Abstract
Posterior urethral valves are the most common cause of partial bladder outlet obstruction (PBOO) in the pediatric population. Pathological changes in the bladder developed during PBOO are responsible for long-lasting voiding dysfunction in this population despite early surgical interventions. Increasing evidence showed PBOO induces an upregulation of hypoxia-inducible factors (HIFs) and their transcriptional target genes, and they play a role in pathophysiological changes in the obstructed bladders. We hypothesized that blocking HIF pathways can prevent PBOO-induced bladder dysfunction. PBOO was surgically created by ligation of the bladder neck in male C57BL/6J mice for 2 wk. PBOO mice received intraperitoneal injection of either saline or 17-DMAG (alvespimycin, 3 mg/kg) every 48 h starting from day 1 postsurgery. Sham-operated animals received injection of saline on the same schedule as PBOO mice and served as controls. The bladders were harvested after 2 wk, and basal activity and evoked contractility of the detrusor smooth muscle (DSM) were evaluated in vitro. Bladder function was assessed in vivo by void spot assay and cystometry in conscious, unrestrained mice. Results indicated the 17-DMAG treatment preserved DSM contractility and partially prevented the development of detrusor over activity in obstructed bladders. In addition, PBOO caused a significant increase in the frequency of micturition, which was significantly reduced by 17-DMAG treatment. The 17-DMAG treatment improved urodynamic parameters, including increases in the bladder pressure at micturition and nonvoid contractions observed in PBOO mice. These results demonstrate that treatment with 17-DMAG, a HIF inhibitor, significantly alleviated PBOO-induced bladder pathology in vivo.
Keywords: bladder outlet obstruction, hypoxia-inducible factor, HIF inhibitor, bladder dysfunction, 17-DMAG
posterior urethral valves (PUV) are the most common reason for partial bladder outlet obstruction (PBOO) in children with an estimated incidence of 1 in every 3,000–8,000 births (45). With the improvements in diagnosis and treatments, prognosis for PUV patients has improved. However, the long-term deteriorating effects of PUV on both bladder and renal functions have been challenging for pediatric urologists. The main characteristics of this bladder dysfunction include decreased compliance and detrusor overactivity that turn into myogenic failure after adolescence. Consequently, despite the apparent success of early valve ablation, almost two-thirds of PUV patients develop permanent bladder dysfunction, which can worsen the renal outcome, and more than one-third can face with end stage renal disease (45). Many PUV patients exhibit the anatomical disturbances of the lower urinary tract including enlarged posterior urethra, hypertrophic bladder neck, and thick-walled bladder with fibrosis and trabeculation (5, 45). In addition, physiological changes such as dysregulation of spontaneous rhythmic contractions produced by detrusor smooth muscle (DSM) under basal conditions (myogenic tone) have been linked to the bladder dysfunction in PUV and PBOO patients (37, 45). These anatomical and physiological changes developed in the bladder during the period of PBOO appear to contribute to long-term sequlae from PUV.
Currently, there are very limited treatment options for bladder dysfunction due to PBOO. With anticholinergics being the most commonly used, none of the current treatment options include a targeted therapy for histopathological changes (45). Proactive management in addition to valve ablation, which prevents the bladder pathology, including inflammation, decreases in detrusor contractility, fibrosis, and denervation, can support normal bladder growth and renal function in children with PUV and PBOO. Although not fully understood, there is growing evidence that bladder dysfunction due to PBOO is related to ischemic hypoxia and subsequent induction of hypoxia-inducible factors (HIFs). HIF is a transcription factor responsible for activation/transcription of many genes that are involved in a wide variety of physiological process including cellular metabolism, angiogenesis, extracellular matrix remodeling, and cell survival (40). HIF is a heterodimer that consists of hypoxia-regulated α- and oxygen-insensitive β-subunits. Currently, three isoforms of α-subunits, HIF-1α, HIF-2α, and HIF-3α, and one β-subunit, HIF-1β (also known as ARNT), are identified. HIF-1 and HIF-2 are closely related and regulate overlapping but distinct target genes. HIF-3 differs from the other two members in protein structure and has been considered as a negative mediator of HIF-regulated genes (40). HIF activity depends on the availability of α-subunit, which is continuously expressed at a low level in the cell, but it is rapidly degraded via ubiquitin-proteasomal pathway under normoxic conditions (40). Studies revealed an upregulation of HIF-1 and HIF-2 in obstructed bladders and suggested an involvement of HIF pathways in multiple PBOO-induced bladder histopathology including fibrosis, inflammation, and DSM hyperplasia/hypertrophy (12, 21, 22, 26). Accordingly, we hypothesized that HIF pathways could be a target for treating PBOO-related bladder pathophysiology.
Considering their central roles in cancer biology, different therapeutic approaches targeting HIF pathways have been developed: through antisense strategies, through inhibition of the ability of HIF-α to interact with proteins that modulate its activity, or through inhibition of signal transduction pathways. Among them, inhibitors of heat-shock protein 90 (HSP90) such as geldanamycin were shown to abolish HIF-α (40). HSP90 is a molecular chaperone that facilitates proper folding, stability, interactions, and intracellular trafficking of many proteins, including HIF-α subunits (4, 40). 17-DMAG is a less toxic water-soluble analogue of geldanamycin and has been revealed to inhibit HIF pathways by destabilizing HIF-α subunits through blocking its interaction with HSP90 in different cell types and tissues (4, 22, 27). In previous study, we demonstrated that administration of 17-DMAG suppressed HIF activation and decreased histopathological changes in the bladders in a murine model of PBOO in vitro (21, 22). In the current study, we sought to further our understanding of the mechanism underlying PBOO-induced bladder pathophysiology and to evaluate in vivo as well as in vitro effects of 17-DMAG as a potential treatment agent for bladder dysfunction after 2 wk of PBOO in the mouse model.
MATERIALS AND METHODS
Animals.
Male C57BL/6J mice (8 wk old, n = 80) were utilized in this study. Mice were excluded from the studies when adverse events occurred. These included ≥15% reduction in body weight, lethargy, pain, or distress not relieved by our Institutional Animal Care and Use Committee-approved regimen of analgesics after the surgery. All procedures using animals were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Colorado Anschutz Medical Campus.
Creation of PBOO and experimental groups.
Mice underwent surgical ligation of the proximal urethra to induce PBOO, as previously described (21). Briefly, the mice underwent a lower midline incision with exposure of the bladder neck and proximal urethra under isoflurane inhalational anesthesia. A 1-Fr silicon tubing was placed next to the urethra, a 5–0 silk suture was passed behind vesicourethral junction, and then urine was extruded from the bladder with a gentle pressure of the fingers. The suture was tied snugly around the tubing with the urethra, which was narrowed to 0.61 mm as the outside diameter of the tubing. The tubing was removed and the abdomen was closed. Sham-operated mice serving as controls were subjected to an identical surgical procedure as PBOO animals, except without creating suture ligature of the urethra. All animals received a single dose of carprofen (5 mg/kg) subcutaneously for analgesia daily 0–3 days postsurgery. The mice had ad libitum access to food and water postsurgery. PBOO mice were divided into two subgroups: PBOO + P (placebo) and PBOO + T (treatment), receiving 100 μl of either saline (vehicle) or 17-DMAG (3 mg/kg of body wt; LC Laboratories, Woburn, MA) by intraperitoneal injection every 48 h from 1 to 13 days postsurgery. Control mice received 100 μl of saline as same as PBOO + P group.
Histological Analyses.
Paraformaldehyde-fixed paraffin sections (5-µm thickness) of the bladders from each group were used. Collagen fiber was visualized and imaged with second harmonic generation microscopy (Carl Zeiss Microscopy, Thornwood, NY) (6). Sections from at least three animals in each group were analyzed for reproducibility. Areas of whole tissue, DSM, and collagen fibers (pseudo colored in red) in second harmonic generation images of bladder sections were measured using Adobe Photoshop (Adobe Systems, San Jose, CA). Collagen measurement was performed in the entire tissue section and in the DSM layer, separately, and expressed as a proportion of collagen level as collagen-to-total and collagen-to-DSM.
Gene Expression Analyses.
Total RNA was isolated from the bladders (n = 4–5 per group) using QIAzol lysis reagent (Qiagen, Germantown, MD) and transcribed into cDNA (Bio-Rad, Hercules, CA). Quantitative real-time PCR (qRT-PCR) was performed using a Light Cycler 480 (Roche Diagnostics, Indianapolis, IN). Expression levels of each gene were calculated as fold changes based on ΔΔCt values. Data were normalized to 18S rRNA.
In vitro detrusor contractility measurements.
