolate treatment (with pyrimethamine and sulfadoxine) and antibiotics followed by long-term, secondary antifolate prophylaxis on the rate of toxoplasmic retinochoroiditis recurrence. PLoS Negl Trop Dis 10:e0004892. doi: 10.1371/journal.pntd.0004892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Silveira C, Belfort R Jr, Muccioli C, Holland GN, Victora CG, Horta BL, Yu F, Nussenblatt RB. 2002. The effect of long-term intermittent trimethoprim/sulfamethoxazole treatment on recurrences of toxoplasmic retinochoroiditis. Am J Ophthalmol 134:41–46. doi: 10.1016/S0002-9394(02)01527-1. [DOI] [PubMed] [Google Scholar]
- 141.Felix JP, Lira RP, Zacchia RS, Toribio JM, Nascimento MA, Arieta CE. 2014. Trimethoprim-sulfamethoxazole versus placebo to reduce the risk of recurrences of Toxoplasma gondii retinochoroiditis: randomized controlled clinical trial. Am J Ophthalmol 157:762.e1–766.e1. doi: 10.1016/j.ajo.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 142.Fernandes Felix JP, Cavalcanti Lira RP, Cosimo AB, Cardeal da Costa RL, Nascimento MA, Leite Arieta CE. 2016. Trimethoprim-sulfamethoxazole versus placebo in reducing the risk of toxoplasmic retinochoroiditis recurrences: a three-year follow-up. Am J Ophthalmol 170:176–182. doi: 10.1016/j.ajo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 143.Reich M, Mackensen F. 2015. Ocular toxoplasmosis: background and evidence for an antibiotic prophylaxis. Curr Opin Ophthalmol 26:498–505. doi: 10.1097/ICU.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 144.Jasper S, Vedula SS, John SS, Horo S, Sepah YJ, Nguyen QD. 2013. Corticosteroids as adjuvant therapy for ocular toxoplasmosis. Cochrane Database Syst Rev 2013:CD007417. doi: 10.1002/14651858.CD007417.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gordon DM. 1970. The treatment of toxoplasmic uveitis. Int Ophthalmol Clin 10:639–646. [PubMed] [Google Scholar]
- 146.Nozik RA. 1977. Results of treatment of ocular toxoplasmosis with injectable corticosteroids. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol 83:811–818. [PubMed] [Google Scholar]
- 147.Bosch-Driessen EH, Rothova A. 1998. Sense and nonsense of corticosteroid administration in the treatment of ocular toxoplasmosis. Br J Ophthalmol 82:858–860. doi: 10.1136/bjo.82.8.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.de-la-Torre A, Rios-Cadavid AC, Cardozo-Garcia CM, Gomez-Marin JE. 2009. Frequency and factors associated with recurrences of ocular toxoplasmosis in a referral centre in Colombia. Br J Ophthalmol 93:1001–1004. doi: 10.1136/bjo.2008.155861. [DOI] [PubMed] [Google Scholar]
- 149.Jasper S, Vedula SS, John SS, Horo S, Sepah YJ, Nguyen QD. 2017. Corticosteroids as adjuvant therapy for ocular toxoplasmosis. Cochrane Database Syst Rev 1:CD007417. doi: 10.1002/14651858.CD007417.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Desmonts G, Couvreur J. 1974. Toxoplasmosis in pregnancy and its transmission to the fetus. Bull N Y Acad Med 50:146–159. [PMC free article] [PubMed] [Google Scholar]
- 151.Oniki S. 1983. Prognosis of pregnancy in patients with toxoplasmic retinochoroiditis. Jpn J Ophthalmol 27:166–174. [PubMed] [Google Scholar]
- 152.Garweg JG, Scherrer J, Wallon M, Kodjikian L, Peyron F. 2005. Reactivation of ocular toxoplasmosis during pregnancy. BJOG 112:241–242. doi: 10.1111/j.1471-0528.2004.00302.x. [DOI] [PubMed] [Google Scholar]
- 153.Kump LI, Androudi SN, Foster CS. 2005. Ocular toxoplasmosis in pregnancy. Clin Exp Ophthalmol 33:455–460. doi: 10.1111/j.1442-9071.2005.01061.x. [DOI] [PubMed] [Google Scholar]
- 154.Vogel N, Kirisits M, Michael E, Bach H, Hostetter M, Boyer K, Simpson R, Holfels E, Hopkins J, Mack D, Mets MB, Swisher CN, Patel D, Roizen N, Stein L, Stein M, Withers S, Mui E, Egwuagu C, Remington J, Dorfman R, McLeod R. 1996. Congenital toxoplasmosis transmitted from an immunologically competent mother infected before conception. Clin Infect Dis 23:1055–1060. doi: 10.1093/clinids/23.5.1055. [DOI] [PubMed] [Google Scholar]
- 155.Vergani P, Ghidini A, Ceruti P, Strobelt N, Spelta A, Zapparoli B, Rescaldani R. 1998. Congenital toxoplasmosis: efficacy of maternal treatment with spiramycin alone. Am J Reprod Immunol 39:335–340. doi: 10.1111/j.1600-0897.1998.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 156.Dunn D, Wallon M, Peyron F, Petersen E, Peckham C, Gilbert R. 1999. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet 353:1829–1833. doi: 10.1016/S0140-6736(98)08220-8. [DOI] [PubMed] [Google Scholar]
