Here, a novel nondestructive real-time approach for monitoring intrinsic indicators of cardiac metabolism and oxygenation is described using a catheter-based transillumination of the left ventricular free wall together with complete spectral analysis of transmitted light. This approach is a significant improvement in the quality of cardiac optical absorbance spectroscopic metabolic analyses.
Keywords: mitochondria, Langendorff heart, cytochromes, broad-band spectral analysis
Abstract
Absorbance spectroscopy of intrinsic cardiac chromophores provides nondestructive assessment of cytosolic oxygenation and mitochondria redox state. Isolated perfused heart spectroscopy is usually conducted by collecting reflected light from the heart surface, which represents a combination of surface scattering events and light that traversed portions of the myocardium. Reflectance spectroscopy with complex surface scattering effects in the beating heart leads to difficulty in quantitating chromophore absorbance. In this study, surface scattering was minimized and transmural path length optimized by placing a light source within the left ventricular chamber while monitoring transmurally transmitted light at the epicardial surface. The custom-designed intrachamber light catheter was a flexible coaxial cable (2.42-Fr) terminated with an encapsulated side-firing LED of 1.8 × 0.8 mm, altogether similar in size to a Millar pressure catheter. The LED catheter had minimal impact on aortic flow and heart rate in Langendorff perfusion and did not impact stability of the left ventricule of the working heart. Changes in transmural absorbance spectra were deconvoluted using a library of chromophore reference spectra to quantify the relative contribution of specific chromophores to the changes in measured absorbance. This broad-band spectral deconvolution approach eliminated errors that may result from simple dual-wavelength absorbance intensity. The myoglobin oxygenation level was only 82.2 ± 3.0%, whereas cytochrome c and cytochrome a + a3 were 13.3 ± 1.4% and 12.6 ± 2.2% reduced, respectively, in the Langendorff-perfused heart. The intracardiac illumination strategy permits transmural optical absorbance spectroscopy in perfused hearts, which provides a noninvasive real-time monitor of cytosolic oxygenation and mitochondria redox state.
NEW & NOTEWORTHY Here, a novel nondestructive real-time approach for monitoring intrinsic indicators of cardiac metabolism and oxygenation is described using a catheter-based transillumination of the left ventricular free wall together with complete spectral analysis of transmitted light. This approach is a significant improvement in the quality of cardiac optical absorbance spectroscopic metabolic analyses.
INTRODUCTION
Optical absorbance spectroscopy for monitoring intact organ biochemistry is a widely used approach due to its intrinsic, nondestructive nature as well as extensive information content without the use of exogenous probes (2, 3, 9, 15, 23, 24, 28, 43, 44). Myoglobin (Mb) absorbance provides a measure of the average cytosolic O2 tension (40, 47, 48), whereas mitochondrial cytochromes provide information regarding the redox state of oxidative phosphorylation (7, 30, 41), from substrate entry at the level of NADH and flavin, membrane potential from cytochrome bL (27), and oxygenation from cytochrome oxidase (33). More recently, using metabolic rate and mitochondrial membrane potential measures, the individual activities of the complexes of oxidative phosphorylation have been estimated using optical spectroscopy data in isolated mitochondria (21). Thus, in a single setting, optical absorbance spectroscopy can provide a wealth of information on a variety of metabolic parameters in the intact heart. The purpose of the present study was to develop a robust quantitative method of monitoring the optical absorbance spectrum of the isolated perfused heart.
Reflectance absorption spectroscopy has been successfully used to measure the redox state of cytochromes (16, 17, 23–25) and oxygenation of cytoplasmic Mb in perfused hearts (23, 24, 37) and in vivo (2). Reflectance spectroscopy of the intact heart consists of impinging light on the surface of the heart and collecting the light scattered through the heart as well as diffuse and specular reflected light from the surface of the heart. Thus, the collected “transmitted light” in this geometry is a composite of multiple scattering mechanisms significantly influenced by where the light is impinged on the heart, heart motion, and the chromophore absorbance that contains the information of interest. In addition, the optical path length under these conditions is a complicated factor involving absorbance and scattering phenomena in a wavelength-dependent fashion (19).
In this study, we attempted to overcome the limitations of cardiac reflectance spectroscopy on the intact heart by performing transmission spectroscopy across the heart wall (29, 44) by placing a catheter tip light source directly in the left ventricular (LV) chamber, in the same manner as the widely used Millar pressure probes, which are inserted in the LV in working heart studies (39, 42, 49), or catheter NMR probes (26), and detecting from the outside, light that traversed the LV free wall. Using a ventricular cavity light source and collecting photons from the outside provides a true absorbance spectroscopy measure as every photon collected migrates through the tissue. This maximized optical path length through the tissue while minimizing surface-scattering effects and associated motion artifacts. In addition, we applied full-multicomponent spectral analysis to improve the accuracy of the spectroscopic determination of cardiac chromophores as previously described (12). Using these approaches, we approximated the redox potential of cytochrome c and cytochrome a + a3 as well as the oxygenation level of Mb for a Krebs-Henseleit (KH) solution-perfused rabbit heart.
MATERIALS AND METHODS
LED catheter design.
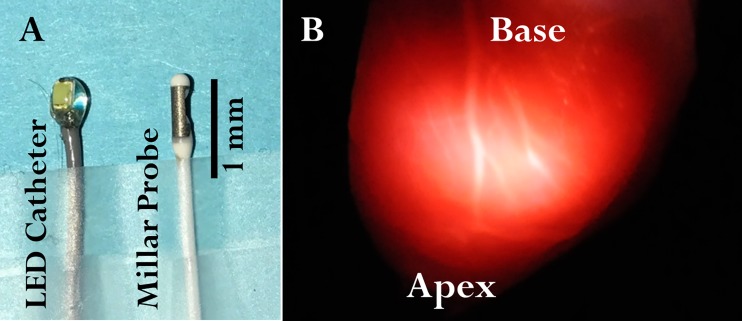
The LED-tipped catheter was constructed from a flexible coaxial cable (outer diameter 2.42-Fr CXN 3011, W. L. Gore & Associates) with a commercially available inexpensive LED nano chip (LED 1.8 × 0.8 mm, Evans Designs). The “side-firing” chip geometry was selected over the “end-firing” bulb geometry since a side-firing device impinges the light through the free wall and not the apex of the heart on the tip of a catheter. The LED was directly soldered onto the end of the coaxial cable. The exposed end of the coaxial cable, solder joint, and LED were thinly coated with epoxy (Double/Bubble Epoxy extra fast setting, Hardman) as an insulator. The LED was powered with a regulated direct current power supply (2.8 V). The LED catheter was designed mirroring the commercially available and widely used intraventricular Millar pressure probe, as shown in Fig. 1A. An image of the illumination of an arrested heart with the LED catheter is shown in Fig. 1B. Even though an LED is considered a “cold light source,” we measured the heat production from the probe to assure it would not heat the ventricular space. LED catheter heat generation was evaluated by placing it in a static 3-ml water beaker at room temperature and continuously recording temperature. This was considered a worst-case scenario for the perfused heart with constant flow.
Fig. 1.
LED catheter geometry and illumination from within the left ventricle (LV). A: the LED catheter is approximately the same size as that of a Millar intracardiac pressure probe. The size of the LED catheter is 2.42-Fr and that of the Millar catheter (SPR-542) is 2.3-Fr. The size of the tip of the LED catheter is 6.3-Fr and that of the tip of the Millar catheter is 3.5-Fr. B: photo of the LV free wall of a perfused rabbit heart with the LED catheter positioned in the LV chamber. The heart wall was highly heterogeneous with regard to the amplitude of transmitted light.
Heart excision and perfusion.