Freshly isolated bladders from mice in each group (n = 10–12) were cut into two halves longitudinally. Each strip (approximately 3 × 6 mm, n = 17) was placed in organ baths (Radnoti, Monrovia, CA) filled with oxygenated Tyrode’s buffer (in mM; 125 NaCl, 2.5 KCl, 23.8 NaHCO3, 0.5 MgCl2, 0.4 NaH2PO4, 1.8 CaCl2, and 5.5 glucose) at 37°C. One end of the strip was secured to a glass rod at the bottom of the organ chamber (Radnoti), and the other end was attached to a force displacement transducer. Tissues were equilibrated for 45 min and then stretched to their optimum length for muscle contraction (Lo) in which the maximum force for muscle contraction produced by electrical field stimulation (EFS; 70 V, 1 ms, and 32 Hz). Once Lo was determined, each muscle strip was allowed to equilibrate a further 30 min in fresh Tyrode’s buffer.
Detrusor muscle contractility was examined as the responses to a series of EFS (70 V, 0.5–32 Hz), the acetylcholine receptor agonist carbachol (CCh; 0.1–100 µM), and high KCl solution (125 mM, substituted for NaCl in Tyrode’s buffer). To confirm 17-DMAG itself does not affect detrusor contractility, 17-DMAG prepared in saline (3 mg/ml) was added to the organ bath at 1,000 dilution (final concentration of 3 mg/l) in Tyrode’s solution to match the amount administered to animals (3 mg/kg of body wt). Bladder strips from sham and PBOO + P (N = 3, n = 3 per group) were tested the responses to EFS, CCh, and KCl after a 30-min incubation in Tyrode’s buffer containing 17-DMAG. Stimulus-response curves were calculated in grams of tension per weight of individual muscle strip.
To assess the effect of PBOO and 17-DMAG treatment on basal bladder activity, each strip was thoroughly washed after all contractile recordings to EFS, CCh, and KCl and equilibrated for 45 min in fresh Tyrode’s solution. Then, 15 min of spontaneous contractions under steady state were sampled. Three parameters of spontaneous contractions were analyzed; average amplitude (g, grams of tension), frequency (Hz), and tone (g). Tone was defined as the mean spontaneous rhythmic contractile force produced by the bladder strips (37). As protein kinase C (PKC) and Rho-associated kinase (ROCK) signaling pathways have been shown to play a role in the regulation of myogenic bladder tone and bladder compliance (20); next, the spontaneous contractions of the strips was assessed with the presence of one of the following three drugs: cumulatively increasing concentrations of a PKC activator, phorbol 12,13-dibutyrate (PDBu; Tocris Bioscience, Avonmouth, Bristol, UK) at 1–300 nM; a PKC inhibitor, bisindolylmaleimide-I (BIM-1; Cayman Chemical, Ann Arbor, MI) at 16 nM; or a selective inhibitor of ROCK, Y-27632 (Tocris Bioscience) at 30 nM (20). The bladder strips were treated with each concentration of PDBu for 15 min or either BIM-1 or Y-27632 for 30 min. The last 15-min interval within each observation period was used to analyze spontaneous contractions. All data analyses were performed using PowerLab Lab-Chart version 7.3.7 (AD Instruments, Colorado Springs, CO). Changes in amplitude and frequency of spontaneous contractions were expressed as percent changes before and after the administration of test compounds. Changes in tone were shown as the difference between the average force generated by the bladder strips with drugs compared with that before adding testing drugs (taken as 0 g).
Spontaneous void spot assay.
To evaluate voiding habits, void spot assays were conducted at 3, 7, and 14 days after the surgery as previously described (n = 12 per group) (25, 47). Briefly, mice were individually placed onto a steel wire mesh (0.64-cm2 opening) floor elevated from the cage bottom by 1 cm in a clean cage. A precut Whatman grade filter paper (Fisher Scientific, Hampton, NH) was placed to cover the entire surface of the bottom of the cage. Each mouse had free access to water but no food during 3-h test period. All void spot assay (VSA) experiments were started from between 9 and 10 AM. The urine spots on the filter paper were imaged using ultraviolet light on a transilluminator, and the number of all urine spots on each filter was counted. Areas of urine spots were converted to volumes by constructing a standard curve that relates known volumes of mouse urine spotted on the filter paper using Adobe Photoshop, and urine spots of <0.1 cm2 (corresponding to 3 μl of urine) were excluded from analysis as previously described (47). The number of large (≥50 μl) and small (<50 μl) micturition spots on the filter paper was counted, and the volume of each spot was summed to determine total volume of void. Mean volume of large voids was determined by averaging the volume of large (≥50 μl) voids on each filter paper.
Surgical procedure to catheterize the urinary bladder.
A subset of mice from each group underwent surgical catheter implantation in the bladder for cystometry studies at the time of PBOO or sham surgery. Briefly, immediately after the surgery creating PBOO or sham operation, a flared-end polyethylene catheter (PE-10) was inserted through a puncture on the bladder dome and secured with a 7–0 Prolene suture (Ethicon, Somerville, NJ). The catheter was tunneled subcutaneously and exteriorized at the scapula region, where it was sutured to the skin. The catheter was filled with sterile saline after confirming no leakage at the bladder and then plugged to prevent leakage (25). The abdominal incision was closed, and animals were transferred to individual cages after the recovery from anesthesia. A single dose of carprofen (5 mg/kg) and penicillin (40,000 IU/kg) was given subcutaneously daily 0–3 days postsurgery.
Urodynamic evaluation of bladder function.
Urodynamic evaluation was performed at 2 wk after the surgery (n = 6 per group) (33). Conscious mice were placed in cystometry cages (16-cm width, 12-cm height, and 16-cm length) without any restraint and allowed to acclimate for 30 min. The tip of the exteriorized bladder catheter located at the base of the mouse neck was connected to a pressure transducer and an infusion pump of the cystometry station (Small Animal Laboratory Cystometry Lab Station, Catamount Research and Development, St. Albans, VT). Room temperature saline solution (0.9% NaCl) was infused into the bladder at a rate of 10 μl/min. Voided urine was monitored with the analytical balance connected to a force displacement transducer integrated into the data acquisition system. Each animal was observed for up to six to eight voiding cycles. Urodynamic values recorded continuously during testing, and the following urodynamic parameters were analyzed using Cystometry Analysis Software (SOF-552, Catamount Research and Development): maximum intravesical pressure at micturition, functional bladder capacity, voided volume, number of nonvoid contractions per voiding cycle, and intermicturition interval. The nonvoid contractions were defined as intravesical pressure rises greater than 5 mmHg from baseline pressure, which was ~40% of the maximal voiding pressure in control group (0.5- to 10-s total duration) without triggering micturition (39).
Statistical Analysis.
All data were analyzed using one-way repeated-measures ANOVA among three groups using GraphPad InStat (GraphPad Software, La Jolla, CA). Differences those showed significance by ANOVA tests were analyzed by the Tukey-Kramer multiple comparisons test. The GraphPad outlier calculator (GraphPad Software) was used to detect outliers, which were excluded from the analysis in VSA. P < 0.05 was regarded as significant.
RESULTS
17-DMAG treatment alleviated an increase in bladder weight and fibrosis induced by PBOO.
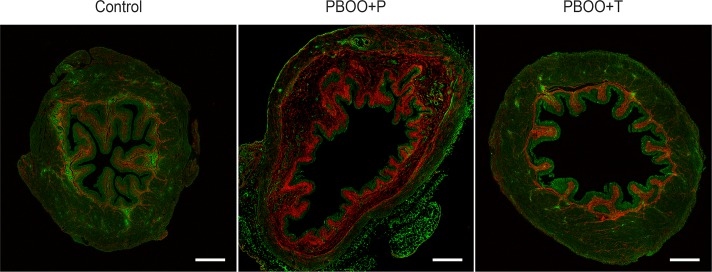
PBOO caused a significant increase in bladder weight in comparison with control group, while 17-DMAG treatment alleviated the bladder enlargement by 34% (169 ± 8% in PBOO + P vs. 135 ± 11% in PBOO + T, control group taken as 100%) (Table 1). No difference in body weight was detected among the groups (n = 20 per group). Enhanced collagen deposition was observed in mucosal and detrusor muscle layers in the bladders from PBOO + P mice; the distribution and proportional amount of collagen fibers were similar in the control and PBOO + T groups (Fig. 1). Total collagen in the entire tissue as well as the collagen-to-DSM ratio was significantly increased in PBOO + P group compared with control mice (P = 0.013 and P = 0.047), while the change in PBOO + T mice was smaller and insignificant to control mice (P = 0.431 and 0.166) (Table 2). These results indicate that the 17-DMAG regimen utilized in this study alleviated the fibrosis in the obstructed bladders.
Table 1.
Comparison of body and bladder weight
| Group | Control | PBOO + P | PBOO + T |
|---|---|---|---|
| Body, g | 23.7 ± 0.4 | 22.8 ± 0.4 | 23.6 ± 0.4 |
| Bladder, mg | 21.2 ± 0.8 | 34.5 ± 1.8* | 28.2 ± 2.2*† |
| Relative bladder weight, %control | 100 ± 3 | 169 ± 8* | 135 ± 11*† |
Values represent means ± SE. Partial bladder outlet obstruction (PBOO) caused a significant increase in bladder weight, while 17-DMAG treatment ameliorated the bladder enlargement. No change was observed in the body weight among groups. P, placebo; T, treatment.
