Abstract
Background
Why resistance to specific antibiotics emerges and spreads rapidly in some bacteria confronting these drugs but not others remains a mystery. Resistance to erythromycin in the respiratory pathogens Staphylococcus aureus and Streptococcus pneumoniae emerged rapidly and increased problematically. However, resistance is uncommon amongst the classic Bordetella species despite infections being treated with this macrolide for decades.
Objectives
We examined whether the apparent progenitor of the classic Bordetella spp., Bordetella bronchiseptica, is able to rapidly generate de novo resistance to antibiotics and, if so, why such resistance might not persist and propagate.
Methods
Independent strains of B. bronchiseptica resistant to erythromycin were generated in vitro by successively passaging them in increasing subinhibitory concentrations of this macrolide. Resistant mutants obtained were evaluated for their capacity to infect mice, and for other virulence properties including adherence, cytotoxicity and induction of cytokines.
Results
B. bronchiseptica rapidly developed stable and persistent antibiotic resistance de novo. Unlike the previously reported trade-off in fitness, multiple independent resistant mutants were not defective in their rates of growth in vitro but were consistently defective in colonizing mice and lost a variety of virulence phenotypes. These changes rendered them avirulent but phenotypically similar to the previously described growth phase associated with the ability to survive in soil, water and/or other extra-mammalian environments.
Conclusions
These observations raise the possibility that antibiotic resistance in some organisms results in trade-offs that are not quantifiable in routine measures of general fitness such as growth in vitro, but are pronounced in various aspects of infection in the natural host.
Introduction
On exposure to sublethal levels of antibiotics, some bacteria are known to readily develop antibiotic resistance de novo.1,2 Once resistance has emerged it can spread and amplify especially rapidly in antibiotic-treated hosts that are relatively free of natural competitors. Thus, we observe outbreaks of resistance in environments in which antibiotic use is prevalent, such as amongst patients and staff in hospitals and amongst farm animals mass treated with antibiotics.3,4 The potential speed and progress of this process is demonstrated by the case of erythromycin, the prototypical macrolide. Erythromycin binds 23S rRNA, inhibiting protein synthesis by affecting the exit of nascent peptides,5 and was hailed as a wonder drug. But within a few years of its first commercial use in 1952, reports of erythromycin-resistant Staphylococcus aureus and Streptococcus pneumoniae surfaced.6 Since then, resistant strains have increased in prevalence and dispersed globally, contributing to a worldwide problem of increasing concern.6,7
The highly publicized pattern by which antibiotic resistance rapidly evolves and disseminates8 has been followed by several important pathogens, but not by others. The Bordetella species are one of the most prominent of these exceptions. Although erythromycin has been the drug of choice for the treatment of whooping cough (pertussis) for decades,9,10 compared with the wide prevalence of resistant S. aureus or S. pneumoniae, large-scale resistance in Bordetella pertussis has been historically rare and continues to be so with the exception of a more recent outbreak reported in northern China.11,12 Similarly, the apparent progenitor of the classic Bordetella spp., Bordetella bronchiseptica,13 which causes respiratory infections in a wide range of mammals (including humans) and birds and is similarly erythromycin treated en masse, has been and largely remains susceptible to erythromycin, demonstrating a counter-example to the rapid rise of erythromycin resistance in Staphylococcus, and suggesting that the relative failure to rapidly evolve and disperse resistance is common to, and may be related to shared characteristics of, these species.14
Although the observation that some pathogens rapidly evolve resistance to antibiotics and disseminate widely has been extensively studied as a worrisome example of evolution in real time,15 the fact that other pathogens do not has garnered less attention. One compelling explanation has been that there is a trade-off: de novo acquired resistance is linked to a loss of fitness, measured as a decreased growth rate in vitro.16 However, the generality of this trade-off is speculative and its relevance to the specific case of the Bordetella spp. is unknown.
Therefore, the low prevalence of resistance among Bordetella spp. remains enigmatic. From in vitro culture in the presence of increasing sub-MIC concentrations of erythromycin we observed that B. bronchiseptica can rapidly evolve resistance to this macrolide. As measured by their growth rates in vitro, no fitness costs were detected among independent resistant strains isolated in this way, and the high levels of resistance of these strains were maintained through multiple passages (many generations) in the absence of erythromycin. By examining their various characteristics in vitro and in vivo we observed that erythromycin-resistant strains were severely defective in a variety of virulence-associated characteristics. All of these erythromycin-resistant strains were defective in their expression of virulence-associated antigens, and all failed to successfully colonize and persist in the respiratory tracts of their hosts. These results suggest that erythromycin resistance can be readily generated de novo by Bordetella spp. but at a cost to their ability to infect their animal hosts.
Materials and methods
Microbiology
Growth and culture of bacteria
Liquid cultures of B. bronchiseptica strain RB50 and derivative mutants RB54 and RB50i were grown in Stainer Scholte medium (SS medium) supplemented with 0.5% (w/v) heptakis(2,6-di-O-methyl)-β-cyclodextrin (Sigma H0513). Liquid cultures were plated on Bordet Gengou (BG) agar supplemented with 10% (v/v) defibrinated sheep blood (Hemstat, Hemostat Laboratories, USA) and streptomycin (20 mg/L).
