Abstract
Nuclear distribution and migration of herpes simplex virus type 1 Us11 transcripts were studied in transient expression at the ultrastructural level and compared to that of RNA polymerase II protein. Transcription was monitored by autoradiography following a short pulse with tritiated uridine. Us11 transcripts accumulated mainly over the foci of intermingled RNP fibrils as demonstrated by the presence of silver grains localizing incorporated radioactive uridine superimposed to these structures in which the presence of Us11 RNA and poly(A) tails was previously demonstrated. Silver grains were also scattered over the remaining nucleoplasm but not in the clusters of interchromatin granules, and over the dense fibrillar component of the nucleolus as in control, nontransfected HeLa cells. Pulse-chase experiments revealed the transient presence of migrating RNA in the clusters of interchromatin granules. RNA polymerase II was revealed by immunogold labeling following the use of two monoclonal antibodies: mAb H5, which recognizes the hyperphosphorylated form of the carboxy-terminal domain (CTD) of the molecule, and mAb 7C2, which recognizes both its hyperphosphorylated and unphosphorylated forms. The two mAbs bind to the newly formed Us11 transcription factories and the clusters of interchromatin granules of transfected cells. In control cells, however, clusters of interchromatin granules were labeled with mAb H5 but not with mAB 7C2. Taken together, our data demonstrate the involvement of the clusters of interchromatin granules in the intranuclear migration of Us11 RNA in transient expression. They also suggest the occurrence of changes in the accessibility of the RNA polymerase II CTD upon expression of the Us11 gene after transfection by exposing some epitopes, otherwise masked in nontransfected cells.
Keywords: Autoradiography, Clusters of interchromatin granules, Electron microscopy, In situ hybridization, HeLa cells, H5 monoclonal antibody, 7C2 monoclonal antibody, RNA polymerase II, Transcription factories, Transfection, Tritiated uridine, Us11 gene
THE combination at the electron microscope level of various techniques for visualizing and detecting the nuclear components (40) has dramatically increased our knowledge of structure-function relationships in the cell nucleus. It is now generally accepted that transcription of premessenger RNA (pre-mRNA) takes place within a narrow zone at the border of condensed chromatin clumps (15,17,35,36). In addition, a number of studies including parallel biochemical and morphological approaches demonstrated that the perichromatin fibrils present at the transcription sites correspond to nascent transcripts (1,15,17,19,35,36). It was also shown that following their synthesis the perichromatin fibrils migrate towards the interchromatin space while their RNA is being matured (18,34), indicating that these structures also correspond to the morphological support of splicing of pre-mRNA. This was more recently confirmed by the demonstration at the electron microscope level that spliceosome components, including small nuclear antigens (16,37,47–49,51) and small nuclear RNA (51), are associated with perichromatin fibrils. However, spliceosome components also accumulate within three other nuclear compartments that are not directly involved in transcription and splicing: the clusters of interchromatin granules, the interchromatin granule-associated zones, and the coiled bodies, suggesting that these structures participate in some pre- and/or postsplicing events (51). In addition, the recent detection of poly(A)+ RNA in the clusters of interchromatin granules of normal (21,52) and DNA virus-infected cells (4,39) raised the question of the involvement of these structures in some regulation of nuclear postsplicing events (36).
Because they exhibit a high level of transcription of one specific gene, transfected cells provide a suitable system to study many aspects of gene expression, including processing of primary transcript and transport of messenger RNA. However, compared to normal nontransfected cells, some modifications cannot be excluded. Up to now, only a few ultrastructural studies have been devoted to the morphological and functional changes induced in the nuclei after transfection. Special attention has been paid to the behavior of herpes simplex virus type 1 (HSV-1) Us11 gene in transfected cells because of the possible involvement of its product in the nucleo-cytoplasmic transport of viral RNA (12). In lytically HSV-1-infected cells, Us11 protein is the product of a true late gene and is incorporated into virions (27). Us11 protein also exhibits a nucleolar localization in HSV-1-infected cells as well as in cells expressing Us11 alone, either transiently or constitutively (3,11,30,38,44). Site- and conformation-specific RNA binding abilities (41–43) and possible posttranscriptional transactivation of human retrovirus gene expression (11) are among the major properties of this viral protein. Us11 protein is able to act similarly on HTLV-I-Rex and HIV-1-Rev proteins by binding to Rex- and Rev-responsive elements, leading to the subsequent synthesis of retroviral envelope glycoproteins. In situ hybridization visualized by electron microscope demonstrated that Us11 RNA and poly(A) tails accumulated into two distinct nucleoplasmic structures, that is, newly formed foci consisting of intermingled RNP fibrils, and well-identifiable preexisting clusters of interchromatin granules (3) in HeLa cells transiently expressing Us11 gene.
To determine the precise sites of transcription of Us11 gene and the functional significance of Us11 RNA accumulation in these two nucleoplasmic structures, we used similarly transfected cells to undertake at the ultrastructural level the precise localization of viral transcripts. First, the immunogold distribution of RNA polymerase II molecules was visualized using two monoclonal antibodies (mAb) directed toward the carboxy-terminal domain (CTD) of the large subunit of RNA polymerase II. One of these antibodies, mAb H5, is specific for the 240-kDa hyperphosphorylated form of the CTD of the large subunit of RNA polymerase II (IIo) (7) whereas a second antibody, mAb 7C2, recognizes both its phosphorylated and unphosphorylated forms (4). The detection of RNA polymerase II was carried out with or without concomitant detection of Us11 RNA by in situ hybridization. Second, nascent RNA and migrating RNA were localized by electron microscope autoradiography following incorporation of pulsed and pulse-chased tritiated uridine, respectively. We report here evidence that the foci of intermingled Us11 RNP fibrils contain growing Us11 RNA together with RNA polymerase II molecules, indicating that they correspond to transcription factories, whereas the clusters of interchromatin granules contain Us11 RNA that has left its transcription site. Both RNA polymerase II antibodies detect sites of active transcription of transfected genes. However, whereas mAb H5 intensely labels each cluster of interchromatin granules in transfected and nontransfected cells, a significant binding of mAb 7C2 occurs only over some clusters of interchromatin granules in Us11 transfected cells but not in control cells. These data might suggest changes in the accessibility of some epitopes of the CTD domain of the RNA polymerase II molecule in the clusters of interchromatin granules under expression of Us11 gene after transfection.
MATERIALS AND METHODS
Vectors and Transfection of Hela Cells
Preparation of plasmid DNA and procedures of transfection are described in detail in our previous studies (3,11,20). Briefly, the pCMV-Us11 vector was built by subcloning the entire Us11 coding sequence in pBC12/CMV in order to place the Us11 coding sequence under the control of the CMV immediate-early promoter (10,11). The polyadenylation signal was brought by pBC12/ CMV. The 3′ untranslated region of Us11 primary transcript contains the intron (500 nucleotides) of rat preproinsulin II primary transcript encoded by pBC12/CMV original vector. Therefore, the insert consisted of the Us11 gene of HSV-1, which is a gene without intron, and the intronic 500-nucleotide sequence of a cellular gene. Transfections were carried out on mono-layers of HeLa cells (15 μg of pCMV-Us11 vector was added to 106 cells) by using the calcium phosphate precipitation procedure (46). Cells were fixed 48 h after transfection. Transfection efficiency was monitored by detecting Us11 protein by indirect immunofluorescence. In a typical experiment, Us11 protein was detected in about 50% of the transfected cells. As control, cells were transfected with pCMV vector, which is pCMV-Us11 devoid of Us11 coding sequence (11). pCMV-Us11 and pCMV were also previously called pG-9 and pG-8, respectively (3).
Antibody and Probe
Two polymerase II large subunit monoclonal antibodies (mAb) were used. The IgM mAb H5 was produced and characterized as described previously (54). It is specific for the 240-kDa hyper-phosphorylated form of the largest subunit of RNA polymerase II (54,56). The IgG mAb 7C2, directed against the carboxy-terminal domain (CTD) of RNA polymerase II, was produced as previously described (4). Briefly, it was raised against a synthetic polypeptide corresponding to a threefold repetition of the consensus heptapeptide, which is repeated 52 times at the C-terminal end of the largest subunit of the mammalian RNA polymerase II. Positive hybridomas were sub-cloned twice on soft agar. The selected clone 7C2 produced IgG1(κ) antibodies in the supernatant of the hybridoma culture.
