Abstract
Background
Acidic juices such as lemon juice, vinegar and Verjuice are popular beverages regularly consumed by both children and adults. Various brands of different acidic juices in Iran markets are packaged in disposable plastic bottles. Some evidence suggests that phthalates may immigrate from plastic bottles.
Methods
In this research the influence of sunlight, type of container and storage time on the leaching of phthalates from packaging materials into selected juices was investigated, by analyzing the samples kept in different conditions, before and throughout 2, 4 and 6 months storage time.
Results
The mean phthalate concentrations of the examined samples were determined to be between <LOD and 0.521 μg/L in verjuice, <LOD and 0.261 μg/L in lemon juice, <LOD and 0.599 μg/L in vinegar. DEP and DEHP showed the highest level of migration into acidic juices packed in plastic bottles.
Conclusions
Results of analyses before and after storage show that some storage conditions can increase the concentrations of DBP, DEHP and DEP in acidic juices. The results of this study indicate the possible leaching of phthalates from packages made of plastic materials into the contents.
Keywords: Phthalate esters, Migration, Storage condition, Plastic
Background
Plastics are transforming every day; usage is increasing daily and annual production has increased up to 300 million tons by 2010. It is evident that using plastics packages in food industries bring many advantages such as reduction of weight and volume, the ability to be presented in suitable forms due to desirable transparency, low permeability, high flexibility, inhibiting gas outflow, and simple transportation [1]. However, concerns about usage and discarding are diverse and include: accumulation of waste in landfills and in environment, the migration of chemicals from plastic products and the potential for plastics to transfer chemicals to wildlife and human and their adverse health effects. Virgin plastic polymers are rarely used alone and usually the polymer resins are mixed with different additives to improve plastic performance. These additives include some inorganic fillers such as silica and carbon that reinforce the plastic, plasticizers to render the material flexibility, ultraviolet and thermal stabilizers, colorings and flame retardants [2]. Some additive chemicals are potentially toxic (for example plasticizers in high density polyethylene (HDPE) and polyethylene terephthalate (PET)), but there is remarkable controversy about the extent to which additives leached from plastic products (like bisphenol A and phthalates) have adverse health effects in animal or human populations [3–6]. Phthalic acid esters (PAEs) are found in many mass produced products such as food packages and cans represent a notable content of the plastic. Phthalates can immigrate from plastic packages into food or beverages because they are not chemically bound to the polymer matrix [5, 6], and they have attracted particular attention because of their serious side effects on human health. A relationship between the presences of PAE plasticizers in packaging and contamination of food was hypothesized by Page and Lacroix [7]. In a study carried out by Casajuana and Lacorte, five PAEs were considered in retail milk packed in two types of containers –Tetra pak and high-density polyethylene. Diethyl phthalate (DEP), Dibutyl phthalate (DBP) and Diethylhexyl phthalate (DEHP) were the PAEs mainly identified, suggesting the probable influence of the packaging on PAE levels in milk [8]. The PAEs’ migration process depends on their concentrations in the packaging polymer, on the acidity of content in contact with packages, on the lipid content and on the time and temperature of the contact [9]. Verjuice, vinegar, and lemon juice are among highly consumed acidic juices in Iran. However, for communities, disposable containers made out of different materials are available, but in Iran, PET and HDPE are typically used. Both PET and HDPE should not contain PAEs as plasticizers; however, the literature shows that PET can release PAEs [9–11]. Because in some study Significant amounts of phthalate esters were found in some kinds of beverage such as lemon juice and vinegar [12, 13], the goal of this study was evaluating the influence of natural sunlight irradiation, packaging type and storage time on PAEs releases from packages into acidic juice which are generally stored for a long time under the direct sunlight in Iran.