Development of resistance and MIC determination
Development of erythromycin resistance in B. bronchiseptica RB50 was assessed starting with four replicate cultures (lineages) of ∼104 cfu of RB50 grown in 96-well microtitre plates with 200 μL of SS medium per well and 2-fold diluted gradients of erythromycin (0.625–20 mg/L). Bacteria were incubated statically at 37°C for 2 days in a humidified incubator with 5% CO2. Growth was assessed by OD600 readings using a microtitre plate reader. A 10 μL portion of each bacterial culture replicate exceeding an OD600 value of 0.5 was then used to seed a fresh erythromycin-gradient microtitre plate containing 190 μL of SS medium per well. Growth in the second plate [passage 2 (p2)] was recorded after 2 days and further passaged. Erythromycin concentration gradients for later passages were increased to accommodate growing resistance. MIC ranges of erythromycin (Sigma PHR1039) for B. bronchiseptica [RB50 (WT), RB54, RB50i and antibiotic-resistant strains] were conducted in microtitre plates using 100 μL of SS medium containing 2-fold dilutions of the antibiotic, at the appropriate concentration ranges. Inhibitory ranges were estimated by identifying the two concentrations of antibiotic between which bacterial cells showed the largest decrease in OD600 after 20 h of growth at 37°C.
Mouse experiments
Mouse experiments were performed following recommendations from the Guide for the Care and Use of Laboratory Animals of the NIH. Protocols were approved by the Institutional Animal Care and Use Committee at the University of Georgia, Athens, GA, USA (A2016 02-010-Y2-A3 ‘Bordetella–Host Interactions’). Mice were anaesthetized using 5% isoflurane and euthanized by carbon dioxide inhalation followed by cervical dislocation. Experiments were conducted on 5-week-old female C57BL/6 mice obtained from the Jackson Laboratory, Bar Harbor, ME, USA; for the duration of the experiments, the mice were housed in groups of three or four in home cages at the Specific Pathogen Free facilities of the Central Animal Facility, University of Georgia.
Inoculation and harvesting
Mice were inoculated intranasally by pipetting 5 μL of PBS containing 150 cfu (1× standard dose) of bacteria into the nares of mice lightly anaesthetized with isoflurane/oxygen. To test colonization by the antibiotic-resistant mutants, 1500 cfu (10×) and 15 000 cfu (100×) of bacteria were used to inoculate groups of three or four mice. Bacterial numbers in the inoculum were validated by dilution plating on BG agar plates. At indicated timepoints, nasal cavities, trachea and lungs excised from the mice were homogenized in PBS using a tissue disruptor (BeadMill24, Thermo Scientific). Samples were then serially diluted (10-fold) in PBS, plated on BG agar with streptomycin and incubated at 37°C for 2 days for bacterial enumeration. To obtain serum, blood was collected by cardiac puncture 30 days post-inoculation and centrifuged at 3000 rpm for 20 min, and serum was collected and stored at –20˚C until further use.
In vivo complementation
Groups of four mice were inoculated with ∼500 cfu of either RB50 or an erythromycin-resistant clone from lineage 1, p6 (EmR1 p6), or a 1: 1 mixture of the two (∼250 cfu each). At 7 days post-inoculation the ability of the EmR1 p6 strain to colonize the nasal cavity was assessed by plating the nasal cavity homogenates on BG agar plates with and without erythromycin (20 mg/L). Erythromycin concentrations were chosen to differentiate between the WT and p6 resistant mutant colonies.
In vitro experiments
Cytotoxicity
The cytotoxicity of WT and mutant bacteria against mouse RAW 264.7 cells (moi 10:1) was tested using the CytoTox Nonradioactive Cytotoxicity Assay Kit (Promega) following supplied protocols. Cytotoxicity was recorded as the percentage activity of lactate dehydrogenase release compared with that observed from fully lysed cells.
Adherence
Adherence of bacteria to human lung epithelial cell line A549 (ATCC) was assessed in 24-well plates at an moi of 10:1 (bacteria:cell). Plates were centrifuged at 300 g for 10 min to synchronize exposure of the cells to bacteria. Following incubation for 5 min at 37°C, the supernatant was aspirated, and the epithelial cells washed (four times) with 1 mL of PBS. Cells were then lysed with 0.1% sodium deoxycholate. Adherent bacteria associated with cells were enumerated by dilution plating on BG agar.
Cytokine assays
RAW 264.7 cells were cultured in RPMI 1640 (Gibco, Life Technologies) supplemented with 10% FBS (Gibco, Life Technologies) and 100 U/mL penicillin/streptomycin (Gibco, Life Technologies) to 90% confluency. Macrophages were washed twice with PBS and fresh medium was added. B. bronchiseptica strain RB50 grown in SS medium to the mid-log phase, as well as the four strains isolated from p1, were added to the cells at an moi of 100. At 4 h post-inoculation, TNF-α and IFN-γ were measured using a DuoSet ELISA system (R&D Systems) following the manufacturer’s protocols.
RT–PCR
Transcript expression of virulent-phase genes (cyaA and prn) and non-virulent-phase genes (cheZ and flhD) was analysed using RNA extracted from mid-log phase cultures grown by shaking at 200 rpm at 37°C in SS medium (virulence-inducing conditions). Expression levels were calculated with the ΔΔCt method using recA for normalization and B. bronchiseptica RB50 as the reference. Data were analysed using DataAssistTM version 3.0 (Applied Biosystems).
