Abstract
Despite recent advances in the knowledge of the neural control of cardiovascular function, the cause of sympathetic overactivity in neurogenic hypertension remains unknown. Studies from our laboratory point out that rats submitted to chronic intermittent hypoxia (CIH), an experimental model of neurogenic hypertension, present changes in the central respiratory network that impact the pattern of sympathetic discharge and the levels of arterial pressure. In addition to the fine coordination of respiratory muscle contraction and relaxation, which is essential for O2 and CO2 pulmonary exchanges, neurons of the respiratory network are connected precisely to the neurons controlling the sympathetic activity in the brain stem. This respiratory-sympathetic neuronal interaction provides adjustments in the sympathetic outflow to the heart and vasculature during each respiratory phase according to the metabolic demands. Herein, we report that CIH-induced sympathetic over activity and mild hypertension are associated with increased frequency discharge of ventral medullary presympathetic neurons. We also describe that their increased frequency discharge is dependent on synaptic inputs, mostly from neurons of the brain stem respiratory network, rather than changes in their intrinsic electrophysiological properties. In perspective, we are taking into consideration the possibility that changes in the central respiratory rhythm/pattern generator contribute to increased sympathetic outflow and the development of neurogenic hypertension. Our experimental evidence provides support for the hypothesis that changes in the coupling of respiratory and sympathetic networks might be one of the unrevealed secrets of neurogenic hypertension in rats.
Keywords: neurogenic hypertension, sympathetic overactivity, presympathetic neurons, respiratory neurons, autonomic and respiratory networks
in this review, we explore how breathing modulates sympathetic activity and cardiovascular function under physiological and pathophysiological conditions. The concept of respiratory-sympathetic interaction is not novel in the cardiovascular field, and it was originated from the fundamental work and thoughtful observations by Carl Ludwig and colleagues in Germany (see Seller; Ref 53) during the second half of the 19th century. Although Ludwig and colleagues were not able to record sympathetic nerves due to technological limitations at that time, they performed simultaneous recordings of the intrathoracic pressure and the changes in arterial blood pressure using their own developed kymograph drums. Based on these recordings, Ludwig and colleagues proposed a correlation between blood pressure and breathing, which was the base for the original concept of the coupling between the respiratory and cardiovascular functions (53). In addition to this concept, Ludwig’s laboratory introduced other key fundamental concepts in cardiovascular physiology, including 1) the description of the sinus arrhythmia and its dependency of the vagus nerve integrity, 2) the experimental evidence about the activity of depressor nerve (Cyon), and 3) the description of ventral medullary neurons that send projections to the spinal cord and contribute to the generation of sympathetic tone. Therefore, the experiments performed in the Ludwig laboratory brought to the field several concepts that remain as the groundwork for the contemporary cardiovascular physiology.
The original concepts by Ludwig’s laboratory (53) related to cardiovascular and respiratory interactions are discussed in this review in the context of physiological and pathophysiological conditions, such as neurogenic hypertension. We hope that the experimental data presented here, obtained using new technologies and revealing the mechanisms of correlation between ventral medullary sympatho-excitatory and respiratory neurons, extend Ludwig’s findings into a contemporary view that improves our understanding of the mechanisms underlying development of neurogenic hypertension associated with chronic intermittent hypoxia. Our recent data summarized here are additional bricks in a huge building construction by the community of autonomic, respiratory, and cardiovascular physiologists initiated by Carl Ludwig.
Traube-Hering Waves, Sinus Arrhythmia, and Sympathetic Oscillations
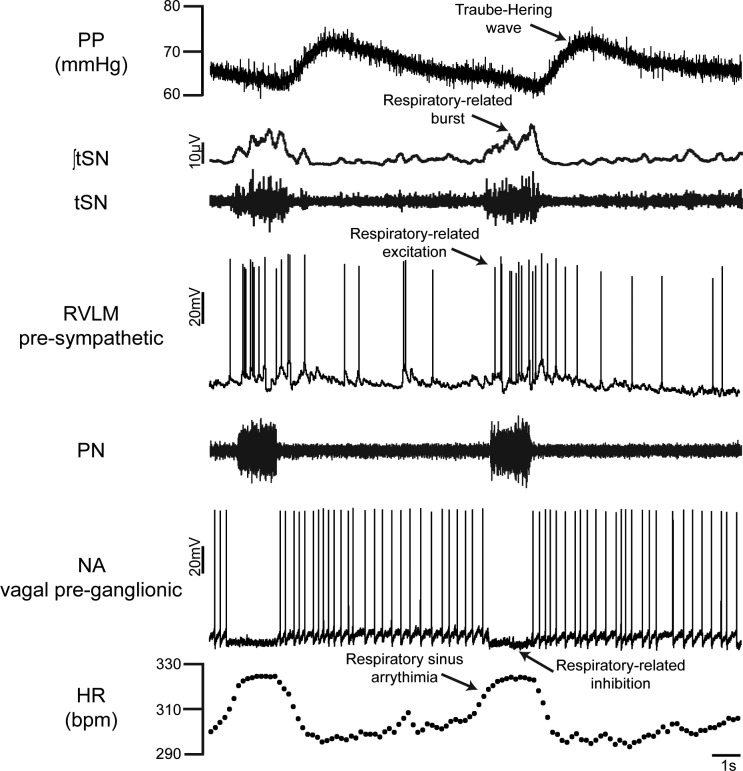
In the context that respiration influences the cardiovascular system, Ludwig Traube (58) and Ewald Hering (24), during the second half of the 19th century, reported the existence of respiratory-related oscillations in arterial blood pressure, which were later named as Traube-Hering waves. Some of the original observations indicated that these waves were not the consequence of changes in the intrathoracic pressure but were due to respiratory-related fluctuations in sympathetic outflow promoting phasic constrictions of the arterial vasculature. In fact, as observed in the recordings of the thoracic sympathetic nerve (tSN) and perfusion pressure (PP) of the decerebrated, arterially perfused in situ working heart/brain stem preparation (WHBP) of rat, a constant latency is observed between the respiratory-related peak of sympathetic activity and the correspondent peak in PP (Fig. 1). Such respiratory-related sympathetic oscillations play an important role in the maintenance of arterial blood pressure levels, because central respiratory arrest significantly reduces levels of PP and the magnitude of the sympathetic outflow oscillations, which are restored only when respiration returns to a eupneic pattern, as documented by Simms et al. (55).
Fig. 1.
