Abstract
Purpose
Measures of white matter (WM) microstructure inferred from diffusion magnetic resonance imaging (dMRI) are useful for studying brain development. There is uncertainty about agreement between FA and MD values obtained from region-of-interest (ROI) versus whole tract approaches. We investigated agreement between dMRI measures using ROI and Probabilistic Neighbourhood Tractography (PNT) in genu of corpus callosum (gCC) and corticospinal tracts (CST).
Materials and Methods
81 neonates underwent 64 direction DTI at term equivalent age. FA and MD values were extracted from a 8 mm3 ROI placed within the gCC, right and left posterior limbs of internal capsule. PNT was used to segment gCC and CSTs to calculate whole tract-averaged FA and MD. Agreement between values obtained by each method was compared using Bland–Altman statistics and Pearson's correlation.
Results
Across the 3 tracts the mean difference in FA measured by PNT and ROI ranged between 0.13 and 0.17, and the 95% limits of agreement did not include the possibility of no difference. For MD, the mean difference in values obtained from PNT and ROI ranged between 0.101 and 0.184 mm2/s × 10−3 mm2/s: the mean difference in gCC was 0.101 × 10−3 mm2/s with 95% limits of agreement that included the possibility of no difference, but there was significant disagreement in MD values measured in the CSTs.
Conclusion
Agreement between dMRI measures of neonatal WM microstructure calculated from ROI and whole tract averaged methods is weak. ROI approaches may not provide sufficient representation of tract microstructure at the level of neural systems in newborns.
Keywords: Neonatal, Diffusion, Tractography, Region-of-interest, Corticospinal, Corpus callosum
Abbreviations: CST, corticospinal tract; GA, gestational age; gCC, Genu of corpus callosum; ICC, Intraclass correlation; OFC, Occipital-frontal head circumference; PLIC, posterior limb of internal capsule; PNT, probabilistic neighbourhood tractography
Highlights
-
•
Agreement is poor between dMRI parameters measured in regions of interest within tracts versus whole tract averaged values.
-
•
ROI approaches may not provide sufficient representation of whole tract microstructure in the developing brain.
-
•
These observations have implications for the development of neonatal MR image biomarkers.
1. Introduction
Structural and diffusion magnetic resonance imaging of the brain during the newborn period contributes to understanding the neural systems that underpin typical and atypical development. Preterm birth is closely associated with injury and/or maldevelopment of cerebral white matter,1 and with several long-term adverse outcomes including cerebral palsy, neurocognitive impairment, social difficulties and vulnerability to psychiatric disease.2, 3, 4, 5
Quantitative parameters derived from diffusion magnetic resonance imaging (dMRI) include fractional anisotropy (FA) and mean diffusivity (MD), which enable inference about the microstructural organisation of brain tissue and white matter tracts.6 A consistent finding is that FA is decreased and MD is increased in the white matter of preterm infants at term equivalent age.7, 8, 9, 10 The corpus callosum (CC) and corticospinal tracts (CST) are of particular interest in the context of developing biomarkers for later neurodevelopmental outcome after preterm birth, because dMRI parameters in these tracts are influenced by gestational age, and their microstructural properties have been associated with later function.11, 12, 13
Two widely used methods to measure dMRI parameters in these tracts include region-of-interest (ROI) analysis and tractography, but the agreement of values obtained using these methods is uncertain; for example, ROI measures show an inconsistent correlation with values of the white matter skeleton at corresponding sites using tract-based spatial statistics.14 ROI approaches are often used in clinical settings because they provide absolute quantification of MD and FA in selected regions without the need for substantial post-processing but they are labour intensive, prone to operator bias, and the volume and shape of the ROI can influence results.15, 16, 17
Probabilistic Neighbourhood Tractography (PNT) is an automatic segmentation method, which provides whole tract-averaged measures of FA and MD in major white matter fasciculi. This method, first described by Clayden et al.,18, 19 has been optimized for neonatal data.20 It involves single seed point tractography to segment a tract of interest by modelling the length and shape variation of individual tracts compared with a pre-defined reference tract.