P < 0.05, vs. control group;
P < 0.05, PBOO + P vs. PBOO + T groups.
Fig. 1.
Histological analysis of collagen deposition in the bladders. Representative second harmonic generation image of the bladders from sham control (left), partial bladder outlet obstruction (PBOO) + placebo (P; middle), or PBOO + treatment (T; right) groups at 2 wk postsurgery. Enhanced collagen deposition (red) was observed in mucosa, and both between and within detrusor muscles in the bladders from the PBOO + P mice, while the bladders from the PBOO + T mice appeared comparable with those from controls. Scale bars = 500 μm.
Table 2.
Comparison of proportional collagen in the bladders from 3 groups
| Collagen | Control | PBOO + P | PBOO + T |
|---|---|---|---|
| To DSM | 15.1 ± 3.0 | 28.4 ± 3.6* | 21.9 ± 4.4 |
| To total | 25.3 ± 2.4 | 41.5 ± 5.5* | 27.8 ± 1.8† |
Values represent means ± SE (%). Areas of detrusor smooth muscle (DSM) layer or a whole section (total area) were taken as 100%. PBOO caused a significant increase in collagen level, while 17-DMAG treatment suppressed the collagen deposition in the obstructed bladders.
P < 0.05, vs. control group;
P < 0.05, PBOO + P vs. PBOO + T groups.
17-DMAG treatment suppressed PBOO-induced upregulation of HIF target genes.
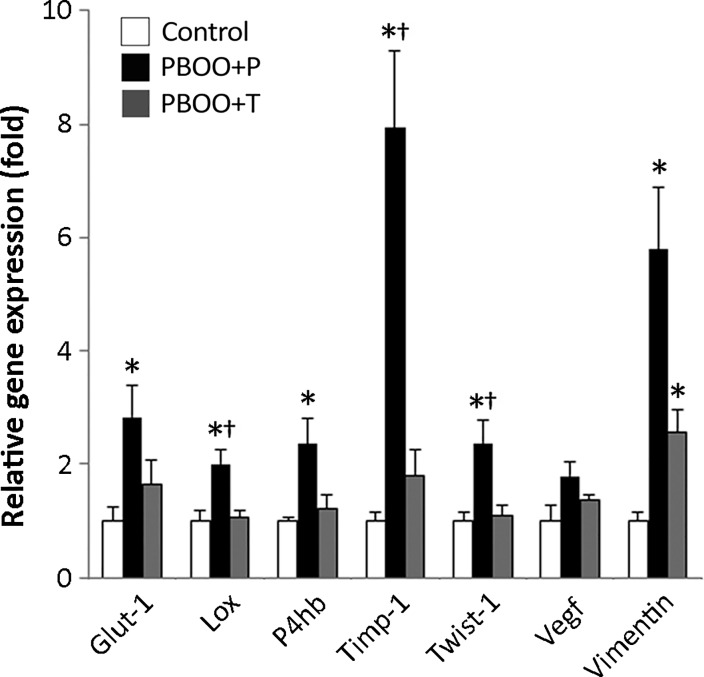
The multiple HIF transcription target genes glucose transporter 1 (Glut-1/Slc2a1), lysyl oxidase (Lox), prolyl 4-hydroxylase β (P4hb), tissue inhibitor of metalloproteinase-1 (TIMP-1), Twist-1, and vimentin were significantly upregulated in PBOO + P mice compared with those in control group (2.0- to 7.9-fold, P < 0.05) (Fig. 2). An increase in VEGF expression was also observed in PBOO + P mice, while the change was statistically insignificant (1.8-fold, P = 0.08). All of these genes have been reported to play a role in bladder pathogenesis following PBOO (12, 21, 22). Treatment with 17-DMAG decreased the degree of PBOO-induced upregulation of HIF target genes by 25 to 77%, supporting that an inhibitory effect of 17-DMAG on HIF transcriptional activity in the bladders following PBOO.
Fig. 2.
Expression analyses of hypoxia-inducible factor (HIF) target genes in bladders. Significant increases in mRNA expression levels of HIF target genes were detected in bladders from PBOO + P mice compared with control mice. Values represent means ± SE fold difference (control group taken as 1) in expression level of each gene in bladders. *P < 0.05 vs. control group; †P < 0.05, between PBOO + P and PBOO + T groups.
17-DMAG treatment preserved detrusor contractility in the obstructed bladders.
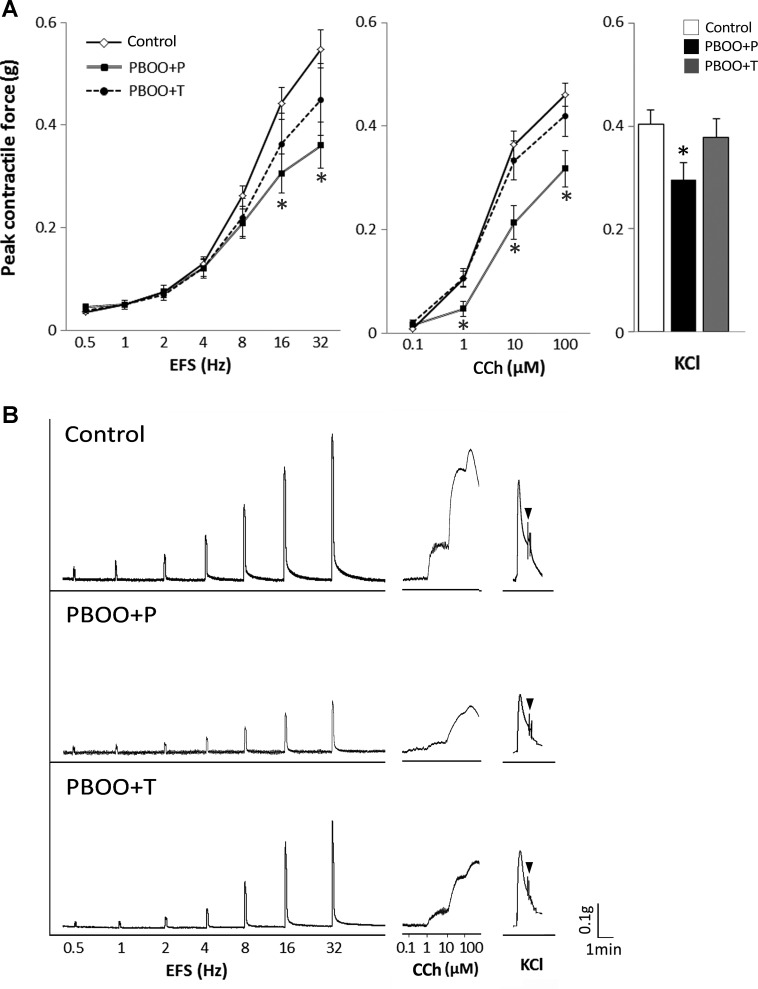
The bladder strips from PBOO + P mice demonstrated a significant decrease in detrusor contractility in response to EFS (16 and 32 Hz), CCh (1–100 μM), and KCl compared with those from control mice upon normalization of the response to the strip weight (Fig. 3). 17-DMAG treatment partially preserved the muscle contractility in the obstructed bladders in response to all stimuli: EFS (82 ± 13 vs. 66 ± 8% in PBOO + P at 32 Hz), 100 μM CCh (91 ± 9 vs. 69 ± 8% in PBOO + P), and KCl (93 ± 9 vs. 73 ± 8% in PBOO + P) (control group taken as 100%). We confirmed that 17-DMAG itself did not affect the DSM contractility by testing the bladder strips from control and PBOO + P groups in Tyrode’s buffer containing 17-DMAG.
Fig. 3.
Peak contractile force of bladder strips. A: control, PBOO + P, and PBOO + T groups in response to: electric field stimulation (EFS; left), carbachol (CCh; middle), and high concentration of KCl (right). B: representative raw traces of contractions in response to EFS (left), CCh (middle), and KCl (right) from each group. The force was normalized with tissue weight. Arrowheads indicate the end point of KCl. Values are means ± SE; n = 14 in each group. *P < 0.05 vs. control group.
17-DMAG treatment improved basal detrusor overactivity in the obstructed bladders.