Materials and methods
PET and HDPE bottles were purchased from Iran Petrochemical Co. (Tehran, Iran). A standard solution of phthalic acid esters, containing six compounds (diethyl phthalate (DEP), dimethyl phthalate (DMP), butyl benzyl phthalate (BBP), di-n-octyl phthalate (DNOP), dibutyl phthalate (DBP), and bis (2-ethylhexyl) phthalate (DEHP) at 2.0 mg/mL in n-hexane (purity 99.8%)), was purchased from Sigma-Aldrich (St. Louis, MO, USA). The phthalic acid esters calibration and quality control samples were prepared by serial dilution of the stock solution in methanol-water (50: 50, v/v). Benzyl benzoate (internal standard, IS) was added to each sample at a final concentration of 1 μg/L. A mixture of acetonitrile, isopropanol and water (40:40:20 v/v) was applied as extraction solution. All the stock and working solutions were kept at 4 °C, in the dark until analysis. The magnetic multi-wall carbon nanotube-polydimethylsiloxane nanocomposite (PDMS/MWCNTs-OH) particles were prepared based on a two-step reaction as explained previously [14].
Preparation of samples
Fresh lemon and sour grape samples were obtained during harvest period from the main field of vegetables and fruits in Tehran, Iran. After washing, they were fully dried and their juice was extracted by using an industrial juicer and transferred to the laboratory in glass bottles. At the laboratory, the initial concentrations of PAEs in bulk samples of juices were measured as the zero time (control). Then, the bulk samples were divided and poured into PET and HDPE bottles, which had already been purchased, and then stored according to predefined irradiation and time conditions. The bulk vinegar sample was also obtained from a local producer before packaging and initial concentrations of phthalate esters were measured and then packed in PET and HDPE containers, and stored under preservation conditions. The samples were kept at room temperature (25 °C) and under natural sun light for 2, 4, and 6 months. One glass bottle of vinegar, lemon juice and verjuice was kept as control samples. Measurement and analysis of the concentration of the compounds of interest were carried out using Magnetic Solid-Phase Extraction and gas chromatography with a mass detector.
Extraction and measurement of phthalate esters
Using the stock solutions prepared out of standard solutions of each of PAEs, calibration curve was plotted at different concentrations (0.02, 0.1, 0.5,1 and 5 μg/L) all over the five compounds (DEP, DBP, DEHP, DnOP and BBP). A solution of benzyl benzoate with a concentration of 2 ng/μL was used as the internal standard. After completion of extraction stages, samples were injected into mass detector gas chromatography device. All of the stages were replicated three times for every concentration. A method developed in a previous study was used for the quantification of phthalate [14]. In order to extract the compounds of interest, at first, 10 mg magnetic PDMS/MWCNTs-OH particles were accurately weighed and washed with methanol and water separately in sequence. Then, the activated MPs and 1 g NaCl were added to the 10 mL of each acidic juice sample. The mixture was shaken vigorously by vortex to extract the analytes for 5.0 min. An external magnet was applied to gather the magnetic adsorbent to the side of the vial (within ~80 s). The supernatant was then discarded and followed by addition of acetone (2 mL) along with 3.0 min of vigorous shaking to elute phthalates from the adsorbent. Then the magnetic adsorbent was gathered to the side of the vial again. The desorption solvent was collected and evaporated until dry at 37 °C under gentle stream of nitrogen followed by reconstitution in 0.1 mL methanol for the subsequent GC–MS analysis. Agilent gas chromatograph 6890 plus (Agilent Technologies, Palo Alto, CA, USA) equipped with a 5973 quadruple mass spectrometer was applied for GC–MS analysis. The gas chromatograph was fitted with an DP-5 ms capillary column (30 m, 0.25 mm i.d., 0.25 μm film thickness) and the following heating program was used: injector temperature, 290 °C and initial column temperature, 50 °C held for 1 min, 1st Ramp: 30 °C/ min to 280 °C and finally increased to 310 °C at a rate of 15 °C/min held for 4 min. The inlet was operated in splitless mode. The temperature of the transfer line was maintained at 310 °C. As carrier gas, helium (99.9999%) was used at 1 mL/min (constant flow). The mass selective detector was operated in electron impact (EI) mode, using selected ion monitoring (SIM).