We aimed to compare FA and MD values measured from geometric ROIs with tract-averaged values obtained using PNT in the genu of the corpus callosum (gCC) and the CST. The secondary aim was to evaluate intra-rater variation in FA and MD values obtained by ROI placement within these tracts.
2. Materials and methods
2.1. Participants
Ethical approval was granted from the National Research Ethics Service (South East Scotland Research Ethics Committee) and informed parental consent was obtained. The study was conducted in accordance with the 18th World Medical Assembly, Helsinki 1964 and later revisions. Infants were recruited from the Neonatal Intensive Care Unit and postnatal wards between June 2012 and June 2014. The group consists of a subset of infants recruited to a longitudinal study of the effect of preterm birth on the developing brain and neurodevelopmental outcome. Preterm infants (birth weight <1500 g or gestational age <32 completed weeks) and term infants (birth after 37 weeks gestation) were recruited. All infants underwent MRI at term equivalent age (38–42 weeks' gestational age). Infants with chromosomal or congenital abnormalities and those with major parenchymal lesions including haemorrhagic parenchymal infarction and cystic periventricular leucomalacia were excluded.
2.2. Magnetic resonance imaging
A Siemens Magnetom Verio 3 T MRI clinical scanner (Siemens Healthcare GMbH, Erlangen, Germany) and 12-channel phased-array head coil were used to acquire: T1-weighted MPRAGE volume (∼1 mm3 resolution), T2-weighted STIR (∼0.9 mm3 resolution), T2-weighted FLAIR (∼1 mm3 resolution), and diffusion MRI (11 T2-and 64 diffusion encoding direction (b = 750 s/mm2) single-shot spin-echo echo planar imaging (EPI) volumes with 2 mm isotropic voxels) data.
Infants were examined in natural sleep with pulse oximetry, temperature and electrocardiography data monitoring. Ear protection was used for each infant, comprising earplugs placed in the external ear and neonatal earmuffs (MiniMuffs, Natus Medical Inc., CA).
2.3. Diffusion MRI data processing
After conversion from DICOM to NIfTI-1 format, dMRI data were pre-processed using FSL tools (FMRIB, Oxford, UK; http://www.fmrib.ox.ac.uk). This included brain extraction and removal of bulk infant motion and eddy current induced artifacts by registering subsequent diffusion-weighted volumes to the first T2-weighted EPI volume for each subject. Using DTIFIT, MD and FA volumes were generated for every subject. Underlying connectivity data was provided by FSL's BedpostX/ProbTrackX algorithm run with its default parameters: 2-fiber model per voxel, 5000 probabilistic streamlines for each tract with a fixed separation distance of 0.5 mm between successive points.21, 22
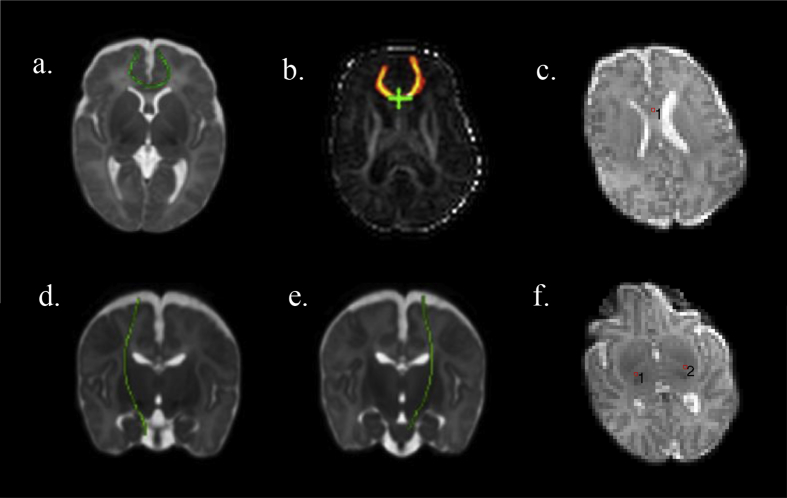
For ROI analysis, values were extracted from a 8 mm3 cubic ROI placed at the centre of the genu of corpus callosum (manual visual placement in axial plane being midline between the superior margins of the lateral ventricles, below the cerebral cortex; Fig. 1c), and right and left PLIC (manual visual placement in axial plane being inferior-lateral on the PLIC, seen as 2 diagonal bands of increased signal intensity within the basal ganglia; Fig. 1f) on the T2-weighted EPI volume. ’In-house’ MATLAB (http://uk.mathworks.com) code was written to allow the user to interactively place these cubic ROIs using a mouse-driven GUI and generate FA and MD values from them; FA and MD parametric volumes are, by definition, co-registered to the T2-weighted volume for each subject.