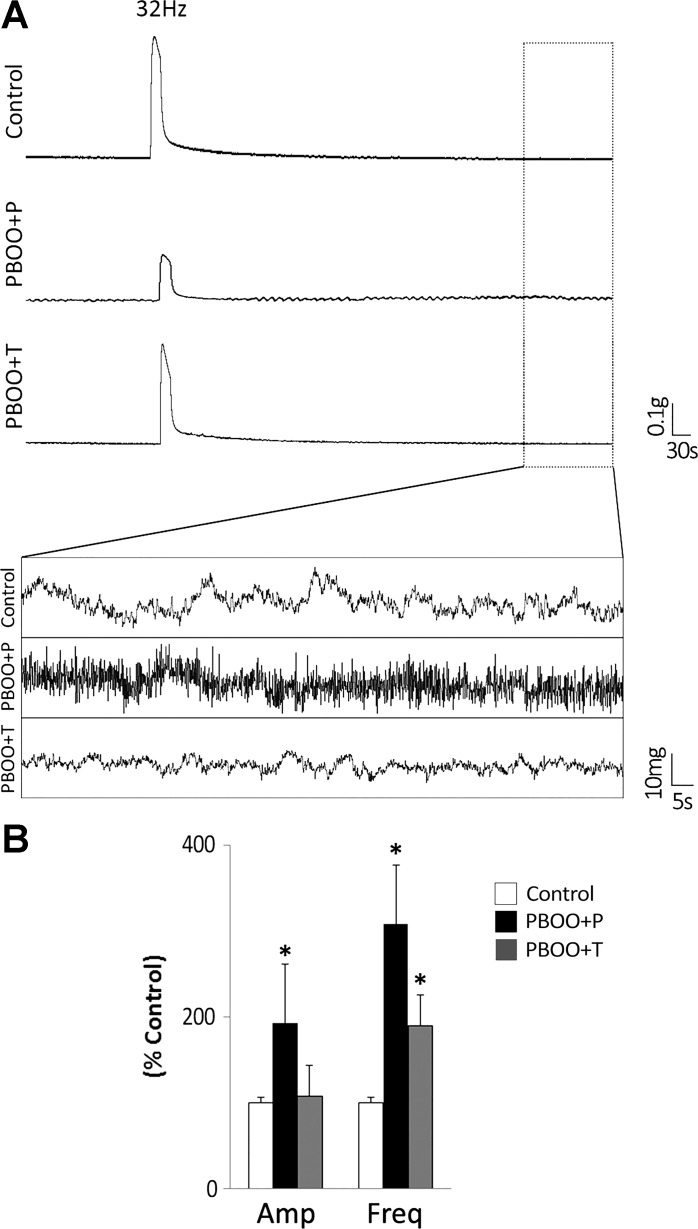
The amplitude of the spontaneous contractions was approximately twofold larger in PBOO + P mice (26 ± 3 mg, P = 0.001) compared with other groups (14 ± 1 in control, and 15 ± 1 in PBOO + T groups). The frequency of spontaneous contractions without any stimuli was 1.1 ± 0.1 contractions/min in control group. A significantly high frequency of spontaneous contraction was observed in both PBOO groups (3.3 ± 0.8 in PBOO + P and 2.0 ± 0.4 in PBOO + T, P < 0.005). Figure 4 shows the representative traces of the basal activity of bladder strips from each group and the summary of the amplitude and the frequency of spontaneous contraction relative to those in control group.
Fig. 4.
Comparison of basal activity of bladder strips from each group. A: the representative trace of spontaneous contractile activity of detrusor smooth muscle (DSM) strips from control, PBOO + P, and PBOO + T groups without any stimuli. B: the amplitude and the frequency of spontaneous contractions of the bladder strip in PBOO + P and PBOO + T groups relative to those in control group (taken as 100%). Both PBOO groups showed a significant increase in the frequency compared with control mice. In addition, the amplitude was significantly larger in PBOO + P group compared with that in control. Values are means ± SE. *P < 0.05 vs. control group.
PBOO altered the mechanisms of maintenance of detrusor tone.
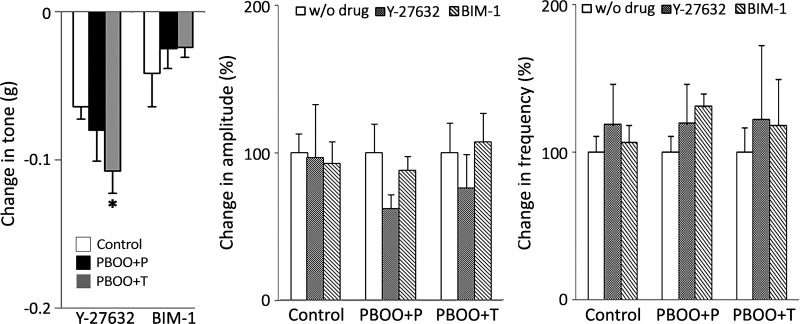
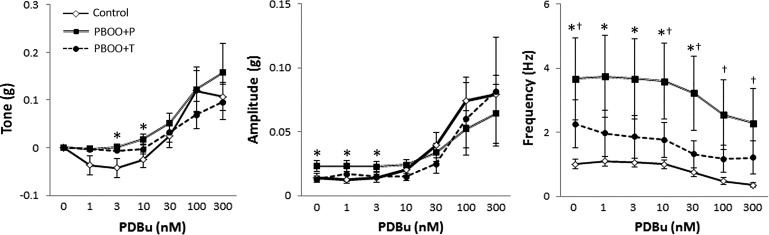
PKC and ROCK have been suggested to have a role in the regulation of myogenic tone and spontaneous contractions (7, 20, 31, 36). We examined the effects of the PKC and ROCK inhibitors BIM-1 and Y-27632, respectively, as well as the PKC agonist PDBu on the tone and spontaneous contractions using bladder strips. BIM-1 lowered the detrusor tone in all groups, while the effect tended to be smaller in PBOO groups compared with control mice. Y-26732 caused a larger decrease (1.5 to 4.5-fold) in tone than that by BIM-1 in all groups. The relaxation effect of tone by blocking ROCK was more apparent in PBOO groups (Fig. 5). This observation is consistent with the recent report that PBOO enhanced ROCK pathway and disturbed the PKC pathway (41). PDBu induced a biphasic effect on tone in control group and lowered the tone at low concentrations (1–10 nM), whereas it increased the tone at higher concentrations (30–300 nM) as previously reported (Fig. 6) (20). This reciprocal effect of PKC activation might be attributed to the dual function of PKC of regulating both relaxation and contraction of DSM (2, 20). PDBu did not induce any relaxation on the bladder strips from the PBOO + P mice; instead, the tone showed a steady increase from 10 nM (Fig. 6). PBOO + T showed a minimal decrease in the tone with PDBu at 1–10 nM. An insignificant trend of the decrease in the amplitude of spontaneous contractions was observed with Y-27632, specifically in PBOO groups, whereas BIM-1 did not affect the amplitude in any groups (Fig. 5). PDBu induced a steady increase in the amplitude of spontaneous contractions, starting from 10 and 30 nM in control and both PBOO groups, respectively (Fig. 6). There was no significant difference among the groups. There was no change was observed in the frequency of spontaneous contractions by Y-27632 and BIM-1 within and among three groups compared with that without these drugs (Fig. 5). The frequency was continuously decreased in PBOO + T mice with PDBu at all tested concentrations (Fig. 6). In control and PBOO + P mice, there was no change in the frequency of spontaneous contractions up to 10 nM of PDBu, and the frequency started declining with higher doses (30–300 nM) of PDBu. The frequency was lowered notably in PBOO groups with PDBu compared with that in control, yet the frequency of the obstructed bladders stayed higher than that in control mice (approximately 3.0- and 1.7-fold in PBOO + P and PBOO + T groups, respectively).
Fig. 5.
Effects on the basal activity in the bladder strips with the inhibitors of Rho-associated kinase (ROCK) and PKC. Left: changes in tone. PBOO + T mice showed a significant decrease in tone with Y-27632 compared with that in control group. The change in PBOO + P mice was similar to PBOO + T mice but statistically insignificant. Neither amplitude nor frequency of spontaneous contractions showed a significant difference among groups and between without drug and Y-27632 or bisindolylmaleimide-I (BIM-1) within each group. Changes in amplitude (middle) and frequency (right) are depicted as the percentage to the value without drug (taken as 100%). Values are means ± SE. *P < 0.05 vs. control group.
Fig. 6.
Effects on the basal activity in the bladder strips with the PKC activator phorbol 12,13-dibutyrate (PDBu). Tone (left), and amplitude (middle), and frequency (right) of spontaneous contractions. PDBu showed a biphasic effect on the tone in control group, which was not observed in PBOO + P mice. A notable decrease in the frequency was observed in PBOO groups compared with those in control mice. Values are means ± SE. *P < 0.05, between control and PBOO + P groups; †P < 0.05, between control and PBOO + T groups.
17-DMAG treatment improved the bladder dysfunction following PBOO.
