Abstract
Disruption of the alveolar-capillary barrier is a hallmark of acute respiratory distress syndrome (ARDS) that leads to the accumulation of protein-rich edema in the alveolar space, often resulting in comparable protein concentrations in alveolar edema and plasma and causing deleterious remodeling. Patients who survive ARDS have approximately three times lower protein concentrations in the alveolar edema than nonsurvivors; thus the ability to remove excess protein from the alveolar space may be critical for a positive outcome. We have recently shown that clearance of albumin from the alveolar space is mediated by megalin, a 600-kDa transmembrane endocytic receptor and member of the low-density lipoprotein receptor superfamily. In the currents study, we investigate the molecular mechanisms by which transforming growth factor-β (TGF-β), a key molecule of ARDS pathogenesis, drives downregulation of megalin expression and function. TGF-β treatment led to shedding and regulated intramembrane proteolysis of megalin at the cell surface and to a subsequent increase in intracellular megalin COOH-terminal fragment abundance resulting in transcriptional downregulation of megalin. Activity of classical protein kinase C enzymes and γ-secretase was required for the TGF-β-induced megalin downregulation. Furthermore, TGF-β-induced shedding of megalin was mediated by matrix metalloproteinases (MMPs)-2, -9, and -14. Silencing of either of these MMPs stabilized megalin at the cell surface after TGF-β treatment and restored normal albumin transport. Moreover, a direct interaction of megalin with MMP-2 and -14 was demonstrated, suggesting that these MMPs may function as novel sheddases of megalin. Further understanding of these mechanisms may lead to novel therapeutic approaches for the treatment of ARDS.
Keywords: alveolar protein transport, megalin, matrix metalloproteinases, receptor shedding, regulated intramembrane proteolysis
acute respiratory distress syndrome (ARDS) is a severe clinical condition that is associated with impaired alveolar-capillary barrier permeability leading to formation of protein-rich edema and compromised alveolar gas exchange (50). The therapy of ARDS remains entirely supportive, and no pharmacological means exist (23). It is very well established that the clearance of pulmonary edema is essential for survival (51). In the acute phase of ARDS, protein concentrations in the alveolar edema fluid may reach up to 90% of plasma levels, which may further rise as pulmonary edema is reabsorbed, causing deleterious remodeling and impairing resolution of lung injury (27). Importantly, survivors of ARDS present on average a three times lower protein concentration in the bronchoalveolar lavage (BAL) than patients who do not survive (1). Thus the ability to remove excess protein content from the distal airways may promote the resolution of ARDS.
Several mechanisms have been implicated in the removal of excess protein from the alveolar space, including mucociliary clearance, phagocytosis by macrophages, protein degradation, as well as passive and active alveolar epithelial transport processes (18). Initial studies showed that protein clearance across the alveolar epithelium is a slow process, and although protein uptake could be attenuated by inhibitors of endocytosis in alveolar epithelial cells, when distal airspaces of rabbits were filled with a protein-rich solution, clearance of immunoglobulin G and surfactant protein-A remained unaffected (2, 20, 21). More recent studies utilizing intact rabbit lungs in which clearance of radio-labeled albumin was measured in real time, suggested that the removal of excess protein content from the alveolar compartment is an active, receptor-mediated, high-capacity transport process with velocities that may have a (patho)physiological relevance, which required clathrin-mediated endocytosis of the protein (6, 17, 55). Furthermore, we have previously demonstrated that transport of albumin across the alveolar epithelial barrier is mediated by megalin, a 600-kDa transmembrane endocytic receptor and a member of the low-density lipoprotein (LDL) receptor family (6, 17, 33). Additionally, most recent data from our group showed that alveolar protein clearance is impaired in a murine model of acute lung injury and that inhibition of transforming growth factor-β (TGF-β), an important mediator of the pathogenesis of ARDS (39, 40), partially restores megalin function and enhances alveolar protein removal (49).
Regulated intramembrane proteolysis (RIP) represents an evolutionary conserved process that connects receptor function with transcriptional regulation (42). A well-characterized example is the Notch signaling pathway where RIP is stimulated by shedding of the receptor ectodomain by matrix metalloproteinases (MMPs) followed by γ-secretase-mediated intramembrane proteolysis of the resulting membrane-associated fragment. This leads to the release of the COOH-terminal cytosolic domain, which translocates into the nucleus where it affects gene expression (42). There is increasing evidence that megalin may also be a target of ectodomain shedding and RIP in different cell types (3, 32, 56) that are regulated by metalloprotease and γ-secretase activity.
During the acute phase of ARDS and along the reconstitution of the alveolar-capillary barrier, MMPs play a critical role in remodeling of the extracellular matrix (ECM) (10). However, the function of MMPs is not limited to the ECM. Recent studies have shown that secreted MMPs may be re-endocytosed by interacting with cell surface proteins such as members of the LDL receptor-related protein family, including megalin (36). Reciprocal regulation between MMPs and TGF-β has been extensively investigated (26, 30). Importantly, expression and activity of TGF-β, MMP-2, and MMP-9 are markedly upregulated in BAL samples from patients with ARDS (39, 41, 45). Furthermore, in the context of tumor progression and epithelial-to-mesenchymal transition, the above mentioned metalloproteinases have been shown to be activated by TGF-β. Also, expression of MMP-14, an enzyme that drives the shedding of LDL receptor-related protein 1 (which is structurally very similar to megalin) in lung fibroblasts (52), may be upregulated by TGF-β during progression of angiogenesis (53).
In the present study, we demonstrate that TGF-β impairs alveolar protein transport by inducing megalin shedding and RIP in alveolar epithelial cells, thereby inhibiting megalin-mediated uptake of albumin. Moreover, we show that PKC and γ-secretase activities are required for these TGF-β-induced effects, and identify MMP-2 and -14 as novel megalin sheddases.
MATERIALS AND METHODS
Reagents.
Human recombinant transforming growth factor β1 (hr-TGF-β1) was purchased from R&D Systems (Wiesbaden-Nordenstadt, Germany). siRNAs were obtained from Santa Cruz Biotechnology (Heidelberg, Germany). Gö6976 (PKC inhibitor) and compound E (CE, γ-secretase activity inhibitor) were from Calbiochem (San Diego, CA). Megalin antibody was obtained from Proteintech (Manchester, UK); MMP-2, MMP-9, PKCα/β, and presenilin-1 antibodies were from Santa Cruz Biotechnology; MMP-14 antibody was from Thermo Fisher (Schwerte, Germany); transferrin receptor antibody was purchased from Invitrogen (Karlsruhe, Germany), and Na, K-ATPase antibody was from Millipore (Darmstadt, Germany). The antibody against fibrillarin was from Cell Signaling (Leiden, The Netherlands). EZ-link NHS-LC-biotins, streptavidin-agarose beads, and the ECL detection kit were purchased form Thermo Fisher Scientific (Darmstadt, Germany). Bradford reagent was from Bio-Rad (Munich, Germany). All other reagents including β-actin antibodies were purchased from Sigma (Seelze, Germany).
Cell culture and maintenance.
The rat lung epithelial-T-antigen negative (RLE-6TN) cell line was obtained from the American Type Culture Collection and used for experiments at passages 9 to 13. Primary ATII cells were isolated from male Sprague-Dawley rats not older than 3 wk (140–160 g). Cells were cultured in DMEM 1 g/ml glucose containing 10% FBS and 1% penicillin/streptomycin mix (PAN-Biotech, Aidenbach, Germany), in a humidified atmosphere 5% CO2 at 37°C.
Primary ATII cells isolation from rat lungs.
Alveolar type II cells were isolated as previously described (6, 47). The day of isolation and plating was designated as day 0. The cells were processed 24 or 72 h after isolation.
SDS-PAGE and Western blotting.
Protein concentration was quantified by Bradford assay. Equal amounts of proteins were cracked in Laemmli buffer and resolved in 10 or 16% polyacrylamide gels. For detection of megalin, 4 to 16% or 4 to 10% gradient gels were prepared. Proteins were transferred to nitrocellulose membranes (Bio-Rad) and blocked in T-TBS 5% skim milk. Membranes were incubated overnight with specific antibodies at 4°C. Blots were developed with SuperSignal West Pico or Femto Chemiluminescent Substrate detection kit in a CP 1000 automatic film processor (AGFA, Mortsel, Belgium). The bands were quantified by densitometry (Image J, National Institutes of Health, Bethesda, MD).
mRNA analysis.
Total RNA was isolated from primary ATII and RLE-6TN cells by using a Qiagen RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) following the instructions of the manufacturer. cDNA was prepared with the iScript cDNA Synthesis kit (Bio-Rad). Real-time quantitative PCR was performed with SYBR Green Master Mix (Bio-Rad) in a real-time thermocycler device from Stratagene (San Diego, CA). The following primer sequences were used for amplification: megalin sense, 5′-AAAGTGGCCCTGGCAGTTC-3′; megalin antisense, 5′-GAAGGATCTATGGGCCTTCCA-3′; NHE3 sense, 5′-ACTGCTTAATGACGCGGTGACTGT-3′; NHE3 antisense, 5′-AAAGACGAAGCCAGGCTCGATGAT-3′; GAPDH sense, 5′-GGCAAGTTCAAGGGCACAGT-3′; GAPDH antisense, 5′-TGGTGAAGACGCCAGTAGACTC-3′. ΔCt values were calculated as CtGAPDH – Ctgene of interest.
Specific mRNA knockdown.
RLE-6TN cells were cultured to reach 60 to 80% confluence on the day of transfection. Megalin (50 nM) MMP-2 (50 nM), MMP-9 (50 nM), MMP-14 (40 nM), presenilin (PS)-1 (50 nM), and PKC α/β (50 nM) siRNA were transfected using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s instructions. Scrambled (Scr) siRNA (Santa Cruz Biotechnology) was used as control. Megalin cell surface stability and FITC-labeled albumin binding and uptake were measured 72 h after transfection.
Biotinylation of cell surface proteins.
Primary ATII and RLE-6TN cells were labeled with 1 mg/ml of EZ-link NHS-LC-biotin in PBS for 20 min, and cell surface proteins were precipitated with streptavidin-agarose beads and detected by SDS-PAGE and Western blot analysis, as previously described (47).
Plasma membrane purification.