- 157.Gilbert R, Gras L, European Multicentre Study on Congenital Toxoplasmosis. 2003. Effect of timing and type of treatment on the risk of mother to child transmission of Toxoplasma gondii. BJOG 110:112–120. [DOI] [PubMed] [Google Scholar]
- 158.Gilbert RE, Gras L, Wallon M, Peyron F, Ades AE, Dunn DT. 2001. Effect of prenatal treatment on mother to child transmission of Toxoplasma gondii: retrospective cohort study of 554 mother-child pairs in Lyon, France. Int J Epidemiol 30:1303–1308. doi: 10.1093/ije/30.6.1303. [DOI] [PubMed] [Google Scholar]
- 159.Hotop A, Hlobil H, Gross U. 2012. Efficacy of rapid treatment initiation following primary Toxoplasma gondii infection during pregnancy. Clin Infect Dis 54:1545–1552. doi: 10.1093/cid/cis234. [DOI] [PubMed] [Google Scholar]
- 160.Wallon M, Peyron F, Cornu C, Vinault S, Abrahamowicz M, Kopp CB, Binquet C. 2013. Congenital toxoplasma infection: monthly prenatal screening decreases transmission rate and improves clinical outcome at age 3 years. Clin Infect Dis 56:1223–1231. doi: 10.1093/cid/cit032. [DOI] [PubMed] [Google Scholar]
- 161.Cortina-Borja M, Tan HK, Wallon M, Paul M, Prusa A, Buffolano W, Malm G, Salt A, Freeman K, Petersen E, Gilbert RE, European Multicentre Study on Congenital Toxoplasmosis. 2010. Prenatal treatment for serious neurological sequelae of congenital toxoplasmosis: an observational prospective cohort study. PLoS Med 7:e1000351. doi: 10.1371/journal.pmed.1000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Prusa AR, Kasper DC, Pollak A, Olischar M, Gleiss A, Hayde M. 2015. Amniocentesis for the detection of congenital toxoplasmosis: results from the nationwide Austrian prenatal screening program. Clin Microbiol Infect 21:191.e1–191.e8. doi: 10.1016/j.cmi.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 163.SYROCOT (Systematic Review on Congenital Toxoplasmosis) Study Group, Thiebaut R, Leproust S, Chene G, Gilbert R. 2007. Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patients' data. Lancet 369:115–122. doi: 10.1016/S0140-6736(07)60072-5. [DOI] [PubMed] [Google Scholar]
- 164.Daffos F, Forestier F, Capella-Pavlovsky M, Thulliez P, Aufrant C, Valenti D, Cox WL. 1988. Prenatal management of 746 pregnancies at risk for congenital toxoplasmosis. N Engl J Med 318:271–275. doi: 10.1056/NEJM198802043180502. [DOI] [PubMed] [Google Scholar]
- 165.Berrebi A, Kobuch WE. 1994. Toxoplasmosis in pregnancy. Lancet 344:950. [DOI] [PubMed] [Google Scholar]
- 166.Patel DV, Holfels EM, Vogel NP, Boyer KM, Mets MB, Swisher CN, Roizen NJ, Stein LK, Stein MA, Hopkins J, Withers SE, Mack DG, Luciano RA, Meier P, Remington JS, McLeod RL. 1996. Resolution of intracranial calcifications in infants with treated congenital toxoplasmosis. Radiology 199:433–440. doi: 10.1148/radiology.199.2.8668790. [DOI] [PubMed] [Google Scholar]
- 167.Wallon M, Liou C, Garner P, Peyron F. 1999. Congenital toxoplasmosis: systematic review of evidence of efficacy of treatment in pregnancy. BMJ 318:1511–1514. doi: 10.1136/bmj.318.7197.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Peyron F, Wallon M, Liou C, Garner P. 2000. Treatments for toxoplasmosis in pregnancy. Cochrane Database Syst Rev 2000:CD001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Foulon W, Pinon JM, Stray-Pedersen B, Pollak A, Lappalainen M, Decoster A, Villena I, Jenum PA, Hayde M, Naessens A. 1999. Prenatal diagnosis of congenital toxoplasmosis: a multicenter evaluation of different diagnostic parameters. Am J Obstet Gynecol 181:843–847. doi: 10.1016/S0002-9378(99)70311-X. [DOI] [PubMed] [Google Scholar]
- 170.Gras L, Wallon M, Pollak A, Cortina-Borja M, Evengard B, Hayde M, Petersen E, Gilbert R, European Multicenter Study on Congenital Toxoplasmosis. 2005. Association between prenatal treatment and clinical manifestations of congenital toxoplasmosis in infancy: a cohort study in 13 European centres. Acta Paediatr 94:1721–1731. doi: 10.1111/j.1651-2227.2005.tb01844.x. [DOI] [PubMed] [Google Scholar]
- 171.Gras L, Gilbert RE, Ades AE, Dunn DT. 2001. Effect of prenatal treatment on the risk of intracranial and ocular lesions in children with congenital toxoplasmosis. Int J Epidemiol 30:1309–1313. doi: 10.1093/ije/30.6.1309. [DOI] [PubMed] [Google Scholar]
- 172.Kieffer F, Wallon M, Garcia P, Thulliez P, Peyron F, Franck J. 2008. Risk factors for retinochoroiditis during the first 2 years of life in infants with treated congenital toxoplasmosis. Pediatr Infect Dis J 27:27–32. doi: 10.1097/INF.0b013e318134286d. [DOI] [PubMed] [Google Scholar]