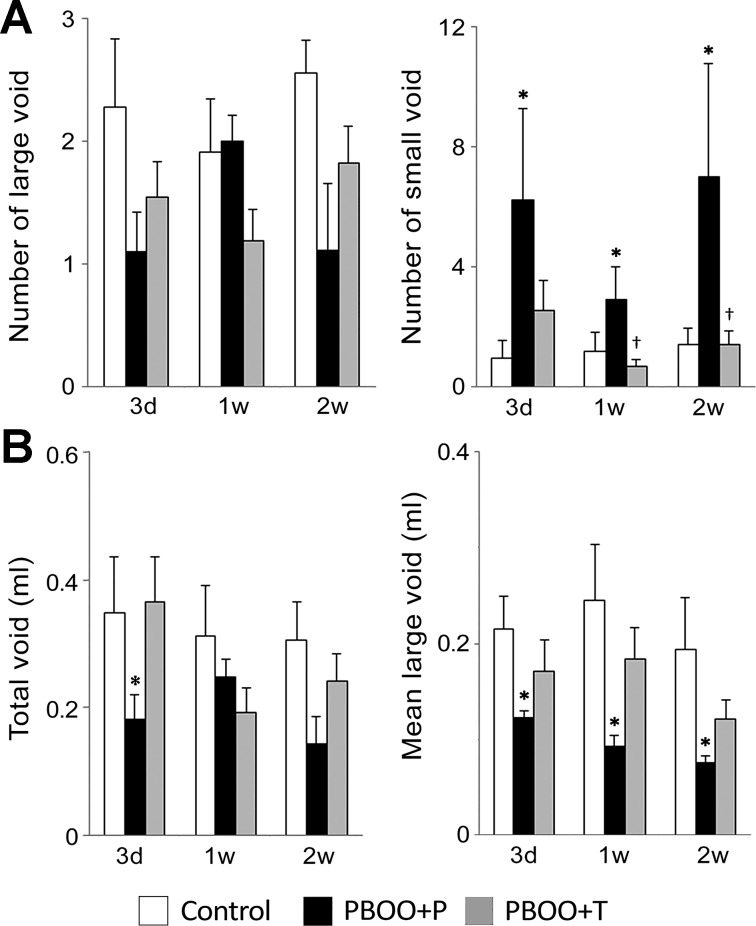
To assess bladder function, we first used the VSA to examine spontaneous micturition pattern of unrestrained animals (47). The number of large voids (≥50 µl per void) was similar among all groups. The number of small voids (<50 µl) was significantly increased in PBOO + P group compared with other groups (Fig. 7A). A significant decrease in the volume of total void was observed in PBOO + P group compared with that in control group at 3 days after the surgery (43% of the volume in control mice, P = 0.037). The mean volume of large voids was significantly decreased in PBOO + P mice compared with that in control mice (Fig. 7B). Even though a similar trend was observed in PBOO + T group, the change was smaller compared with that in PBOO + P group, suggesting 17-DMAG treatment improved the voiding pattern in PBOO animals.
Fig. 7.
Micturition pattern analyses in spontaneous void spot assays. Micturition patterns were assessed on 3 days and 1 and 2 wk after the surgery. A: number of the urine spots of large void (≥50 μl, left) and small void (<50 μl, right). B: volume of total void (left) and mean volume of the large void spots (right). Values are means ± SE; n = 10–12 per group. *P < 0.05, vs. control group; †P < 0.05, between PBOO + P and PBOO + T groups.
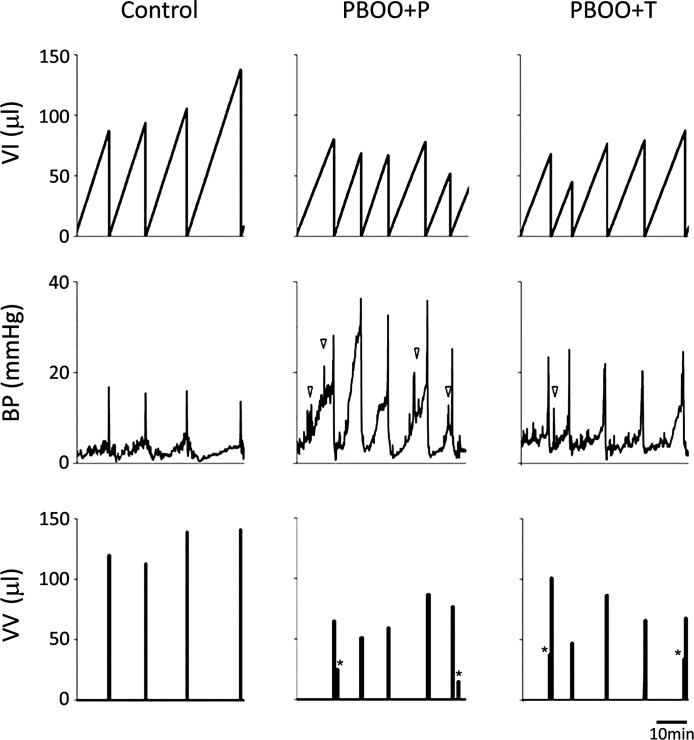
To further evaluate the changes in micturition patterns using an independent assay, we performed cystometry in nonanesthetized mice at 2 wk after PBOO or sham surgery. During the period of cystometry, reproducible micturition patterns were obtained. Urodynamic parameters in PBOO mice were apparently distinguished from those in control group (Table 3 and Fig. 8). Control mice showed the maximum intravesical pressure at voiding of 12.8 ± 0.5 mmHg with the micturition interval of 10.3 ± 1.1 min and the bladder capacity of 112 ± 11 μl. PBOO + P mice, however, had 2.3-fold higher maximum intravesical pressure (30.1 ± 2.5 mmHg, P < 0.001) than in control group and occasionally displayed a concurrent steady increase in intravesical pressure during filling phase, a sign of decreased compliance. In PBOO + T mice, the increase in maximum intravesical pressure was smaller (1.3-fold) compared with PBOO + P group (16.6 ± 0.8 mmHg, P > 0.05 vs. control mice, and P < 0.001 vs. PBOO + P mice). The micturition interval, voided volume, and the functional bladder capacity regarded as the infused volume at each cycle were decreased by approximately 20 and 10% in PBOO + P and PBOO + T groups, respectively, compared with control group although statistically insignificant. In PBOO mice, approximately 33 and 15% of a micturition cycle were separated in two parts of void in placebo and 17-DMAG treatment groups, respectively, as depicted in Fig. 5 (asterisks in the bottom panels; VV); the first and the last voids in both groups. Nonvoiding bladder contractions were consistently recorded in PBOO + P mice (4.3 ± 1.0 per cycle), while they were was absent or minimal in control and PBOO + T mice (0.2 ± 0.1 and 0.4 ± 0.2 per cycle, respectively, P < 0.001 vs. PBOO + P group).
Table 3.
Comparison of urodynamic parameters in each group
| Interval, s | Infused, μl | Void, μl | Void/Infused, % | Voiding Pressure, mmHg | Nonvoid Contractions | |
|---|---|---|---|---|---|---|
| Control | 617 ± 66 | 112 ± 11 | 107 ± 8 | 99 ± 7 | 12.8 ± 0.5 | 0.2 ± 0.1 |
| PBOO + P | 460 ± 52 | 94 ± 11 | 84 ± 11 | 85 ± 6 | 30.1 ± 2.5* | 4.3 ± 1.0* |
| PBOO + T | 503 ± 38 | 103 ± 4 | 94 ± 4 | 99 ± 5 | 16.6 ± 0.8† | 0.4 ± 0.2† |
Values represent means ± SE. A significant increase in the bladder pressure at micturition and nonvoid contractions in PBOO + P mice compared with those in control and PBOO + T groups was observed; n = 6.
P < 0.05, vs. control group;
P < 0.05, PBOO + P vs. PBOO + T groups.
Fig. 8.
Functional bladder analyses in cystometry. Representative cystometrogram trace from unanesthetized, unrestrained control (left), PBOO + P (middle), and PBOO + T (right) mice during a continuous intravesical infusion (10 μl/min) of room temperature saline. Volume infused (VI; top), bladder pressure (BP; middle), and voided volume (VV; bottom) are shown. Arrowheads in the bladder pressure traces (middle) indicate examples of nonvoiding bladder contractions. Asterisks in the volume of micturition traces (bottom) indicate the micturition composed of in 2 parts of void.
