Streptococcus agalactiae, or group B streptococcus (GBS), is a major neonatal pathogen. Recent data have elucidated the global prevalence of maternal and neonatal colonization, but gaps still remain in the epidemiology of this species.
KEYWORDS: group B streptococcus, Streptococcus agalactiae, epidemiology, serotype, sequence type, clonal complex, surveillance, vaccine, immunization, perinatal infections
SUMMARY
Streptococcus agalactiae, or group B streptococcus (GBS), is a major neonatal pathogen. Recent data have elucidated the global prevalence of maternal and neonatal colonization, but gaps still remain in the epidemiology of this species. A number of phenotypic and genotypic classifications can be used to identify the diversity of GBS strains, and some are more discriminatory than others. This review explores the main schemes used for GBS epidemiology and further details the targets for epidemiological surveillance. Current screening practices across the world provide a unique opportunity to gain detailed information on maternal colonizing strains and neonatal disease-causing strains, which is vital for monitoring and therapeutics, if sufficient detail can be extracted. Deciphering which isolates are circulating within specific populations and recording targets within invasive strains are crucial steps in monitoring the implementation of therapeutics, such as vaccines, as well as developing novel therapies against prevalent GBS strains. Having a detailed understanding of global GBS epidemiology will prove invaluable for understanding the pathogenesis of this organism and equipping future prevention strategies for success.
INTRODUCTION
Streptococcus agalactiae is the sole member of the Lancefield group, i.e., group B streptococcus (GBS), and therefore is commonly referred to as GBS (1). This Gram-positive coccus resides as a commensal of the gastrointestinal and genitourinary tracts of humans; however, it has the capacity to cause serious infections. These are often opportunistic in nature, affecting the elderly, the immunocompromised, and particularly neonates, for which GBS is a major cause of morbidity and mortality (2, 3). Recently, infections of healthy adults have also been reported (4, 5). In the affected populations, the spectrum of disease ranges from sepsis, pneumonia, and meningitis to endocarditis. GBS disease in neonates can be classified based on the time of onset: early-onset disease (EOD) occurs in the first week of life (6), while any disease presentation after this to up to 3 months of life is classified as late-onset disease (LOD) (7). In neonates, the majority of early-onset GBS disease occurs as a result of transmission from a colonized mother, either through ascending infection or during the process of vaginal birth (8). In contrast, late-onset sepsis is believed to be contracted via nosocomial or community-acquired means (9). Due to the potential for transmission from the mother, universal screening of pregnant women by either risk-based or culture-based methods has been implemented in most countries. In such countries, intrapartum antibiotic prophylaxis (IAP) is administered before delivery to women at risk of GBS disease (delivery at <37 weeks of gestation, intrapartum temperature of ≥38.0°C, or rupture of membranes for ≥18 h) or with GBS colonization (10). This widespread treatment strategy has been successful in reducing EOD significantly but has had no effect on LOD (10). Screening for GBS by culture currently involves vaginal and rectal swabs collected at 35 to 37 weeks of gestation and cultured in selective enrichment media (11). Additionally, diagnostic molecular techniques to detect GBS have also been described (12). The outcome of this screening is either GBS colonization or absence. However, there is a plethora of other information about these organisms that could be obtained during routine screening that may prove vital in furthering our understanding of their epidemiology; as such, we are currently underutilizing this potentially valuable surveillance source. Expanding our knowledge of current targets through the use of phenotypic and molecular assays to identify and differentiate isolates within the population will likely prove essential for future GBS interventions.
IDENTIFICATION AND CLASSIFICATION
Several methods for identifying and characterizing GBS for epidemiological and diagnostic purposes have been described (13). These include serotyping (based on the capsular polysaccharide), typing of surface proteins, multilocus sequence typing (MLST), multilocus variable-number tandem-repeat analysis (MLVA), and, more recently, clustered regularly interspaced short palindromic repeat (CRISPR)-locus assays. All have advantages and disadvantages, and all convey different levels of epidemiologic data in the context of surveillance.
Serotyping
The capsular polysaccharide (CPS) that encapsulates GBS is a major virulence factor, but it also determines the serotype. A total of 10 serotypes have been identified, with CPS IX being the most recently proposed one (14). Serotypes can be identified through latex agglutination and molecular assays, such as multiplex PCR (13, 15–17). As one of the first discriminating factors for GBS, the serotypes have been described in the literature as a way of grouping isolates in an attempt to understand their prevalence in maternal colonization and neonatal disease. Serotyping provides valuable information that allows classification into serologically similar groups, and patterns have emerged based on global prevalence. The most common global serotypes include CPS Ia, Ib, II, III, and V. In a perinatal context, however, many studies describe limited serotype testing (18–20), and others were completed prior to inclusion of CPS IX (21–23). These are major limitations in such studies, but there are still numerous studies that have included all known serotypes, and these generally support the overarching predominance of the five main CPS types in a perinatal context (24, 25).
Global serotype distribution.
A recent meta-analysis of maternal colonization described a global data set of serotype prevalences (24). Russell and colleagues highlighted the regional variation for the less common serotypes, such as CPS IV, VI, VII, VIII, and IX. Prior to this analysis, the general consensus was that approximately 10 to 30% of pregnant women were colonized with GBS; this analysis supports previous estimates and suggests that the global colonization rate in pregnant women is 18%, with regional estimates varying from 11 to 35%. South and East Asian countries had the lowest reported prevalences (11 to 12.5%), while the Caribbean was observed to have the highest prevalence (34.7%) (24). The subsequently estimated neonatal disease rate for GBS is 0.49 per 1,000 live births globally, with the highest incidence in Africa (1.12 per 1,000 live births) and the lowest in Asia (0.30 per 1,000 live births) (25). Five serotypes (Ia, Ib, II, III, and V) have been estimated to encompass approximately 98% and 97% of the serotypes identified during maternal colonization and neonatal disease, respectively. This leaves a small proportion of colonization/neonatal disease cases attributable to the remaining five serotypes; however, it is important to highlight these serotypes, as the dynamic has the potential to shift. Several studies have highlighted the variation in serotype prevalence globally, and the results are summarized here, along with general trends, for each serotype (Table 1).
TABLE 1.
Summary of various serotyping distribution studies that highlight serotype variation, showing the variety of patients, specimens, clinical presentations, and methods useda
| Study authors (yr) | Location | Patients | Specimen type(s) | Clinical presentation | Serotyping method(s) | Serotypes | Reference |
|---|---|---|---|---|---|---|---|
| Lin et al. (2016) | Central Taiwan | Neonates to adults | Blood | Invasive | PCR | All 10 | 28 |
| Pregnant women | Vaginal and rectal | Carriage | PCR | All 10 | 28 | ||
| Uh et al. (2005) | South Korea | Adults | NS | Infection | Latex agglutination | Only Ia, Ib, II to VIII | 29 |
| Turner et al. (2012) | Thai-Myanmar border | Pregnant women | Vaginal and rectal | Carriage | Latex agglutination and PCR | All 10 | 30 |
| Lopez et al. (2017) | Catalonia, Spain | Pregnant women and neonates | NS | Carriage | NS | NS | 31 |
| NS | Invasive | NS | NS | 31 | |||
| Elikwu et al. (2016) | Nigeria | Pregnant women | Vaginal and rectal | Carriage | PCR | All 10 | 32 |
| Foster-Nyarko et al. (2016) | Gambia | Infants | Nasopharyngeal | Carriage | Latex agglutination and PCR | All 10 | 33 |
| Romain et al. (2017) | France | Neonates and infants | Blood and cerebrospinal fluid | Invasive | NS (French National Reference Centre for Streptococci) | Only Ia to V | 35 |
| Bjorsdottir et al. (2016) | Iceland | Adults | Blood and synovial fluid | Invasive | Latex agglutination | All 10 | 36 |
| Teatero et al. (2017) | Toronto, Canada | Pregnant women | Vaginal and rectal | Carriage | PCR and latex agglutination | All 10 | 37 |
| Teatero et al. (2015) | Toronto, Canada | Neonates to adults | Blood, cerebrospinal fluid, and tissue | Invasive | PCR and latex agglutination | All 10 | 38 |
| Ferrieri et al. (2013) | Minnesota, USA | Infants | Blood, cerebrospinal fluid, bone, joint, and postmortem samples from liver and lung | Invasive | Immunoprecipitation | All 10 | 39 |
| Blumberg et al. (1996) | Atlanta, GA, USA | Infants to adults | Blood, peritoneal fluid, and cerebrospinal fluid | Invasive | Capillary precipitin | Only Ia, Ib, II to V | 41 |
| Belard et al. (2015) | Gabon | Pregnant women | Vaginal and rectal | Carriage | Latex agglutination | All 10 | 42 |
| Suara et al. (1994) | Gambia | Pregnant women | Vaginal and rectal | Carriage | Capillary precipitin | Only I to VI | 43 |
| Neonates | Throat, rectal, and umbilical | Carriage | Capillary precipitin | Only I to VI | 43 | ||
| Shabayek et al. (2014) | Egypt | Pregnant and nonpregnant women | Vaginal | Carriage | PCR and latex agglutination | All 10 | 44 |
| Lachenauer et al. (1999) | Japan | Pregnant women | Vaginal | Carriage | Inhibition enzyme-linked immunosorbent assay | Only Ia, Ib, II to VIII | 45 |
| Eskandrian et al. (2015) | Malaysia | Neonates and adults | Vaginal and urine | Carriage | PCR and latex agglutination | Only Ia, Ib, II to VII | 18 |
| Blood, wound and skin ulcers, tracheal and gastric aspirates | Invasive/infection | PCR and latex agglutination | Only Ia, Ib, II to VII | 18 | |||
| Suhaimi et al. (2017) | Malaysia | Neonates to adults (80+ yr) | Vaginal, urine, and placental | Carriage | PCR and latex agglutination | All 10 | 46 |
| Blood and tissue | Invasive | PCR and latex agglutination | All 10 | 46 | |||
| Dutra et al. (2014) | Brazil | Pregnant women | Vaginal and perianal | Carriage | Capillary precipitin | Only Ia, Ib, II to VIII | 19 |
| Adults | Blood, urine, and male genital tract discharge | Invasive/infection | Capillary precipitin | Only Ia, Ib, II to VIII | 19 | ||
| Chaudhary et al. (2017) | India | Pregnant women | Vaginal and rectal | Carriage | Latex agglutination | All 10 | 49 |
| Islam et al. (2016) | Bangladesh | Neonates | Umbilical cord | Carriage | PCR | All 10 | 50 |
| Ekelund et al. (2003) | Denmark | Neonates to adults (80+ yr) | Blood | Invasive | Precipitin test | Only Ia, Ib, II to VIII | 51 |
| Paoletti et al. (1999) | Boston, MA, USA | Neonates and adults | Genitourinary tract, others NS | NS | Precipitin test | Only Ia, Ib, II, III, V, VI, VIII | 52 |
| Yan et al. (2016) | China | Pregnant women | NS | Carriage | PCR | All 10 | 53 |
| Toyofuku et al. (2017) | Japan | Neonates | Nasopharyngeal and rectal | Carriage | PCR | Only Ia, Ib, II to VIII | 54 |
| Slotved et al. (2017) | Ghana | Pregnant women | Vaginal and rectal | Carriage | Latex agglutination | All 10 | 47 |
| Gudjonsdottir et al. (2015) | Sweden | Neonates and adults | Blood, cerebrospinal fluid, and synovial fluid | Invasive | Latex agglutination | All 10 | 55 |
| Takahara et al. (2015) | Japan | Neonate (case study) | Blood and cerebrospinal fluid | Invasive | Latex agglutination | All 10 | 56 |
NS, not stated.