Representative simultaneous recordings of phrenic (PN) and thoracic sympathetic (tSN) nerve activities, perfusion pressure (PP), intracellular recordings of the rostral ventrolateral medulla (RVLM) presympathetic neuron and nucleus ambiguus (NA) vagal preganglionic neuron, and heart rate (HR) from 1 in situ working heart/brain stem preparation of rat. Note the respiratory-related excitation of RVLM presympathetic neuron during inspiration (coincident with PN discharge), the associated burst in tSN, and the consequent Traube-Hering wave, as well as the respiratory sinus arrhythmia of HR (increase in the heartbeats during PN discharge) mediated by respiratory-related inhibition of vagal preganglionic neuron in NA.
The respiratory sinus arrhythmia is another important aspect of respiratory modulation of the autonomic activity to cardiovascular function. Original studies by Anrep et al. (8) documented an increased number of heartbeats during the inspiratory phase of intact animals, which is also observed in the recordings of heart rate (HR) in different phases of the respiratory cycle performed in the in situ preparations (Fig. 1). Later, it was demonstrated that this inspiratory-related increase in HR is mainly the consequence of inspiratory inhibition of the cardiac vagal preganglionic neurons in the nucleus ambiguus (NA; Fig. 1), thus reducing the parasympathetic tone to the heart (50). Functionally, respiratory sinus arrhythmia represents a precise mechanism to increase cardiac output to the pulmonary and systemic circulations during a period of lung expansion and favorable conditions of blood gas exchange. An elegant demonstration of this concept was provided by experiments demonstrating that respiratory sinus arrhythmia optimizes the pulmonary perfusion-to-ventilation ratio in dogs, with consequent reduction in the pulmonary shunt (23). It has also been proposed that cardiorespiratory coupling may reduce cardiac work while maintaining the blood gas partial pressure at normal levels (10) as well as buffer variations in left cardiac output and arterial pressure at each respiratory cycle (19). In studies from our laboratory using simultaneous recordings of the diaphragmatic electromyogram and the pulsatile arterial pressure, we demonstrated clearly the physiological impacts of respiratory movements on pulsatile arterial pressure (Traube-Hering waves) and HR (respiratory sinus arrhythmia) in rats, as illustrated by Moraes et al. (41).
Since the pioneering work by Adrian et al. (6), respiration has been considered an important modulator of sympathetic outflow. This classical study by Adrian et al. (6) was the first to report the presence of respiratory-related oscillations in the sympathetic activity, and they suggested that “the respiratory grouping of sympathetic activity is due to a direct action of the respiratory centre on the vaso-motor centre.” The respiratory modulation of sympathetic outflow is in part the consequence of changes in venous return due to intrathoracic pressure oscillations and also activation of pulmonary stretch receptors. However, respiratory-related sympathetic bursts persist after decerebration, vagotomy, baroreceptor denervation, and bilateral pneumonectomy (9). These findings support the concept that central respiratory modulation of sympathetic outflow occurs primarily at the level of the medulla oblongata. In fact, the neurons that are part of the respiratory and cardiovascular neuronal networks are intermingled in the brain stem, especially at the ventral medullary region. More than just a close location, the synaptic connectivity between these neuronal networks suggests the existence of a common cardiorespiratory oscillator (50). However, the identification of a specific pattern of interaction between these networks has been difficult to investigate due to technical limitations in the past. This important issue was targeted by several recent studies from our laboratory (37, 39, 43, 60, 61).
Modulation of Ventral Medullary Presympathetic Neurons By the Respiratory Network
The ventral medullary presympathetic neurons of the rostral ventrolateral medulla (RVLM) were identified as a key region generating the ongoing excitatory drive to vasomotor preganglionic sympathetic neurons in the spinal cord. Pioneering studies during the second half of the last century demonstrated that excitation of this region with microinjections of l-glutamate increases the arterial pressure (14, 15), whereas bilateral lesion reduces the sympathetic outflow to levels observed after spinal transection (51). The RVLM contains two major groups of glutamatergic neurons projecting to vasomotor preganglionic neurons: 1) one expressing all the enzymes required for synthetizing epinephrine, which is identified as the C1 cell group; and 2) the other noncatecholaminergic neurons (52, 57). Because of the advancement in electrophysiological techniques, it became possible to record presympathetic neurons simultaneously with the phrenic nerve (PN) activity and characterize their patterns of discharge according to the respiratory cycle. With this approach, studies by Haselton and Guyenet (22) documented different subtypes of sympatho-excitatory bulbo-spinal RVLM presympathetic neurons that display distinct patterns of firing frequency (excitation, inhibition, or no change) according to the phase of the respiratory cycle. Among the subpopulations of neurons affected by respiration, some of them are classified as those increasing their frequency discharge during the early inspiratory phase (early-I; see the RVLM presympathetic neuron increasing its firing frequency at inspiration, PN, generating the respiratory-related burst in tSN in Fig. 1) or during the postinspiratory phase (post-I). There is also a subpopulation of presympathetic neurons that is inhibited during the inspiration, as illustrated by the extracellular recordings by Haselton and Guyenet (22) or by the intracellular recordings by Moraes et al. (37).
Challenges to Sympathetic-Respiratory Coupling
The pattern of respiratory modulation of sympathetic outflow varies according to the sympathetic branch recorded. Under baseline conditions, cervical and lumbar sympathetic nerve activities are least active during early inspiration and present maximal activity postinspiration (44). In contrast, the splanchnic, cardiac, renal, and adrenal sympathetic nerves exhibit their peak activity during early inspiration (44). These observations suggest that the distinct subpopulations of presympathetic neurons described previously in the RVLM generate the different respiratory-related activity profiles among sympathetic nerves. At the same time, the resting respiratory pattern in PN activity is characterized by an active inspiratory phase, with the contraction of pumping muscles that drives inspiratory flow, whereas expiration is mostly passive, with the expiratory flow generated by the recoil forces of the lungs (20, 26). Under conditions of low respiratory drive (e.g., hypocapnia), the respiratory-related bursts in sympathetic activity disappear (40), indicating that baseline respiratory-sympathetic coupling, at least to resistance vascular beds, is generated mainly by inputs from the respiratory network. On the other hand, during metabolic challenges such as hypoxia and hypercapnia, there is an enhancement in the sympathetic-respiratory coupling, with the emergence of novel expiratory-related bursts in the sympathetic activity (40). Under these challenges the abdominal expiratory motor activity is recruited, and expiration becomes an active process. Interestingly, the expiratory bursts in sympathetic activity occur phase-locked with the peaks in abdominal activity (34, 41), indicating that the sympathetic activity during hypoxia and hypercapnia is coupled not only with the inspiratory but also with the expiratory neurons. This suggests that the respiratory-sympathetic coupling mechanisms dynamically regulate sympathetic activity according to the demands of O2 and CO2 homeostasis. Moreover, this idea raises the possibility that these mechanisms are susceptible to plasticity and may introduce abnormal respiratory-related modulation in sympathetic outflow in pathological conditions, especially in the context of chronic cardiovascular diseases such as hypertension, heart failure, and obstructive sleep apnea (OSA). The potential role of altered respiratory modulation of sympathetic activity in the pathogenesis of hypertension is presented below.