Fig. 1.
a: Reference tract of genu of corpus callosum calculated from term controls displayed in green and overlaid on an age-specific standard space template b: illustration of segmentation of whole genu for an individual subject calculated using PNT c: ROI placement in genu of corpus callosum d: reference tract of right CST e: reference tract of left CST f. ROI placement in right and left posterior limb of internal capsule.
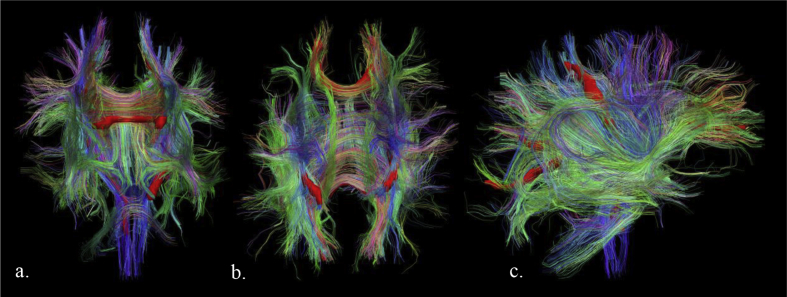
For PNT analysis, we used the method first described by Clayden et al.18, 19 and optimised by Anblagan et al.20 for the neonatal brain. Reference tracts for the gCC (Fig. 1a) and CST (Fig. 1d and e) were generated from a previous study of 20 term-control infants.20 Individual tracts of interest (gCC, right and left CST) were segmented using PNT as implemented in TractoR (http://www.tractor-mri.org.uk). The resulting tractography mask was applied to each subject's MD and FA maps and tract-averaged values were calculated (see Fig. 1b for an example segmentation of the genu in a representative subject, and Fig. 2a–c for examples of segmented CC and CST in 3D space).
Fig. 2.
Axial (a), coronal (b) and sagittal (c) views showing genu, splenium and bilateral corticospinal tracts (scarlet) segmented using PNT within 3D space for a representative subject. Smaller tracts in 3D space running left/right are indicated in red, superior/inferior in blue and anterior/posterior in green.
2.4. Statistical analysis
Pearson correlation was used to investigate the strength and direction of linear relationship between both FA and MD derived from ROI and PNT for each tract; correlation coefficient (r) values of 0.00–0.39, 0.40–0.59, and >0.6 were defined as weak, moderate and strong respectively, and p < 0.05 was considered statistically significant. Bland–Altman statistics were used to investigate agreement between FA and MD values derived from ROI and tract-averaged approaches for each tract; the mean difference (95% limits of agreement) is reported. Intraclass correlation coefficient (ICC) was used to estimate intra-rater variability by a single observer who repeated ROI procedures on two occasions (S.A.S.) for each tract; strength of correlation was classified as weak, moderate and strong using the thresholds stated above. Statistical analyses were carried out using SPSS v21 (SPSS Inc., Chicago, USA).
3. Results
3.1. Participants
The study group consisted of 81 neonates (41 female, 40 male) of which 15 were born at term. Participants underwent MRI at term equivalent age, and characteristics are shown in Table 1.
Table 1.