Statistical analysis
Statistical analyses of differences between the WT and mutant groups were performed using Student’s t-test of significance, with P < 0.05 considered to be statistically significant.
Results
B. bronchiseptica rapidly develops high levels of antibiotic resistance
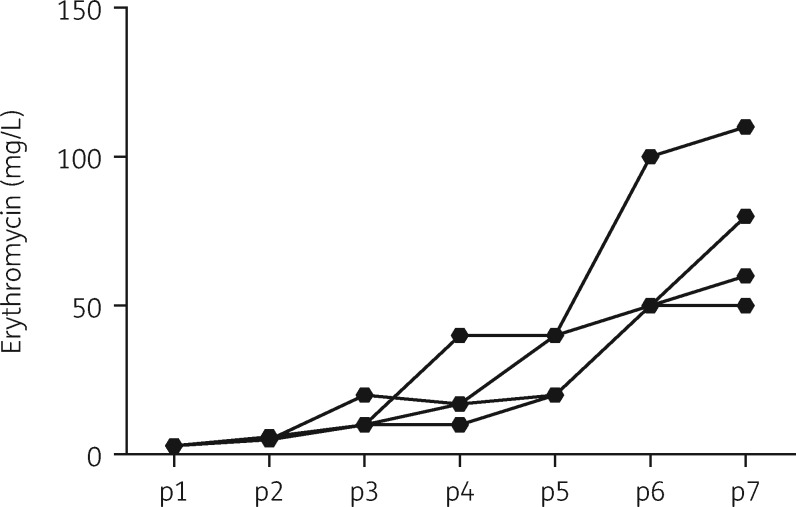
To examine if the low reported incidence of erythromycin-resistant B. bronchiseptica was due to an inherently low ability to develop resistance de novo, we exposed erythromycin-susceptible B. bronchiseptica RB50 (WT strain, isolated from a rabbit) to gradually increasing concentrations of erythromycin. We started with four independent cultures to establish four antibiotic-resistant lineages (L1–L4) by serially transferring bacteria that resisted the antibiotic to fresh medium with increasing concentrations of antibiotic (see the Materials and methods section). Our results showed that with each transfer (passage), B. bronchiseptica increased its resistance to erythromycin (Figure 1). Estimated MIC ranges demonstrated corresponding increases between the passages (Table 1). Resistance continued to rise to much higher levels over five further passages (data not shown).
Figure 1.
Increase in erythromycin resistance of B. bronchiseptica RB50 in vitro. The graph traces the average increase in resistance to erythromycin (OD600 ≥0.5) of four lineages of B. bronchiseptica RB50 serially passaged (p1–p7) in the presence of increasing concentrations of the antibiotic. Standard deviation bars are removed for clarity.
Table 1.
MIC ranges of erythromycin-resistant mutants
| Lineage | Erythromycin MIC values (mg/L) |
||
|---|---|---|---|
| p1 | p3 | p6 | |
| L1 | 2.5–5 | 10–20 | 70–80 |
| L2 | 2.5–5 | 30–40 | 90–100 |
| L3 | 2.5–5 | 10–20 | 90–100 |
| L4 | 2.5–5 | 10–20 | 90–100 |
MIC ranges for randomly chosen B. bronchiseptica colonies from p1, p3 and p6 isolated from four independent antibiotic-resistant lineages (L1–L4).
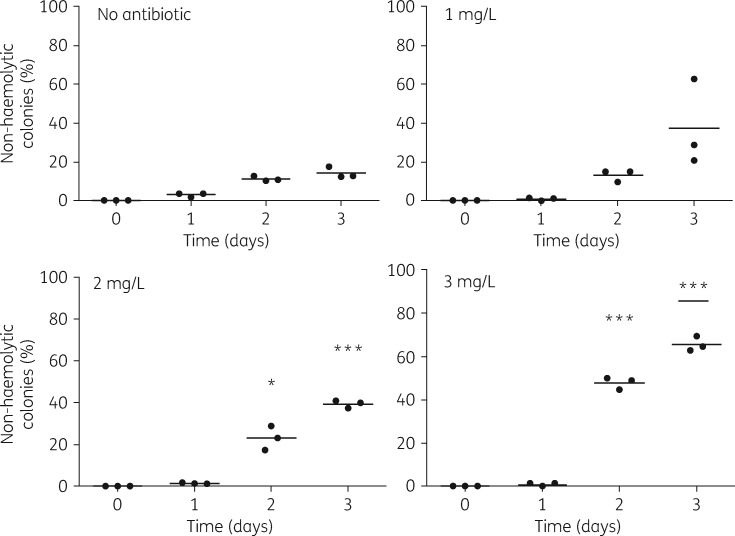
The four independent lineages displayed modest variability in their resistance levels, which is likely a reflection of variation in the specific nature of mutations from which their resistances are derived. The resistant clones were all genetically stable, with the mutants retaining their respective resistance thresholds even after several passages without the antibiotic (data not shown). Growth of resistant bacteria on BG blood agar at 37°C revealed that all four lineages of antibiotic-resistant mutants lacked the characteristic haemolytic zone of clearance that normally surrounds WT bacterial colonies. Non-haemolytic colonies are known to arise spontaneously among WT cultures of B. bronchiseptica at frequencies of ∼1/10 000 cfu.17 To assess the contribution of erythromycin exposure to the recovery of non-haemolytic colonies, we grew liquid cultures of WT bacteria in the presence of sublethal amounts of erythromycin (0, 1, 2 or 3 mg/L). After plating the bacteria on three consecutive days of growth at 37°C, we observed a significant dose-dependent increase in numbers of non-haemolytic colonies for 2 and 3 mg/L starting from the second day (Figure 2).
