Abstract
Hypoxia results in decreased arterial Po2, arterial chemoreflex activation, and compensatory increases in breathing, sympathetic outflow, and neuroendocrine secretions, including increased secretion of AVP, corticotropin-releasing hormone (CRH), adrenocorticotropin hormone (ACTH), and corticosterone. In addition to a brain stem pathway, including the nucleus tractus solitarius (nTS) and the rostral ventrolateral medulla (RVLM), medullary pathways to the paraventricular nucleus of the hypothalamus (PVN) contribute to chemoreflex responses. Experiments evaluated activation of specific cell phenotypes within the PVN following an acute hypoxic stimulus (AH; 2 h, 10% O2) in conscious rats. Retrograde tracers (from spinal cord and RVLM) labeled presympathetic (PreS) neurons, and immunohistochemistry identified AVP- and CRH-immunoreactive (IR) cells. c-Fos-IR was an index of neuronal activation. Hypoxia activated AVP-IR (~6%) and CRH-IR (~15%) cells, but not PreS cells in the PVN, suggesting that sympathoexcitation during moderate AH is mediated mainly by a pathway that does not include PreS neurons in the PVN. Approximately 14 to 17% of all PVN cell phenotypes examined expressed neuronal nitric oxide synthase (nNOS-IR). AH activated only nNOS-negative AVP-IR neurons. In contrast ~23% of activated CRH-IR neurons in the PVN contained nNOS. In the median eminence, CRH-IR terminals were closely opposed to tanycyte processes and end-feet (vimentin-IR) in the external zone, where vascular NO participates in tanycyte retraction to facilitate neuropeptide secretion into the pituitary portal circulation. Results are consistent with an inhibitory role of NO on AVP and PreS neurons in the PVN and an excitatory role of NO on CRH secretion in the PVN and median eminence.
Keywords: vasopressin, AVP, corticotropin-releasing hormone, CRH, median eminence, nitric oxide
the arterial chemoreflex is important for initiating adaptive changes in breathing and the autonomic nervous system to maintain adequate oxygenation of tissues throughout the body under a variety of environmental and physiological conditions. During hypoxia, the arterial chemoreflex is initiated by activation of peripheral chemoreceptors, increased carotid sinus afferent nerve discharge, and subsequent integration at the nucleus tractus solitarius (nTS) in the dorsal medulla (32). In turn, the nTS sends projections to the rostral ventrolateral medulla (RVLM) and the ventral respiratory group in the medulla and to the paraventricular nucleus of the hypothalamus (PVN), regions that project directly or indirectly to preganglionic sympathetic neurons and the phrenic motor nucleus in the spinal cord (21, 59). Compensatory chemoreflex responses to a decrease in arterial Po2 include increases in sympathetic outflow, breathing, and neuroendocrine secretions (21).
The PVN, which is critical for neuroendocrine and autonomic function, is composed of two morphologically distinct types of neurons: magnocellular and smaller parvocellular cells (19). Magnocellular neurons in the PVN and the supraoptic nucleus of the hypothalamus project to the posterior pituitary gland and comprise the major source of circulating AVP and oxytocin (OXY) (52). A subpopulation of neuroendocrine parvocellular neurons in the medial PVN synthesizes corticotropin-releasing hormone (CRH) and sends projections to the median eminence, where CRH is released into the portal vessels to the anterior pituitary gland. CRH stimulates secretion of ACTH, which then promotes glucocorticoid synthesis and release from the adrenal cortex (64). The endocrine response to an acute bout of hypoxia in conscious rats includes increased plasma concentrations of AVP, OXY, ACTH, and corticosterone (9, 20, 53), implicating activation of neuroendocrine cells in the PVN.
Other parvocellular neurons in the PVN send long descending projections to regions important for autonomic regulation, including the nTS, RVLM, and the intermediolateral cell column of the spinal cord (IML) (19). A subgroup of preautonomic neurons in the PVN is termed “presympathetic” (PreS) because they innervate spinal preganglionic sympathetic neurons in the IML through either a direct projection or a pathway that includes a synapse in the RVLM (4, 67). Interestingly, a portion of the preautonomic neurons in the PVN contains CRH, and indirect evidence suggests that neurosecretory and preautonomic populations of CRH-immunoreactive (IR) neurons are regulated independently (58).
The PVN has been implicated in increased arterial pressure and sympathetic outflow in response to physiological challenges, such as dehydration (68) and in cardiovascular diseases, such as heart failure (38, 72) and hypertension (1). Importantly, increased sympathetic nerve activity in these diseases is associated with augmented chemoreflex function (60, 61, 69). Our group has shown that brain stem neurons in the nTS and caudal ventrolateral medulla (CVLM) that project to the PVN are activated acutely, even with a moderate hypoxic stimulus, suggesting that the PVN may be more important to normal chemoreflex responses than previously thought (28, 29).
Within the PVN inhibitory effects of local production of the neuromodulator nitric oxide (NO) are well documented. Blockers of nitric oxide synthase (NOS) excite, while NO donors inhibit, magnocellular AVP and OXY neurons, an effect mediated by a presynaptic action of NO to increase release of the inhibitory neurotransmitter, GABA (5, 66). NO inhibits sympathetic outflow at the level of the PVN (73, 74) by a similar mechanism (38). Blunted NO-mediated GABAergic inhibition of the PVN in pathophysiological conditions, such as heart failure (38), contributes to hyperactivity of the sympathetic nervous system.
In contrast to the inhibitory role of NO on magnocellular and PreS neurons, NO is primarily excitatory within the hypothalamic-pituitary-adrenal (HPA) axis and includes positive modulation of CRH secretion at the level of both the PVN and the median eminence (7, 34, 49). Although the major isozyme of NOS in the PVN is neuronal NOS (nNOS) (52), both nNOS and endothelial NOS (eNOS) are potential sources of NO in the median eminence (49). Importantly, hypoxia is associated with increased production of NO by the vascular endothelium (40). Furthermore, type 2 vesicular glutamate transporter (VGLUT2) mRNA is expressed in CRH-containing PVN neurons, and VGLUT2-IR and CRH-IR are colocalized in the median eminence (25), suggesting that glutamate is a cotransmitter in CRH neurons. Partly through actions on tanycytes, specialized ependymoglial cells in the median eminence, both NO and glutamate have been proposed as positive modulators of CRH secretion into the portal circulation (47).
While the PVN is a major CNS site for the regulation of both neuroendocrine and autonomic function (3, 6), and hypoxia affects both, little is known regarding specific PVN cell populations targeted or their regulation. We hypothesized that, in addition to AVP-IR neurons, acute hypoxia (AH) would activate identified CRH-IR and retrogradely labeled PreS cells within the PVN. Also, we hypothesized that expression of nNOS within distinct cell types would correlate differentially with neuronal activation during hypoxia. Presympathetic IML- and RVLM-projecting neurons were identified by retrograde fluorescent tracers and immunohistochemistry (IHC) was used to identify AVP-IR, CRH-IR, and nNOS-IR neurons in the PVN. Expression of the early gene c-fos in cell nuclei was used as an index of neuronal activation (8). Additionally, since CRH secretion may also be regulated at the level of the median eminence, the relationships among neurosecretory CRH nerve fibers (prelabeled with enhanced green fluorescent protein (eGFP) by viral transfection of CRH neurons in the PVN), tanycytes, CRH-IR, nNOS-IR, and VGLUT2-IR were evaluated.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats (250–320 g) used in these experiments were purchased from ENVIGO (Madison, WI) and housed on a 12:12-h light-dark cycle at room temperature (22°C) and 40% humidity. All experiments were performed according to the American Physiological Society Guidelines for Research Involving Animals, and experimental protocols were approved by the institutional Laboratory Animal Care and Use Committee at the University of Missouri.
Drugs and Solutions
Isoflurane was purchased from Aerane, Baxter (Deerfield, IL); heparin sodium was purchased from Fresenius Kabi (Schaumburg, IL); dexamethasone was purchased from Bimed-MTC Animal Health, (Cambridge, ON, Canada); Buprenex was purchased from Reckitt Benckiser Pharmaceuticals (Richmond, VA); Baytril was purchased from Bayer Health Care (Shawnee Mission, KS), and SomnaSol euthanasia solution was purchased from Butler Schein Animal Health (Dublin, OH). DMEM, paraformaldehyde (PFA), and, unless otherwise noted, all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). All antibodies were dissolved in 0.01 M PBS [0.387 M NaCl, 0.02 M monobasic NaH2PO4 (anhydrous), and 0.8 M dibasic Na2HPO4 in distilled H2O; pH 7.4]. Normal donkey serum and all secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA). Cryoprotectant solution was composed of 0.1 M sucrose, 0.05 M polyvinylpyrrolidone-40 (MW 40,000), and 5.4 M ethylene glycol in PBS.
Recovery Surgery
Rats were anesthetized with isoflurane (2 l/min, 5% in 100% O2 for induction and maintained at 2–3%), given dexamethasone (50 μg im) to limit swelling, and placed into a stereotaxic frame (Kopf, Tujunga, CA). Rectal body temperature was maintained at 37 ± 0.5°C, with a water-circulating heating pad (model K-20; American Pharmaseal, Valencia, CA). Using aseptic procedures, we performed microinjections in specific CNS sites, and wounds were closed. Buprenex (0.5 mg/kg sc) and Baytril (2.5 mg/kg im) were given postoperatively for pain management and prevention of infection. Rats were returned to their home cage and monitored following surgery until ambulatory (2–3 h).
Retrograde labeling of presympathetic neurons in the PVN.
The retrograde tracer Alexa Fluor 555-conjugated cholera toxin subunit B (CT-B-Alexa Fluor 555, 0.5% in deionized H2O; Molecular Probes, Grand Island, NY) was microinjected 6 to 8 days before experiments to label presympathetic cells in the PVN. In 10 rats, the tracer was injected into the left dorsal lateral sulcus containing the IML (between T1 and T2; 180 nl total over three rostrocaudal sites, 0.8–0.9 mm ventral to the dorsal surface). In 10 additional rats, the tracer was injected into the left RVLM (30 nl). As previously described (33), the rat’s head was deflected downward to level the brain stem in the horizontal plane; the pipette was positioned perpendicular to the dorsal surface, and the following stereotaxic coordinates were used to inject into the RVLM: +0.7 mm anterior to calamus scriptorius, 1.8 mm lateral to midline, and −3.7 mm from the dorsal surface.
In rats that received tracer injections, the spinal cord (IML injections) or brain stem (RVLM injections) was harvested following perfusion of the rat (see below), and tissue was sectioned (50 μm). The injection sites were verified by the presence of CT-B-Alexa Fluor 555 fluorescence in the appropriate region (IML or RVLM), based on comparison with a stereotaxic atlas (46). Representative examples of tracer injection sites in the IML region of the spinal cord and the RVLM are shown in Fig. 1, A and B, respectively. Proper anatomical placement of injections and retrograde labeling were verified for all rats included in the data analysis.
Fig. 1.