Ia.
The frequency of different serotypes as colonizers makes determination of disease development an extremely difficult task. The asymptomatic colonization in some yet invasive disease progression in others emphasizes the complexity of the host-GBS interaction. Maternal colonization by serotype Ia is seen globally (24), and it is the most prevalent serotype in maternal disease, according to a limited meta-analysis (including only the United States, United Kingdom, and France) (26). Regarding neonatal disease, a previous meta-analysis found Ia to be the most frequent serotype contributing to EOD; however, this global estimate lacked data from Asia (27). The latest analysis includes Asia, and while overall trends of prevalence remain similar, South America is the only region in which CPS Ia predominates over CPS III in cases of EOD (25). In contrast, in East Asia, unlike other regions, the prevalence of Ib surpasses that of Ia (25).
Ib.
East Asia has the largest proportion of neonatal disease attributable to CPS Ib, and within the Asian region, CPS Ib is second only to CPS III in predominance (25). In Taiwan, Lin and colleagues found CPS Ib to account for 21.6% of the isolates collected from a mixed population of pregnant women, neonates, and nonpregnant adults, representing the most common serotype in the study (28). This frequency, however, was largely accounted for by nonpregnant adult invasive disease isolates, which represented 26/29 CPS Ib isolates. Similarly, in South Korea, 22% of GBS serotypes causing infection in adults were identified as Ib (29). Differences in distribution have been observed within different cohorts, such as pregnant, neonatal, and adult populations. Maternal colonization with CPS Ib in Mexico and South America has been reported to be considerably higher than that in other countries (24). On the opposite end of the spectrum, CPS Ib was the least prevalent serotype, after nontypeable (NT) isolates, to account for maternal disease (in the United States, United Kingdom, and France) (26).
II.
Serotype II is the fourth most prevalent serotype in maternal disease, according to a study including the United States, United Kingdom, and France (26), and is the fifth most prevalent in neonatal disease worldwide (25). The predominance of this serotype was observed in pregnant women at the Thai-Myanmar border, where 24% of isolates recovered were CPS II, as reported by Turner and others (30). A study in Catalonia, Spain, assessed GBS isolates from pregnant women and pathogenic strains isolated from neonates with sepsis and found that serotypes Ia and II were significantly more frequent than others as colonizing strains (31). Colonization of Nigerian pregnant women also showed a predominance of serotype Ia, while serotype II accounted for 71.4% of the neonatal colonizing isolates (32). Likewise, GBS isolated from the nasopharynxes of Gambian neonatal carriers found the highest prevalences of CPS V and II (33).
III.
CPS III is well known for its association with disease, in particular EOD, and it has been reported thoroughly worldwide and remains the leading cause of neonatal disease (25). Maternal prevalences differ by continent or region, as Africa, Australia, and New Zealand have much higher prevalences, while the Middle East, Eastern Europe, South Asia (including India and Bangladesh), and Central America have lower rates (24). In coastal Kenya, CPS III accounted for the majority of maternal colonizing isolates and 70% of the neonatal disease strains (34). In France, serotype III accounted for 83.9% of infant meningitis cases caused by GBS infection (35). The temporal dynamics of this serotype were reported for adult invasive infections in Iceland, where the only significant decline in serotype prevalence over the 39-year period was for CPS III (36). This observation contrasts with those for other populations, such as neonates, in which it is attributed to the majority of invasive disease cases (25).
IV.
Serotype IV is less prevalent globally than the other main serotypes (Ia, Ib, II, III, and V). Recent studies, however, have noticed the emergence of this serotype, particularly in Canada and the United States (37–39). These studies also reported evidence of capsular switching relevant to this serotype, a concern for targeted therapies. The presence of serotype IV within a predominantly CPS III cluster of isolates from France was suggested to have occurred through capsular switching (40), and capsular switching to serotype IV within clonal complex 17 was also reported in Kenya (34), which further supports this as a cause for concern.
V.
Early studies reporting the emergence of CPS V noted a close genetic relationship among the strains of this serotype and its recovery from all populations, but more so from nonpregnant adults (41). It is recognized as the third most prevalent serotype associated with maternal disease in the United States, United Kingdom, and France (26). Africa, more specifically West Africa, has a higher frequency of CPS V maternal colonization than those of other regions, such as North America, Australia, and New Zealand, which have much lower prevalences (24). In Gabon, approximately one-third of strains isolated from pregnant women were CPS V (42). This supports previous observations of CPS V predominance by other studies of Gambian mothers and their infants and of pregnant women in Egypt (43, 44).
VI.
Considered one of the rarer serotypes (with VII, VIII, and IX), CPS VI is often reported in Asian countries, in particular Japan (45) and Malaysia (18, 46). While global colonization estimates place CPS VI in the minority, this serotype is thought to account for as much as 20% of serotypes observed in Southeast Asian pregnant women (24).
VII.
Similarly, CPS VII is less well represented across the globe (24). Interestingly, it was found to be the most common serotype in southern Ghana, in the first study to report such serotype prevalence in pregnant women from West Africa (47). A number of Malaysian studies observed serotype VII isolates in their cohorts, ranging from 1.7% to 21.4% of serotypes (18, 46, 48). This supports the observation of intracountry variation that was similarly reported (although not for CPS VII) for serotypes in different regions of Brazil (19). A study of maternal colonization in India observed CPS VII accounting for 6.7% of the serotypes and having a higher prevalence than that of CPS Ib, which is indicative of Asian countries yet shows a unique distribution (49). More recently, Islam et al. observed that 37.1% of the GBS specimens isolated in Bangladesh from newborn umbilical cord areas (colonizing isolates) belonged to CPS VII (50).
VIII.
Serotype VIII is strongly associated with Japan, with an early study reporting a high frequency in pregnant women (45). That study found a 35.6% prevalence of CPS VIII among serotypes, followed by CPS VI (also considered rare globally), among colonizing isolates from Japanese pregnant women (45). Thereafter, incidences of its presence began to be described outside Japan. In Denmark, seven instances of invasive disease isolates belonging to CPS VIII among nonpregnant adults were reported from 1999 to 2002 (51). Also, one isolate colonizing a pregnant woman in Maryland was recognized as CPS VIII (52). Lately, the frequency of CPS VIII is generally low outside Japan, and studies often describe few isolates (53, 54).
IX.
The newest serotype, described in 2007, has had fewer chances for description in the literature; however, in the last decade, studies have adopted new techniques, and now most test for all serotypes. As mentioned above, a study in southern Ghana observed CPS IX as having the second highest prevalence, behind CPS VII (47). A Swedish study assessed changes in serotype distribution over time for invasive disease (neonatal and adult populations) and suggested that circulating serotypes remained stable from 1990 onwards; however, the study noted an increase in CPS VII to IX (55). Additionally, a case study in Japan described an incidence of ultra-late-onset sepsis caused by CPS IX, and this demonstrates its capacity to play a role in disease (56).
Nontypeable isolates.
Numerous serotyping studies result in isolates that do not represent any of the defined types and are thus considered nontypeable (NT). It is not known whether this is a result of technique specificity or whether such isolates may represent novel serotypes; either way, NT isolates may potentially skew the available data. Capsule loss and lack of expression are both potential contributors to this NT category (57, 58). One study assessed a collection of GBS isolates classified as NT by serological methods and went on to characterize them by a number of different techniques (immunodiffusion, pulsed-field gel electrophoresis [PFGE], and multilocus sequence typing) (59). This resulted in all isolates being serotyped and found to represent all types, excluding IV and VII to IX, with instances of multiple serotypes per isolate, which has been described previously (52). This may represent an incidence of capsular switching, a phenomenon that involves the genetic exchange of capsular genes and thus enables expression of alternative capsule types, and it is proposed that such horizontal transfer may be responsible for the capsular locus diversity (60–62). In a former study by Ramaswamy and colleagues, 80% of isolates containing multiple serotypes contained a type V CPS-specific gene, and the authors proposed that this serotype may have an association with increased competency and ability to acquire DNA (59). This is an interesting notion, but given that GBS is not thought to be naturally transformable, the idea that capsular switching may result from increased competence of CPS V seems unlikely; however, capsular switching has been reported by others (37, 40, 63, 64). This is a concern for development of vaccines targeting the capsule, as this has been an issue for the naturally transformable organism Streptococcus pneumoniae due to capsular switching (65, 66).
One of the major limiting factors in discussing GBS serotype distribution is that a direct comparison between studies can be difficult due to the number of different study parameters assessed, such as specimen type and study population, and the added complexity of various serotyping methodologies. For optimal surveillance outcomes, data on all types of patient cohorts are vital to completing the broader picture of GBS epidemiology. Increasing our understanding of prevalent isolates associated with specific body sites and their potential to cause disease by monitoring the serotypes is the first step toward narrowing down invasive strain targets and surveying the serotype distribution dynamics.
Surface Proteins
In addition to the CPS, proteins are also present on the surfaces of GBS cells, and studies have shown that antibodies against both CPS and surface proteins are protective against GBS infection (67–69). The alpha-like protein (Alp) family proteins are among the well-characterized GBS surface proteins and are significant virulence factors. These include alpha-C protein, Rib, Alp2, Alp3, Alp4, and epsilon (Alp1) and are encoded by the bca, rib, alp2, alp3, alp4, and epsilon/alp1 genes, respectively (70). Detection of the expressed surface proteins by serology or the presence of their genes by molecular techniques, such as PCR or sequencing, can be used as another grouping tool. The C antigen was the first identified surface protein antigen and has been found in numerous GBS strains, except for serotype III strains (71). Alps have been present in the majority of GBS isolates, but non-Alp isolates do exist. A total of 1.1% of isolates in one study were non-Alp isolates, and all were associated with invasive bloodstream infections (72). Smith and others examined associations between the common serotypes (Ia, Ib, II, III, and V) and specific genes. They found that most genes tested (sbp1, bca, bac, rib, brp, pag, and psp) were common in only one or two serotypes, except for bca, which was present in >50% of all serotype Ib, II, and V strains (73). A review of several surface proteins reported correlations for alpha protein presence in CPS Ia, Ib, and II strains, yet it was found almost never in CPS III and only rarely in CPS V strains. Rib is expressed by the large majority of CPS III, many CPS II, and a few CPS V strains. Alp3 is expressed by CPS V and VIII strains and Alp2, more rarely, by CPS Ia, III, and V strains (70).
Pulsed-Field Gel Electrophoresis
Pulsed-field gel electrophoresis (PFGE) is another molecular method that distinguishes isolates with greater discrimination than that of serotyping for evaluation of genetic relatedness. It involves nucleic acid digestion with one or more restriction enzymes (often SmaI in the case of GBS) to produce fragment profiles on an agarose gel, after which the profiles are then compared for similarity and grouped accordingly (74). Fasola and colleagues described this method for use in GBS in 1993, and Benson and Ferrieri later reported an updated and more rapid PFGE technique for GBS, reducing the workflow from 6 to 8 days to 3 days total (75, 76). PFGE profile correlations with serotypes have shown variation; Skjaervold et al. observed homogeneity for Ib, III, and V strains, while IV, Ia, and II strains were heterogenous (77). This suggests that serotype grouping does not permit an understanding of close genetic relationships. While a greater depth of genetic relationship can be observed using PFGE than using serological typing, there are limitations with this method. Due to the lack of a uniform typing system with respect to naming, PFGE analyses can be difficult to compare between studies: what may be PFGE clone A in one study could be clone 37 in another (78, 79). Therefore, pattern and band sizes must be used for comparison between studies. In this respect, it is mainly useful for comparison within a study to see diversity among isolates and modes of transmission. For example, mother and infant paired GBS specimen fragment profiles were observed to have common patterns, highlighting vertical transmission (80). In the past, identifying fragment banding on gels was subjective and lacked accuracy in the reporting of band sizes, making comparison across studies difficult. The idea of using restriction enzymes with minimal cutting sites aims to reduce this by producing fewer fragments for visualization on the gel (74). Regardless of any limitations, PFGE has identified variation within and across serotypes and has led to the progression of the molecular epidemiology of GBS.