DISCUSSION
Despite the fact that valve ablation surgery can considerably improve voiding dysfunction in most PUV patients, the symptoms including enuresis, urinary frequency, and poor bladder emptying persisted in some patients and required further and often long-term treatment (19, 43). Currently, anticholinergics, clean intermittent catheterization, intradetrusor Botulinum toxin injections, overnight drainage, α-adrenergic blockers, and, finally, bladder augmentation are the treatment modalities used to manage bladder dysfunction in PUV patients. It has been suggested that irreversible damage occurred in the urinary system during the period of obstruction is a perpetrator of the continued bladder dysfunction, and proactive management would be desired (14, 16). Previous studies have shown DSM hypertrophy, denervation, remodeling of extracellular matrix components, and alterations to urothelium are the histological changes responsible for voiding dysfunction (1, 23, 26). A line of studies revealed that HIF pathways are, in part, responsible for those histopathological changes (12, 21, 26). Our previous study revealed that 17-DMAG reduced the expression of HIFs and their target in the obstructed bladders, and improved PBOO-induced bladder pathology in terms of histophysiology in vitro without any apparent adverse effects at the daily dose of 3 mg/kg (22). It has been reported that 17-DMAG inhibits the HIF activity by blocking the interaction between HSP90 and HIF-α subunits, which is essential for HIF activation (4, 28). Consistent with previous reports, we observed a decrease in PBOO-induced upregulation of multiple HIF target genes including Glut-1, Lox, TIMP-1, and VEGF in the bladders by 17-DMAG treatment, suggesting that 17-DMAG blocked HIF transcriptional activity in the bladders following PBOO (22, 27). As HSP90 mediates the folding and activation of diverse client proteins including HIFs, we examined the expression level of nuclear factor-κB (NF-κB), which was shown to be a target of 17-DMAG (18). There was no statistically significant change in NF-κB expression in the bladders from the PBOO + P (1.7-fold, P = 0.14) and PBOO + T groups (0.7-fold, P = 0.41) compared with that in control group (taken as 1.0). However, the difference between the PBOO + P and PBOO + T groups was statistically significant (P < 0.01), indicating that 17-DMAG suppressed NF-κB expression in the obstructed bladders. NF-κB has been identified as a downstream molecule of HIFs as well as I-κ-B kinase (IKK), which is also a HSP90 client protein (18), suggesting that 17-DMAG affects NF-κB expression through both HIF-dependent and -independent pathways in the obstructed bladders. Altogether, we consider that HIF pathways are the major target of 17-DMAG in the bladder following PBOO as seen in the other systems (3, 4, 22, 27, 34, 48). Our current study provides an important step to underline the feasibility of HIF inhibitors to be used for treating PBOO-associated bladder dysfunction in vivo. PBOO caused an enlargement of the bladder with enhanced collagen deposition, which was partially relieved by 17-DMAG treatment. We reason that 17-DMAG alleviated histopathological changes in the obstructed bladders through reducing the level of HIF target genes, which play key role for fibrosis and hyperplasia/hypertrophy (22, 29, 46). Yet, a smaller but significant increase in the bladder weight in PBOO + T mice could have resulted from HIF target genes transactivated by other transcription factors as well as mediated by other signaling pathways such as transforming growth factor-β, PDGF, and connective tissue growth factor, which are known to contribute PBOO-induced bladder pathology (22, 26).
The PBOO + P group showed decreased contractility in response to EFS, CCh, and KCl stimulations when compared with the control group, suggesting that PBOO induced alterations in the contractility of DSM itself as well as of nerve-dependent responses. We speculate that PBOO-induced fibrosis is at least partly responsible for these problems by inducing cellular and myofibrillar disorganization, reducing the compliance of the bladder wall, and hindering the efficient communication between the neurons and DSM as well as among muscle cells. This notion is supported by the observation of improved DSM contractile function in the bladders from the 17-DMAG treatment group, which showed no apparent fibrosis following the same period of PBOO. DSM contractility was well preserved in PBOO + T mice, especially in response to CCh and KCl (91–93% of that in control mice), while the contractile response to EFS was less maintained (83% of that in control) by 17-DMAG treatment. We consider that HIF inhibition by 17-DMAG preserved overall bladder architecture and muscle cell physiological property including activation of acetylcholine receptors and the contractile machinery; however, minor nerve damage and/or disturbance of neuromuscular communication may have developed in the obstructed bladders even with 17-DMAG treatment. Further study is required to address the detailed mechanism of protective effects of a HIF inhibitor on PBOO-induced pathophysiology.
One of the main aspects of bladder dysfunction due to PBOO is detrusor overactivity leading to urinary incontinence and/or upper urinary tract deterioration. Studies revealed that spontaneous contractions are elevated in detrusor strips from patients with overactive bladder and highly active DSM tone in low compliant bladders (8, 11). It has been shown that PBOO causes bladder stiffness, at least partly caused by an impairment of relaxation property in the bladder (12). Our results demonstrated that both amplitude and frequency of spontaneous contractions were significantly increased in PBOO + P mice compared with other groups, indicating that PBOO caused detrusor overactivity. The PBOO + T group also caused a significant increase in the frequency of spontaneous contractions compared with control mice, while the change was smaller than that in PBOO + P mice. These results indicated that 17-DMAG treatment partially prevented the development of detrusor overactivity in the obstructed bladders.
PKC and ROCK are the most well described molecules linked to the bladder tone during filling phase (20). We utilized the inhibitors and the activator for PKC and ROCK to assess the effect of 17-DMAG on basal activity in obstructed bladders, specifically, spontaneous contractions and relaxation property, which are important for compliance. It has been shown an upregulation of ROCK and enhanced ROCK pathway in obstructed bladders (44), which is consistent with our observation of the increased relaxation of bladder strips by the ROCK inhibitor Y-27632 in PBOO mice compared with the control group. Previous studies have shown HIFs (HIF-1 and HIF-2) mediate the formation of stress fibers and myosin light chain phosphorylation through the ROCK pathway and also extracellular matrix (ECM) contraction (15). In obstructed bladders, HIFs promote ROCK, which leads to 1) DSM contraction through the inhibition of myosin light chain phosphatase (MLCP), and 2) bladder stiffness through stress fiber-derived ECM contraction. Therefore, inhibition of ROCK by Y-27632 could relieve the inhibition of MLCP and resulted in a larger relaxation of bladder strips in PBOO groups than control mice. However, Y-27632 was not effective for relieving bladder stiffness, which was caused by contraction of increased ECM, and resulted in a smaller relaxation of bladder strips in PBOO + P mice compared with PBOO + T group, which did not develop fibrosis.
The trend of the increased effect of the ROCK inhibitor and the decreased effect of the PKC inhibitor on relaxing DSM in PBOO mice suggests the proportional change of the contribution from each pathway to regulate tone in the obstructed bladders. The observation of the loss of the relaxation of DSM tone by low concentrations (1–10 nM) of PDBu in PBOO + P mice without changing the effect of high concentrations (≥30 nM) of PDBu suggests that the PKC pathway was active in the obstructed bladders. These results suggest that 1) the PKC pathway involved in relaxation of DSM was disturbed in the obstructed bladders, and 2) 17-DMAG treatment partially preserved the PKC pathway; however, the enhanced force maintenance activity of ROCK pathway overrode the DSM-relaxing effect by PKC pathway. The differences among PKC isozymes have been well documented, including the distinct PDBu binding affinity, contributing to different aspects of muscle contractility (contraction, sustaining contraction, or relaxation), involving multiple cellular functions, and coupling with a variety of cofactors (9, 20, 30, 42). In addition, a sequential activation of individual PKC isozymes during muscle cell spreading has been reported (10). DSM hyperplasia/proliferation, which occurs during the initial compensatory stage of PBOO, leads to spreading of newly generated DSM cells in obstructed bladders (21, 23, 26). In light of these facts, we speculate that the PKC pathways involved in DSM activity were altered following PBOO, such as downregulation of the PKC isozymes responsible for DSM relaxation and/or upregulation of PKC pathways that induce muscle contraction with high dose of PDBu. Currently, very little is known about the relation between PKC and HIFs. It has been reported that PKC induces HIF-1α through phosphatidylinositol 3-kinase pathway (32), but there is no evidence of HIF-mediated PKC activation to the best of our knowledge. Since the PKC pathway is active in normal bladders regulating both contractility and relaxation of DSM, it is unlikely that PKC mediated HIF activation in bladders in general. This notion is supported by the previous studies showing no detectable HIF-1α or hypoxia in normal bladders (12, 21, 22, 26). Therefore, we consider that PBOO induced alteration of the PKC pathways likely in a HIF-independent manner.
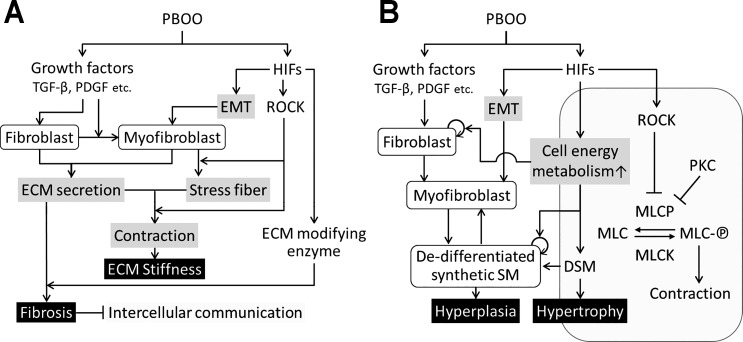
Based on our current and previous findings, we propose a model of signaling pathways that involve in pathogenesis in bladders secondary to PBOO (Fig. 9). PBOO activates two major signaling pathways and growth factors including transforming growth factor-β, PDGF, and HIFs (12, 21). Driven primarily by growth factors, PBOO results in activation of fibroblast leading to differentiation of myofibroblasts. The HIF-induced epithelial mesenchymal transition also contributes to myofibroblast formation (21). Fibroblasts and myofibroblasts secrete extracellular matrix (ECM) including collagen and fibronectin. HIFs upregulate ECM-modifying enzymes such P4hb and Lox, which promote ECM remodeling and ultimately lead to fibrosis (21, 22). Intercellular communication would be interfered with by remodeled ECM, which contributes to symptoms such as bladder overactivity in obstructed bladders. In myofibroblasts, ROCK induced by HIFs promotes stress fiber production and contraction of stress fibers, which connect to the ECM resulting in bladder stiffness (15, 44). HIFs induce genes, increasing cell energy metabolism (12, 40), which promotes proliferation of fibroblast and dedifferentiated synthetic smooth muscle cells derived from myofibroblasts and DSM, resulting in hyperplasia, while DSM undergoes hypertrophy. ROCK and PKC inhibit MLCP, which prevents dephosphorylation of MLC and leads DSM contraction (7, 20). In obstructed bladders, ROCK induced by HIFs can cause sustained phosphorylation of MLC, which contributes to lower compliance together with ECM stiffness.