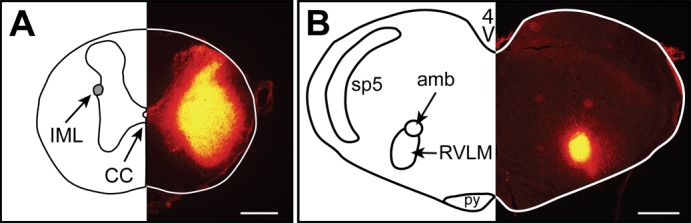
Representative retrograde tracer injection sites (Alexa Fluor 555-conjugated CT-B). Landmarks in traces (left side) were determined by comparison with a rat brain atlas (46). Bright yellow fluorescence in sections (right side) indicates the tracer injection site; scale bars = 100 µm. A. spinal injection site encompassing the intermediolateral cell column (IML) in one rat (~T1). CC denotes central canal. B: rostral ventrolateral medulla (RVLM) injection site (~12 mm caudal to bregma) in one rat. sp5, spinal trigeminal tract; amb, ambiguus nucleus; py, pyramidal tract.
Chemoreflex Activation
On the experimental day, 6 to 8 days after retrograde tracer injection, an acute hypoxia protocol was performed in conscious rats similar to that previously described (32). Briefly, rats from each tracer group (IML or RVLM) remained in their home cage, which was placed in a hypoxia chamber, and 30 min was allowed for acclimation to the internal environment of the chamber (room air = 21% O2). Rats were then exposed for 2 h to either a gas mixture of nitrogen balanced 21% O2 (N = normoxia; n = 9) or 10% O2 (AH = acute hypoxia; n = 11).
Immunohistochemistry
Immediately after exposure to N or AH, rats were deeply anesthetized (Somnasol, 390 mg pentobarbital sodium + 50 mg phenytoin sodium in 1 ml ip) and transcardially perfused first with 200 ml of heparinized (50 U/ml) DMEM (previously bubbled with O2, pH = 7.4) followed by 4% PFA (250 ml, pH = 7.4), and the brain and spinal cord were removed. Tissue was postfixed in 4% PFA overnight and then placed in a 30% sucrose solution in 0.01 M PBS for 3–5 days. Thirty-five-micrometer coronal sections of the forebrain were cut in a 1:6 series, and IHC was then performed on free-floating forebrain sections separated by ~175 μm. Tissue from one N and one AH rat was processed at the same time, and subsequent images were obtained with identical camera settings. The remaining tissue was stored in cryoprotectant at 4°C. In all IHC protocols, control sections from each rat were processed in the absence of primary antibodies.
To determine whether AH activated PreS, AVP, and nNOS cells in the PVN, a series of PVN sections from rats with IML (N, n = 5; AH, n = 5) and RVLM (N, n = 4; AH, n = 6) tracer injections underwent IHC evaluation of Fos-IR, nNOS-IR, and AVP-IR (study 1). On day 1, endogenous peroxidase was quenched by 30-min incubation in 0.3% hydrogen peroxide in PBS. Sections then were rinsed in 0.01 M PBS (3 × 10 min) and preblocked with 10% normal donkey serum (NDS) in 0.3% Triton-PBS for 30 min. Tissue sections were rinsed and then incubated at 4°C overnight with rabbit anti-c-Fos polyclonal antibody (1:6,000; Calbiochem, EMD Millipore Chemicals, Gibbstown, NJ) in PBS containing 0.3% Triton and 3% NDS. One control section from each animal was incubated similarly but without primary antibody for c-Fos. On day 2, sections were rinsed, incubated with a biotinylated donkey anti-rabbit IgG secondary antibody (1:200) in PBS containing 3% NDS and 0.3% Triton-PBS for 2 h at room temperature; then they were rinsed (3 × 5 min) and incubated (1 h) with an avidin-biotin complex solution (ABC kit; Vectastain, Vector Laboratories, Burlingame, CA) in PBS with 3% NDS. After rinsing was completed, sections were submerged in a nickel-enhanced 3,3′-diaminobenzidine solution (DAB; peroxidase substrate kit; Vector Laboratories) per the manufacturer’s instructions. After ~2 min, when a dark brown reaction product was formed, sections were rinsed, incubated overnight with primary antibodies—mouse anti-nNOS (1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-AVP (1:3,000; Calbiochem, EMD Millipore Chemicals, Gibbstown, NJ)—rinsed again and incubated for 2 h in IgG secondary antibodies donkey anti-mouse Alexa Fluor 488 and anti-rabbit Alexa Fluor 647 (1:200). After rinsing was completed, sections were mounted on gel-coated slides (0.5% gelatin in dH2O), placed in the dark to dry, and then dehydrated in 3 × 3 min washes of ethanol (70, 90, and 100%) followed by 2 × 5 min washes of xylene and coverslipped with Cytoseal (Thermo Fisher Scientific, Waltham, MA).
To evaluate the relationship among PreS, CRH, and nNOS neurons and their potential activation by AH, additional PVN sections from IML-injected rats underwent evaluation of c-Fos-IR, nNOS-IR, and CRH-IR (study 2; N, n = 4; AH, n = 5). Antibodies and procedures for c-Fos-IR and nNOS-IR were the same as for study 1. CRH-IR was assessed using guinea pig anti-CRF primary antibody (1:1,000; Peninsula Laboratories, Bachem, San Carlos, CA) and donkey anti-guinea pig Alexa Fluor 647 secondary antibody.
Viral transfection of CRH neurons in the PVN and IHC in the median eminence.
CRH secretion can be regulated by altering activity of CRH cell bodies in the PVN and also by altering CRH release at the level of the median eminence (7, 34, 49). To evaluate the anatomical relationship among elements in the median eminence, which may participate in regulation of peptide secretion, terminal fields for CRH and the cotransmitter glutamate, tanycytes, and nNOS within the median eminence were assessed. Since the main goal of these experiments was to evaluate terminal fields, c-Fos-IR was not assessed, and rats were not exposed to the AH protocol.
To visualize CRH fibers projecting from the PVN to the median eminence, an adeno-associated viral vector containing a rat CRH promoter-enhanced green fluorescent protein plasmid (pCRH-AAV-eGFP, titer > 1 × 1012; GeneDetect, Sarasota, FL) was microinjected (250 nl) bilaterally into the PVN of 16 rats. Stereotaxic coordinates were 1.8 mm caudal to bregma, 0.5 mm lateral to the midline, and −7.4 mm from the dorsal surface. Four to seven weeks following AAV injection, rats were perfused, and brain tissue was harvested, as described above. Nine rats were eliminated from further study after initial histological evaluation revealed injection sites outside the PVN (n = 3) or minimal eGFP expression in brains obtained 6–7 wk following AAV injection (n = 6). In the remaining 7 rats, expression of eGFP was clearly identified in the PVN (Fig. 2, C1 and C2) and coincided with CRH-IR in both spinally projecting (Fig. 2, D1–D3) and medial parvocellular neurons (Fig. 2, E1–E3) in forebrains harvested 4 wk (n = 3) or 5 wk (n = 4) after injection of pCRH-AAV-eGFP into the PVN. eGFP expression in forebrain tissue from these seven rats was comparable and was used for further IHC evaluation of CRH fibers and terminal fields in the median eminence. No eGFP fluorescence was visualized in the PVN 4 wk after injection of a control empty (null) vector (n = 3; example Fig. 2, B1 and B2; camera settings equivalent to Fig. 2, C1 and C2).
Fig. 2.
Specificity of corticotrophin-releasing hormone (CRH) antibody and expression of adeno-associated viral vector containing rat CRH promoter-enhanced green fluorescent protein plasmid (pCRH-AAV-eGFP). The third ventricle is outlined on the left; spinally projecting neurons (pseudo-colored white) are included to orient location within the PVN (~1.7 to 1.9 mm caudal to bregma). Higher-magnification images of dashed-line boxes are on bottom right of A1, A2, and B2. A1: CRH-immunoreactive (IR) cells in PVN (pseudo-colored green; antibody dilution = 1:6,000). A2: preabsorption with the peptide immunogen (×200 antibody concentration by weight) greatly reduced CRH staining in an adjacent section from the same rat. B1: absence of labeling following prior PVN injection of null AAV (4 wk). B2: CRH-IR (green) in same section as B1. C1: Prior PVN injection of pCRH-AAV-eGFP (4 wk) resulted in eGFP expression (pseudo-colored red). C2: merged image showing colocalization of pCRH-AAV-eGFP (red) and CRH-IR (green). D1–D3: higher-magnification images of dashed-line box in C2 (presympathetic region). Arrows denote examples of pCRH-AAV-eGFP expression (red, D1) in CRH-IR cells (green, D2). Arrowhead indicates colabeling in a spinally projecting cell (white). D3 shows merged image. E1–E3: higher magnification of solid-line box in C2 (medial parvocellular region). pCRH-AAV-eGFP was colocalized with CRH-IR cells (arrows). E3 shows merged image. Scale bars: A–C = 200 µm; D and E = 25 µm.
eGFP-expressing fibers in the median eminence were identified in rats in which CRH neurons in the PVN had been transfected by prior injection of pCRH-AAV-eGFP. eGFP expression was evaluated in combination with CRH-IR (n = 5) or in combination with vimentin-IR (marker for tanycytes; mouse anti-vimentin; 1:2,000; EMD Millipore, Temecula, CA) and VGLUT2-IR (guinea pig anti-VGLUT2; 1:1,000; EMD Millipore) (n = 4). Secondary antibodies were donkey anti-guinea pig Alexa Fluor 647 and anti-mouse Cy3. IHC was also performed on sections containing the median eminence from naïve rats without prior AAV injections. Tanycytes (vimentin-IR) were evaluated in combination with CRH-IR and nNOS-IR (primary = rabbit anti-nNOS, 1:1,000, EMD Millipore; secondary = anti-rabbit Alexa Fluor 488) (n = 6). Sections were mounted on gel-coated slides, dried, and coverslipped with ProLong Diamond Antifade Mountant (Invitrogen, Molecular Probes, Life Technologies).
Image Analysis
To evaluate regions of the PVN in which the majority of PreS and neuroendocrine cells are contained (see Fig. 3), images of the PVN were acquired (×20) corresponding to −1.4 to −2.1 mm caudal to bregma. An Olympus BX51 microscope containing a three-axis motorized stage (Ludl Electronic Products, Hawthorne, NY) with a monochrome digital camera (ORCA, Hamamatsu, Bridgewater, NJ) along with Neurolucida software (version 10, MicroBrightField, Willston, VT) was used to obtain Z-stack images (10 every 2 µm). Z-stack images (10 every 0.5 µm) of the median eminence (−1.8 to −3.3 caudal to bregma) were obtained at magnifications of ×10 to ×60. Appropriate camera settings and filter cubes for the cell markers were used: Bright-field, Alexa Fluor 488 [excitation (ex.): λ480 nm; emission (em.): λ510 nm], Alexa Fluor 555 [ex. λ555 nm; em. λ588], and Alexa Fluor 647 [ex. λ650 nm; em. λ670 nm]. Image stacks were merged, and only brightness and contrast were adjusted for optimization. In studies 1 and 2, for each section of the PVN, cells were counted independently by two individuals using ImageJ software. Counts were then averaged and used for statistical analyses.
Fig. 3.