This protocol was adapted from previously published studies (11, 24, 43, 44). Briefly, RLE-6TN cells were plated to reach 90% confluence. Cells were incubated with homogenization buffer [HBr; 0.25 M sucrose, 3 mM Imidazol, 1 mM EDTA, and protease inhibitors (Complete, Roche, Penzberg, Germany) at a pH of 7.4] and passed through a 26-gauge needle syringe to homogenize the cells. One aliquot was centrifuged at 100,000 g for 30 min to remove remaining debris (cytoplasmic fraction); the rest of the homogenate was centrifuged at 9,800 g for 10 min. The pellet was resuspended in HBr buffer and passed through a 26-gauge needle syringe. Two milliliters of high-sucrose buffer (50 mM Tris pH 7.5, 1.8 M sucrose; 3 mM MgCl2, and protease inhibitors) were placed at the bottom of a centrifuge bottle, and 2 ml of low-sucrose buffer (50 mM Tris pH 7.5, 0.3 M sucrose, 3 mM MgCl2, and protease inhibitors) premixed with the pellet suspension were gently placed on top. Centrifugation followed at 71,000 g for 90 min in an Optima MAX XP ultracentrifuge (Beckman Coulter, Brea, CA). The interphase and the pellet were recentrifuged under the same conditions for better purification. Purified plasma membrane proteins were extracted from the interphase with HBr buffer plus 0.5% NP-40, and nuclear pellets were resuspended in five volumes of nuclear extraction buffer (20 mM HEPES pH 7.5, 1.5 mM MgCl2, 0.5 M NaCl, 0.2 mM EDTA, and 20% glycerol) for nuclear proteins isolation. Samples were then centrifuged at 100,000 g for 30 min. Supernatants were kept as purified nuclear (N) and plasma membrane proteins, respectively. All fractions were separated by SDS-PAGE in 4 to 16% gradient gels. Fibrillarin, β-actin, and Na, K-ATPase were used as markers of nuclear, cytoplasm, and plasma membrane fractions, respectively. Coomassie brilliant blue staining was used as loading control.
Immunofluorescence and confocal microscopy.
RLE-6TN cells were plated 24 h before the experiments in eight-well Laboratory-Tek chamber slides with Permanox (Thermo Fisher, Waltham, MA) to reach a confluency of 40 to 60%. Cells were incubated with TGF-β (20 ng/ml) for 10 h, washed, and fixed in 1:1 methanol:acetone for 5 min. After fixation, cells were permeabilized with 0.1% Triton X-100 in PBS without Ca++/Mg++ for 10 min and blocked for 1 h with 1% BSA in 0.1% Tween 20 (Sigma, St. Louis, MO) in PBS without Ca++/Mg++. Specific primary antibodies against megalin or β-actin were incubated overnight at 4°C, and anti-rabbit secondary antibodies Alexa-fluor 488 or anti-mouse secondary antibodies Alexa-fluor 594, respectively, were subsequently added for 1 h. Finally, nuclear staining was performed with DAPI for 10 min. Cells were mounted with Vectashield mounting medium for fluorescence (Vector, Burlingame, CA). Fluorescence was detected with a laser scanning confocal microscope LSM 710 (Leica Microsystems, Wetzlar, Germany).
Inhibition of PKC and γ-secretase activities.
RLE-6TN cells were pretreated with 1.3 µM gö6976, 1 µM Compound E (CE), or vehicle (DMSO) for 1.5 h in conditioned low-glucose DMEM at 37°C. After pretreatment, cells were incubated with TGF-β in the presence of the inhibitors for maximal 4 h (for gö6976) or 48 h (for CE). Megalin COOH-terminal fragment was detected from whole cell homogenates by SDS-PAGE and Western blot analysis0000.
Enzyme-linked immunosorbent assay.
Cell culture supernatants (SNs) from RLE-6TN cells were centrifuged to remove debris. SNs were concentrated using 6-ml Vivaspin concentrators (Sartorius, Göttingen, Germany) up to 500 µl. MMPs and megalin ectodomain abundance in the SNs were measured by specific ELISA kits from R&D Systems and LSBio (Seattle, WA), respectively, following the manufacturer’s recommendations. The optical density was determined at 450 nm by an Infinite M200 ELISA reader (TECAN, Männedorf, Switzerland). Concentration values in nanograms per milliliter were normalized to the concentration values of total proteins in the SNs.
Zymography.
MMP-2 and MMP-9 proteolytic activity in cell culture supernatants form RLE-6TN was evaluated by in-gel-digestion following a previously established protocol (46). Gels were scanned with the molecular imager ChemiDoc XRS+ (Bio-Rad).
Transfection of the megalin intracellular domain.
RLE-6TN or rat primary ATII cells were plated on 60-mm Petri dishes or six-well plates to reach 70% confluence. Transfection of 2 to 4 µg (1.2 ng/µl) of the synthetic megalin intracellular domain (MICD) (Biomatik, Ontario, Canada) was performed 24 h after seeding of cells by using a protein delivery reagent (PULSin, Polyplus, Illkirch, France) according to the manufacturer’s instructions. Controls were sham transfected with PULSin alone.
FITC-albumin binding and uptake.
Megalin functionality was tested by cell surface binding and cellular uptake of FITC-albumin as reported previously (6, 55). Briefly, RLE-6TN or rat primary ATII cells were preincubated with DPBS-G (0.1mM CaCl2, 0.5mM MgCl2, 5mM glucose PBS) for 15 min at 37°C. Immediately after, 50 µg/ml of FITC-labeled albumin or FITC-labeled dextran (70 kDa) in DPBS-G was added and incubated for 1 h at 37°C. Bound FITC-labeled compounds were removed by digestion with 500 µl of Solution X [1× Trypsin, 0.5 mg/ml proteinase K (Qiagen, Hilden, The Netherlands), 0.5 mM EDTA; DPBS-G] for 10 min and centrifugation at 9,800 g for 5 min at 4°C. Supernatants were saved on ice (bound fraction), and the pellets were resuspended in 0.1% Triton X-100 in PBS before centrifugation at 10,000 g for 5 min. Supernatants were collected (taken-up fraction). Fluorescence was measured by duplicates with an ELISA reader setting excitation at 490 nm and emission at 520 nm. Total amounts of proteins were measured from the taken-up fraction by Bradford assay and used for normalization of fluorescence values.
Statistical analysis.
All data sets were plotted as means ± SE. For statistical analysis and plotting of the data, GraphPad Prism 5 for Windows software was employed (GraphPad Software, San Diego, CA). Comparisons between two groups were done by paired (matched samples) or unpaired (independent samples) two-tailed Student’s t-test. Comparisons between more than two groups were done by one- or two-way ANOVA and Tukey’s or Šidák’s multiple comparisons or Dunnett’s multiple comparisons when each group was compared with a control group. Statistical significance was defined as P < 0.05, P < 0.01, P < 0.001, and P < 0.0001.
RESULTS
TGF-β-induced release of MICD reduces gene expression and cell surface stability of megalin.
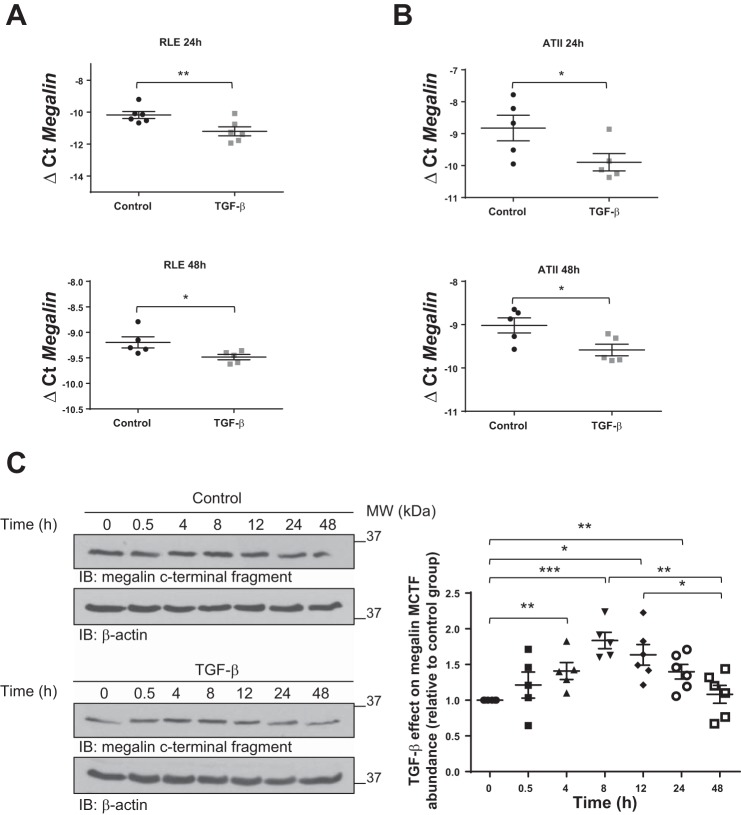
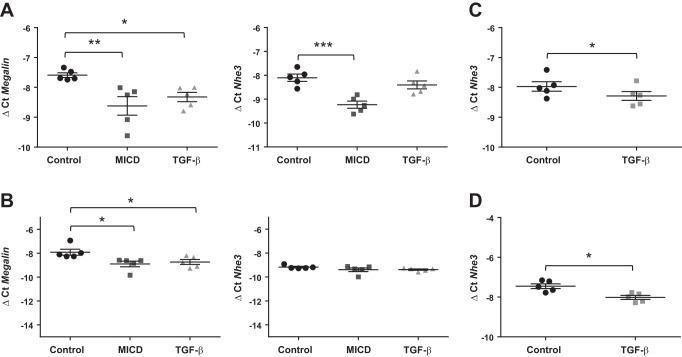
To study whether TGF-β regulates megalin expression, we first evaluated mRNA expression of megalin 24 and 48 h after TGF-β treatment and observed a significant downregulation in both RLE-6TN and primary rat ATII cells (Fig. 1, A and B, respectively). It has been previously suggested that in response to certain stimuli megalin may be shed from the plasma membrane, initiating a signaling cascade that by promoting RIP leads to the release of the MICD, which may translocate into the nucleus and specifically downregulate gene expression of megalin (3, 32, 33). To study whether MICD release is regulated by TGF-β, we next measured the abundance of the MICD precursor, megalin COOH-terminal fragment (MCTF), in whole cell homogenates from RLE-6TN cells (detection of MICD was not feasible because of its very short half-life) and detected a time-dependent increase in intracellular MCTF abundance 4 to 24 h after TGF-β treatment, showing a maximum between 8 and 12 h after TGF-β exposure (Fig. 1C). To establish a link between the TGF-β-induced MICD release and downregulation of megalin expression, we next measured gene expression of the Na+/H+ exchanger channel 3 (NHE3), which has previously been described to be a target of MICD (32), in the presence or absence of TGF-β or synthetic MICD for 24 h. A significant reduction of megalin mRNA levels was seen both in RLE-6TN and ATII cells when treated either with TGF-β or synthetic MICD; however, the effect of TGF-β treatment on NHE3 expression was less prominent (Fig. 2, A and B). Considering that synthetic MICD was added in excess and that it targets the pathway clearly downstream of TGF-β, we next asked whether this unexpected lack of effect of TGF-β on NHE3 gene expression was due to an insufficient time of TGF-β exposure. To answer this question, we repeated these measurements 48 h after TGF-β treatment and detected a significant downregulation of NHE3 expression in both cell types (Fig. 2, C and D). Collectively, these results suggest that TGF-β may exert an effect on megalin downregulation by inducing release of MICD into the cytoplasm, which may subsequently translocate into the nucleus and inhibit gene expression of megalin.