Multilocus Sequence Typing
Jones and colleagues developed the multilocus sequence typing (MLST) method for GBS by using seven defined housekeeping genes (adhP, atr, glcK, glnA, pheS, sdhA, and tkt) (81). The MLST database was produced by Jolley and colleagues and currently includes over 4,000 GBS isolates (82). Allelic variations among housekeeping genes allow the determination of different sequence types (STs). These STs can then be clustered into clonal complexes (CCs), which are conservatively defined by members having no more than one allelic difference from other members of the cluster.
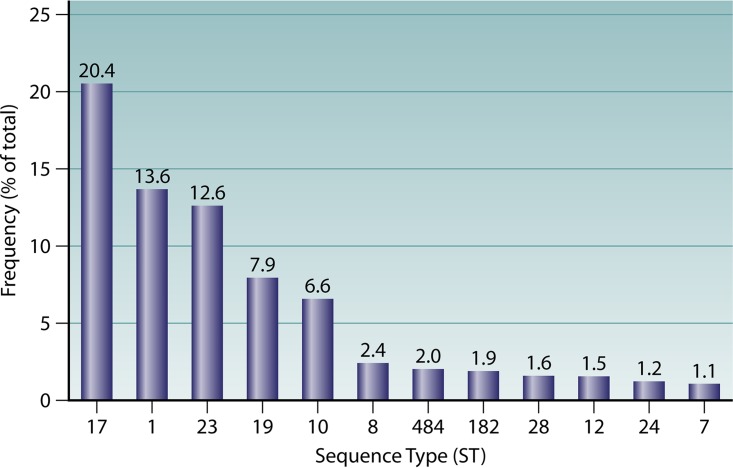
The MLST database is a valuable resource that enables sharing of data; the publicly available information for GBS includes a range of criteria to assess, including the country of origin, serotype, and specimen type. Although there remains a reporting bias, in that only submitted information is available, the data represent a mix of global information which is very useful. An audit of the GBS MLST database (https://pubmlst.org/sagalactiae/) found a total of 4,131 isolates from 38 different countries. Of these, 3,949 isolates had been assigned an ST (n = 635). Twelve STs account for 73% of the GBS isolates with available MLST data (Fig. 1). The remaining STs (27%) each account for less than 1% of all isolates, and 525 STs have a single isolate submitted to the database.
FIG 1.
Frequencies of the 12 most prevalent GBS sequence types available from the MLST database, which includes a total of 635 sequence types (remaining STs account for <1% each).
Having access to the location of each isolate's origin enabled categorization of STs by continent (Fig. 2). This highlighted gaps in the global data, with no isolates from Australia and New Zealand available, unless they are not stated (categorized as “unknown”). Examining the main contributors within each continent, Netherlands (n = 1,230/1,610 isolates), Kenya (n = 1,133/1,172 isolates), Japan (n = 490/550 isolates), United States (n = 325/491 isolates), and Brazil (n = 3/5 isolates) have submitted the most isolates within Europe, Africa, Asia, North America, and South America, respectively.
FIG 2.
Contributions by continent of GBS isolates available in the MLST database, as percentages of all isolates (South America represents 0.1% and is therefore not visible).
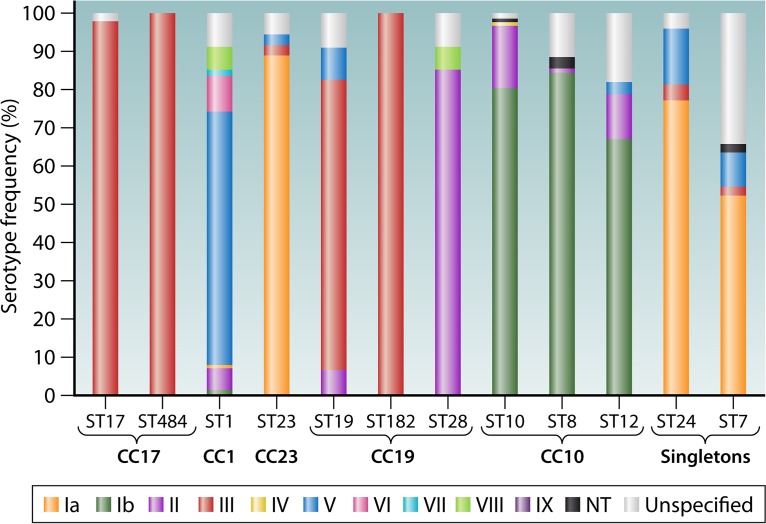
Associations between ST and serotype have been reported in the literature, with some showing a strong correlation and others very little; this is also observed in the MLST database (Fig. 3). Ramaswamy et al. observed a correlation between CPS III and ST-17 and between CPS Ib and ST-12, and they found that CPS V was represented in all STs except for ST-17 (59). Similar trends are observed within the MLST database, where each of the most prevalent STs appears to be dominated by a particular serotype (Fig. 3). The serotype variability in some instances, for example, ST-1, may be suggestive of discordance with serotype profiling. Trends observed by various studies reveal a variable serotype presence within ST-1, variation between CPS Ib and II within ST-10, and a predominance of CPS III within ST-17 and ST-19 and of CPS Ia within ST-23 (83, 84). Within the clonal complexes, homogeneity of serotypes is evident in the CC-17 group, with all isolates representing CPS III, except for a small proportion with unspecified serotypes. Similarly, within CC-19, ST-182 includes CPS III only, but other STs in this complex show variation, especially ST-28, which is predominantly CPS V (Fig. 3). From an evolutionary perspective, caution must be taken when inferring phylogenetic relationships, as suggested by Sorensen et al., due to the indication that different segments of the GBS genome evolved independently (85). With this in mind, MLST is perhaps not the best method for describing evolutionary relationships, but for the purposes of epidemiology at the current scale, it should be acceptable now that we are aware of these factors.
FIG 3.
Serotype distribution within each sequence type, using the public MLST database for GBS, for the 12 most represented sequence types within the database, as well as their corresponding clonal complexes (CCs), where appropriate.
Recurring themes in the literature include the numerous studies that have described the association between ST-17 and invasive neonatal disease (83, 86–90) and, further, the relationship between this lineage and meningitis (91). Neonatal invasive isolates collected over a 10-year period in Sweden showed variation in genotype prevalence over time and in different regions, in particular the emergence of CC-1 and subsequent disappearance of CC-23 (88). These temporal changes emphasize the importance of understanding GBS epidemiology and tracking changes in prevalence worldwide: ST-17 may expand and/or other STs may become hypervirulent over time.
The MLST profiling scheme is publicly available and curated, making it an invaluable epidemiology resource. If the database were treated like a repository, the epidemiological surveillance would be greatly enhanced, which would be desirable for targeted therapeutics assessment.
Random Amplified Polymorphic DNA and Repetitive-Sequence-Based PCRs
Random amplified polymorphic DNA PCR (RAPD-PCR) and repetitive-sequence-based PCR (rep-PCR) are techniques that rely on the amplification of DNA via random primers (RAPD-PCR) (92) or repetitive sequence target primers (rep-PCR) and result in genomic fingerprinting profiles, as the generated amplicons lead to strain-specific band patterns on an electrophoretic gel.
The RAPD assay has been used in a number of genotyping contexts, such as evolution and genetic relatedness (93), epidemiology (46, 94), transmission (95), and isolate variation within individual patients (96–98). One study examined RAPD profiles of GBS isolates from a number of sites, such as blood, cerebrospinal fluid, and maternal breast milk, in cases of LOD in neonates (n = 4) (96). It found identical banding profiles for all sample types from individual patients and differing types between patients, but this was representative of a small sample size. Likewise, Brzychczy-Wloch et al. used RAPD-PCR to suggest that GBS isolates from colonized neonates originated from the colonized mothers, regardless of mode of delivery, as they had identical genotypic profiles (99). While detection of identical clones via RAPD profiles can suggest vertical transmission, other studies have used this to demonstrate horizontal transmission in neonates born to noncolonized mothers that likely acquired GBS from the hospital environment, as identical profiles were observed in other hospital wards (100).
Toresani et al. found 16 profiles for 21 GBS isolates tested by RAPD assay, and furthermore, successive samples from two patients showed different genotype profiles, suggesting reinfection (97). In contrast, El Aila et al. compared culture methods and genotyped multiple isolates collected from individual patients. RAPD testing of 118 isolates from 32 pregnant women resulted in 66 genotype profiles, including cases of multiple different genotypes at each site (vagina and rectum), demonstrating that assumed reinfection may be due to a lack of initial identification (98). This highlights the use of RAPD assay for single-center studies and its integration with high-resolution capillary electrophoresis, which enhances accuracy and provides digital fingerprints (98).
Numerous studies have observed correlations between RAPD profiles and clustering of strains with resistant phenotypes (101, 102). The results from a study of macrolide-resistant GBS isolates suggested that PFGE was a more precise tool than RAPD-PCR for analyzing molecular epidemiology (95). In contrast, Zhang and colleagues (103) found that the clustering of the isolates by RAPD-PCR was in concordance with previous PFGE findings suggesting that clusters could be serotype specific (80).
The great advantages of RAPD assay are a relatively short procedure time and a simple methodology, which make it a suitable tool for suggesting clonal relatedness in clinical settings and for monitoring transmission. Comparison between studies can be difficult, however, particularly if different primers are used. The random nature of this profiling technique provides variation and enables discrimination between clones, but similar to the PFGE technique, due to the lack of specific targets, the information this provides beyond strain similarity is somewhat limited unless further genetic analysis is undertaken.
The literature assessing rep-PCR for GBS is limited, with one study comparing the rep-PCR Diversilab system with MLST and PFGE by using clinical adult and neonatal GBS isolates (n = 179) (104). The clustering concordances of PFGE and rep-PCR were similarly low, but PFGE had a greater discriminatory power than that of rep-PCR (104). One study compared both RAPD-PCR and rep-PCR techniques to genotype GBS isolates from fish and observed RAPD-PCR to have a greater level of variability than that of rep-PCR between strains, with 13 and 9 profiles, respectively (105). This is not supportive of the use of rep-PCR over other methods tested, including MLST and PFGE. rep-PCR typing is notorious for its susceptibility to minor variations in experimental conditions and reagents, resulting in poor reproducibility (106, 107).
Multilocus Variant-Number Tandem-Repeat Assay
Various bacterial species have been typed through the detection of variable-number tandem repeats (VNTRs), which is based on the repeated sequences that make up DNA at different loci and that are found throughout bacterial genomes (108). As the name suggests, these VNTR loci vary in repeat number, allowing for an assay of a combination of several VNTR loci to generate different strain-specific profiles. This is known as a multilocus variant-number tandem-repeat analysis (MLVA) and has a principle similar to that of MLST; however, rather than housekeeping genes, MLVA uses VNTR loci (109). This typing scheme was developed for GBS by Radtke and colleagues and includes five of the most diverse loci (SATR1 [110], SATR2, SATR3 [111], SATR4 [112], and SATR5 [113]) (109). The authors found clustering by CPS/surface protein typing or MLST to generally correspond with the MLVA profiles, but the latter was more discriminatory. A total of 126 GBS isolates tested were profiled as having 70 different MLVA types (MTs), compared to 36 STs by MLST and 19 CPS/protein types. A specific number of 15 repeats within SATR2 was observed to correspond with serotype IX. Additionally, for one of the three described SATR3 alleles, all isolates of that allele (except one) belonged to ST-17, known to account for a large proportion of neonatal infections (81, 88). Other MLST groups were more dispersed, including CPS V/ST-1, for which SATR5 resolved into a number of profiles. This is interesting due to the often homogeneous reports of this subgroup in other studies (28, 77). The SATR1 locus is considered to be composed of clustered regularly interspaced short palindromic repeats (CRISPR), and this worked efficiently as part of the MLVA scheme.
