Abstract
The objective of this study was to evaluate oxidation products of arachidonic acid and linoleic acid in lipoproteins and synovial fluid (SF) from patients with active rheumatoid arthritis (RA) compared to non-RA controls. High-density lipoproteins (HDL) and low-density lipoproteins (LDL) were isolated from plasma using fast protein liquid chromatography and HDL was isolated from SF using dextran sulfate precipitation. 5-Hydroxyeicosatetraenoic acid (HETE), 12-HETE, 15-HETE, 9 hydroxyoctadecadienoic (HODE), and 13-HODE levels were measured in HDL, LDL, and SF by liquid chromatography–tandem mass spectrometry. HDL’s anti-inflammatory function, cholesterol levels, myeloperoxidase (MPO) and paraoxonase 1 (PON1) activities were determined as previously. 5-HETE, 15-HETE, 9-HODE, and 13-HODE levels were significantly increased in HDL and LDL from patients with active RA (n = 10) compared to healthy controls (n = 8) and correlated significantly with measures of systemic inflammation, particularly in HDL (r = 0.65–0.80, p values < 0.004). Higher HETES and HODES in HDL were also significantly correlated with impaired HDL function as measured by the HDL inflammatory index (HII) (r = 0.54–0.58; p values < 0.03). 15-HETE levels and MPO activity were higher in RA SF (n = 10) compared to osteoarthritis (OA) SF(n = 11), and HDL from RA SF had worse function compared to OA SF HDL (HII = 2.1 ± 1.9 and 0.5 ± 0.1), respectively (p < 0.05). Oxidation products of arachidonic acid and linoleic acid are increased in HDL and LDL from patients with active RA compared to healthy controls, and are associated with worse anti- oxidant function of HDL. These results suggest a potential mechanism by which oxidative stress from active RA increases oxidized fatty acids in HDL, promoting HDL dysfunction, and thereby increasing atherosclerotic risk.
Keywords: Fatty acids, High density lipoprotein, Low density lipoprotein, Rheumatoid arthritis
Patients with rheumatoid arthritis (RA) suffer significantly increased cardiovascular (CV) morbidity and mortality [1–3], which has been closely linked to high levels of systemic inflammation and RA disease activity [4, 5]. Better understanding of the interaction between systemic inflammation and vascular pathophysiology is needed for adequate CV risk assessment and development of targeted CV therapeutics in these high risk patients.
Oxidative stress is increased in RA patients and has been directly implicated in the pathogenesis of RA. Excess reactive oxygen species (ROS) produced by activated phagocytic cells during oxidative bursts directly damage tissues as well as amplify the inflammatory response [6]. Increased lipid oxidation products have been reported in RA synovial fluid (SF) and cartilage [6, 7].
Oxidative stress has also been directly implicated in the pathogenesis of atherosclerosis. Oxidation products of arachidonic acid and linoleic acid including hydroxyeicosatetraenoic acids (HETES) and hydroxyoctadecadienoic acids (HODES) contribute to the oxidation of LDL, and their accumulation in high-density lipoprotein (HDL) has been proposed to inhibit HDL function, increasing atherosclerotic risk [8–10]. Previous work has shown increased HETES and HODES in HDL of patients with diabetes and atherosclerotic vascular disease [11] as well as in plasma of humans and rodents with pulmonary hypertension [12, 13].
The HDL particle normally functions in an anti-atherogenic, anti-inflammatory capacity by preventing oxidation of LDL and promoting cholesterol efflux from the arterial wall [14–16]. High levels of RA disease activity have been associated with impairment in both of these protective functions [17, 18]. In the current work, we evaluated whether oxidation products of arachidonic acid and linoleic acid are increased in HDL and LDL from patients with active RA compared to healthy controls. We further investigated the levels of HETES and HODES in SF from patients with active RA compared to OA controls. We hypothesized that HETES and HODES are increased in lipoproteins from active RA patients and are associated with abnormal HDL anti-oxidant function.
Methods
Study design
RA patients and healthy controls were recruited from the rheumatology offices at the University of California, Los Angeles (UCLA). All RA patients met the American College of Rheumatology criteria for RA, which was verified by chart review. All subjects gave written informed consent for the study under a protocol approved by the Human Research Subject Protection Committee at UCLA.