Fig. 1.
Transforming growth factor-β (TGF-β) reduces transcriptional expression of megalin and regulates megalin COOH-terminal fragment (MCTF) abundance. Total RNA from rat lung epithelial-T-antigen negative (RLE-6TN) (A) or primary rat ATII cells (B) was isolated after TGF-β (20 ng/ml) treatment for 24 or 48 h. Real-time PCR was performed after cDNA preparation. GAPDH was used as a housekeeping gene. Paired t-test, *P < 0.05; **P < 0.01; n = 5. C: RLE-6TN cells were treated with TGF-β (20 ng/ml) up to 48 h. Whole cell homogenates were processed by SDS-PAGE and immunoblot (IB). One-way ANOVA and Tukey’s multiple comparisons, *P < 0.05; **P < 0.01; ***P < 0.001; n ≥ 5. Results are shown as means ± SE. Representative blots are shown.
Fig. 2.
TGF-β impairs both megalin and Na+/H+ exchanger 3 (NHE3) mRNA expression by promoting megalin intracellular domain (MICD) release into the cytoplasm. A: RLE-6TN cells treated for 24 h, n = 5. B: ATII cells treated for 24 h, n = 5. C: NHE3 expression measured from RLE-6TN cells treated for 48 h, n = 5. D: NHE3 expression measured from ATII cells treated for 48 h, n = 5. Cells were treated with MICD (1.2 µg/µl) or TGF-β (20 ng/ml), and total RNA was isolated. Megalin and NHE3 expression was measured by real-time PCR with specific primers after reverse transcription. Results are shown as means ± SE. One-way ANOVA and Dunnett’s multiple comparisons, *P < 0.05; **P < 0.01; ***P < 0.001.
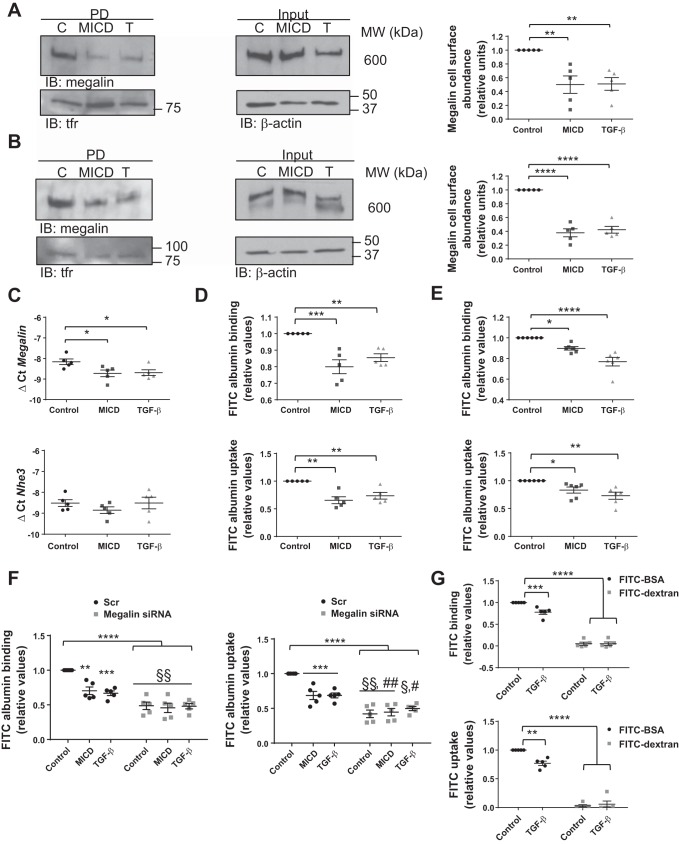
To further characterize the effects of MICD on megalin downregulation, we evaluated the cell surface stability of megalin in the presence or absence of synthetic MICD and TGF-β for 10 h (a time point between 8 and 12 h where maximum intracellular abundance of MCTF was detected) by applying streptavidin pulldown assays. Of note, exogenous MICD reduced megalin cell surface stability markedly and comparably to TGF-β treatment in both RLE-6TN and ATII cells (Fig. 3, A and B). Importantly, both treatments also significantly reduced megalin mRNA levels after 10 h of exposure (Fig. 3C). Considering that albumin is the most abundant protein in plasma that can bind to megalin, binding and uptake of FITC-albumin were also measured to test the function of cell surface megalin in the presence or absence of MICD or TGF-β. RLE-6TN or ATII cells were treated as described above and subsequently incubated with FITC-albumin for 1 h. Receptor-bound and taken-up fractions were collected, and fluorescence intensity was measured. Consistent with the effects of MICD and TGF-β on megalin cell surface abundance, both binding and uptake of FITC-albumin showed a reduction when RLE-6TN or ATII cells were treated with either MICD or TGF-β (Fig. 3, D and E). These effects, albeit significant, were not as pronounced as the effects of MICD peptide or TGF-β on the cell surface abundance of megalin. A possible explanation for this may be the existence of other putative albumin receptors at the plasma membrane of alveolar epithelial cells, such as other members of the low-density lipoprotein receptor-related protein (LRP) family, which may also be able to bind and endocytose the protein. To further test the requirement of megalin in alveolar protein clearance, we repeated the above mentioned experiments after silencing the expression of megalin. In line with our previous findings, the effects of both MICD and TGF-β treatments were lost after megalin knockdown (Fig. 3F). Additionally, binding and uptake of FITC-albumin were reduced to ~50% of control levels. Collectively, these data suggest that the clearance of the bulk of total albumin across alveolar epithelial cells requires megalin function and MICD and TGF-β impair albumin transport by inhibiting megalin. Controls of specificity of albumin transport were performed with 70 kDa FITC-dextran, which has a similar molecular weight as albumin, but is not actively transported across the alveolar epithelium (Fig. 3G) (6).
Fig. 3.
Application of exogenous MICD reduces megalin cell surface abundance thereby altering binding and uptake of albumin by alveolar epithelial cells. RLE-6TN (A) or primary ATII (B) cells were treated with a synthetic protein containing megalin intracellular domain (MICD) sequence (1.2 µg/µl) or with TGF-β (20 ng/ml) for 10 h. Cell surface proteins were labeled with biotin and pulled down (PD) with streptavidin beads. Pulled-down proteins were separated by SDS-PAGE and blotted with specific antibodies. C: RLE-6TN cells were treated as previously described, and total RNA was isolated. Megalin and NHE3 expression was measured by real-time PCR. RLE-6TN (D) or primary ATII (E) cells were treated with synthetic MICD (1.2 µg/µl) or with TGF-β (20 ng/ml) for 10 h and FITC-albumin binding, and uptake assays were performed. Fluorescence readouts were normalized to total protein amount from the taken-up fraction. Results (A–E) are shown as means ± SE. One-way ANOVA and Tukey’s multiple comparisons, *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; n = 5. F: RLE-6TN cells were transfected with specific siRNA for megalin knockdown or with control siRNA (Scr) for 48 h before treatment with MICD or TGF-β as described above. FITC-albumin binding and uptake assays were performed as previously described. Results are shown as means ± SE. Two-way ANOVA and Tukey’s multiple comparisons, **P < 0.01 (compared with control Scr); ***P < 0.001; ****P < 0.0001; §P < 0.05 (compared with MICD Scr); §§P < 0.01; #P < 0.05 (compared with TGF-β Scr); ##P < 0.01; n = 5. G: control experiments showing specificity of TGF-β on albumin binding and uptake. RLE-6TN cells were incubated with TGF-β (20 ng/ml) for 10 h and assayed for binding and uptake of FITC-albumin or FITC-dextran. Results are shown as means ± SE. One-way ANOVA and Tukey’s multiple comparisons, **P < 0.01; ***P < 0.001; ****P < 0.0001; n = 5.
TGF-β-induced megalin downregulation requires PKC and γ-secretase activity.