Additional MLVA schemes have been reported, one of which used six VNTR loci, including three described by Radtke et al. (109, 114). Similar results were observed in that study, with greater isolate discrimination reported with the MLVA technique (98 types) than with MLST (51 types). The clustering patterns observed for MLVA types showed similarity to MLST CCs; in particular, all human strains of MLST CC-17 appeared in MLVA cluster 9 (114). This scheme was able to categorize MLST CC-23 into two clusters, one accounting for CPS III and the other for CPS Ia, which highlights this scheme's ability to distinguish strain variability within lineages (114).
A study of commensal GBS colonization of nonpregnant women of reproductive age utilized MLVA for genotyping and observed 15 types among the 86 GBS isolates (115). For pregnant women, 30 MTs from a total of 41 GBS isolates were observed, which suggested a highly diverse population structure (116). The MTs are difficult to compare between studies, though, as there is no mention of a database in which naming conventions are uniformly enforced; however, a database has been created for this purpose (http://www.mlva.net) but unfortunately does not include GBS.
CRISPR Locus 1
Lastly, the molecular target which was mentioned above as one of the loci of the MLVA scheme is CRISPR as well as Cas (CRISPR-associated proteins). These are known for their role in bacterial host defense and adaptive immunity against foreign invading DNA, such as bacteriophages and mobile genetic elements (117). The genetic arrangement that this system follows results in a polarized orientation, which allows tracking of ancestral invasions over time (118). In GBS, two CRISPR-Cas systems have been identified, including a type II-A system (ubiquitous and functional) and a type I-C system (rare and often incomplete), associated with CRIPSR locus 1 (CRISPR1) and CRISPR2, respectively (119, 120). The ubiquitous nature of CRISPR1 makes it an attractive molecular marker with which to track changes in the genome. The highly conserved direct repeats form the spacers between introduced genetic materials and can therefore be monitored for changes. Using this method, Beauruelle and others were able to monitor the vaginal GBS colonization of 100 women over an 11-year period (121). This extensive study highlights the influence of genetic variables that alter these organisms over time, for example, bacteriophage infection. This is an example of new epidemiological markers that may assist in understanding these bacteria, their colonization, and their pathogenic tendencies.
FROM TARGETS TO TREATMENT
The purpose of the targets and molecular assays presented above is to understand the population of GBS with respect to colonization and disease; by distinguishing differences between strains, we can begin to associate specific features with clinical outcomes. Equally, by identifying similarities that are represented across the majority or the entire population of GBS strains, we can target these bacteria more broadly. Within antenatal settings, the current preventative measures for GBS include antenatal GBS screening (risk or culture based) and subsequent intrapartum antibiotic prophylaxis prior to delivery. This has resulted in reductions in EOD rates as reported by the Centers for Disease Control and Prevention and is also recommended in other countries (10, 11, 122, 123). Unfortunately, no change in LOD has been observed with such preventative strategies; however, this is likely due to the nature of transmission (nosocomial or community acquired) (124).
Due to the recommended IAP, antibiotic exposure has become widespread, but the potential for resulting adverse events is unclear, as highlighted in a recent systematic review (125). While consistency is lacking for data on development of antimicrobial resistance and long-term adverse events as a result of IAP for GBS, infant microbiome disruption has been observed by numerous studies (126–132). However, due to the lack of high-level (species) taxonomic data and the myriad other methodological problems that are still rampant in microbiome studies (lack of negative controls and proper selection of primer sets) (133), with the current data it remains unclear what impact this may have on neonates. With the potential implications in mind, however, it is important to examine alternative preventative and therapeutic strategies.
Maternal GBS vaccination has been proposed as an alternative preventative strategy due to the ability of maternal IgG to cross the placenta and provide immunity for the fetus (134). Baker and Kasper identified an association between deficient maternal antibody levels and the occurrence of neonatal GBS infection. That study also detected GBS antibody titers in the umbilical cord sera of three healthy neonates born to mothers with demonstrable antibody levels (60 to 80% of the maternal levels were detected), suggesting transplacental transfer and illustrating the potential for a maternal vaccine (135). The benefit of maternal vaccination has since been described, and a number of GBS vaccines have been developed (134, 136–138).
While no vaccines against GBS are currently licensed and available, several polysaccharide conjugate and surface protein vaccines are under trial (137, 138). Through comparison of the vaccine targets with the global GBS epidemiology, we can identify areas of vaccine coverage. Numerous studies that have identified the less commonly recognized serotypes within their populations have raised concern about vaccinations targeting specific serotypes, such as Ia, Ib, and III, all of which are included in the trivalent vaccine currently furthest along in progress, with completion of a phase Ib/2 clinical trial (ClinicalTrials.gov number NCT01193920) (139, 140). A review by Lin et al. describes such challenges faced by capsule-based conjugate vaccines against GBS, in addition to dealing with the potential for serotype replacement and capsular switching (138). More recently, a pentavalent vaccine targeting serotypes Ia, Ib, II, III, and V commenced a phase I trial on healthy volunteers (ClinicalTrials.gov number NCT03170609); if successful, this may be an effective GBS vaccine outside Asia, as it covers the five major circulating serotypes in the United States, Europe, and Australia (24).
Alternative vaccination strategies have focused on proteins. Surface proteins have been included as vaccine targets in addition to capsule types, and given the variety of circulating serotypes described, these may provide a broader, more universal target to exploit, which would be ideal for vaccine development (137). Although few surface proteins are conserved across all strains, Maione and colleagues provided evidence of protein vaccination in female mice with the ability to elicit protection in 43 to 80% of cases of GBS challenge to pups born to the vaccinated mother (141). Four proteins, including a conserved Sip protein (SAG0032) encoded within the core genome of strains, were included in this study, in addition to three other antigens (SAG1404, SAG0645, and SAG0649) which had a variable presence in the genome (141). Studies in humans have been described by Heath (137) and include multiple studies of monovalent, one bivalent, and several trivalent CPS-based vaccines, all in the proof-of-concept stage involving trials in pregnant women. A bivalent vaccine including the N-terminal domains of the surface proteins alpha-C and Rib is currently in development by MinervaX and has completed phase I clinical trials (ClinicalTrials.gov number NCT02459262). Larsson et al. reported the association between low protein alpha and Rib antibodies and the occurrence of neonatal invasive infection (142). While the type of vaccine is important in terms of coverage, the implementation is equally important in considering access, especially in low-income settings and developing countries, where screening and widespread prophylaxis are often not practical (143). Based on the World Health Organization guidelines, GBS vaccination, particularly in South Africa, would be very cost-effective (144). In developed countries, such as the United States, Kim and others evaluated the cost-effectiveness of a maternal vaccine compared to screening/IAP (145). They concluded that ≥70% vaccine efficacy in addition to IAP for unvaccinated women would prevent more disease than current strategies, with a similar economic burden (145). Epidemiological data about GBS are invaluable not only for vaccine development and success but also for other future targeted therapeutic agents, such as bacteriophage therapy (146).
CONCLUSIONS
This review describes different commonly used and emerging techniques to define GBS epidemiology and phylogeny. Some traditional targets, such as serotypes, are widely reported but lack the level of definition that other molecular targets can provide with respect to genetic relatedness and pathogenic features. The difficulty lies in recognizing these targets and widely reporting them to provide a comparable basis for global surveillance. It is likely that future epidemiological efforts to monitor GBS will include the reporting of a combination of these targets and will in turn refine the association between these different components. The epidemiology of GBS provides insight into the adaptation and evolution of these organisms through examination of their genetic relatedness and population structure. Understanding GBS as an opportunistic pathogen is one aspect to arise from the proposed surveillance efforts; another is guidance for development of future therapeutics. Vaccines based on serotypes commonly found in one area may not include prevalent serotypes of another due to the geographic variation observed even at the regional level. To vet these coverage issues, surveillance of different targets within GBS is required to monitor prevalent isolates in all populations. Additionally, following vaccine implementation, this will provide a platform to monitor shifts in GBS dynamics and to identify emerging pathogen types before outbreaks occur. Broader coverage may result from protein vaccine development, which further emphasizes the importance of characterization of a range of targets. The targets outlined here each have individual relevance, although uniformity in reporting profiles would be ideal for global comparisons. Currently, MLST, while less discriminatory than MLVA, comprises the only method with an established database to enable this comparison and provide the added confidence of curation.
ACKNOWLEDGMENTS
L.L.F. is supported by an Australian Government Research Training Program Scholarship and a Professor Gordon King Postgraduate Scholarship, provided by the Women and Infants Research Foundation. M.S.P. is supported by an NHMRC project grant (grant 1077931).
We have no conflicts of interest.
Biographies

Lucy L. Furfaro is currently completing her Ph.D. at the University of Western Australia and previously completed her B.S. and Honors programs at the University of Western Australia. Her research interests include perinatal microbiology, with a focus on group B streptococcus and treatment alternatives to antibiotics, such as bacteriophage therapy. This area of research has been pursued over the last 4 years as part of Honors and postgraduate studies.

Barbara J. Chang received her Ph.D. from Monash University, Australia, followed by postdoctoral positions at the University of Calgary, Canada. Currently, she is the Director of Postgraduate Programs in Infectious Diseases at the School of Biomedical Sciences at the University of Western Australia. Her research interests include the biology and genetics of bacteriophages, anti-quorum-sensing agents, and bacterial pathogens, including Clostridium difficile, Moraxella catarrhalis, and group B streptococcus.

Matthew S. Payne was awarded his Ph.D. in molecular microbiology from the University of Queensland, Australia, and has since conducted postdoctoral research at Kings College London, United Kingdom, and La Trobe University, Australia. Dr. Payne is currently at The University of Western Australia, where he is focused on preventing infection-mediated preterm birth. His research interests include the microbiology of preterm birth, microbial metagenomics, Ureaplasma and Mycoplasma spp., and antibiotic and nonantibiotic treatment regimens for prevention of preterm birth and neonatal sepsis.