Stimulation of the peripheral chemoreceptors by acute hypoxia or intravenous injection of potassium cyanide (KCN) produces autonomic and respiratory changes such as increases in the inspiratory and expiratory motor activities (active expiration), respiratory frequency, and sympathetic outflow (11, 38). The first synapse of peripheral chemoreceptor afferents is located in the nucleus tractus solitarius (NTS), where higher-order neurons may send projections to ventral medulla neurons involved with generation of sympathetic activity and to neurons of the respiratory network (3, 5, 31). The increase in sympathetic activity occurs by two distinct components: a respiratory-related oscillation that is dependent on inhibitory connections between neurons from RVLM and caudal ventral lateral medulla (CVLM) and another tonic, respiratory-independent component of which its changes are probably mediated by direct glutamatergic projections from NTS to RVLM presympathetic neurons (27, 28, 32, 42). The enhancement of expiratory activity in response to chemoreflex activation also induces additional increases in sympathetic nerve activity (38). This pattern of coupled respiratory and autonomic responses is also observed during physical activity to provide appropriated respiratory responses and simultaneous increase in the sympathetic activity to the cardiovascular system (2).
One important question addressed in several studies from our laboratory refers to the consequences of chronic activation of peripheral chemoreceptors on the modulation of the cardiovascular-sympathetic activity. To produce a chronic and intermittent activation of the peripheral chemoreceptors, a hallmark of OSA, we use the experimental model of chronic intermittent hypoxia in rats (60, 61). Although we are using chronic intermittent hypoxia (CIH) in our laboratory to produce intermittent hypoxia, it is clear for us that it is not a complete experimental model for OSA observed in humans, since rats submitted to CIH respond with hyperventilation and the level of CO2 in the arterial blood is reduced and different from OSA patients in which the upper airway obstruction also induces hypercapnia. Therefore, CIH is an experimental model that reproduces only the intermittent hypoxia stimulus observed in OSA patients. In the present review, we are focusing on the effect of intermittent hypoxia on the respiratory-sympathetic coupling.
OSA patients present recurrent obstruction of the upper airways during the natural sleep cycle, causing hypoxemia and consequent stimulation of the peripheral chemoreceptors that produces arousal, tachypnea, and increase in the sympathetic activity (13). Even during the wake period, the sympathetic activity of OSA patients remains increased, and most of them are hypertensive (12, 13). The cause of hypertension in these patients is multifactorial, but the increase in baseline sympathetic activity, consequent to intermittent hypoxia, may contribute to the hypertensive state (18, 56). This possibility is supported by clinical evidence indicating that the use of devices to produce continuous positive airflow pressure in OSA patients prevents the episodes of hypoxemia and lowers in the majority of the patients the sympathetic activity levels and the high blood pressure (46).
In our experimental model of chronic intermittent hypoxia (CIH), juvenile rats are submitted to episodes of hypoxia (6%) every 9 min for 8 h each day for 10 days, whereas control rats are kept in similar cages under normoxic conditions. We found that rats exposed to this paradigm presented, 1 day after the end of CIH protocol, a significant increase in mean arterial pressure (MAP) when compared with their respective controls, indicating that these animals developed hypertension (60, 61). We also verified that the increased MAP of CIH rats was due to augmented sympathetic activity because the depressor response to intravenous injection of hexamethonium, a ganglionic blocker, was greater in CIH than in control rats (59). In agreement with this finding, the magnitude of oscillations at low frequency range of systolic arterial pressure of CIH rats was enhanced (59), providing additional support to the concept that CIH promotes sympathetic-mediated hypertension.
Considering that previous studies described changes in the carotid body sensitization after intermittent hypoxia (47) as well in hypertensive rats (49), we should also take into account the possibility that the observed changes in the respiratory-sympathetic coupling are due to the tonicity of the glomus cells in the carotid body. However, the observed changes of the sympathetic-respiratory coupling in CIH rats were not altered after the removal of carotid chemoreceptors, indicating that these central changes seem to be initiated by intermittent activation of the chemoreflex, but they are not driven by sensitization of the carotid body (61).
Changes in Respiratory Modulation of Sympathetic Outflow Contribute to Hypertensive State in CIH Rats
Several studies described in this review were performed in the decerebrated WHBP, which is used in our laboratory due to its feasibility for nerve and intracellular neuronal recordings in the absence of anesthetics. Although the WHBP presents advantages for this kind of experimental approach, it also presents some limitations, since it is not a whole animal under the ideal physiological condition. However, we do not have available, at least in our laboratory, technologies to perform intracellular recording in the ventral medulla of conscious, freely moving rats. Therefore, we are taking advantage of this in situ WHBP preparation.
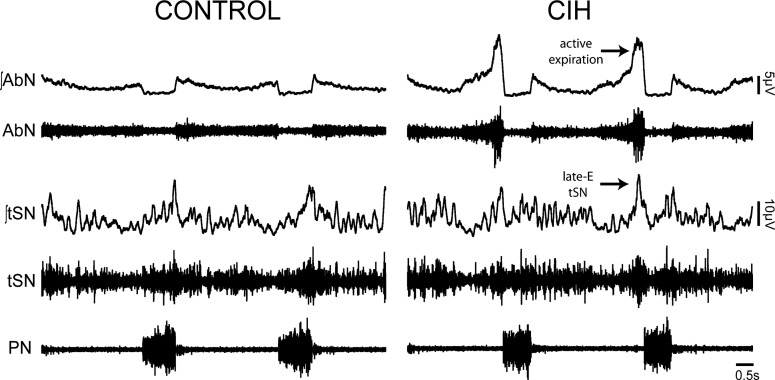
From direct recordings of the lower thoracic sympathetic chain of the in situ WHBP preparations, we documented that hypertensive CIH rats also show higher levels of baseline vasomotor activity (61). Moreover, as illustrated in the original recordings of tSN in Fig. 2, we found that the sympathetic overactivity in CIH rats was associated with additional high-amplitude bursts during the late part of expiratory phase, named late-expiration (61), and was also associated with larger Traube-Hering pressure waves (41). Thus the maximal sympathetic activity and the peak in abdominal expiratory motor activity in CIH rats were phase-locked during late expiration, indicating a correlation between sympathetic overactivity and abdominal nerve (AbN) active expiration (Fig. 2) (61). Moreover, the lowering of respiratory activity with hypocapnia discontinued AbN hyperactivity and reduced sympathetic nerve activity (34). These findings allow us to suggest that augmented sympathetic activity during expiration in CIH rats was dependent on changes of respiratory pattern and the presence of active expiration at rest (34, 61).