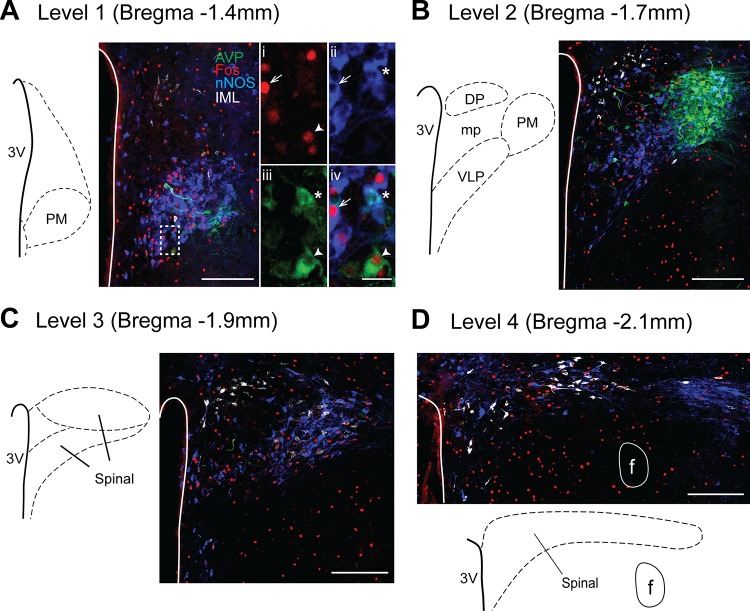
Distribution of AVP-, neuronal nitric oxide synthase (nNOS)-, Fos-IR, and spinally projecting neurons in the PVN from one rat exposed to acute hypoxia (AH). A–D: images of four rostrocaudal levels of the PVN examined. Traces on left indicate regions of interest and approximate rostrocaudal coordinates relative to bregma (46). Images are pseudo-colored: AVP-IR is green, nNOS-IR is blue, Fos-IR is red, and spinally projecting neurons are white. Scale bars = 200 µm. Higher-magnification images of dashed-line box in A are shown on right (scale bar = 25 µm): i, c-Fos; ii, nNOS; iii, AVP; and iv, merged image. Arrow, nNOS-Fos cell; arrowhead, AVP-Fos cell; *AVP-nNOS. PM, posterior magnocellular; DP, dorsal parvocellular; VLP, ventrolateral parvocellular; and mp, medial parvocellular regions; 3V, 3rd cerebral ventricle; f, fornix.
Immunopositive Cells
Immunoreactivity for c-Fos (Fos-IR), which could be seen as a dark nuclear reaction product under bright-field microscopy was used as a marker for cellular activation (8). Retrogradely labeled (CT-B-Alexa Fluor 555) RVLM- and IML-projecting neurons were identified by the presence of granular, cytoplasmic labeling devoid of nuclear staining. Cells were positively identified for AVP, CRH, and nNOS in the presence of dense staining of the cytoplasm with an empty nuclear region. Colabeling of cells was identified when two or more fluorophores met the above criteria when cells were viewed in the same focal plane. Tanycytes were identified extending from the ventral surface of the third ventricle to the dorsal external zone of the median eminence by their glial-like appearance and vimentin-IR. Punctate labeling of CRH and VGLUT2 was identified in both the internal and external zones of the median eminence.
Antibody Specificity
With the use of identical camera settings and filter cubes as for sections exposed to primary antibodies, absence of visible staining in sections in which primary antibody was withheld, served as a control for nonspecific staining. Western blot analysis previously verified specificity of rabbit anti-Fos (18), mouse anti-vimentin (45), and rabbit anti- nNOS antibodies (56). Mouse anti-nNOS was validated by Western blot analysis by the manufacturer (Santa Cruz Biotechnology) and by full correspondence of IHC labeling with the rabbit anti-nNOS antibody (56). Preadsorption experiments verified specificity of rabbit anti-vasopressin (39) and guinea pig anti-VGLUT2 (41). With the use of a preadsorption protocol similar to that previously described (12), specificity of guinea pig anti-CRH antibody was verified in PVN sections from the current experiments (Fig. 2, A1 and A2). Specificity of CT-B retrograde labeling was confirmed by the distribution of staining in regions with known projections to injection sites and an absence of staining in other brain regions.
Statistical Analyses
Data were analyzed by two-way repeated-measures (RM) ANOVA (SigmaPlot v. 12.5, Systat Software, San Jose, CA) for each cellular phenotype evaluated in the PVN to determine rostrocaudal distribution and effects of AH. To meet criteria of normality and equal variance, in some instances, square root or log transformations were performed before statistical comparison. When appropriate, post hoc analysis was performed using Student-Newman-Keuls test. Student’s t-tests were used to compare total counts across all levels of the PVN between N and AH rats. Data are presented as means ± SE, and significance was accepted at P ≤ 0.05.
RESULTS
Presympathetic Projecting Cells Are Located Primarily in the Caudal PVN, While AVP-IR, nNOS-IR, and CRH-IR Cells Are Located Primarily in the Rostral PVN
Figure 3 contains examples of the four rostrocaudal levels of the PVN evaluated from one AH-treated rat in which activation (Fos-IR) of AVP-IR, nNOS-IR, and spinally projecting cells was examined. AVP-IR cells were located in the posterior magnocellular subregion of the PVN in levels 1 and 2. Higher-magnification images (Fig. 3A, right) contain examples of activated nNOS, activated AVP, and colabeled AVP-nNOS cells.
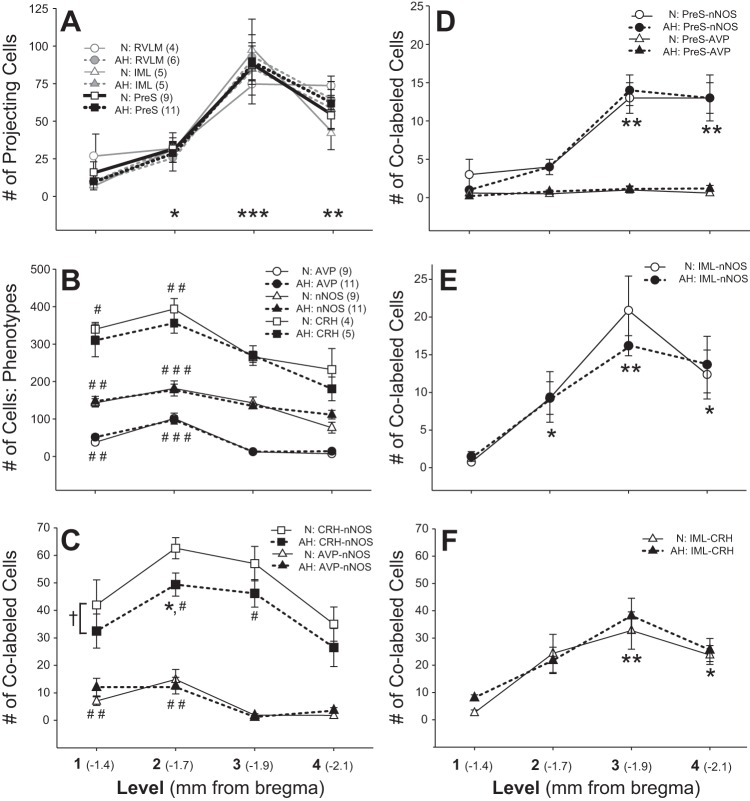
Mean data (Fig. 4A) show a similar number and distribution of retrogradely labeled spinal IML- and RVLM-projecting cells in the four levels of the PVN. Data from these subgroups of rats were pooled (presympathetic, PreS) for further analysis. Two-way RM ANOVA revealed no effect of AH and a main effect of level. Consistent with previous reports (67), the majority of PreS cells were located caudally (level 3 > 4 > 2 > 1).
Fig. 4.
Rostrocaudal distribution of PVN cell phenotypes. A: number and distribution of RVLM- and IML-projecting neurons were equivalent and located primarily in caudal levels of the PVN. Data were pooled as presympathetic cells (PreS). B: the numbers of AVP-, nNOS-IR, and CRH-IR cells were highest in rostral regions of the paraventricular nucleus (PVN). C: nNOS colabeled with AVP rostrally, while nNOS-CRH cells were located throughout the PVN with highest numbers in levels 2 and 3. D: PreS-nNOS cells were highest in caudal regions of the PVN and AVP-IR was not present in PreS cells. IML-projecting cells colabeled for nNOS (E) and CRH (F), and these colabeled cells were located more caudally. Numbers of the various cell types (A–F) were similar between normoxia (N) and acute hypoxia (AH) groups, with the exception of CRH-nNOS colabeled cells, where there was a main effect of AH to decrease the number of CRH-nNOS cells (†). Significant differences: * > level 1; ** > level 1 and 2; *** > levels 1, 2, and 4; # > level 4; ## > levels 3 and 4; and ### > levels 1, 3, and 4.
Similarly, there was no effect of AH on the number or distribution of AVP-IR, nNOS-IR, or CRH-IR cells (Fig. 4B). Cells with these three phenotypes were most prevalent in the rostral PVN. AVP-IR cells were located almost exclusively in rostral levels (levels 1 and 2), while nNOS-IR and CRH-IR cells were found throughout the rostrocaudal extent of the PVN with the greatest number in levels 1 and 2 (Fig. 4B).
nNOS Colocalized with AVP-IR and CRH-IR and Presympathetic Cells
nNOS was present in both AVP-IR and CRH-IR cells (Fig 4C). Because nNOS was distributed throughout all levels of the PVN (Fig 4B), it is not surprising that AVP-IR cells containing nNOS (AVP-nNOS) were located primarily in levels 1 and 2 (where most AVP-IR cells are located). Across all levels of the PVN, the total number (Table 1) and percentage of AVP-IR cells, which colabeled with nNOS [(AVP-nNOS)/AVP·100], were similar between N (17 ± 2%) and AH rats (15 ± 2%).
Table 1.
Total cell counts across four levels of the PVN in N and AH rats (study 1)
| Phenotypes Study 1 |
PreS | nNOS | AVP | PreS-nNOS | PreS-AVP | AVP-nNOS |
|---|---|---|---|---|---|---|
| N (8) | 183 ± 22 | 532 ± 34 | 162 ± 22 | 32 ± 6 | 3 ± 1 | 27 ± 5 |
| AH (10) | 189 ± 24 | 571 ± 27 | 174 ± 19 | 31 ± 4 | 3 ± 1 | 27 ± 6 |
| Activated Study 1 |
PreS-Fos | nNOS-Fos | AVP-Fos | PreS-nNOS-Fos | PreS-AVP-Fos | AVP-nNOS-Fos |
|---|---|---|---|---|---|---|
| N (8) | 7 ± 2 | 29 ± 6 | 1 ± 0.3 | 1 ± 0.5 | 0 ± 0 | 0.3 ± 0.2 |
| AH (10) | 9 ± 2 | 86 ± 12* | 11 ± 3* | 3 ± 1 | 0 ± 0 | 2 ± 1 |
Acute hypoxia (AH) > normoxia (N). Other abbreviations are described in text.