The Us11 DNA probe was biotinylated by nick translation of whole plasmid DNA using biotinylated dATP (Bethesda Research Laboratory, Bethesda, MD) as previously described (3). The extent of the reaction was monitored in parallel by incubating an aliquot of the reaction mixture in the presence of [α-35S]dATP. The level of replacement of dAMP residues by radioactive dAMP in DNA plasmid reached about 40%. It reflects the extent of biotinylation of the probe because the incorporation is fully dependent on the presence of biotinylated dATP. The biotinylated double-stranded DNA probe was added to salmon sperm DNA (200 μg/ml) before ethanol precipitation. After a second round of ethanol precipitation, the biotinylated probe was resuspended in distilled water (60 μg/ml) and stored at –20°C.
Immunolabeling and In Situ Hybridization
Immunolabeling of RNA polymerase II molecules and in situ hybridization of Us11 RNA were carried out on nonradioactive cultures of pCMV-Us11 transfected cells previously fixed for 1 h at 4°C with 1.6% glutaraldehyde (Taab Lab. Equip. Ltd, Reading, UK) or 4% formaldehyde (Merck, Darmstadt, Germany) in 0.1 M phosphate buffer, pH 7.3, dehydrated in methanol and embedded in Lowicryl K4M (Polysciences Europe GmbH, Ep-pelheim, Germany) at low temperature according to Roth (45), as previously described (3). Untreated HeLa cells and pCMV transfected cells were used as controls. Ultrathin sections of transfected and nontransfected HeLa cells were collected on formvar-carbon-coated gold grids (200 mesh).
For detection of the RNA polymerase II molecules, grids bearing Lowicryl sections of normal cells and of cells previously transfected with either pCMV-Us11 vector or pCMV control vector were floated at the surface of 10-μl drops of mAb H5 [1/100 in phosphate-buffered saline (PBS)] and mAb 7C2 (1/10 in PBS) (3) at room temperature, for 30 min and 1 h, respectively. After washing in PBS, grids were floated for 30 min, at room temperature, on 10-μl drops of secondary antibody conjugated to 10-nm-diameter gold particles (Biocell Res. Lab., Cardiff, UK) and diluted 1/25 in PBS. Goat anti-mouse IgM and goat anti-mouse IgG were used for the labeling of H5 and 7C2 antibody, respectively. After washing in PBS, grids were rinsed in distilled water, air dried, and stained for 10 min with 5% aqueous uranyle acetate. For controls, grids were incubated in the presence of nonimmune mouse serum (1/10 in PBS) instead of mAb H5 and mAb 7C2 before incubation with gold-labeled secondary antibody.
Sequential double labeling was performed by incubating sections of cells transfected with pCMV-Us11 vector successively over drops of mAb H5 (IgM), mAb 7C2 (IgG), and finally over a cocktail of gold-labeled goat anti-mouse IgM and anti-mouse IgG, 10 and 5 nm in diameter, respectively.
For the simultaneous detection of Us11 RNA and RNA polymerase II molecules, grids bearing sections of cells transfected with pCMV-Us11 vector were incubated for 3 h at 37°C over minidrops (1–2 μl) of freshly heat-denatured hybridization solution as previously described (3). The latter consisted of 50% deionized formamide, 2 × SSC (1 × SSC = 0.15 M sodium chloride, 0.015 M sodium citrate), 10% dextran sulfate, 400 μg/ml of salmon sperm DNA, and 10 μg/ml of biotinylated plasmid. After hybridization, grids were rinsed at the surface of PBS drops. They were then floated at room temperature over 10-μl drops of either mAb H5 (1/100 in PBS) or mAb 7C2 (1/10 in PBS) for 30 min and 1 h, respectively, and rapidly rinsed over drops of PBS. Finally, they were incubated for 30 min at room temperature over a mixture of gold-labeled goat anti-biotin antibody and goat anti-mouse antibody, either 5 and 10 nm or 10 and 5 nm in diameter, respectively (Biocell Research Lab.), each 1/25 in PBS. These double labeling experiments performed, of course, in the presence of the proteins of the section, resulted in a rather moderate efficiency of Us11 RNA detection as already reported (3).
Autoradiography
To localize nascent RNA molecules, cells were transfected with pCMV-Us11 vector for 48 h then pulse-labeled for 6 min with 3.7 × 106 Bq/ml of [5,6-3H]uridine (sp. act. 1.30TBq/mmol) (Amersham International pic. Amersham, UK) in Eagle’s minimum essential medium supplemented with 10% heat-inactivated newborn calf serum. Then they were fixed for 1 h at 4°C with the 1.6% glutaraldehyde solution, dehydrated in ethanol, and embedded in Epon.
To visualize the migration of the newly synthesized molecules, cells were incubated in the presence of tritiated uridine as above, then incubated in fresh medium containing 10–4 M of cold uridine for 30, 60, and 90 min before being dehydrated and embedded as above.
Epon sections were recovered onto Formvarcarbon-coated copper grids (mesh 200). Deposit of Ilford L4 emulsion was performed by the loop technique (5). Development of labeled material was performed with phenidon after 5-month exposure. Sections were stained with uranyl acetate with or without lead citrate or EDTA regressive staining (2).
RESULTS
Distribution of RNA Polymerase II Molecules
To study the fate of the RNA polymerase II molecules during transfection, two corresponding antibodies, mAb 7C2 and mAb H5, were tested on Lowicryl sections of pCMV-Us11 transfected cells and control cells. The specificity of mAb 7C2 was demonstrated by immunoblot analysis of a crude enzyme preparation from HeLa cell extracts (Fig. 1). As revealed after high-resolution SDS-gel electrophoresis, only two bands were labeled by the antibody, corresponding to the multiphosphory-lated (Ho, 240 kDa) and unphosphorylated (Ha, 214 kDa) forms of the largest subunit of RNA polymerase II, respectively (14,33). These results strongly suggest that both transcribing and non-transcribing polymerase molecules could be visualized with mAb 7C2. In contrast, mAb H5 recognizes only a hyperphosphorylated form of the polymerase largest subunit (54). With this in mind, the two antibodies were applied on sections of cells prepared for electron microscopy and processed as described in Materials and Methods.
FIG. 1.
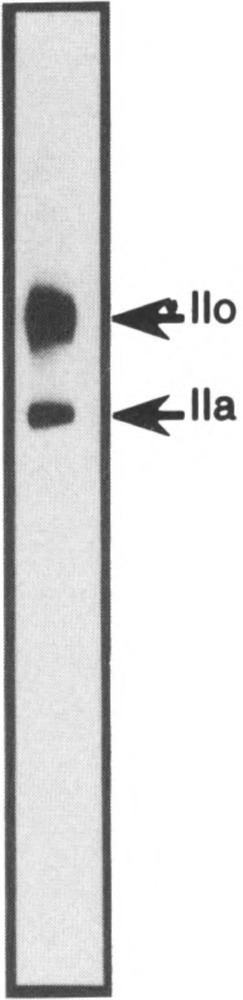
Specificity of mAb 7C2. About 1 μg of DE1.0, a 1 M KCl eluate from a DEAE-5PW chromatography corresponding to a partially purified RNA polymerase II fraction from HeLa cells [see (25)], was loaded on a 5% polyacrylamide SDS gel (29). After electrophoresis, the proteins were transferred to a nitrocellulose membrane and incubated with the mAb 7C2. Specific immune complexes were revealed by the ECL Western blotting detection system (Amersham International pic, Buckinghamshire, UK), as previously described (8). The positions of the phosphorylated (Ho) and unphosphorylated (11a) largest subunit of RNA polymerase II are indicated.
In the nuclei of control cultures in which no foreign gene was expressed (i.e., in untreated cells and in cells transfected with the pCMV vector), mAb H5 intensely labeled the clusters of interchromatin granules (Fig. 2a). In addition, gold particles were present over the perichromatin fibrils, which were scattered in the nucleoplasm or constituted small clusters of intermingled fibrils (Fig. 2b). The coiled bodies (Fig. 2c), interchromatin granule-associated zones (Fig. 2d), and nucleolus were entirely devoid of labeling. Following the use of mAb 7C2 gold particles were associated with the perichromatin fibrils of the interchromatin space; however, they were never found in the clusters of interchromatin granules. These data are identical to those previously described (4) and are not shown.
FIG. 2.