Fig. 2.
Recordings from representative in situ preparations illustrating the pattern of raw and integrated (∫) activities of abdominal (AbN), thoracic sympathetic (tSN), and phrenic nerves (PN) from control and chronic intermittent hypoxic (CIH) rats. Note that the control rat presents a low amplitude in AbN, indicating that the respiratory pattern is composed of active inspiration and passive expiration. The CIH rat presents enhanced AbN activity at the late part of expiration, indicating that both inspiration and expiration are active processes. As a consequence of the AbN active expiration, the tSN of CIH rats exhibits a correlated peak of discharge during the same phase of respiratory cycle/late-expiratory (late-E) tSN peak.
To verify whether the changes in the respiratory pattern after CIH were also present in conscious, freely moving rats, we recorded the electromyogram of the abdominal and diaphragm muscles simultaneously with arterial pressure (37). Under this experimental condition, we confirmed that rats exposed to CIH presented high levels of arterial pressure and a clear increase in the abdominal muscle contraction at the end of expiration. Therefore, the changes in the pattern of expiration after CIH were not an epiphenomenon observed in the in situ WHBP preparations of rats. It is important to note that the observed changes in the pattern of respiratory modulation of sympathetic activity in in situ WHBP preparation of CIH rats also correlate with larger Traube-Hering waves, indicating that the enhanced respiratory-modulated sympathetic outflow of CIH rats is clearly impacting the vasomotor control and amplifying the sympathetic activity reaching the blood vessels (37). Importantly, our previous spectral analysis of systolic blood pressure of conscious CIH and control rats also demonstrated an increase in the amplitude of high frequency variability, frequency range of Traube-Hering waves in conscious animals (59), providing additional support to the concept that the changes in respiratory-sympathetic coupling are not restricted to experiments performed in in situ rat WHBP preparations. Although we are describing several correlations between changes in the respiratory-sympathetic coupling and the baseline sympathetic activity in CIH rats, we must emphasize that these correlations do not imply a direct causal relationship, and new experiments are required to prove our complex working hypothesis.
Cardiac and Sympatho-Inhibitory Baroreflex Gains are Increased in CIH Rats
To explore the origin of sustained increase in the sympathetic activity and hypertension in CIH rats, initially we considered a possible reduction in the gain of the cardiac and sympathetic components of the baroreflex in this experimental model. In this regard, previous studies (21, 30, 48) proposed that reduction in baroreflex gain plays an important role in the development of sympathetic overactivity and hypertension after several days of CIH. On the other hand, studies by Lai et al. (29) demonstrated that rats submitted to CIH present an increase in arterial pressure after the fifth day of exposure, whereas a reduction in spontaneous cardiac baroreflex gain was not observed until the seventeenth day. Experiments performed in our laboratory using conscious rats documented that the cardiac component of the baroreflex was, in fact, increased in rats kept in the CIH protocol for 10 days (59). Similar results were also observed in experiments performed in our laboratory using in situ WHBP preparations of rats, which demonstrated that the neuronal CVLM activation and sympatho-inhibitory component of the baroreflex was also enhanced after CIH exposure (36). Therefore, these previous data support the new concept that the early phase of sympathetic overactivity and hypertension developed in juvenile rats exposed to CIH for 10 days are not secondary to reduction in both sympatho-inhibitory and cardiac components of baroreflex. However, these findings do not imply that the baroreflex control of sympathetic activity and cardiac function may be reduced after a long-term exposure to CIH (21, 29, 30), because different mechanisms might occur between the onset of hypertension and the long-term sustained increase in arterial pressure induced by CIH.
How Does Sympathetic Activity Increase During the Late-Expiratory Activity in CIH Rats?
The first possibility for explaining the sympathetic overactivity in CIH rats was an increase in the frequency of discharge of the bulbospinal presympathetic neurons located in the RVLM due to changes in their intrinsic electrophysiological properties, since there is a tendency among authors in the field to link the cause of neurogenic hypertension to electrophysiological changes in brain stem presympathetic neurons, as discussed by Accorsi-Mendonça et al. (4). To check this possibility, we intracellularly recorded the activity of the bulbo-spinal presympathetic RVLM neurons using the whole cell patch clamp technique in slices of brain stem as well in in situ WHBP preparations of CIH rats. Our findings indicated that despite the increased frequency discharge of RVLM presympathetic neurons from CIH rats in the intact respiratory and sympathetic networks (37), we verified that their intrinsic firing frequency and electrophysiological properties after blockade of fast synaptic transmission were similar to those from control rats (7), indicating that the intrinsic electrophysiological properties of presympathetic neurons were not affected by CIH. These findings also indicated that the increased frequency discharge of RVLM presympathetic neurons was probably driven by excitatory synaptic inputs to these neurons.
To explore the mechanisms underlying the increased synaptic excitatory inputs to RVLM presympathetic neurons after CIH, we recorded the spontaneous excitatory postsynaptic potentials (sEPSPs) and currents (sEPSCs) to these neurons in the in situ WHBP preparations of rats. In the current-clamp mode recordings, we verified a significant increase in sEPSP frequency during late expiration (late-E). In the voltage-clamp mode recordings, we verified a significant increase in sEPSC frequency during the same respiratory phase in RVLM presympathetic neurons from CIH rats (37). These findings indicate that presympathetic neurons from CIH rats receive additional excitatory synaptic inputs probably from neurons of the respiratory network mostly during the end of the expiratory phase (37). Therefore, the next question was: What are the possible sources of additional excitatory inputs to RVLM presympathetic neurons at the end of expiration in CIH rats?