All animal protocols were approved by the National Heart, Lung, and Blood Institute Animal Care and Use Committee and performed in accordance with guidelines described in the Animal Care and Welfare Act (7 USC 2142 § 13). New Zealand White rabbits (n = 20, 2.9 ± 0.1 kg) of either sex were anesthetized via an intramuscular injection of ketamine-xylazine mix (10:1). Heparin (1,000 units) was administered by an intravenous ear vein injection, and isoflurane (4−5%) was administered via inhalation. After 3 min of heparin circulation and proper depth of anesthesia was determined, the animal was euthanized with 6 mEq KCl iv bolus. The chest was then opened; hearts were rapidly excised and retrograde perfused in the constant pressure mode using a heavily modified perfused heart system (120101BEZ, Radnoti) for use with a rabbit heart. The perfusate used was a modified KH crystalloid solution containing (in mmol/l) 137 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 1.0 Na2HPO4, 10 glucose, 1 lactate, and 10 HEPES (pH 7.4) oxygenated with 100% O2 and maintained at 38°C. Hypoxic perfusion medium was created by bubbling this medium extensively with 100% N2 rather than O2. Temperature was measured continuously by inserting a T-type Implantable Thermocouple Probe (IT-18, MLT1401) attached to the T-type Pod temperature transducer (ML312, AD Instruments) in the cannula right above the aorta. Diastolic aortic pressure was set at 65 mmHg. Aortic pressure was measured continuously with the MLT844 Physiological Pressure Transducer (AD Instruments). The vena cavae were ligated, and the pulmonary artery was cannulated. Flow into the aorta and flow from the pulmonary artery (coronary flow rate) were measured continuously using a flow-through probe (TS410) coupled with 4PXN sensors (Transonic). Perfusate oxygenation into and out of the heart was measured with custom-design flow-through optical oxygen sensors connected to the NeoFox-GT phase fluorimeter (Ocean Optics). Signals were acquired via a PowerLab unit (AD Instruments).
Myocardial optical absorbance spectroscopy.
Changes in cardiac chromophores absorbance were measured from the LV free wall by inserting the LED catheter inside the LV. After the hearts had been cannulated, a stabilization period of at least 10 min occurred before the LED insertion. In Langendorff experiments, this was accomplished by cutting a small opening in the left atrium and inserting the LED catheter through the mitral valve. In left working heart experiments, the LED catheter was fed through the perfusion system proximal to the heart, via the aortic cannula, and then through the aorta and aortic valve. The LED catheter was rotated to maximize light transmission through the LV free wall by visual inspection (see Fig. 1B). The transmitted light was collected with a fiber optic light guide (2 mm diameter, Thor Laboratories) placed ~1 cm from the heart’s LV perpendicular to the maximum transmitted light intensity. The fiber optic was connected to a detector, a cooled rapid-scanning spectrometer (model QE65PRO, Ocean Optics). Emitted light intensity within the entire spectral range of the spectrometer (1,044 points covering a bandwidth of 348.82−742.03 nm) was recorded. Transmitted light spectra were collected at 2 Hz (i.e., 2 samples/s) using a custom LabVIEW-based program for spectral acquisition and analysis installed on a PC using LabVIEW drivers provided by Ocean Optics. With a heart rate of 153 ± 10 beats/min under control conditions, this acquisition rate corresponded to an average of ~1.3 beats/spectrum during the control periods. The LabVIEW code is available by request to the authors. With more rapid acquisitions across the cardiac cycle 100 Hz (i.e., 100 samples/s) coupled with cycle-specific averaging, we found no difference in heart chromophore absorbances through the cardiac cycle, consistent with prior reports in vivo and in vitro (2, 28). Thus, averaging over a cardiac cycle does not lose any useful information under our conditions.
To attempt to quantitate Mb oxygenation level and cytochrome redox state, the fully oxygenated and oxidized spectrum along with the fully deoxygenated and reduced spectrum needed to be collected to establish the full range. This is a challenging task in whole organs, and any method applied must only be considered an estimate of the full range. These experiments also permitted a wide dynamic range to evaluate our spectral fitting routines outlined below. After considering and attempting several different approaches, we settled on using myxothiazol to block electron flow at the level of complex III (1, 10) to oxidize cytochromes c and a + a3 and fully oxygenate Mb by inhibiting O2 consumption. Thus, with a single treatment, we can oxidize the cytochromes of interest as well as oxygenate Mb. Dose responses of myxothiazol were obtained using a micro-syringe pump injecting concentrated myxothiazol close to the cardiac cannula and calculating concentration from the perfusion flow rate. Cytochromes redox state and Mb oxygenation state were measured along with perfusate flow and myocardial O2 consumption.
We also explored several different methods of achieving maximum reduction of the cytochromes and deoxygenation of Mb. Surprisingly, we found that common strong reducing agents such as sodium dithionite and sodium sulfite resulted in the generation of new chromophores or alteration of existing chromophores not observed in simple anoxia/ischemia protocols. Similar observations have been made with sodium sulfide (44). Cyanide produces a reduction spectrum of the cytochromes while causing oxygenation of Mb (9, 12). However, over long incubation times, we have observed an interaction of cyanide with Mb potentially interfering with subsequent quantitation in the deoxygenated state. Thus, we elected to simply use anoxic perfusion followed by total ischemia to approximate the fully reduced cytochromes and deoxygenated Mb in the paired experiments with myxothiazol-treated hearts. We did separate myxothiazol-treated and anoxia/ischemia-treated hearts since myxothiazol, especially at higher doses, resulted in a residual decrease in O2 consumption making the attainment of hypoxia difficult. We were comfortable with this paired analysis as the total absorbance for the cytochromes [0.38 ± 0.03 optical density (OD) for cytochrome a + a3 and 0.23 ± 0.01 OD for cytochrome c] and Mb (0.34 ± 0.03 OD) was highly reproducible for the anoxia/ischemia protocol from heart to heart (n = 8) using this transmission optical geometry.
Spectral data analysis.
Spectral data were analyzed using a custom-developed LabVIEW-based program. Technically, the difference absorbance [ΔODλ(t)] was obtained by removing dark current (IDark,λ) from both the intensity of the light without the tissue present (I0,λ) and intensity of light transmitted through the tissue at any time t [Iλ(t)], compiled into a single matrix as follows:
| (1) |
By calculating and expanding the formula above, the term I0,λ − IDark,λ cancels out and the formula, as used in the program, becomes as follows:
| (2) |
The baseline condition at time t0 was selected after the heart became stable after LED catheter insertion and before any perturbation. The difference absorbance between any two spectra was calculated by selecting two regions within the different steady-state conditions. To estimate the signal to noise of this measure, the unfiltered difference spectrum between two single spectra (500 ms/spectrum) within a control period, separated in time by >1 min, was taken as the noise spectrum, whereas the signal spectrum was taken between the difference of single spectra from the fully oxidized and reduced conditions over the same time range. The signal to noise, 5,687 ± 763 (n = 3), was estimated from the root mean square (RMS) using the following equation: (signal RMS/noise RMS)2 over the bandwidth of the spectral analysis (535−630 nm).