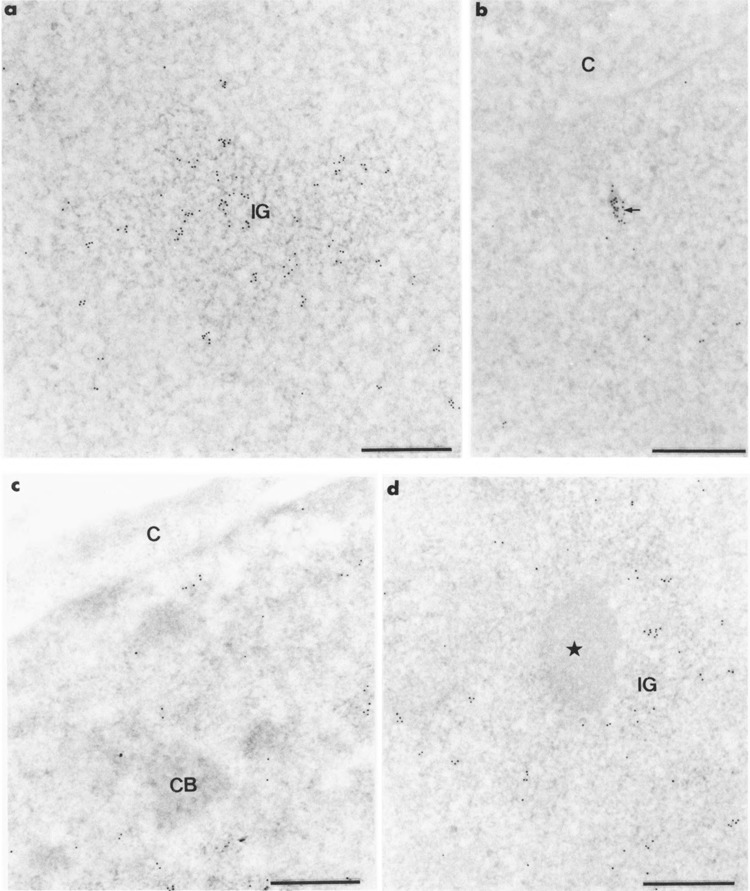
Intranuclear distribution of RNA polymerase II molecules in HeLa cells following the use of mAb H5. Formaldehyde fixation, Lowicryl embedding, and uranyl acetate staining. Bars represent 0.5 μm. (a, b) Gold particles are numerous over a cluster of interchromatin granules (IG) in (a) and over a small cluster of RNP fibrils (arrow) in (b). They are scattered over the surrounding nucleoplasm, (c, d) The coiled body (CB) in (c) and the interchromatin granule-associated zone (star) in (d) are devoid of gold particles. C: cytoplasm; IG: cluster of interchromatin granules.
When immunogold labelings with mAb H5 and mAb 7C2 were performed on cell cultures transfected with pCMV-Us11 vector, about 95% of the nuclei showed a labeling pattern identical to that described above for control cells. The remaining nuclei showed an additional labeling over a new structure of the nucleoplasm. Whatever the anti-body used, gold particles accumulated over more or less roundish foci of up to 0.8 jinn in diameter, consisting of intermingled, 25-nm-thick RNP fibrils (Figs. 3 and 4a). Some foci of gold-labeled fibrils were located close to the clusters of interchromatin granules (Fig. 4b), the latter always being labeled with mAb H5 as in control cells (Fig.3). Interestingly, following the use of mAb 7C2, many gold particles were found over some clusters of interchromatin granules (Fig. 5a). It must be pointed out that labeled and unlabeled clusters of interchromatin granules were observed concomitantly in the same nucleus. Favorable, but unusual, planes of section clearly demonstrated a concomitant labeling of clusters of interchromatin granules and of foci of perichromatin fibrils with mAb 7C2 (Fig. 5b).
FIG. 3.
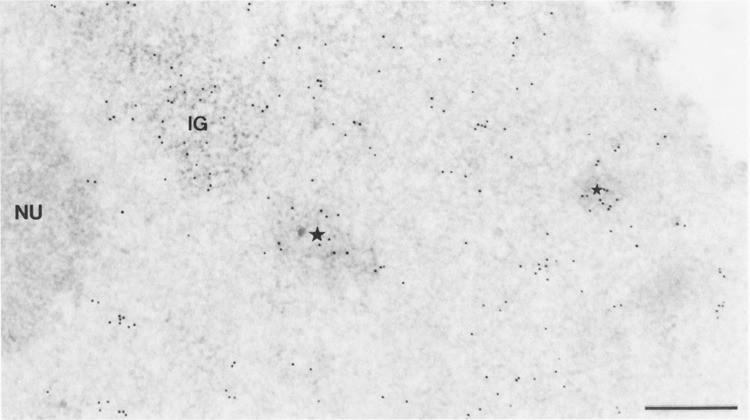
Intranuclear distribution of RNA polymerase II molecules in a cell transiently expressing the Us11 gene, following the use of mAb H5. Formaldehyde fixation, Lowicryl embedding, and uranyl acetate staining. Bars represent 0.5 μm. Gold particles are mainly observed over the cluster of interchromatin granules (IG) and the clumps of intermingled RNP fibrils induced by transfection (stars). They are scattered over the surrounding nucleoplasm and absent over the nucleolus (NU).
FIG. 4.
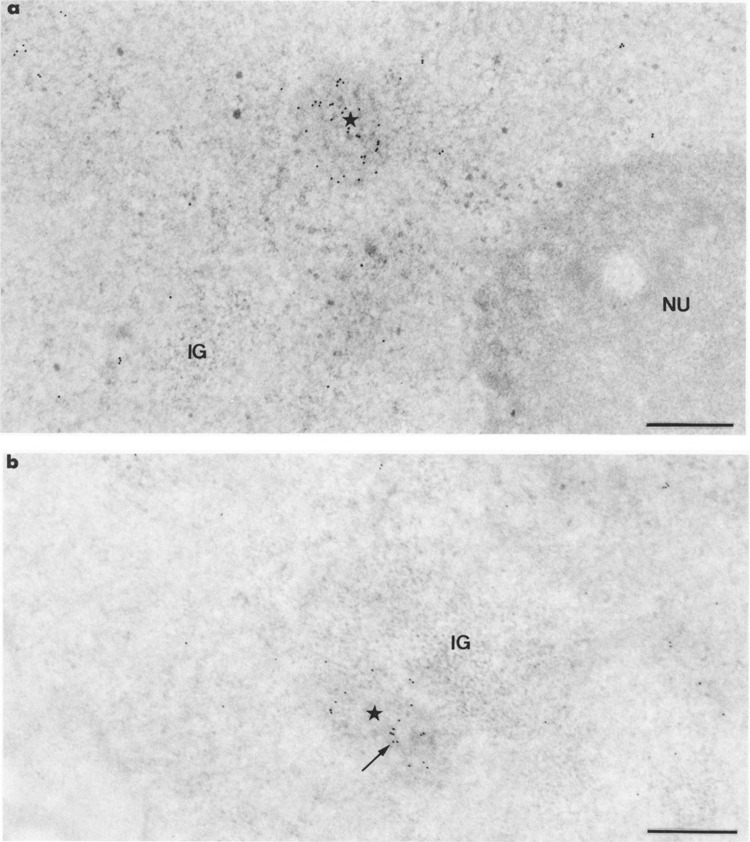
Intranuclear distribution of RNA polymerase II molecules in cells transiently expressing the Us11 gene, following the use of mAb 7C2. Formaldehyde fixation, Lowicryl embedding, and uranyl acetate staining. Bars represent 0.5 μm. (a) Gold particles accumulate over a roundish focus of intermingled RNP fibrils (star) whereas they are scattered over the surrounding nucleoplasm. IG: cluster of interchromatin granules; NU: nucleolus, (b) The focus of intermingled viral RNP fibrils (star) is located near a cluster of interchromatin granules (IG). Labeling over the focus of RNP fibrils is exactly superimposed to its individual fibrils (arrow).
FIG. 5.