- 173.Mandelbrot L, Kieffer F, Sitta R, Laurichesse-Delmas H, Winer N, Mesnard L, Berrebi A, Le Bouar G, Bory JP, Cordier AG, Ville Y, Perrotin F, Jouannic JM, Biquard F, d'Ercole C, Houfflin-Debarge V, Villena I, Thiebaut R. 2018. Prenatal therapy with pyrimethamine + sulfadiazine vs spiramycin to reduce placental transmission of toxoplasmosis: a multicenter, randomized trial. Am J Obstet Gynecol 2018:S0002-9378(18)30441-1. doi: 10.1016/j.ajog.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 174.Maldonado YA, Read JS, Committee On Infectious Diseases. 2017. Diagnosis, treatment, and prevention of congenital toxoplasmosis in the United States. Pediatrics 139:e20163860. doi: 10.1542/peds.2016-3860. [DOI] [PubMed] [Google Scholar]
- 175.Valentini P, Annunziata ML, Angelone DF, Masini L, De Santis M, Testa A, Grillo RL, Speziale D, Ranno O. 2009. Role of spiramycin/cotrimoxazole association in the mother-to-child transmission of toxoplasmosis infection in pregnancy. Eur J Clin Microbiol Infect Dis 28:297–300. doi: 10.1007/s10096-008-0612-5. [DOI] [PubMed] [Google Scholar]
- 176.Valentini P, Buonsenso D, Barone G, Serranti D, Calzedda R, Ceccarelli M, Speziale D, Ricci R, Masini L. 2015. Spiramycin/cotrimoxazole versus pyrimethamine/sulfonamide and spiramycin alone for the treatment of toxoplasmosis in pregnancy. J Perinatol 35:90–94. doi: 10.1038/jp.2014.161. [DOI] [PubMed] [Google Scholar]
- 177.Olariu TR, Remington JS, McLeod R, Alam A, Montoya JG. 2011. Severe congenital toxoplasmosis in the United States: clinical and serologic findings in untreated infants. Pediatr Infect Dis J 30:1056–1061. doi: 10.1097/INF.0b013e3182343096. [DOI] [PubMed] [Google Scholar]
- 178.Peyron F, McLeod R, Ajzenberg D, Contopoulos-Ioannidis D, Kieffer F, Mandelbrot L, Sibley LD, Pelloux H, Villena I, Wallon M, Montoya JG. 2017. Congenital toxoplasmosis in France and the United States: one parasite, two diverging approaches. PLoS Negl Trop Dis 11:e0005222. doi: 10.1371/journal.pntd.0005222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wilson CB, Remington JS, Stagno S, Reynolds DW. 1980. Development of adverse sequelae in children born with subclinical congenital Toxoplasma infection. Pediatrics 66:767–774. [PubMed] [Google Scholar]
- 180.Phan L, Kasza K, Jalbrzikowski J, Noble AG, Latkany P, Kuo A, Mieler W, Meyers S, Rabiah P, Boyer K, Swisher C, Mets M, Roizen N, Cezar S, Remington J, Meier P, McLeod R, Toxoplasmosis Study Group. 2008. Longitudinal study of new eye lesions in treated congenital toxoplasmosis. Ophthalmology 115:553.e8–559.e8. doi: 10.1016/j.ophtha.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 181.Guerina NG, Hsu HW, Meissner HC, Maguire JH, Lynfield R, Stechenberg B, Abroms I, Pasternack MS, Hoff R, Eaton RB, Grady GF. 1994. Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. The New England Regional Toxoplasma Working Group. N Engl J Med 330:1858–1863. [DOI] [PubMed] [Google Scholar]
- 182.McLeod R, Boyer K, Karrison T, Kasza K, Swisher C, Roizen N, Jalbrzikowski J, Remington J, Heydemann P, Noble AG, Mets M, Holfels E, Withers S, Latkany P, Meier P, Toxoplasmosis Study Group. 2006. Outcome of treatment for congenital toxoplasmosis, 1981–2004: the national collaborative Chicago-based, congenital toxoplasmosis study. Clin Infect Dis 42:1383–1394. doi: 10.1086/501360. [DOI] [PubMed] [Google Scholar]
- 183.McAuley J, Boyer KM, Patel D, Mets M, Swisher C, Roizen N, Wolters C, Stein L, Stein M, Schey W, Remington J, Meier P, Johnson D, Heydeman P, Holfels E, Withers S, Mack D, Brown C, Patton D, McLeod R. 1994. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial. Clin Infect Dis 18:38–72. doi: 10.1093/clinids/18.1.38. [DOI] [PubMed] [Google Scholar]
- 184.Teil J, Dupont D, Charpiat B, Corvaisier S, Vial T, Leboucher G, Wallon M, Peyron F. 2016. Treatment of congenital toxoplasmosis: safety of the sulfadoxine-pyrimethamine combination in children based on a method of causality assessment. Pediatr Infect Dis J 35:634–638. doi: 10.1097/INF.0000000000001120. [DOI] [PubMed] [Google Scholar]
- 185.Opsteegh M, Kortbeek TM, Havelaar AH, van der Giessen JW. 2015. Intervention strategies to reduce human Toxoplasma gondii disease burden. Clin Infect Dis 60:101–107. doi: 10.1093/cid/ciu721. [DOI] [PubMed] [Google Scholar]