As these were primarily steady-state analysis, the signal to noise of the measurement was increased by averaging multiple consecutive spectra for each of the steady-state conditions [usually 100 spectra (i.e., 50 s of data collection)]. Again, no spectral or temporal filtering was applied to the data presented. The contribution of each chromophore was estimated with a linear least-square regression fitting technique in the 535- to 630-nm region using isolated reference spectra (reduced – oxidized or deoxygenated – oxygenated) of the chromophores of interest. This spectral range was found to provide the best discrimination of the cardiac chromophores with the transmitted light used (12). A similar approach was taken with a limited number of chromophores by Hoffman et al. (25), who analyzed perfused heart reflection spectra The data and references were zeroed at 630 nm, which can be considered as an anoxia isobestic point with minimal absorbance from the tissue. The references for cytochromes a + a3 (a605), cytochrome blow (bL), cytochrome bhigh (bH), cytochrome c (c), and cytochrome c1 (c1) have been previously described (12). The Mb reference was acquired following previous procedures (38). A line was included as a reference for fitting for baseline and potential sample scattering events. In addition, the LED’s spectrum (I0) was used as a reference to capture any light that traversed to the detector without interacting with an absorber, that is, sieved photons. Fitted ΔOD* spectra were computed using the following equation:
| (3) |
Considering the close absorption maxima of c1 and c along with the fact that c1 and c are at redox equilibrium (22), we fixed the ratio of c1 and c to the value detected in the difference of fully reduced and fully oxidized spectrum of a detergent extract of the whole heart (5). The goodness of fit was evaluated mainly by visual inspection of the linearity of the fit residuals (raw data – fit data). We also used the weighted mean square error (WMSE) in the LabVIEW fitting routine. The range, mean, and SE of this WMSE for all experiments are reported.
Mb oxygenation level as well as cytochrome c and cytochrome a + a3 redox potential were calculated using ΔOD* at specific wavelengths, representing characteristic peaks (582 nm for Mb, 550 nm for cytochrome c, and 605 nm for cytochrome a + a3 in the reference spectrum used to fit the complex heart spectrum, not in the observed spectrum). This was done by multiplying the fitting coefficient of a given chromophore by the ΔOD of the chromophore in the reference at the specific wavelength, as follows:
| (4) |
| (5) |
| (6) |
The percentages were calculated as follows:
| (7) |
In the present study, the true magnitude of the incident light (I0) was too high to directly measure with the dynamic range of our spectrometer. However, for difference spectra, the I0 term cancels (see Eq. 1) permitting calculation of a true absorbance difference, as discussed above. To present the relative spectral absorbance (RSA) of the heart for this presentation (see Fig. 2C), we “normalized” the transmission data to the spectrum of the light source by taking the ratio of transmitted light spectrum from the heart to the incident light model () shown in Fig. 2A as follows:
| (8) |
where , or RSA, is the log ratio of the transmitted to incident light model spectrum.
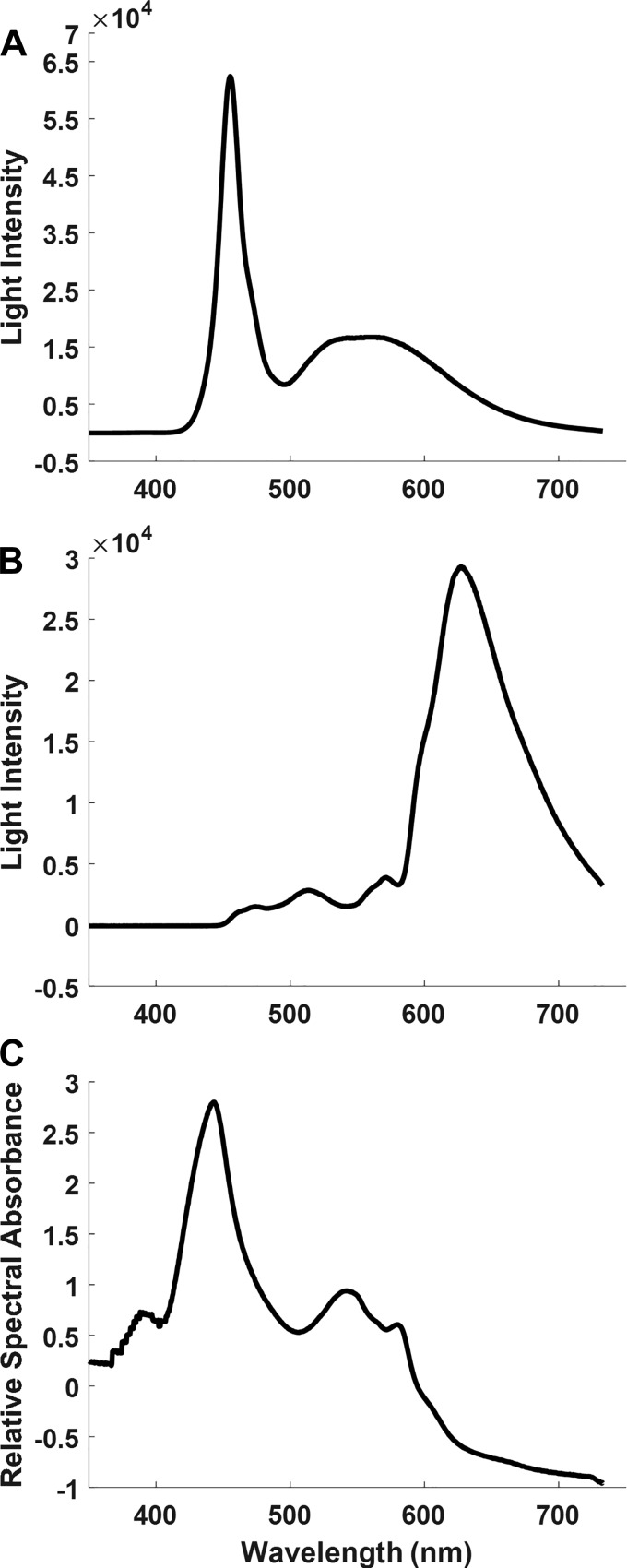
Fig. 2.
Spectral content and intensity of light emitted by the LED. A: raw spectrum of the light () emitted by the LED. Incident light intensity was decreased to avoid detector saturation at the peak that occurs at ~450 nm. B: intensity of LED light emitted transmurally from the left ventricular (LV) chamber through the LV free wall. The detector was positioned 1 cm away from the epicardial surface of the LV and centered at the position of maximum intensity of transmitted light. C: relative spectral absorbance of LED light through the LV free wall calculated using the spectra in A and B and in Eq. 6.
To estimate the overall absorbance/scattering of the rabbit heart, the LV free wall (~3.5 mm) and right ventricular free wall (~2.5 mm) were dissected from a perfused heart and mounted flat in a cuvette. The reported thicknesses were measured just before the optical measures and were thicker than the expanded wall occurring during perfusion. Total absorbance/scattering was measured in a conventional spectrophotometer (Schimadzu UV-2700 Spectrophotometer). The LV had an apparent absorbance of ~5 OD at 550 nm, approaching the limits of the instrument dynamic range, whereas the right ventricle had an apparent absorbance of ~3.5 OD. This suggests that the apparent absorbance (i.e., scattering and absorbance) of the heart wall is roughly ~1.4 OD/mm tissue in the absence of blood. Naturally, having blood in the wall will dramatically increase the scattering and absorbance (19). These data are consistent with the large attenuation of the transmitted light observed through the heart wall in these experiments (Fig. 2B).
Path length determination.
The path length of light was calculated per the Beer-Lambert law (A = εLc, where A is the absorbance, ε is the extinction coefficient, L is the path length, and c is the chromophore’s concentration, in this case Mb). The rabbit heart Mb content is low at 53 nmol/g, and its extinction coefficient is 12.8 g·mmol−1·cm−1 at 582 nm (4). The between fully oxygenated and fully deoxygenated was used as A.
The scattered path length was assumed to be the same in the fully oxidized and reduced hearts and relatively independent of the gross cross-section of the heart. To confirm this assumption, we infused an inert reference dye (Alexa Fluor NHS Ester, ThermoFisher Scientific), which absorbs light outside of our normal region of interest (~700 nm) into the heart until it reached a steady state. The absorbance of this inert chromophore was used as an independent gauge of changes in tissue path length.
Statistical methods.
Goodness-of-fit statistics for the spectral fitting were established using the LabVIEW weighted mean square error in the General Linear Fit function. All summary data are reported as means ± SE of the data series. RMS was determined by the following equation:
RESULTS
LED catheter heating.