Figure 2.
Formation of non-haemolytic mutants on exposure to erythromycin. The graphs depict the percentage of non-haemolytic colonies detected over time in triplicate cultures of B. bronchiseptica RB50 grown at 37°C (200 rpm) in SS medium with 0, 1, 2 and 3 mg/L erythromycin. The numbers of non-haemolytic and haemolytic colonies were separately noted to calculate the percentage of non-haemolytic colonies detected. As the graphs show, the percentage of non-haemolytic colonies increased incrementally in response to the antibiotic concentrations. Student’s t-test significance values were determined by comparing the percentage of non-haemolytic colonies observed with antibiotics on each day with corresponding percentage values observed without antibiotics. *P < 0.05; ***P < 0.0001 (P values of <0.05 were considered statistically significant).
We tested whether the haemolytic phenotype of the passaged erythromycin-resistant mutants would be restored via reversion or compensatory mutations by serially transferring the mutants on plates without antibiotic. Five consecutive passages without antibiotic failed to restore haemolysis for any of the lineages, indicating that the phenotype was stable (data not shown).
Erythromycin-resistant mutants are deficient in colonizing mice
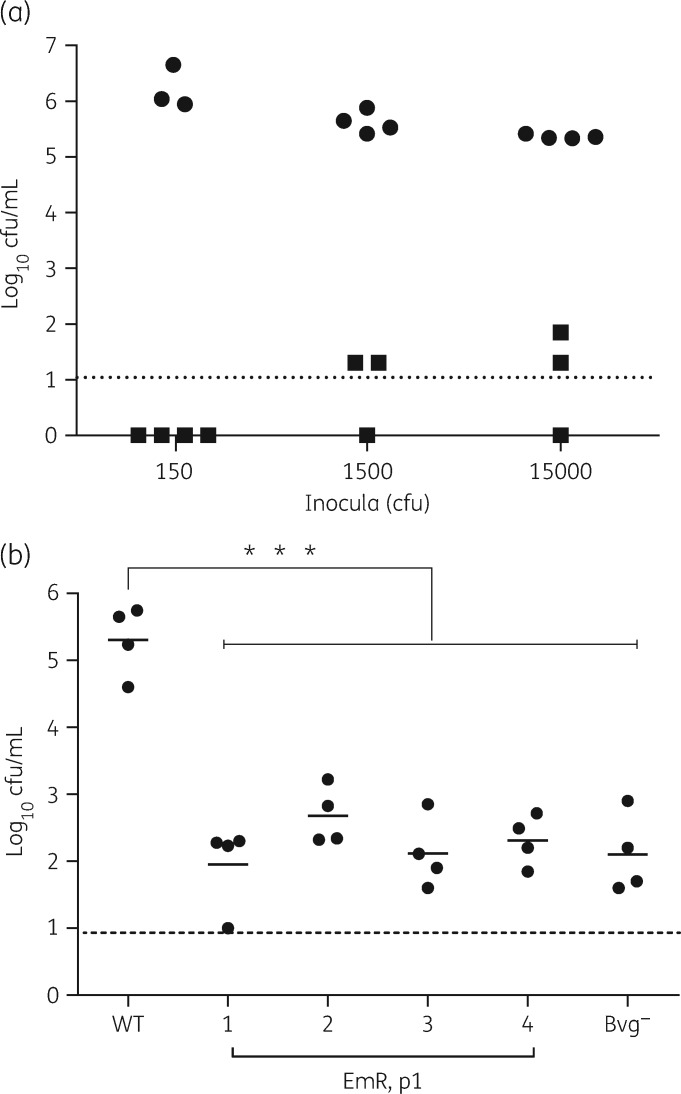
The in vitro haemolysis of blood by B. bronchiseptica is mediated by adenylate cyclase toxin (ACT), one of the several virulence factors18,19 under the control of a major virulence regulator of Bordetella, the BvgAS system.20 The BvgAS system senses ambient conditions and induces a virulent (Bvg+), intermediate (Bvgi) or avirulent (Bvg–) phase in the bacteria. Whole-cell antigenic profiles of the antibiotic-resistant mutants from different lineages were similar to each other and to a previously described avirulent Δbvg mutant derivative (RB54),21 although differences between the latter and the resistant mutants were also evident (Figure S1, available as Supplementary data at JAC Online), raising the question of how these differences would affect colonization and growth in mice. We examined the ability of a resistant clone (EmR1) from the first passage (p1) to infect mice by inoculating groups with 150, 1500 or 15 000 cfu by delivery to the external nares in 5 μL of PBS. The results showed that WT bacteria successfully colonized mice even when inoculated with as few as 150 cfu, while the mutants were barely detectable even when mice were inoculated with 15 000 cfu (100 times more) (Figure 3a). We further compared the colonization efficiencies of randomly chosen clones from all the four lineages of p1 mutants with RB54 in mice inoculated with a high dose of 15 000 cfu of each strain. As expected, WT bacteria grew in numbers ∼10-fold higher than inoculation levels. In contrast, the Bvg– mutant and EmR strains colonized the mice at levels 100- to 1000-fold lower than WT, indicating that the severity of the colonization defect in all four antibiotic-resistant lineages was similar to the severe defect of the Bvg– mutant (Figure 3b). Using growth as an index of general fitness, we tested whether reduced fitness of the mutants could explain the failure to efficiently colonize mice. However, we observed that the resistant mutants grew more vigorously than the WT strain, excluding this possibility (Figure S2).