Clinical characteristics of participants.
| Characteristics | Patient Group (n = 81) |
|---|---|
| Birthweight/kg (range) | 1.582 (0.55–4.26) |
| Mean gestational age at birth/weeks (range) | 31.28 (23.28–41.71) |
| Mean gestational age at MRI scan/weeks (range) | 40.28 (37.28–45.43) |
| Mean OFC at time of MRI scan/cm (range) | 34.9 (31.0–38.0) |
| Mean weight at time of MRI scan/kg (range) | 2.994 (2.06–4.26) |
| Vaginal delivery (%) | 31 (38.3) |
| Histological evidence of chorioamnionitis (%) | 19 (23.5) |
| Exposure to any antenatal steroids (%) | 63 (77.8) |
| Exposure to antenatal magnesium sulphate (%) | 30 (37.8) |
3.2. Tract-averaged versus region-of-interest measurements for genu of corpus callosum and corticospinal tracts
FA and MD values generated from PNT and ROI placement in gCC and left and right CST are shown in Table 2. For each tract, FA and MD derived from both methods had weak to moderate correlation (Pearson coefficient [r] 0.24–0.54, all p-values < 0.05).
Table 2.
dMRI measurements generated by PNT and ROI methods in genu of the corpus callosum and the corticospinal tracts.
| gCC |
Right CST |
Left CST |
||||
|---|---|---|---|---|---|---|
| FA | MD × 10−3 mm2/s | FA | MD × 10−3 mm2/s | FA | MD × 10−3 mm2/s | |
| Mean tract-averaged value (95% CI) | 0.22 (0.14–0.30) | 1.473 (1.287–1.659) | 0.27 (0.21–0.33) | 1.167 (1.038–1.296) | 0.29 (0.23–0.35) | 1.216 (1.073–1.359) |
| Mean ROI value (95% CI) | 0.38 (0.22–0.54) | 1.371 (1.050–1.692) | 0.42 (0.30–0.54) | 1.032 (0.930–1.130) | 0.43 (0.33–0.53) | 1.031 (0.949–1.113) |
| Mean difference | −0.17 | 0.101 | −0.15 | 0.136 | −0.13 | 0.184 |
| 95% limits of agreement | −0.01 to −0.32 | −0.21 to 0.415 | −0.24 to −0.05 | 0.005 to 0.267 | −0.22 to −0.05 | 0.062 to 0.307 |
| Pearson correlation r | 0.24 | 0.34 | 0.54 | 0.38 | 0.47 | 0.53 |
| p value for correlation | 0.032 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 |
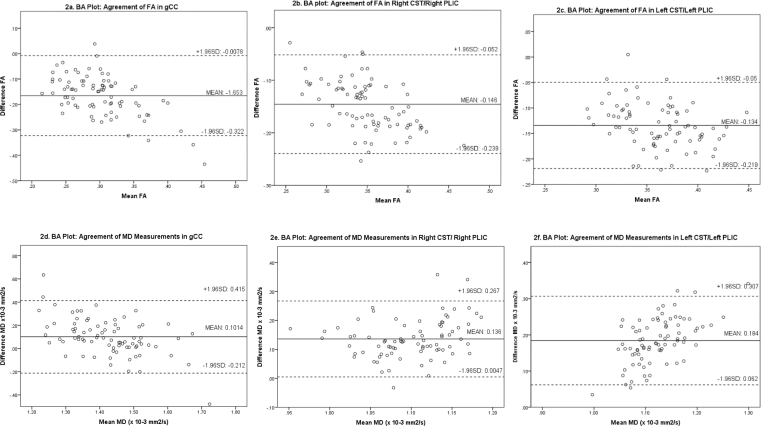
The magnitude of difference in FA measured using PNT or ROI ranged between 0.13 and 0.17 for the 3 tracts, and none had 95% limits of agreement that included the possibility of no difference. Therefore, for FA, ROI measures extracted from the center of the gCC and the PLIC do not reflect whole tract-averaged values from the gCC or CST respectively (Fig. 3a–c).
Fig. 3.
Bland–Altman plots of tract-averaged and ROI dMRI measurements in genu of corpus callosum and right and left corticospinal tracts.
The magnitude of difference in MD measured from PNT and ROI ranged between 0.101 and 0.184 × 10−3 mm2/s across the studied tracts. The mean difference in MD in gCC derived from PNT and ROI placement was low (0.101 × 10−3 mm2/s) and included the possibility of no mean difference, but tract-averaged MD values for right and left CST disagreed with values obtained from ROI placement in the right and left PLIC respectively (mean difference 0.136 [95% limits 0.005–0.267], right; mean difference 0.184, [0.062–0.307], left), Fig. 3d–f.