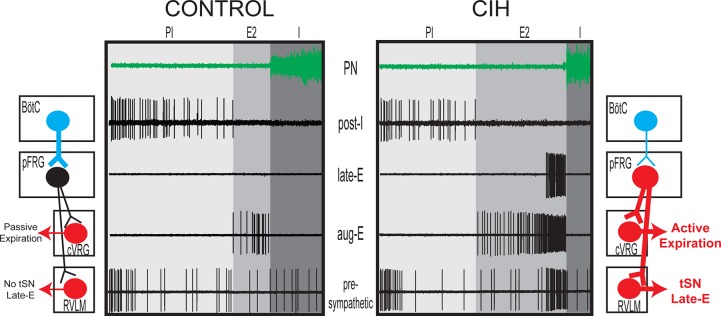
There is evidence that RVLM presympathetic neurons are modulated by synaptic inputs from a large diversity of respiratory neurons, including the ventral medullary [parafacial respiratory group (pFRG), Bötzinger (BötC), and pre-Bötzinger complexes] and pontine respiratory neurons (17). Several recent studies have suggested that pFRG neurons present characteristics consistent with a late-E oscillator, producing active expiration in juvenile/adult rats only under conditions of elevated central respiratory drive (1, 25, 33). At normocapnia, in the absence of active expiration, pFRG neurons in juvenile/adult animals are silent (1, 38, 45). When disinhibited or photoactivated, these neurons exhibit rhythmical late-E activity and generate expiratory burst in abdominal activity (16, 25, 45). The activation of the peripheral chemoreflex with KCN also activates late-E neurons in the pFRG, which correlates with the increase in the AbN and tSN activities (38). Based on these observations, we hypothesized that the pFRG expiratory neurons may be a potential source of excitatory drive to the RVLM. Using an in silico approach (34), we considered the possibility that CIH promotes a long-lasting activation of pFRG late-E neurons. As a consequence, the hyperactivity of pFRG late-E neurons after CIH would result in excessive excitatory drive to both ventral respiratory group (VRG) bulbo-spinal expiratory neurons and RVLM presympathetic neurons, resulting in the emergence of coupled late-E bursts in AbN and sympathetic neurons (34). In agreement with these model predictions, we verified that pFRG late-E neurons of in situ WHBP preparations of CIH rats, but not of controls, were active (presenting spontaneous action potentials; see the extracellular recordings of pFRG neurons from CIH rats in Fig. 3) under normocapnic conditions and correlated with the late-E bursts in sympathetic activity (43). These findings suggest that the pFRG is a potential source of excitatory inputs to RVLM at the end of expiration.
Fig. 3.
Schematic model and original extracellular recordings of interactions between ventral medullary respiratory and sympathetic neurons in control rats and in rats submitted to chronic intermittent hypoxia (CIH). In control rats at resting conditions, the inhibitory postinspiratory (post-I) neurons (blue) of the Bötzinger complex (BötC) inhibit expiratory activity by inhibiting the parafacial respiratory group (pFRG) late-expiratory (late-E) neurons. Therefore, expiration is a passive process associated with the absence of sympathetic overactivity at the end of the second half of expiration (E2 phase). CIH decreases the firing frequency and intrinsic excitability of BötC post-I neurons, thus releasing pFRG late-E neurons from inhibition and allowing them to fire at the end of E2 phase in conditions of normoxia and normocapnia. The pFRG late-E neurons are the common source of excitatory inputs (red) to the augmenting-expiratory (aug-E) neurons of the caudal ventral respiratory group (cVRG) and to the presympathetic neurons in the rostral ventrolateral medulla (RVLM) at the end of the E2 phase, generating coupled active expiration and sympathetic overactivity. I, inspiration; PN, phrenic nerve; tSN, thoracic sympathetic nerve.
The next step was to understand the mechanisms underlying the generation of active expiration after CIH exposure and the possible changes in the bulbospinal augmenting-expiratory (aug-E) neurons of the caudal VRG (cVRG). These neurons receive excitatory inputs from pFRG neurons and seem to generate late-E bursts in abdominal activity during active expiration (54). The extracellular recordings of cVRG aug-E neurons from CIH rats showed a large increase in their firing frequency when compared with neurons from control rats (Fig. 3). However, their intrinsic frequency discharge after synaptic blockade was similar between control and CIH rats (35), indicating that 1) the electrophysiological properties of cVRG aug-E neurons are not affected by CIH, and 2) the increase in the frequency discharge of aug-E neurons is probably driven by the increased activity of pFRG late-E neurons, impacting on the expiratory function after CIH.
Taking into consideration the evidence that postinspiratory neurons (post-I) located in the BötC may send inhibitory projections to the late-E neurons in the pFRG (1, 33), we explored the hypothesis that reductions of intrinsic excitability of these post-I neurons affect their inhibitory synaptic inputs to late-E in pFRG. The reduction of these inhibitory synaptic inputs may release the pFRG late-E neurons to fire at the end of expiration, which in turn produces enhanced synaptic excitation of cVRG aug-E and RVLM presympathetic neurons. The extracellular recordings of BötC post-I neurons from CIH rats showed a clear reduction in their firing frequency (Fig. 3), which was maintained lower after the synaptic blockade when compared with neurons from control rats (35). Using intracellular recordings, we also documented that resting membrane potential of the BötC post-I neurons from CIH rats was more negative, i.e., hyperpolarized, and their intrinsic input resistance was significantly reduced when compared with neurons from control rats (35). Using single-cell RT-PCR, we verified that the hyperpolarized post-I neurons of CIH rats were glycinergic, which is consistent with the neurochemical profile of these inhibitory neurons (35). These findings indicated that CIH hyperpolarizes and decreases the intrinsic excitability of BötC post-I neurons, which may be a cause of the increased activity of late-E neurons in the pFRG and the consequent increase in the frequency discharge of cVRG aug-E and RVLM presympathetic neurons, as hypothesized by Molkov et al. (34).
With respect to the electrophysiological changes observed in the BötC post-I neurons from CIH rats (hyperpolarization and reductions in input resistance), these changes are consistent with a possible increase in resting potassium conductance. In this context, we explored different channels mediating potassium currents, and we verified that CIH increased the conductance of potassium leak channels in post-I neurons (35). Based upon this finding, we suggest that the BötC post-I neurons and their potassium leak channels may be a major target of CIH, which promotes relevant changes in the expression and conductance of ionic channels, impacting on synaptic excitation to cVRG aug-E and RVLM presympathetic neurons from pFRG late-E neurons (Fig. 3). Therefore, the BötC may be a major site of interaction between the two complex neuronal networks (sympathetic and expiratory) in the context of CIH.