The LED temperature test in the static 3-ml sample resulted in <0.5°C maximum drift for both control (i.e., LED OFF) and LED ON conditions. The drift was attributed to room temperature changes, as the time trace for both conditions were similar. It was concluded that the LED would not contribute to heating of the rabbit ventricular cavity.
LED catheter spectrum.
The LED catheter’s spectrum is shown in Fig. 2A. This spectrum was collected with much less light than used in the heart to avoid saturating the detector at the peak that occurs at 454 nm and should not be considered a magnitude spectrum of incident light but simply represents the spectral properties of the light source. The power of the LED catheter was clearly adequate to transilluminate the heart LV free wall with a predicted ~5 OD absorbance/scattering in the ~400- to 735-nm bandwidth for saline-perfused rabbit heart, as shown in the transmittance spectrum in Fig. 2B. The RSA shown in Fig. 2C reveals the baseline absorbance of light in the heart primarily from Mb and cytochromes in the 550- and 440-nm regions. Note the difference in RSA absorbance peak for the heart absorbance at 442 nm is distinct from the light source peak at 454 nm consistent with this approach representing the heart absorbance and not the light source characteristics. Little light is transmitted through the heart below 400 nm.
An image of the transmitted light across the wall is shown in Fig. 1B in a heart arrested by anoxia/ischemia. The motion of the beating heart and low light level prevented the imaging of the beating heart. Note that the heart is not of uniform thickness, with the larger vessels indicating a thinner wall displaying a higher transmitted light level. Indeed, we have found that the inclusion of a LED light spectrum model was needed in the fitting routine to compensate for light that sieved through the tissue without being absorbed. The change in this component of the spectrum was largest in the arrested state (see below) consistent with the heterogeneity of the wall seen in the image shown in Fig. 1B.
Stability of cardiac performance with the LED catheter.
The performance stability of the heart with the LED catheter in the cavity was evaluated by assessing temporal changes in functional data. Figure 3A shows aortic flow and heart rate before and after insertion of the LED catheter at time 0 during Langendorff perfusion. A functional disruption was observed at time 0, but 6.5 ± 2.5 min later the heart recovered to a slightly different steady state. Figure 3B shows the stability of the heart’s cardiac output and heart rate during 60 min with and without the LED catheter. No major difference was observed. These data are consistent with the extensive experience in using Millar-style pressure catheters, introduced to the heart in a similar manner, that have minimal impact on heart function in perfused heart studies.
Fig. 3.
Functional stability of perfused rabbit hearts with the tip of the LED catheter positioned inside the left ventricular chamber. A: after the left atrium was cut at time 0 in the Langendorff experiments, the LED catheter was inserted into the left ventricle via the mitral valve. Cardiac function recovers from this perturbation after 6.5 ± 0.9 min to a new steady state with aortic flow (which is equal to total coronary flow) slightly lower than that before insertion of the LED catheter. B: functional stability over 60 min in left working hearts after insertion of the LED catheter (n = 3; *) was similar to that of left working hearts without the LED catheter (n = 7; ○), indicating that the LED catheter does not impose time-dependent effects on heart rate and cardiac output. BPM, beats/min.
Spectral fitting of absorbance difference spectra.
The multicomponent linear regression fit of the heart absorption spectrum’s goodness of fit was based on both visual inspection of the residuals and statistical evaluation. We have previously established the main chromophores that dominate myocardial absorbance (12, 38). We have now added a light source reference spectrum model to compensate for sieved light present in transmural illumination.
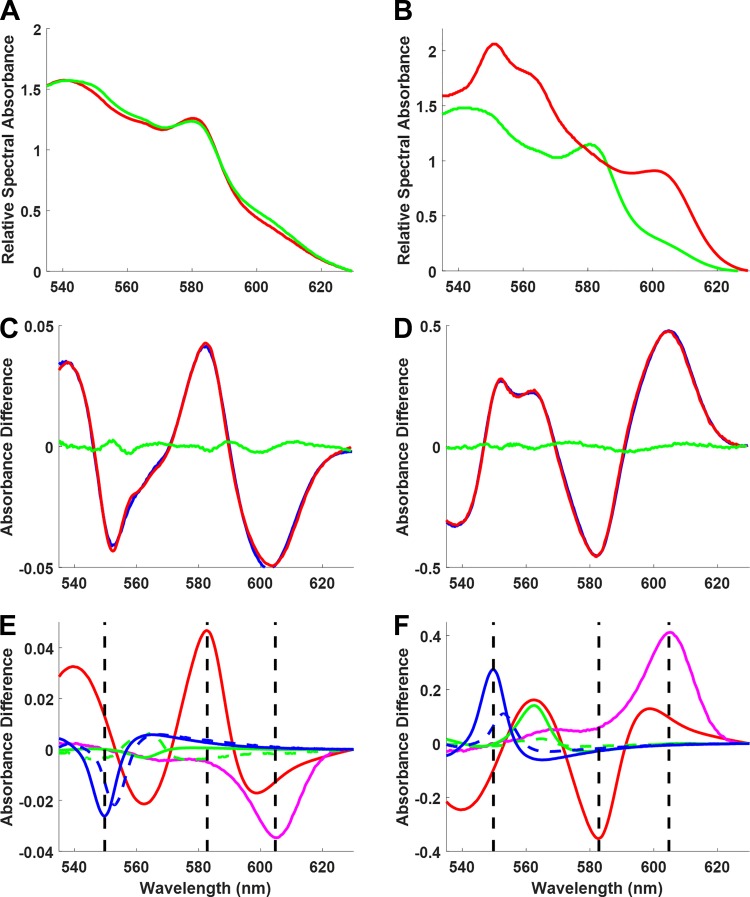
The absorbance difference spectrum was calculated by taking the log difference of two conditions (Eq. 2). For reference, the RSA spectra for control and 10 μM myxothiazol conditions are shown in Fig. 4A, with RSA spectra for myxothiazol and anoxia/ischemia conditions shown in Fig. 4B. In Fig. 4, bottom, the absorbance difference spectrum and multicomponent deconvolutions are shown for control versus myxothiazol (Fig. 4, C and E) and control versus anoxia/ischemia (fully reduced; Fig. 4, D and F). Over the wide dynamic range of these two perturbations (0.1 ΔOD control vs. myxothiazol and 1 ΔOD control vs. ischemia), the fits for these spectra generated minimal residuals. The WMSE for both fits ranged from 1.5 × 10−6 to 3.5 × 10−4 with a mean of 4.5 × 10−5 ± 1.8 × 10−5. The light sieved () and scattering (line) contributions are not shown as they do not represent biochemical information.
Fig. 4.
Transmural myocardial absorbance spectra deconvolution. A: relative spectral absorbance of baseline (green) and fully oxidized with myxothiazol (red) in a perfused rabbit heart. B: relative spectral absorbance of baseline (green) and fully reduced at ischemia (red) in a perfused rabbit heart. C and D: fitted spectra (red) and residuals (green) of the absorbance difference (blue) of myxothiazol – control (from A) or ischemia – control (from B) spectra, respectively. E and F: contribution of each reference in the fitted data [myoglobin (red), cytochrome a + a3 (magenta), cytochrome bH (green), cytochrome bL (dashed green), cytochrome c (blue), and cytochrome c1 (dashed blue)]. The light leak () and scattering (line) contributions are not shown and hence were subtracted from both the raw and fitted data in C and D). The dashed vertical lines represent the peaks of cytochrome c (550 nm), myoglobin (582 nm), and cytochrome a + a3 (605 nm). The value of each peak in the raw data is a linear combination of chromophores with absorbance at that wavelength. This shows that the dual-wavelength measurement technique, which consists of measuring the change in optical density of a chromophore by taking the value of its representative peak and subtracting the value of an isobestic point, is not an accurate measurement approach.