REFERENCES
- 1.Lancefield RC. 1933. A serological differentiation of human and other groups of hemolytic streptococci. J Exp Med 57:571–595. doi: 10.1084/jem.57.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heath PT, Schuchat A. 2007. Perinatal group B streptococcal disease. Best Pract Res Clin Obstet Gynaecol 21:411–424. doi: 10.1016/j.bpobgyn.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Schuchat A. 1998. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev 11:497–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujita H, Nakamura I, Tsukimori A, Sato A, Ohkusu K, Matsumoto T. 2015. Severe infective endocarditis in a healthy adult due to Streptococcus agalactiae. Int J Infect Dis 38:43–45. doi: 10.1016/j.ijid.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Li LQ, Cheema S, Goel N. 2016. Group B streptococcal meningitis in a previously healthy man. BMJ Case Rep 2016:bcr2015213999. doi: 10.1136/bcr-2015-213999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oddie S, Embleton ND. 2002. Risk factors for early onset neonatal group B streptococcal sepsis: case-control study. BMJ 325:308. doi: 10.1136/bmj.325.7359.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin F-YC, Weisman LE, Troendle J, Adams K. 2003. Prematurity is the major risk factor for late-onset group B Streptococcus disease. J Infect Dis 188:267–271. doi: 10.1086/376457. [DOI] [PubMed] [Google Scholar]
- 8.Berardi A, Rossi C, Guidotti I, Vellani G, Lugli L, Bacchi Reggiani ML, Ferrari F, Facchinetti F, Ferrari F. 2014. Factors associated with intrapartum transmission of group B Streptococcus. Pediatr Infect Dis J 33:1211–1215. doi: 10.1097/INF.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 9.Aber RC, Allen N, Howell JT, Wilkenson HW, Facklam RR. 1976. Nosocomial transmission of group B streptococci. Pediatrics 58:346–353. [PubMed] [Google Scholar]
- 10.Verani JR, McGee L, Schrag SJ. 2010. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep 59:1–36. [PubMed] [Google Scholar]
- 11.Cagno CK, Pettit JM, Weiss BD. 2012. Prevention of perinatal group B streptococcal disease: updated CDC guideline. Am Fam Physician 86:59–65. [PubMed] [Google Scholar]
- 12.Rosa-Fraile M, Spellerberg B. 2017. Reliable detection of group B Streptococcus in the clinical laboratory. J Clin Microbiol 55:2590–2598. doi: 10.1128/JCM.00582-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imperi M, Pataracchia M, Alfarone G, Baldassarri L, Orefici G, Creti R. 2010. A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of Streptococcus agalactiae. J Microbiol Methods 80:212–214. doi: 10.1016/j.mimet.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Slotved HC, Kong F, Lambertsen L, Sauer S, Gilbert GL. 2007. Serotype IX, a proposed new Streptococcus agalactiae serotype. J Clin Microbiol 45:2929–2936. doi: 10.1128/JCM.00117-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furfaro LL, Chang BJ, Payne MS. 2017. A novel one-step real-time multiplex PCR assay to detect Streptococcus agalactiae presence and serotypes Ia, Ib and III. Diagn Microbiol Infect Dis 89:7–12. doi: 10.1016/j.diagmicrobio.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Yao K, Poulsen K, Maione D, Rinaudo CD, Baldassarri L, Telford JL, Sorensen UB, Kilian M. 2013. Capsular gene typing of Streptococcus agalactiae compared to serotyping by latex agglutination. J Clin Microbiol 51:503–507. doi: 10.1128/JCM.02417-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng X, Kong F, Wang H, Darbar A, Gilbert GL. 2006. Simultaneous detection of nine antibiotic resistance-related genes in Streptococcus agalactiae using multiplex PCR and reverse line blot hybridization assay. Antimicrob Agents Chemother 50:204–209. doi: 10.1128/AAC.50.1.204-209.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eskandarian N, Ismail Z, Neela V, van Belkum A, Desa MN, Amin Nordin S. 2015. Antimicrobial susceptibility profiles, serotype distribution and virulence determinants among invasive, non-invasive and colonizing Streptococcus agalactiae (group B streptococcus) from Malaysian patients. Eur J Clin Microbiol Infect Dis 34:579–584. doi: 10.1007/s10096-014-2265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutra VG, Alves VM, Olendzki AN, Dias CA, de Bastos AF, Santos GO, de Amorin EL, Sousa MA, Santos R, Ribeiro PC, Fontes CF, Andrey M, Magalhaes K, Araujo AA, Paffadore LF, Marconi C, Murta EF, Fernandes PC Jr, Raddi MS, Marinho PS, Bornia RB, Palmeiro JK, Dalla-Costa LM, Pinto TC, Botelho AC, Teixeira LM, Fracalanzza SE. 2014. Streptococcus agalactiae in Brazil: serotype distribution, virulence determinants and antimicrobial susceptibility. BMC Infect Dis 14:323. doi: 10.1186/1471-2334-14-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Florindo C, Damiao V, Silvestre I, Farinha C, Rodrigues F, Nogueira F, Martins-Pereira F, Castro R, Borrego MJ, Santos-Sanches I. 2014. Epidemiological surveillance of colonising group B Streptococcus epidemiology in the Lisbon and Tagus Valley regions, Portugal (2005 to 2012): emergence of a new epidemic type IV/clonal complex 17 clone. Euro Surveill 19:20825. doi: 10.2807/1560-7917.ES2014.19.23.20825. [DOI] [PubMed] [Google Scholar]
- 21.Tyrrell GJ, Senzilet LD, Spika JS, Kertesz DA, Alagaratnam M, Lovgren M, Talbot JA. 2000. Invasive disease due to group B streptococcal infection in adults: results from a Canadian, population-based, active laboratory surveillance study—1996. Sentinel Health Unit Surveillance System Site Coordinators. J Infect Dis 182:168–173. [DOI] [PubMed] [Google Scholar]
- 22.Tsolia M, Psoma M, Gavrili S, Petrochilou V, Michalas S, Legakis N, Karpathios T. 2003. Group B streptococcus colonization of Greek pregnant women and neonates: prevalence, risk factors and serotypes. Clin Microbiol Infect 9:832–838. doi: 10.1046/j.1469-0691.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 23.Davies HD, Raj S, Adair C, Robinson J, McGeer A. 2001. Population-based active surveillance for neonatal group B streptococcal infections in Alberta, Canada: implications for vaccine formulation. Pediatr Infect Dis J 20:879–884. doi: 10.1097/00006454-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Russell NJ, Seale AC, O'Driscoll M, O'Sullivan C, Bianchi-Jassir F, Gonzalez-Guarin J, Lawn JE, Baker CJ, Bartlett L, Cutland C, Gravett MG, Heath PT, Le Doare K, Madhi SA, Rubens CE, Schrag S, Sobanjo-ter Meulen A, Vekemans J, Saha SK, Ip M, Asturias E, Gaind R, Kumar P, Anthony B, Madrid L, Bassat Q, Zhu C, Luo M, Nagarjuna D, Majumder S. 2017. Maternal colonization with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis 65:S100–S111. doi: 10.1093/cid/cix658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madrid L, Seale AC, Kohli-Lynch M, Edmond KM, Lawn JE, Heath PT, Madhi SA, Baker CJ, Bartlett L, Cutland C, Gravett MG, Ip M, Le Doare K, Rubens CE, Saha SK, Sobanjo-ter Meulen A, Vekemans J, Schrag S, Agarwal R, da Silva ARA, Bassat Q, Berkley JA, Dangor Z, Dhaded S, Giannoni E, Hammoud M, Kobayahsi M, O'Sullivan C, Sakata H, Sridhar S, Sigaúque B, Tyrrell G, Paul V. 2017. Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin Infect Dis 65:S160–S172. doi: 10.1093/cid/cix656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall J, Adams NH, Bartlett L, Seale AC, Lamagni T, Bianchi-Jassir F, Lawn JE, Baker CJ, Cutland C, Heath PT, Ip M, Le Doare K, Madhi SA, Rubens CE, Saha SK, Schrag S, Sobanjo-ter Meulen A, Vekemans J, Gravett MG. 2017. Maternal disease with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis 65:S112–S124. doi: 10.1093/cid/cix660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edmond KM, Kortsalioudaki C, Scott S, Schrag SJ, Zaidi AK, Cousens S, Heath PT. 2012. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet 379:547–556. doi: 10.1016/S0140-6736(11)61651-6. [DOI] [PubMed] [Google Scholar]
- 28.Lin HC, Chen CJ, Chiang KH, Yen TY, Ho CM, Hwang KP, Su BH, Lin HC, Li TC, Lu JJ. 2016. Clonal dissemination of invasive and colonizing clonal complex 1 of serotype VI group B Streptococcus in central Taiwan. J Microbiol Immunol Infect 49:902–909. doi: 10.1016/j.jmii.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Uh Y, Kim HY, Jang IH, Hwang GY, Yoon KJ. 2005. Correlation of serotypes and genotypes of macrolide-resistant Streptococcus agalactiae. Yonsei Med J 46:480–483. doi: 10.3349/ymj.2005.46.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner C, Turner P, Po L, Maner N, De Zoysa A, Afshar B, Efstratiou A, Heath PT, Nosten F. 2012. Group B streptococcal carriage, serotype distribution and antibiotic susceptibilities in pregnant women at the time of delivery in a refugee population on the Thai-Myanmar border. BMC Infect Dis 12:34. doi: 10.1186/1471-2334-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez Y, Parra E, Cepas V, Sanfeliu I, Juncosa T, Andreu A, Xercavins M, Perez J, Sanz S, Vergara A, Bosch J, Soto SM. 2017. Serotype, virulence profile, antimicrobial resistance and macrolide-resistance determinants in Streptococcus agalactiae isolates in pregnant women and neonates in Catalonia, Spain. Enferm Infecc Microbiol Clin 2017:S0213-005X(17)30226-4. doi: 10.1016/j.eimc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Elikwu CJ, Oduyebo O, Ogunsola FT, Anorlu RI, Okoromah CN, Konig B. 2016. High group B streptococcus carriage rates in pregnant women in a tertiary institution in Nigeria. Pan Afr Med J 25:249. doi: 10.11604/pamj.2016.25.249.9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster-Nyarko E, Kwambana B, Aderonke O, Ceesay F, Jarju S, Bojang A, McLellan J, Jafali J, Kampmann B, Ota MO, Adetifa I, Antonio M. 2016. Associations between nasopharyngeal carriage of group B Streptococcus and other respiratory pathogens during early infancy. BMC Microbiol 16:97. doi: 10.1186/s12866-016-0714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seale AC, Koech AC, Sheppard AE, Barsosio HC, Langat J, Anyango E, Mwakio S, Mwarumba S, Morpeth SC, Anampiu K, Vaughan A, Giess A, Mogeni P, Walusuna L, Mwangudzah H, Mwanzui D, Salim M, Kemp B, Jones C, Mturi N, Tsofa B, Mumbo E, Mulewa D, Bandika V, Soita M, Owiti M, Onzere N, Walker AS, Schrag SJ, Kennedy SH, Fegan G, Crook DW, Berkley JA. 2016. Maternal colonization with Streptococcus agalactiae and associated stillbirth and neonatal disease in coastal Kenya. Nat Microbiol 1:16067. doi: 10.1038/nmicrobiol.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romain AS, Cohen R, Plainvert C, Joubrel C, Bechet S, Perret A, Tazi A, Poyart C, Levy C. 2018. Clinical and laboratory features of group B Streptococcus meningitis in infants and newborns: study of 848 cases in France from 2001–2014. Clin Infect Dis 66:857–864. doi: 10.1093/cid/cix896. [DOI] [PubMed] [Google Scholar]