- 186.Rajapakse S, Weeratunga P, Rodrigo C, de Silva NL, Fernando SD. 2017. Prophylaxis of human toxoplasmosis: a systematic review. Pathog Glob Health 111:333–342. doi: 10.1080/20477724.2017.1370528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Boyer KM, Holfels E, Roizen N, Swisher C, Mack D, Remington J, Withers S, Meier P, McLeod R, Toxoplasmosis Study Group. 2005. Risk factors for Toxoplasma gondii infection in mothers of infants with congenital toxoplasmosis: implications for prenatal management and screening. Am J Obstet Gynecol 192:564–571. doi: 10.1016/j.ajog.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 188.Berghold C, Herzog SA, Jakse H, Berghold A. 2016. Prevalence and incidence of toxoplasmosis: a retrospective analysis of mother-child examinations, Styria, Austria, 1995 to 2012. Euro Surveill 21:30317. doi: 10.2807/1560-7917.ES.2016.21.33.30317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Stillwaggon E, Carrier CS, Sautter M, McLeod R. 2011. Maternal serologic screening to prevent congenital toxoplasmosis: a decision-analytic economic model. PLoS Negl Trop Dis 5:e1333. doi: 10.1371/journal.pntd.0001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Prusa AR, Kasper DC, Sawers L, Walter E, Hayde M, Stillwaggon E. 2017. Congenital toxoplasmosis in Austria: prenatal screening for prevention is cost-saving. PLoS Negl Trop Dis 11:e0005648. doi: 10.1371/journal.pntd.0005648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Derouin FLC, Sumyuen MH, Romand S, Garin YJ. 1995. Experimental models of toxoplasmosis. Pharmacological applications. Parasite 2:243–256. [DOI] [PubMed] [Google Scholar]
- 192.Tsunoda K, Suzuki K, Ito S, Tsutsumi Y. 1966. Isolation of toxoplasma from experimental pigs medicated with sulfamonomethoxine. Natl Inst Anim Health Q (Tokyo) 6:83–88. [PubMed] [Google Scholar]
- 193.Huldt G. 1966. Experimental toxoplasmosis. Effect of cortico-steroids on rabbits with varying degree of immunity. Acta Pathol Microbiol Scand 68:605–621. [DOI] [PubMed] [Google Scholar]
- 194.Harper JS III, London WT, Sever JL. 1985. Five drug regimens for treatment of acute toxoplasmosis in squirrel monkeys. Am J Trop Med Hyg 34:50–57. doi: 10.4269/ajtmh.1985.34.50. [DOI] [PubMed] [Google Scholar]
- 195.Dukaczewska A, Tedesco R, Liesenfeld O. 2015. Experimental models of ocular infection with Toxoplasma gondii. Eur J Microbiol Immunol 5:293–305. doi: 10.1556/1886.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Munoz M, Liesenfeld O, Heimesaat MM. 2011. Immunology of Toxoplasma gondii. Immunol Rev 240:269–285. doi: 10.1111/j.1600-065X.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 197.Innes EA. 1997. Toxoplasmosis: comparative species susceptibility and host immune response. Comp Immunol Microbiol Infect Dis 20:131–138. doi: 10.1016/S0147-9571(96)00038-0. [DOI] [PubMed] [Google Scholar]
- 198.Dubey JP, Frenkel JK. 1998. Toxoplasmosis of rats: a review, with considerations of their value as an animal model and their possible role in epidemiology. Vet Parasitol 77:1–32. doi: 10.1016/S0304-4017(97)00227-6. [DOI] [PubMed] [Google Scholar]
- 199.Benavides J, Fernandez M, Castano P, Ferreras MC, Ortega-Mora L, Perez V. 2017. Ovine toxoplasmosis: a new look at its pathogenesis. J Comp Pathol 157:34–38. doi: 10.1016/j.jcpa.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 200.Behnke MS, Khan A, Lauron EJ, Jimah JR, Wang Q, Tolia NH, Sibley LD. 2015. Rhoptry proteins ROP5 and ROP18 are major murine virulence factors in genetically divergent South American strains of Toxoplasma gondii. PLoS Genet 11:e1005434. doi: 10.1371/journal.pgen.1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liesenfeld O. 2002. Oral infection of C57BL/6 mice with Toxoplasma gondii: A new model of inflammatory bowel disease? J Infect Dis 185:S96–S101. doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- 202.Brun-Pascaud M, Chau F, Garry L, Jacobus D, Derouin F, Girard PM. 1996. Combination of PS-15, epiroprim, or pyrimethamine with dapsone in prophylaxis of Toxoplasma gondii and Pneumocystis carinii dual infection in a rat model. Antimicrob Agents Chemother 40:2067–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Piketty C, Derouin F, Rouveix B, Pocidalo JJ. 1990. In vivo assessment of antimicrobial agents against Toxoplasma gondii by quantification of parasites in the blood, lungs, and brain of infected mice. Antimicrob Agents Chemother 34:1467–1472. doi: 10.1128/AAC.34.8.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Eyles DE, Coleman N. 1952. Tests of 2,4-diaminopyrimidines on toxoplasmosis. Public Health Rep 67:249–252. doi: 10.2307/4588050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Petrovicky O, Styblova V. 1955. Pyrimethamine (daraprim) in therapy of human toxoplasmosis. Cas Lek Cesk 94:937–939. [PubMed] [Google Scholar]