We compared this approach with the common dual-wavelength analysis where a single wavelength is used as a reference at a putative isobestic point and subtracted from a readout frequency representing a specific chromophore absorbance. For example, cytochrome c is often measured with a reference at 568 nm and readout at 550 nm, while the Mb readout is typically at 582 nm. For cytochrome a + a3, the reference is often 630 nm with a readout at 605 nm (24). We were surprised that isobestic points seemed to be constant over many of the perturbations we have used even though they occur due to the fortuitous cancelation of several chromophore overlaps in these spectral regions (Fig. 4, E and F). However, the readout frequencies did not provide unique information on any given chromophore for most of the conditions we have studied. This is illustrated by the vertical lines (Fig. 4, E and F) for the typical readout frequencies for cytochrome c (550 nm), Mb (582 nm), and cytochrome a + a3 (605 nm), which clearly show that more than the target chromophore is significantly contributing to the absorbance at these frequencies. Thus, the dual-wavelength approach does not isolate the contribution of the different chromophores in this complex system. These data illustrate the necessity of multicomponent fitting to extract quantitative data on these chromophores in the intact heart.
Estimated control redox state of cytochrome c and oxygenation of Mb.
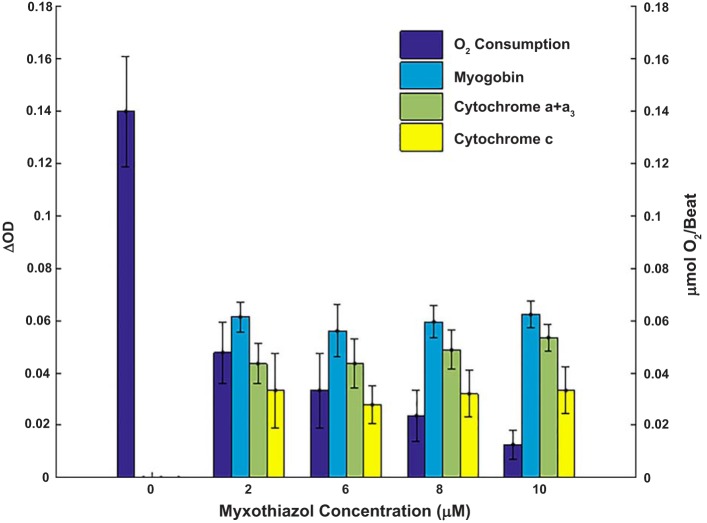
As discussed above, we selected myxothiazol to attempt to fully oxidize cytochrome c and oxygenate Mb. To optimize this effect, dose-dependent experiments were conducted on the effect of myxothiazol on O2 consumption, Mb, and cytochrome absorbance. These data are shown in Fig. 5 and Table 1. Mb oxygenation and cytochrome oxidation state increased to maximum levels with the lowest dose (2 μM) of myxothiazol even though the respiratory rate was not fully inhibited until higher doses of myxothiazol were applied (10 μM maximum). Coronary flow also increased with the 2 μM dose of myxothiazol from 29 ± 2 to 77 ± 1 ml/min and remained essentially constant at higher doses, consistent with the inhibition of mitochondrial function. Note that despite the increase in coronary flow and inhibition of respiration, the heart rate remained nearly constant in this nonworking Langendorff-perfused heart. This residual activity is consistent with glycolysis supporting ATP and heart rate in this nonworking condition, as found with cyanide treatment in other systems (14, 31). These data imply that the lowest dose (2 μM) of myxothiazol sufficiently restricted reducing equivalent delivery to the cytochromes that resulted in a highly oxidized state but still permitted some respiration. While the inhibition of respiration and increase in coronary flow with 2 μM myxothiazol was sufficient to increase O2 tension to saturate Mb with oxygen, subsequent additions of myxothiazol further reduced respiration with no detectable change in the cytochrome c and + a3 redox state, Mb oxygenation, or coronary flow. Thus, any changes in redox state associated with the higher inhibition of respiration were beyond our current detection limits. We concluded from these results that the maximal attainable oxidation of cytochromes and oxygenation of Mb was attained even with 2 μM myxothiazol. Consistent with this conclusion, in eight hearts, cyanide (1 mM) generated the same oxygenation of Mb as found with myxothiazol (Mb ΔOD* with cyanide was 0.07 ± 0.01 and ΔOD* with myxothiazol was 0.07 ± 0.01). Data from hearts treated with 2 or 10 μM myxothiazol were pooled for the quantitation of the fully oxidized condition.
Fig. 5.
O2 consumption during myxothiazol titration. O2 consumption, myoglobin oxygenation, cytochrome a + a3 oxidation, and cytochrome c oxidation are shown during myxothiazol titration in perfused rabbit hearts (n = 3). The point at 0 μM is taken during the control phase before myxothiazol injection. The reported concentration was calculated from the myxothiazol stock in the syringe and coronary flow and was controlled by stepwise changes in speed of injection. ΔOD, change in optical density.
Table 1.
Cardiac function before and after administration of myxothiazol
| Control | Myxothiazol (2 μM) | Myxothiazol (10 μM) | |
|---|---|---|---|
| Heart rate, beats/min | 155.95 ± 7.51 | 157.75 ± 9.78 | 139.47 ± 14.31 |
| Aortic flow, ml/min | 25.82 ± 1.45 | 62.30 ± 3.54 | 75.47 ± 1.94 |
| Peak aortic pressure, mmHg | 78.07 ± 2.13 | 69.66 ± 1.92 | 50.59 ± 0.00 |
| O2 consumption, μmol·min−1·g wet wt−1 | 3.05 ± 0.17 | 2.44 ± 0.18 | 0.61 ± 0.25 |
| O2 consumption, μmol/beat | 0.16 ± 0.01 | 0.12 ± 0.01 | 0.03 ± 0.01 |
Data are reported as means ± SE for control conditions (no myxothiazol) as well as for 2 μM (n = 9) and 10 μM (n = 3) myxothiazol concentrations.
Full cytochrome reduction and Mb deoxygenation were attained with ischemia after an anoxic perfusion to avoid residual oxygen in the tissue. We confirmed that this approach fully reduced the cytochromes by comparing cytochrome redox states with the cyanide treatment discussed above. ΔOD* for cytochromes c and a + a3 were 0.13 ± 0.01 and 0.25 ± 0.02 (n = 8) with cyanide, which are less than 0.23 ± 0.01 and 0.38 ± 0.02 with anoxia/ischemia (n = 7). This discrepancy could be due to the inability to fully inhibit complex IV in this complex system as well as the change in the extinction coefficient of cytochrome a + a3 with cyanide bound. In any event, anoxia/ischemia treatment resulted in an apparent higher reduction level of the cytochromes than detected with 1 mM cyanide. These data suggest that anoxia/ischemia was removing oxygen from the tissue (fully reduced cytochrome a + a3), which should easily fully deoxygenate Mb. Based on these data, we used anoxia/ischemia to estimate the fully reduced cytochromes and fully deoxygenated Mb.
Using the fully oxidized (myxothaizol) and reduced states (anoxia/ischemia) generated in this preparation, we can estimate the absolute redox state of cytochromes and oxygenation of Mb. Due to the uncertainties in quantitating the complex spectral and redox properties of cytochrome bL and cytochrome bH, we focused these initial efforts on cytochrome c and cytochrome a + a3 as well as the oxygenation of Mb. Using Eqs. 4−7, we calculated the oxygenation level of Mb to be 82.2 ± 3.0%, the resting redox state of cytochrome c to be 12.6 ± 2.2%, and the resting redox state of cytochrome a + a3 to be 13.3 ± 1.4% in the isolated saline-perfused Langendorff rabbit heart.