3.3. Intra-rater agreement for region of interest measurements
Intraclass correlation for intra-rater variation in ROI measurements of FA and MD was moderate to strong across all tracts (ICC 0.56–0.94, p < 0.001 for all), Table 3. Intra-rater ROI measurements for FA and MD showed that the mean difference for each tract was marginal, with difference in FA values between 0.0005 and 0.004 and difference in MD values between 0.003 and 0.005 × 10−3 mm2/s. The 95% limits of agreement for both FA and MD in all tracts included the possibility of no difference.
Table 3.
Intra-rater agreement for dMRI parameters generated by ROI placement in genu of corpus callosum and corticospinal tracts.
| gCC |
Right CST |
Left CST |
||||
|---|---|---|---|---|---|---|
| FA | MD × 10−3 mm2/s | FA | MD × 10−3 mm2/s | FA | MD × 10−3 mm2/s | |
| ROI Measure 1/Mean (95% CI) | 0.38 (0.22–0.54) | 1.38 (1.01–1.75) | 0.423 (0.31–0.54) | 1.033 (0.94–1.13) | 0.43 (0.33–0.53) | 1.031 (0.95–1.11) |
| ROI Measure 2/Mean (95% CI) | 0.39 (0.21–0.57) | 1.39 (1.08–1.70) | 0.421 (0.30–0.54) | 1.031 (0.93–1.13) | 0.42 (0.32–0.52) | 1.024 (0.93–1.12) |
| Mean difference | −0.001 | −0.005 | −0.0005 | 0.003 | 0.004 | 0.002 |
| 95% limits of agreement | −0.08 to 0.08 | −0.3 to 0.29 | 0.08 to 0.08 | −0.08 to 0.08 | −0.06 to 0.07 | −0.1 to 0.1 |
| ICC/Mean (95% CI) | 0.94 (0.9–0.96) | 0.78 (0.65–0.86) | 0.86 (0.78–0.91) | 0.79 (0.67–0.86) | 0.86 (0.78–0.91) | 0.56 (0.32–0.72) |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
4. Discussion
The results of this study indicate that FA and MD values estimated from regions of interest within the genu of the corpus callosum (gCC) and the corticospinal tracts (CST) show weak correlation and low agreement with tract-averaged values. Since intra-rater variability in ROI measurement of FA and MD was low (mean ICC > 0.75 for five out of six tracts), these observations do not appear to be substantially explained by operator bias in ROI placement. The study suggests that sampling ROIs within major fasciculi does not provide sufficient representation of white matter tract microstructure in the newborn. The magnitude of disagreement in FA and MD was large and this has implications for research designed to evaluate neonatal image biomarker qualification and validation because relatively modest changes in FA and MD in the CC and CST in the newborn period are associated with neurodevelopmental outcome in childhood.9, 11, 23 Therefore, the differences observed between dMRI parameters derived from ROI and PNT values may include clinically significant values.
The differences in dMRI parameters observed in ROIs versus tract-averaged values could be explained by within-tract variation in maturational processes that influence tissue microstructure. In early development, white matter tract myelination proceeds in phases: the first is characterized by proliferation and maturation of oligodendrocytes in contact with axons, an increase in cell membrane density and a reduction in brain water content; and the second involves ensheathment of oligodendroglial processes around axons.6 Each phase is associated with changes in the molecular motion of water; specifically, changes in anisotropy precede the appearance of myelin.24, 25, 26 Our data are consistent with the observation that these processes show considerable spatial variation within tracts. As the genu of the corpus callosum is unmyelinated in the neonatal period,27 the higher FA and lower MD values sampled from the ROI placement (Fig. 1c) compared to tract averaged values could be attributable to microstructural differences within the tract that precede myelination. The CST is partially myelinated at the end of gestation27 with MRI evidence of myelin in posterior limb of the internal capsule and tracts of the pre- and post-central gyri apparent by term equivalent age,28 although myelination of the whole tract is not complete until the second year of life. The high FA and low MD observed in the PLIC ROI compared with whole CST could therefore represent variation of myelination within the tract, or it could be attributable to other properties of tract maturation that influence dMRI parameters described above.