- 206.Petrovicky O. 1955. Meningoencephalitis toxoplasmatica acuta cured with pyrimethamine. Cas Lek Cesk 94:486–490. [PubMed] [Google Scholar]
- 207.Beverley JK, Fry BA. 1957. The treatment of experimental toxoplasmosis in rabbits. Br J Pharmacol Chemother 12:185–188. doi: 10.1111/j.1476-5381.1957.tb00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Eyles DE, Jones FE. 1955. The chemotherapeutic effect of pyrimethamine and sulfadiazine on toxoplasmosis of the Norway rat. Antibiot Chemother (Northfield) 5:731–734. [PubMed] [Google Scholar]
- 209.Eyles DE, Coleman N. 1955. An evaluation of the curative effects of pyrimethamine and sulfadiazine, alone and in combination, on experimental mouse toxoplasmosis. Antibiot Chemother (Northfield) 5:529–539. [PubMed] [Google Scholar]
- 210.Frenkel JK, Weber RW, Lunde MN. 1960. Acute toxoplasmosis. Effective treatment with pyrimethamine, sulfadiazine, leucovorin calcium, and yeast. JAMA 173:1471–1476. [DOI] [PubMed] [Google Scholar]
- 211.Giles CL, Jacobs L, Melton ML. 1964. Experimental use of folinic acid in the treatment of toxoplasmosis with pyrimethamine. Arch Ophthalmol 72:82–85. doi: 10.1001/archopht.1964.00970020084019. [DOI] [PubMed] [Google Scholar]
- 212.Thiermann E, Apt W, Atias A, Lorca M, Olguin J. 1978. A comparative study of some combined treatment regimens in acute toxoplasmosis in mice. Am J Trop Med Hyg 27:747–750. doi: 10.4269/ajtmh.1978.27.747. [DOI] [PubMed] [Google Scholar]
- 213.Derouin F, Piketty C, Chastang C, Chau F, Rouveix B, Pocidalo JJ. 1991. Anti-Toxoplasma effects of dapsone alone and combined with pyrimethamine. Antimicrob Agents Chemother 35:252–255. doi: 10.1128/AAC.35.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Biocca E, Pasqualin R. 1942. A acao terapeutica de alguns compostos sulfanilamidicos na infeccao experimental por Toxoplasma. Arq Inst Biol (Sao Paulo) 26:107–109. [Google Scholar]
- 215.Nguyen BT, Stadtsbaeder S. 1983. Comparative effects of cotrimoxazole (trimethoprim-sulphamethoxazole), pyrimethamine-sulphadiazine and spiramycin during avirulent infection with Toxoplasma gondii (Beverley strain) in mice. Br J Pharmacol 79:923–928. doi: 10.1111/j.1476-5381.1983.tb10537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Alvarado-Esquivel C, Niewiadomski A, Schweickert B, Liesenfeld O. 2011. Antiparasitic treatment suppresses production and avidity of Toxoplasma gondii-specific antibodies in a murine model of acute infection. Eur J Microbiol Immunol 1:249–255. doi: 10.1556/EuJMI.1.2011.3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Seah SK. 1975. Chemotherapy in experimental toxoplasmosis: comparison of the efficacy of trimethoprim-sulfur and pyrimethamine-sulfur combinations. J Trop Med Hyg 78:150–153. [PubMed] [Google Scholar]
- 218.Coradello H, Kretschmer S. 1978. A comparison of Ultrax, diazil, Bactrim and spiramycin in experimental toxoplasmosis in mice (author's translation). Wien Klin Wochenschr 90:25–29. [PubMed] [Google Scholar]
- 219.Grujic J, Djurkovic-Djakovic O, Nikolic A, Klun I, Bobic B. 2005. Effectiveness of spiramycin in murine models of acute and chronic toxoplasmosis. Int J Antimicrob Agents 25:226–230. doi: 10.1016/j.ijantimicag.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 220.Chew WK, Segarra I, Ambu S, Mak JW. 2012. Significant reduction of brain cysts caused by Toxoplasma gondii after treatment with spiramycin coadministered with metronidazole in a mouse model of chronic toxoplasmosis. Antimicrob Agents Chemother 56:1762–1768. doi: 10.1128/AAC.05183-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mack DG, McLeod R. 1984. New micromethod to study the effect of antimicrobial agents on Toxoplasma gondii: comparison of sulfadoxine and sulfadiazine individually and in combination with pyrimethamine and study of clindamycin, metronidazole, and cyclosporin A. Antimicrob Agents Chemother 26:26–30. doi: 10.1128/AAC.26.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Harris C, Salgo MP, Tanowitz HB, Wittner M. 1988. In vitro assessment of antimicrobial agents against Toxoplasma gondii. J Infect Dis 157:14–22. doi: 10.1093/infdis/157.1.14. [DOI] [PubMed] [Google Scholar]