Path length of transmural light.
The thickness of the rabbit LV free wall was determined by gross dissection to be 3.5 mm immediately after dissection, consistent with Doppler measures of the beating rabbit heart of 2.2 ± 0.3 mm in diastole and 3.5 ± 0.6 mm in systole (18). However, path length calculated using Mb absorbance was 6.1 ± 0.1 mm, significantly longer than the transmural thickness of the ventricular wall. This implies that light scattering within the wall dominated the average light path as found in other biological systems (13, 20, 32) and also implies that the transmural thickness of the wall, within limits, will have a minimal impact on observed optical path length (32). We confirmed this notion by adding an inert stable dye, Alexa Fluor 700 succinimidyl ester (ThermoFisher Scientific), which absorbed outside of our region of interest (700 nm). The Alexa Fluor 700-nm absorbance should only change with a change in tissue geometry or optical path length independent of the biochemistry. Monitoring the absorbance of this probe during our standard protocols revealed no significant change in absorbance despite the changes in wall thickness occurring during the cardiac cycle and more notably with anoxia. These data are consistent with an insignificant change in overall path length with these perturbations, suggesting that path length is more dependent on scattering distances than net thickness of the wall. It is interesting to note that using the transmural approach, we have observed a greater than fourfold increase in path length compared with conventional reflectance spectroscopy on rabbit hearts and approximately twofold higher than the complicated illumination through the base of the rabbit heart from our laboratory (24).
DISCUSSION
A novel catheter-based side-firing LED light source was developed for transmural absorption spectroscopy of perfused hearts. The light source is introduced into the ventricular cavity, as done with ventricular pressure monitoring catheters, with modest impact on cardiac function. The LED generates adequate power and bandwidth through the optically opaque heart wall to observe the intrinsic chromophores from the cytosol (Mb) and mitochondria (cytochromes) with excellent temporal and spectral resolution with a fiber optic light collector outside of the heart. It is important to note that this approach maximizes the path length, and associated signal-to-noise ratio, while minimizing surface reflection artifacts obtained with more common reflection spectroscopy methods. This advantage is accomplished with no significant heat from this “cool” LED source within the ventricular cavity. This simple device, used much like a pressure catheter in the ventricle, provides a wealth of online nondestructive information on the metabolic status of the heart, such as the cytosolic O2 content and the redox state of multiple sites along the oxidative phosphorylation cascade. This approach should also be useful for exogenous optical probes for pH, Ca2+, and membrane potentials.
The estimated path length of this optical interrogation of the rabbit free wall at ~6 mm is twice the physical thickness of the wall at ~3 mm. This implies that the majority of the light transmitted through the wall is highly scattered with a path length roughly doubled with respect to what is expected from the few ballistic photons traversing directly through the tissue. From our own experience with refection spectroscopy on this preparation, transmural illumination increases the path length by approximately fourfold. This corresponds to an approximately fourfold increase in signal to noise based on the Beer-Lambert law. The dominance of scattering on path length reduces the impact of changes in wall thickness on the transmural optical path length (32), as confirmed with inert dyes in this study. Thus, modest changes in wall thickness do not result in significant changes in path length and therefore observed absorbance of chromophores. Since the transmural illumination detects chromophores across the entire heart wall, the interpretation of these data must be tempered in the sense that large changes in small regions, such as the endocardium, could be missed or underestimated using this approach. This is analogous to whole heart 31P, 13C, and 1H NMR spectroscopy studies of perfused heart metabolism. Finally, the transmural illumination likely makes the measure more susceptible to optical sieving through the tissue. That is, some regions of the heart might be effectively translucent, especially in a thin wall region around the large vessels and paravascular fat (see Fig. 1B), which permit light to pass through the tissue essentially unabsorbed. Due to the high absorbance of the tissue (see Fig. 2, A and B), a small fraction of this sieved light would have a large impact on the spectral density of the collected light. This light sieving phenomenon was corrected for in our spectral analysis, as discussed further below.
It is tempting to speculate that by using the epicardium weighted surface reflectance and the transmission approach that potentially differences in the epicardium and whole heart wall might be extracted from the data. However, as the field of diffuse optical tomography has established (8), the intrinsic spatial resolution of optical spectroscopy in scattering media is very poor even with multiple transmitters and detectors capable of time of flight determination. Thus, to create even a partial transmural image of these chromophores across the thin heart wall would involve a much more complicated system than presented here.
This intracardiac light catheter approach could be applied to other perfused heart systems. This approach provides ample light for application to smaller hearts (rats, guinea pigs, etc.) but, however, might require using a smaller LED and coaxial cable. We determined that the effective optical absorbance of the heart is roughly 1.4 OD/mm tissue. Since heart composition is very constant over a mammalian allometry series (34, 35), it is reasonable to assume that this value scales across mammalian hearts. As shown in Fig. 2, the ~5 OD of the rabbit LV (~0.35 mm) was close to the limits that this approach could likely be applied. Taking this up to a 0.5−0.75 cm thickness of a larger animal (canine, porcine, etc.) is likely not feasible but might work over a very limited spectral bandwidth, as previously described by Arai et al. (2).
Another approach might be to use a side-firing optical fiber where the light source is generated externally and delivered into the ventricle via the optical fiber. An example of this type of fiber is the 1.65-Fr DuoTome SideLite fiber by Lumenis. A portion of the light emitted from the fiber is directed nominally at a 70° angle and can deliver up to 100 W of power. Preliminary work with this fiber suggests that it may be appropriate for smaller hearts but is much more rigid than our custom-designed system based on the CXN 3011 coaxial cable. Moving this technology to in vivo applications is likely not feasible, even in small animals, as the blood absorbance, both in the ventricular cavity and capillary system, would likely prevent many photons from escaping from the tissue, resulting in a very high OD (19). However, again, limited bandwidth studies might be feasible (2).
Another important aspect of this study was analysis of the transmission data using full spectral analysis. This was accomplished using reference absorption spectra for each of the major heart chromophores, as previously described (12, 38), as well as the inclusion as references of sieved light that scattered through the tissue with no absorbance. The spectral fitting using this approach was adequate based on the residuals and statistical analysis of the fit. Naturally, when using multiple absorbances for a linear regression fit, care must be taken to assure the unique solution is reached. In this study, we collected light over a broad bandwidth, but selected the range of 535−630 nm to optimize the fitting sensitivity of the known myocardial chromophores (12, 38), since this is the bandwidth where the discrimination between the different chromophores was maximized. Using this approach and evaluating the fit solutions, it became clear that the common use of a single peak wavelength and an “isobestic” (i.e., zero crossing points in difference spectra) reference wavelength to quantitate a given chromophore (2, 23, 24) is problematic. First, although the isobestic points determined at anoxia are reproducible, they are the result of cancellation of multiple chromophores, resulting in a fortuitous zero crossing point (see Fig. 4, E and F). Thus, these isobestic points determined at anoxia, common in the literature, might not be appropriate to account for more selective changes in the chromophore absorbance or for scattering and sieved light. More importantly, a close inspection of the actual spectra used for these fits reveals that no single wavelength can be used to represent a given chromophore due to the extensive overlap of the absorption spectra (see Fig. 4, E and F). Thus, the most reliable method of quantitating a given chromophore is to fit the entire spectrum using known reference spectra.