Summary
The experimental data described in this review allow us to state that the sympathetic overactivity observed at the end of expiration in CIH rats is mostly associated with changes in the intrinsic electrophysiological properties of the BötC post-I neurons rather than changes in the intrinsic properties of the RVLM presympathetic neurons. In fact, the RVLM presympathetic neurons are receiving increased excitatory synaptic inputs probably from pFRG late-E neurons. This possibility is supported by the observation that the intrinsic excitability of post-I neurons is reduced, which in turn may produce less inhibition on the pFRG late-E neurons. Although we do not yet have direct experimental evidence, our findings suggest that pFRG late-E neurons drive additional excitatory inputs that increase the frequency discharge of the RVLM presympathetic and cVRG aug-E neurons. The overall findings described in this review suggest that changes in neurons of the respiratory network are a main cause of sympathetic overactivity observed in CIH rats. However, it is worthy to note that our working hypothesis is complex and that some gaps remain open. For these reasons, several important unanswered questions are currently under investigation in our laboratory.
To conclude, we want to highlight that the original observations by Carl Ludwig and several other autonomic, cardiovascular, and respiratory physiologists, ever since the second half of the 19th century, about the coupling of arterial pressure and breathing remain as the groundwork for the findings and ideas described in this review. We hope that our contribution in revealing the secrets of the functioning respiratory network and its interaction with sympathetic nervous system will shed light on the mechanisms underlying neurogenic hypertension.
Perspectives and Significance
Despite several decades of investigation, we still do not have a clear picture about the mechanisms underlying the neurogenic hypertension. Neurogenic hypertension is a consequence of multiple factors affecting the sympathetic outflow to the cardiovascular system. Dysfunction in the respiratory neuronal network must be considered another natural candidate among those modulating the level of sympathetic activity. In this scenario, the full understanding of the mechanisms involved in the coupling of respiratory and sympathetic neurons at the brain stem level will be a critical step for new knowledge about interactions between cardiovascular and respiratory systems. On one side, it is true that changes of this precise coupling, as observed in the CIH experimental model, may contribute to hypertension. However, on the other side, which is supposed to be the right way, the restoration of an appropriated coupling of these two neuronal networks in case of previous dysfunction may have the power to reduce, at least in part, the sympathetic outflow and the level of high blood pressure in hypertensive patients. Therefore, the possibility that the voluntary breath pattern control may contribute to modulate sympathetic activity and be used as a natural adjuvant for lowering high blood pressure must be considered. Accordingly, the effect of respiratory control on the sympathetic outflow cannot be considered an exotic cultural and philosophical issue anymore.
GRANTS
All publications and the correspondent experiments performed in our laboratory were supported by Fundação de Amparo à Pesquisa do Estado de São Paulo and Conselho Nacional de Desenvolvimento Científico e Tecnológico.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.H.M., D.B.Z., and D.J.M. conceived and designed research; B.H.M., D.B.Z., and D.J.M. analyzed data; B.H.M., D.B.Z., and D.J.M. interpreted results of experiments; B.H.M., D.B.Z., and D.J.M. drafted manuscript; B.H.M., D.B.Z., and D.J.M. edited and revised manuscript; B.H.M., D.B.Z., and D.J.M. approved final version of manuscript; D.B.Z. and D.J.M. performed experiments; D.J.M. prepared figures.
ACKNOWLEDGMENTS
This review was presented by B. H. Machado as the Carl Ludwig Distinguished Lectureship of the American Physiological Society Neural Control and Autonomic Regulation Section during the Experimental Biology-2016 Meeting, San Diego, CA.
We thank Dr. Melina Pires da Silva Moraes for contributing to this article.
REFERENCES
- 1.Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol : 3539–3559, 2009. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham KA, Feingold H, Fuller DD, Jenkins M, Mateika JH, Fregosi RF. Respiratory-related activation of human abdominal muscles during exercise. J Physiol : 653–663, 2002. doi: 10.1113/jphysiol.2001.013462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Accorsi-Mendonça D, Castania JA, Bonagamba LG, Machado BH, Leão RM. Synaptic profile of nucleus tractus solitarius neurons involved with the peripheral chemoreflex pathways. Neuroscience : 107–120, 2011. doi: 10.1016/j.neuroscience.2011.08.054. [DOI] [PubMed] [Google Scholar]
- 4.Accorsi-Mendonça D, da Silva MP, Souza GM, Lima-Silveira L, Karlen-Amarante M, Amorim MR, Almado CE, Moraes DJ, Machado BH. Pacemaking property of RVLM presympathetic neurons. Front Physiol : 424, 2016. doi: 10.3389/fphys.2016.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Accorsi-Mendonça D, Machado BH. Synaptic transmission of baro- and chemoreceptors afferents in the NTS second order neurons. Auton Neurosci : 3–8, 2013. doi: 10.1016/j.autneu.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Adrian ED, Bronk DW, Phillips G. Discharges in mammalian sympathetic nerves. J Physiol : 115–133, 1932. doi: 10.1113/jphysiol.1932.sp002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almado CE, Leão RM, Machado BH. Intrinsic properties of rostral ventrolateral medulla presympathetic and bulbospinal respiratory neurons of juvenile rats are not affected by chronic intermittent hypoxia. Exp Physiol : 937–950, 2014. doi: 10.1113/expphysiol.2013.077800. [DOI] [PubMed] [Google Scholar]
- 8.Anrep GV, Pascual W, Rössler R. Respiratory variations of the heart rate. I. The reflex mechanism of the respiration arrhythmia. Proc R Soc Lond B Biol Sci : 191–217, 1936. doi: 10.1098/rspb.1936.0005. [DOI] [Google Scholar]