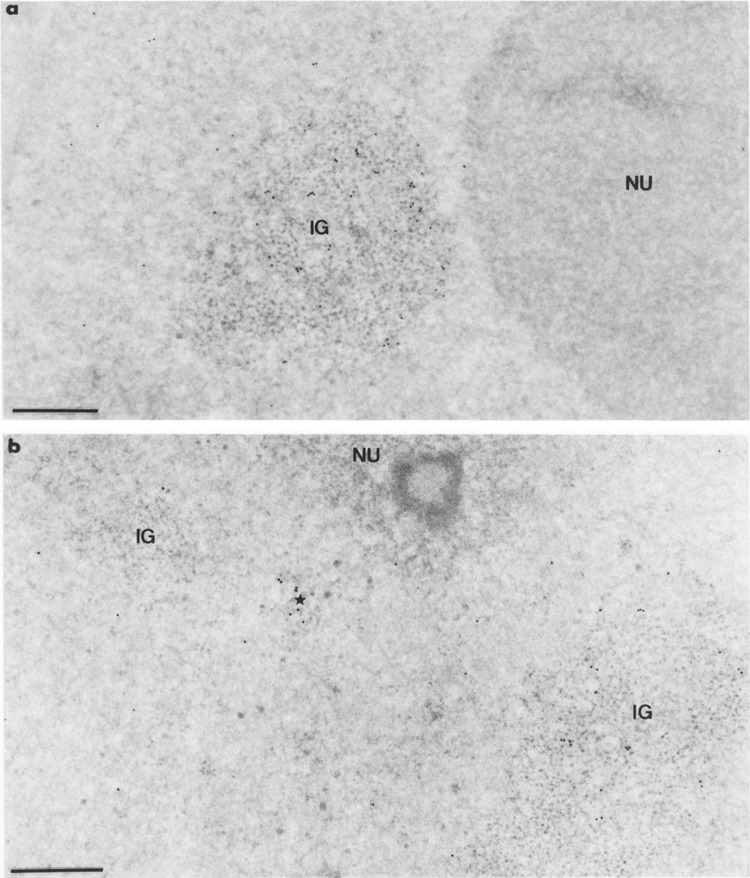
Intranuclear distribution of RNA polymerase II molecules in cells transiently expressing the Us11 gene, following the use of mAb 7C2. Formaldehyde fixation, Lowicryl embedding, and uranyl acetate staining. Bars represent 0.5 μm. (a) Gold particles are numerous over the large cluster of interchromatin granules (IG) and are markedly rare in the surrounding nucleoplasm. The nucleolus (NU) is entirely devoid of labeling, (b) Two clusters of interchromatin granules (IG) are present, only one being decorated with gold particles (IG on the right). Between these two clusters is a small focus of intermingled RNP fibrils (star) that is labeled as in Fig. 4a, b. Once again the nucleolus (NU) is unlabeled.
Following concomitant detection of mAb H5 and mAb 7C2 antigens with 10- and 5-nm gold particles, respectively, both gold particles were present within the roundish foci of intermingled RNP fibrils, which occurred in cells transfected with pCMV-Us11 vector, indicating that the corresponding epitopes of the largest subunit of the RNA polymerase II are accessible in these structures (Fig. 6a). The two sizes of gold particles also were observed over the perichromatin fibrils, which were scattered in the nucleoplasm of each cell. Regarding the clusters of interchromatin granules, some contained the two sizes of gold particles (Fig. 6b) whereas the others were decorated by the 10-nm gold particles only.
FIG. 6.
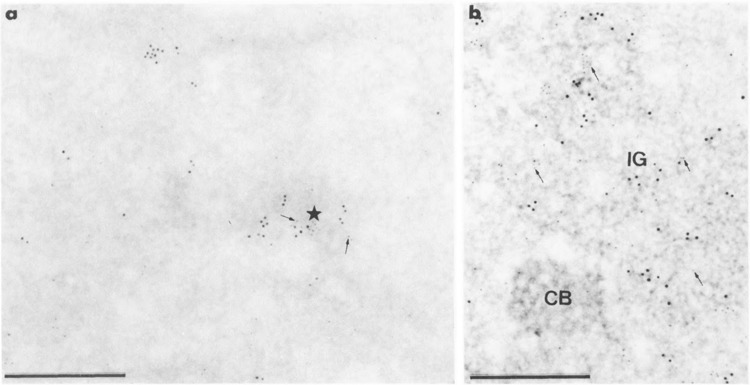
Concomitant detection of mAb H5 and mAb 7C2 antigens of RNA polymerase II molecules with 10- and 5-nm gold particles, respectively, in cells transiently expressing the Us11 gene. Formaldehyde fixation, Lowicryl embedding, and uranyl acetate staining. Bars represent 0.5 μm. (a, b) Mixed 5-nm (arrows) and 10-nm gold particles are present over the clump of intermingled viral RNP fibrils (star) in (a) and the cluster of interchromatin granules (IG) in (b). Individual 5- and 10-nm gold particles are present in the surrounding nucleoplasm. CB: coiled body.
Concomitant detection of RNA polymerase II molecules by immunocytology and Us11 RNA by in situ hybridization was undertaken to identify clearly cells expressing Us11 gene. Double labeling of sections for mAb H5 antigens and Us11 RNA was accomplished using 10- and 5-nm gold particles, respectively. In contrast, concomitant detection of mAb 7C2 and Us11 RNA was obtained following the use of 5- and 10-nm gold particles, respectively. Both 5- and 10-nm gold particles were present over foci of intermingled perichromatin fibrils and were scattered over the nucleoplasm whatever the combination used (Fig. 7a, c). In experiments related to the concomitant detection of mAb 7C2 antigens and Us11 RNA, although most clusters of interchromatin granules were devoid of labeling, some were labeled by the two sizes of gold particles (Fig. 7b) and some others (Fig. 7a) only contained 10-nm gold particles. In experiments related to the concomitant detection of mAb H5 antigens and Us11 RNA, most clusters of interchromatin granules were decorated with 10-nm gold particles only; however, a few contained the two sizes of gold particles.
FIG. 7.
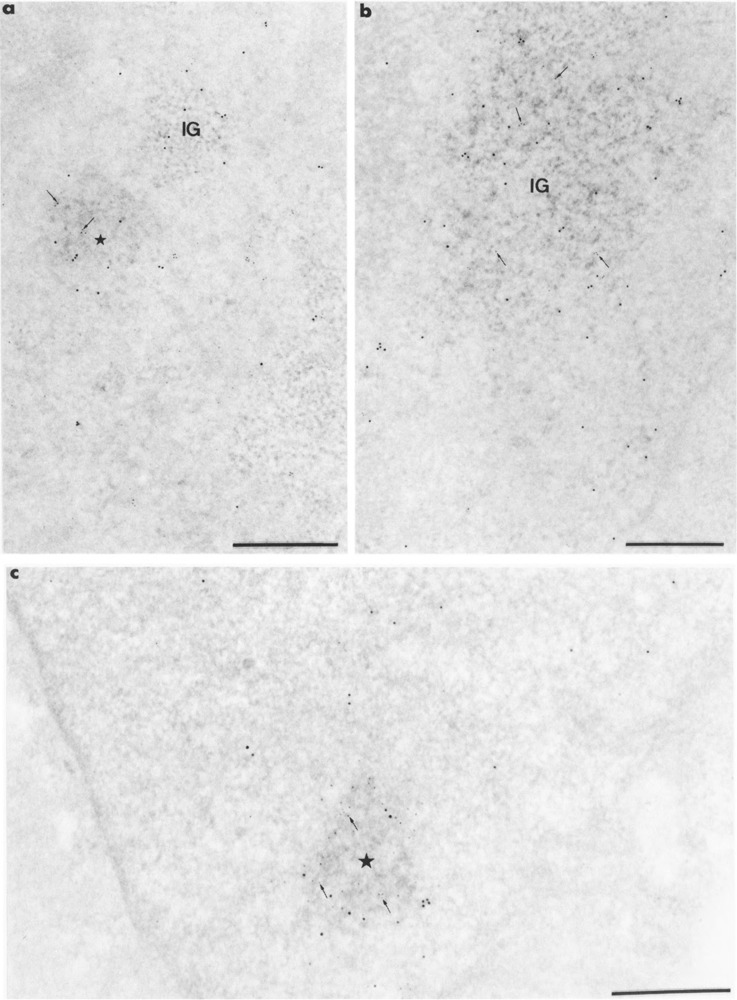
Colocalization of RNA polymerase II molecules and Us11 RNA on Lowicryl sections of cells transiently expressing Us11 gene. Formaldehyde in (a, b) and glutaraldehyde in (c) fixation. Uranyl acetate staining. Bars represent 0.5 μm. (a, b) mAb 7C2 and biotinylated Us11 DNA probe combination. Gold particles of 5-nm diameter label the protein whereas the 10-nm gold particles label the viral RNA. (a) Mixed 5-nm (arrows) and 10-nm gold particles are present over the focus of intermingled viral RNP fibrils (star) whereas 10-nm gold particles are present only over the small cluster of interchromatin granules (IG). Individual 5- and 10-nm gold particles are present in the surrounding nucleoplasm, (b) Mixed 5-nm (arrows) and 10-nm gold particles are present over the large cluster of interchromatin granules (IG). Individual 5- and 10-nm gold particles are present in the surrounding nucleoplasm, (c) mAb H5 and biotinylated Us11 DNA probe combination. Gold particles of 10-nm diameter label the protein, whereas the 5-nm gold particles label the viral RNA. Mixed 5-nm (arrows) and 10-nm gold particles are present over the focus of intermingled viral RNP fibrils (star).