We studied the CST because injury to the developing brain is strongly associated with motor impairment,3 and the development of early image markers of injury to motor systems could enable more effective stratification of high-risk groups for trials of early interventions. The CST is readily segmented using a range of neonatal tractography techniques29, 30, 31, 32, 33; and we observed comparable values of FA and MD to those reported by others, suggesting that the study group is representative. The posterior limb of the internal capsule is of particular interest because of its close association with motor outcome following different forms of perinatal brain injury.34 The corpus callosum provides the neural substrate for transfer of interhemispheric information, and segmentations from neonatal MRI have shown that its macro- and micro-structure are influenced by perinatal events, and are linked to outcome.23, 32, 33, 35, 36, 37, 38 The weak agreement we observed for FA and MD values derived from ROIs in CST and gCC compared with whole tract-averaged values from the respective tracts, suggests that ROI approaches do not represent microstructural characteristics of the whole tract unit, and that the reliability of neonatal DTI biomarkers is strongly influenced by site of measurement.
We found that intra-observer agreement of FA and MD values obtained by manual ROI placement in gCC and PLICs was high, which is consistent with agreement studies reported in the literature17 for these specific structures. However, ROI analysis is a subjective method and variation in measurements may be explained by slice choice and manual ROI placement. Angulation between slices may alter tract volume and ROI placement close to the edge of a tract may capture CSF or grey matter, which have different FA and MD values to white matter structures.39 Therefore, ROI analysis requires an operator with experience in neonatal neuroanatomy to maximize the validity of results, and difficulties ensuring accurate placement of ROIs within white matter networks limits application of this approach.
The study has some limitations. First, we studied ROI versus tract-averaged agreement of FA and MD values in 3 major tracts because ROI placement was accurate and consistent for these tracts. Further work using high resolution DTI and rigorous protocols for consistent ROI placement would be required to determine whether ROI measurements achieve better representation of whole tracts in other neural systems. Second, we did not include specific myelin related imaging protocols such as magnetization transfer ratio26, 40 or myelin water fraction,41 or methods for resolving multiple fiber orientations within voxels. In future work the use of these imaging techniques may be informative for understanding sources of within-tract variation in microstructure and, by inference, maturation observed in this study.
5. Conclusions
In conclusion, FA and MD values derived from regions of interest show weak agreement with tract-averaged values in the corpus callosum and corticospinal tracts. ROI approaches may not provide sufficient representation of whole tract microstructure in the developing brain. These observations have implications for the development of neonatal MR image biomarkers of perinatal brain injury and disease.
Conflicts of interest
The authors declare no real or potential conflicts of interest.
Acknowledgements
We are grateful to the families who consented to take part in the study and to the nursing and radiography staff at the Clinical Research Imaging Centre, the University of Edinburgh (http://www.cric.ed.ac.uk) who scanned the infants. We thank Thorsten Feiweier at Siemens Healthcare for collaborating with DTI acquisitions (Works-in-Progress Package for Advanced EPI Diffusion Imaging).