Quantification of phthalate esters
The linearity of the calibration curves was assessed in the range of 0.01–10 μg/L. For all the analytes the correlation coefficients were determined in the range from 0.993 to 0.997. The limits of detection (LODs) were estimated to be three times of the standard deviation of the baseline noise (N = 6). The limits of quantification (LOQs) were determined by the analysis of spiked samples. The lowest concentration of the analytes on the calibration curves with a precision of less than 20% coefficient of variation and an accuracy of 80–120% was considered as LOQ [14]. Based on the results, the LODs and LOQs of the target analytes were in the range from 0.01 to 0.02 and 0.03 to 0.06 μg/L, respectively.
Statistical analysis
In the present work, we used SPSS (version 22.0) to analyze the results statistically. Means and standard deviations for the concentrations of all esters of phthalate (DEP, DBP, DEHP, DnOP and BBP) in acidic juice samples were reported. Normality of data of each group was checked by the Kolmogorov–Smirnov test before performing this statistical analysis. The nonparametric Kruskal–Wallis H test was used for comparing concentrations from different storage conditions because the distribution of data was not normal. The concentrations from the exposure periods were compared using the Friedman test. The analyses were considered statistically significant when the p value was <0.05.
Data Availability
The data will not be shared with a reason.
Results
The mean phthalate concentrations of the examined samples were determined to be between <LOD and 0.521 μg/L in verjuice, <LOD and 0.261 μg/L in lemon juice, <LOD and 0.599 μg/L in vinegar. The results of the analyses of the initial concentrations of PAEs in bulk samples of acidic juices before storage (exactly after production) proved that the initial DEHP and DEP levels were very low, although detectable levels of DBP was found in none of the samples prior to storage (Table 1). The effect of time duration (2, 4 and 6 months) at different storage conditions on the phthalate concentrations (μg/L) for each phthalate is shown in Figs. 1, 2 and 3. However, as shown in the figures, the migration of these compounds in different kind of polymeric-bottled after 2 months storage under the examined conditions was in the order DEHP > DEP > DBP and DEP > DEHP >DBP in HDPE and PET containers respectively. Therefore, DBP migration was the lowest in both kinds of bottles.
Table 1.
Results of phthalate levels (mean ± SD) in μg/L from polymeric bottles into acidic juices under different storage conditions
| Types of bottles | Storage condition | Acidic beverage | DEP | DBP | DEHP |
|---|---|---|---|---|---|
| HDPE | Control | Verjuice | 0.031 ± 0.011 | <LOD | 0.042 ± 0.021 |
| Lemon juice | <LOD | <LOD | <LOD | ||
| Vinegar | 0.215 ± 0.038 | 0.112 ± 0.023 | 0.105 ± 0.032 | ||
| 25 °C | Verjuice | 0.252 ± 0.148 | 0.142 ± 0.085 | 0.299 ± 0.182 | |
| Lemon juice | 0.091 ± 0.051 | 0.097 ± 0.058 | 0.121 ± 0.068 | ||
| Vinegar | 0.334 ± 0.081 | 0.206 ± 0.068 | 0.395 ± 0.127 | ||
| Sunlight | Verjuice | 0.204 ± 0.138 | 0.139 ± 0.101 | 0.342 ± 0.216 | |
| Lemon juice | 0.081 ± 0.058 | 0.083 ± 0.066 | 0.165 ± 0.103 | ||
| Vinegar | 0.317 ± 0.087 | 0.197 ± 0.074 | 0.441 ± 0.165 | ||
| PET | Control | Verjuice | 0.031 ± 0.011 | <LOD | 0.042 ± 0.021 |
| Lemon juice | <LOD | <LOD | <LOD | ||
| Vinegar | 0.215 ± 0.038 | 0.112 ± 0.023 | 0.105 ± 0.032 | ||
| 25 °C | Verjuice | 0.305 ± 0.183 | 0.135 ± 0.081 | 0.158 ± 0.092 | |
| Lemon juice | 0.127 ± 0.071 | 0.108 ± 0.065 | 0.105 ± 0.061 | ||
| Vinegar | 0.403 ± 0.125 | 0.211 ± 0.071 | 0.351 ± 0.092 | ||
| Sunlight | Verjuice | 0.287 ± 0.182 | 0.129 ± 0.091 | 0.212 ± 0.130 | |
| Lemon juice | 0.095 ± 0.071 | 0.081 ± 0.056 | 0.120 ± 0.070 | ||
| Vinegar | 0.382 ± 0.132 | 0.201 ± 0.079 | 0.387 ± 0.120 |
LOD Limit of detection
Fig. 1.