Figure 3.
Antibiotic-resistant mutants fail to colonize the host. (a) Colonization of nasal cavities of C57BL/6 mice 7 days after being inoculated with 150, 1500 or 15 000 cfu of B. bronchiseptica RB50 WT (circles) or the p1 antibiotic-resistant mutant EmR1 (squares). The dotted line represents the limit of detection. (b) Colonization profiles of B. bronchiseptica WT (RB50), antibiotic-resistant p1 mutants (EmR1–EmR4) and Bvg– (RB54) in mouse nasal cavities 7 days after being inoculated with 15 000 cfu of each strain. ***P < 0.0001 (P values of <0.05 were considered statistically significant). The dotted line represents the limit of detection.
Antibiotic-resistant mutants have decreased virulence
The ability of B. bronchiseptica to colonize mice is dependent on its ability to coordinate the activity of many virulence factors to mediate its interaction with the host. We tested the mutants for any alterations to several established phenotypes in vitro. We noted differences in two critical phenotypes associated with in vivo virulence (Figure 4a and b). First, in vitro cytotoxicity of B. bronchiseptica directed against mammalian cells is closely associated with its virulence.22 We observed a significant decrease (P < 0.001) in the cytotoxicity of the mutants compared with the WT when tested against mouse RAW 264.7 macrophage-like cells. Second, the mutants exhibited reduced adherence (P < 0.01) towards cultured human lung epithelial cells (A549), adherence being an important phenotype required for establishing colonization of the respiratory tract and immunomodulation.23,24 We further noted a significantly reduced ability of the mutants to induce the pro-inflammatory cytokine TNF-α and modestly reduced amounts of IFN-γ from RAW 264.7 cells (Figure 4c). Together, these observations suggest that the antibiotic-resistant mutants have lost their robust virulence phenotype.
Figure 4.
In vitro phenotype comparisons of B. bronchiseptica RB50 and erythromycin-resistant mutant derivatives. (a) Cytotoxicity of B. bronchiseptica RB50 WT and p1 resistant mutants against RAW 264.7 macrophages. Relative percentage cytotoxicity of WT and mutant bacteria against cultured RAW 264.7 mouse macrophages exposed to the bacterial strains for 4 h at 37°C (moi 10:1). The bars represent the mean ± SEM (n = 3). (b) Adherence of WT B. bronchiseptica RB50 and mutant bacteria EmR1 and Δfha to A459 human lung epithelial cells. (c) Induction of cytokines from RAW 264.7 mouse macrophages by B. bronchiseptica and antibiotic-resistant mutants from p1. The bars represent the mean ± SEM (n = 3). *P < 0.05; **P < 0.001; ***P < 0.0001 (P values of <0.05 were considered statistically significant).
Interestingly, although the average MIC range for the resistant strains in the early passages falls within the same range as that for RB50 (Figure 1) but lower than that for RB54 (5–10 mg/L), continued exposure to the antibiotic further increased the MIC values, indicating that in vitro resistance increases independent of the ability to colonize mice. The MIC range of RB50 Bvgi, a mutant strain25 that exhibits a phenotype intermediate to the Bvg+ and Bvg– phases, was observed to fall between 1.25 and 2.5 mg/L, lower than the p1 resistant strains and WT.