CRH-IR and nNOS-IR were evaluated in a separate series of PVN sections from N (n = 4) and AH rats (n = 5) with retrogradely labeled spinally (IML)-projecting cells. Two-way RM ANOVA indicated that CRH- and nNOS-colabeled cells (CRH-nNOS) were most prevalent in intermediate levels of the PVN (levels 2 and 3), and there was a main effect of AH to decrease the number of CRH-nNOS cells (Fig. 4C). Across all levels of the PVN, AH had no significant effect on the total number of nNOS or CRH cells individually, but the total number of CRH-nNOS-colabeled cells was less in AH compared with N (Table 2). However, the percentage of CRH cells colabeled with nNOS [(CRH-nNOS)/CRH·100] was not different between N (16 ± 1%) and AH rats (14.5 ± 1%). Thus, although not significant, it is likely that the trend (P = 0.19) for a lower total number of CRH-IR cells in AH rats, contributed to the decrease in the number of CRH-nNOS colabeled cells, which were counted (Table 2).
Table 2.
Total cell counts across four levels of the PVN in N and AH rats (study 2)
| Phenotypes Study 2 |
IML | nNOS | CRH | IML-nNOS | IML-CRH | CRH-nNOS |
|---|---|---|---|---|---|---|
| N (4) | 200 ± 39 | 449 ± 34 | 1232 ± 33 | 43 ± 8 | 84 ± 14 | 197 ± 12 |
| AH (5) | 223 ± 23 | 454 ± 34 | 1100 ± 79 | 41 ± 5 | 93 ± 8 | 156 ± 5* |
| Activated Study 2 |
IML-Fos | nNOS-Fos | CRH-Fos | IML-nNOS-Fos | IML-CRH-Fos | CRH-nNOS-Fos |
|---|---|---|---|---|---|---|
| N (4) | 9 ± 2 | 19 ± 6 | 64 ± 23 | 3 ± 1 | 6 ± 1 | 14 ± 4 |
| AH (5) | 10 ± 1 | 77 ± 9* | 167 ± 31* | 3 ± 1 | 7 ± 1 | 38 ± 8* |
AH > N. Abbreviations are described in text.
PreS cells from study 1 (Fig. 4D) and study 2 (Fig. 4E) colabeled similarly with nNOS, primarily in caudal regions of the PVN. Across all levels of the PVN, the total numbers (Tables 1 and 2) and the percentages of PreS cells, which colabeled with nNOS [(PreS-nNOS)/PreS·100] was similar between N (17 ± 2 and 22 ± 2%) and AH groups (17 ± 1 and 18 ± 2%).
Presympathetic Cells in PVN Colabel With CRH but not AVP
Very few PreS cells in the PVN contained AVP (Fig. 4D). In both the N and AH groups, across all four levels of the PVN, only 3 ± 1 PreS-AVP-colabeled cells were identified per rat (Table 1). In contrast, a substantial number of PreS cells expressed CRH (IML-CRH, Table 2). The percentage of spinally projecting cells that coexpressed CRH [(IML-CRH)/IML·100] was not different between N (43 ± 3%) and AH rats (42 ± 3%), and these were located in more caudal levels of the PVN (Fig. 4F).
AH Activates AVP-IR, CRH-IR, and nNOS-IR Cells, but not Presympathetic Cells, in the PVN
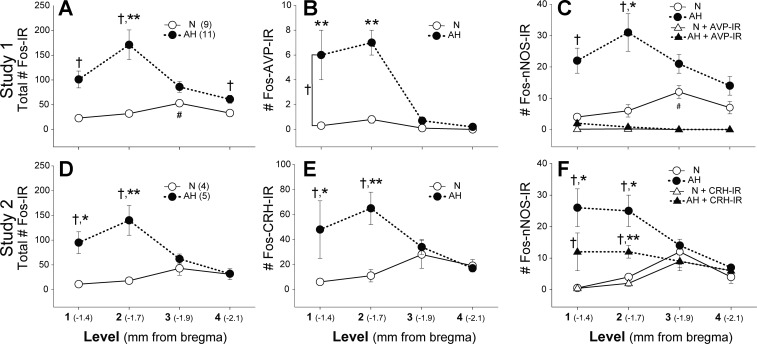
Neuronal activation of the PVN, as assessed by the number of c-Fos-IR cells, was greater in AH compared with N rats. c-Fos-IR was most prevalent rostrally and AVP-IR, CRH-IR, and nNOS-IR cells that were activated by AH were located in levels 1 and 2 of the PVN (Fig. 5). Interestingly, AVP-nNOS colabeled cells were not activated (Fig. 5C and Table 1), but CRH-nNOS cells were activated by AH (Fig. 5F and Table 2). Expressed as percentages, across all levels of the PVN, AH resulted in activation of nNOS-IR (AH = 15 ± 2% > N = 6 ± 1%), AVP-IR (AH = 6 ± 1% > N = 0.9 ± 0.3%), and CRH-IR cells (AH = 14 ± 2% > N = 5 ± 2%) [% activated = (phenotype-Fos)/phenotype·100]. The percentage of CRH-nNOS cells that were activated [(CRH-nNOS-Fos)/(CRH-nNOS)·100] was greater in AH (25 ± 6%) compared with N rats (7 ± 2%).
Fig. 5.
Rostral-caudal distribution of activated PVN cells. A: (study 1). D: (study 2). AH increased the total number of Fos-IR cells primarily in the rostral PVN. Within AH, Fos-IR was highest in level 2. B: there was a main effect of AH to increase activation of AVP cells in the rostral PVN. C: AH activated nNOS cells rostrally, but the activated nNOS cells were not AVP-IR (Fos-nNOS-AVP). E: AH-activated CRH cells in rostral levels of the PVN. F: approximately half of the Fos-nNOS cells activated by AH also contained CRH and followed a similar pattern of activation in AH rats, with greatest activation in level 2. Significant differences: †AH > N; * > level 4; ** > level 3 and 4; # > level 1.
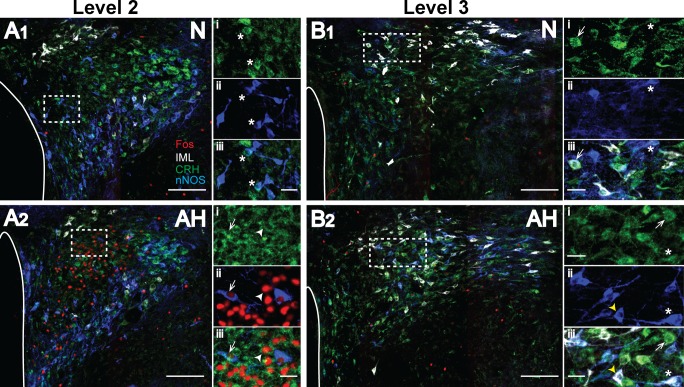
As discussed above, CRH colabeled with both nNOS-IR and IML-projecting cells in levels 2 and 3 of the PVN. Figure 6 shows photomicrographs of PVN levels 2 and 3 from one N and one AH rat. Compared with AVP-IR cells, which were located specifically within the posterior magnocelluar subregion (Fig. 3), CRH-IR and nNOS-IR cells were more broadly distributed within a given level of the PVN, and nNOS colabeling was evident in both medial and IML-projecting CRH-IR cells (Fig. 6). The medial parvocellular region of the PVN contains the majority of CRH neurosecretory cells (35) and is readily identifiable in level 2. As shown in these examples, CRH-IR cells in the medial parvocellular region, including CRH-nNOS-IR cells, were activated (Fos-IR) by AH (Fig. 6A2), while IML-projecting cells containing CRH were not activated by AH (Fig. 6B2).
Fig. 6.
Acute hypoxia activates CRH-IR cells in the medial parvocellular region of the PVN. Images are levels 2 and 3 from one N (A1 and B1) and one AH rat (A2 and B2), respectively. Neurons are pseudo-colored: CRH-IR, green; nNOS-IR, blue; Fos-IR, red; spinally projecting neurons, white. Scale bars = 200 µm. The third cerebral ventricle is outlined on the left side of PVN images. Higher-magnification image of dashed line box in each image are shown to the right; scale bars = 25 µm. A1: i, CRH; ii, nNOS; iii, merged image from medial parvocellular region of an N rat. Asterisk denotes CRH-nNOS cells. A2: i, CRH; ii, Fos-nNOS; iii, merged image from medial parvocellular region of an AH rat. Arrowhead denotes CRH-Fos cell, and arrow denotes CRH-nNOS-Fos cell. B1: i, CRH; ii, nNOS; iii, merged image, including IML-labeled cells in an N rat. Arrow denotes a CRH-IML cell, while asterisks denote a CRH-nNOS-IML cell. B2: i, CRH; ii, nNOS; iii, merged image, including IML-labeled cells in an AH rat. Arrow denotes a CRH-IML cell, while an arrowhead denotes an nNOS-IML cell, and an asterisk denotes a CRH-nNOS-IML cell.
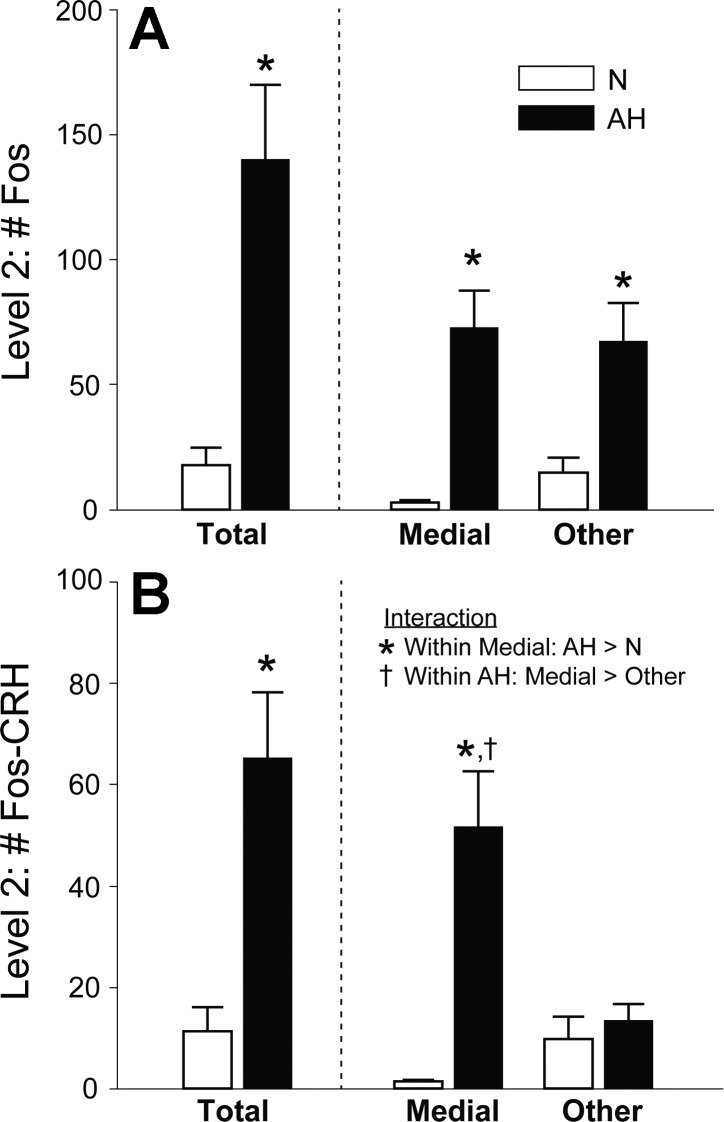
Figure 7 contains a more detailed analysis of level 2 of the PVN. Analysis of Fos-IR in level 2 revealed that AH produced equivalent activation of cells within and outside of the medial parvocellular region (Fig. 7A, right). Of all Fos-IR cells in level 2, 47 ± 3% were also immunopositive for CRH {[(c-Fos − CRH)/c-Fos·100]; compare left panels, Fig. 7, A and B)}. The majority of Fos-CRH neurons in level 2 were located in the medial neurosecretory region [(c-Fos-CRH-medial)/(c-Fos − CRH)·100 = 78 ± 3%], and only CRH-IR cells within the medial region were activated by AH (Fig. 7B, right). The number of nNOS-IR cells in the medial parvocellular region was not different between N (40 ± 5) and AH rats (33 ± 8), and approximately half of these nNOS cells contained CRH (nNOS-CRH: N = 16 ± 2; AH = 20 ± 4). Activation of nNOS-IR (AH = 11 ± 3 > N = 0) and nNOS-CRH colabeled cells (AH = 5 ± 1 > N = 0) occurred only in the AH group. Thus, different from magnocellular AVP-nNOS cells, which were not activated by AH, 27 ± 6% of medial parvocellular CRH-nNOS cells were activated by AH [(Fos-CRH-nNOS)/(CRH-nNOS)·100].