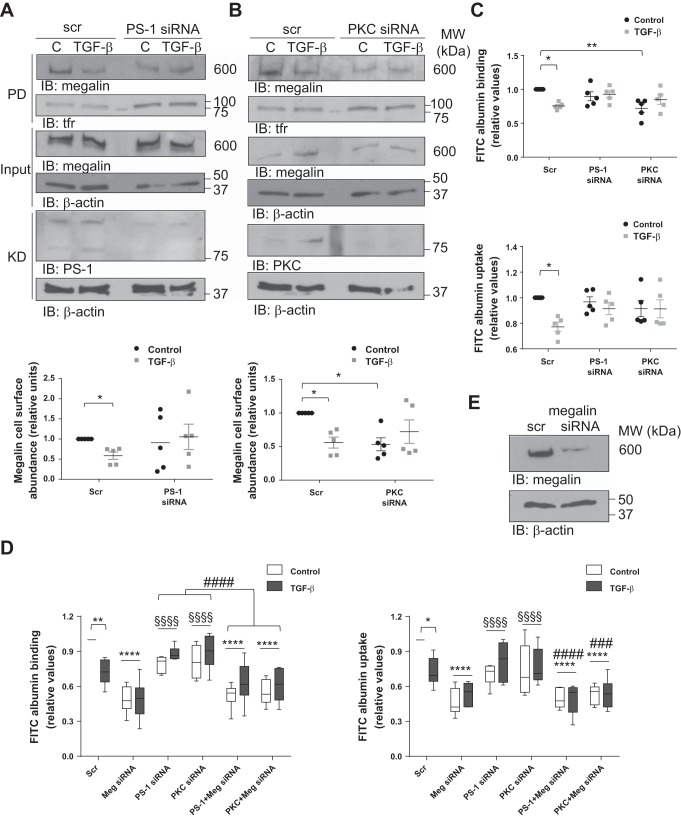
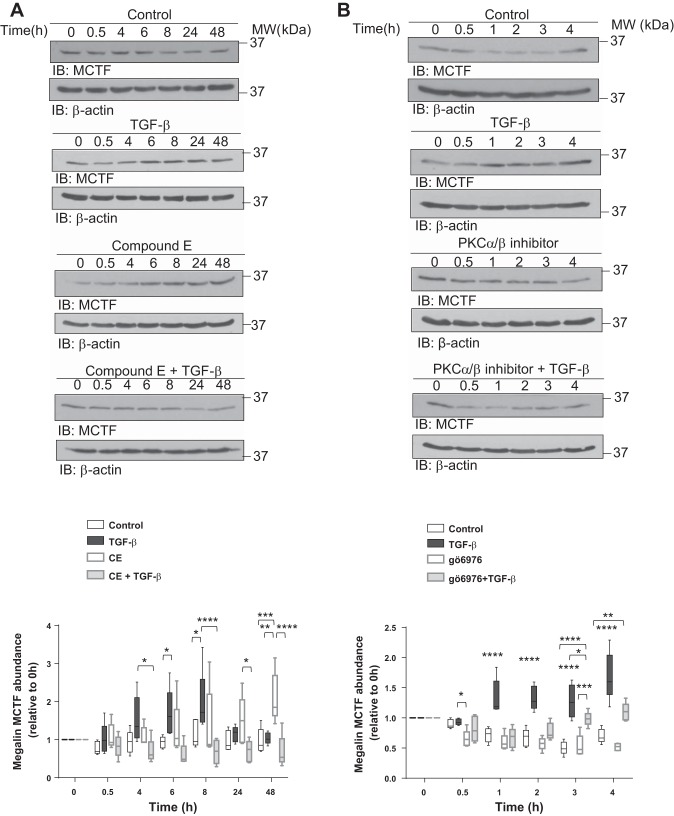
As it has been previously reported that shedding and RIP of megalin in other cell types are dependent on activities of PKC and γ-secretase, and that the regulation of these two processes determines the intracellular amounts of MCTF and MICD (3, 32). We next investigated the potential regulatory role of TGF-β in these processes by specific knockdown (KD) of the enzymes. RLE-6TN cells were transfected with siRNA against PS-1, an enzyme responsible for the γ-secretase activity in the proteolytic complex, and megalin cell surface stability was evaluated after 10 h of TGF-β treatment (a time point where maximum intracellular abundance of MCTF was detected). Indeed, silencing PS-1 abolished the TGF-β-induced impairment of megalin cell surface stability (Fig. 4A). Similar data on TGF-β-induced reduction of megalin cell surface expression and activity were obtained after silencing PKCα/β (Fig. 4, B and C). Furthermore, specific KD of PS-1 also prevented the negative effects of TGF-β on megalin-mediated binding and uptake of FITC-albumin (Fig. 4C). Additionally, silencing of megalin expression significantly reduced binding and uptake of FITC-albumin with no rescue after PS-1 or PKC knockdown (Fig. 4, D and E), further proving that both enzymes regulate megalin cell surface abundance in response to TGF-β and confirming that albumin transport depends on megalin function. Finally, chemical inhibition of either γ-secretase activity or classical PKCs prevented the effects of TGF-β on intracellular MCTF abundance (Fig. 5, A and B), thereby further supporting the hypothesis that classical PKCs and γ-secretase are key regulators of the TGF-β-induced megalin shedding and RIP. Of note, we elected to reduce the duration of the experiments in which classical PKCs were inhibited, as the half-life of gö6976 appeared to be significantly shorter than the half-life of the γ-secretase inhibitor CE.
Fig. 4.
Inhibition of γ-secretase and classical PKCs prevent the effect of TGF-β on megalin cell surface stability increasing megalin functionality. RLE-6TN cells were transfected with presenilin-1 (PS-1) or PKCα/β siRNA for 72 h and treated with TGF-β (20 ng/ml) for 10 h. Cells were then processed to detect megalin cell surface abundance by biotin-streptavidin pulldown assay (A and B) or to measure FITC-albumin binding and uptake (C). Results are shown as means ± SE. Two-way ANOVA and Šidák’s multiple comparisons, *P < 0.05; **P < 0.01; n = 5. D: RLE-6TN cells were co-transfected with PS-1 or PKC siRNA in the presence or absence of megalin (Meg) siRNA and FITC-albumin binding, and uptake were assessed. Boxes represent the median + quartiles; the ends of the whiskers show the minimum and maximum of all data. Two-way ANOVA and Tukey’s multiple comparisons, *P < 0.05; **P < 0.01; ****P < 0.0001 (compared with Scr control); §§§§P < 0.0001 (compared with Meg siRNA); ###P < 0.001; ####P < 0.0001 (compared with the corresponding single knockdown); n = 5. E: a representative Western blot of megalin knockdown in RLE-6TN cells. Scr, scrambled siRNA; PD, pulldown; KD, knockdown.
Fig. 5.
Chemical inhibition of γ-secretase and PKC activities prevents the effects of TGF-β on MCTF abundance. A: RLE-6TN cells were pretreated for 4 h with 1 µM of a γ-secretase activity inhibitor, compound E (CE), and subsequently treated with TGF-β (20 ng/ml) in the presence of the inhibitor for up to 48 h. B: RLE-6TN cells were pretreated for 4 h with 1.3 µM of a PKC inhibitor, gö6976, and subsequently treated with TGF-β (20 ng/ml) in the presence of the inhibitor for up to 48 h. In all the cases, whole cell homogenates were processed by SDS-PAGE and IB. Boxes represent the median + quartiles; the ends of the whiskers show the minimum and maximum of all data. Two-way ANOVA Tukey’s multiple comparisons test, *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; n = 6.
TGF-β-induced megalin shedding is regulated by expression, activity, and subcellular localization of MMPs.
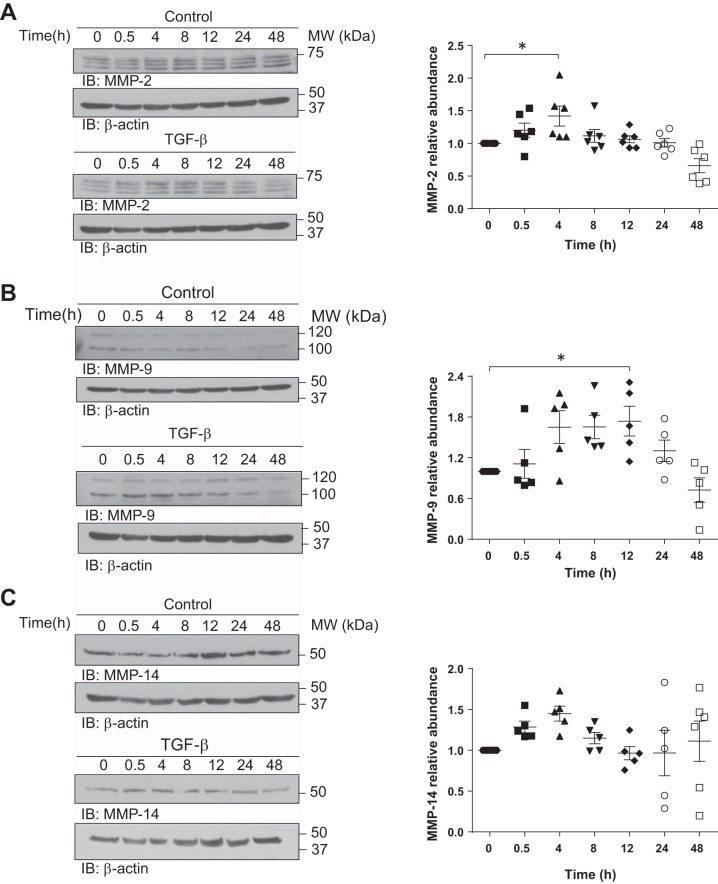
As PKCs have been previously proposed as regulators of shedding of plasma membrane receptors by activation of membrane-associated MMPs (3, 33), we next asked whether these enzymes are implicated in the TGF-β-induced megalin shedding. To address this question, we first evaluated the intracellular protein expression of MMP-2 and MMP-9, which are characteristic matrix remodelers in acute lung injury (10), and MMP-14, which was found to be responsible for shedding of LRP-1, which is structurally similar to megalin (52). Therefore, RLE-6TN cells were incubated with TGF-β for up to 48 h, and protein expression of the above mentioned MMPs was assessed. Whereas TGF-β increased MMP-2 and MMP-14 protein levels in the first 4 h after treatment, an enhanced protein expression of MMP-9 was evident 12 h after TGF-β exposure (Fig. 6, A–C), These results indicate that TGF-β increases expression of MMPs before the peak of MCTF release occurs (8–12 h), supporting the idea that either of these enzymes or even all of them might be implicated in megalin shedding.
Fig. 6.
TGF-β regulates intracellular expression of MMP-2, -9, and -14. RLE-6TN cells were treated with TGF-β (20 ng/ml) up to 48 h. Whole cell homogenates were processed by SDS-PAGE and IB for MMP-2 (A), MMP-9 (B), and MMP-14 (C). Representative blots are shown. Results are shown as means ± SE. One-way ANOVA and Šidák’s or Dunnett’s multiple comparisons, *P < 0.05; n = 6. Control and treated samples were derived from the same cellular passage and processed in parallel.