Patients provided a blood sample and completed questionnaires as described below. Assessment of inflammatory markers including high-sensitivity C-reactive protein (HSCRP) and Westergren erythrocyte sedimentation rate (ESR), fasting lipid profiles, and synovial fluid cell counts were measured in the UCLA clinical laboratory using standard methods. SF cholesterol levels were measured by standard as-says (Thermo DMA Co., San Jose, CA). Additional blood was collected in heparinized tubes (Becton Dickinson) and stored at −80 °C for HETES and HODES and HDL function assays. Synovial fluid was collected in heparinized tubes from patients undergoing arthrocentesis of the knee as part of routine clinical care and stored at −80 °C prior to testing; 5/11 patients with OA had primary OA and 6/11 patients had secondary OA with medial meniscal tear as the most common etiology in 5/6 patients. Imaging of the knees (either x-ray, MRI, or both) had been done to document disease. Cardiovascular risk and health information was obtained by questionnaire and chart review.
Evaluation of HDL’s anti-oxidant function
The cell free assay (CFA) was a modification of a previously published method [19] using LDL as the fluorescence-inducing agent [19]. HDL was isolated from plasma using FPLC as above and from SF by dextran bead precipitation. To determine the anti-inflammatory properties of HDL, the change in fluorescence intensity as a result of the oxidation of dihydrodichlorofluorescein (DCFH) in incubations with a standard LDL in the absence or presence of the test HDL was assessed and the HDL inflammatory index (HII) calculated. In brief, 25 μl of LDL-cholesterol (100 μg/ml) was mixed with 50 μl of test HDL (100 μg HDL-cholesterol/ml) in black, flat bottom polystyrene microtiter plates and incubated at 37 °C with rotation for 30 min. Twenty-five microliters of DCFH solution (0.2 mg/ml) was added to each well, mixed, and incubated at 37 °C for 1 h with rotation. Fluorescence was determined with a plate reader (Spectra Max, Gemini XS; Molecular Devices) at an excitation wavelength of 485 nm, emission wavelength of 530 nm, and cutoff of 515 nm with photomultiplier sensitivity set at medium. Readings with DCFH and LDL-C were normalized to 1.0. Readings equal or greater than 1.0 after the addition of test HDL-C indicated pro-inflammatory HDL and values less than 1.0 indicated anti-inflammatory HDL. Values for intra- and interassay variability were 0.5 ± 0.37 and 3.0 ± 1.7%, respectively [20].
Determination of PON1 activity
PON1 activity was quantified as previously [21] using paraoxon as the substrate and measuring the increase in the absorbance at 405 nm due to the formation of 4-nitrophenol over a period of 12 min (at 20-s intervals). Paraoxon was purchased from Sigma (St. Louis, MO) and further purified using chloroform extraction. A unit of PON1 activity was defined as the formation of 1 nmol of 4-ntirophenol per minute per milliliter of sample used. Arylesterase activity was quantified using phenylacetate as the substrate and measuring the increase in the absorbance at 270 nm spectrophotometry over a 2 min period at 15 s intervals as described previously [22].
Determination of HETES and HODES in HDL and LDL
LDL and HDL were fractionated from plasma by fast-performance liquid chromatography (FPLC). Liquid chromatography–electrospray ionization, tandem mass spectroscopy (LCESI-MS/MS) was performed with a mass spectrometer (4000 QTRAP; Applied Biosystems, Foster City, CA) equipped with an electrospray ionization source as previously described [8].
Determination of HETES and HODES in synovial fluid
Synovial fluid was thawed and centrifuged at 10 k for 15 min at 4°; 150 μl of synovial fluid was combined with 850 acidified water and 20 μl of 5× IS, and a final concentration of 20 μM BHT. The solution was vortexed and kept on ice for lipid extraction followed by mass spectrometry, which was performed as above.
MPO activity
The activity of MPO was measured in synovial fluid using the InnoZyme MPO activity assay kit (EMD Chemicals, Darmstadt, Germany). In brief, patient synovial fluid was added to a 96-well plate with an immobilized polyclonal antibody specific for human MPO. Activity of captured MPO was measured using a detection reagent that includes tetramethyl benzi-dine (TMB) and hydrogen peroxide. Following color development, the reaction was stopped with sulfuric acid and the absorbance of the oxidized TMB detected at 450 nm.
Statistical analysis
Data were analyzed using JMP IN 10.0 (SAS Institute Inc., Cary, NC, USA). Patient groups were compared using Student’s t test for continuous variables and the chi-square test of association for categorical variables, along with Fisher’s exact test for small sample sizes. When needed, nonparametric Wilcoxon rank-sum tests were used to analyze continuous variables. Correlations between variables were evaluated using the Pearson’s correlation coefficient for normally distributed data and Spearman’s correlation coefficient for nonparametric data. The significance level was pre-specified at p < 0.05.
