We determined the optimal conditions of puromycin incorporation in cardiac myocyte culture. We took advantage of this approach to identify the growth dynamics of cardiac myocytes in vitro. We went further to discover the protein synthesis rate in vivo, which provides novel insights about cardiac temporal growth dynamics in response to pressure overload.
Keywords: cardiac myocyte, pathological cardiac hypertrophy, pressure overload, puromycin, growth dynamics
Abstract
Hypertension is one of the most important risk factors of heart failure. In response to high blood pressure, the left ventricle manifests hypertrophic growth to ameliorate wall stress, which may progress into decompensation and trigger pathological cardiac remodeling. Despite the clinical importance, the temporal dynamics of pathological cardiac growth remain elusive. Here, we took advantage of the puromycin labeling approach to measure the relative rates of protein synthesis as a way to delineate the temporal regulation of cardiac hypertrophic growth. We first identified the optimal treatment conditions for puromycin in neonatal rat ventricular myocyte culture. We went on to demonstrate that myocyte growth reached its peak rate after 8–10 h of growth stimulation. At the in vivo level, with the use of an acute surgical model of pressure-overload stress, we observed the maximal growth rate to occur at day 7 after surgery. Moreover, RNA sequencing analysis supports that the most profound transcriptomic changes occur during the early phase of hypertrophic growth. Our results therefore suggest that cardiac myocytes mount an immediate growth response in reply to pressure overload followed by a gradual return to basal levels of protein synthesis, highlighting the temporal dynamics of pathological cardiac hypertrophic growth.
NEW & NOTEWORTHY We determined the optimal conditions of puromycin incorporation in cardiac myocyte culture. We took advantage of this approach to identify the growth dynamics of cardiac myocytes in vitro. We went further to discover the protein synthesis rate in vivo, which provides novel insights about cardiac temporal growth dynamics in response to pressure overload.
INTRODUCTION
Heart failure is a leading cause of mortality worldwide (4). In the United States, ~6 million people suffer from heart failure, which has created a tremendous healthcare and economic burden on our society. Heart failure occurs when the heart muscle is weakened and cannot pump sufficiently to meet the body’s needs for oxygen and blood (22). Clinical presentations include fatigue, shortness of breath, and fluid accumulation. Hypertension, currently affecting one-third of our population (23, 24), is one of the most prominent risk factors of heart failure (4). In response to high blood pressure, the heart manifests hypertrophic growth to ameliorate ventricular wall stress (9). This response is considered to be adaptive, with the increases in ventricular wall thickness helping to compensate for the increased load. This concentric hypertrophy may progress into symptomatic heart failure with a preserved ejection fraction and diastolic dysfunction. On the other hand, likely in the presence of a second stress, i.e., myocardial infarction, the left ventricular hypertrophy may deteriorate, and dilated heart failure may develop with dysfunctions at both systole and diastole (9). Despite the clinical importance and tremendous interests, our understanding of the mechanisms of hypertrophic growth and the signaling pathways governing the transition remain incomplete (1, 2, 9, 10, 20, 33).
Cardiac hypertrophic growth, in response to pressure overload, is highly dynamic (15). A combination of mechanical stress and growth factor signaling leads to activation of cellular growth, which combined with the Ca2+ signaling, metabolism, and inflammation, participates in the remodeling of the heart (13). Moreover, degradation processes, such as autophagy and the ubiquitin-proteasome system, play critical roles in reconstruction of cellular organelles and cytoskeleton (35, 37). Most of the studies on cardiac hypertrophic growth, however, examine late end points of the response to pressure overload for functional and molecular analysis. Our knowledge on the temporal dynamics of cardiac hypertrophy is therefore limited.
Puromycin is an aminonucleoside antibiotic that is a structural analog of aminoacyl tRNAs (8). Puromycin can enter the acceptor site of active ribosomes and be incorporated into the growing end of polypeptides, which terminate protein translation. The covalent link of puromycin to nascent polypeptide chains, when used at limiting amounts, provides a label to track newly synthesized proteins. Compared with the traditional means of incorporating radioactive-labeled amino acids (38), the puromycin method is advantageous for reproducibility, safety, and resolution (11). Indeed, this limited incorporation mechanism has been exploited to quantify protein synthesis rate and identify newly synthesized proteins under various conditions (11).
Here, we took advantage of the puromycin approach and set out to determine the temporal dynamics of cardiac hypertrophy in response to pressure overload at both in vitro and in vivo levels.
MATERIALS AND METHODS
Animals.
Mice of the C57BL/6 background were maintained on a 12:12-h dark-light cycle (6 AM to 6 PM) and housed in a group of no more than 4 mice/cage with unlimited access to water and chow (2916, Teklad, Envigo, Indianapolis, IN). The Institutional Animal Care and the Use Committee of the University of Texas Southwestern Medical Center approved all animal experiments.
Thoracic aortic constriction.
Aortic banding was performed according to standard procedures (14). Neither food nor water was withheld before surgery. Preanesthesia was achieved using ketamine (100 mg/kg ip) plus xylazine (5 mg/kg ip). Sterile instruments, supplies, and suture material were used. Intubation was achieved orally. A small incision in the anterior neck was made to reveal the trachea, facilitating cannulation of the upper airway. Respiratory rate, body temperature, and heart rate were monitored continuously during the procedure. The aortic arch was accessed via a left lateral thoracotomy, and the suture material (5-0 silk) was used to ligate the aorta (between the innominate and left carotid arteries) and an overlying 27-gauge needle. After ligation, the 27-gauge needle was removed immediately, leaving a discrete region of stenosis in the aorta. The chest was then closed, and animals were observed during the recovery from anesthesia.
Male mice at 8–12 wk old were randomized into two groups for sham and thoracic aortic constriction (TAC) surgery, respectively. After 2, 4, 7, 10, 14, and 21 days postsurgery, food was first removed for 2 h. Puromycin was administered intraperitoneally 30 min before euthanasia. Hearts were harvested, immediately frozen in liquid nitrogen, and stored at −80°C until use.
Neonatal rat ventricular myocyte isolation and culture.
Ventricles from 1- to 2-day-old Sprague-Dawley neonatal rats were collected and subjected to cardiomyocyte isolation using the neonatal rat/mouse cardiomyocyte isolation kit (NC-6031, Cellutron Life Technologies, Baltimore, MD). After clearance of fibroblasts, neonatal rat ventricular myocytes (NRVMs) were plated at a density of 1,250 cells/mm2 in medium with 5% FBS, 10% horse serum, and bromodeoxyuridine (100 μM).
After 24 h, NRVMs were incubated with 1% FBS medium (DMEM-medium 199 at 3:1) with bromodeoxyuridine. Twenty-four hours later, NRVMs were washed twice with 1× PBS, and the medium was changed to serum-free DMEM (high glucose)-medium 199 at 3:1. Cells were then treated by phenylephrine (PE; 50 μM). Puromycin was added 2 h before cells were harvested. To compare different hypertrophic stimuli, the following treatments were used: PE (50 μM), insulin-like growth factor-1 (10 nM), endothelin-1 (100 nM), 50% hyposmotic solution, and 3% FBS.
Cytotoxicity of puromycin in NRVMs was determined by a lactate dehydrogenase release assay with the CytoTox 96 nonradioactive cytotoxicity assay kit according to the manufacturer’s instructions (G1780, Promega, Madison, WI).
Radioactive measurement of protein synthesis.