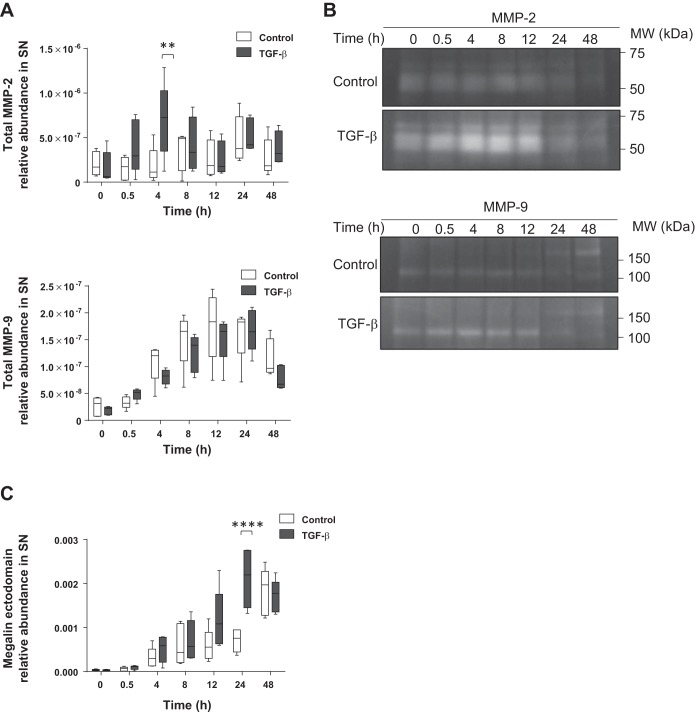
To further test our hypothesis, we measured the effects of TGF-β on MMP-2 and MMP-9 exocytosis by a specific ELISA from cell culture supernatants by using the same time course as above. As shown in Fig. 7A, we observed a similar time course of enhanced MMP-2 release into the extracellular space in the presence of TGF-β as we detected before for the intracellular protein levels. In contrast, in the case of MMP-9, although there was a continuous exocytosis of the enzyme, differences between the control conditions and TGF-β treatment group were indistinguishable. Moreover, to study extracellular activity of MMP-2 and MMP-9, zymography assays were performed from the supernatants of the above mentioned experiments. Consistent with the data obtained in the ELISA assays, MMP-2 activity was increased 4 to 12 h after TGF-β treatment. In contrast, MMP-9 activity remained unaltered after TGF-β treatment, comparable to control values (Fig. 7B). Finally, these data were complemented by detection of the release of the shed megalin ectodomain into the cell culture supernatant under the above mentioned conditions. For this purpose, cell culture supernatants were applied to an ELISA plate precoated with specific antibodies binding the megalin ectodomain. After chemiluminescence detection, we observed a higher abundance of the megalin ectodomain in the supernatant of cells treated with TGF-β (Fig. 7C), indicating that exposure to this cytokine increases megalin shedding and release of the ectodomain into the extracellular space.
Fig. 7.
TGF-β regulates extracellular activation of MMP-2 and promotes shedding of megalin ectodomain. RLE-6TN cells were treated with TGF-β (20 ng/ml) up to 48 h. Supernatants (SN) were collected, centrifuged to remove debris, and concentrated for subsequent analysis. A: specific ELISA for MMP-2 or MMP-9. Chemiluminescent units were normalized to protein concentration in the SN. Boxes represent the median + quartiles; the ends of the whiskers show the minimum and maximum of all data. Two-way ANOVA and Šidák’s multiple comparisons, **P < 0.01; n = 5. B: representative zymography from SN processed in A. Control and treated samples were derived from the same cellular passage and processed in parallel. MMP-2 and MMP-9 blots proceed from the same gels, even if shown separately; n = 5. C: specific ELISA for megalin ectodomain. Boxes represent the median + quartiles; the ends of the whiskers show the minimum and maximum of all data. Two-way ANOVA and Šidák’s multiple comparisons, ****P < 0.0001; n = 5.
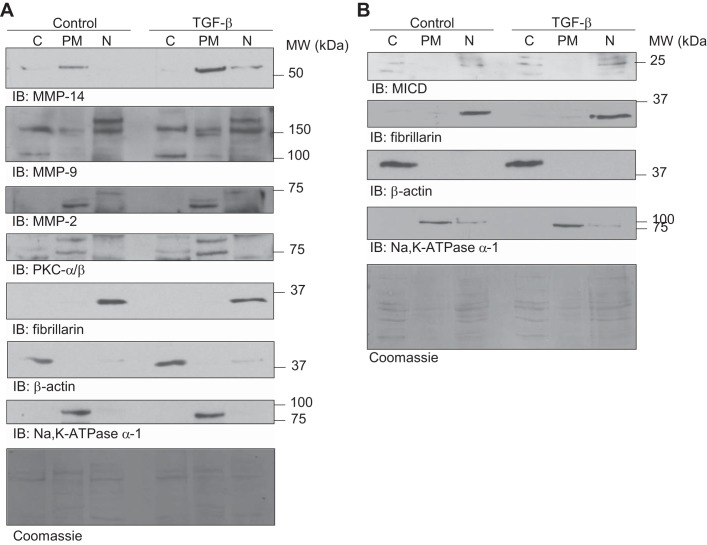
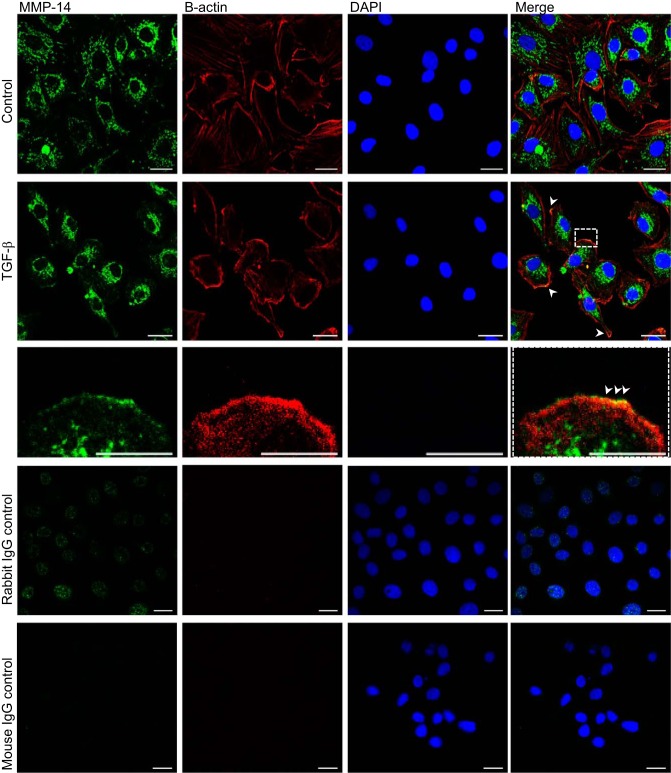
Because MMP-14 remains attached to the cell surface, it is not feasible to detect this protease in cell culture supernatants. Therefore, we studied MMP-14 translocation to the plasma membrane after treatment with TGF-β. RLE-6TN cells were exposed to TGF-β for 10 h, and the plasma membrane fraction was purified from whole cell lysates. Of note, we observed a translocation of MMP-14 into the plasma membrane fraction after TGF-β exposure (Fig. 8A), which was confirmed by immunofluorescence and confocal microscopy (Fig. 9). Interestingly, we also detected an increased abundance of MMP-9 and PKC-α and -β at the cell surface (Fig. 8A), suggesting that TGF-β may promote translocation of these proteins to the plasma membrane, although exocytosis and extracellular activation of MMP-9 were not evident. To further correlate these results with the hypothesis that TGF-β promotes the release of MICD, which induces megalin downregulation through translocation into the nucleus and subsequent transcriptional silencing, we performed identical cell fractionation experiments and assayed MICD co-localization with the purified nuclear fraction. In line with our previous findings, an increase of megalin COOH-terminal domain in the nucleus was observed in the presence of TGF-β (Fig. 8B), further supporting our hypothesis.
Fig. 8.
TGF-β promotes translocation of PKC, MMP-9, and MMP-14 to the plasma membrane and an increase of MICD in the nuclear fraction. A: co-localization of PKC, MMP-9, and MMP-14 with the purified plasma membrane fraction. B: co-localization of MICD with the purified nuclear fraction. Representative blots are shown; n = 5. RLE-6TN cells were treated with TGF-β (20 ng/ml) for 10 h, and whole cell homogenates were fractionated by isopycnic (sucrose gradient) and differential centrifugation. Cytoplasm (endosomes-free) (C), plasma membrane (PM), and nucleus (N) were purified. Fractions were processed by SDS-PAGE in 4–16% gradient gels followed by IB. Total proteins were stained with Coomassie brilliant blue.
Fig. 9.
MMP-14 co-localizes with the plasma membrane in the presence of TGF-β. RLE-6TN cells were treated with TGF-β for 10 h and processed for detection of MMP-14 by immunofluorescence staining and confocal microscopy. Staining of the cytoskeleton with β-actin specific antibodies was performed to detect the cellular boundaries. The nuclei were stained with DAPI. IgG controls were performed with naïve rabbit or mouse primary antibodies. Scale bar = 20 μm; scale bar of the zoom = 10 μm. Green: MMP-14; red: β-actin; blue: DAPI.
MMP-2 and MMP-14 physically interact with megalin at the plasma membrane in presence of TGF-β.
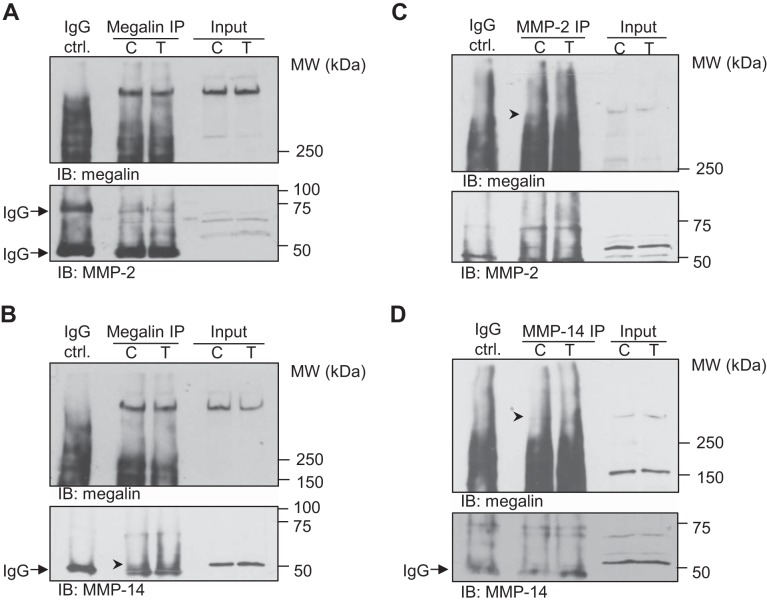
As we found that MMP-2 and -14 activities were upregulated by TGF-β, and considering that physical interaction of proteins might be necessary for proteolysis, we studied the potential interaction of MMP-2 and -14 with megalin by co-immunoprecipitation (co-IP) assays from plasma-membrane-enriched fractions after 10 h of treatment with TGF-β. As shown in Fig. 10, A and B, both MMP-2 and -14 were pulled down with megalin when specific antibodies for the receptor were used for IP. Importantly, reverse co-IPs (in which MMP-2 or -14 were immunoprecipitated) also pulled down megalin (Fig. 10, C and D). These data suggest that MMP-2 and -14 physically interact with megalin at the cell surface. Together with the finding that MMP-2 and -14 are upregulated in the presence of TGF-β, our data suggest that these two MMPs may be novel candidates responsible for megalin shedding.