- 36.Bjornsdottir ES, Martins ER, Erlendsdottir H, Haraldsson G, Melo-Cristino J, Kristinsson KG, Ramirez M. 2016. Changing epidemiology of group B streptococcal infections among adults in Iceland: 1975–2014. Clin Microbiol Infect 22:379.e9–379.e16. doi: 10.1016/j.cmi.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Teatero S, Ferrieri P, Martin I, Demczuk W, McGeer A, Fittipaldi N. 2017. Serotype distribution, population structure, and antimicrobial resistance of group B Streptococcus strains recovered from colonized pregnant women. J Clin Microbiol 55:412–422. doi: 10.1128/JCM.01615-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teatero S, McGeer A, Li A, Gomes J, Seah C, Demczuk W, Martin I, Wasserscheid J, Dewar K, Melano RG, Fittipaldi N. 2015. Population structure and antimicrobial resistance of invasive serotype IV group B Streptococcus, Toronto, Ontario, Canada. Emerg Infect Dis 21:585–591. doi: 10.3201/eid2014.140759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrieri P, Lynfield R, Creti R, Flores AE. 2013. Serotype IV and invasive group B Streptococcus disease in neonates, Minnesota, USA, 2000–2010. Emerg Infect Dis 19:551–558. doi: 10.3201/eid1904.121572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellais S, Six A, Fouet A, Longo M, Dmytruk N, Glaser P, Trieu-Cuot P, Poyart C. 2012. Capsular switching in group B Streptococcus CC17 hypervirulent clone: a future challenge for polysaccharide vaccine development. J Infect Dis 206:1745–1752. doi: 10.1093/infdis/jis605. [DOI] [PubMed] [Google Scholar]
- 41.Blumberg HM, Stephens DS, Modansky M, Erwin M, Elliot J, Facklam RR, Schuchat A, Baughman W, Farley MM. 1996. Invasive group B streptococcal disease: the emergence of serotype V. J Infect Dis 173:365–373. doi: 10.1093/infdis/173.2.365. [DOI] [PubMed] [Google Scholar]
- 42.Belard S, Toepfner N, Capan-Melser M, Mombo-Ngoma G, Zoleko-Manego R, Groger M, Matsiegui PB, Agnandji ST, Adegnika AA, Gonzalez R, Kremsner PG, Menendez C, Ramharter M, Berner R. 2015. Streptococcus agalactiae serotype distribution and antimicrobial susceptibility in pregnant women in Gabon, Central Africa. Sci Rep 5:17281. doi: 10.1038/srep17281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suara RO, Adegbola RA, Baker CJ, Secka O, Mulholland EK, Greenwood BM. 1994. Carriage of group B streptococci in pregnant Gambian mothers and their infants. J Infect Dis 170:1316–1319. doi: 10.1093/infdis/170.5.1316. [DOI] [PubMed] [Google Scholar]
- 44.Shabayek S, Abdalla S, Abouzeid AM. 2014. Serotype and surface protein gene distribution of colonizing group B streptococcus in women in Egypt. Epidemiol Infect 142:208–210. doi: 10.1017/S0950268813000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lachenauer CS, Kasper DL, Shimada J, Ichiman Y, Ohtsuka H, Kaku M, Paoletti LC, Ferrieri P, Madoff LC. 1999. Serotypes VI and VIII predominate among group B streptococci isolated from pregnant Japanese women. J Infect Dis 179:1030–1033. doi: 10.1086/314666. [DOI] [PubMed] [Google Scholar]
- 46.Suhaimi MES, Desa MNM, Eskandarian N, Pillay SG, Ismail Z, Neela VK, Masri SN, Nordin SA. 2017. Characterization of a group B Streptococcus infection based on the demographics, serotypes, antimicrobial susceptibility and genotypes of selected isolates from sterile and non-sterile isolation sites in three major hospitals in Malaysia. J Infect Public Health 10:14–21. doi: 10.1016/j.jiph.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Slotved HC, Dayie N, Banini JAN, Frimodt-Moller N. 2017. Carriage and serotype distribution of Streptococcus agalactiae in third trimester pregnancy in southern Ghana. BMC Pregnancy Childbirth 17:238. doi: 10.1186/s12884-017-1419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karunakaran R, Raja NS, Hafeez A, Puthucheary SD. 2009. Group B Streptococcus infection: epidemiology, serotypes, and antimicrobial susceptibility of selected isolates in the population beyond infancy (excluding females with genital tract- and pregnancy-related isolates) at the University Malaya Medical Centre, Kuala Lumpur. Jpn J Infect Dis 62:192–194. [PubMed] [Google Scholar]
- 49.Chaudhary M, Rench MA, Baker CJ, Singh P, Hans C, Edwards MS. 2017. Group B streptococcal colonization among pregnant women in Delhi, India. Pediatr Infect Dis J 36:665–669. doi: 10.1097/INF.0000000000001514. [DOI] [PubMed] [Google Scholar]
- 50.Islam MS, Saha SK, Islam M, Modak JK, Shah R, Talukder RR, El Arifeen S, Baqui AH, Darmstadt GL, Mullany LC. 2016. Prevalence, serotype distribution and mortality risk associated with group B Streptococcus colonization of newborns in rural Bangladesh. Pediatr Infect Dis J 35:1309–1312. doi: 10.1097/INF.0000000000001306. [DOI] [PubMed] [Google Scholar]
- 51.Ekelund K, Slotved HC, Nielsen HU, Kaltoft MS, Konradsen HB. 2003. Emergence of invasive serotype VIII group B streptococcal infections in Denmark. J Clin Microbiol 41:4442–4444. doi: 10.1128/JCM.41.9.4442-4444.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paoletti LJ, Bradford J, Paoletti LC. 1999. A serotype VIII strain among colonizing group B streptococcal isolates in Boston, Massachusetts. J Clin Microbiol 37:3759–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan Y, Hu H, Lu T, Fan H, Hu Y, Li G, Zhang X, Shi Y, Xia R. 2016. Investigation of serotype distribution and resistance genes profile in group B Streptococcus isolated from pregnant women: a Chinese multicenter cohort study. APMIS 124:794–799. doi: 10.1111/apm.12570. [DOI] [PubMed] [Google Scholar]
- 54.Toyofuku M, Morozumi M, Hida M, Satoh Y, Sakata H, Shiro H, Ubukata K, Murata M, Iwata S. 2017. Effects of intrapartum antibiotic prophylaxis on neonatal acquisition of group B streptococci. J Pediatr 190:169.e1–173.e1. doi: 10.1016/j.jpeds.2017.07.039. [DOI] [PubMed] [Google Scholar]
- 55.Gudjonsdottir MJ, Hentz E, Berg S, Backhaus E, Elfvin A, Kawash S, Trollfors B. 2015. Serotypes of group B streptococci in western Sweden and comparison with serotypes in two previous studies starting from 1988. BMC Infect Dis 15:507. doi: 10.1186/s12879-015-1266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahara T, Matsubara K, Maihara T, Yamagami Y, Chang B. 2015. Ultra-late-onset meningitis caused by serotype IX group B Streptococcus. Pediatr Infect Dis J 34:801. doi: 10.1097/INF.0000000000000732. [DOI] [PubMed] [Google Scholar]
- 57.Creti R, Imperi M, Pataracchia M, Alfarone G, Recchia S, Baldassarri L. 2012. Identification and molecular characterization of a S. agalactiae strain lacking the capsular locus. Eur J Clin Microbiol Infect Dis 31:233–235. doi: 10.1007/s10096-011-1298-7. [DOI] [PubMed] [Google Scholar]
- 58.Rosini R, Campisi E, De Chiara M, Tettelin H, Rinaudo D, Toniolo C, Metruccio M, Guidotti S, Sorensen UB, Kilian M, Ramirez M, Janulczyk R, Donati C, Grandi G, Margarit I. 2015. Genomic analysis reveals the molecular basis for capsule loss in the group B Streptococcus population. PLoS One 10:e0125985. doi: 10.1371/journal.pone.0125985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramaswamy SV, Ferrieri P, Flores AE, Paoletti LC. 2006. Molecular characterization of nontypeable group B streptococcus. J Clin Microbiol 44:2398–2403. doi: 10.1128/JCM.02236-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cieslewicz MJ, Chaffin D, Glusman G, Kasper D, Madan A, Rodrigues S, Fahey J, Wessels MR, Rubens CE. 2005. Structural and genetic diversity of group B streptococcus capsular polysaccharides. Infect Immun 73:3096–3103. doi: 10.1128/IAI.73.5.3096-3103.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martins ER, Melo-Cristino J, Ramirez M. 2007. Reevaluating the serotype II capsular locus of Streptococcus agalactiae. J Clin Microbiol 45:3384–3386. doi: 10.1128/JCM.01296-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martins ER, Melo-Cristino J, Ramirez M. 2010. Evidence for rare capsular switching in Streptococcus agalactiae. J Bacteriol 192:1361–1369. doi: 10.1128/JB.01130-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meehan M, Cunney R, Cafferkey M. 2014. Molecular epidemiology of group B streptococci in Ireland reveals a diverse population with evidence of capsular switching. Eur J Clin Microbiol Infect Dis 33:1155–1162. doi: 10.1007/s10096-014-2055-5. [DOI] [PubMed] [Google Scholar]
- 64.Neemuchwala A, Teatero S, Athey TB, McGeer A, Fittipaldi N. 2016. Capsular switching and other large-scale recombination events in invasive sequence type 1 group B Streptococcus. Emerg Infect Dis 22:1941–1944. doi: 10.3201//eid2211.152064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nesin M, Ramirez M, Tomasz A. 1998. Capsular transformation of a multidrug-resistant Streptococcus pneumoniae in vivo. J Infect Dis 177:707–713. doi: 10.1086/514242. [DOI] [PubMed] [Google Scholar]
- 66.Ramirez M, Tomasz A. 1999. Acquisition of new capsular genes among clinical isolates of antibiotic-resistant Streptococcus pneumoniae. Microb Drug Resist 5:241–246. doi: 10.1089/mdr.1999.5.241. [DOI] [PubMed] [Google Scholar]
- 67.Stålhammar-Carlemalm M, Stenberg L, Lindahl G. 1993. Protein rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J Exp Med 177:1593–1603. doi: 10.1084/jem.177.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bevanger L, Naess AI. 1985. Mouse-protective antibodies against the Ibc proteins of group B streptococci. Acta Pathol Microbiol Immunol Scand B 93:121–124. [DOI] [PubMed] [Google Scholar]
- 69.Wessels MR, Rubens CE, Benedi VJ, Kasper DL. 1989. Definition of a bacterial virulence factor: sialylation of the group B streptococcal capsule. Proc Natl Acad Sci U S A 86:8983–8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindahl G, Stalhammar-Carlemalm M, Areschoug T. 2005. Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clin Microbiol Rev 18:102–127. doi: 10.1128/CMR.18.1.102-127.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferrieri P, Flores AE. 1997. Surface protein expression in group B streptococcal invasive isolates. Adv Exp Med Biol 418:635–637. doi: 10.1007/978-1-4899-1825-3_148. [DOI] [PubMed] [Google Scholar]
- 72.Gabrielsen C, Mæland JA, Lyng RV, Radtke A, Afset JE. 2017. Molecular characteristics of Streptococcus agalactiae strains deficient in alpha-like protein encoding genes. J Med Microbiol 66:26–33. doi: 10.1099/jmm.0.000412. [DOI] [PubMed] [Google Scholar]