NRVMs were cultured in serum-free medium with l-[3,4,5-3H]leucine (NET460A001MC, Perkin-Elmer, Billerica, MA) at a final concentration of 2 μCi/ml. PE treatment was then initiated. At the end of the treatment, cells were washed twice with 1× ice-cold PBS. Then, 2 ml trichloroacetic acid [TCA; 10% (wt/vol), LC262302, LabChem, Zelienople, PA] was added to each well of the six-well plates for 30 min with agitation followed by two washes with 95% ice-cold ethanol. NaOH (1 ml, 0.5 N) was then added to each well. The plate was sealed and incubated at 37°C for 6 h or overnight while shaken. An equal volume of HCl (1 ml, 0.5 N) was then added to neutralize the pH, and the entire content was transferred to scintillation vials followed by mixing with 18 ml scintillation solution (EcoLite, 882475, MP Biomedicals, Santa Ana, CA) for counting.
Monitor growth rate in adult mouse ventricular myocytes.
Adult mouse ventricular myocytes (AMVMs) were isolated from 6- to 8-wk-old C57BL/6 mice. AMVMs were plated in the six-well cell culture plate for 2 h. After two washes with 1× PBS, the medium was changed to serum-free MEM, 1% antibiotics, and 0.1% BSA and incubated for 1 h. AMVMs were then treated with PE (50 μM) for 24 h, and puromycin, at a final concentration of 1 μg/ml, was added 1 h before cells were harvested.
Immunoblot analysis.
When being harvested, NRVMs were washed once with ice-cold 1× PBS, and ice-cold radioimmunoprecipitation assay buffer (80 μl) supplemented with protease and phosphatase inhibitors (88669, ThermoFisher Scientific, Waltham, MA) was added to each well. Cells were then stored at −80°C. During processing, 5× SDS-PAGE loading buffer (20 μl) was added to each well, and cells were scraped on ice and transferred to tubes. Proteins were boiled for 10 min at 95°C, centrifuged briefly, and filtered by glass wool. Samples were then subjected to SDS-PAGE using Criterion gels (Bio-Rad Laboratories, Hercules, CA) followed by transfer to the nitrocellulose membrane. After being bloted, the membrane was scanned by an Odyssey scanner (LI-COR, Lincoln, NE). The following antibodies were used: puromycin (clone 12D10, MABE343, Millipore Sigma, Burlington, MA), GAPDH (10R-G109A, Fitzgerald, Acton, MA), regulator of calcineurin 1.4 (Rcan1.4; D6694, Millipore Sigma), β-myosin heavy chain (β-MHC; ab50967, Abcam, Cambridge, MA), goat anti-mouse secondary antibody Alexa fluor 680 (A21057, ThermoFisher Scientific), goat anti-mouse secondary antibody Alexa fluor 680 IgG2a (A31563, ThermoFisher Scientific), and goat anti-rabbit secondary antibody 800 CW (925-32211, LI-COR).
Total cardiac proteins were isolated from heart tissues using homogenizers. Approximately 30 mg tissues was used for each heart with 500 μl radioimmunoprecipitation assay buffer. After centrifugation at 14,000 rpm for 10 min, supernatants were collected for a bicinchoninic assay (Promega). Equal total proteins (30 μg) for each sample were loaded onto a Criterion gel and subjected to immunoblot analysis.
Immunofluorescence staining.
Glass coverslips were sterilized by ultraviolet exposure for 20 min and put into the 12-well plates. Gelatin (1 ml) was added for coating overnight. NRVMs were then cultured in the 12-well plates with coverslips. The treatment conditions were the same for six-well plates. For immunofluorescence staining, NRVMs were first washed twice with ice-cold 1× PBS and fixed by 4% paraformaldehyde for 30 min at 4°C. Cells were then washed once with ice-cold 1× PBS before permeabilization with 0.1% Triton X-100 in PBS on ice for 10 min. After being washed, NRVMs were blocked with 1.5% normal goat serum in 1× PBS with 1% BSA at room temperature for 30 min followed by incubation with primary antibodies in blocking buffer in a humid chamber at 4°C overnight. The next day, NRVMs were washed six times and incubated with secondary antibodies at room temperature for 1 h. After being washed, NRVMs were sealed with ProLong Gold Antifade Mountant (P36931, ThermoFisher Scientific) on tissue slides. The following antibodies were used: troponin I (1:100; H-170, Santa Cruz Biotechnology, Dallas, TX), puromycin (1:100; Millipore Sigma), goat anti-rabbit IgG Alexa fluor 488 (1:300, A32731, ThermoFisher Scientific), and goat anti-mouse IgG Alexa fluor 568 (1:300, A11004, ThermoFisher Scientific).
RNA-Seq analysis.
Total RNA was isolated from animal hearts at days 2, 4, 7, and 21 after sham or TAC surgery by an Aurum total RNA fatty and fibrous tissue kit (7326830, Bio-Rad Laboratories). The library was generated with an Ovation RNA sequencing kit (RNA-Seq, NuGEN, San Carlos, CA) and subjected for RNA-Seq analysis on a NextSeq 500 system (Illumina, San Diego, CA). Differential gene expression was detected by differential gene expression analysis (DESeq; cutoff: fold change ≥1.5, P < 0.05) and analyzed by gene set enrichment analysis (GSEA). The data are available in the Gene Expression Omnibus, GEO: GSE101977.
Statistical analysis.
Data are expressed as means ± SE. A Student’s t-test (two-tailed) was used for comparison between two groups. P < 0.05 was considered statistically significant.
RESULTS
Labeling cellular proteins with puromycin.
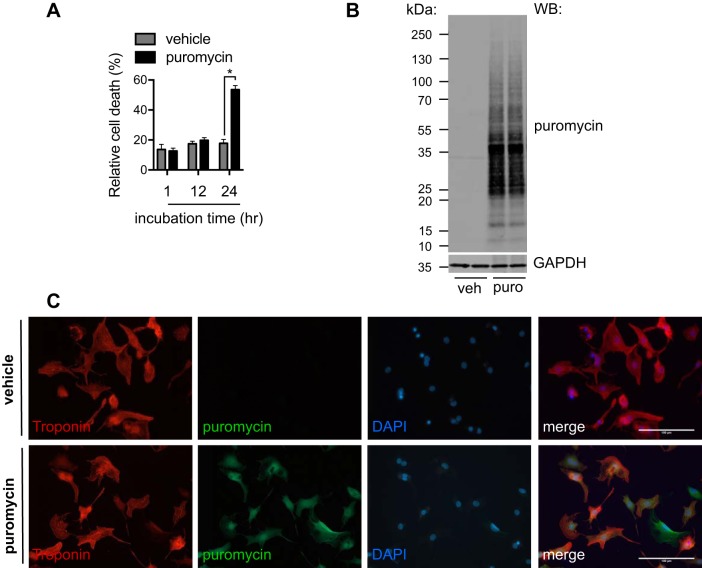
The temporal dynamics of hypertrophic growth in response to pressure overload remains an unanswered question. Puromycin can enter the accepting site of translating ribosomes (8), which provides a method to label nascent peptides universally and, when analyzed, quantify the protein synthesis rate at a given time. Puromycin treatment leads to translation inhibition, which may affect cell viability. We first tested the cytotoxicity of puromycin. We chose the primary culture of NRVMs from day 1- to day 2-born rats. We administered puromycin at 1 μg/ml for different times. We then collected culture medium and cells to measure the release of lactate dehydrogenase as an indication of cell death (36). We found that treatment of puromycin, for up to 12 h, did not affect cell viability (Fig. 1A), whereas cell death increased significantly after 24 h of incubation. We therefore conclude that short-term treatment of <12 h with puromycin does not interfere with cell survival.