- 223.Nikolic T, Djurkovic-Djakovic O, Bobic B, Nikolic A, Babic D. 1999. Treatment protocol determines the efficacy of clindamycin in acute murine toxoplasmosis. Int J Antimicrob Agents 11:145–149. doi: 10.1016/S0924-8579(98)00064-8. [DOI] [PubMed] [Google Scholar]
- 224.Hofflin JM, Conley FK, Remington JS. 1987. Murine model of intracerebral toxoplasmosis. J Infect Dis 155:550–557. doi: 10.1093/infdis/155.3.550. [DOI] [PubMed] [Google Scholar]
- 225.Djurkovic-Djakovic O, Nikolic T, Robert-Gangneux F, Bobic B, Nikolic A. 1999. Synergistic effect of clindamycin and atovaquone in acute murine toxoplasmosis. Antimicrob Agents Chemother 43:2240–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Araujo FG, Huskinson J, Remington JS. 1991. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob Agents Chemother 35:293–299. doi: 10.1128/AAC.35.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Araujo FG, Huskinson-Mark J, Gutteridge WE, Remington JS. 1992. In vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against the cyst form of Toxoplasma gondii. Antimicrob Agents Chemother 36:326–330. doi: 10.1128/AAC.36.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Romand S, Pudney M, Derouin F. 1993. In vitro and in vivo activities of the hydroxynaphthoquinone atovaquone alone or combined with pyrimethamine, sulfadiazine, clarithromycin, or minocycline against Toxoplasma gondii. Antimicrob Agents Chemother 37:2371–2378. doi: 10.1128/AAC.37.11.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Araujo FG, Lin T, Remington JS. 1993. The activity of atovaquone (566C80) in murine toxoplasmosis is markedly augmented when used in combination with pyrimethamine or sulfadiazine. J Infect Dis 167:494–497. doi: 10.1093/infdis/167.2.494. [DOI] [PubMed] [Google Scholar]
- 230.Djurkovic-Djakovic O, Milenkovic V, Nikolic A, Bobic B, Grujic J. 2002. Efficacy of atovaquone combined with clindamycin against murine infection with a cystogenic (Me49) strain of Toxoplasma gondii. J Antimicrob Chemother 50:981–987. doi: 10.1093/jac/dkf251. [DOI] [PubMed] [Google Scholar]
- 231.Mitchell SM, Zajac AM, Davis WL, Lindsay DS. 2004. Efficacy of ponazuril in vitro and in preventing and treating Toxoplasma gondii infections in mice. J Parasitol 90:639–642. doi: 10.1645/GE-250R. [DOI] [PubMed] [Google Scholar]
- 232.Mitchell SM, Zajac AM, Kennedy T, Davis W, Dubey JP, Lindsay DS. 2006. Prevention of recrudescent toxoplasmic encephalitis using ponazuril in an immunodeficient mouse model. J Eukaryot Microbiol 53(Suppl 1):S164–S165. doi: 10.1111/j.1550-7408.2006.00217.x. [DOI] [PubMed] [Google Scholar]
- 233.Schultz TL, Hencken CP, Woodard LE, Posner GH, Yolken RH, Jones-Brando L, Carruthers VB. 2014. A thiazole derivative of artemisinin moderately reduces Toxoplasma gondii cyst burden in infected mice. J Parasitol 100:516–521. doi: 10.1645/13-451.1. [DOI] [PubMed] [Google Scholar]
- 234.Ferreira RA, Oliveira AB, Ribeiro MF, Tafuri WL, Vitor RW. 2006. Toxoplasma gondii: in vitro and in vivo activities of the hydroxynaphthoquinone 2-hydroxy-3-(1′-propen-3-phenyl)-1,4-naphthoquinone alone or combined with sulfadiazine. Exp Parasitol 113:125–129. doi: 10.1016/j.exppara.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 235.Doggett JS, Nilsen A, Forquer I, Wegmann KW, Jones-Brando L, Yolken RH, Bordon C, Charman SA, Katneni K, Schultz T, Burrows JN, Hinrichs DJ, Meunier B, Carruthers VB, Riscoe MK. 2012. Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc Natl Acad Sci U S A 109:15936–15941. doi: 10.1073/pnas.1208069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Benmerzouga I, Checkley LA, Ferdig MT, Arrizabalaga G, Wek RC, Sullivan WJ Jr. 2015. Guanabenz repurposed as an antiparasitic with activity against acute and latent toxoplasmosis. Antimicrob Agents Chemother 59:6939–6945. doi: 10.1128/AAC.01683-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Vidadala RS, Rivas KL, Ojo KK, Hulverson MA, Zambriski JA, Bruzual I, Schultz TL, Huang W, Zhang Z, Scheele S, DeRocher AE, Choi R, Barrett LK, Siddaramaiah LK, Hol WG, Fan E, Merritt EA, Parsons M, Freiberg G, Marsh K, Kempf DJ, Carruthers VB, Isoherranen N, Doggett JS, Van Voorhis WC, Maly DJ. 2016. Development of an orally available and central nervous system (CNS) penetrant Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) inhibitor with minimal human Ether-a-go-go-related gene (hERG) activity for the treatment of toxoplasmosis. J Med Chem 59:6531–6546. doi: 