Fig. 10.
Megalin interacts with MMP-2 and MMP-14 at the plasma membrane. RLE-6TN cells were treated with TGF-β (20 ng/ml) for 10 h and crude plasma membrane fractions were obtained by centrifugation from whole cell lysates. Proteins from the crude plasma membrane fraction were extracted and immunoprecipitation (IP) with specific antibodies for megalin performed. Co-IP was assessed by immunoblotting with specific antibodies for MMP-2 (A) or MMP-14 (B). Reverse co-IP experiments were also performed as described above but with an IP with MMP-2 (C) or MMP-14 (D) specific antibodies. Co-IP was assessed by immunoblotting with a specific antibody for megalin. Representative blots are shown; n = 5.
Megalin downregulation induced by TGF-β is impaired by MMP knockdown.
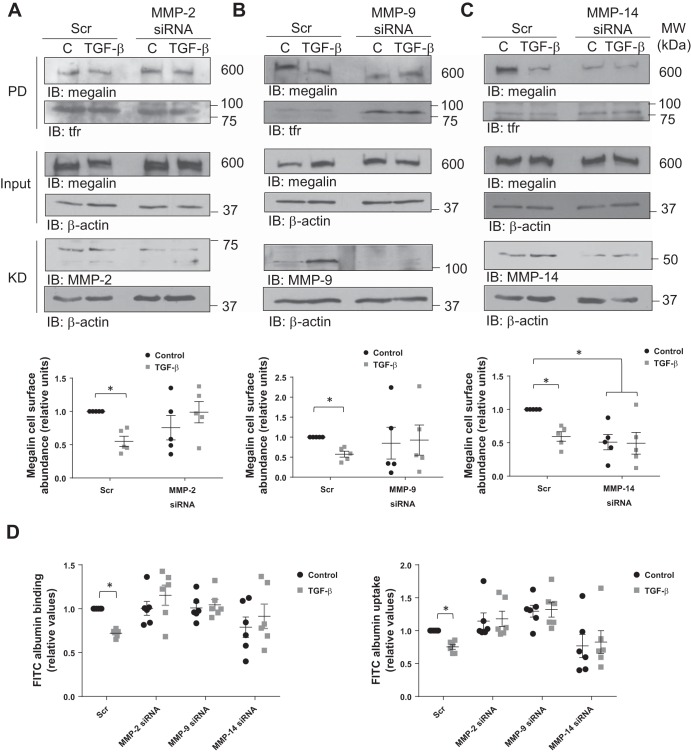
Because it has been demonstrated that MMPs are tightly regulated by TGF-β (30), the effects of TGF-β on megalin cell surface stability and function in the absence of MMP-2, -9, or -14 were next studied. RLE-6TN cells were transfected with siRNA sequences to specifically silence MMP-2, -9, or -14 mRNA levels for 72 h. TGF-β treatment was conducted for 10 h before the end of the silencing period, and cells were immediately processed for megalin cell surface detection or FITC-albumin binding and uptake experiments (Fig. 11, A–C and D, respectively). As expected, loss of MMP-2 or -14 expression rescued megalin cell surface stability in the presence of TGF-β and consequently restored control levels of albumin binding and uptake. Interestingly, downregulation of MMP-9 expression had the same effect on TGF-β-induced megalin endocytosis and function as the other two enzymes, even when TGF-β showed no effect on MMP-9 extracellular release and activity, although it clearly promoted translocation of the metalloprotease to the plasma membrane. Taken together, these results suggest that TGF-β promotes megalin shedding and subsequent downregulation by modulating localization and activity of MMP-2, -9, and -14.
Fig. 11.
Silencing of MMP-2, -9, or -14 prevents TGF-β-induced reduction of megalin cell surface abundance and restores albumin binding and uptake. RLE-6TN cells were transfected with MMP-2 (A), -9 (B), or -14 (C) siRNA for 72 h and treated with TGF-β (20 ng/ml) for 10 h. Megalin cell surface stability was measured by biotin-streptavidin pulldown assay and detected by SDS-PAGE and IB. D: RLE-6TN cells were treated as described above and subsequently incubated with FITC-albumin, cell surface-bound and taken-up fractions were collected, and FITC fluorescence was quantified. Fluorescence readouts were normalized to total protein amount from the taken-up fraction. Results are shown as means ± SE. Two-way ANOVA and Šidák’s multiple comparisons, *P < 0.05; n = 5. Scr, scrambled siRNA; PD, pulldown; KD, knockdown.
DISCUSSION
Removal of excess proteins from the alveolar space is of high clinical importance as protein-rich edema in the distal airways significantly contributes to exacerbated inflammation and disruption of the alveolar-capillary barrier, both hallmarks of ARDS (1, 14, 23, 27). Here we provide evidence that TGF-β, a key player in the pathogenesis of ARDS (7, 39, 40), significantly impairs alveolar protein clearance by downregulation of the endocytic receptor megalin in alveolar epithelial cells. Our novel data suggest that TGF-β promotes shedding of the megalin ectodomain and RIP of the resulting membrane-associated fragment, followed by release of a soluble variant of the megalin COOH-terminal tail that translocates into the nucleus and downregulates gene expression. We also demonstrate that the TGF-β-induced megalin shedding and RIP require activities of classical PKCs and γ-secretase, as well as regulation of the expression, activity, and localization of MMP-2, -9, and -14.
The pathophysiological relevance of active protein removal in the setting of acute lung injury is controversial and a topic of intense debate. Early studies suggested that excess alveolar protein can be cleared by various mechanisms, and it appeared that at low concentrations an active, receptor-mediated epithelial transport mechanism is at play and that to some extent macrophages contributed to protein removal by phagocytosis; however, at higher protein concentrations in vivo protein clearance was not affected by inhibitors of endocytosis (18–21). In contrast, we and others have recently demonstrated that albumin clearance by alveolar epithelial cells is a unidirectional, clathrin-dependent, and megalin-mediated process that occurs at comparable rates across alveolar epithelial type II- and type-I-like cells and is impaired in acute lung injury (6, 27, 28, 49, 55). Indeed, in intact, isolated, ventilated, and perfused rabbit lungs, we observed that the velocity of active albumin clearance is high and can be markedly attenuated by reducing temperature, by competition, or by inhibitors of endocytosis (6). Clearly, assessing the exact contribution of these active transport processes to the overall protein clearance in vivo in uninjured and injured lungs as well as in the setting of ARDS will require further research.
Function of megalin is critical in multiple endocytic transport processes in various organs, such as the kidney and the gut, which has been shown to be negatively regulated by TGF-β (16, 33); however, the exact molecular mechanisms underlying the TGF-β-dependent megalin downregulation were not fully elucidated. Notch-like processing of megalin has been previously investigated in the context of kidney diseases, where release of its extracellular domain is a consequence of MMP-dependent shedding, followed by RIP of MCTF and cytoplasmic delivery of the remaining protein (3, 56). This cytoplasmic variant of megalin (MICD) has been suggested to translocate into the nucleus and negatively regulate gene expression of megalin (32). Furthermore, TGF-β has been associated with Smad-independent downregulation of the megalin promoter in diabetic nephropathy (16). These previous studies led us to hypothesize that megalin downregulation in response to TGF-β may be a consequence of shedding and RIP of megalin, with subsequent “self-downregulation” at the level of transcription.
We started to evaluate this hypothesis by measuring mRNA levels of megalin after long-term treatment with TGF-β (for up to 2 days) and observed that megalin gene expression is significantly reduced in the presence of TGF-β. To further test our hypothesis, we aimed to find a link between the TGF-β-induced mRNA downregulation and the release of MCTF and found that a time-dependent release of MCTF upon TGF-β exposure, showing a peak between 8 and 12 h after treatment, which correlated with a marked reduction of megalin cell surface abundance and nuclear co-localization of MICD after 10 h of exposure to TGF-β. These findings were complemented by detection of higher amounts of the megalin ectodomain in cell culture supernatants of TGF-β-treated cells. Furthermore, we confirmed the MICD-mediated effects of TGF-β on transcriptional downregulation of megalin in studies where cells treated with either exogenous MICD or TGF-β showed comparable reduced levels of megalin mRNA.
Other targets of MICD transcriptional regulation have also been described in the proximal tubules (32). One of them is NHE3 that is expressed in the apical surface of various epithelia, including the renal proximal tubules and the alveolar epithelium (5, 38), where it may co-localize with megalin and is associated with regulation of pH (4). In the present study, we were able to show that both megalin and NHE3 transcriptional levels are reduced in the presence of excess MICD or TGF-β. The physiological relevance of MICD-induced downregulation of NHE3 gene expression or how these proteins are functionally connected to each other remain unknown; however, it has been reported that NHE3 is mostly sequestered into recycling endosomes, suggesting that it might be implicated in pH-dependent release of megalin ligands before their lysosomal degradation or transepithelial transport (9). Alternatively, regulation of NHE3 transcriptional levels by MICD has also been considered to be an indirect process, given that inhibition of megalin RIP probably does not prevent downregulation of NHE3 (32).
Ectodomain shedding of transmembrane receptors is regulated by different isoforms of PKC that present selective affinity for specific substrates. These protein kinases are responsible for activating sheddases through phosphorylation of their cytoplasmic tails and promote substrate recognition by conformational changes (22). Ectodomain shedding represents a regulatory mechanism of γ-secretase activity at the plasma membrane; however, ligand-dependent recruitment of these enzymes to the cell surface was reported as well (29). Megalin shedding and RIP are dependent on the coordinated function of classical PKCs and γ-secretase activity (3, 56). Although some evidence regarding reciprocal regulation between these enzymes and TGF-β is available, the signaling cascade that specifically relates these factors with megalin processing in ARDS is not yet clear. To address this, we hypothesized that TGF-β regulates megalin shedding through modulation of the activity of the PKC-α/β and γ-secretase complex in alveolar epithelial cells.