Fig. 1.
Labeling cellular proteins by puromycin (puro). A: cytotoxic effects of puromycin treatment. Neonatal rat ventricular myocytes (NRVMs) were incubated with 1 μg/ml puromycin for different times. Relative cell death was determined by release of lactate dehydrogenase. Puromycin treatment was well tolerated until 24 h. B: the labeling of cellular proteins by puromycin. NRVMs were treated by puromycin for 6 h. Total proteins were isolated for immunoblot analysis [Western blot (WB)]. There was no detectable signal in vehicle (veh)-treated groups, highlighting the specificity of the puromycin method. C: immunofluorescence staining to confirm the incorporation of puromycin into NRVMs. The cells were similarly treated, and immunostaining for puromycin was conducted. Note that there was no puromycin signal in the vehicle-treated group. Troponin was used as a marker of cardiac myocytes. DAPI, 4′,6′-diamidino-2-phenylindole. Original scale bars = 100 μm. n = 3. *P < 0.05.
Puromycin, when conjugated to the COOH-terminal of nascent peptide chains, can be detected by immunoblot analysis with monoclonal antibody against puromycin (12D10) (28). We treated NRVMs with puromycin (1 μg/ml) for 6 h and isolated cell lysates for Western blot analysis. Puromycin-labeled proteins were readily detected compared with vehicle controls (Fig. 1B). Consistently, immunofluorescent staining showed a robust signal of puromycin in NRVMs (Fig. 1C).
Optimizing the incubation condition of puromycin.
We confirmed that puromycin could be easily detected in NRVMs, which may be exploited to quantify protein synthesis rate and cell growth. However, puromycin is a protein translation inhibitor, which may interfere with protein synthesis and cell growth. An optimal treatment regime is therefore needed to ensure detection sensitivity while not adversely affecting the growth response.
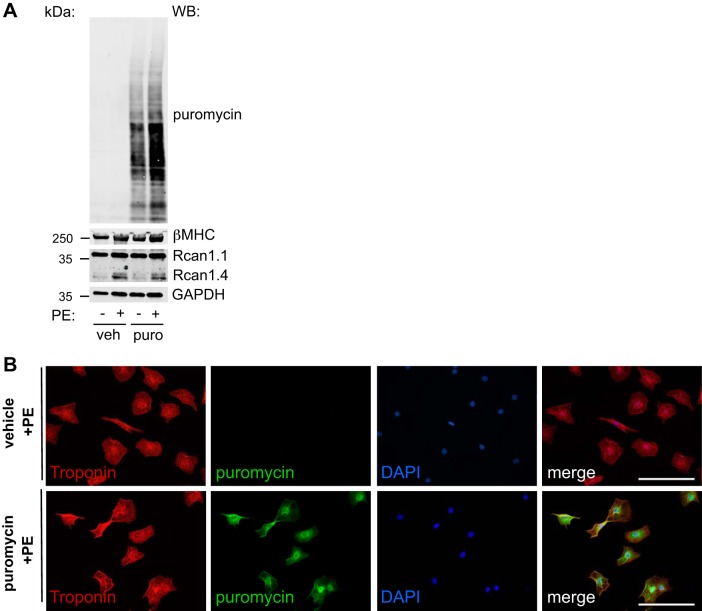
We first examined the incorporation of puromycin in NRVMs under hypertrophic growth conditions. We treated NRVMs with PE (50 μM) for 12 h in the presence or absence of puromycin. We found that PE treatment led to increases in puromycin incorporation (Fig. 2A). The induction of Rcan1.4 and β-MHC expression, markers of hypertrophy (5, 27, 34), was indistinguishable between vehicle- and puromycin-treated groups, suggesting that hypertrophic signaling was not affected by puromycin administration. These findings were confirmed by immunofluorescence staining (Fig. 2B).
Fig. 2.
Puromycin (puro) labeling under hypertrophic growth. A: hypertrophic growth in neonatal rat ventricular myocytes (NRVMs). Puromycin was introduced to monitor cell growth, which did not affect the induction of β-myosin heavy chain (β-MHC) or regulator of calcineurin 1.4 (Rcan1.4), two markers of hypertrophic growth. B: immunofluorescence staining showed incorporation of puromycin after phenylephrine (PE) treatment in NRVMs. WB, Western blot analysis; veh, vehicle; DAPI, 4′,6′-diamidino-2-phenylindole. Original scale bars = 100 μm.
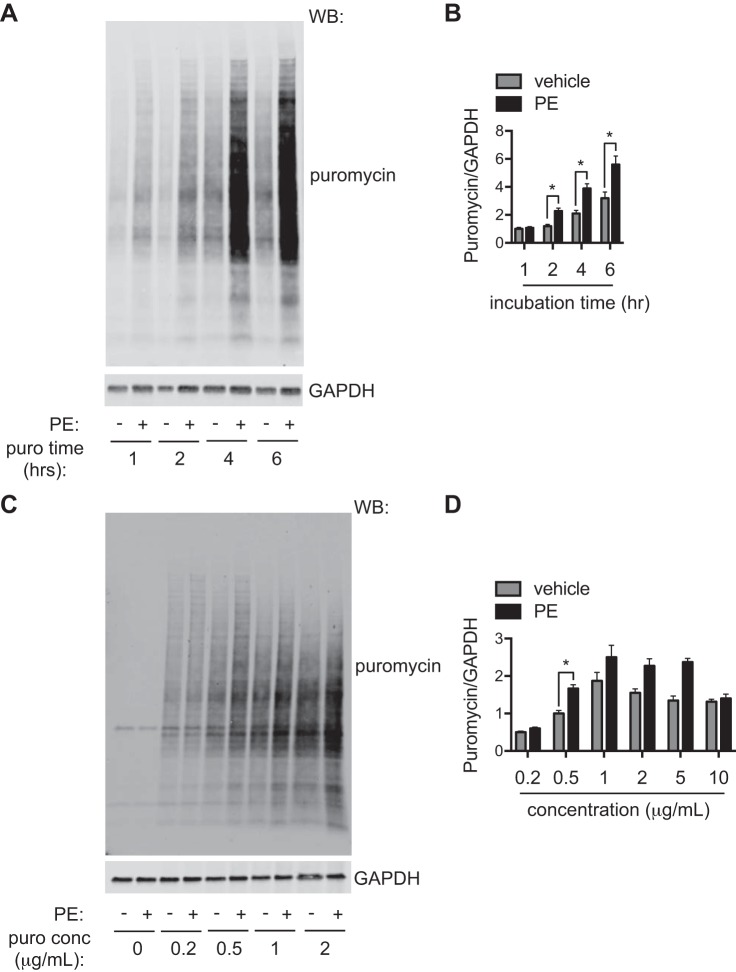
We then went on to determine the optimal incubation time of puromycin. We treated NRVMs with PE for 48 h. In the end of this treatment, we introduced puromycin (1 μg/ml) for 1, 2, 4, and 6 h. We found that the puromycin signal was proportionally elevated to the incubation time (Fig. 3A). However, longer incubation also led to increases in puromycin incorporation in the vehicle-treated groups. Furthermore, prolonged incubation of puromycin of >24 h led to suppression of protein synthesis (data not shown). We found that an incubation of 2 h showed the highest sensitivity (Fig. 3B).
Fig. 3.