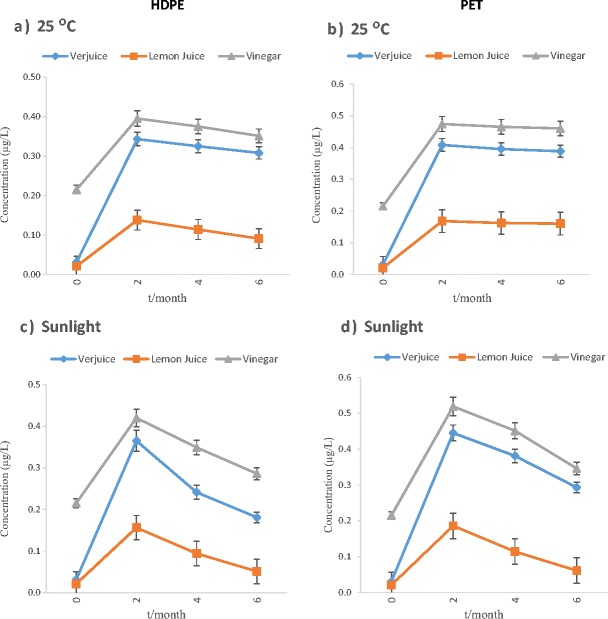
The effect of time duration (0, 2, 4, and 6 months) at different storage conditions on DEP concentration (μg/L) in acidic juices packed in polymeric bottles
Fig. 2.
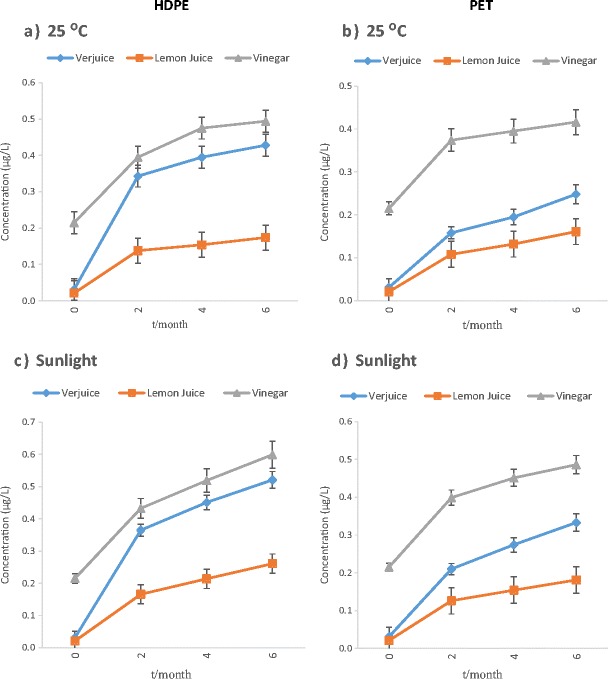
The effect of time duration (0, 2, 4, and 6 months) at different storage conditions on DEHP concentration (μg/L) in acidic juices packed in polymeric bottles
Fig. 3.