Fig. 7.
CRH-IR neurons in the medial parvocellular neurosecretory region were activated by AH. A: of all activated cells in level 2 of the PVN (left, Fos-IR), approximately half were located in the medial neurosecretory region (right). B: CRH-IR neurons that were activated by AH (left) were located in the medial region of the PVN (right). Significant differences: *AH > N; †Medial AH > other AH.
Relationships Among CRH Fibers/Terminals and Other Cell Phenotypes in Median Eminence
CRH-IR cells in the medial PVN were activated by AH, and these neuroendocrine cells are known to project to the median eminence, where secretion of CRH can be further regulated. To examine the relationship of CRH projections from the PVN to other cell types, CRH fibers and terminal fields in the median eminence were labeled by prior injections of pCRH-AAV-eGFP in the PVN and/or by using anti-CRH antibody. Images in Fig. 8 from one rat 4 wk following injection of pCRH-AAV-eGFP in the PVN demonstrate CRH-IR in eGFP-expressing fibers, which are distributed across both the internal and external zones of the median eminence. CRH-IR is especially prevalent in the distal external zone of the median eminence (Fig. 8A). A similar labeling pattern was observed in the median eminence of four additional rats. Thus, these experiments verify that injection of pCRH-AAV-eGFP into the PVN resulted in expression of eGFP in neurosecretory CRH cells in the medial parvocellular region, which was then transported down cellular processes to the median eminence. CRH-IR occurred along the length of eGFP fibers, where it exhibited an interrupted pattern of labeling, and punctate CRH-IR labeling extended beyond eGFP labeling in the external zone of the median eminence.
Fig. 8.
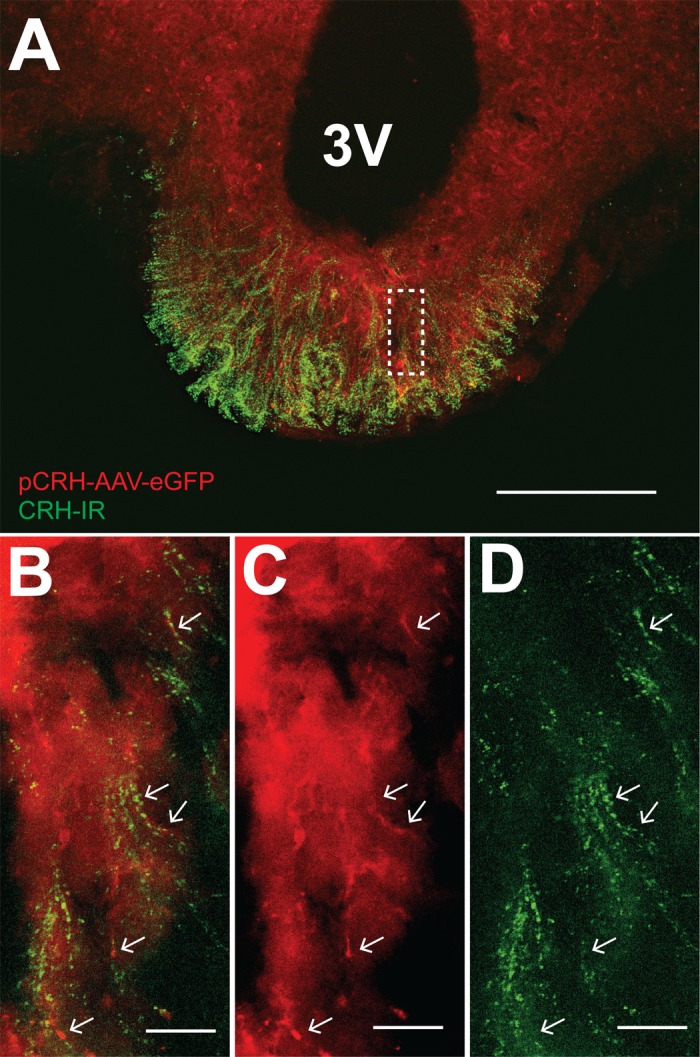
eGFP is expressed in CRH-IR fibers in the median eminence following transfection of the PVN with pCRH-AAV-eGFP. A: presumed CRH fibers from the PVN (pseudo-colored red) displayed CRH-IR (pseudo-colored green). Scale bar = 200 µm. B: higher-magnification image of region in dashed line box in A. Arrows indicate pCRH-AAV-eGFP fibers (C) coincident with CRH-IR (D). Scale bars for B–D = 15 µm.
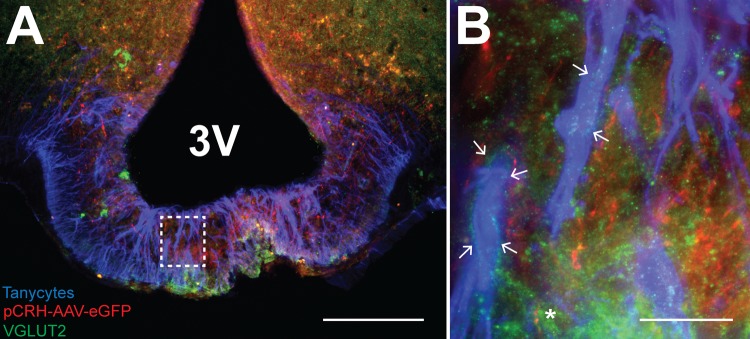
Glutamate, a proposed cotransmitter with CRH, may initiate retraction of tanycytes in the median eminence, facilitating access of CRH nerve terminals to the portal circulation, which is adjacent to the distal border of the median eminence (17, 42). In tissue from four rats, we evaluated the relationship among tanycyte processes, VGLUT2 and eGFP-expressing CRH fibers originating from the PVN. Figure 9 contains images of the median eminence from one rat. Punctate VGLUT2-IR is concentrated in the distal external zone of the median eminence (Fig. 9A), a distribution similar to that seen with CRH-IR (Fig. 8A). Higher magnification (Fig. 9B) shows punctate VGLUT2-IR in close proximity, and often outlining, tanycyte processes, which were labeled with the tanycyte marker vimentin. eGFP-expressing fibers from the PVN tended to run parallel to tanycytes.
Fig. 9.
VGLUT2-IR terminals were located in close apposition to tanycytes in the median eminence. A: vimentin-IR tanycyte processes (pseudo-colored blue) extend into the external zone of the median eminence in close proximity to CRH fibers (pseudo-colored red) and VGLUT2-IR (pseudo-colored green). Scale bar = 100 µm. B: higher-magnification image of dashed box in A. Arrows indicate VGLUT2 terminals surrounding tanycyte processes. An asterisk denotes close association of CRH fiber (pCRH-AAV-eGFP from PVN) with VGLUT2-IR. Scale bar = 25 µm.
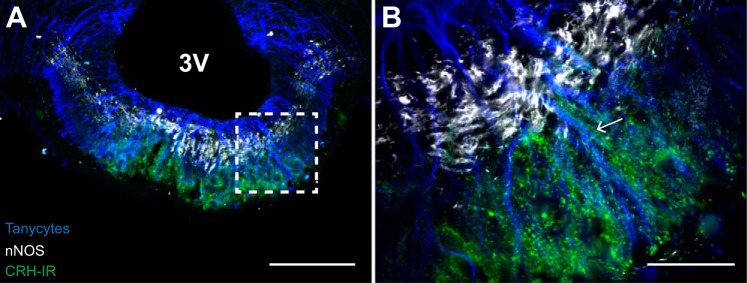
Nitric oxide also has been implicated in tanycyte retraction, and a close association between the distal external zone of the median eminence and pituitary portal capillaries containing eNOS is well known (42, 48, 49). Since nNOS is also present in the median eminence, we evaluated the relative distribution of tanycytes, nNOS-IR and CRH-IR terminals (n = 6). Figure 10 contains a representative photomicrograph of the median eminence from one rat and clearly shows that nNOS-IR is restricted to the internal zone of the median eminence, while tanycyte end-feet and CRH-IR are concentrated in the distal external zone. Similar to VGLUT2-IR (Fig. 9B), punctate CRH-IR can be identified outlining tanycyte processes (Fig. 10B).
Fig. 10.
CRH-IR and tanycyte end-feet are prevalent in the external zone of the median eminence. A: punctate CRH-IR (pseudo-colored green) and tanycyte terminations (pseudo-colored blue) are concentrated in the external zone of the median eminence, while nNOS-IR (pseudo-colored white) is concentrated in the internal zone. 3V, third ventricle. B: higher magnification of dashed-line box in A. CRH-IR surrounds tanycyte processes (arrow). Scale bars: A = 200 µm; B = 50 µm.