Optimizing the conditions of puromycin (puro) labeling. A: optimizing the incubation time of puromycin. Phenylephrine (PE) was used to trigger hypertrophic growth in neonatal rat ventricular myocytes (NRVMs) for 48 h. At the end of the treatment, puromycin was introduced for 1, 2, 4, or 6 h. Immunoblot analysis was conducted to examine puromycin incorporation. B: puromycin inclusion for 2, 4, or 6 h showed significant increases in PE-treated groups compared with control samples. However, puromycin incorporation was increased in vehicle-treated samples after 4 and 6 h of incubation. We conclude that 2 h of puromycin administration is the most optimal incubation time. C: optimizing the concentration of puromycin. Various doses of puromycin were used at the end of PE treatment for 2 h. Western blot (WB) analysis was used to analyze puromycin incorporation. D: higher doses of puromycin led to increases in the puromycin signal. However, the basal incorporation rate was also elevated in vehicle-treated groups. We conclude that 0.5 μg/ml is the best dose to label cellular proteins. n = 6–9. *P < 0.05.
We next continued to examine the optimal concentration of puromycin. Similarly, we treated NRVMs with PE for 48 h. In the last 2 h of the treatment, we included different concentrations of puromycin in the culture. Cellular lysates were subjected to immunoblot analysis for puromycin incorporation. The puromycin signal intensity was upregulated proportionally along with the increase of concentration (Fig. 3C). At the highest concentration of 10 μg/ml, however, puromycin incorporation was not enhanced by PE treatment, which was probably due to translation inhibition by this concentration of puromycin. We found that 0.5 μg/ml puromycin showed the best signal-to-noise ratio (Fig. 3D). In aggregate, our results indicate that puromycin incorporation is an applicable method in NRVMs to quantify relative rates of protein synthesis and cellular hypertrophic growth and that 2 h of incubation at 0.5 μg/ml concentration represents the most optimal condition.
Direct comparison of puromycin incorporation and radioactive amino acid labeling methods.
Classical methods of measuring the increased protein synthesis indicative of hypertrophy rely on quantifying changes in incorporation of a radiolabeled amino acid over an incubation period of 24–48 h. The puromycin approach gives a snapshot of relative protein synthesis rates at the time tested. To compare directly this puromycin incorporation method with the classical radioactive amino acid labeling assay, we treated NRVMs with PE for 24 h. At the last 2 h of treatment, puromycin was introduced, and cell lysates were prepared for immunoblot analysis (Fig. 4A). For the radioactive assay, [3H]leucine was included in culture medium when PE treatment was initiated. Leucine uptake and incorporation were then determined by a scintillation counter (7). The puromycin method showed similar sensitivity compared with the conventional radioactive leucine assay (Fig. 4B), which supports the puromycin incorporation assay as a nonradioactive alternative to measure cellular hypertrophic growth.
Fig. 4.
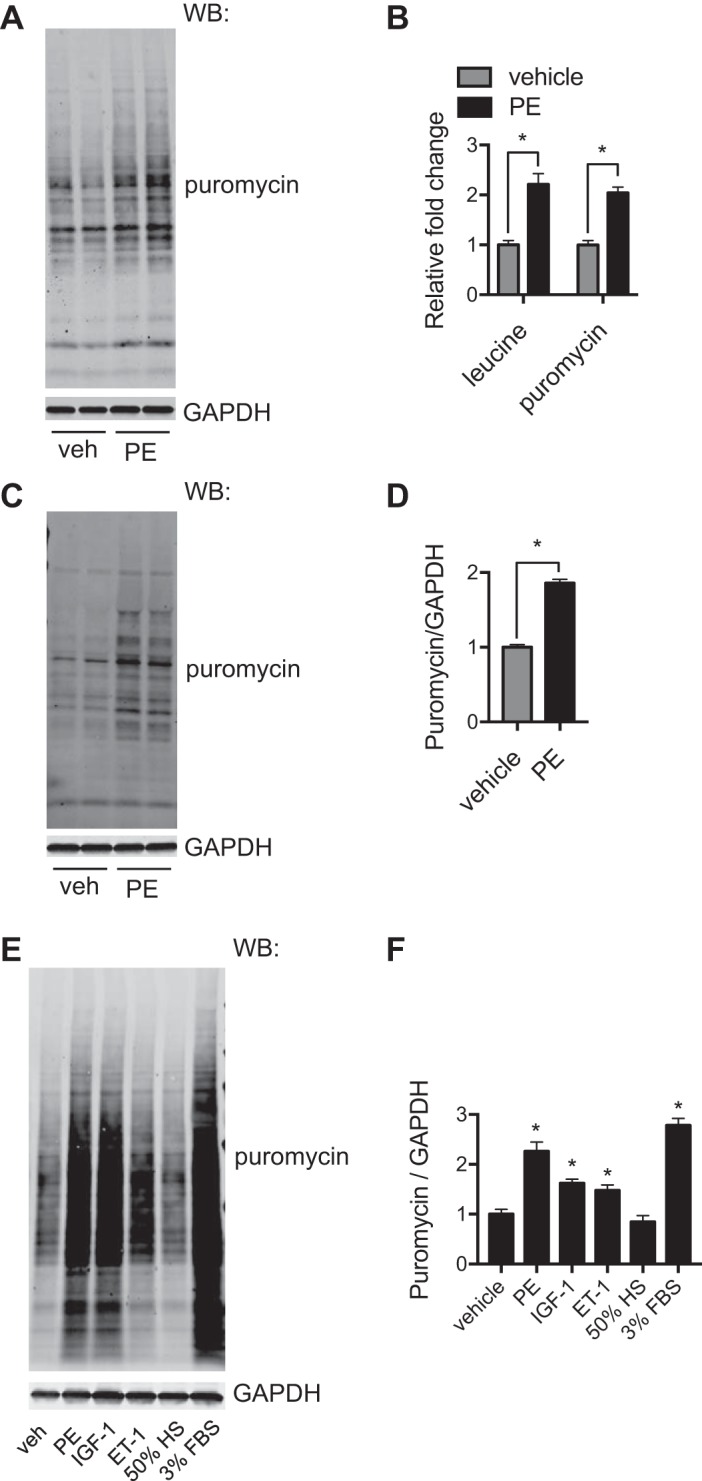
Comparison of the puromycin (puro) method with the radioactive amino acid labeling assay. A: puromycin incorporation under the hypertrophic growth condition. Neonatal rat ventricular myocytes (NRVMs) were treated with phenylephrine (PE) for 24 h. At the last 2 h, puromycin was included, and cellular lysates were subjected to immunoblot analysis. B: NRVMs with the same treatment condition were used for [3H]leucine labeling. Comparison between these two methods showed similar increases after PE treatment. We conclude that the puromycin method is equally sensitive compared with the conventional radioactive amino acid labeling approach. C: puromycin labeling was used to monitor hypertrophic growth in adult mouse ventricular myocytes (AMVMs). Here, AMVMs were treated by PE for 24 h. Puromycin was introduced at the last hour of treatment. Protein lysates were used for immunoblot analysis. D: quantification showed that PE induced significant hypertrophy in AMVMs. E: puromycin labeling was conducted to examine hypertrophic effects of different stimuli. NRVMs were subjected to various treatments and puromycin incorporation, which were later visualized by immunoblot analysis. F: quantification of the puromycin signal over controls showed significant increases in puromycin incorporation in most hypertrophic treatments. WB, Western blot analysis; veh, vehicle; IGF-1, insulin-like growth factor 1; ET-1, endothelin-1; HS, hyposmotic solution. n = 3–4/group. *P < 0.05.