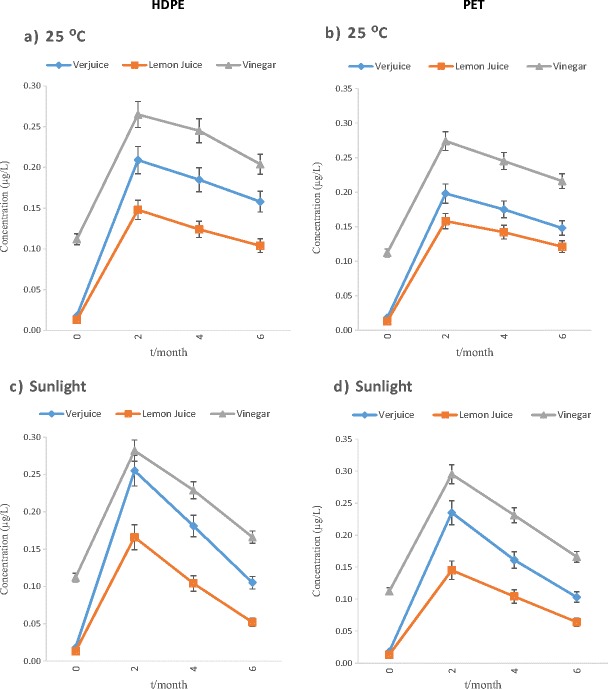
The effect of time duration (0, 2, 4, and 6 months) at different storage conditions on DBP concentration (μg/L) in acidic juices packed in polymeric bottles
Discussion
As shown in Table 1, phthalates presence in vinegar exactly after production in the factory can be accused to usage of plastic equipment during vinegar production processes. However, DnOP and BBP were found at detectable levels in none of the samples before and after storage. The average pH of vinegar, verjuice and lemon juice in this research was approximately 2.56, ranging from 2.7 to 2.8, 2.5 to 2.7 and 2.3 to 2.4, respectively. The findings of this study showed that type of the container, type of acidic juice, contact time and storage conditions are factors that may explain the release of PAEs from polymeric containers into their contents. The effects of various conditions of storage on the migration of phthalates into acidic juices packed in polymeric container, regardless of exposure period, are summarized in Table 1. DEHP and DEP showed the highest level of migration into acidic juices packed in PET and HDPE bottles, respectively. The concentration of DEHP in all samples which were classified by their storage conditions determined that the rate of migration increased by increasing of storage time, but some decreasing can be seen in concentrations of DEP and DBP under natural sunlight irradiation after a significant increasing of the concentrations after 2 months. The maximum level of DEHP (0.599 μg/L) was detected in vinegar packed in HDEP container and kept under sunlight irradiation for 6 months. However, as shown in the figures, the migration of these compounds in different kind of polymeric-bottled after 2 months storage under the examined conditions was in the order DEHP > DEP > DBP and DEP > DEHP >DBP in HDPE and PET bottles respectively. Thus, DBP migration was the lowest in both kinds of bottles. Besides sunlight and span of exposure, the type of juices which were stored in container affected PAEs migration. The statistical analysis demonstrated a significant difference among the concentration of all kinds of phthalates in lemon juice with vinegar and verjuice. The lowest levels of contamination were found in lemon juice. By comparing the results of analyses of DEHP, DEP and DBP concentrations in acidic juices before and after storage, this study concluded that unsuitable storage conditions may cause a perceptible increase in the concentrations of DEP, DBP specially DEHP in these kinds of juices. Similar to our results, in the study accomplished by Yılmaz and et al. (2014), levels of PAEs in the lemon juice and vinegar samples in PET bottles were between <LOD and 0.44 μg/L [12]. Higher levels of phthalates (ND – 140 μg/L) were detected in lemon juice and vinegar samples in plastic containers by Farajzadeh and Khoshmaram [13]. Samples of previous studies [12, 13] were provided from public market or initial levels of phthalates were may not be measured before storage; thus, previous improper storage condition and exposure of these samples with environmental factors (e.g., duration of storage, sunlight, etc.) from production up to consuming should not be excluded. Hence, the particular effect of each storage condition on phthalate migration could not be seen. Because of the above-mentioned points, it is unsure to represent the reason behind the presence of phthalates in the juices; as the result, in this study, the increase observed in phthalate concentrations was related to leaching from the polymeric matters in different conditions of storage. Concentration of DEHP in all samples categorized by their storage conditions showed that the rate of migration increased by increasing of storage time, but some decreasing can be seen in concentrations of DEP and DBP under sunlight after increasing of the concentrations after 2 months. These results are similar to the results of the abiotic degradation tests of the phthalates which were directed by Lertsirisopon and et al. [15]. The phthalates concentrations in the acidic solution were affected by hydrolysis and photolysis. Degradation of all the phthalates proceeded slowly, but the rate of degradation was increased under sunlight irradiation. Lau et al. (2005) revealed that the rates of DEP and DBP degradation (hydrolysis and photolysis) were high at both the highest pH value (pH = 10) and lowest pH (pH = 3) [16]. DEP and DBP were degraded by hydrolysis and photolysis more than 20% under acidic condition and sunlight irradiation. However, hydrolysis and photolysis was not effective for DEHP at acidic/alkaline pH and degradation of DEHP was negligible at the same condition.