- 73.Smith TC, Roehl SA, Pillai P, Li S, Marrs CF, Foxman B. 2007. Distribution of novel and previously investigated virulence genes in colonizing and invasive isolates of Streptococcus agalactiae. Epidemiol Infect 135:1046–1054. doi: 10.1017/S0950268806007515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fasola E, Livdahl C, Ferrieri P. 1993. Molecular analysis of multiple isolates of the major serotypes of group B streptococci. J Clin Microbiol 31:2616–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benson JA, Ferrieri P. 2001. Rapid pulsed-field gel electrophoresis method for group B Streptococcus isolates. J Clin Microbiol 39:3006–3008. doi: 10.1128/JCM.39.8.3006-3008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Skjaervold NK, Bergh K, Bevanger L. 2004. Distribution of PFGE types of invasive Norwegian group B streptococci in relation to serotypes. Indian J Med Res 119(Suppl):201–204. [PubMed] [Google Scholar]
- 78.Souza VC, Kegele FC, Souza SR, Neves FP, de Paula GR, Barros RR. 2013. Antimicrobial susceptibility and genetic diversity of Streptococcus agalactiae recovered from newborns and pregnant women in Brazil. Scand J Infect Dis 45:780–785. doi: 10.3109/00365548.2013.810814. [DOI] [PubMed] [Google Scholar]
- 79.Diedrick MJ, Flores AE, Hillier SL, Creti R, Ferrieri P. 2010. Clonal analysis of colonizing group B Streptococcus, serotype IV, an emerging pathogen in the United States. J Clin Microbiol 48:3100–3104. doi: 10.1128/JCM.00277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ellis S, Kotiw M, Garland SM. 1996. Restriction endonuclease analysis of group B streptococcal isolates from two distinct geographical regions. J Hosp Infect 33:279–287. doi: 10.1016/S0195-6701(96)90014-6. [DOI] [PubMed] [Google Scholar]
- 81.Jones N, Bohnsack JF, Takahashi S, Oliver KA, Chan MS, Kunst F, Glaser P, Rusniok C, Crook DW, Harding RM, Bisharat N, Spratt BG. 2003. Multilocus sequence typing system for group B streptococcus. J Clin Microbiol 41:2530–2536. doi: 10.1128/JCM.41.6.2530-2536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jolley KA, Chan MS, Maiden MC. 2004. mlstdbNet—distributed multi-locus sequence typing (MLST) databases. BMC Bioinformatics 5:86. doi: 10.1186/1471-2105-5-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kang HM, Lee HJ, Lee H, Jo DS, Lee HS, Kim TS, Shin JH, Yun KW, Lee B, Choi EH. 2017. Genotype characterization of group B Streptococcus isolated from infants with invasive diseases in South Korea. Pediatr Infect Dis J 36:e242–e247. doi: 10.1097/INF.0000000000001531. [DOI] [PubMed] [Google Scholar]
- 84.Lo CW, Liu HC, Lee CC, Lin CL, Chen CL, Jeng MJ, Chiu CH. 2017. Serotype distribution and clinical correlation of Streptococcus agalactiae causing invasive disease in infants and children in Taiwan. J Microbiol Immunol Infect 2017:S1684-1182(17)30228-1. doi: 10.1016/j.jmii.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 85.Sorensen UB, Poulsen K, Ghezzo C, Margarit I, Kilian M. 2010. Emergence and global dissemination of host-specific Streptococcus agalactiae clones. mBio 1:e00178-10. doi: 10.1128/mBio.00178-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bisharat N, Jones N, Marchaim D, Block C, Harding RM, Yagupsky P, Peto T, Crook DW. 2005. Population structure of group B streptococcus from a low-incidence region for invasive neonatal disease. Microbiology 151:1875–1881. doi: 10.1099/mic.0.27826-0. [DOI] [PubMed] [Google Scholar]
- 87.Jones N, Oliver KA, Barry J, Harding RM, Bisharat N, Spratt BG, Peto T, Crook DW. 2006. Enhanced invasiveness of bovine-derived neonatal sequence type 17 group B streptococcus is independent of capsular serotype. Clin Infect Dis 42:915–924. doi: 10.1086/500324. [DOI] [PubMed] [Google Scholar]
- 88.Luan SL, Granlund M, Sellin M, Lagergard T, Spratt BG, Norgren M. 2005. Multilocus sequence typing of Swedish invasive group B streptococcus isolates indicates a neonatally associated genetic lineage and capsule switching. J Clin Microbiol 43:3727–3733. doi: 10.1128/JCM.43.8.3727-3733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martins ER, Pessanha MA, Ramirez M, Melo-Cristino J. 2007. Analysis of group B streptococcal isolates from infants and pregnant women in Portugal revealing two lineages with enhanced invasiveness. J Clin Microbiol 45:3224–3229. doi: 10.1128/JCM.01182-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin FY, Whiting A, Adderson E, Takahashi S, Dunn DM, Weiss R, Azimi PH, Philips JB III, Weisman LE, Regan J, Clark P, Rhoads GG, Frasch CE, Troendle J, Moyer P, Bohnsack JF. 2006. Phylogenetic lineages of invasive and colonizing strains of serotype III group B streptococci from neonates: a multicenter prospective study. J Clin Microbiol 44:1257–1261. doi: 10.1128/JCM.44.4.1257-1261.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Manning SD, Springman AC, Lehotzky E, Lewis MA, Whittam TS, Davies HD. 2009. Multilocus sequence types associated with neonatal group B streptococcal sepsis and meningitis in Canada. J Clin Microbiol 47:1143–1148. doi: 10.1128/JCM.01424-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Limansky AS, Guardati MC, Sutich EG, Toresani IE, Bogado IE, Joannas GM, Viale AM. 1995. Characterization of clinical isolates of Streptococcus agalactiae by random amplified polymorphic DNA using degenerate oligonucleotides. Medicina (B Aires) 55:681–684. [PubMed] [Google Scholar]
- 93.Martinez G, Harel J, Higgins R, Lacouture S, Daignault D, Gottschalk M. 2000. Characterization of Streptococcus agalactiae isolates of bovine and human origin by randomly amplified polymorphic DNA analysis. J Clin Microbiol 38:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chatellier S, Ramanantsoa C, Harriau P, Rolland K, Rosenau A, Quentin R. 1997. Characterization of Streptococcus agalactiae strains by randomly amplified polymorphic DNA analysis. J Clin Microbiol 35:2573–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brzychczy-Wloch M, Gosiewski T, Bodaszewska M, Pabian W, Bulanda M, Kochan P, Strus M, Heczko PB. 2010. Genetic characterization and diversity of Streptococcus agalactiae isolates with macrolide resistance. J Med Microbiol 59:780–786. doi: 10.1099/jmm.0.018176-0. [DOI] [PubMed] [Google Scholar]
- 96.Brandolini M, Corbella M, Cambieri P, Barbarini D, Sassera D, Stronati M, Marone P. 2014. Late-onset neonatal group B streptococcal disease associated with breast milk transmission: molecular typing using RAPD-PCR. Early Hum Dev 90(Suppl 1):S84–S86. doi: 10.1016/S0378-3782(14)70025-8. [DOI] [PubMed] [Google Scholar]
- 97.Toresani I, Limansky A, Bogado I, Guardati MC, Viale A, Sutich EG. 2001. Phenotypic and genotypic study of Streptococcus agalactiae in vagina of pregnant women in Argentina. Medicina (B Aires) 61:295–300. [PubMed] [Google Scholar]
- 98.El Aila NA, Tency I, Claeys G, Saerens B, De Backer E, Temmerman M, Verhelst R, Vaneechoutte M. 2009. Genotyping of Streptococcus agalactiae (group B streptococci) isolated from vaginal and rectal swabs of women at 35–37 weeks of pregnancy. BMC Infect Dis 9:153. doi: 10.1186/1471-2334-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brzychczy-Wloch M, Strus M, Pawlik D, Gosiewski T, Krzysztof R, Drzewiecki A, Lauterbach R, Heczko PB. 2008. Studies on the possible application of molecular methods in diagnosing carriers and in similarity analysis of group B streptococci (Streptococcus agalactiae). Med Dosw Mikrobiol 60:91–99. [PubMed] [Google Scholar]
- 100.Strus M, Pawlik D, Brzychczy-Włoch M, Gosiewski T, Rytlewski K, Lauterbach R, Heczko PB. 2009. Group B streptococcus colonization of pregnant women and their children observed on obstetric and neonatal wards of the University Hospital in Krakow, Poland. J Med Microbiol 58:228–233. doi: 10.1099/jmm.0.002865-0. [DOI] [PubMed] [Google Scholar]
- 101.Hannoun A, Shehab M, Khairallah MT, Sabra A, Abi-Rached R, Bazi T, Yunis KA, Araj GF, Matar GM. 2009. Correlation between group B streptococcal genotypes, their antimicrobial resistance profiles, and virulence genes among pregnant women in Lebanon. Int J Microbiol 2009:796512. doi: 10.1155/2009/796512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muller AE, Valkenburg-van den Berg AW, Kreft D, Oostvogel PM, Sprij AJ, van Belkum A. 2008. Low rate of carriage of macrolide-resistant group B streptococci in pregnant women in The Netherlands. Eur J Obstet Gynecol Reprod Biol 137:17–20. doi: 10.1016/j.ejogrb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 103.Zhang GW, Kotiw M, Daggard G. 2002. A RAPD-PCR genotyping assay which correlates with serotypes of group B streptococci. Lett Appl Microbiol 35:247–250. doi: 10.1046/j.1472-765X.2002.01177.x. [DOI] [PubMed] [Google Scholar]
- 104.Al Nakib M, Longo M, Tazi A, Billoet A, Raymond J, Trieu-Cuot P, Poyart C. 2011. Comparison of the Diversilab(R) system with multi-locus sequence typing and pulsed-field gel electrophoresis for the characterization of Streptococcus agalactiae invasive strains. J Microbiol Methods 85:137–142. doi: 10.1016/j.mimet.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 105.Amal MN, Zamri-Saad M, Siti-Zahrah A, Zulkafli AR, Nur-Nazifah M. 2013. Molecular characterization of Streptococcus agalactiae strains isolated from fishes in Malaysia. J Appl Microbiol 115:20–29. doi: 10.1111/jam.12210. [DOI] [PubMed] [Google Scholar]
- 106.Tyler KD, Wang G, Tyler SD, Johnson WM. 1997. Factors affecting reliability and reproducibility of amplification-based DNA fingerprinting of representative bacterial pathogens. J Clin Microbiol 35:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deplano A, Schuermans A, Van Eldere J, Witte W, Meugnier H, Etienne J, Grundmann H, Jonas D, Noordhoek GT, Dijkstra J, van Belkum A, van Leeuwen W, Tassios PT, Legakis NJ, van der Zee A, Bergmans A, Blanc DS, Tenover FC, Cookson BC, O'Neil G, Struelens MJ. 2000. Multicenter evaluation of epidemiological typing of methicillin-resistant Staphylococcus aureus strains by repetitive-element PCR analysis. The European Study Group on Epidemiological Markers of the ESCMID. J Clin Microbiol 38:3527–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yazdankhah SP, Lindstedt BA. 2007. Variable number tandem repeat typing of bacteria. Methods Mol Biol 396:395–405. doi: 10.1007/978-1-59745-515-2_25. [DOI] [PubMed] [Google Scholar]
- 109.Radtke A, Lindstedt BA, Afset JE, Bergh K. 2010. Rapid multiple-locus variant-repeat assay (MLVA) for genotyping of Streptococcus agalactiae. J Clin Microbiol 48:2502–2508. doi: 10.1128/JCM.00234-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dmitriev AV, Shen AD, Totolian AA. 2007. Streptococcus agalactiae sak0192 gene contains direct repeats and spacers that are genetic markers for characterizing the strains. Mol Gen Mikrobiol Virusol 2007:37–42. [PubMed] [Google Scholar]