Fig. 9.
Schematic model of hypothetical mechanisms involve in PBOO-induced pathology in bladders. PBOO activates 2 major signaling pathways; growth factors including TGF-β, PDGF, and HIFs. Growth factors contribute to PBOO-induced pathogenesis through activation of fibroblast and myofibroblast, while HIFs activate genes in different types of cells, involving in multiple stage of bladder pathology secondary to PBOO. A: ECM remodeling through 2 signaling pathways leads to fibrosis and bladder stiffness. B: an enhancement of cell energy metabolism in the activated fibroblast, myofibroblast, synthetic smooth muscle, and DSM result in DSM hyperplasia/hypertrophy. Inhibition of MLCP by ROCK and PKC promotes sustained contraction of DSM. ECM, extracellular matrix; EMT, epithelial mesenchymal transition; SM, smooth muscle; DSM, detrusor smooth muscle; MLC, myosin light chain; MLC-℗, phosphorylated myosin light chain; MLCP, myosin light chain phosphatase; MLCK, myosin light chain kinase; TGF-β, transforming growth factor-β.
The DSM relaxation mechanism is essential to accommodate increasing volumes of urine during filling phase of the micturition cycle. Our data from in vitro physiological tests are in good agreement with the phenotype observed in the in vivo examination of bladder function by VSA and cystometry. The lack of DSM relaxation and the detrusor overactivity was manifested as smaller voided volume and frequent small voids in PBOO + P group, indicating that the bladder became hypocompliant with a reduced functional capacity. The improvement of these symptoms in PBOO + T mice could be explained by the relatively stable basal bladder activity and sustained responsivity to different stimuli tested in the DSM contractility study. The cystometric findings of PBOO + P group indicated 1) an increased intravesical pressure at micturition, 2) a decreased bladder capacity, 3) detrusor overactivity, and 4) a decreased compliance. These symptoms have been commonly observed in urodynamic tests in PUV patients, verifying that our PBOO models recapitulated the clinical phenotype (13, 17, 24, 35). The 17-DMAG treatment group showed an improvement especially in terms of maximal bladder pressure and nonvoid contractions. As maximal bladder pressure during filling is a determiner of upper urinary tract deterioration, 17-DMAG may have a potential protective effect for the upper urinary tract as well.
In this study, we demonstrated that the same total dose of 17-DMAG (21 mg/kg) for 2 wk (twice longer period of PBOO from our previous study) could preserve bladder function and prevent pathophysiological alterations in the obstructed bladders. Even though the 17-DMAG regimen employed in our current and previous studies did not cause any noticeable undesired phenotype in major organs, and overall health and behavior in animals, one of the concerns that may rise is the practicality of 17-DMAG, especially for treatment in the pediatric population, and for longer durations, as it is a cytotoxic agent originally developed to treat cancer (38). Future studies involving less toxic pharmaceuticals that inhibit HIF pathways will lead to the development of better pharmacological treatment options.
The limitations for this study are the lack of a PBOO model mimicking PUV where the obstruction begins in utero with technical difficulties and the lack of longer follow-up. Future studies focusing on the long-term effects of HIF inhibitors regarding outcomes after elimination of obstruction will help to clarify the implementation of those molecules into clinical practice.
In conclusion, this study provided direct evidence that a HIF inhibitor, 17-DMAG, significantly prevented the development of PBOO-induced bladder pathology and preserved bladder function in vivo. The effect of 17-DMAG to block HIF pathways can be a potential target for novel pharmacological therapies to treat PBOO-associated pathology.
GRANTS
This study was supported by University of Colorado, School of Medicine Academic Enrichment Seed Funds (to D. T. Wilcox) and Ponzio Family Endowment Fund (to D. T. Wilcox). Histological study was supported by University of Colorado Denver Research Histology Shared Resource funded by Cancer Center Support Grant P30-CA-046934.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.I. and D.T.W. conceived and designed research; N.I. and A.C. performed experiments; N.I. analyzed data; N.I., M.İ.D., A.C., and D.T.W. interpreted results of experiments; N.I. prepared figures; N.I. and M.İ.D. drafted manuscript; N.I., M.İ.D., A.P.M., A.C., and D.T.W. edited and revised manuscript; D.T.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Kentaro Takezawa from Osaka University, Osaka, Japan, for technical support for the urodynamic study.
REFERENCES
- 1.Aitken KJ, Bägli DJ. The bladder extracellular matrix. Part I: architecture, development and disease. Nat Rev Urol : 596–611, 2009. doi: 10.1038/nrurol.2009.201. [DOI] [PubMed] [Google Scholar]
- 2.Andrea JE, Walsh MP. Protein kinase C of smooth muscle. Hypertension : 585–595, 1992. doi: 10.1161/01.HYP.20.5.585. [DOI] [PubMed] [Google Scholar]
- 3.Berger EA, McClellan SA, Vistisen KS, Hazlett LD. HIF-1α is essential for effective PMN bacterial killing, antimicrobial peptide production and apoptosis in Pseudomonas aeruginosa keratitis. PLoS Pathog : e1003457, 2013. doi: 10.1371/journal.ppat.1003457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burroughs SK, Kaluz S, Wang D, Wang K, Van Meir EG, Wang B. Hypoxia inducible factor pathway inhibitors as anticancer therapeutics. Future Med Chem : 553–572, 2013. doi: 10.4155/fmc.13.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caione P, Nappo SG. Posterior urethral valves: long-term outcome. Pediatr Surg Int : 1027–1035, 2011. doi: 10.1007/s00383-011-2946-9. [DOI] [PubMed] [Google Scholar]
- 6.Cox G, Kable E, Jones A, Fraser I, Manconi F, Gorrell MD. 3-dimensional imaging of collagen using second harmonic generation. J Struct Biol : 53–62, 2003. doi: 10.1016/S1047-8477(02)00576-2. [DOI] [PubMed] [Google Scholar]
- 7.de Godoy MA, Rattan S. Role of rho kinase in the functional and dysfunctional tonic smooth muscles. Trends Pharmacol Sci : 384–393, 2011. doi: 10.1016/j.tips.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean GE, Cargill RS 3rd, Macarak E, Snyder HM, Duckett JW, Levin R. Active and passive compliance of the fetal bovine bladder. J Urol : 1094–1099, 1997. doi: 10.1016/S0022-5347(01)64396-9. [DOI] [PubMed] [Google Scholar]
- 9.Dimopoulos GJ, Semba S, Kitazawa K, Eto M, Kitazawa T. Ca2+-dependent rapid Ca2+ sensitization of contraction in arterial smooth muscle. Circ Res : 121–129, 2007. doi: 10.1161/01.RES.0000253902.90489.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Disatnik MH, Boutet SC, Lee CH, Mochly-Rosen D, Rando TA. Sequential activation of individual PKC isozymes in integrin-mediated muscle cell spreading: a role for MARCKS in an integrin signaling pathway. J Cell Sci : 2151–2163, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Drake MJ, Harvey IJ, Gillespie JI, Van Duyl WA. Localized contractions in the normal human bladder and in urinary urgency. BJU Int : 1002–1005, 2005. doi: 10.1111/j.1464-410X.2005.05455.x. [DOI] [PubMed] [Google Scholar]
- 12.Ekman M, Uvelius B, Albinsson S, Swärd K. HIF-mediated metabolic switching in bladder outlet obstruction mitigates the relaxing effect of mitochondrial inhibition. Lab Invest : 557–568, 2014. doi: 10.1038/labinvest.2014.48. [DOI] [PubMed] [Google Scholar]
- 13.Emir H, Eroğlu E, Tekant G, Büyükünal C, Danişmend N, Söylet Y. Urodynamic findings of posterior urethral valve patients. Eur J Pediatr Surg : 38–41, 2002. doi: 10.1055/s-2002-25093. [DOI] [PubMed] [Google Scholar]
- 14.Farrugia MK. Fetal bladder outlet obstruction: embryopathology, in utero intervention and outcome. J Pediatr Urol : 296–303, 2016. doi: 10.1016/j.jpurol.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 15.Gilkes DM, Xiang L, Lee SJ, Chaturvedi P, Hubbi ME, Wirtz D, Semenza GL. Hypoxia-inducible factors mediate coordinated RhoA-ROCK1 expression and signaling in breast cancer cells. Proc Natl Acad Sci USA : E384–E393, 2014. doi: 10.1073/pnas.1321510111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Glassberg KI. The valve bladder syndrome: 20 years later. J Urol : 1406–1414, 2001. doi: 10.1016/S0022-5347(05)65796-5. [DOI] [PubMed] [Google Scholar]