References
- 1.Volpe J.J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodward L.J., Anderson P.J., Austin N.C., Howard K., Inder T.K. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. NEJM. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 3.Moore T., Hennessy E.M., Myles J. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. doi: 10.1136/bmj.e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boardman J.P., Craven C., Valappil S. A common neonatal image phenotype predicts adverse neurodevelopmental outcome in children born preterm. Neuroimage. 2010;52:409–414. doi: 10.1016/j.neuroimage.2010.04.261. [DOI] [PubMed] [Google Scholar]
- 5.Johnson S., Hollis C., Kochhar P., Hennessy E., Wolke D., Marlow N. Psychiatric disorders in extremely preterm children: longitudinal finding at age 11 years in the EPICure study. J Am Acad Child Adolesc Psychiatry. 2010;49:453–463. [PubMed] [Google Scholar]
- 6.Dubois J., Dehaene-Lambertz G., Kulikova S. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276:48–71. doi: 10.1016/j.neuroscience.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 7.Counsell S.J., Shen Y., Boardman J.P., Poupon C., Hüppi P.S., Hertz-Pannier L. Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term-equivalent age. Pediatrics. 2006;117:376–386. doi: 10.1542/peds.2005-0820. [DOI] [PubMed] [Google Scholar]
- 8.Counsell S.J., Edwards A.D., Chew A.T. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131:3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- 9.van Kooij B.J., de Vries L.S., Ball G. Neonatal tract-based spatial statistics findings and outcome in preterm infants. AJNR Am J Neuroradiol. 2012;33:188–194. doi: 10.3174/ajnr.A2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Telford E.J., Cox S.R., Fletcher-Watson S. A latent measure explains substantial variance in white matter microstructure across the newborn human brain. Brain Struct Funct. 2017 Dec;222(9):4023–4033. doi: 10.1007/s00429-017-1455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arzoumanian Y., Mirmiran M., Barnes P.D. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR Am J Neuroradiol. 2003;24:1646–1653. [PMC free article] [PubMed] [Google Scholar]
- 12.Anjari M., Srinivasan L., Allsop J.M. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. NeuroImage. 2007;35:1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Braga R.M., Roze E., Ball G. Development of the corticospinal and callosal tracts from extremely premature birth up to 2 Years of age. Zhou J., editor. PLoS One. 2015;10(5):e0125681. doi: 10.1371/journal.pone.0125681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo Y., Wang Z.J., Ball G., Rollins N.K. Diffusion tensor imaging metrics in neonates—a comparison of manual region-of-interest analysis vs. tract-based spatial statistics. Pediatr Radiol. 2013;43(1):69–79. doi: 10.1007/s00247-012-2527-7. [DOI] [PubMed] [Google Scholar]
- 15.Oouchi H., Yamada K., Sakai K. Diffusion anisotropy measurement of brain white matter is affected by voxel size: underestimation occurs in areas with crossing fibers. AJNR Am J Neuroradiol. 2007;28(6):1102–1106. doi: 10.3174/ajnr.A0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bisdas S., Bohning D.E., Besenski N., Nicholas J.S., Rumboldt Z. Reproducibility, interrater agreement and age-related changes of fractional anisotropy measures at 3 T in healthy subjects: effect of the applied b-value. AJNR Am J Neuroradiol. 2008;29:1128–1133. doi: 10.3174/ajnr.A1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lepomäki V.K., Paavilainen T.P., Hurme S.A., Komu M.E., Parkkola R.K., PIPARI Study Group Fractional anisotropy and mean diffusivity parameters of the brain white matter tracts in preterm infants: reproducibility of region-of-interest measurements. Pediatr Radiol. 2012;42(2):175–182. doi: 10.1007/s00247-011-2234-9. [DOI] [PubMed] [Google Scholar]
- 18.Clayden J.D., Bastin M.E., Storkey A.J. Improved segmentation reproducibility in group tractography using a quantitative tract similarity measure. Neuroimage. 2006;33(2):482–492. doi: 10.1016/j.neuroimage.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Clayden J.D., Storkey A.J., Bastin M.E. A probabilistic model-based approach to consistent white matter tract segmentation. IEEE Trans Med Imag. 2007;26(11):1555–1561. doi: 10.1109/TMI.2007.905826. [DOI] [PubMed] [Google Scholar]