- 111.Lamy MC, Dramsi S, Billoet A, Reglier-Poupet H, Tazi A, Raymond J, Guerin F, Couve E, Kunst F, Glaser P, Trieu-Cuot P, Poyart C. 2006. Rapid detection of the “highly virulent” group B Streptococcus ST-17 clone. Microbes Infect 8:1714–1722. doi: 10.1016/j.micinf.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 112.Reinscheid DJ, Gottschalk B, Schubert A, Eikmanns BJ, Chhatwal GS. 2001. Identification and molecular analysis of PcsB, a protein required for cell wall separation of group B streptococcus. J Bacteriol 183:1175–1183. doi: 10.1128/JB.183.4.1175-1183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rosenau A, Martins K, Amor S, Gannier F, Lanotte P, van der Mee-Marquet N, Mereghetti L, Quentin R. 2007. Evaluation of the ability of Streptococcus agalactiae strains isolated from genital and neonatal specimens to bind to human fibrinogen and correlation with characteristics of the fbsA and fbsB genes. Infect Immun 75:1310–1317. doi: 10.1128/IAI.00996-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haguenoer E, Baty G, Pourcel C, Lartigue MF, Domelier AS, Rosenau A, Quentin R, Mereghetti L, Lanotte P. 2011. A multi locus variable number of tandem repeat analysis (MLVA) scheme for Streptococcus agalactiae genotyping. BMC Microbiol 11:171. doi: 10.1186/1471-2180-11-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Otaguiri ES, Morguette AE, Tavares ER, dos Santos PM, Morey AT, Cardoso JD, Perugini MR, Yamauchi LM, Yamada-Ogatta SF. 2013. Commensal Streptococcus agalactiae isolated from patients seen at University Hospital of Londrina, Parana, Brazil: capsular types, genotyping, antimicrobial susceptibility and virulence determinants. BMC Microbiol 13:297. doi: 10.1186/1471-2180-13-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beigverdi R, Jabalameli F, Mirsalehian A, Hantoushzadeh S, Boroumandi S, Taherikalani M, Emaneini M. 2014. Virulence factors, antimicrobial susceptibility and molecular characterization of Streptococcus agalactiae isolated from pregnant women. Acta Microbiol Immunol Hung 61:425–434. doi: 10.1556/AMicr.61.2014.4.4. [DOI] [PubMed] [Google Scholar]
- 117.Barrangou R, Horvath P. 2012. CRISPR: new horizons in phage resistance and strain identification. Annu Rev Food Sci Technol 3:143–162. doi: 10.1146/annurev-food-022811-101134. [DOI] [PubMed] [Google Scholar]
- 118.Shariat N, Dudley EG. 2014. CRISPRs: molecular signatures used for pathogen subtyping. Appl Environ Microbiol 80:430–439. doi: 10.1128/AEM.02790-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lopez-Sanchez MJ, Sauvage E, Da Cunha V, Clermont D, Ratsima Hariniaina E, Gonzalez-Zorn B, Poyart C, Rosinski-Chupin I, Glaser P. 2012. The highly dynamic CRISPR1 system of Streptococcus agalactiae controls the diversity of its mobilome. Mol Microbiol 85:1057–1071. doi: 10.1111/j.1365-2958.2012.08172.x. [DOI] [PubMed] [Google Scholar]
- 120.Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, Barrangou R, Brouns SJ, Charpentier E, Haft DH, Horvath P, Moineau S, Mojica FJ, Terns RM, Terns MP, White MF, Yakunin AF, Garrett RA, van der Oost J, Backofen R, Koonin EV. 2015. An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol 13:722–736. doi: 10.1038/nrmicro3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beauruelle C, Pastuszka A, Horvath P, Perrotin F, Mereghetti L, Lanotte P. 2017. CRISPR: a useful genetic feature to follow vaginal carriage of group B Streptococcus. Front Microbiol 8:1981. doi: 10.3389/fmicb.2017.01981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Di Renzo GC, Melin P, Berardi A, Blennow M, Carbonell-Estrany X, Donzelli GP, Hakansson S, Hod M, Hughes R, Kurtzer M, Poyart C, Shinwell E, Stray-Pedersen B, Wielgos M, El Helali N. 2015. Intrapartum GBS screening and antibiotic prophylaxis: a European consensus conference. J Matern Fetal Neonatal Med 28:766–782. doi: 10.3109/14767058.2014.934804. [DOI] [PubMed] [Google Scholar]
- 123.Agence Nationale d'Accréditation et d'Evaluation en Santé 2003. Antenatal prevention of early neonatal bacterial infection (September 2001). J Gynecol Obstet Biol Reprod (Paris) 32:68–74. [PubMed] [Google Scholar]
- 124.Jordan HT, Farley MM, Craig A, Mohle-Boetani J, Harrison LH, Petit S, Lynfield R, Thomas A, Zansky S, Gershman K, Albanese BA, Schaffner W, Schrag SJ. 2008. Revisiting the need for vaccine prevention of late-onset neonatal group B streptococcal disease: a multistate, population-based analysis. Pediatr Infect Dis J 27:1057–1064. doi: 10.1097/INF.0b013e318180b3b9. [DOI] [PubMed] [Google Scholar]
- 125.Seedat F, Stinton C, Patterson J, Geppert J, Tan B, Robinson ER, McCarthy ND, Uthman OA, Freeman K, Johnson SA, Fraser H, Brown CS, Clarke A, Taylor-Phillips S. 2017. Adverse events in women and children who have received intrapartum antibiotic prophylaxis treatment: a systematic review. BMC Pregnancy Childbirth 17:247. doi: 10.1186/s12884-017-1432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aloisio I, Mazzola G, Corvaglia LT, Tonti G, Faldella G, Biavati B, Di Gioia D. 2014. Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl Microbiol Biotechnol 98:6051–6060. doi: 10.1007/s00253-014-5712-9. [DOI] [PubMed] [Google Scholar]
- 127.Aloisio I, Quagliariello A, De Fanti S, Luiselli D, De Filippo C, Albanese D, Corvaglia LT, Faldella G, Di Gioia D. 2016. Evaluation of the effects of intrapartum antibiotic prophylaxis on newborn intestinal microbiota using a sequencing approach targeted to multi hypervariable 16S rDNA regions. Appl Microbiol Biotechnol 100:5537–5546. doi: 10.1007/s00253-016-7410-2. [DOI] [PubMed] [Google Scholar]
- 128.Corvaglia L, Tonti G, Martini S, Aceti A, Mazzola G, Aloisio I, Di Gioia D, Faldella G. 2016. Influence of intrapartum antibiotic prophylaxis for group B streptococcus on gut microbiota in the first month of life. J Pediatr Gastroenterol Nutr 62:304–308. doi: 10.1097/MPG.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 129.Jaureguy F, Carton M, Panel P, Foucaud P, Butel MJ, Doucet-Populaire F. 2004. Effects of intrapartum penicillin prophylaxis on intestinal bacterial colonization in infants. J Clin Microbiol 42:5184–5188. doi: 10.1128/JCM.42.11.5184-5188.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arboleya S, Sanchez B, Milani C, Duranti S, Solis G, Fernandez N, de los Reyes-Gavilan CG, Ventura M, Margolles A, Gueimonde M. 2015. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr 166:538–544. doi: 10.1016/j.jpeds.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 131.Arboleya S, Sanchez B, Solis G, Fernandez N, Suarez M, Hernandez-Barranco AM, Milani C, Margolles A, de Los Reyes-Gavilan CG, Ventura M, Gueimonde M. 2016. Impact of prematurity and perinatal antibiotics on the developing intestinal microbiota: a functional inference study. Int J Mol Sci 17:E649. doi: 10.3390/ijms17050649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mazzola G, Murphy K, Ross RP, Di Gioia D, Biavati B, Corvaglia LT, Faldella G, Stanton C. 2016. Early gut microbiota perturbations following intrapartum antibiotic prophylaxis to prevent group B streptococcal disease. PLoS One 11:e0157527. doi: 10.1371/journal.pone.0157527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, Turner P, Parkhill J, Loman NJ, Walker AW. 2014. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol 12:87. doi: 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. 2012. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baker CJ, Kasper DL. 1976. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med 294:753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- 136.Englund JA. 2007. The influence of maternal immunization on infant immune responses. J Comp Pathol 137(Suppl 1):S16–S19. doi: 10.1016/j.jcpa.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 137.Heath PT. 2016. Status of vaccine research and development of vaccines for GBS. Vaccine 34:2876–2879. doi: 10.1016/j.vaccine.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 138.Lin SM, Zhi Y, Ahn KB, Lim S, Seo HS. 2018. Status of group B streptococcal vaccine development. Clin Exp Vaccine Res 7:76–81. doi: 10.7774/cevr.2018.7.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Madhi SA, Cutland CL, Jose L, Koen A, Govender N, Wittke F, Olugbosi M, Meulen AS, Baker S, Dull PM, Narasimhan V, Slobod K. 2016. Safety and immunogenicity of an investigational maternal trivalent group B streptococcus vaccine in healthy women and their infants: a randomised phase 1b/2 trial. Lancet Infect Dis 16:923–934. doi: 10.1016/S1473-3099(16)00152-3. [DOI] [PubMed] [Google Scholar]
- 140.Madhi SA, Koen A, Cutland CL, Jose L, Govender N, Wittke F, Olugbosi M, Sobanjo-Ter Meulen A, Baker S, Dull PM, Narasimhan V, Slobod K. 2017. Antibody kinetics and response to routine vaccinations in infants born to women who received an investigational trivalent group B Streptococcus polysaccharide CRM197-conjugate vaccine during pregnancy. Clin Infect Dis 65:1897–1904. doi: 10.1093/cid/cix666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maione D, Margarit I, Rinaudo CD, Masignani V, Mora M, Scarselli M, Tettelin H, Brettoni C, Iacobini ET, Rosini R, D'Agostino N, Miorin L, Buccato S, Mariani M, Galli G, Nogarotto R, Nardi-Dei V, Vegni F, Fraser C, Mancuso G, Teti G, Madoff LC, Paoletti LC, Rappuoli R, Kasper DL, Telford JL, Grandi G. 2005. Identification of a universal group B streptococcus vaccine by multiple genome screen. Science 309:148–150. doi: 10.1126/science.1109869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Larsson C, Lindroth M, Nordin P, Stalhammar-Carlemalm M, Lindahl G, Krantz I. 2006. Association between low concentrations of antibodies to protein alpha and Rib and invasive neonatal group B streptococcal infection. Arch Dis Child Fetal Neonatal Ed 91:F403–F408. doi: 10.1136/adc.2005.090472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cutland CL, Madhi SA, Zell ER, Kuwanda L, Laque M, Groome M, Gorwitz R, Thigpen MC, Patel R, Velaphi SC, Adrian P, Klugman K, Schuchat A, Schrag SJ. 2009. Chlorhexidine maternal-vaginal and neonate body wipes in sepsis and vertical transmission of pathogenic bacteria in South Africa: a randomised, controlled trial. Lancet 374:1909–1916. doi: 10.1016/S0140-6736(09)61339-8. [DOI] [PubMed] [Google Scholar]
- 144.Kim S-Y, Russell LB, Park J, Verani JR, Madhi SA, Cutland CL, Schrag SJ, Sinha A. 2014. Cost-effectiveness of a potential group B streptococcal vaccine program for pregnant women in South Africa. Vaccine 32:1954–1963. doi: 10.1016/j.vaccine.2014.01.062. [DOI] [PubMed] [Google Scholar]
- 145.Kim SY, Nguyen C, Russell LB, Tomczyk S, Abdul-Hakeem F, Schrag SJ, Verani JR, Sinha A. 2017. Cost-effectiveness of a potential group B streptococcal vaccine for pregnant women in the United States. Vaccine 35:6238–6247. doi: 10.1016/j.vaccine.2017.08.085. [DOI] [PubMed] [Google Scholar]
- 146.Furfaro LL, Chang BJ, Payne MS. 2018. Applications for bacteriophage therapy during pregnancy and the perinatal period. Front Microbiol 8:2660. doi: 10.3389/fmicb.2017.02660. [DOI] [PMC free article] [PubMed] [Google Scholar]