Conclusions
In this study, two terms of common consumption of acidic juices in polymeric bottles were inquired to evaluate the release of phthalates in to juices. This research has established that the amount of PAEs found in bottled juices due to the type of the bottle, the period of storage (the time of contact with packaging materials) and sunlight irradiation. DEP, DBP and DEHP are observed in all of the samples, but, in all conditions, phthalates migration were not considerable and all tested samples had levels of DEHP below 6 μg/L, the maximum contaminant level in drinking water established by the US Environmental Protection Agency [17]. Hence, the original conclusion of this study is that bottled acidic juices do not represent a main ingestion source of phthalate esters for consumers, and the levels of observed PAEs in these kinds of juices are not a matter of concern from the viewpoint of adverse health effects.
Acknowledgments
This research has been supported by Tehran University of Medical Sciences grant (project No. 92-01-46-21405). Hereby, the cooperation of the University and also Institute for Environmental Research (IER) is highly appreciated.
Abbreviations
- PET
Polyethylene terephthalate
- HDPE
High density polyethylene
- PAEs
Phthalic acid esters
- DEP
Diethyl phthalate
- DBP
Dibutyl phthalate
- DEHP
Diethylhexyl phthalate
- DMP
Dimethyl phthalate
- BBP
Butyl benzyl phthalate
- DNOP
di-n-octyl phthalate
- IS
Internal standard
Authors’ contributions
NR, YM and MZJ participated in the design of the study. RA did the analyses, YM and MZJ interpreted the analyzed results. MZJ participated in sample collection. NR was the main investigator, supervised the work, drafted and revised the paper critically for important intellectual content and compiled the work in accordance to journal format. All authors have read and approved the final manuscript.
Funding
This research has been supported by the Tehran University of Medical Sciences (TUMS) and the Health Services Grant (Project no. 92–01–46-21405).
Declaration
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Contributor Information
Noushin Rastkari, Phone: +98 21 88978395, Email: nrastkari@tums.ac.ir, Email: nr_rastkari@yahoo.com.
Masud Yunesian, Email: yunesian@tums.ac.ir.
Reza Ahmadkhaniha, Email: r-ahmadkhaniha@tums.ac.ir.