Antibiotic-resistant mutants resemble an engineered bvgS mutant
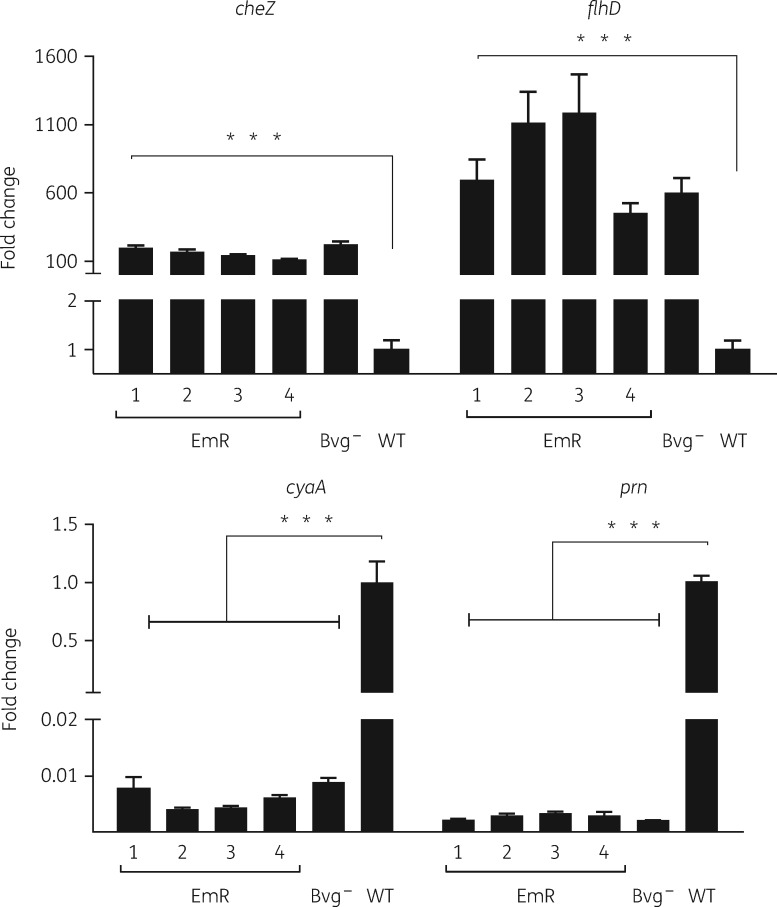
The loss of the ability to colonize mice along with the loss of virulence-associated phenotypes in the mutants closely resembles the avirulent phenotype of B. bronchiseptica in the Bvg– state.26 To evaluate this similarity, we compared EmR mutants with a B. bronchiseptica mutant (strain RB54) in which the bvgS gene is deleted, locking the mutant in the Bvg– state. We examined the expression profiles of the WT and EmR and Bvg– phase-locked bacterial mutants grown at 37°C in liquid medium for genes expressed in the Bvg+ phase [cyaA (adenylate cyclase toxin) and prn (pertactin)] and Bvg– phase [cheZ (chemotactin) and flhD (flagella)].27 WT RB50 repressed transcription of cheZ and flhD but induced expression of cyaA and prn, whereas the bvgS deletion strain RB54 showed the reverse pattern. All four EmR mutants resembled the bvgS mutant, rather than the WT, with reduced expression of virulence genes and higher expression of motility/chemotaxis genes (Figure 5). These results demonstrate a pattern of altered expression of virulence genes that consistently accompanies increased antibiotic resistance in these four independent lineages, which may explain their defects in mouse infections.
Figure 5.
Comparison of WT B. bronchiseptica RB50 and Bvg– phase-locked and antibiotic-resistant mutants. RT–PCR analysis of genes induced in Bvg– [cheZ (chemotactin) and flhD (flagella)] and Bvg+ [cyaA (adenylate cyclase toxin) and prn (pertactin)] phases of growth in RB50 WT, RB54 (Bvg– phase locked) and EmR1 p1 antibiotic-resistant mutant growing at 37°C. The bars represent the mean ± SEM (n = 3). ***P < 0.0001 (P values of <0.05 were considered statistically significant).
Overall, these results indicate that in the course of antibiotic exposure and development of resistance, B. bronchiseptica attains a phenotype similar, though not identical, to the Bvg– phase.
WT bacteria fail to complement mutants for host colonization
Since many of the known virulence factors, such as ACT, in Bordetella species are secreted,28 WT bacteria could potentially complement such defects, allowing the resistant mutants to colonize and spread among their hosts. To test this hypothesis, we inoculated separate groups of mice with 500 cfu of WT RB50, the EmR1-resistant mutant from p6, or a 1:1 mixed population of the two. At 7 days post-inoculation, we found that WT bacteria had colonized the nasal cavity at the expected levels; however, no erythromycin-resistant bacteria were detected, indicating that the presence of WT bacteria did not facilitate the colonization of the resistant mutant (Figure 6). Bacterial colonization levels in the trachea were ∼1000-fold lower than in the nasal cavity, but here too no antibiotic-resistant mutants could be detected. Similarly, the lungs, which had low and inconsistent levels of colonization, showed no resistant mutants. These results indicate that production of factors such as ACT by the WT bacteria did not correct the colonization deficiency of the antibiotic-resistant mutant, suggesting a more complex defect, and indicating that emerging resistant mutants would be unlikely to survive in their host when they arise in response to antibiotic treatment.
Figure 6.
WT B. bronchiseptica RB50 are unable to complement an antibiotic-resistant mutant. The graphs show the number of bacteria recovered from nasal cavities from separate groups of mice 7 days after being inoculated with 500 cfu of RB50 WT, the EmR1 erythromycin-resistant mutant from p6, or a 1:1 mixed population of WT and mutant (250 cfu each). Bacteria recovered from mixed-population inoculations were plated on plates either without (–) or with (+) antibiotic. The dotted lines represent the limit of detection.
Discussion
Although erythromycin has been used extensively in veterinary and medical practice for treating Bordetella spp. infections for decades, the frequency of erythromycin resistance among Bordetella spp. is conspicuously low. Here, we have investigated erythromycin resistance in B. bronchiseptica, a species that, though having close phylogenetic links to the human-restricted B. pertussis, has a broad host range infecting various wild and domesticated animals. In vitro experiments show that B. bronchiseptica readily acquires resistance when exposed to subinhibitory levels of erythromycin. However, the resistant strains that arose were severely deficient in colonizing mice even when inoculated with 15 000 cfu, 100-fold above our standard inoculation dose. All four independent lineages of the mutants that we investigated were similar to the Bvg– phase-locked B. bronchiseptica mutant (RB54), which lacks the ability to switch to the virulent Bvg+ phase required for host colonization. Deficient host colonization would likely impede transmission and spread of antibiotic-resistant bacteria in a population.