- 9.Barman SM, Gebber GL. Sympathetic nerve rhythm of brain stem origin. Am J Physiol : R42–R47, 1980. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Tal A, Shamailov SS, Paton JF. Evaluating the physiological significance of respiratory sinus arrhythmia: looking beyond ventilation-perfusion efficiency. J Physiol : 1989–2008, 2012. doi: 10.1113/jphysiol.2011.222422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braga VA, Soriano RN, Braccialli AL, de Paula PM, Bonagamba LG, Paton JF, Machado BH. Involvement of L-glutamate and ATP in the neurotransmission of the sympathoexcitatory component of the chemoreflex in the commissural nucleus tractus solitarii of awake rats and in the working heart-brainstem preparation. J Physiol : 1129–1145, 2007. doi: 10.1113/jphysiol.2007.129031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calhoun DA, Harding SM. Sleep and hypertension. Chest : 434–443, 2010. doi: 10.1378/chest.09-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med : 187–197, 2005. doi: 10.7326/0003-4819-142-3-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 14.Dampney RA. Brain stem mechanisms in the control of arterial pressure. Clin Exp Hypertens : 379–391, 1981. doi: 10.3109/10641968109033672. [DOI] [PubMed] [Google Scholar]
- 15.Dampney RA, Goodchild AK, Robertson LG, Montgomery W. Role of ventrolateral medulla in vasomotor regulation: a correlative anatomical and physiological study. Brain Res : 223–235, 1982. doi: 10.1016/0006-8993(82)90056-7. [DOI] [PubMed] [Google Scholar]
- 16.de Britto AA, Moraes DJ. Non-chemosensitive parafacial neurons simultaneously regulate active expiration and airway patency under hypercapnia in rats. J Physiol : 2043–2064, 2017. doi: 10.1113/JP273335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dempsey B, Le S, Turner A, Bokiniec P, Ramadas R, Bjaalie JG, Menuet C, Neve R, Allen AM, Goodchild AK, McMullan S. Mapping and analysis of the connectome of sympathetic premotor neurons in the rostral ventrolateral medulla of the rat using a volumetric brain atlas. Front Neural Circuits : 9, 2017. doi: 10.3389/fncir.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drager LF, Polotsky VY, O’Donnell CP, Cravo SL, Lorenzi-Filho G, Machado BH. Translational approaches to understanding metabolic dysfunction and cardiovascular consequences of obstructive sleep apnea. Am J Physiol Heart Circ Physiol : H1101–H1111, 2015. doi: 10.1152/ajpheart.00094.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elstad M. Respiratory variations in pulmonary and systemic blood flow in healthy humans. Acta Physiol (Oxf) : 341–348, 2012. doi: 10.1111/j.1748-1716.2012.02419.x. [DOI] [PubMed] [Google Scholar]
- 20.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol : 423–452, 2013. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu H, Lin M, Liu J, Gozal D, Scrogin KE, Wurster R, Chapleau MW, Ma X, Cheng ZJ. Selective impairment of central mediation of baroreflex in anesthetized young adult Fischer 344 rats after chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol : H2809–H2818, 2007. doi: 10.1152/ajpheart.00358.2007. [DOI] [PubMed] [Google Scholar]
- 22.Haselton JR, Guyenet PG. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol Regul Integr Comp Physiol : R739–R750, 1989. [DOI] [PubMed] [Google Scholar]
- 23.Hayano J, Yasuma F, Okada A, Mukai S, Fujinami T. Respiratory sinus arrhythmia. A phenomenon improving pulmonary gas exchange and circulatory efficiency. Circulation : 842–847, 1996. doi: 10.1161/01.CIR.94.4.842. [DOI] [PubMed] [Google Scholar]
- 24.Hering E. Über den Einflulß der Atmung auf den Kreislauf. Erste Mitteilung. Über Atembewegungen der Gefäßsysteme. Sitzungsber Akad Wiss PVien Math Naturw : 829–856, 1869. [Google Scholar]
- 25.Huckstepp RT, Cardoza KP, Henderson LE, Feldman JL. Role of parafacial nuclei in control of breathing in adult rats. J Neurosci : 1052–1067, 2015. doi: 10.1523/JNEUROSCI.2953-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkin SE, Milsom WK. Expiration: breathing’s other face. Prog Brain Res : 131–147, 2014. doi: 10.1016/B978-0-444-63488-7.00008-2. [DOI] [PubMed] [Google Scholar]
- 27.Koshiya N, Guyenet PG. NTS neurons with carotid chemoreceptor inputs arborize in the rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol : R1273–R1278, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Koshiya N, Huangfu D, Guyenet PG. Ventrolateral medulla and sympathetic chemoreflex in the rat. Brain Res : 174–184, 1993. doi: 10.1016/0006-8993(93)90871-J. [DOI] [PubMed] [Google Scholar]
- 29.Lai CJ, Yang CC, Hsu YY, Lin YN, Kuo TB. Enhanced sympathetic outflow and decreased baroreflex sensitivity are associated with intermittent hypoxia-induced systemic hypertension in conscious rats. J Appl Physiol (1985) : 1974–1982, 2006. doi: 10.1152/japplphysiol.01051.2005. [DOI] [PubMed] [Google Scholar]
- 30.Lin M, Liu R, Gozal D, Wead WB, Chapleau MW, Wurster R, Cheng ZJ. Chronic intermittent hypoxia impairs baroreflex control of heart rate but enhances heart rate responses to vagal efferent stimulation in anesthetized mice. Am J Physiol Heart Circ Physiol : H997–H1006, 2007. doi: 10.1152/ajpheart.01124.2006. [DOI] [PubMed] [Google Scholar]
- 31.Machado BH. Neurotransmission of the cardiovascular reflexes in the nucleus tractus solitarii of awake rats. Ann NY Acad Sci : 179–196, 2001. doi: 10.1111/j.1749-6632.2001.tb03676.x. [DOI] [PubMed] [Google Scholar]
- 32.Mandel DA, Schreihofer AM. Modulation of the sympathetic response to acute hypoxia by the caudal ventrolateral medulla in rats. J Physiol : 461–475, 2009. doi: 10.1113/jphysiol.2008.161760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molkov YI, Abdala AP, Bacak BJ, Smith JC, Paton JF, Rybak IA. Late-expiratory activity: emergence and interactions with the respiratory CpG. J Neurophysiol : 2713–2729, 2010. doi: 10.1152/jn.00334.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molkov YI, Zoccal DB, Moraes DJ, Paton JF, Machado BH, Rybak IA. Intermittent hypoxia-induced sensitization of central chemoreceptors contributes to sympathetic nerve activity during late expiration in rats. J Neurophysiol : 3080–3091, 2011. doi: 10.1152/jn.00070.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moraes D, da Silva MP, Zoccal DB, Varanda WA Machado BH. Changes in ionic currents of respiratory neurons produce sympathetic overactivity in chronic intermittent hypoxic rats. FASEB J : 1135.6, 2013. [Google Scholar]