DISCUSSION
Previous studies from our group found that acute hypoxic stimuli produced intensity-dependent activation of catecholaminergic-brain stem neurons that projected to the PVN (28, 29). Adrenergic receptors are expressed throughout the PVN (13, 14), and lesion of cells in the A1 catecholaminergic region of the medulla attenuates hypoxia-induced activation of cells in the medial parvocellular region and in AVP- and oxytocin-IR cells in the PVN (63). However, the phenotype of medial parvocellular cells activated by acute hypoxia, effects of hypoxia on identified preautonomic cells, and the potential role of NO, an important neuromodulator in the hypothalamus, on responses to hypoxia have not been studied systematically. In the present experiments, the arterial chemoreflex was stimulated by acute exposure to hypoxia, and activation of major cell types within the PVN was evaluated. In addition, since CRH containing medial parvocellular cells in the PVN project to the median eminence, and CRH secretion may be regulated in this region, the relationships among CRH projections and other prominent cellular elements within the median eminence were evaluated. Nitric oxide is increased by different stress paradigms (15, 31), and potentially has differential effects among magnocellular (5), parvocellular PreS (36, 37), and parvocellular neuroendocrine cells and terminals (7, 34). Thus, a potential role for NO in the response to acute hypoxia was evaluated. We hypothesized that 1) AH would activate magnocellular AVP-IR, medial parvocellular CRH-IR, and PreS neurons (RVLM and IML projecting), and 2) the presence of nNOS in these PVN cell phenotypes would correlate with their activation by AH. Major new findings include 1) AH produced a modest activation of AVP-IR magnocellular neurons, which occurred only in nNOS-negative cells; 2) medial parvocellular CRH-IR cells, including a portion of those colabeled with nNOS-IR, were activated by AH; 3) nNOS-IR was not present in the external zone of the median eminence, where tanycyte end-feet and punctate CRH-IR were prominent; and 4) a significant portion of PreS parvocellular neurons contained either CRH (42%), nNOS (17%), or both (9%), but regardless of colabeling phenotype, PreS cells in the PVN were not activated by AH.
On the basis of our observations that PVN-projecting brain stem neurons were activated by an acute hypoxic stimulus (28, 29), evidence that a chemical chemoreflex stimulus [intravenous potassium cyanide (KCN)] activated RVLM-projecting presympathetic PVN neurons in rats (10) and reports that hypoxia increased sympathetic nerve activity in conscious humans (55, 57) and rats (43), we predicted that PreS neurons in the PVN would be activated by AH in our experiments. However, contrary to our original hypothesis, PreS neurons were not activated by exposure to 10% O2 for 2 h. Cruz et al. (10) used repeated intravenous injections of KCN rather than continuous hypoxia to activate the arterial chemoreflex in conscious rats, which could possibly account for the difference in our findings. In this regard, it is worth noting that other experiments implicating a role for the PVN in sympathoexcitation due to arterial chemoreflex stimulation also have used intravenous KCN to activate carotid body chemoreceptors. For example, electrolytic lesion of the PVN (44), or blockade of neurotransmission with lidocaine in the PVN (54), attenuated increases in arterial pressure, sympathetic nerve activity, and phrenic nerve activity in response to intravenous KCN, which led authors to conclude that the PVN is required for full expression of the arterial chemoreflex. Compared with intravenous KCN, hypoxia is considered a more physiologically relevant chemoreflex stimulus (21, 54), but it is possible that more severe hypoxia could result in activation of PreS neurons in the PVN. In this regard, compared with 10% O2, exposure to 8% O2 produced greater activation of both nTS and CVLM cells that project to the PVN (28, 29).
A previous study in which conscious rabbits were exposed to 10% O2 for 1 h supports our current findings. Although not quantitated, Hirooka et al. (24) noted that neurons from suprapontine regions, including the hypothalamus, appeared to be activated by hypoxia. However, neurons that projected directly to presumed presympathetic neurons in the RVLM and were activated by acute hypoxia were located exclusively in the lower brain stem, primarily within the nTS and Kölliker-Fuse nucleus in the pons. Thus, sympathoexcitation, due to a moderate acute hypoxic stimulus, is likely mediated by activation of pathways that do not involve PreS neurons in the PVN, such as the direct nTS to RVLM pathway (24, 32).
nNOS is the major NOS isozyme in the PVN (52), and it is well known that, through a presynaptic mechanism to increase GABA release, NO inhibits IML-projecting neurons in the PVN (37). In addition, blocking the synthesis of endogenous NO in the PVN results in elevated renal sympathetic nerve activity (73, 74). Similar to a previous report, we found that a portion of PreS cells in the PVN contained nNOS (37) and showed that the percentage of PreS cells colabeled with nNOS did not change with AH. Overall, across the four levels of the PVN evaluated, a total of ~15% of nNOS-IR neurons were activated by AH, which is consistent with reports of elevated NO levels in the PVN following acute stress (26). NO is highly diffusible and may affect targets up to a distance of 150 µm from its initial source of production (70). In addition, nNOS is expressed in dendrites (2), and dendrites from neurons in the posterior magnocellular region have been identified in close apposition to PreS neurons in the PVN (65). Thus, in the current experiments, it is plausible that during acute hypoxia, NO produced by activation of nNOS-IR cells in the vicinity of PreS neurons could limit their activation. Inhibition of PreS neurons by NO would prevent the PVN from contributing to increases in sympathetic nerve activity that have been observed during moderate hypoxic stimuli (43, 55, 57).
AH-mediated activation of neuroendocrine AVP neurons in the current experiments was modest and is consistent with a small, but significant, increase in plasma AVP concentrations in conscious rats after exposure to 10% O2 for 1 h (20). It is possible that projections from the nTS to the PVN contributed to activation of AVP-IR PVN cells. However, a major contribution for ascending catecholaminergic projections from the CVLM to the PVN is supported by experiments showing that prior excitotoxic lesions of a brain stem region that contains catecholaminergic A1 neurons (CVLM), greatly reduced Fos-IR in AVP-IR cells during a hypoxic stimulus similar to that used in our experiments (63). An interesting finding of the current experiments is that, even though a portion of AVP-IR cells colabeled with nNOS, the AVP-IR cells in which Fos-IR was detected did not contain nNOS. It is known that activation of magnocellular neurons stimulates NO production, which then diffuses out of the cell, promotes GABA release from presynaptic terminals, and feeds back to suppress neuronal discharge (5). Since c-Fos expression requires a strong and sustained stimulus (11), it is possible that locally produced NO in AVP-nNOS cells buffered activation of these cells, so that c-Fos expression did not reach a level sufficient for detection of c-Fos-IR in our experiments.
In addition to increasing AVP secretion, acute hypoxia increases plasma ACTH levels in conscious rats (53) and, similar to a previous report (63), we found that AH activated cells in the medial parvocellular region of the PVN. However, cell types other than CRH neuroendocrine cells (e.g., thyrotropin-releasing hormone cells) are present in the medial parvocellular region (19). The current experiments extended previous findings to indicate that the majority of activated cells in the medial parvocellular region of the PVN were immunopositive for CRH. In addition, different from PreS and magnocellular AVP-IR cells, a substantial portion of neurosecretory CRH cells in the medial parvocellular region of the PVN, which contained nNOS-IR, were activated by hypoxia.
As discussed above, there is good evidence that NO inhibits sympathetic outflow and AVP secretion at the level of the PVN, but the effect of NO on CRH secretion has been controversial, with reports of both excitatory and inhibitory effects (49). Most applicable to the current experiments, literature from intact conscious rats suggests that NO activates the HPA axis by promoting secretion of CRH. The net effect of intracerebroventricular injection of an NO donor in conscious rats is upregulation of heteronuclear transcripts for CRH in cell bodies in the medial parvocellular region of the PVN and increased circulating levels of ACTH, an effect abolished by intracerebroventricular injection of antibodies to CRH (34). Conversely, intracerebroventricular injection of the NOS inhibitor, NG-nitro-l-arginine methyl ester, attenuates catecholamine-induced ACTH and corticosterone secretion (7). Compounds injected intracerebroventricularly may affect CRH cell bodies in the PVN, as well as CRH terminal fields in the median eminence, and there is evidence for a facilitatory role of NO at both of these sites. For example, levels of NO metabolites in the PVN are elevated, and plasma ACTH is increased within 15 min of initiating microinfusion of an NO donor directly into the PVN of chronically instrumented conscious rats (62). Application of an NO donor to median eminence fragments, which contain CRH neuroendocrine terminals, but not the CRH cell bodies, results in a robust increase in CRH release (51). Taken together, results of these studies suggest that NO may increase secretion of ACTH through actions on both CRH neurons in the PVN and CRH nerve terminals in the median eminence (49).
At the median eminence, secretion of neuropeptides into the pituitary portal circulation is regulated by complex mechanisms, which involve reversible retraction of specialized ependymoglial cells, tanycytes, and subsequent formation of neurohemal junctions, a process that facilitates secretion of neuropeptides into the portal capillaries and can occur within a few hours (48). Although most studies evaluating mechanisms for morphological plasticity and neuropeptide secretion at the median eminence have focused on hormonal regulation of gonadotropin-releasing hormone secretion, similar processes are likely to be involved in the release of other neuropeptides, such as CRH (27).
NO has been implicated in tanycyte plasticity to facilitate secretion of neuropeptides (47, 48). Herbison et al (23) previously showed that nNOS-IR was located in the internal zone of the median eminence, and our experiments demonstrate a clear anatomical distinction between nNOS-IR in the internal zone and CRH-IR puncta in the external zone. Different from nNOS, eNOS-IR has been identified at the distal border of the external zone of the median eminence in pituitary portal capillaries, which are in close proximity to neurosecretory terminals (42, 49). This anatomical arrangement is consistent with the concept that eNOS, rather than nNOS, may be the major source of NO that interacts with tanycyte end-feet (49). The importance of eNOS as a source of NO in the median eminence is supported by experiments in which knock-down of eNOS blocked tanycyte plasticity in median eminence cell cultures, which included both tanycytes and endothelial cells (16). Important to the current study, it is known that hypoxia increases plasma levels of CRH (22) and also stimulates vascular production of NO (40). Given the close anatomical association of CRH terminals and the pituitary portal vessels at the external zone of the median eminence, it is plausible to propose that hypoxia-induced increases in vascular NO could contribute to increased CRH secretion through an action at the median eminence.
In parallel with actions of NO, activation of glutamate receptors in the median eminence can initiate processes that also promote tanycyte plasticity. Both metabotropic and ionotropic glutamate receptors are present on astrocytes and tanycytes in the median eminence (27), and glutamate initiates a cascade of events, which leads to the formation of the downstream effector PGE2, stimulation of metalloproteinase activity, remodeling of the extracellular matrix, and tanycyte retraction. In addition, PGE2 can directly stimulate neuropeptide secretion from nerve terminals (47, 50). Over 90% of CRH neurons in the PVN express mRNA for type 2 vesicular glutamate transporter (VGLUT2), and CRH-IR axon varicosities in the median eminence colabel with VGLUT2-IR (25), suggesting that glutamate is an important cotransmitter in CRH neurons. In the current experiments, we used an AAV with a CRH promoter to overexpress eGFP in CRH neurons in the PVN and verified that PVN eGFP fibers projecting to the median eminence were immunopositive for CRH. We demonstrated for the first time that CRH-IR and VGLUT2-IR had a similar distribution, with punctate labeling in the median eminence that often outlined tanycyte processes. Thus, our experiments in the PVN found that hypoxia activated CRH neurosecretory neurons, which likely contain glutamate, and these initial findings in the median eminence provide an anatomical substrate for a potential role of glutamate, as a cotransmitter, to promote tanycyte retraction and CRH secretion. To definitively demonstrate morphological plasticity due to hypoxia, electron microscopy experiments will be required, similar to those that demonstrated tanycyte remodeling in relationship to GnRH nerve terminals during the proestrous stage of the estrous cycle (48).