Distribution of Tritiated-Labeled RNA
Following a short-pulse with tritiated uridine after 48 h of transfection with pCMV-Us11 vector, silver grains localizing nascent RNA were concentrated over roughly roundish foci of intermingled fibrils (Fig. 8a–c). Some of these foci were close to a cluster of interchromatin granules (Fig. 8c) or near the nucleolus (Fig. 8a). In addition, a few silver grains were observed throughout the nucleoplasm and over the dense fibrillar component of the nucleolus but not over its granular component (Fig. 8a). The clusters of interchromatin granules and the cytoplasm were always entirely devoid of silver grains.
FIG. 8.
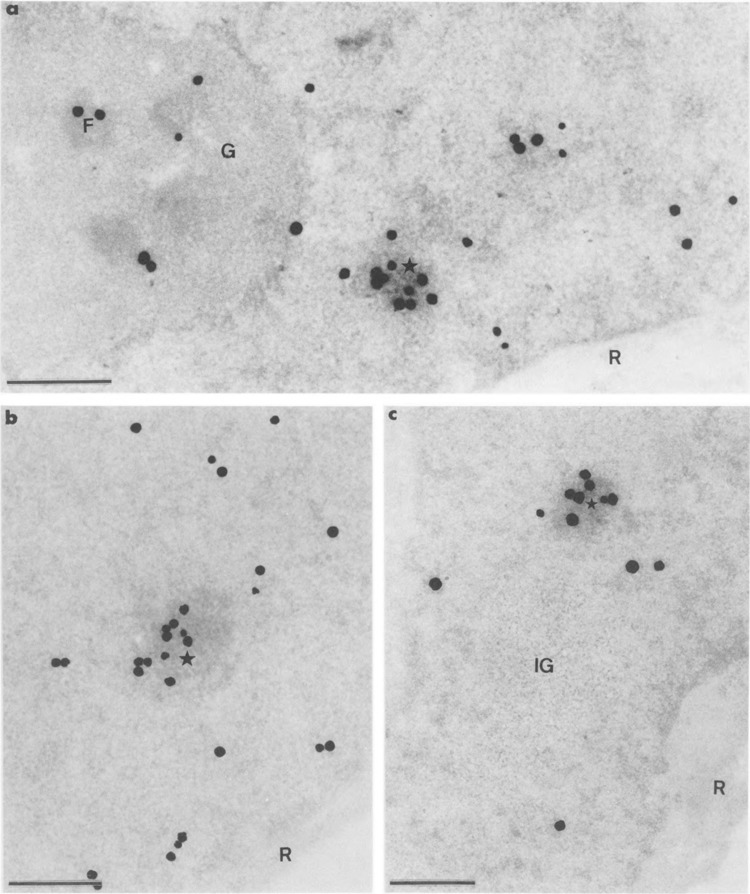
Autoradiograms of cells transiently expressing Us11 gene and pulse-labeled during 6 min with tritiated uridine just before fixation. Epon embedding and uranyl acetate staining. Bars represent 0.5 μm. (a) Silver grains that localize the sites of transcription of both Us11 genes and cellular genes are numerous over the focus of intermingled RNP fibrils (star) and are also present over areas of the surrounding nucleoplasm and over the dense fibrillar component (F) of the nucleolus. This indicates the persistent transcriptional activity of the cellular genes, including the ribosomal RNA genes. The granular component (G) of the nucleolus and the cytoplasmic ribosomes (R) are devoid of labeling, (b) Part of nucleoplasm. The focus of intermingled RNP fibrils (star) is intensely labeled. The concomitant presence of silver grains over the surrounding nucleoplasm demonstrates once again that transcription of cellular genes is still occurring. Cytoplasmic ribosomes (R) are devoid of silver grains, (c) Part of nucleoplasm in which the intensely labeled focus of intermingled RNP fibrils (star) is located near an unlabeled large cluster of interchromatin granules (IG). Once again the cytoplasmic ribosomes (R) are devoid of silver grains.
When pCMV-Us11 transfected cells labeled with tritiated uridine were submitted to a chase of 30 min, the distribution of silver grains was changed when compared to that observed immediately after the pulse of labeling. Indeed, foci of RNP fibrils were free of silver grains, except at their periphery (Fig. 9a), and some clusters of interchromatin granules became labeled (Fig. 9a, b). In the nucleolus, silver grains were located over the granular component and were absent from the dense fibrillar one. Areas of cytoplasm containing the ribosomes were also labeled (Fig. 9b). After a chase of 60 min, the distribution of silver grains was similar to that obtained after a chase of 30 min except for the periphery of the foci of RNP fibrils, which was now entirely devoid of labeling. Once again, some clusters of interchromatin granules were labeled (Fig. 10a, b). After a chase of 90 min, no silver grains were found over the clusters of interchromatin granules or over the foci of RNP fibrils (not shown). Silver grains were never observed over the clusters of interchromatin granules of the cells, which did not express the Us11 gene whatever the duration of the chase period.
FIG. 9.
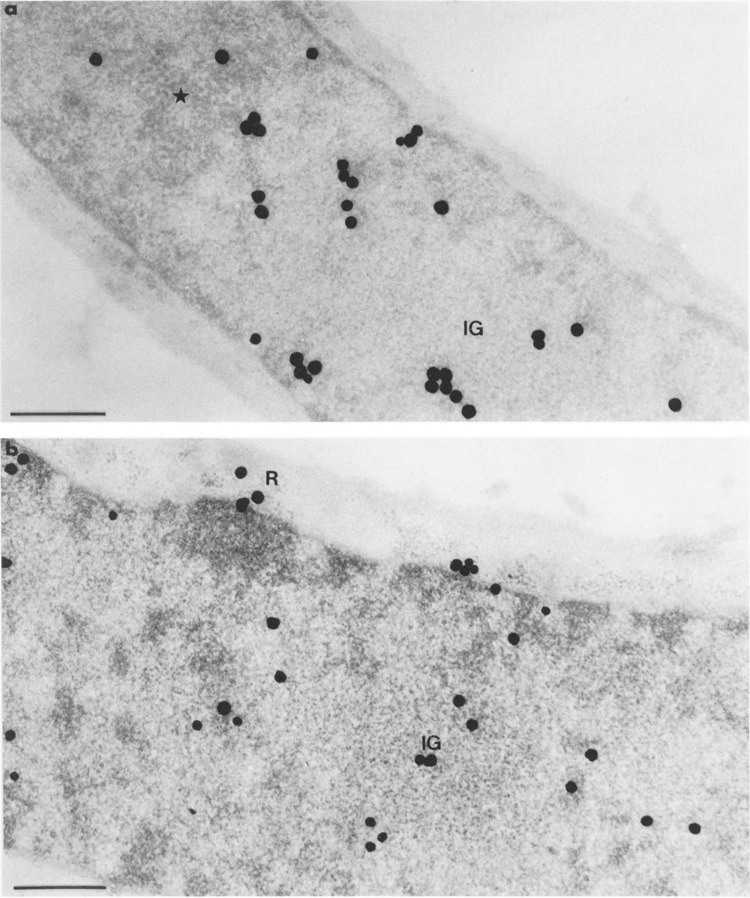
Autoradiograms of cells transiently expressing Us11 gene and pulse-labeled with tritiated uridine as in Fig. 8, then submitted to a chase of 30 min before fixation. Epon embedding. Bars represent 0.5 μm. (a) Uranyl acetate staining. Silver grains are present at the border of the focus of intermingled viral RNP fibrils (star), but not inside. Silver grains are also present in the large cluster of interchromatin granules (IG) as well as in the remaining nucleoplasm, (b) Uranyl acetate and lead citrate staining. The cluster of interchromatin granules (IG) is clearly labeled. Silver grains are also scattered over the nucleoplasm and some grains are present over the cytoplasmic ribosomes (R).