In this study, we focused on quantitating the redox states of cytochrome c and cytochrome a + a3 as a measure of mitochondrial redox state and the oxygenation level of Mb as a measure of mean cytosolic O2 tension. Using the full range of oxidized and reduced cytochrome c and cytochrome a + a3 in these hearts, we determined the resting cytochrome c redox state to be 13.3 ± 1.4% reduced and cytochrome a + a3 to be 12.6 ± 2.2% reduced, consistent with prior measures in intact systems (45, 46). Mb oxygenation was only 82.2 ± 3.0% oxygenated, consistent with a prior reflection spectroscopy study (36), suggesting that cytosolic O2 is lower than that of the resting heart in vivo where the Mb oxygenation is close to 100% (2, 11). It has been suggested that the deoxygenation of low-O2 affinity Mb and reduction of high-O2 affinity cytochrome a + a3 occurs in the same small regions in the perfused heart and is caused by steep, localized gradients of O2 tension (44). These small regions, estimated to be on the order of 15% of the heart in the present study, are nearly completely anoxic, generating the simultaneous deoxygenation of Mb and reduction of cytochrome. Theoretical models of the microvascular structure are also consistent with a localized O2 deficit in the saline-perfused heart (6).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.N.F., S.K.-G., R.C., M.W.K., and R.S.B. conceived and designed research; A.N.F., A.V.G., and R.S.B. performed experiments; A.N.F., S.K.-G., R.C., M.W.K., and R.S.B. analyzed data; A.N.F., S.K.-G., R.C., M.W.K., and R.S.B. interpreted results of experiments; A.N.F. prepared figures; A.N.F. and R.S.B. drafted manuscript; A.N.F., S.K.-G., R.C., A.V.G., M.W.K., and R.S.B. edited and revised manuscript; A.N.F., S.K.-G., R.C., A.V.G., M.W.K., and R.S.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the surgical team at the National Heart, Lung, and Blood Institute for support with the animal preparation and heart excision. We appreciate Shawn Kozlov for the suggestion of going through the mitral valve with the LED catheter in the Langendorff experiments.
REFERENCES
- 1.Alexandre A, Lehninger AL. Bypasses of the antimycin a block of mitochondrial electron transport in relation to ubisemiquinone function. Biochim Biophys Acta 767: 120–129, 1984. doi: 10.1016/0005-2728(84)90086-0. [DOI] [PubMed] [Google Scholar]
- 2.Arai AE, Kasserra CE, Territo PR, Gandjbakhche AH, Balaban RS. Myocardial oxygenation in vivo: optical spectroscopy of cytoplasmic myoglobin and mitochondrial cytochromes. Am J Physiol 277: H683–H697, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Arakaki LS, Burns DH, Kushmerick MJ. Accurate myoglobin oxygen saturation by optical spectroscopy measured in blood-perfused rat muscle. Appl Spectrosc 61: 978–985, 2007. doi: 10.1366/000370207781745928. [DOI] [PubMed] [Google Scholar]
- 4.Bailey JR, Driedzic WR. Lack of correlation between cardiac myoglobin concentration and in vitro metmyoglobin reductase activity. J Exp Biol 173: 301–306, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Balaban RS, Mootha VK, Arai A. Spectroscopic determination of cytochrome c oxidase content in tissues containing myoglobin or hemoglobin. Anal Biochem 237: 274–278, 1996. doi: 10.1006/abio.1996.0239. [DOI] [PubMed] [Google Scholar]
- 6.Beard DA, Schenkman KA, Feigl EO. Myocardial oxygenation in isolated hearts predicted by an anatomically realistic microvascular transport model. Am J Physiol Heart Circ Physiol 285: H1826–H1836, 2003. doi: 10.1152/ajpheart.00380.2003. [DOI] [PubMed] [Google Scholar]
- 7.Benard G, Faustin B, Passerieux E, Galinier A, Rocher C, Bellance N, Delage JP, Casteilla L, Letellier T, Rossignol R. Physiological diversity of mitochondrial oxidative phosphorylation. Am J Physiol Cell Physiol 291: C1172–C1182, 2006. doi: 10.1152/ajpcell.00195.2006. [DOI] [PubMed] [Google Scholar]
- 8.Boas DA, Brooks DH, Miller EL, DiMarzio CA, Kilmer M, Gaudette RJ, Quan Z. Imaging the body with diffuse optical tomography. IEEE Signal Process Mag 18: 57–75, 2001. doi: 10.1109/79.962278. [DOI] [Google Scholar]
- 9.Bose S, French S, Evans FJ, Joubert F, Balaban RS. Metabolic network control of oxidative phosphorylation: multiple roles of inorganic phosphate. J Biol Chem 278: 39155–39165, 2003. doi: 10.1074/jbc.M306409200. [DOI] [PubMed] [Google Scholar]
- 10.Camougrand NM, Zniber S, Guérin MG. The antimycin-A-insensitive respiratory pathway of Candida parapsilosis: evidence for a second quinone involved specifically in its functioning. Biochim Biophys Acta 1057: 124–130, 1991. doi: 10.1016/S0005-2728(05)80092-1. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Zhang J, Eljgelshoven MH, Zhang Y, Zhu XH, Wang C, Cho Y, Merkle H, Uĝurbil K. Determination of deoxymyoglobin changes during graded myocardial ischemia: an in vivo 1H NMR spectroscopy study. Magn Reson Med 38: 193–197, 1997. doi: 10.1002/mrm.1910380206. [DOI] [PubMed] [Google Scholar]
- 12.Chess DJ, Billings E, Covian R, Glancy B, French S, Taylor J, de Bari H, Murphy E, Balaban RS. Optical spectroscopy in turbid media using an integrating sphere: mitochondrial chromophore analysis during metabolic transitions. Anal Biochem 439: 161–172, 2013. doi: 10.1016/j.ab.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delpy DT, Cope M, van der Zee P, Arridge S, Wray S, Wyatt J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol 33: 1433–1442, 1988. doi: 10.1088/0031-9155/33/12/008. [DOI] [PubMed] [Google Scholar]
- 14.Elliott AC, Smith GL, Allen DG. Simultaneous measurements of action potential duration and intracellular ATP in isolated ferret hearts exposed to cyanide. Circ Res 64: 583–591, 1989. doi: 10.1161/01.RES.64.3.583. [DOI] [PubMed] [Google Scholar]
- 15.Epstein FH, Balaban RS, Ross BD. Redox state of cytochrome aa3 in isolated perfused rat kidney. Am J Physiol Renal Physiol 243: F356–F363, 1982. [DOI] [PubMed] [Google Scholar]
- 16.Fabel H, Lubbers DW. Measurements of reflection spectra of beating rabbit heart in situ. Biochemische Zeitschrift 341: 351−356, 1965. [Google Scholar]
- 17.Figulla HR, Hoffmann J, Lübbers DW. Evaluation of reflection spectra of the isolated heart by multicomponent spectra analysis in comparison to other evaluating methods. Adv Exp Med Biol 169: 821–830, 1984. doi: 10.1007/978-1-4684-1188-1_75. [DOI] [PubMed] [Google Scholar]
- 18.Fontes-Sousa AP, Brás-Silva C, Moura C, Areias JC, Leite-Moreira AF. M-mode and Doppler echocardiographic reference values for male New Zealand white rabbits. Am J Vet Res 67: 1725–1729, 2006. doi: 10.2460/ajvr.67.10.1725. [DOI] [PubMed] [Google Scholar]
- 19.Gandjbakhche AH, Bonner RF, Arai AE, Balaban RS. Visible-light photon migration through myocardium in vivo. Am J Physiol Heart Circ Physiol 277: H698–H704, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Gandjbakhche AH, Nossal R, Agah R, Motamedi M, Bonner RF. Random walk methodology for determining optical properties of tissue from reflection and transmission measurements. P Soc Photo-Opt Ins 2391: 273–283, 1995. [Google Scholar]