The association between acquisition of antibiotic resistance and loss of virulence has been described in other pathogens in vitro29 and is typically ascribed to a fitness cost. Such costs are believed to be overcome by compensatory mutations that restore fitness, antibiotic susceptibility or virulence.30–32 However, we observed no loss of resistance and we did not observe the reversion of the haemolytic phenotype among the B. bronchiseptica mutants even after several passages without antibiotics. In addition, the resistant mutants grew robustly in liquid medium, indicating that no obvious fitness cost accompanied the acquisition of resistance.
Growth in the presence of low concentrations of erythromycin has been previously reported to induce resistance in S. aureus,33–36 attaining MIC levels up to 30-fold higher than for uninduced bacteria.7 However, such high ‘induced resistance’37 is typically associated with horizontally acquired elements and characteristically decreases once the antibiotic is removed.33 Studies conducted on B. pertussis exposed to erythromycin also reported reversible changes between virulent and avirulent phases, along with concomitant increases in resistance.38 However, the resistance observed in our study is clonally preserved, retained in the absence of antibiotics and likely associated with genomic changes. Finally, the recent observation that B. bronchiseptica has an alternate and independent life cycle associated with amoebae39,40 raises the possibility that infections coming from an environmental source could be having a diluting and buffering effect on the animal-to-animal transmission. Such an effect would affect the rapid spread of antibiotic-resistant B. bronchiseptica arising from animals treated with antibiotics.
The processes by which resistance arises and spreads in some pathogens but not in others remain enigmatic. Although the previously proposed trade-off in fitness may partly explain the lack of resistance in other organisms, our results suggest something else might be going on in Bordetella spp. These organisms have recently been shown to have environmental sources including soil and water.39,41 In evolutionary terms, these are the environments in which chemicals similar to antibiotics are likely to be encountered. It may be that Bordetella spp. are relatively well adapted to survive such chemical threats, but that resistance is associated with expression of other factors that promote survival in soil and water but are detrimental during respiratory tract infection. Ectopic expression of Bvg– phase genes during infection has previously been shown to be sufficient to disrupt the infectious process.42 For Bordetella spp., and perhaps other pathogens, the acquisition of antibiotic resistance may not be accompanied by loss of fitness, as measured by in vitro growth, but by an altered gene expression pattern associated with surviving antibiotics in their evolutionary context. Thus, antibiotic resistance arises rapidly, but leads consistently to a dead-end lineage that cannot propagate and transmit amongst mammalian hosts.
Supplementary Material
Acknowledgements
We thank the members of the Central Animal Facility, College of Veterinary Medicine, University of Georgia for providing the Specific Pathogen Free facilities for working with mice used in this study.
Funding
This work was supported by the National Institutes of Health (grant numbers GM083113, AI1107016, AI116186 and GM113681 to E. T. H.) and in part by the National Institute of Allergy and Infectious Diseases (contract number HHSN272200900007C to E. T. H.) and the National Institute of General Medical Science (grant number GM19875 to B. R. L.).
Transparency declarations
None to declare.
Supplementary data
Figures S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. Gullberg E, Cao S, Berg OG. et al. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog 2011; 7: e1002158.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kohanski MA, DePristo MA, Collins JJ.. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol Cell 2010; 37: 311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pendleton JN, Gorman SP, Gilmore BF.. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther 2013; 11: 297–308. [DOI] [PubMed] [Google Scholar]
- 4. Tacconelli E, Carrara E, Savoldi A. et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18: 318–27. [DOI] [PubMed] [Google Scholar]
- 5. Kannan K, Kanabar P, Schryer D. et al. The general mode of translation inhibition by macrolide antibiotics. Proc Natl Acad Sci USA 2014; 111: 15958–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arthur M, Brisson-Noel A, Courvalin P.. Origin and evolution of genes specifying resistance to macrolide, lincosamide and streptogramin antibiotics: data and hypotheses. J Antimicrob Chemother 1987; 20: 783–802. [DOI] [PubMed] [Google Scholar]
- 7. Nicola FG, McDougal LK, Biddle JW. et al. Characterization of erythromycin-resistant isolates of Staphylococcus aureus recovered in the United States from 1958 through 1969. Antimicrob Agents Chemother 1998; 42: 3024–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Normark BH, Normark S.. Evolution and spread of antibiotic resistance. J Intern Med 2002; 252: 91–106. [DOI] [PubMed] [Google Scholar]
- 9. Pertusis (Whooping Cough) 2017. https://www.cdc.gov/pertussis/clinical/treatment.html.