- 36.Moraes DJ, Bonagamba LG, da Silva MP, Mecawi AS, Antunes-Rodrigues J, Machado BH. Respiratory network enhances the sympathoinhibitory component of baroreflex of rats submitted to chronic intermittent hypoxia. Hypertension : 1021–1030, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07731. [DOI] [PubMed] [Google Scholar]
- 37.Moraes DJ, da Silva MP, Bonagamba LG, Mecawi AS, Zoccal DB, Antunes-Rodrigues J, Varanda WA, Machado BH. Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats. J Neurosci : 19223–19237, 2013. doi: 10.1523/JNEUROSCI.3041-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moraes DJ, Dias MB, Cavalcanti-Kwiatkoski R, Machado BH, Zoccal DB. Contribution of the retrotrapezoid nucleus/parafacial respiratory region to the expiratory-sympathetic coupling in response to peripheral chemoreflex in rats. J Neurophysiol : 882–890, 2012. doi: 10.1152/jn.00193.2012. [DOI] [PubMed] [Google Scholar]
- 39.Moraes DJ, Machado BH, Paton JF. Specific respiratory neuron types have increased excitability that drive presympathetic neurones in neurogenic hypertension. Hypertension : 1309–1318, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02283. [DOI] [PubMed] [Google Scholar]
- 40.Moraes DJ, Machado BH, Zoccal DB. Coupling of respiratory and sympathetic activities in rats submitted to chronic intermittent hypoxia. Prog Brain Res : 25–38, 2014. doi: 10.1016/B978-0-444-63488-7.00002-1. [DOI] [PubMed] [Google Scholar]
- 41.Moraes DJ, Zoccal DB, Machado BH. Medullary respiratory network drives sympathetic overactivity and hypertension in rats submitted to chronic intermittent hypoxia. Hypertension : 1374–1380, 2012. doi: 10.1161/HYPERTENSIONAHA.111.189332. [DOI] [PubMed] [Google Scholar]
- 42.Moraes DJ, Zoccal DB, Machado BH. Sympathoexcitation during chemoreflex active expiration is mediated by L-glutamate in the RVLM/Bötzinger complex of rats. J Neurophysiol : 610–623, 2012. doi: 10.1152/jn.00057.2012. [DOI] [PubMed] [Google Scholar]
- 43.Moraes DJ, Zoccal DB, Machado BH. Intracellular recordings of respiratory and pre-sympathetic neurons in the ventrolateral medulla of rats submitted to chronic intermittent hypoxia. FASEB J : 899–891, 2012. [Google Scholar]
- 44.Numao Y, Koshiya N, Gilbey MP, Spyer KM. Central respiratory drive-related activity in sympathetic nerves of the rat: the regional differences. Neurosci Lett : 279–284, 1987. doi: 10.1016/0304-3940(87)90396-X. [DOI] [PubMed] [Google Scholar]
- 45.Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci : 2895–2905, 2011. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pedrosa RP, Drager LF, de Paula LK, Amaro AC, Bortolotto LA, Lorenzi-Filho G. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest : 1487–1494, 2013. doi: 10.1378/chest.13-0085. [DOI] [PubMed] [Google Scholar]
- 47.Peng Y, Kline DD, Dick TE, Prabhakar NR. Chronic intermittent hypoxia enhances carotid body chemoreceptor response to low oxygen. Adv Exp Med Biol : 33–38, 2001. doi: 10.1007/978-1-4615-1375-9_5. [DOI] [PubMed] [Google Scholar]
- 48.Peng YJ, Nanduri J, Zhang X, Wang N, Raghuraman G, Seagard J, Kumar GK, Prabhakar NR. Endothelin-1 mediates attenuated carotid baroreceptor activity by intermittent hypoxia. J Appl Physiol (1985) : 187–196, 2012. doi: 10.1152/japplphysiol.00529.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pijacka W, Moraes DJ, Ratcliffe LE, Nightingale AK, Hart EC, da Silva MP, Machado BH, McBryde FD, Abdala AP, Ford AP, Paton JF. Purinergic receptors in the carotid body as a new drug target for controlling hypertension. Nat Med : 1151–1159, 2016. doi: 10.1038/nm.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richter DW, Spyer KM. Cardiorespiratory control In: Central Regulation of Autonomic Functions, edited by Loewy AD and Spyer KM. Oxford, UK: Oxford University, 1990, p. 189–207. [Google Scholar]
- 51.Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandez-Pardal J, Saavedra JM, Reis DJ. Tonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. J Neurosci : 474–494, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruggiero DA, Cravo SL, Golanov E, Gomez R, Anwar M, Reis DJ. Adrenergic and non-adrenergic spinal projections of a cardiovascular-active pressor area of medulla oblongata: quantitative topographic analysis. Brain Res : 107–120, 1994. doi: 10.1016/0006-8993(94)90468-5. [DOI] [PubMed] [Google Scholar]
- 53.Seller H. Carl Ludwig and the localization of the medullary vasomotor center: old and new concepts of the generation of sympathetic tone. Pflügers Arch , Suppl: R94–R98, 1996. [PubMed] [Google Scholar]
- 54.Silva JN, Tanabe FM, Moreira TS, Takakura AC. Neuroanatomical and physiological evidence that the retrotrapezoid nucleus/parafacial region regulates expiration in adult rats. Respir Physiol Neurobiol : 9–22, 2016. doi: 10.1016/j.resp.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension? J Physiol : 597–610, 2009. doi: 10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest : 1897–1904, 1995. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. J Comp Neurol : 207–220, 2002. doi: 10.1002/cne.10142. [DOI] [PubMed] [Google Scholar]
- 58.Traube L. Über periodische Thätigkeits-Aeusscrungen desvasomotorischen und Hemmungs-Nervencentrums. Centralhl Medic : 881–885, 1865. [Google Scholar]
- 59.Zoccal DB, Bonagamba LG, Paton JF, Machado BH. Sympathetic-mediated hypertension of awake juvenile rats submitted to chronic intermittent hypoxia is not linked to baroreflex dysfunction. Exp Physiol : 972–983, 2009. doi: 10.1113/expphysiol.2009.048306. [DOI] [PubMed] [Google Scholar]
- 60.Zoccal DB, Paton JF, Machado BH. Do changes in the coupling between respiratory and sympathetic activities contribute to neurogenic hypertension? Clin Exp Pharmacol Physiol : 1188–1196, 2009. doi: 10.1111/j.1440-1681.2009.05202.x. [DOI] [PubMed] [Google Scholar]
- 61.Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol : 3253–3265, 2008. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]