Our studies demonstrate that both PKC and γ-secretase activity are required for TGF-β-induced megalin processing, as specific silencing of either PKC-α/β or presenilin-1 (responsible of γ-secretase activity) restored megalin abundance at the plasma membrane after TGF-β exposure and rescued megalin function. Furthermore, chemical inhibition of either of these factors impaired the effect of TGF-β on MCTF abundance. In the present study no experiments addressing the exact molecular mechanisms involved in the regulation of these events were performed. However, considering that both PKC and the γ-secretase complex are located at the plasma membrane, we speculate that transactivation of these proteins in response to TGF-β may be a TβRI/II-Smad-dependent process (34). Additional evidence of the negative effects of TGF-β on E-cadherin expression (15, 48), which also undergoes shedding and RIP in response to injury of the lung epithelium (35), supports our hypothesis that TGF-β may downregulate megalin expression thought induction of shedding and RIP of the receptor. Furthermore, it will be important to evaluate the role of other PKC isoforms in the TGF-β-dependent megalin shedding since inhibition of PKC-θ and –ζ, but not –δ, have been described to reduce the expression and activity of two typical sheddases, MMP-2 and MMP-9 (8, 54).
Activity of MMPs represents another critical regulatory step of megalin shedding. PKC isoforms regulate ectodomain shedding of transmembrane receptors by phosphorylation and subsequent activation of various MMPs that mediate numerous responses to acute lung injury, such as cell elongation, migration, tissue remodeling, and repair (22). Recent studies have shown that secreted MMPs not only act pericellularly within the extracellular matrix (ECM) but also bind to specific cell surface receptors, membrane-anchored proteins, or cell-associated ECM. It has also been suggested that secreted MMPs may be recruited back to the local cell environment by interactions with cell surface proteins (36). Mutual regulation between MMPs and TGF-β has been already reported in other systems (25, 26, 30). Thus we hypothesized that TGF-β not only regulates megalin shedding and RIP through PKC and γ-secretase activity but also through modulation of MMP function.
In the present study we show that TGF-β upregulates total protein expression of MMP-2 and -9 after 4 h of treatment in alveolar epithelial cells. Of note, a correlation between MMP-2 levels and megalin ectodomain release into the extracellular space was found as well. Consistently with our findings, even if both MMP-2 and -9 have been reported to be highly expressed in BAL samples of patients with ARDS (12, 41, 45), TGF-β treatment of A549 cells caused only an increase of MMP-2 expression and activity, leaving MMP-9 levels unaltered (26). To further support our findings, the effects of TGF-β on megalin cell surface stability and function were measured after transfection of specific siRNA against MMP-2 or MMP-9. As expected, MMP-2 silencing prevented TGF-β-induced reduction in megalin cell surface abundance, restoring its functionality; however, MMP-9 silencing appeared to have the same effect regardless of the fact that neither its extracellular release nor its extracellular activity was regulated by TGF-β. It is possible that MMP-9 is indirectly implicated in megalin shedding by activating other MMPs directly involved in the process; thus TGF-β may be responsible of regulating MMP-9 interaction with other MMPs instead of regulating its expression or activity. Moreover, MMP-9 has been already reported to bind the ectodomain of megalin since this receptor mediates MMP-9 clearance from the alveolar space (13). TGF-β may also enhance such an interaction, thereby promoting megalin shedding. These last two notions are supported by our observations that TGF-β promotes translocation of MMP-9 to the plasma membrane, potentially facilitating its interaction with megalin and other MMPs. Finally, taking into account that MMPs should physically bind the receptor to catalyze its shedding, we assessed the interaction of MMP-2 with megalin at the plasma membrane of alveolar epithelial cells. In line with our previous findings, we observed that megalin and MMP-2 interact with each other. The data reported here serves also as a confirmation of the autocrine role of MMP-2 and MMP-9 catalytic activity in view of the absence of any additional source of these enzymes other than the alveolar epithelial cells cultured on the plates.
MMP-14 is a membrane-anchored MMP that has been described to activate MMP-2 in response to bleomycin in alveolar epithelial cells (31). Additionally, MMP-14 mediates the shedding of another member of the LRP family, LRP-1, in human lung fibroblasts (52). In the present study, we demonstrate that TGF-β treatment not only upregulates MMP-2 and -9 total protein levels but MMP-14 expression as well. Furthermore, MMP-14 translocation to the plasma membrane was increased in the presence of TGF-β, and the effect of TGF-β on megalin endocytosis was impaired when silencing of MMP-14 was performed. In line with these results and similarly to MMP-2, direct physical interaction between MMP-14 and megalin was proven at the plasma membrane of alveolar epithelial cells. Considering that MMP-14 is required to proteolytically activate MMP-2, these findings may suggest that megalin could serve as a scaffold that allows interaction and activation of the enzymes that later mediate shedding of its own ectodomain.
Collectively, our data provide evidence for a regulatory role of TGF-β in megalin shedding by modulation of MMPs. Importantly, we identify two novel mediators of megalin shedding, MMP-2 and MMP-14. Both enzymes are required for TGF-β-induced megalin downregulation and directly interact with the receptor at the plasma membrane. Although the further signaling pathways responsible of coordinating these enzymes to remove the ectodomain of megalin still need to be elucidated, based on previously published data, we propose that PKC-dependent MMP-14 activation may enhance pro-MMP-2 proteolytic processing, thereby leading to its activation (22, 31, 37). In the present study, we describe a novel regulatory mechanism of protein clearance from the alveolar space. Elevated levels of TGF-β by promoting downregulation of megalin contribute to impaired alveolar protein clearance, leading to persistence of protein-rich edema in the distal airways, thereby further disrupting the alveolar-capillary barrier and impairing gas exchange. Thus, interfering with the molecular mechanisms underlying the TGF-β-induced megalin downregulation may hold a therapeutic promise.
GRANTS
This work was supported by grants from the Excellence Cluster “Cardio Pulmonary System” (ECCPS); the German Center for Lung Research (DZL); the Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz (LOEWE) of the Hessen State Ministry of Higher Education, Research and the Arts; and the Deutsche Forschungsgemeinschaft (Clinical Research Unit KFO309/1) (for K. Mayer, S. Herold, R. Morty, W. Seeger, and I. Vadász). I. Vadász was supported by a Clinical Scientist Career Program Grant from the ECCPS.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.C.M., W.S., and I.V. conceived and designed research; L.C.M. performed experiments; L.C.M., C.U.V., K.M., S.H., R.E.M., and I.V. analyzed data; L.C.M., C.U.V., K.M., S.H., R.E.M., W.S., and I.V. interpreted results of experiments; L.C.M. prepared figures; L.C.M. drafted manuscript; L.C.M., C.U.V., K.M., S.H., R.E.M., W.S., and I.V. approved final version of manuscript; W.S. and I.V. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Miriam Wessendorf and Jordi Ruiz Camp (Universities of Giessen and Marburg Lung Center) for excellent technical assistance.
REFERENCES
- 1.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med : 35–56, 1982. [PubMed] [Google Scholar]
- 2.Berthiaume Y, Albertine KH, Grady M, Fick G, Matthay MA. Protein clearance from the air spaces and lungs of unanesthetized sheep over 144 h. J Appl Physiol (1985) : 1887–1897, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Biemesderfer D. Regulated intramembrane proteolysis of megalin: linking urinary protein and gene regulation in proximal tubule? Kidney Int : 1717–1721, 2006. doi: 10.1038/sj.ki.5000298. [DOI] [PubMed] [Google Scholar]
- 4.Biemesderfer D, DeGray B, Aronson PS. Active (9.6 s) and inactive (21 s) oligomers of NHE3 in microdomains of the renal brush border. J Biol Chem : 10161–10167, 2001. doi: 10.1074/jbc.M008098200. [DOI] [PubMed] [Google Scholar]
- 5.Biemesderfer D, Pizzonia J, Abu-Alfa A, Exner M, Reilly R, Igarashi P, Aronson PS. NHE3: a Na+/H+ exchanger isoform of renal brush border. Am J Physiol : F736–F742, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Buchäckert Y, Rummel S, Vohwinkel CU, Gabrielli NM, Grzesik BA, Mayer K, Herold S, Morty RE, Seeger W, Vadász I. Megalin mediates transepithelial albumin clearance from the alveolar space of intact rabbit lungs. J Physiol : 5167–5181, 2012. doi: 10.1113/jphysiol.2012.233403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budinger GR, Chandel NS, Donnelly HK, Eisenbart J, Oberoi M, Jain M. Active transforming growth factor-β1 activates the procollagen I promoter in patients with acute lung injury. Intensive Care Med : 121–128, 2005. doi: 10.1007/s00134-004-2503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborti S, Mandal A, Das S, Chakraborti T. Role of MMP-2 in PKCδ-mediated inhibition of Na+ dependent Ca2+ uptake in microsomes of pulmonary smooth muscle: involvement of a pertussis toxin sensitive protein. Mol Cell Biochem : 107–117, 2005. doi: 10.1007/s11010-005-8237-9. [DOI] [PubMed] [Google Scholar]
- 9.Chow CW. Regulation and intracellular localization of the epithelial isoforms of the Na+/H+ exchangers NHE2 and NHE3. Clin Invest Med : 195–206, 1999. [PubMed] [Google Scholar]
- 10.Corbel M, Boichot E, Lagente V. Role of gelatinases MMP-2 and MMP-9 in tissue remodeling following acute lung injury. Braz J Med Biol Res : 749–754, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Cox B, Emili A. Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat Protoc : 1872–1878, 2006. doi: 10.1038/nprot.2006.273. [DOI] [PubMed] [Google Scholar]