10.1021/acs.jmedchem.6b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rutaganira FU, Barks J, Dhason MS, Wang Q, Lopez MS, Long S, Radke JB, Jones NG, Maddirala AR, Janetka JW, El Bakkouri M, Hui R, Shokat KM, Sibley LD. 2017. Inhibition of calcium dependent protein kinase 1 (CDPK1) by pyrazolopyrimidine analogs decreases establishment and reoccurrence of central nervous system disease by Toxoplasma gondii. J Med Chem 60:9976–9989. doi: 10.1021/acs.jmedchem.7b01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Couzin-Frankel J. 2013. Breakthrough of the year 2013. Cancer immunotherapy. Science 342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 240.Werner H, Masihi KN, Tischer I, Adusu E. 1977. The effect of chemo-immunotherapy with SDDS, pyrimethamine and anti-Toxoplasma serum on Toxoplasma gondii cysts in latent infected NMRI mice. Tropenmed Parasitol 28:528–532. [PubMed] [Google Scholar]
- 241.Araujo FG, Remington JS. 1991. Synergistic activity of azithromycin and gamma interferon in murine toxoplasmosis. Antimicrob Agents Chemother 35:1672–1673. doi: 10.1128/AAC.35.8.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Khan IA, Matsuura T, Kasper LH. 1994. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect Immun 62:1639–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hoover DR, Saah AJ, Bacellar H, Phair J, Detels R, Anderson R, Kaslow RA. 1993. Clinical manifestations of AIDS in the era of pneumocystis prophylaxis. Multicenter AIDS Cohort Study. N Engl J Med 329:1922–1926. doi: 10.1056/NEJM199312233292604. [DOI] [PubMed] [Google Scholar]
- 244.Brun-Pascaud M, Chau F, Simonpoli AM, Girard PM, Derouin F, Pocidalo JJ. 1994. Experimental evaluation of combined prophylaxis against murine pneumocystosis and toxoplasmosis. J Infect Dis 170:653–658. doi: 10.1093/infdis/170.3.653. [DOI] [PubMed] [Google Scholar]
- 245.Brun-Pascaud M, Rajagopalan-Levasseur P, Chau F, Bertrand G, Garry L, Derouin F, Girard PM. 1998. Drug evaluation of concurrent Pneumocystis carinii, Toxoplasma gondii, and Mycobacterium avium complex infections in a rat model. Antimicrob Agents Chemother 42:1068–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Maenz M, Schluter D, Liesenfeld O, Schares G, Gross U, Pleyer U. 2014. Ocular toxoplasmosis past, present and new aspects of an old disease. Prog Retin Eye Res 39:77–106. doi: 10.1016/j.preteyeres.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 247.Gormley PD, Pavesio CE, Minnasian D, Lightman S. 1998. Effects of drug therapy on Toxoplasma cysts in an animal model of acute and chronic disease. Invest Ophthalmol Vis Sci 39:1171–1175. [PubMed] [Google Scholar]
- 248.Tabbara KF, Nozik RA, O'Connor GR. 1974. Clindamycin effects on experimental ocular toxoplasmosis in the rabbit. Arch Ophthalmol 92:244–247. doi: 10.1001/archopht.1974.01010010252017. [DOI] [PubMed] [Google Scholar]
- 249.Davidson MG, Lappin MR, Rottman JR, Tompkins MB, English RV, Bruce AT, Jayawickrama J. 1996. Paradoxical effect of clindamycin in experimental, acute toxoplasmosis in cats. Antimicrob Agents Chemother 40:1352–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Rollins DF, Tabbara KF, Ghosheh R, Nozik RA. 1982. Minocycline in experimental ocular toxoplasmosis in the rabbit. Am J Ophthalmol 93:361–365. doi: 10.1016/0002-9394(82)90541-4. [DOI] [PubMed] [Google Scholar]
- 251.Lopes CD, Silva NM, Ferro EAV, Sousa RA, Firmino ML, Bernardes ES, Roque-Barreira MC, Pena JDO. 2009. Azithromycin reduces ocular infection during congenital transmission of toxoplasmosis in the Calomys callosus model. J Parasitol 95:1005–1010. doi: 10.1645/GE-1765.1. [DOI] [PubMed] [Google Scholar]
- 252.Araujo FG, Remington JS. 1974. Effect of clindamycin on acute and chronic toxoplasmosis in mice. Antimicrob Agents Chemother 5:647–651. doi: 10.1128/AAC.5.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Schoondermark-van de Ven E, Galama J, Vree T, Camps W, Baars I, Eskes T, Meuwissen J, Melchers W. 1995. Study of treatment of congenital Toxoplasma gondii infection in rhesus monkeys with pyrimethamine and sulfadiazine. Antimicrob Agents Chemother 39:137–144. doi: 10.1128/AAC.39.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Schoondermark-van de Ven E, Melchers W, Camps W, Eskes T, Meuwissen J, Galama J. 1994. Effectiveness of spiramycin for treatment of congenital Toxoplasma-gondii infection in rhesus-monkeys. Antimicrob Agents Chemother 38:1930–1936. doi: 10.1128/AAC.38.9.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol 149:175–180. [PubMed] [Google Scholar]