- 20.Anblagan D., Bastin M.E., Sparrow S. Tract shape modelling detects changes associated with preterm birth and neuroprotective treatment effects. NeuroImage Clin. 2015;8:51–58. doi: 10.1016/j.nicl.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behrens T.E.J., Johansen-Berg H., Woolrich M.W. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6(7):750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 22.Behrens T.E.J., Berg H.J., Jbabdi S., Rushworth M.F., Woolrich M.W. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson D.K., Inder T.E., Faggian N. Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage. 2012;59:3571–3581. doi: 10.1016/j.neuroimage.2011.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wimberger D.M., Roberts T.P., Barkovich A.J., Prayer L.M., Moseley M.E., Kucharczyk J. Identification of ‘‘premyelination’’ by diffusion-weighted MRI. J Comput Assist Tomogr. 1995;19(1):28–33. doi: 10.1097/00004728-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Zanin E., Ranjeva J.P., Confort-Gouny S. White matter maturation of normal human fetal brain. An in vivo diffusion tensor tractography study. Brain Behav. 2011;1(2):95–108. doi: 10.1002/brb3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nossin-Manor R., Card D., Morris D. Quantitative MRI in the very preterm brain: assessing tissue organization and myelination using magnetization transfer, diffusion tensor and T(1) imaging. Neuroimage. 2013;64:505–516. doi: 10.1016/j.neuroimage.2012.08.086. [DOI] [PubMed] [Google Scholar]
- 27.Gilles F.H., Nelson . The developing human brain: growth and adversities. Mac Keith Press; London: 2012. “Myelinated tracts: growth patterns”; pp. 94–151. [Google Scholar]
- 28.Counsell S.J., Maalouf E.F., Fletcher A.M. MR imaging assessment of myelination in the very preterm brain. AJNR Am J Neuroradiol. 2002;23(5):872–881. [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Baleriaux D., Kavec M. Structural asymmetries in motor and language networks in a population of healthy preterm neonates at term-equivalent age: a diffusion tensor imaging and probabilistic tractography study. Neuroimage. 2010;51:783–788. doi: 10.1016/j.neuroimage.2010.02.066. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y., Metens T., Absil J. Gender differences in language and motor-related fibres in a population of healthy preterm neonates at term-equivalent age: a diffusion tensor and probabilistic tractography study. AJNR Am J Neuroradiol. 2011;32:2011–2016. doi: 10.3174/ajnr.A2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Bruine F.T., van Wezel-Meijler G., Leijser L.M. Tractography of developing white matter of the internal capsule and corpus callosum in very preterm infants. Eur Radiol. 2011;21:538–547. doi: 10.1007/s00330-010-1945-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Pul C., van Kooij B.J., de Vries L.S., Benders M.J., Vilanova A., Groenendaal F. Quantitative fibre tracking in the corpus callosum and internal capsule reveals microstructural abnormalities in preterm infants at term-equivalent age. AJNR Am J Neuroradiol. 2012;33:678–684. doi: 10.3174/ajnr.A2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y., Aeby A., Baleriaux D. White matter abnormalities are related to microstructural changes in preterm neonates at term-equivalent age: a diffusion tensor imaging and probabilistic tractography study. AJNR Am J Neuroradiol. 2012;33:839–845. doi: 10.3174/ajnr.A2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowan F.M., deVries L.S. The internal capsule in neonatal imaging. Semin Fetal Neonatal Med. 2005;10(5):461–474. doi: 10.1016/j.siny.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Thompson D.K., Inder T.E., Faggian N. Characterization of the corpus callosum in very preterm and full-term infants utilizing MRI. Neuroimage. 2011;55:479–490. doi: 10.1016/j.neuroimage.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasegawa T., Yamada K., Morimoto M. Development of corpus callosum in preterm infants is affected by the prematurity: in vivo assessment of diffusion tensor imaging at term-equivalent age. Pediatr Res. 2011;69:249–254. doi: 10.1203/PDR.0b013e3182084e54. [DOI] [PubMed] [Google Scholar]
- 37.Bassi L., Chew A., Merchant N. Diffusion tensor imaging in preterm infants with punctate white matter lesions. Pediatr Res. 2011;69:561–566. doi: 10.1203/PDR.0b013e3182182836. [DOI] [PubMed] [Google Scholar]
- 38.De Bruıne F.T., Van Wezel-Meijler G., Leijser L.M. Tractography of white-matter tracts in very preterm infants: a 2-year follow-up study. Dev Med Child Neurol. 2013;55:427–433. doi: 10.1111/dmcn.12099. [DOI] [PubMed] [Google Scholar]
- 39.Bonekamp D., Nagae L.M., Degaonkar M. Diffusion tensor imaging in children and adolescents: repeatability, hemispheric, and age-related differences. Neuroimage. 2007;34:733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGowan J.C. The physical basis of magnetization transfer imaging. Neurology. 1999;53(5 Suppl 3):S3–S7. [PubMed] [Google Scholar]
- 41.Deoni S.C., Dean D.C., 3rd, O'Muircheartaigh J., Dirks H., Jerskey B.A. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage. 2012;63(3):1038–1053. doi: 10.1016/j.neuroimage.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]