- 17.Guerra L, Leonard M, Castagnetti M. Best practice in the assessment of bladder function in infants. Ther Adv Urol : 148–164, 2014. doi: 10.1177/1756287214528745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hertlein E, Wagner AJ, Jones J, Lin TS, Maddocks KJ, Towns WH III, Goettl VM, Zhang X, Jarjoura D, Raymond CA, West DA, Croce CM, Byrd JC, Johnson AJ. 17-DMAG targets the nuclear factor-kappaB family of proteins to induce apoptosis in chronic lymphocytic leukemia: clinical implications of HSP90 inhibition. Blood : 45–53, 2010. doi: 10.1182/blood-2010-01-263756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes N, Harrison MR, Baskin LS. Fetal surgery for posterior urethral valves: long-term postnatal outcomes. Pediatrics : E7, 2001. doi: 10.1542/peds.108.1.e7. [DOI] [PubMed] [Google Scholar]
- 20.Hypolite JA, Chang S, Wein AJ, Chacko S, Malykhina AP. Protein kinase C modulates frequency of micturition and non-voiding contractions in the urinary bladder via neuronal and myogenic mechanisms. BMC Urol : 34, 2015. doi: 10.1186/s12894-015-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iguchi N, Hou A, Koul HK, Wilcox DT. Partial bladder outlet obstruction in mice may cause E-cadherin repression through hypoxia induced pathway. J Urol : 964–972, 2014. doi: 10.1016/j.juro.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Iguchi N, Malykhina AP, Wilcox DT. Inhibition of HIF reduces bladder hypertrophy and improves bladder function in murine model of partial bladder outlet obstruction. J Urol : 1250–1256, 2016. doi: 10.1016/j.juro.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juan YS, Chuang SM, Jang MY, Huang CH, Chou YH, Wu WJ, Long CY. Basic research in bladder outlet obstruction. Incont Pelvic Floor Dysfunct : 1–6, 2011. [Google Scholar]
- 24.Kim YH, Horowitz M, Combs AJ, Nitti VW, Borer J, Glassberg KI. Management of posterior urethral valves on the basis of urodynamic findings. J Urol : 1011–1016, 1997. doi: 10.1016/S0022-5347(01)64377-5. [DOI] [PubMed] [Google Scholar]
- 25.McMillan MT, Pan XQ, Smith AL, Newman DK, Weiss SR, Ruggieri MR Sr, Malykhina AP. Coronavirus-induced demyelination of neural pathways triggers neurogenic bladder overactivity in a mouse model of multiple sclerosis. Am J Physiol Renal Physiol : F612–F622, 2014. doi: 10.1152/ajprenal.00151.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metcalfe PD, Wang J, Jiao H, Huang Y, Hori K, Moore RB, Tredget EE. Bladder outlet obstruction: progression from inflammation to fibrosis. BJU Int : 1686–1694, 2010. doi: 10.1111/j.1464-410X.2010.09445.x. [DOI] [PubMed] [Google Scholar]
- 27.Milkiewicz M, Doyle JL, Fudalewski T, Ispanovic E, Aghasi M, Haas TL. HIF-1alpha and HIF-2alpha play a central role in stretch-induced but not shear-stress-induced angiogenesis in rat skeletal muscle. J Physiol : 753–766, 2007. doi: 10.1113/jphysiol.2007.136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minet E, Mottet D, Michel G, Roland I, Raes M, Remacle J, Michiels C. Hypoxia-induced activation of HIF-1: role of HIF-1alpha-Hsp90 interaction. FEBS Lett : 251–256, 1999. doi: 10.1016/S0014-5793(99)01359-9. [DOI] [PubMed] [Google Scholar]
- 29.Misra S, Fu AA, Misra KD, Shergill UM, Leof EB, Mukhopadhyay D. Hypoxia-induced phenotypic switch of fibroblasts to myofibroblasts through a matrix metalloproteinase 2/tissue inhibitor of metalloproteinase-mediated pathway: implications for venous neointimal hyperplasia in hemodialysis access. J Vasc Interv Radiol : 896–902, 2010. doi: 10.1016/j.jvir.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murthy KS, Grider JR, Kuemmerle JF, Makhlouf GM. Sustained muscle contraction induced by agonists, growth factors, and Ca2+ mediated by distinct PKC isozymes. Am J Physiol Gastrointest Liver Physiol : G201–G210, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Ozaki H, Yasuda K, Kim YS, Egawa M, Kanzaki H, Nakazawa H, Hori M, Seto M, Karaki H. Possible role of the protein kinase C/CPI-17 pathway in the augmented contraction of human myometrium after gestation. Br J Pharmacol : 1303–1312, 2003. doi: 10.1038/sj.bjp.0705552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagé EL, Robitaille GA, Pouysségur J, Richard DE. Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J Biol Chem : 48403–48409, 2002. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- 33.Pandita RK, Fujiwara M, Alm P, Andersson KE. Cystometric evaluation of bladder function in non-anesthetized mice with and without bladder outlet obstruction. J Urol : 1385–1389, 2000. doi: 10.1016/S0022-5347(05)67204-7. [DOI] [PubMed] [Google Scholar]
- 34.Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J : 439–453, 2008. doi: 10.1042/BJ20071640. [DOI] [PubMed] [Google Scholar]
- 35.Peters CA, Bolkier M, Bauer SB, Hendren WH, Colodny AH, Mandell J, Retik AB. The urodynamic consequences of posterior urethral valves. J Urol : 122–126, 1990. doi: 10.1016/S0022-5347(17)39388-6. [DOI] [PubMed] [Google Scholar]
- 36.Rajasekaran M, Wilkes N, Kuntz S, E Albo M. Rho-kinase inhibition suppresses bladder hyperactivity in spontaneously hypertensive rats. Neurourol Urodyn : 295–300, 2005. doi: 10.1002/nau.20122. [DOI] [PubMed] [Google Scholar]
- 37.Ratz PH, Miner AS. Length-dependent regulation of basal myosin phosphorylation and force in detrusor smooth muscle. Am J Physiol Regul Integr Comp Physiol : R1063–R1070, 2003. doi: 10.1152/ajpregu.00596.2002. [DOI] [PubMed] [Google Scholar]
- 38.Sausville EA, Tomaszewski JE, Ivy P. Clinical development of 17-allylamino, 17-demethoxygeldanamycin. Curr Cancer Drug Targets : 377–383, 2003. doi: 10.2174/1568009033481831. [DOI] [PubMed] [Google Scholar]
- 39.Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol : R534–R547, 2010. doi: 10.1152/ajpregu.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer : 721–732, 2003. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 41.Shahab N, Kajioka S, Takahashi-Yanaga F, Onimaru M, Matsuda M, Seki N, Naito S. Obstruction enhances rho-kinase pathway and diminishes protein kinase C pathway in carbachol-induced calcium sensitization in contraction of α-toxin permeabilized guinea pig detrusor smooth muscle. Neurourol Urodyn : 593–599, 2012. doi: 10.1002/nau.21193. [DOI] [PubMed] [Google Scholar]
- 42.Shindo M, Irie K, Nakahara A, Ohigashi H, Konishi H, Kikkawa U, Fukuda H, Wender PA. Toward the identification of selective modulators of protein kinase C (PKC) isozymes: establishment of a binding assay for PKC isozymes using synthetic C1 peptide receptors and identification of the critical residues involved in the phorbol ester binding. Bioorg Med Chem : 2073–2081, 2001. doi: 10.1016/S0968-0896(01)00100-6. [DOI] [PubMed] [Google Scholar]
- 43.Smith-Harrison LI, Hougen HY, Timberlake MD, Corbett ST. Current applications of in utero intervention for lower urinary tract obstruction. J Pediatr Urol : 341–347, 2015. doi: 10.1016/j.jpurol.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi N, Shiomi H, Kushida N, Liu F, Ishibashi K, Yanagida T, Shishido K, Aikawa K, Yamaguchi O. Obstruction alters muscarinic receptor-coupled RhoA/Rho-kinase pathway in the urinary bladder of the rat. Neurourol Urodyn : 257–262, 2009. doi: 10.1002/nau.20625. [DOI] [PubMed] [Google Scholar]
- 45.Taskinen S, Heikkilä J, Rintala R. Effects of posterior urethral valves on long-term bladder and sexual function. Nat Rev Urol : 699–706, 2012. doi: 10.1038/nrurol.2012.196. [DOI] [PubMed] [Google Scholar]
- 46.Vesely ED, Heilig CW, Brosius FC III. GLUT1-induced cFLIP expression promotes proliferation and prevents apoptosis in vascular smooth muscle cells. Am J Physiol Cell Physiol : C759–C765, 2009. doi: 10.1152/ajpcell.00213.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu W, Ackert-Bicknell C, Larigakis JD, MacIver B, Steers WD, Churchill GA, Hill WG, Zeidel ML. Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. Am J Physiol Renal Physiol : F1296–F1307, 2014. doi: 10.1152/ajprenal.00074.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuehlke A, Johnson JL. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers : 211–217, 2010. doi: 10.1002/bip.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]