- 256.Suzuki Y, Conley FK, Remington JS. 1990. Treatment of toxoplasmic encephalitis in mice with recombinant gamma interferon. Infect Immun 58:3050–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Miedouge M, Bessieres MH, Cassaing S, Swierczynski B, Seguela JP. 1997. Parasitemia and parasitic loads in acute infection and after anti-gamma-interferon treatment in a toxoplasmic mouse model. Parasitol Res 83:339–344. doi: 10.1007/s004360050258. [DOI] [PubMed] [Google Scholar]
- 258.Nicoll S, Wright S, Maley SW, Burns S, Buxton D. 1997. A mouse model of recrudescence of Toxoplasma gondii infection. J Med Microbiol 46:263–266. doi: 10.1099/00222615-46-3-263. [DOI] [PubMed] [Google Scholar]
- 259.McCabe RE, Luft BJ, Remington JS. 1986. The effects of cyclosporine on Toxoplasma gondii in vivo and in vitro. Transplantation 41:611–615. doi: 10.1097/00007890-198605000-00012. [DOI] [PubMed] [Google Scholar]
- 260.Sumyuen MH, Garin YJ, Derouin F. 1996. Effect of immunosuppressive drug regimens on acute and chronic murine toxoplasmosis. Parasitol Res 82:681–686. doi: 10.1007/s004360050185. [DOI] [PubMed] [Google Scholar]
- 261.Huo XX, Wang L, Chen ZW, Chen H, Xu XC, Zhang AM, Song XR, Luo QL, Xu YH, Fu Y, Wang H, Du J, Cai YH, Lun ZR, Lu FL, Wang Y, Shen JL. 2013. Preventive effect of pidotimod on reactivated toxoplasmosis in mice. Parasitol Res 112:3041–3051. doi: 10.1007/s00436-013-3488-y. [DOI] [PubMed] [Google Scholar]
- 262.Denkers EY, Gazzinelli RT. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev 11:569–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lieberman LA, Hunter CA. 2002. The role of cytokines and their signaling pathways in the regulation of immunity to Toxoplasma gondii. Int Rev Immunol 21:373–403. doi: 10.1080/08830180213281. [DOI] [PubMed] [Google Scholar]
- 264.Taylor GA, Feng CG, Sher A. 2007. Control of IFN-gamma-mediated host resistance to intracellular pathogens by immunity-related GTPases (p47 GTPases). Microbes Infect 9:1644–1651. doi: 10.1016/j.micinf.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 265.Suzuki Y, Kang H, Parmley S, Lim S, Park D. 2000. Induction of tumor necrosis factor-alpha and inducible nitric oxide synthase fails to prevent toxoplasmic encephalitis in the absence of interferon-gamma in genetically resistant BALB/c mice. Microbes Infect 2:455–462. doi: 10.1016/S1286-4579(00)00318-X. [DOI] [PubMed] [Google Scholar]
- 266.Scholer N, Krause K, Kayser O, Muller RH, Borner K, Hahn H, Liesenfeld O. 2001. Atovaquone nanosuspensions show excellent therapeutic effect in a new murine model of reactivated toxoplasmosis. Antimicrob Agents Chemother 45:1771–1779. doi: 10.1128/AAC.45.6.1771-1779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Shubar HM, Lachenmaier S, Heimesaat MM, Lohman U, Mauludin R, Mueller RH, Fitzner R, Borner K, Liesenfeld O. 2011. SDS-coated atovaquone nanosuspensions show improved therapeutic efficacy against experimental acquired and reactivated toxoplasmosis by improving passage of gastrointestinal and blood-brain barriers. J Drug Target 19:114–124. doi: 10.3109/10611861003733995. [DOI] [PubMed] [Google Scholar]
- 268.Dunay IR, Heimesaat MM, Bushrab FN, Muller RH, Stocker H, Arasteh K, Kurowski M, Fitzner R, Borner K, Liesenfeld O. 2004. Atovaquone maintenance therapy prevents reactivation of toxoplasmic encephalitis in a murine model of reactivated toxoplasmosis. Antimicrob Agents Chemother 48:4848–4854. doi: 10.1128/AAC.48.12.4848-4854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Shubar HM, Dunay IR, Lachenmaier S, Dathe M, Bushrab FN, Mauludin R, Muller RH, Fitzner R, Borner K, Liesenfeld O. 2009. The role of apolipoprotein E in uptake of atovaquone into the brain in murine acute and reactivated toxoplasmosis. J Drug Target 17:257–267. doi: 10.1080/10611860902718680. [DOI] [PubMed] [Google Scholar]
- 270.Sauer I, Dunay IR, Weisgraber K, Bienert M, Dathe M. 2005. An apolipoprotein E-derived peptide mediates uptake of sterically stabilized liposomes into brain capillary endothelial cells. Biochemistry 44:2021–2029. doi: 10.1021/bi048080x. [DOI] [PubMed] [Google Scholar]
- 271.Dunay IR, Chan WC, Haynes RK, Sibley LD. 2009. Artemisone and artemiside control acute and reactivated toxoplasmosis in a murine model. Antimicrob Agents Chemother 53:4450–4456. doi: 10.1128/AAC.00502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]