One caveat to the current experiments is that, although we demonstrated that microinjection of pCRH AAV-eGFP into the PVN infected CRH-IR neurons in the PVN and that eGFP fibers in the median eminence contained CRH-IR, we rarely saw evidence of colabeling of eGFP and CRH-IR in what were likely CRH terminal fields. The pCRH-AAV-eGFP construct targets CRH cells in the PVN, but the molecule that is overexpressed in the CRH neurons is eGFP. Similar to other neuropeptides such as AVP (71), it is possible that recognition of specific elements in the CRH molecule may be necessary to initiate packaging of the peptide into secretory granules. It is likely that eGFP is not recognized endogenously for vesicular packaging, and, therefore, would not be present in CRH neurosecretory granules. The interrupted pattern of CRH-IR labeling seen along eGFP fibers in the median eminence would be consistent with concentration of CRH-IR, but not eGFP, in secretory boutons. This potential technical limitation in using viral constructs for overexpression of fluorescent markers is important to recognize.
Perspectives and Significance
Although sympathetic nerve activity is increased following hypoxia (43), presympathetic neurons in the PVN were not activated, which suggests that sympathoexcitation in response to moderate hypoxia is mediated by a chemoreflex pathway that does not include presympathetic neurons in the PVN. Hypoxia activated nNOS neurons throughout the PVN, and a portion of AVP neurons contained nNOS. However, only AVP neurons that did not contain nNOS were activated by acute hypoxia. These findings are consistent with negative modulation of PreS and AVP-IR neurons by NO. In contrast, CRH-nNOS colabeled cells in the PVN were activated by hypoxia, consistent with literature suggesting that NO may facilitate CRH secretion by an action in the PVN (62). Hypoxia increases NO production in the vasculature (40), and, along with glutamate, NO promotes morphological plasticity at the median eminence (48), which would facilitate CRH secretion into the portal circulation. Data from the median eminence in the current experiments provide an anatomical substrate for an interaction of tanycytes and glutamate, a cotransmitter with CRH (25), which could promote tanycyte retraction, access to the portal circulation (and vascular NO) and facilitate CRH secretion. Our results suggesting a differential role of NO on AVP vs. CRH secretion are consistent with the observation that, although moderate hypoxia activates both AVP and medial parvocellular neurons in the PVN, increasing severity of hypoxia results in further activation of putative CRH, but not AVP-IR neurons (63). It is possible that as NO levels increase with severity of hypoxia, differential modulation of neurosecretory neurons by NO becomes more evident. Potentiating effects of NO on CRH neurons would contribute to further increases in CRH secretion, while inhibitory effects of NO on AVP neurons would prevent further increases in secretion of AVP.
Finally, it should be noted that in the current experiments, we specifically focused on effects of a moderate hypoxic stimulus on presympathetic and neuroendocrine cells in the PVN. However, the PVN has both direct and indirect connections to the phrenic motor nucleus and the diaphragm, the activation of which increases ventilation (59). Breathing was not evaluated in the current study, but recent experiments from our group demonstrated that a hypoxic stimulus with a level of O2 deprivation similar to that used in the current experiments increased respiratory rate and tidal volume, and lesion of catecholaminergic inputs to the PVN attenuated ventilatory responses to hypoxic stimuli (30).
GRANTS
Support for this work was provided by a National Heart, Lung, and Blood Institute Multi-PI grant to C. M. Heesch, E. M. Hasser, and D. D. Kline (R01-HL-098602).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M.C., D.-P.L., D.D.K., E.M.H., and C.M.H. conceived and designed research; K.M.C., D.-P.L., and C.M.H. performed experiments; K.M.C. and C.M.H. analyzed data; K.M.C. and C.M.H. interpreted results of experiments; K.M.C. and C.M.H. prepared figures; K.M.C. and C.M.H. drafted manuscript; K.M.C., D.-P.L., D.D.K., E.M.H., and C.M.H. edited and revised manuscript; K.M.C., D.-P.L., D.D.K., E.M.H., and C.M.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Luise King for advice on immunohistochemistry techniques at the inception of these experiments and Sean McCalmon and Charles Berka for assistance in conducting the immunohistochemistry protocols. In addition, we acknowledge Glenn Phaup for excellent technical contributions to central nervous system microinjection procedures.
REFERENCES
- 1.Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension : 275–280, 2002. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson L, Batten TF, Corbett EK, Sinfield JK, Deuchars J. Subcellular localization of neuronal nitric oxide synthase in the rat nucleus of the solitary tract in relation to vagal afferent inputs. Neuroscience : 115–122, 2003. doi: 10.1016/S0306-4522(02)00946-6. [DOI] [PubMed] [Google Scholar]
- 3.Badoer E. Proceedings of the Australian Physiological and Pharmacological Society Symposium: The Hypothalamus. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol : 95–99, 2001. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- 4.Badoer E, Merolli J. Neurons in the hypothalamic paraventricular nucleus that project to the rostral ventrolateral medulla are activated by haemorrhage. Brain Res : 317–320, 1998. doi: 10.1016/S0006-8993(98)00140-1. [DOI] [PubMed] [Google Scholar]
- 5.Bains JS, Ferguson AV. Nitric oxide regulates NMDA-driven GABAergic inputs to type I neurones of the rat paraventricular nucleus. J Physiol : 733–746, 1997. doi: 10.1113/jphysiol.1997.sp021965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benarroch EE. Paraventricular nucleus, stress response, and cardiovascular disease. Clin Auton Res : 254–263, 2005. doi: 10.1007/s10286-005-0290-7. [DOI] [PubMed] [Google Scholar]
- 7.Bugajski J, Gadek-Michalska A, Głód R, Borycz J, Bugajski AJ. Blockade of nitric oxide formation impairs adrenergic-induced ACTH and corticosterone secretion. J Physiol Pharmacol : 327–334, 1999. [PubMed] [Google Scholar]
- 8.Chan RK, Sawchenko PE. Spatially and temporally differentiated patterns of c-fos expression in brainstem catecholaminergic cell groups induced by cardiovascular challenges in the rat. J Comp Neurol : 433–460, 1994. doi: 10.1002/cne.903480309. [DOI] [PubMed] [Google Scholar]
- 9.Chen XQ, Du JZ. Hypoxia induces oxytocin release in the rat. Neuro Endocrinol Lett : 373–378, 1999. [PubMed] [Google Scholar]
- 10.Cruz JC, Bonagamba LG, Machado BH, Biancardi VC, Stern JE. Intermittent activation of peripheral chemoreceptors in awake rats induces Fos expression in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus. Neuroscience : 463–472, 2008. doi: 10.1016/j.neuroscience.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dampney RA, Horiuchi J. Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog Neurobiol : 359–384, 2003. doi: 10.1016/j.pneurobio.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Das M, Vihlen CS, Legradi G. Hypothalamic and brainstem sources of pituitary adenylate cyclase-activating polypeptide nerve fibers innervating the hypothalamic paraventricular nucleus in the rat. J Comp Neurol : 761–776, 2007. doi: 10.1002/cne.21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day HE, Campeau S, Watson SJ Jr, Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat : 115–139, 1997. doi: 10.1016/S0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- 14.Day HE, Campeau S, Watson SJ Jr, Akil H. Expression of alpha(1b) adrenoceptor mRNA in corticotropin-releasing hormone-containing cells of the rat hypothalamus and its regulation by corticosterone. J Neurosci : 10,098–10,106, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Oliveira RM, Aparecida Del Bel E, Mamede-Rosa ML, Padovan CM, Deakin JF, Guimarães FS. Expression of neuronal nitric oxide synthase mRNA in stress-related brain areas after restraint in rats. Neurosci Lett : 123–126, 2000. doi: 10.1016/S0304-3940(00)01287-8. [DOI] [PubMed] [Google Scholar]
- 16.de Seranno S, d’Anglemont de Tassigny X, Estrella C, Loyens A, Kasparov S, Leroy D, Ojeda SR, Beauvillain JC, Prevot V. Role of estradiol in the dynamic control of tanycyte plasticity mediated by vascular endothelial cells in the median eminence. Endocrinology : 1760–1772, 2010. doi: 10.1210/en.2009-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Seranno S, Estrella C, Loyens A, Cornea A, Ojeda SR, Beauvillain JC, Prevot V. Vascular endothelial cells promote acute plasticity in ependymoglial cells of the neuroendocrine brain. J Neurosci : 10353–10363, 2004. doi: 10.1523/JNEUROSCI.3228-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deurveilher S, Lo H, Murphy JA, Burns J, Semba K. Differential c-Fos immunoreactivity in arousal-promoting cell groups following systemic administration of caffeine in rats. J Comp Neurol : 667–689, 2006. doi: 10.1002/cne.21084. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus—a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets : 717–727, 2008. doi: 10.1517/14728222.12.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffen SC, Raff H. Vasopressin responses to hypoxia in conscious rats: interaction with water restriction. J Endocrinol : 61–66, 1990. doi: 10.1677/joe.0.1250061. [DOI] [PubMed] [Google Scholar]
- 21.Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol : 1511–1562, 2014. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao K, Kong FP, Gao YQ, Tang JW, Chen J, Evans AM, Lightman SL, Chen XQ, Du JZ. Inactivation of corticotropin-releasing hormone-induced insulinotropic role by high-altitude hypoxia. Diabetes : 785–795, 2015. doi: 10.2337/db14-0500. [DOI] [PubMed] [Google Scholar]
- 23.Herbison AE, Simonian SX, Norris PJ, Emson PC. Relationship of neuronal nitric oxide synthase immunoreactivity to GnRH neurons in the ovariectomized and intact female rat. J Neuroendocrinol : 73–82, 1996. doi: 10.1111/j.1365-2826.1996.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 24.Hirooka Y, Polson JW, Potts PD, Dampney RA. Hypoxia-induced Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla. Neuroscience : 1209–1224, 1997. doi: 10.1016/S0306-4522(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 25.Hrabovszky E, Wittmann G, Turi GF, Liposits Z, Fekete C. Hypophysiotropic thyrotropin-releasing hormone and corticotropin-releasing hormone neurons of the rat contain vesicular glutamate transporter-2. Endocrinology : 341–347, 2005. doi: 10.1210/en.2004-0856. [DOI] [PubMed] [Google Scholar]
- 26.Ishizuka Y, Ishida Y, Jin Q, Kato K, Kunitake T, Mitsuyama Y, Kannan H. Differential profiles of nitric oxide and norepinephrine releases in the paraventricular nucleus region in response to mild footshock in rats. Brain Res : 17–25, 2000. doi: 10.1016/S0006-8993(00)02061-8. [DOI] [PubMed] [Google Scholar]