We next took the puromycin approach to determine hypertrophic growth in adult cardiomyocytes. The primary culturing of adult murine cardiomyocytes is an important method to provide an in vitro approach to examine pathways of cardiac hypertrophy. The drawbacks are in the rapid loss of transverse tubule structures that define the adult myocyte structure that occurs within 24–48 h. To test if the puromycin assay could be sensitive enough with primary adult myocytes, mouse adult cardiomyocytes were isolated from 6- to 8-wk-old male C57BL/6 mice. PE treatment was conducted for 24 h. Puromycin was added 1 h before cells were harvested. Our results showed that PE led to a twofold increase of protein synthesis in adult cardiomyocytes compared with the elevation in NRVMs (Fig. 4, C and D).
Moreover, we used the puromycin incorporation method to compare cellular hypertrophic growth by different stimuli. NRVMs were treated under various conditions. Puromycin incorporation was quantified relative to vehicle controls (Fig. 4, E and F).
Temporal dynamics of hypertrophic growth in NRVMs.
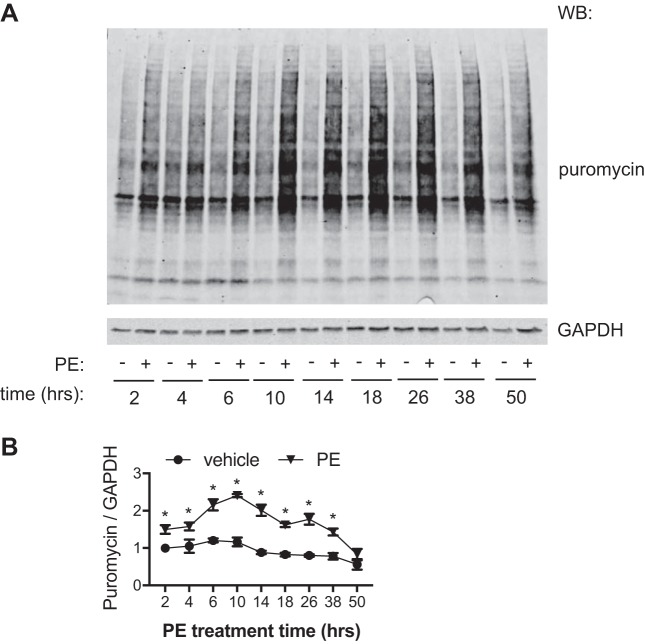
After validation of the puromycin incorporation approach, we then used it to determine the temporal dynamics of hypertrophic growth. Most studies use 24–48 h of treatment when examining hypertrophy and hypertrophic pathways in NRVMs. We wanted to understand how PE-induced changes in protein synthesis occur over this time of treatment. NRVMs were treated by PE for a range of 2–48 h. Puromycin was introduced at the last 2 h of PE treatment. Protein lysates were isolated and subjected for immunoblot analysis (Fig. 5A). We found that the hypertrophic growth rate was continuously increased in the early phase of PE treatment and peaked at 6–10 h. Over time, the protein synthesis rate gradually slowed down, returning to baseline by 48 h (Fig. 5B). The baseline protein synthesis rate (vehicle-treated groups) did not change significantly over the course of the assay. More importantly, the reduction in growth rate was not entirely due to metabolic elimination of PE, as replenishing PE at 12 h did not significantly increase growth rate measured at 24 h (data not shown). Collectively, these results indicate that the hypertrophic growth in response to growth stimulus reaches the peak ~10 h after treatment and gradually returns to the basal level.
Fig. 5.
Temporal dynamics of hypertrophic growth in neonatal rat ventricular myocytes (NRVMs). A: NRVMs were treated with phenylephrine (PE) for 48 h. At different times of the treatment, puromycin was added for 2 h. Cells were then harvested to examine the hypertrophic growth rate by puromycin incorporation. B: quantification showed that the growth rate triggered by PE reached peak at 10 h posttreatment. The growth then slowed down to basal levels at the end of the treatment. WB, Western blot analysis. n = 3–9 for each group. *P < 0.05 for PE-treated groups compared with the respective vehicle control groups.
Determining the pathological cardiac growth rate in vivo.
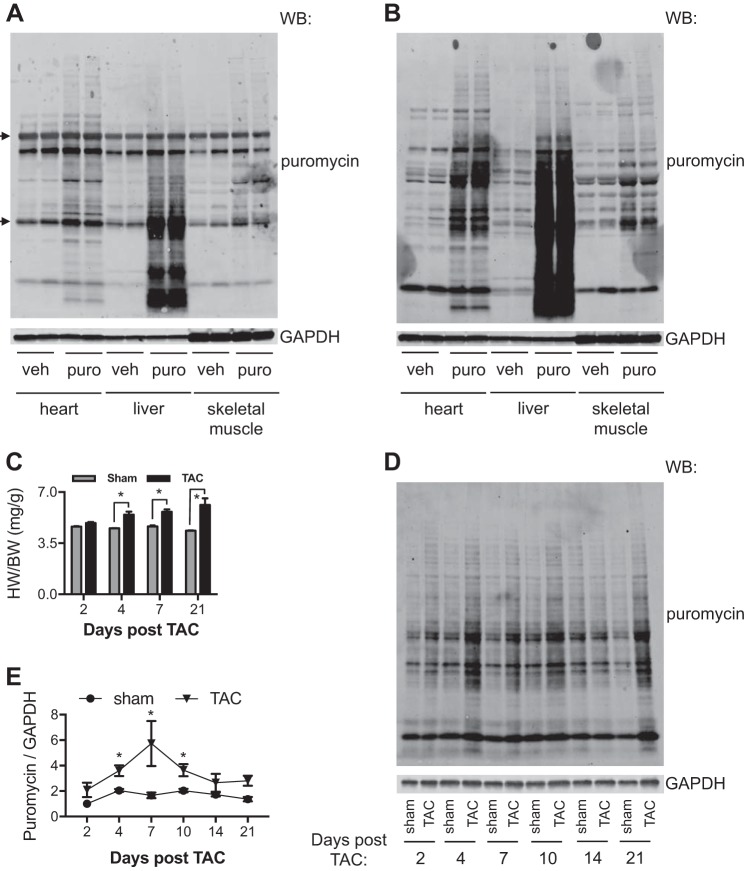
Cardiac hypertrophic growth in vivo is traditionally measured as changes in heart weight-to-body weight ratios and measurements of cellular cross-sectional area. Techniques for examining the temporal dynamics of pathological cardiac hypertrophic growth in vivo are less defined. We first validated the feasibility to detect puromycin incorporation in mice. We injected PBS control or puromycin solution to 8-wk-old C57BL/6 mice at 0.04 μmol/g body weight and harvested the heart, liver, and skeletal muscle 30 min later. This condition has been used before to examine protein synthesis in various organs (12, 18). Immunoblot analysis showed that puromycin was incorporated into these tissues. However, a strong nonspecific signal at 50 and 25 kDa was also detected (Fig. 6A). The primary antibody against puromycin was raised and purified from the mouse (28). These nonspecific bands may therefore be the endogenous mouse IgG heavy and light chains. To eliminate maximally the nonspecific signal, we chose IgG2a-specific secondary antibodies for immunoblot analysis, which only recognize the anti-puromycin monoclonal antibody (clone 12D10) (11). We found a robust and clean signal of puromycin in the heart and liver (Fig. 6B). We therefore decided to use this approach to determine the hypertrophic growth dynamics in vivo.
Fig. 6.