Results
Demographic, laboratory, and clinical characteristics
RA patients and healthy controls were not significantly different in age, sex, or ethnicity. No differences in traditional CV risk factors including diabetes, hypertension, smoking, and family history of cardiovascular disease were noted between the groups. Standard cholesterol profiles were similar including serum levels of TC, LDL-C, HDL-C, and triglycerides. One patient in each group was taking a statin (Table 1).
Table 1.
Clinical and laboratory characteristics of RA patients and healthy controls
| Rheumatoid arthritis (n = 10) | Healthy control (n = 8) | P value | |
|---|---|---|---|
| Age (years) | 49.6 ± 11.8 | 48.4 ± 15.9 | 0.86 |
| Sex (% female) | 80 | 75 | 1.0 |
| Ethnicity (% Caucasian) | 80 | 63 | 0.61 |
| Hypertension (%) | 30 | 0 | 0.22 |
| Diabetes (%) | 10 | 0 | 1.0 |
| Current smoking (%) | 10 | 0 | 1.0 |
| FH of CVD (%) | 37.5 | 12.5 | 0.57 |
| Statin use (%) | 10 | 12.5 | 1.0 |
| Total cholesterol (mg/dL) | 178 ± 52 | 165 ± 22 | 0.52 |
| LDL cholesterol (mg/dL) | 104 ± 35 | 89 ± 22 | 0.31 |
| HDL cholesterol (mg/dL) | 52 ± 15 | 56 ± 14 | 0.58 |
| Triglycerides (mg/dL) | 116 ± 59 | 101 ± 84 | 0.21 |
| ESR (mm/h) | 71 ± 24 | 6 ± 5 | 0.0006 |
| HS-CRP (mg/L) | 42.1 ± 40.1 | 0.91 ± 1.5 | 0.0006 |
| HDL inflammatory index (HII) | 2.21 ± 0.52 | 0.56 ± 0.09 | 0.0092 |
HDL high density lipoprotein, LDL low density lipoprotein, ESR erythrocyte sedimentation rate, HS-CRP high-sensitivity C-reactive protein
Patients with active RA had higher levels of systemic inflammation as measured by the CRP and ESR compared to healthy controls. HDL’s anti-oxidant capacity in preventing oxidation of DCF in the setting of a standard LDL was significantly worse in active RA patients compared to controls. The mean HII in active RA patients was greater than 1, suggesting dysfunctional, pro-inflammatory HDL compared to healthy controls in which the HII was less than 1, suggesting protective, anti-inflammatory HDL (Table 1). Significant correlations were noted between levels of inflammation and the HII. Higher levels of systemic inflammation were associated with worse HDL function: r = 0.77, p = 0.0003, r = 0.82, p < 0.0001 for correlations of HII with ESR and CRP, respectively.
RA patients had a mean disease duration of 14.1 ± 13.5 years. RA treatments included 5/10 patients using methotrexate, 4/10 patients using NSAIDS, 5/10 using low dose prednisone, 2/10 patients using arava, 2/10 using plaquenil, and 1/10 patients using a TNFα inhibitor.
Oxidation products of arachidonic acid are increased in lipoproteins from patients with active RA compared to healthy controls
5-HETE, 15-HETE, 9-HODE, and 13-HODE were significantly higher in HDL and LDL from patients with active RA compared to healthy controls (Table 2). Correlations were noted between the levels of free oxidized fatty acids in HDL and LDL and the levels of systemic inflammation measured by both ESR and CRP (Table 3). Higher levels of inflammation were associated with higher free oxidized fatty acids in the lipoproteins. The strongest correlations were noted between HDL-associated 5-HETE, 15-HETE, 9-HODE, and 13-HODE and ESR (r = 0.70–0.80, p < 0.004) and CRP (r = 0.65–0.74, p < 0.004). 5-HETE, 15-HETE, 9-HODE, and 13-HODE levels in HDL were also significantly associated with HDL’s anti-oxidant function as measured by the HII (r = 0.54–0.58, p values < 0.03). Higher levels of these free oxidized fatty acids in HDL were associated with worse anti-oxidant function of HDL (Table 3).
Table 2.