- 27.Kawakami S. Glial and neuronal localization of ionotropic glutamate receptor subunit-immunoreactivities in the median eminence of female rats: GluR2/3 and GluR6/7 colocalize with vimentin, not with glial fibrillary acidic protein (GFAP). Brain Res : 198–204, 2000. doi: 10.1016/S0006-8993(00)01980-6. [DOI] [PubMed] [Google Scholar]
- 28.King TL, Heesch CM, Clark CG, Kline DD, Hasser EM. Hypoxia activates nucleus tractus solitarii neurons projecting to the paraventricular nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol : R1219–R1232, 2012. doi: 10.1152/ajpregu.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King TL, Kline DD, Ruyle BC, Heesch CM Hasser EM. Acute systemic hypoxia activates hypothalamic paraventricular nucleus-projecting catecholaminergic neurons in the caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol : R1112–R1123, 2013. doi: 10.1152/ajpregu.00280.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King TL, Ruyle BC, Kline DD, Heesch CM, Hasser EM. Catecholaminergic neurons projecting to the paraventricular nucleus of the hypothalamus are essential for cardiorespiratory adjustments to hypoxia. Am J Physiol Regul Integr Comp Physiol : R721–R731, 2015. doi: 10.1152/ajpregu.00540.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kishimoto J, Tsuchiya T, Emson PC, Nakayama Y. Immobilization-induced stress activates neuronal nitric oxide synthase (nNOS) mRNA and protein in hypothalamic-pituitary-adrenal axis in rats. Brain Res : 159–171, 1996. doi: 10.1016/0006-8993(96)00101-1. [DOI] [PubMed] [Google Scholar]
- 32.Kline DD, King TL, Austgen JR, Heesch CM, Hasser EM. Sensory afferent and hypoxia-mediated activation of nucleus tractus solitarius neurons that project to the rostral ventrolateral medulla. Neuroscience : 510–527, 2010. doi: 10.1016/j.neuroscience.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kvochina L, Hasser EM, Heesch CM. Pregnancy increases baroreflex-independent GABAergic inhibition of the RVLM in rats. Am J Physiol Regul Integr Comp Physiol : R2295–R2305, 2007. doi: 10.1152/ajpregu.00365.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S, Kim CK, Rivier C. Nitric oxide stimulates ACTH secretion and the transcription of the genes encoding for NGFI-B, corticotropin-releasing factor, corticotropin-releasing factor receptor type 1, and vasopressin in the hypothalamus of the intact rat. J Neurosci : 7640–7647, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy BH, Tasker JG. Synaptic regulation of the hypothalamic-pituitary-adrenal axis and its modulation by glucocorticoids and stress. Front Cell Neurosci : 24, 2012. doi: 10.3389/fncel.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li DP, Chen SR, Pan HL. Nitric oxide inhibits spinally projecting paraventricular neurons through potentiation of presynaptic GABA release. J Neurophysiol : 2664–2674, 2002. doi: 10.1152/jn.00540.2002. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Zhang W, Stern JE. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: role of GABA. Neuroscience : 585–601, 2003. doi: 10.1016/S0306-4522(03)00042-3. [DOI] [PubMed] [Google Scholar]
- 38.Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand : 17–26, 2003. doi: 10.1046/j.1365-201X.2003.01043.x. [DOI] [PubMed] [Google Scholar]
- 39.Markakis EA, Palmer TD, Randolph-Moore L, Rakic P, Gage FH. Novel neuronal phenotypes from neural progenitor cells. J Neurosci : 2886–2897, 2004. doi: 10.1523/JNEUROSCI.4161-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marshall JM. The Joan Mott Prize Lecture. The integrated response to hypoxia: from circulation to cells. Exp Physiol : 449–470, 1999. doi: 10.1111/j.1469-445X.1999.01884.x. [DOI] [PubMed] [Google Scholar]
- 41.Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci : 2633–2642, 2004. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mullier A, Bouret SG, Prevot V, Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol : 943–962, 2010. doi: 10.1002/cne.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murasato Y, Hirakawa H, Harada Y, Nakamura T, Hayashida Y. Effects of systemic hypoxia on R-R interval and blood pressure variabilities in conscious rats. Am J Physiol Heart Circ Physiol : H797–H804, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Olivan MV, Bonagamba LG, Machado BH. Involvement of the paraventricular nucleus of the hypothalamus in the pressor response to chemoreflex activation in awake rats. Brain Res : 167–172, 2001. doi: 10.1016/S0006-8993(01)02067-4. [DOI] [PubMed] [Google Scholar]
- 45.Osborn M, Debus E, Weber K. Monoclonal antibodies specific for vimentin. Eur J Cell Biol : 137–143, 1984. [PubMed] [Google Scholar]
- 46.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Elsevier, 2007. [Google Scholar]
- 47.Prevot V. Glial-neuronal-endothelial interactions are involved in the control of GnRH secretion. J Neuroendocrinol : 247–255, 2002. doi: 10.1046/j.0007-1331.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- 48.Prevot V, Bellefontaine N, Baroncini M, Sharif A, Hanchate NK, Parkash J, Campagne C, de Seranno S. Gonadotrophin-releasing hormone nerve terminals, tanycytes and neurohaemal junction remodelling in the adult median eminence: functional consequences for reproduction and dynamic role of vascular endothelial cells. J Neuroendocrinol : 639–649, 2010. doi: 10.1111/j.1365-2826.2010.02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prevot V, Bouret S, Stefano GB, Beauvillain J. Median eminence nitric oxide signaling. Brain Res Brain Res Rev : 27–41, 2000. doi: 10.1016/S0165-0173(00)00035-7. [DOI] [PubMed] [Google Scholar]
- 50.Prevot V, Cornea A, Mungenast A, Smiley G, Ojeda SR. Activation of erbB-1 signaling in tanycytes of the median eminence stimulates transforming growth factor β1 release via prostaglandin E2 production and induces cell plasticity. J Neurosci : 10,622–10,632, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prevot V, Rialas CM, Croix D, Salzet M, Dupouy JP, Poulain P, Beauvillain JC, Stefano GB. Morphine and anandamide coupling to nitric oxide stimulates GnRH and CRF release from rat median eminence: neurovascular regulation. Brain Res : 236–244, 1998. doi: 10.1016/S0006-8993(98)00066-3. [DOI] [PubMed] [Google Scholar]
- 52.Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation. J Chem Neuroanat : 197–208, 2009. doi: 10.1016/j.jchemneu.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 53.Raff H, Fagin KD. Measurement of hormones and blood gases during hypoxia in conscious cannulated rats. J Appl Physiol : 1426–1430, 1984. [DOI] [PubMed] [Google Scholar]
- 54.Reddy MK, Patel KP, Schultz HD. Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes. Am J Physiol Regul Integr Comp Physiol : R789–R797, 2005. doi: 10.1152/ajpregu.00222.2005. [DOI] [PubMed] [Google Scholar]
- 55.Rowell LB, Johnson DG, Chase PB, Comess KA, Seals DR. Hypoxemia raises muscle sympathetic activity but not norepinephrine in resting humans. J Appl Physiol (1985) : 1736–1743, 1989. [DOI] [PubMed] [Google Scholar]
- 56.Russo D, Clavenzani P, Sorteni C, Bo Minelli L, Botti M, Gazza F, Panu R, Ragionieri L, Chiocchetti R. Neurochemical features of boar lumbosacral dorsal root ganglion neurons and characterization of sensory neurons innervating the urinary bladder trigone. J Comp Neurol : 342–366, 2013. doi: 10.1002/cne.23177. [DOI] [PubMed] [Google Scholar]
- 57.Saito M, Mano T, Iwase S, Koga K, Abe H, Yamazaki Y. Responses in muscle sympathetic activity to acute hypoxia in humans. J Appl Physiol (1985) : 1548–1552, 1988. [DOI] [PubMed] [Google Scholar]
- 58.Sawchenko PE. Evidence for differential regulation of corticotropin-releasing factor and vasopressin immunoreactivities in parvocellular neurosecretory and autonomic-related projections of the paraventricular nucleus. Brain Res : 253–263, 1987. doi: 10.1016/0006-8993(87)91641-6. [DOI] [PubMed] [Google Scholar]
- 59.Schlenker EH. Integration in the PVN: another piece of the puzzle. Am J Physiol Regul Integr Comp Physiol : R653–R655, 2005. doi: 10.1152/ajpregu.00386.2005. [DOI] [PubMed] [Google Scholar]
- 60.Schultz HD, Li YL, Ding Y. Arterial chemoreceptors and sympathetic nerve activity: implications for hypertension and heart failure. Hypertension : 6–13, 2007. doi: 10.1161/HYPERTENSIONAHA.106.076083. [DOI] [PubMed] [Google Scholar]
- 61.Schultz HD, Sun SY. Chemoreflex function in heart failure. Heart Fail Rev : 45–56, 2000. doi: 10.1023/A:1009846123893. [DOI] [PubMed] [Google Scholar]
- 62.Seo DO, Rivier C. Microinfusion of a nitric oxide donor in discrete brain regions activates the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol : 925–933, 2001. doi: 10.1046/j.1365-2826.2001.00690.x. [DOI] [PubMed] [Google Scholar]
- 63.Smith DW, Buller KM, Day TA. Role of ventrolateral medulla catecholamine cells in hypothalamic neuroendocrine cell responses to systemic hypoxia. J Neurosci : 7979–7988, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci : 383–395, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Son SJ, Filosa JA, Potapenko ES, Biancardi VC, Zheng H, Patel KP, Tobin VA, Ludwig M, Stern JE. Dendritic peptide release mediates interpopulation crosstalk between neurosecretory and preautonomic networks. Neuron : 1036–1049, 2013. doi: 10.1016/j.neuron.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stern JE. Nitric oxide and homeostatic control: an intercellular signalling molecule contributing to autonomic and neuroendocrine integration? Prog Biophys Mol Biol : 197–215, 2004. doi: 10.1016/j.pbiomolbio.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 67.Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol : R1172–R1183, 2004. doi: 10.1152/ajpregu.00394.2004. [DOI] [PubMed] [Google Scholar]
- 68.Stocker SD, Keith KJ, Toney GM. Acute inhibition of the hypothalamic paraventricular nucleus decreases renal sympathetic nerve activity and arterial blood pressure in water-deprived rats. Am J Physiol Regul Integr Comp Physiol : R719–R725, 2004. doi: 10.1152/ajpregu.00494.2003. [DOI] [PubMed] [Google Scholar]
- 69.Trzebski A, Tafil M, Zoltowski M, Przybylski J. Increased sensitivity of the arterial chemoreceptor drive in young men with mild hypertension. Cardiovasc Res : 163–172, 1982. doi: 10.1093/cvr/16.3.163. [DOI] [PubMed] [Google Scholar]
- 70.Wood J, Garthwaite J. Models of the diffusional spread of nitric oxide: implications for neural nitric oxide signalling and its pharmacological properties. Neuropharmacology : 1235–1244, 1994. doi: 10.1016/0028-3908(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 71.Zhang BJ, Yamashita M, Fields R, Kusano K, Gainer H. EGFP-tagged vasopressin precursor protein sorting into large dense core vesicles and secretion from PC12 cells. Cell Mol Neurobiol : 581–605, 2005. doi: 10.1007/s10571-005-3970-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol : R1006–R1015, 2002. doi: 10.1152/ajpregu.00241.2001. [DOI] [PubMed] [Google Scholar]
- 73.Zhang K, Mayhan WG, Patel KP. Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol : R864–R872, 1997. [DOI] [PubMed] [Google Scholar]
- 74.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol Regul Integr Comp Physiol : R728–R734, 1998. [DOI] [PubMed] [Google Scholar]