Pathological hypertrophic growth in vivo. A: detection of puromycin (puro) incorporation by immunoblot analysis was complicated by endogenous IgG. Puromycin was administered by intraperitoneal injection in C57BL/6 mice. Animal tissues were harvested for Western blot (WB) analysis. The secondary antibodies used also recognized endogenous IgG heavy chains and light chains (arrows). B: specific secondary antibodies against IgG2a, the same isotype of anti-puromycin monoclonal antibody, were used for immunoblot analysis, which showed significantly less background. C: thoracic aortic constriction (TAC) induced cardiac hypertrophy, as shown by increases in ratios of heart weight to body weight (HW/BW). D: temporal dynamics of cardiac hypertrophy was examined by puromycin immunoblot analysis. Puromycin was injected 30 min before euthanasia. Cardiac tissues were subjected to WB analysis to detect the hypertrophic growth rate. E: quantification showed that cardiac hypertrophic growth reached the peak rate at day 7 after TAC surgery. veh, vehicle. *P < 0.05.
We subjected wild-type C57BL/6 male mice at 8 wk of age to TAC to model pathological cardiac hypertrophy (14). We chose a 27-gauge needle to create regular hypertrophic growth and harvested hearts at different time points (27). Puromycin was injected 30 min before euthanasia. TAC triggered progressive cardiac hypertrophy, as shown by increases in heart weight-to-body weight ratios (Fig. 6C). Cardiac tissue lysates were then prepared for immunoblot analysis (Fig. 6D). We found that day 7 was the time of the highest relative increase in protein synthesis (Fig. 6E). The hypertrophic growth slowed down thereafter and reached baseline when approaching 3 wk postsurgery. Although the protein synthesis rate was decreased, the heart continued to grow in the presence of pressure overload, as shown by the higher heart weight-to-body weight ratio at day 21 compared with day 7.
Global analysis of gene expression during cardiac hypertrophic growth.
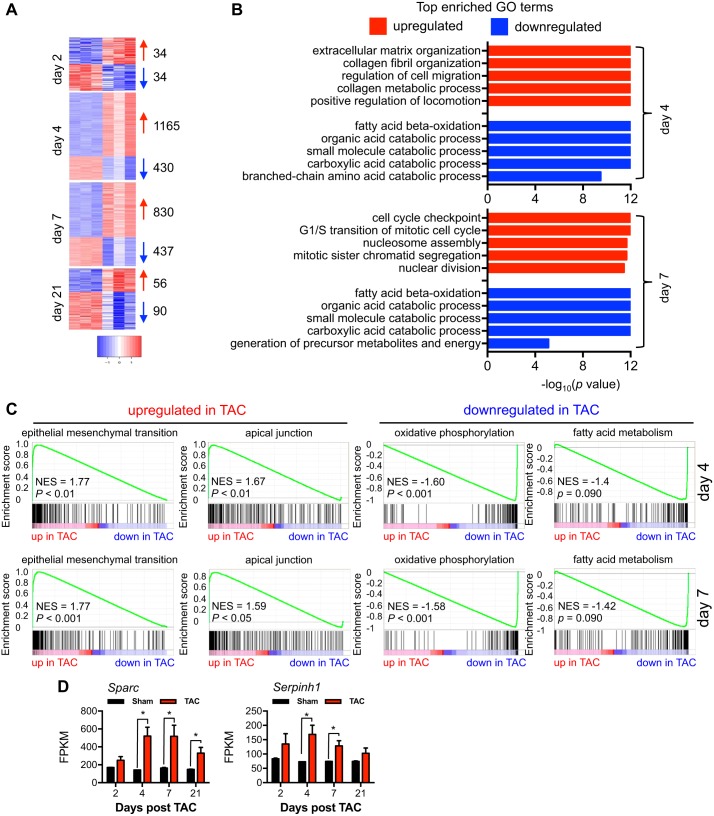
Transcriptional changes underlie the pathological response to pressure overload stress. To understand how these changes relate to the alterations in the relative rate of protein synthesis that we observed in TAC-treated hearts, we set out to investigate the global changes in gene expression during cardiac hypertrophic growth. We isolated total RNA from sham or TAC hearts of different times postsurgery. We then conducted a RNA-Seq experiment to analyze global transcriptomic changes. By DESeq, we observed that days 4 and 7 after TAC had more profound changes in gene expression, with 1,165 and 830 significantly upregulated genes and 430 and 437 significantly downregulated genes, respectively (Fig. 7A). These results were consistent with our findings that the temporal cardiac hypertrophic growth was immediately initiated (day 2), reached peak levels (days 4 and 7), and then slowed down (day 21). We then focused on days 4 and 7, the time with the highest growth rate. There was significant overlapping in pathways between these 2 days, based on top-enriched Gene Ontology (GO) analysis (Fig. 7B). The upregulated pathways consisted of processes in extracellular matrix protein synthesis and cell migration, indicating an active remodeling at this time. On the other hand, the downregulated pathways included mostly catabolic processes, which are consistent with an anabolic phenotype of cardiac hypertrophy. Moreover, by GSEA, we found that most significantly upregulated pathways were related to growth and remodeling, similar to findings by GO analysis (Fig. 7C). We chose two genes in the top upregulated pathways for further analysis. Secreted protein acidic and rich in cysteine (Sparc) is a cysteine-rich acidic protein associated with the matrix (6). Recent studies have shown that Sparc participates in collagen synthesis and deposition and fibrosis in aging-related cardiomyopathy (31) and after myocardial infarction (21). Serine-arginine protease inhibitor peptidase inhibitor, clade H, member 1 (Serpinh1) is an endoplasmic reticulum resident chaperone involved in collagen processing and secretion (16). Here, we found that gene expression of both Sparc and Serpinh1 followed the temporal dynamics of cardiac hypertrophy with peak levels at days 4 and 7 (Fig. 7D), suggesting that fibrosis may play an important role in cardiac remodeling in response to pressure overload.
Fig. 7.
Global analysis of gene expression during cardiac hypertrophic growth. A: RNA-Seq analysis showed that days 4 and 7 were the times with most profound transcriptomic changes. Numbers of genes with significant changes are shown at right. B: the top 5 most enriched Gene Ontology (GO) pathways are shown for both days 4 and 7 after thoracic aortic constriction (TAC). C: based on gene set enrichment analysis analysis, the top up- or downregulated pathways at days 4 and 7 are shown. NES, normalized enrichment score. D: gene expression of secreted protein acidic and rich in cysteine (Sparc) and Serine-arginine protease inhibitor peptidase inhibitor, clade H, member 1 (Serpinh1), two proteins involved in extracellular matrix remodeling and fibrosis, showed a similar temporal dynamics as cardiac hypertrophic growth. FPKM, fragments per kilobases per million fragments. n = 3 for each group. *P < 0.05.
DISCUSSION
Hypertension is one of the two most prominent risk factors of heart failure (4). In the presence of high blood pressure, ventricular wall stress is greatly elevated. The heart responds by increases in cardiac mass to ameliorate wall stress and maintain normal pumping function (9). This once-adaptive process may progress into decompensation and eventually heart failure. However, our understanding of the temporal dynamics remains rather incomplete. Here, we comprehensively characterized the puromycin incorporation method to determine relative changes in protein synthesis at both in vitro and in vivo levels. We validated that brief puromycin administration does not affect the growth response in cardiomyocytes. We went further to demonstrate that cardiac myocyte growth represents a dynamic process. In response to the hypertrophic growth stimulus, cardiomyocytes mount significant growth, which quickly reaches the peak level and returns to baseline. Indeed, at the molecular level, the early phase of hypertrophic growth shows the most genes with significant changes. Our results therefore support a dynamic model of cardiomyocyte growth in response to pressure overload.