- 10. Tiwari T, Murphy TV, Moran J.. Recommended Antimicrobial Agents for the Treatment and Postexposure Prophylaxis of Pertussis: 2005 CDC Guidelines https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5414a1.htm. [PubMed]
- 11. Wang Z, Cui Z, Li Y. et al. High prevalence of erythromycin-resistant Bordetella pertussis in Xi’an, China. Clin Microbiol Infect 2014; 20: 825–30. [DOI] [PubMed] [Google Scholar]
- 12. Liu X, Wang Z, Zhang J. et al. Pertussis outbreak in a primary school in China: infection and transmission of the macrolide-resistant Bordetella pertussis. Pediatr Infect Dis J 2018; 37: e145–8. [DOI] [PubMed] [Google Scholar]
- 13. Parkhill J, Sebaihia M, Preston A. et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet 2003; 35: 32–40. [DOI] [PubMed] [Google Scholar]
- 14. Mattoo S, Cherry JD.. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev 2005; 18: 326–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martinez JL. General principles of antibiotic resistance in bacteria. Drug Discov Today Technol 2014; 11: 33–9. [DOI] [PubMed] [Google Scholar]
- 16. Vogwill T, MacLean RC.. The genetic basis of the fitness costs of antimicrobial resistance: a meta-analysis approach. Evol Appl 2015; 8: 284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van den Akker WM. Bordetella bronchiseptica has a BvgAS-controlled cytotoxic effect upon interaction with epithelial cells. FEMS Microbiol Lett 1997; 156: 239–44. [DOI] [PubMed] [Google Scholar]
- 18. Harvill ET, Cotter PA, Yuk MH. et al. Probing the function of Bordetella bronchiseptica adenylate cyclase toxin by manipulating host immunity. Infect Immun 1999; 67: 1493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buboltz AM, Nicholson TL, Parette MR. et al. Replacement of adenylate cyclase toxin in a lineage of Bordetella bronchiseptica. J Bacteriol 2008; 190: 5502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cummings CA, Bootsma HJ, Relman DA. et al. Species- and strain-specific control of a complex, flexible regulon by Bordetella BvgAS. J Bacteriol 2006; 188: 1775–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cotter PA, Miller JF.. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun 1994; 62: 3381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weyrich LS, Rolin OY, Muse SJ. et al. A Type VI secretion system encoding locus is required for Bordetella bronchiseptica immunomodulation and persistence in vivo. PLoS One 2012; 7: e45892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicholson TL, Brockmeier SL, Loving CL.. Contribution of Bordetella bronchiseptica filamentous hemagglutinin and pertactin to respiratory disease in swine. Infect Immun 2009; 77: 2136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inatsuka CS, Julio SM, Cotter PA.. Bordetella filamentous hemagglutinin plays a critical role in immunomodulation, suggesting a mechanism for host specificity. Proc Natl Acad Sci USA 2005; 102: 18578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cotter PA, Miller JF.. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol Microbiol 1997; 24: 671–85. [DOI] [PubMed] [Google Scholar]
- 26. Mattoo S, Foreman-Wykert AK, Cotter PA. et al. Mechanisms of Bordetella pathogenesis. Front Biosci 2001; 6: E168–86. [DOI] [PubMed] [Google Scholar]
- 27. Nicholson TL, Buboltz AM, Harvill ET. et al. Microarray and functional analysis of growth phase-dependent gene regulation in Bordetella bronchiseptica. Infect Immun 2009; 77: 4221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shrivastava R, Miller JF.. Virulence factor secretion by Bordetella species. Curr Opin Microbiol 2009; 12: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vila-Farrés X, Ferrer-Navarro M, Callarisa AE. et al. Loss of LPS is involved in the virulence and resistance to colistin of colistin-resistant Acinetobacter nosocomialis mutants selected in vitro. J Antimicrob Chemother 2015; 70: 2981–6. [DOI] [PubMed] [Google Scholar]
- 30. Hughes D, Brandis G.. Rifampicin resistance: fitness costs and the significance of compensatory evolution. Antibiotics (Basel) 2013; 2: 206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andersson DI, Levin BR.. The biological cost of antibiotic resistance. Curr Opin Microbiol 1999; 2: 489–93. [DOI] [PubMed] [Google Scholar]
- 32. Björkman J, Hughes D, Andersson DI.. Virulence of antibiotic-resistant Salmonella typhimurium. Proc Natl Acad Sci USA 1998; 95: 3949–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weaver JR, Pattee PA.. Inducible resistance to erythromycin in Staphylococcus aureus. J Bacteriol 1964; 88: 574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weisblum B, Dehmon V.. Erythromycin-inducible resistance in Staphylococcus aureus: survey of antibiotic classes involved. J Bacteriol 1969; 98: 447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weisblum B, Siddhikol C, Lai CJ. et al. Erythromycin-inducible resistance in Staphylococcus aureus: requirements for induction. J. Bacteriol 1971; 106: 835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Allen NE. Macrolide resistance in Staphylococcus aureus: inducers of macrolide resistance. Antimicrob Agents Chemother 1977; 11: 669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chancey ST, Zähner D, Stephens DS.. Acquired inducible antimicrobial resistance in Gram-positive bacteria. Future Microbiol 2012; 7: 959–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weiss AA, Falkow S.. Genetic analysis of phase change in Bordetella pertussis. Infect Immun 1984; 43: 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taylor-Mulneix DL, Bendor L. et al. Bordetella bronchiseptica exploits the complex life cycle of Dictyostelium discoideum as an amplifying transmission vector. PLoS Biol 2017; 15: e2000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Strassmann JE, Shu L.. Ancient bacteria–amoeba relationships and pathogenic animal bacteria. PLoS Biol 2017; 15: e2002460.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hamidou Soumana I, Linz B, Harvill ET.. Environmental origin of the genus Bordetella. Front Microbiol 2017; 8: 28.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Akerley BJ, Cotter PA, Miller JF.. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell 1995; 80: 611–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.