FIG. 10.
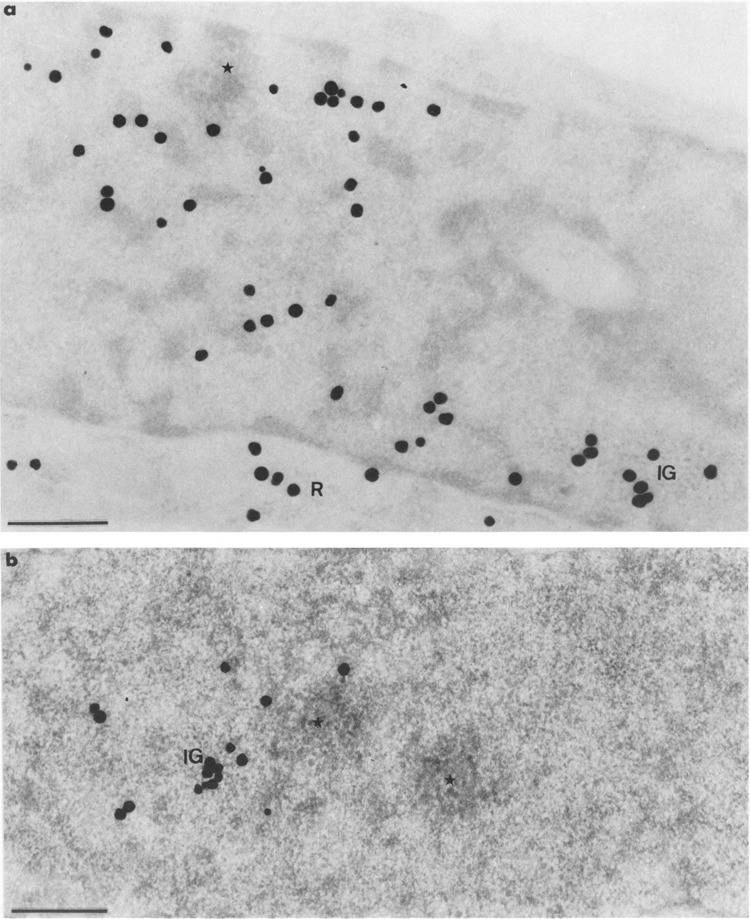
Autoradiograms of cells transiently expressing Us11 gene and pulse-labeled with tritiated uridine as in Fig. 8, then submitted to a chase of 60 min before fixation. Epon embedding. Bars represent 0.5 μm. (a) Uranyl acetate staining. The cluster of interchromatin granules (IG) is labeled whereas there is no labeling over the small focus of intermingled viral RNP fibrils (star). Silver grains are scattered over the remaining nucleoplasm and over the cytoplasmic ribosomes (R). (b) EDTA regressive staining. Part of nucleoplasm showing a labeled cluster of interchromatin granules (IG) and two unlabeled foci of intermingled RNP fibrils (stars), one of which being contiguous to the labeled cluster of interchromatin granules.
DISCUSSION
In this study, we show that in the nuclei of transfected HeLa cells transiently expressing HSV-1 Us11 gene, nascent RNA and RNA polymerase II molecules are concentrated over foci of intermingled RNP fibrils in which we previously demonstrated the presence of Us11 RNA and poly(A) tails (3). The data clearly demonstrate that RNA polymerase II molecules are recruited by Us11 genes active in transcription and, therefore, that the foci of RNP fibrils are transcription factories. The data reveal also that at least a part of the newly synthesized Us11 RNA leaves the sites of transcription to attain the clusters of interchromatin granules for a limited period of time.
It has been shown previously by in situ hybridization that, in transient expression, Us11 RNA is a component of newly formed foci of intermingled fibrils and is also present in preexisting clusters of interchromatin granules (3). A short pulse with tritiated uridine followed by autoradiography at high resolution demonstrate herein that the foci of intermingled fibrils are the sites of synthesis of Us11 RNA. Indeed, the pattern of labeling obtained in the nucleoplasm consists of a few prominent clusters of silver grains superimposed to foci of intermingled RNP fibrils and also of isolated grains scattered throughout the nucleoplasm. Several lines of evidence indicate that the clusters of silver grains represent sites of synthesis of Us11 transcripts. First, such large clusters of silver grains have never been reported in nontransfected cells in which sites of transcription are smaller, more numerous, and found to be associated with the RNP fibrils present within the thin layer of dispersed chromatin, contiguous to the masses of condensed chromatin (1,15,17,18,35). Moreover, in nontransfected cells the transcription sites appear as innumerable spots spread throughout the nucleoplasm when observed by light microscopy (23,24,53). Second, the large radioactive foci observed here are identical in aspect, frequency, and localization in the nucleoplasm to those previously described as containing Us11 RNA and poly(A) tails (3). Therefore, ulstrastructural studies reveal that the high level of transcription of the introduced Us11 genes generates a few, easily identifiable, large clusters of Us11 transcripts. Similar clusters were also observed by Huang and Spector in HeLa cells transfected with constructs expressing both intron-less and intron-containing RNAs (22).
We have compared the localization of nascent Us11 RNA with the distribution of RNA polymerase II molecules by use of the H5 and 7C2 monoclonal antibodies, both raised against the CTD of the largest subunit: mAb H5 revealing its hyperphosphorylated form and mAb 7C2 recognizing both its hyperphosphorylated and unphosphorylated forms. Gold particles localizing these molecules exhibit a widespread nucleoplasmic distribution that probably reflects the transcription of the cellular genes. RNA polymerase II molecules are also present over the entire foci of intermingled Us11 RNP fibrils visible only in transfected cells. Taken together, our data indicate that transcription of Us11 gene occurs in new domains that are viral transcription factories because of the presence of high concentrations of hyperphosphorylated RNA polymerase II molecules in intermingled RNP fibrils together with pulse-labeled Us11 transcripts. Indeed, the significant binding of mAb H5 to the clumps of viral RNP fibrils clearly demonstrates the presence of form IIo of the RNA polymerase II at the transcription sites of transfected Us11 genes. On the other hand, the concomitant binding of mAb 7C2 to these viral clumps suggests that a mixture of IIo and IIa RNA polymerase II might be present at the viral transcription sites. It must be taken into account that heat shock genes in Drosophila polytene chromosomes have been reported to be transcribed by such a mixture (55). Also Zeng et al. (56) demonstrated that RNA polymerase IIo and IIa can be photo-crosslinked to nascent RNA, which suggests that multiple phosphorylated forms of RNA polymerase II molecules are engaged in transcription. Further studies are needed to substantiate this possibility.
Pulse-chase experiments undertaken to visualize the migration route followed by newly synthesized RNA revealed that the RNA molecules synthesized during the pulse with tritiated uridine leave the foci of intermingled Us11 RNP fibrils (i.e., the viral transcription factories) to accumulate for a limited period of time within the clusters of interchromatin granules that are not involved in transcription. The total disappearance of radioactivity within the clusters of interchromatin granules after a chase of 90 min strongly suggests that Us11 RNA and poly(A)+ RNA detected by in situ hybridization in these structures (3) are transiently stored at these sites before being transported to the cytoplasm. However, we cannot exclude that these polyadenylated viral molecules, or only a fraction of them, might be degraded within the clusters of interchromatin granules. The present data are in line with our previous results showing that RNA polymerase II transcripts enter the clusters of interchromatin granules before their transport to the cytoplasm and/or their decay (52). This is true for control cells but also for cells with a high level of RNA polymerase II transcription, such as cells lytically infected with either adenovirus (39) or HSV-1 (4), and for cells transiently expressing Us11 gene [(3), this report].