Accumulating evidence strongly suggests that pathological cardiac hypertrophy is a precursor of heart failure (9, 26). The understanding of the underlying mechanisms therefore has the potential to provide novel insights and pave a way for future therapeutic gain. Over the past decades, numerous pathways and signaling molecules have been identified that play critical roles in the hypertrophic growth (13). These have undoubtedly contributed to our current knowledge of pathological cardiac remodeling. However, cardiac hypertrophy is a dynamic process. Hypertrophic stimuli may trigger distinct responses in the early phase of growth compared with the later time. The majority of studies for pressure overload have focused on the functional and molecular aspects in the final phase of pathological cardiac hypertrophy. Our understanding of the temporal dynamics is therefore limited. To address this, novel approaches to monitor protein synthesis and cell growth are urgently required.
Puromycin is a tRNA analog derived from the Streptomyces alboniger bacterium (8). Puromycin partially resembles the 3′-end of the aminoacylated tRNA that can enter the A site of translating ribosomes. As a consequence, puromycin can cause the formation of a puromycylated nascent chain, premature translational termination, and chain release. Recently, this important feature has been exploited to label growing polypeptides, monitor protein synthesis, and identify nascent proteins (11). Pierre and colleagues (28) developed the surface sensing of translation (SUnSET) approach to quantify protein synthesis rate as a technical alternative to the classical radioactive-labeling method. Aviner et al. (3) went further to take advantage of puromycin to label and precipitate nascent chains for proteomics using cells or frozen tissues. Furthermore, fluorescent puromycin conjugates have been widely adopted to detect protein expression in real time, when combined with flow cytometry and immunostaining (18, 30). Schuman and colleagues (32) even coupled the puromycin labeling with the proximity ligation assay to visualize directly newly synthesized target proteins in situ. Compared with the traditional radioactive amino acid incorporation method, the puromycin strategy is reproducible, safe, user friendly, and versatile, with a potential to become widely used under various physiological and pathological conditions. Furthermore, the puromycin approach may be more accurate, since it only requires a very short time of incubation, which therefore likely provides a more precise snapshot of the protein synthesis rate compared with the conventional radioactive amino acid incorporation method. Stable isotope labeling with amino acids in cell culture (SILAC) is a simple metabolic labeling approach to quantify proteomic changes based on mass spectrometry (25). This method relies on incorporation of nonradioactive amino acids of different isotopes into proteins. Since the labeling does not affect cellular reaction to stimuli, cell cultures from the heavy and light media are mixed and subjected to quantitative proteomics by mass spectrometry. Compared with traditional approaches, SILAC is more accurate, with widespread applications (17). Furthermore, SILAC can also be adapted to identify differentially expressed proteins. Despite these, the puromycin incorporation method is advantageous, since it is simpler and less expensive with easy downstream detection and quantification applications, such as Western blot analysis and immunohistochemistry.
Treatment of NRVMs with PE to induce hypertrophic growth is a classic model to study hypertrophic responses in vitro. Since the pulse time for the puromycin treatment is 2 h, the increased incorporation gives a snapshot of new protein synthesis within that time period. Our findings demonstrate that there is an immediate hypertrophic response within 2 h of PE treatment. We have also uncovered an additional burst of protein synthesis that occurs 6–10 h after treatment. After that time, new protein synthesis begins to decrease until it returns to baseline at 48 h. This decrease may represent a new normalization as the cell responds to the hypertrophic stimulus; likewise, it may also reflect the metabolism of the PE within the cell culture system. However, our results do not support the latter possibility. We provided fresh PE at 12 h of treatment and measured growth rate at 24 h. The puromycin incorporation only showed a trend of increase compared with nonreplenished samples at the same time point. Regardless, these data provide new insights into appropriate time points to examine the hypertrophic pathways.
The ability to measure changes in relative rates of protein synthesis in vitro provides us insight to the timeline of the increases in protein synthesis that drives hypertrophic growth. Our findings suggest that the peak of new protein synthesis, from 4 to 7 days, correlates with the reported increases in hypertrophic growth of the heart. They also mimic what was observed in vitro, with an initial early increase, peaking at a later time and then moving back to baseline. This increase in protein synthesis rates correlates with the RNA-Seq results. Indeed, days 4 and 7 are the time points of the most dramatic transcriptomic changes, with more upregulation than downregulation. The GO term analysis indicates that most of the upregulated pathways belong to extracellular remodeling and cell growth, whereas catabolic pathways are reduced. Interestingly, the fatty acid β-oxidation process is suppressed, which is consistent with the metabolic alterations during cardiac hypertrophic growth (29). These results also suggest that metabolic remodeling is an early event, which may play a causal role in this process (19). Importantly, the GSEA analysis shows similar results as the GO enrichments, with the top-regulated pathways related to tissue remodeling and cellular metabolism, which lends more support to the notion that days 4–7 are the critical phase of pathological cardiac remodeling in response to pressure overload.
Previous work with this animal model of pressure overload-induced hypertrophy has demonstrated that hypertrophic growth persists in these hearts moving from the thickening of the ventricular walls, as in the early response measured here, to an even greater increase in heart size as the walls thin, and the heart becomes decompensated. Further work will examine the relative rates in protein synthesis during this transition from compensated to decompensated cardiac hypertrophy.
In conclusion, to delineate the temporal dynamics of pathological cardiac hypertrophy, we thoroughly characterized the optimal treatment conditions of puromycin in cardiac myocytes and in hypertrophic rodent models. Our results strongly suggest that cardiac hypertrophy follows a dynamic growth pattern, which may provide the basis for future mechanistic studies to identify new players and discover novel pathways to tackle hypertensive heart disease.
GRANTS
Support for this work was provided by American Heart Association Scientist Development Grant 14SDG18440002 and Innovative Research Grant 17IRG33460191 and American Diabetes Association Innovative Basic Science Award 1-17-IBS-120.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.W. and Z.V.W. conceived and designed research; Y.W., G.D., H.I.M., and J.X. performed experiments; Y.W., Y.Z., G.D., and J.X. analyzed data; Y.W., J.X., T.G.G., H.W., and Z.V.W. interpreted results of experiments; Y.W., Y.Z., G.D., and Z.V.W. prepared figures; Y.W. and Z.V.W. drafted manuscript; J.X., T.G.G., H.W., and Z.V.W. edited and revised manuscript; Y.W., Y.Z., G.D., H.I.M., J.X., T.G.G., H.W., and Z.V.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank members of the Z. V. Wang laboratory for valuable discussions. The authors also thank the Molecular Pathology Core (John Shelton) for help with histology.