- 12.Delclaux C, d’Ortho MP, Delacourt C, Lebargy F, Brun-Buisson C, Brochard L, Lemaire F, Lafuma C, Harf A. Gelatinases in epithelial lining fluid of patients with adult respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol : L442–L451, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Emonard H, Marbaix E. Low-density lipoprotein receptor-related protein in metalloproteinase-mediated pathologies: recent insights. Metalloproteinases Med :2: 9–18, 2015. 10.2147/MNM.S63616. [DOI] [Google Scholar]
- 14.Folkesson HG, Matthay MA, Weström BR, Kim KJ, Karlsson BW, Hastings RH. Alveolar epithelial clearance of protein. J Appl Physiol (1985) : 1431–1445, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Fransvea E, Angelotti U, Antonaci S, Giannelli G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology : 1557–1566, 2008. doi: 10.1002/hep.22201. [DOI] [PubMed] [Google Scholar]
- 16.Gekle M, Knaus P, Nielsen R, Mildenberger S, Freudinger R, Wohlfarth V, Sauvant C, Christensen EI. Transforming growth factor-β1 reduces megalin- and cubilin-mediated endocytosis of albumin in proximal-tubule-derived opossum kidney cells. J Physiol : 471–481, 2003. doi: 10.1113/jphysiol.2003.048074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grzesik BA, Vohwinkel CU, Morty RE, Mayer K, Herold S, Seeger W, Vadász I. Efficient gene delivery to primary alveolar epithelial cells by nucleofection. Am J Physiol Lung Cell Mol Physiol : L786–L794, 2013. doi: 10.1152/ajplung.00191.2013. [DOI] [PubMed] [Google Scholar]
- 18.Hastings RH, Folkesson HG, Matthay MA. Mechanisms of alveolar protein clearance in the intact lung. Am J Physiol Lung Cell Mol Physiol : L679–L689, 2004. doi: 10.1152/ajplung.00205.2003. [DOI] [PubMed] [Google Scholar]
- 19.Hastings RH, Folkesson HG, Petersen V, Ciriales R, Matthay MA. Cellular uptake of albumin from lungs of anesthetized rabbits. Am J Physiol Lung Cell Mol Physiol : L453–L462, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Hastings RH, Grady M, Sakuma T, Matthay MA. Clearance of different-sized proteins from the alveolar space in humans and rabbits. J Appl Physiol (1985) : 1310–1316, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Hastings RH, Wright JR, Albertine KH, Ciriales R, Matthay MA. Effect of endocytosis inhibitors on alveolar clearance of albumin, immunoglobulin G, and SP-A in rabbits. Am J Physiol Lung Cell Mol Physiol : L544–L552, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Hayashida K, Bartlett AH, Chen Y, Park PW. Molecular and cellular mechanisms of ectodomain shedding. Anat Rec (Hoboken) : 925–937, 2010. doi: 10.1002/ar.20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herold S, Gabrielli NM, Vadász I. Novel concepts of acute lung injury and alveolar-capillary barrier dysfunction. Am J Physiol Lung Cell Mol Physiol : L665–L681, 2013. doi: 10.1152/ajplung.00232.2013. [DOI] [PubMed] [Google Scholar]
- 24.Holden P, Horton WA. Crude subcellular fractionation of cultured mammalian cell lines. BMC Res Notes : 243, 2009. doi: 10.1186/1756-0500-2-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenkins G. The role of proteases in transforming growth factor-β activation. Int J Biochem Cell Biol : 1068–1078, 2008. doi: 10.1016/j.biocel.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 26.Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-β1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir Res : 56, 2005. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KJ, Malik AB. Protein transport across the lung epithelial barrier. Am J Physiol Lung Cell Mol Physiol : L247–L259, 2003. doi: 10.1152/ajplung.00235.2002. [DOI] [PubMed] [Google Scholar]
- 28.Kim KJ, Matsukawa Y, Yamahara H, Kalra VK, Lee VH, Crandall ED. Absorption of intact albumin across rat alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol : L458–L465, 2003. doi: 10.1152/ajplung.00237.2002. [DOI] [PubMed] [Google Scholar]
- 29.Kopan R, Ilagan MX. γ-Secretase: proteasome of the membrane? Nat Rev Mol Cell Biol : 499–504, 2004. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- 30.Krstic J, Santibanez JF. Transforming growth factor-β and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells. Sci World J : 521754, 2014. doi: 10.1155/2014/521754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunugi S, Fukuda Y, Ishizaki M, Yamanaka N. Role of MMP-2 in alveolar epithelial cell repair after bleomycin administration in rabbits. Lab Invest : 1309–1318, 2001. doi: 10.1038/labinvest.3780344. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Cong R, Biemesderfer D. The COOH terminus of megalin regulates gene expression in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol : C529–C537, 2008. doi: 10.1152/ajpcell.00037.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marzolo MP, Farfán P. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol Res : 89–105, 2011. doi: 10.4067/S0716-97602011000100012. [DOI] [PubMed] [Google Scholar]
- 34.Massagué J. TGF-β signal transduction. Annu Rev Biochem : 753–791, 1998. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 35.McGuire JK, Li Q, Parks WC. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol : 1831–1843, 2003. doi: 10.1016/S0002-9440(10)64318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy G, Nagase H. Localizing matrix metalloproteinase activities in the pericellular environment. FEBS J : 2–15, 2011. doi: 10.1111/j.1742-4658.2010.07918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagase H. Cell surface activation of progelatinase A (proMMP-2) and cell migration. Cell Res : 179–186, 1998. doi: 10.1038/cr.1998.18. [DOI] [PubMed] [Google Scholar]
- 38.Nord EP, Brown SE, Crandall ED. Characterization of Na+-H+ antiport in type II alveolar epithelial cells. Am J Physiol Cell Physiol : C490–C498, 1987. [DOI] [PubMed] [Google Scholar]
- 39.Peters DM, Vadász I, Wujak L, Wygrecka M, Olschewski A, Becker C, Herold S, Papp R, Mayer K, Rummel S, Brandes RP, Günther A, Waldegger S, Eickelberg O, Seeger W, Morty RE. TGF-β directs trafficking of the epithelial sodium channel ENaC which has implications for ion and fluid transport in acute lung injury. Proc Natl Acad Sci USA : E374–E383, 2014. doi: 10.1073/pnas.1306798111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-β is a critical mediator of acute lung injury. J Clin Invest : 1537–1544, 2001. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricou B, Nicod L, Lacraz S, Welgus HG, Suter PM, Dayer JM. Matrix metalloproteinases and TIMP in acute respiratory distress syndrome. Am J Respir Crit Care Med : 346–352, 1996. doi: 10.1164/ajrccm.154.2.8756805. [DOI] [PubMed] [Google Scholar]
- 42.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature : 382–386, 1998. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 43.Song Y, Hao Y, Sun A, Li T, Li W, Guo L, Yan Y, Geng C, Chen N, Zhong F, Wei H, Jiang Y, He F. Sample preparation project for the subcellular proteome of mouse liver. Proteomics : 5269–5277, 2006. doi: 10.1002/pmic.200500893. [DOI] [PubMed] [Google Scholar]
- 44.Stasyk T, Schiefermeier N, Skvortsov S, Zwierzina H, Peränen J, Bonn GK, Huber LA. Identification of endosomal epidermal growth factor receptor signaling targets by functional organelle proteomics. Mol Cell Proteomics : 908–922, 2007. doi: 10.1074/mcp.M600463-MCP200. [DOI] [PubMed] [Google Scholar]
- 45.Torii K, Iida K, Miyazaki Y, Saga S, Kondoh Y, Taniguchi H, Taki F, Takagi K, Matsuyama M, Suzuki R. Higher concentrations of matrix metalloproteinases in bronchoalveolar lavage fluid of patients with adult respiratory distress syndrome. Am J Respir Crit Care Med : 43–46, 1997. doi: 10.1164/ajrccm.155.1.9001287. [DOI] [PubMed] [Google Scholar]
- 46.Toth M, Sohail A, Fridman R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol Biol : 121–135, 2012. doi: 10.1007/978-1-61779-854-2_8. [DOI] [PubMed] [Google Scholar]
- 47.Vadász I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, Tóth PT, Lecuona E, Witters LA, Schumacker PT, Chandel NS, Seeger W, Sznajder JI. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J Clin Invest : 752–762, 2008. doi: 10.1172/JCI29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogelmann R, Nguyen-Tat MD, Giehl K, Adler G, Wedlich D, Menke A. TGFβ-induced downregulation of E-cadherin-based cell-cell adhesion depends on PI3-kinase and PTEN. J Cell Sci : 4901–4912, 2005. doi: 10.1242/jcs.02594. [DOI] [PubMed] [Google Scholar]
- 49.Vohwinkel CU, Buchäckert Y, Al-Tamari HM, Mazzocchi LC, Eltzschig HK, Mayer K, Morty RE, Herold S, Seeger W, Pullamsetti SS, Vadász I. Restoration of megalin-mediated clearance of alveolar protein as a novel therapeutic approach for acute lung injury. Am J Respir Cell Mol Biol. In press. doi: 10.1165/rcmb.2016-0358OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med : 1334–1349, 2000. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 51.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med : 1376–1383, 2001. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 52.Wygrecka M, Wilhelm J, Jablonska E, Zakrzewicz D, Preissner KT, Seeger W, Guenther A, Markart P. Shedding of low-density lipoprotein receptor-related protein-1 in acute respiratory distress syndrome. Am J Respir Crit Care Med : 438–448, 2011. doi: 10.1164/rccm.201009-1422OC. [DOI] [PubMed] [Google Scholar]
- 53.Xiang X, Zhao X, Qu H, Li D, Yang D, Pu J, Mei H, Zhao J, Huang K, Zheng L, Tong Q. Hepatocyte nuclear factor 4 alpha promotes the invasion, metastasis and angiogenesis of neuroblastoma cells via targeting matrix metalloproteinase 14. Cancer Lett : 187–197, 2015. doi: 10.1016/j.canlet.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Xie Z, Singh M, Singh K. Differential regulation of matrix metalloproteinase-2 and -9 expression and activity in adult rat cardiac fibroblasts in response to interleukin-1β. J Biol Chem : 39513–39519, 2004. doi: 10.1074/jbc.M405844200. [DOI] [PubMed] [Google Scholar]
- 55.Yumoto R, Nishikawa H, Okamoto M, Katayama H, Nagai J, Takano M. Clathrin-mediated endocytosis of FITC-albumin in alveolar type II epithelial cell line RLE-6TN. Am J Physiol Lung Cell Mol Physiol : L946–L955, 2006. doi: 10.1152/ajplung.00173.2005. [DOI] [PubMed] [Google Scholar]
- 56.Zou Z, Chung B, Nguyen T, Mentone S, Thomson B, Biemesderfer D. Linking receptor-mediated endocytosis and cell signaling: evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J Biol Chem : 34302–34310, 2004. doi: 10.1074/jbc.M405608200. [DOI] [PubMed] [Google Scholar]