Although they are not transcription sites, clusters of interchromatin granules in control and transfected cells contain large amounts of hyperphosphorylated RNA polymerase II as revealed by their intense labeling following the use of mAb H5. This is in line with recent studies that demonstrate a functional interaction between the hyperphosphorylated CTD of the RNA polymerase II and splicing factors [(13,31) for review see (50)] even though this association can occur without transcription (28). This is also in good agreement with studies carried out at optical level, which reveal that the colocalization of the hyperphosphorylated form of the large subunit of RNA polymerase II with splicing components occurs in interconnected speckle domains (6,7,32) corresponding to the perichromatin fibrils and clusters of interchromatin granules and excluding the coiled bodies (13). Without totally excluding possible masking of the epitopes, we could not detect in the interchromatin granule-associated zones any labeling corresponding to the Ho form of the RNA polymerase II. The latter and splicing components, therefore, apparently only colocalize in one of the three accumulation sites of spliceosome components (51). However, some authors using different monoclonal antibodies only detect a diffuse fluorescence corresponding to perichromatin fibrils and no fluorescence over speckle domains corresponding to clusters of interchromatin granules (9,26). An unexpected and exciting result is the observation of an intense binding of mAb 7C2 to some clusters of interchromatin granules of transfected cells. An explanation for these conflicting results obtained by us and others would be that the different epitopes of the CTD of the RNA polymerase II are not equally accessible to the specific antibodies when the molecule is in the clusters of interchromatin granules. Therefore, the unusual labeling by the mAb 7C2 of clusters of interchromatin granules might reflect the unmasking of the corresponding epitope when cells are expressing the Us11 gene, although the reasons for such a better accessibility in cells transiently expressing Us11 gene, but not in cells lytically infected with HSV-1 (4), remain unknown. Because the mAb 7C2 recognizes both phosphorylated and unphosphorylated CTD of RNA polymerase II, it cannot be excluded that part of the RNA polymerase II molecules that are present in the clusters of interchromatin granules in cells transiently expressing the Us11 gene correspond to the unphosphorylated IIa form, which under normal growth conditions is not associated with splicing components (7,28).
In conclusion, the present study demonstrates that the intermingled RNP fibrils are transcription factories for Us11 gene in transient expression, and brings another evidence for the involvement of clusters of interchromatin granules in some steps of the intranuclear transport of transcripts synthesized by RNA polymerase II. In addition, changes in the accessibility of the carboxy-terminal domain of the molecule under expression of Us11 gene after transfection are suggested by the binding of mAb 7C2 to the clusters of interchromatin granules in such transfected cells but not in control cells.
ACKNOWLEDGMENTS
The authors thank Mrs E. Pichard for her expert technical assistance. This work was supported by the Centre National de la Recherche Scientifique, and by special grants from the Association Nationale de Recherches sur le SIDA, the Association pour la Recherche sur le Cancer (Villejuif, France) and the Ligue Nationale contre le Cancer. S. Besse is a recipient of a fellowship from the Comite de l’Essonne de la Ligue Nationale contre le Cancer. F. Puvion-Dutilleul and J.-J. Diaz are members of the Institut National de la Santé et de la Recherche Médicale (INSERM).
REFERENCES
- 1. Bachellerie J. P.; Puvion E.; Zalta J. P. Ultra-structural organization and biochemical characterization of chromatin-RNA-protein complexes isolated from mammalian cell nuclei. Eur. J. Biochem. 58:327–337; 1975. [DOI] [PubMed] [Google Scholar]
- 2. Bernhard W. A new staining procedure for electron microscopical cytology. J. Ultrastruct. Res. 27:250–265; 1969. [DOI] [PubMed] [Google Scholar]
- 3. Besse S.; Diaz J. J.; Pichard E.; Kindbeiter K.; Madjar J. J.; Puvion-Dutilleul F. In situ hybridization and immuno-electron microscope analyses of herpes simplex virus type 1 US 11 gene during transient expression. Chromosoma 104:434–444; 1996. [DOI] [PubMed] [Google Scholar]
- 4. Besse S.; Vigneron M.; Pichard E.; Puvion-Dutilleul F. Synthesis and maturation of viral transcripts in herpes simplex virus type 1 infected HeLa cells: The role of interchromatin granules. Gene Expr. 4:143–161; 1995. [PMC free article] [PubMed] [Google Scholar]
- 5. Bouteille M. The “Ligop” method for routine ultrastructural autoradiography. A combination of a single-coating gold latensification, and phenidon development. J. Microsc. Biol. Cell 27:121–127; 1976. [Google Scholar]
- 6. Bregman D. B.; Du L.; Ribisi S.; Warren S. L. Cytostellin distributes to nuclear regions enriched with splicing factors. J. Cell Sci. 10:387–396; 1994. [DOI] [PubMed] [Google Scholar]
- 7. Bregman D. B.; Du L.; van der Zee S.; Warren S. L. Transcription-dependent redistribution of the large subunit of RNA polymerase II to discrete nuclear domains. J. Cell Biol. 129:287–298; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chatton B.; Bocco J. L.; Goetz J.; Gaire M.; Lutz Y.; Kedinger C. Jun and Fos heterodimerize with ATFa, a member of the ATF/CREB family and modulate its transcriptional activity. Oncogene 9:375–385; 1994. [PubMed] [Google Scholar]
- 9. Clark R. F.; Cho K. W. Y.; Weinmann R.; Hamkalo B. Preferential distribution of active RNA polymerase II molecules in the nuclear periphery. Gene Expr. 1:61–70; 1991. [PMC free article] [PubMed] [Google Scholar]
- 10. Cullen B. R. Trans-activation of human immunodeficiency virus occurs via a bimodal mechanism. Cell 46:973–982; 1986. [DOI] [PubMed] [Google Scholar]
- 11. Diaz J. J.; Duc Dodon M.; Schaerer-Uthurralt N.; Simonin D.; Kindbeiter K.; Gazzolo L.; Madjar J. J. Post-transcriptional transactivation of human retroviral envelope glycoprotein expression by herpes simplex virus US11 protein. Nature 379:273–277; 1996. [DOI] [PubMed] [Google Scholar]
- 12. Diaz J. J.; Simonin D.; Masse T.; Deviller P.; Kindbeiter K.; Denoroy L.; Madjar J. J. The herpes simplex type 1 US11 gene product is a phosphorylated protein found to be non-specifically with both ribosomal subunits. J. Gen. Virol. 74:397–406; 1993. [DOI] [PubMed] [Google Scholar]
- 13. Du L.; Warren S. L. A functional interaction between the carboxy-terminal domain of RNA polymerase II and pre-mRNA splicing. J. Cell Biol. 136:1–14; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dubois M. F.; Nguyen V. T.; Bellier S.; Bensaude O. Inhibitors of transcription such as 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole and isoquinoline sulfonamide derivatives (H-8 and H-7) promote dephosphorylation of the carboxyl-terminal domain of RNA polymerase II largest subunit. J. Biol. Chem. 269:13331–13339; 1994. [PubMed] [Google Scholar]
- 15. Fakan S. Perichromatin fibrils are in situ forms of nascent transcripts. Trends Cell Biol. 4:8–90; 1994. [DOI] [PubMed] [Google Scholar]
- 16. Fakan S.; Leser G.; Martin T. E. Ultrastructural distribution of nuclear ribonucleoprotein as visualized by immunocytochemistry on thin section. J. Cell Biol. 98:358–363; 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fakan S.; Puvion E. The ultrastructural visualization of nucleolar and extranucleolar RNA synthesis and distribution. Int. Rev. Cytol. 5:255–299; 1980. [DOI] [PubMed] [Google Scholar]
- 18. Fakan S.; Puvion E.; Spohr G. Localization and characterization of newly synthesized nuclear RNA in isolated rat hepatocytes. Exp. Cell Res. 99:155–164; 1976. [DOI] [PubMed] [Google Scholar]
- 19. Gallinaro H.; Puvion E.; Kister L.; Jacob M. Nuclear matrix and hnRNP share a common structural constituent associated with premessenger RNA. EMBO J. 2:953–960; 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greco A.; Simonin D.; Diaz J. J.; Barjhoux L.; Kindbeiter K.; Madjar J. J.; Masse T. The DNA sequence coding for the 5′ untranslated region of herpes simplex virus type 1ICP22 mRNA mediates a high level of gene expression. J. Gen. Virol. 75:1693–1702; 1994. [DOI] [PubMed] [Google Scholar]