REFERENCES
- 1.Akazawa H, Komuro I. Roles of cardiac transcription factors in cardiac hypertrophy. Circ Res 92: 1079–1088, 2003. doi: 10.1161/01.RES.0000072977.86706.23. [DOI] [PubMed] [Google Scholar]
- 2.Ardehali H, Sabbah HN, Burke MA, Sarma S, Liu PP, Cleland JG, Maggioni A, Fonarow GC, Abel ED, Campia U, Gheorghiade M. Targeting myocardial substrate metabolism in heart failure: potential for new therapies. Eur J Heart Fail 14: 120–129, 2012. doi: 10.1093/eurjhf/hfr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aviner R, Geiger T, Elroy-Stein O. Genome-wide identification and quantification of protein synthesis in cultured cells and whole tissues by puromycin-associated nascent chain proteomics (PUNCH-P). Nat Protoc 9: 751–760, 2014. doi: 10.1038/nprot.2014.051. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics−2017 update: a report from the American Heart Association. Circulation 135: e146–e603, 2017. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry JM, Le V, Rotter D, Battiprolu PK, Grinsfelder B, Tannous P, Burchfield JS, Czubryt M, Backs J, Olson EN, Rothermel BA, Hill JA. Reversibility of adverse, calcineurin-dependent cardiac remodeling. Circ Res 109: 407–417, 2011. doi: 10.1161/CIRCRESAHA.110.228452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradshaw AD. The role of secreted protein acidic and rich in cysteine (SPARC) in cardiac repair and fibrosis: does expression of SPARC by macrophages influence outcomes? J Mol Cell Cardiol 93: 156–161, 2016. doi: 10.1016/j.yjmcc.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Cao DJ, Wang ZV, Battiprolu PK, Jiang N, Morales CR, Kong Y, Rothermel BA, Gillette TG, Hill JA. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc Natl Acad Sci USA 108: 4123–4128, 2011. doi: 10.1073/pnas.1015081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darken MA. Puromycin inhibition of protein synthesis. Pharmacol Rev 16: 223–243, 1964. [PubMed] [Google Scholar]
- 9.Drazner MH. The progression of hypertensive heart disease. Circulation 123: 327–334, 2011. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 10.Glembotski CC. Endoplasmic reticulum stress in the heart. Circ Res 101: 975–984, 2007. doi: 10.1161/CIRCRESAHA.107.161273. [DOI] [PubMed] [Google Scholar]
- 11.Goodman CA, Hornberger TA. Measuring protein synthesis with SUnSET: a valid alternative to traditional techniques? Exerc Sport Sci Rev 41: 107–115, 2013. doi: 10.1097/JES.0b013e3182798a95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman CA, Mabrey DM, Frey JW, Miu MH, Schmidt EK, Pierre P, Hornberger TA. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J 25: 1028–1039, 2011. doi: 10.1096/fj.10-168799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589–600, 2006. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 14.Hill JA, Karimi M, Kutschke W, Davisson RL, Zimmerman K, Wang Z, Kerber RE, Weiss RM. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation 101: 2863–2869, 2000. doi: 10.1161/01.CIR.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 15.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med 358: 1370–1380, 2008. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 16.Ito S, Nagata K. Biology of Hsp47 (Serpin H1), a collagen-specific molecular chaperone. Semin Cell Dev Biol 62: 142–151, 2017. doi: 10.1016/j.semcdb.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Kasumov T, Dabkowski ER, Shekar KC, Li L, Ribeiro RF Jr, Walsh K, Previs SF, Sadygov RG, Willard B, Stanley WC. Assessment of cardiac proteome dynamics with heavy water: slower protein synthesis rates in interfibrillar than subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol 304: H1201–H1214, 2013. doi: 10.1152/ajpheart.00933.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Xu Y, Stoleru D, Salic A. Imaging protein synthesis in cells and tissues with an alkyne analog of puromycin. Proc Natl Acad Sci USA 109: 413–418, 2012. doi: 10.1073/pnas.1111561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 90: 207–258, 2010. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 20.Maejima Y, Chen Y, Isobe M, Gustafsson AB, Kitsis RN, Sadoshima J. Recent progress in research on molecular mechanisms of autophagy in the heart. Am J Physiol Heart Circ Physiol 308: H259–H268, 2015. doi: 10.1152/ajpheart.00711.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCurdy SM, Dai Q, Zhang J, Zamilpa R, Ramirez TA, Dayah T, Nguyen N, Jin YF, Bradshaw AD, Lindsey ML. SPARC mediates early extracellular matrix remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol 301: H497–H505, 2011. doi: 10.1152/ajpheart.01070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neubauer S. The failing heart—an engine out of fuel. N Engl J Med 356: 1140–1151, 2007. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 23.Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief 1–8, 2013. [PubMed] [Google Scholar]
- 24.Ong KL, Tso AW, Lam KS, Cheung BM. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension 51: 1142–1148, 2008. doi: 10.1161/HYPERTENSIONAHA.107.105205. [DOI] [PubMed] [Google Scholar]
- 25.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics 1: 376–386, 2002. doi: 10.1074/mcp.M200025-MCP200. [DOI] [PubMed] [Google Scholar]
- 26.Roger VL. Epidemiology of heart failure. Circ Res 113: 646–659, 2013. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothermel BA, Berenji K, Tannous P, Kutschke W, Dey A, Nolan B, Yoo KD, Demetroulis E, Gimbel M, Cabuay B, Karimi M, Hill JA. Differential activation of stress-response signaling in load-induced cardiac hypertrophy and failure. Physiol Genomics 23: 18–27, 2005. doi: 10.1152/physiolgenomics.00061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt EK, Clavarino G, Ceppi M, Pierre P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods 6: 275–277, 2009. doi: 10.1038/nmeth.1314. [DOI] [PubMed] [Google Scholar]
- 29.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 30.Starck SR, Green HM, Alberola-Ila J, Roberts RW. A general approach to detect protein expression in vivo using fluorescent puromycin conjugates. Chem Biol 11: 999–1008, 2004. doi: 10.1016/j.chembiol.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Toba H, de Castro Brás LE, Baicu CF, Zile MR, Lindsey ML, Bradshaw AD. Increased ADAMTS1 mediates SPARC-dependent collagen deposition in the aging myocardium. Am J Physiol Endocrinol Metab 310: E1027–E1035, 2016. doi: 10.1152/ajpendo.00040.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.tom Dieck S, Kochen L, Hanus C, Heumüller M, Bartnik I, Nassim-Assir B, Merk K, Mosler T, Garg S, Bunse S, Tirrell DA, Schuman EM. Direct visualization of newly synthesized target proteins in situ. Nat Methods 12: 411–414, 2015. doi: 10.1038/nmeth.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toral M, Romero M, Pérez-Vizcaíno F, Duarte J, Jiménez R. Antihypertensive effects of peroxisome proliferator-activated receptor-β/δ activation. Am J Physiol Heart Circ Physiol 312: H189–H200, 2017. doi: 10.1152/ajpheart.00155.2016. [DOI] [PubMed] [Google Scholar]
- 34.Vega RB, Rothermel BA, Weinheimer CJ, Kovacs A, Naseem RH, Bassel-Duby R, Williams RS, Olson EN. Dual roles of modulatory calcineurin-interacting protein 1 in cardiac hypertrophy. Proc Natl Acad Sci USA 100: 669–674, 2003. doi: 10.1073/pnas.0237225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Robbins J. Proteasomal and lysosomal protein degradation and heart disease. J Mol Cell Cardiol 71: 16–24, 2014. doi: 10.1016/j.yjmcc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang ZV, Deng Y, Gao N, Pedrozo Z, Li DL, Morales CR, Criollo A, Luo X, Tan W, Jiang N, Lehrman MA, Rothermel BA, Lee AH, Lavandero S, Mammen PPA, Ferdous A, Gillette TG, Scherer PE, Hill JA. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell 156: 1179–1192, 2014. doi: 10.1016/j.cell.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang ZV, Rothermel BA, Hill JA. Autophagy in hypertensive heart disease. J Biol Chem 285: 8509–8514, 2010. doi: 10.1074/jbc.R109.025023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. Hoboken, NJ: Wiley-Liss, 2005. [Google Scholar]