References
- 1.Thompson RC, Moore CJ, Vom Saal FS, Swan SH. Plastics, the environment and human health: current consensus and future trends. Philos Trans R Soc B Biol Sci. 2009;364:2153–2166. doi: 10.1098/rstb.2009.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc B Biol Sci. 2009;364:2097–2113. doi: 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ Sci Technol. 2001;35(2):318–324. doi: 10.1021/es0010498. [DOI] [PubMed] [Google Scholar]
- 4.Oehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, Kusk KO, Wollenberger L, Santos EM, Paull GC, van Look KJW, Tyler CR. A critical analysis of the biological impacts of plasticizers on wildlife. Philos Trans R Soc Lond B Biol Sci. 2009;364:2047–2062. doi: 10.1098/rstb.2008.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talsness CE, Andrade AJ, Kuriyama SN, Taylor JA, vom Saal FS. Components of plastic: experimental studies in animals and relevance for human health. Philos Trans R Soc Lond B Biol Sci. 2009;364(1526):2079–2096. doi: 10.1098/rstb.2008.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner M, Oehlmann J. Endocrine disruptors in bottled mineral water: total estrogenic burden and migration from plastic bottles. Environ Sci Pollut Res. 2009;16(3):278–286. doi: 10.1007/s11356-009-0107-7. [DOI] [PubMed] [Google Scholar]
- 7.Page BD, Lacroix GM. The occurrence of phthalate ester and di-2-ethylhexyl adipate plasticizers in Canadian packaging and food sampled in 1985–1989: a survey. Food Addit Contam. 1995;12(1):129–151. doi: 10.1080/02652039509374287. [DOI] [PubMed] [Google Scholar]
- 8.Casajuana N, Lacorte S. New methodology for the determination of phthalate esters, bisphenol A, bisphenol A diglycidyl ether, and nonylphenol in commercial whole milk samples. J Agric Food Chem. 2004;52(12):3702–3707. doi: 10.1021/jf040027s. [DOI] [PubMed] [Google Scholar]
- 9.Farhoodi M, Emam-Djomeh Z, Ehsani MR, Oromiehie A. Effect of environmental conditions on the migration of di (2-ethylhexyl) phthalate from PET bottles into yogurt drinks: influence of time, temperature, and food simulant. Arabian J Sci Eng. 2008;33(2):279–287. [Google Scholar]
- 10.Montuori P, Jover E, Morgantini M, Bayona JM, Triassi M. Assessing human exposure to phthalic acid and phthalate esters from mineral water stored in polyethylene terephthalate and glass bottles. Food Addit Contam. 2008;25(4):511–518. doi: 10.1080/02652030701551800. [DOI] [PubMed] [Google Scholar]
- 11.Schmid P, Kohler M, Meierhofer R, Luzi S, Wegelin M. Does the reuse of PET bottles during solar water disinfection pose a health risk due to the migration of plasticisers and other chemicals into the water? Water Res. 2008;42(20):5054–5060. doi: 10.1016/j.watres.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Yılmaz PK, Ertaş A, Kolak U. Simultaneous determination of seven phthalic acid esters in beverages using ultrasound and vortex-assisted dispersive liquid–liquid microextraction followed by high-performance liquid chromatography. J Sep Sci. 2014;37(16):2111–2117. doi: 10.1002/jssc.201400408. [DOI] [PubMed] [Google Scholar]
- 13.Farajzadeh MA, Khoshmaram L. Development of dispersive liquid–liquid microextraction technique using ternary solvents mixture followed by heating for the rapid and sensitive analysis of phthalate esters and di (2-ethylhexyl) adipate. J Chromatogr A. 2015;1379:24–33. doi: 10.1016/j.chroma.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 14.Zare Jeddi M, Ahmadkhaniha R, Yunesian M, Rastkari N. Magnetic solid-phase extraction based on modified magnetic nanoparticles for the determination of phthalate diesters in water samples. J Chromatogr Sci. 2015;53(2):385–391. doi: 10.1093/chromsci/bmu058. [DOI] [PubMed] [Google Scholar]
- 15.Lertsirisopon R, Soda S, Sei K, Ike M. Abiotic degradation of four phthalic acid esters in aqueous phase under natural sunlight irradiation. J Environ Sci. 2009;21(3):285–290. doi: 10.1016/S1001-0742(08)62265-2. [DOI] [PubMed] [Google Scholar]
- 16.Lau T, Chu W, Graham N. The degradation of endocrine disruptor di-n-butyl phthalate by UV irradiation: a photolysis and product study. Chemosphere. 2005;60(8):1045–1053. doi: 10.1016/j.chemosphere.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Al-Saleh I, Shinwari N, Alsabbaheen A. Phthalates residues in plastic bottled waters. J Toxicol Sci. 2011;36(4):469–478. doi: 10.2131/jts.36.469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will not be shared with a reason.