- 21. Huang S.; Deerinck T. J.; Ellisman M. H.; Spector D. L. In vivo analysis of the stability and transport of nuclear poly(A)+ RNA. J. Cell Biol. 126:877–899; 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang S.; Spector D. L. Intron-dependent recruitment of pre-mRNA splicing factors to sites of transcription. J. Cell Biol. 133:719–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iborra F. J.; Pombo A.; McManus J.; Jackson D. A.; Cook P. R. The topology of transcription by immobilized polymerases. Exp. Cell Res. 229:167–173; 1996. [DOI] [PubMed] [Google Scholar]
- 24. Jackson D. A.; Hassan A. B.; Errington R. J.; Cook P. R. Visualization of focal sites of transcription within human nuclei. EMBO J. 12:1059–1065; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jalinot P.; Wintzerith M.; Gaire M.; Hauss C.; Egly J.-M.; Kedinger C. Purification and functional characterization of a cellular transcription factor that binds to an enhancer element within the E2a promoter. Proc. Natl. Acad. Sci. USA 85:2484–2488; 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jimenez-Garcia L. F.; Spector D. In vivo evidence that transcription and splicing are coordinated by a recruiting mechanism. Cell 73:47–60; 1993. [DOI] [PubMed] [Google Scholar]
- 27. Johnson P. A.; McLean C.; Marsden H. S.; Dalzeil R. G.; Everett R. D. The product of gene US 11 of herpes simplex virus type 1 is expressed as a true late gene. J. Gen. Virol. 67:871–883; 1986. [DOI] [PubMed] [Google Scholar]
- 28. Kim E.; Du L.; Bregman D. B.; Warren S. L. Splicing factors associate with hyperphosphorylated RNA polymerase II in the absence of pre-mRNA. J. Cell Biol. 136:1–10; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685; 1970. [DOI] [PubMed] [Google Scholar]
- 30. MacLean C.; Rixon F. J.; Marsden H. S. The products of gene US 11 of herpes simplex virus type 1 are DNA-binding and localize to the nucleoli of infected cells. J. Gen. Virol. 68:1921–1937; 1987. [DOI] [PubMed] [Google Scholar]
- 31. McCracken S.; Fong N.; Yankulov K.; Ballantyme S.; Pan G.; Greenblatt J.; Patterson S. D.; Wickens M.; Bentley D. L. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature 385:357–361; 1997. [DOI] [PubMed] [Google Scholar]
- 32. Mortillaro M. J.; Blencowe B. J.; Wei X.; Nakayasu H.; Du L.; Warren S. L.; Sharp P. A.; Berezney R. A hyperphosphorylated form of the large subunit of RNA polymerase II is associated with splicing complexes and the nuclear matrix. Proc. Natl. Acad. Sci. USA 93:8253–8257; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Payne J. M.; Laybourn P. J.; Dahmus M. E. The transition of RNA polymerase from initiation to elongation is associated with phosphorylation of the carboxy-terminal domain of subunit Ha. J. Biol. Chem. 264:19621–19629; 1989. [PubMed] [Google Scholar]
- 34. Puvion E.; Moyne G. Intranuclear migration of newly synthesized extranucleolar ribonucleoproteins. A high resolution quantitative autoradiographical and cytochemical study. Exp. Cell Res. 115:79–88; 1978. [DOI] [PubMed] [Google Scholar]
- 35. Puvion E.; Moyne G. In situ localization of RNA structures. In: Busch H., ed. The cell nucleus, vol. 8 New York: Academic Press; 1981:59–109. [Google Scholar]
- 36. Puvion E.; Puvion-Dutilleul F. Ultrastructure of the nucleus in relation to transcription and splicing: Roles of perichromatin fibrils and interchromatin granules. Exp. Cell Res. 229:217–225; 1996. [DOI] [PubMed] [Google Scholar]
- 37. Puvion E.; Viron A.; Assens C.; Leduc E. H.; Jeanteur Ph. Immunocytochemical identification of nuclear structures containing snRNPs in isolated rat liver cells. J. Ultrastruct. Res. 87:180–189; 1984. [DOI] [PubMed] [Google Scholar]
- 38. Puvion-Dutilleul F. Localization of viral-specific 21 KDa protein in nucleoli of herpes simplex infected cells. Eur. J. Cell Biol. 43:487–498; 1987. [PubMed] [Google Scholar]
- 39. Puvion-Dutilleul F.; Bachellerie J. P.; Visa N.; Puvion E. Rearrangement of intranuclear structures involved in RNA processing in response to adenovirus infection. J. Cell Sci. 13:1457–1468; 1994. [DOI] [PubMed] [Google Scholar]
- 40. Puvion-Dutilleul F.; Puvion E. Immunocytochemistry, autoradiography, in situ hybridization, selective stains: Complementary tools for ultra-structural study of structure-function relationships in the nucleus. Applications to adenovirus-infected cells. Microsc. Res. Tech. 31:22–43; 1995. [DOI] [PubMed] [Google Scholar]
- 41. Roller R. J.; Monk L. L.; Stuart D.; Roizman B. Structure and function in the herpes simplex virus 1 RNA-binding protein US11: Mapping of the domain required for ribosomal and nucleolar association and RNA binding in vitro. J. Virol. 70:2842–2851; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roller R. J.; Roizman B. The herpes simplex virus US 11 open reading frame encodes a sequence-specific RNA-binding protein. J. Virol. 64:3463–3470; 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roller R. J.; Roizman B. Herpes simplex virus 1 RNA-binding protein US 11 negatively regulates the accumulation of a truncated viral mRNA. J. Virol. 65:5873–5879; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roller R. J.; Roizman B. The herpes simplex virus type 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J. Virol. 66:3624–3632; 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roth J. Postembedding labeling on Lowicryl K4M tissue sections: Detection and modification of cellular components. In: Tartakoff A. M., ed. Methods in cell biology. New York: Academic Press; 1989:513–551. [DOI] [PubMed] [Google Scholar]
- 46. Sambrook J.; Fritsh E. E.; Maniatis T. Molecular cloning: A laboratory manual, 2nd ed Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 47. Spector D. L. Macromolecular domains within the cell nucleus. Annu. Rev. Cell Biol. 9:265–315; 1993. [DOI] [PubMed] [Google Scholar]
- 48. Spector D. L.; Fu X. D.; Maniatis T. Associations between distinct pre-mRNA splicing components and the cell nucleus. EMBO J. 10:3465–3481; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spector D. L.; O’Keepe R. T.; Jimenez-Garcia L. F. Dynamics of transcription and pre-mRNA splicing within the mammalian cell nucleus. In: Cold Spring Harbor symposia on quantitative biology, vol. 5 Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993:799–805. [DOI] [PubMed] [Google Scholar]
- 50. Steinmetz E. J. Pre-mRNA processing and the CTD of RNA polymerase II: The tail that wags the dog? Cell 89:491–494; 1997. [DOI] [PubMed] [Google Scholar]
- 51. Visa N.; Puvion-Dutilleul F.; Bachellerie J. P.; Puvion E. Intranuclear distribution of U1 and U2 snRNAs as visualized by high resolution in situ hybridization: Revelation of a novel compartment containing U1 but not U2 snRNA in HeLa cells. Eur. J. Cell Biol. 60:308–321; 1993. [PubMed] [Google Scholar]
- 52. Visa N.; Puvion-Dutilleul F.; Harper F.; Bachellerie J. P.; Puvion E. Intranuclear distribution of poly(A) RNA determined by electron microscope in situ hybridization. Exp. Cell Res. 208:19–34; 1993. [DOI] [PubMed] [Google Scholar]
- 53. Wansink D. G.; Schul W.; van der Kraan I.; van Steensel B.; van Driel R.; de Jong L. Fluorescent labeling of nascent RNA reveals transcription by RNA polymerase II in domains scattered throughout the nucleus. J. Cell Biol. 122:283–293; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Warren S. L.; Landolfi A. S.; Curtis C.; Morrow J. S. Cytostellin: A novel highly conserved protein that undergoes continuous redistribution during the cell cycle. J. Cell Sci. 103:181–188; 1992. [DOI] [PubMed] [Google Scholar]
- 55. Weeks J. R.; Hardin S. E.; Shen J.; Lee J. M.; Greenleaf A. L. Locus-specific variation in phosphorylation of RNA polymerase II in vivo: Correlations with gene activity and transcript processing. Genes Dev. 7:2329–2344; 1993. [DOI] [PubMed] [Google Scholar]
- 56. Zeng C.; Kim E.; Warren S. L.; Berget S. Dynamic relocalization of transcription and splicing factors dependent upon transcriptional activity. EMBO J. 16:1401–1412; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