- 21.Glancy B, Willis WT, Chess DJ, Balaban RS. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry 52: 2793–2809, 2013. doi: 10.1021/bi3015983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupte SS, Hackenbrock CR. The role of cytochrome c diffusion in mitochondrial electron transport. J Biol Chem 263: 5248–5253, 1988. [PubMed] [Google Scholar]
- 23.Hassinen IE, Hiltunen JK, Takala TES. Reflectance spectrophotometric monitoring of the isolated perfused heart as a method of measuring the oxidation-reduction state of cytochromes and oxygenation of myoglobin. Cardiovasc Res 15: 86–91, 1981. doi: 10.1093/cvr/15.2.86. [DOI] [PubMed] [Google Scholar]
- 24.Heineman FW, Kupriyanov VV, Marshall R, Fralix TA, Balaban RS. Myocardial oxygenation in the isolated working rabbit heart as a function of work. Am J Physiol Heart Circ Physiol 262: H255–H267, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann J, Lübbers DW, Heise HM. Applicability of the Kubelka-Munk theory for the evaluation of reflectance spectra demonstrated for haemoglobin-free perfused heart tissue. Phys Med Biol 43: 3571–3587, 1998. doi: 10.1088/0031-9155/43/12/014. [DOI] [PubMed] [Google Scholar]
- 26.Kantor HL, Briggs RW, Balaban RS. In vivo 31P nuclear magnetic resonance measurements in canine heart using a catheter-coil. Circ Res 55: 261–266, 1984. doi: 10.1161/01.RES.55.2.261. [DOI] [PubMed] [Google Scholar]
- 27.Kim N, Ripple MO, Springett R. Measurement of the mitochondrial membrane potential and pH gradient from the redox poise of the hemes of the bc1 complex. Biophys J 102: 1194–1203, 2012. doi: 10.1016/j.bpj.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makino N, Kanaide H, Yoshimura R, Nakamura M. Myoglobin oxygenation remains constant during the cardiac cycle. Am J Physiol Heart Circ Physiol 245: H237–H243, 1983. [DOI] [PubMed] [Google Scholar]
- 29.Marzouki L, Jarry G, Janati-Idrissi R, Amri M. The role of myoglobin in retarding oxygen depletion in anoxic heart. Arch Physiol Biochem 110: 400–407, 2002. doi: 10.1076/apab.110.5.400.11834. [DOI] [PubMed] [Google Scholar]
- 30.Marzulli D, Zanotti F, Lofrumento NE. An indirect and highly sensitive method for the determination of the flow of reducing equivalents in the respiratory chain. Boll Soc Ital Biol Sper 61: 129–135, 1985. [PubMed] [Google Scholar]
- 31.Mateo P, Stepanov V, Gillet B, Beloeil J-C, Hoerter JA. Cardiac performance and creatine kinase flux during inhibition of ATP synthesis in the perfused rat heart. Am J Physiol Heart Circ Physiol 277: H308–H317, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Mupparapu R, Vynck K, Svensson T, Burresi M, Wiersma DS. Path length enhancement in disordered media for increased absorption. Opt Express 23: A1472–A1484, 2015. doi: 10.1364/OE.23.0A1472. [DOI] [PubMed] [Google Scholar]
- 33.Oshino N, Sugano T, Oshino R, Chance B. Mitochondrial function under hypoxic conditions: the steady states of cytochrome alpha+alpha3 and their relation to mitochondrial energy states. Biochim Biophys Acta 368: 298–310, 1974. doi: 10.1016/0005-2728(74)90176-5. [DOI] [PubMed] [Google Scholar]
- 34.Phillips D, Aponte AM, Covian R, Neufeld E, Yu ZX, Balaban RS. Homogenous protein programming in the mammalian left and right ventricle free walls. Physiol Genomics 43: 1198–1206, 2011. doi: 10.1152/physiolgenomics.00121.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips D, Covian R, Aponte AM, Glancy B, Taylor JF, Chess D, Balaban RS. Regulation of oxidative phosphorylation complex activity: effects of tissue-specific metabolic stress within an allometric series and acute changes in workload. Am J Physiol Regul Integr Comp Physiol 302: R1034–R1048, 2012. doi: 10.1152/ajpregu.00596.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schenkman KA. Cardiac performance as a function of intracellular oxygen tension in buffer-perfused hearts. Am J Physiol Heart Circ Physiol 281: H2463–H2472, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Schenkman KA, Beard DA, Ciesielski WA, Feigl EO. Comparison of buffer and red blood cell perfusion of guinea pig heart oxygenation. Am J Physiol Heart Circ Physiol 285: H1819–H1825, 2003. doi: 10.1152/ajpheart.00383.2003. [DOI] [PubMed] [Google Scholar]
- 38.Schenkman KA, Marble DR, Burns DH, Feigl EO. Myoglobin oxygen dissociation by multiwavelength spectroscopy. J Appl Physiol 82: 86–92, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Segreti JA, Polakowski JS, Blomme EA, King AJ. Simultaneous measurement of arterial and left ventricular pressure in conscious freely moving rats by telemetry. J Pharmacol Toxicol Methods 79: 23–33, 2016. doi: 10.1016/j.vascn.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Shibata T, Matsumoto D, Nishimura R, Tai H, Matsuoka A, Nagao S, Matsuo T, Hirota S, Imai K, Neya S, Suzuki A, Yamamoto Y. Relationship between oxygen affinity and autoxidation of myoglobin. Inorg Chem 51: 11955–11960, 2012. doi: 10.1021/ic301848t. [DOI] [PubMed] [Google Scholar]
- 41.Stoner CD. Steady-state kinetics of the overall oxidative phosphorylation reaction in heart mitochondria. Determination of the coupling relationships between the respiratory reactions and miscellaneous observations concerning rate-limiting steps. J Bioenerg Biomembr 16: 115–141, 1984. doi: 10.1007/BF00743044. [DOI] [PubMed] [Google Scholar]
- 42.Suematsu Y, Miura S, Goto M, Matsuo Y, Arimura T, Kuwano T, Imaizumi S, Iwata A, Yahiro E, Saku K. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur J Heart Fail 18: 386–393, 2016. doi: 10.1002/ejhf.474. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi E, Doi K. Visualization of oxygen level inside a single cardiac myocyte. Am J Physiol 268: H2561–H2568, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Tamura M, Oshino N, Chance B, Silver IA. Optical measurements of intracellular oxygen concentration of rat heart in vitro. Arch Biochem Biophys 191: 8–22, 1978. doi: 10.1016/0003-9861(78)90062-0. [DOI] [PubMed] [Google Scholar]
- 45.Wilson DF, Harrison DK, Vinogradov A. Mitochondrial cytochrome c oxidase and control of energy metabolism: measurements in suspensions of isolated mitochondria. J Appl Physiol 117: 1424–1430, 2014. doi: 10.1152/japplphysiol.00736.2014. [DOI] [PubMed] [Google Scholar]
- 46.Wilson DF, Vinogradov SA. Mitochondrial cytochrome c oxidase: mechanism of action and role in regulating oxidative phosphorylation. J Appl Physiol 117: 1431–1439, 2014. doi: 10.1152/japplphysiol.00737.2014. [DOI] [PubMed] [Google Scholar]
- 47.Wright TJ, Davis RW. Myoglobin extraction from mammalian skeletal muscle and oxygen affinity determination under physiological conditions. Protein Expr Purif 107: 50–55, 2015. doi: 10.1016/j.pep.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Wright TJ, Davis RW. Myoglobin oxygen affinity in aquatic and terrestrial birds and mammals. J Exp Biol 218: 2180–2189, 2015. doi: 10.1242/jeb.119321. [DOI] [PubMed] [Google Scholar]
- 49.Zheng XX, Li XY, Lyu YN, He YY, Wan WG, Zhu HL, Jiang XJ. Possible mechanism by which renal sympathetic denervation improves left ventricular remodelling after myocardial infarction. Exp Physiol 101: 260–271, 2016. doi: 10.1113/EP085302. [DOI] [PubMed] [Google Scholar]