Free oxidized fatty acid levels in HDL and LDL fractions from RA patients compared to healthy controls
| Rheumatoid arthritis (n = 10) | Healthy control (n = 8) | P value | |
|---|---|---|---|
| HDL 5-HETE | 14.4 ± 5.8 | 5.8 ± 2.6 | 0.0009 |
| HDL 12-HETE | 155.9 ± 338.6 | 33.3 ± 8.0 | 0.3986 |
| HDL 15-HETE | 3.8 ± 2.2 | 1.4 ± 0.5 | 0.0029 |
| HDL 9-HODE | 27.7 ± 14.8 | 9.5 ± 5.7 | 0.0039 |
| HDL 13-HODE | 38.2 ± 19.9 | 13.7 ± 6.1 | 0.0039 |
| LDL 5-HETE | 0.58 ± 0.28 | 0.22 ± 0.14 | 0.0038 |
| LDL 12-HETE | 7.6 ± 9.5 | 3.4 ± 3.1 | 0.230 |
| LDL 15-HETE | 0.47 ± 0.17 | 0.21 ± 0.14 | 0.003 |
| LDL 9-HODE | 5.3 ± 3.6 | 1.8 ± 1.4 | 0.005 |
| LDL 13-HODE | 6.8 ± 4.5 | 2.3 ± 1.6 | 0.005 |
All units are nanograms per milliliter plasma
HDL high density lipoprotein, LDL low density lipoprotein, HETE hydroxyeicosatetraenoic acid, HODE hydroxyoctadecadienoic acid
Table 3.
Correlations of free oxidized fatty acids in HDL and LDL with measures of systemic inflammation and HDL function
| ESR | P value | HS-CRP | P value | HII | P value | |
|---|---|---|---|---|---|---|
| HDL 5-HETE | 0.78 | 0.0002 | 0.74 | 0.0005 | 0.54 | 0.02 |
| HDL 12-HETE | 0.0037 | 0.99 | 0.25 | 0.33 | 0.35 | 0.16 |
| HDL 15-HETE | 0.80 | 0.0001 | 0.66 | 0.0029 | 0.57 | 0.013 |
| HDL 9-HODE | 0.72 | 0.0010 | 0.65 | 0.0038 | 0.56 | 0.017 |
| HDL 13-HODE | 0.70 | 0.0017 | 0.65 | 0.0032 | 0.58 | 0.01 |
| LDL 5-HETE | 0.39 | 0.12 | 0.65 | 0.0037 | 0.50 | 0.03 |
| LDL 12-HETE | 0.16 | 0.53 | 0.18 | 0.48 | 0.09 | 0.72 |
| LDL 15-HETE | 0.50 | 0.04 | 0.696 | 0.001 | 0.43 | 0.08 |
| LDL 9-HODE | 0.48 | 0.054 | 0.62 | 0.0057 | 0.41 | 0.09 |
| LDL 13-HODE | 0.47 | 0.058 | 0.62 | 0.0064 | 0.29 | 0.24 |
Spearman’s correlation coefficients are shown in the table
HDL high density lipoprotein, LDL low density lipoprotein, HETE hydroxyeicosatetraenoic acid, HODE hydroxyoctadecadienoic acid
HETES and HODES were higher in HDL compared to LDL (Table 2). Correlations were noted between LDL-associated HETES and HODES and measures of systemic inflammation although they were of lesser magnitude than with HDL; (r = 0.30–0.48, p = 0.006–0.25 (ESR), r = 0.34–0.62, p = 0.006–0.16 (CRP)) (Table 3). No differences were noted in HDL or LDL-associated HETES and HODES between RA patients receiving different RA treatments with the exception HDL-5-HETE, which was higher in patients on prednisone (17.4 ± 7.0 ng/ml) compared to patients not on prednisone (11.4 ± 2.1 ng/ml) (p = 0.04).
HDL in RA SF is pro-inflammatory compared to HDL from OA SF
HDL in SF from RA patients had significantly worse anti-inflammatory, anti-oxidant function as measured by a higher mean HDL inflammatory index (HII) compared to SF HDL from patients with OA (Table 4). The mean HII for HDL isolated from SF from RA patients was greater than 1, suggesting dysfunctional, pro-inflammatory HDL compared to HDL isolated from non-inflammatory synovial SF in which the HII was less than 1, suggesting anti-inflammatory HDL.
Table 4.
Characteristics of synovial fluid from patients with rheumatoid arthritis and osteoarthritis
| Rheumatoid arthritis (n = 10) | Osteoarthritis (n = 11) | P value | |
|---|---|---|---|
| Age (years) | 55 ± 13 | 69 ± 19 | 0.055 |
| Female (%) | 60 | 55 | 1.0 |
| SF total WBC | 15,409 ± 6153 | 617 ± 496 | 0.001 |
| SF neutrophils | 11,840 ± 7000 | 120 ± 177 | 0.001 |
| SF monocytes/macrophages | 2074 ± 1490 | 247 ± 135 | 0.001 |
| HDL cholesterol | 18 ± 10 | 12 ± 7 | 0.12 |
| Total cholesterol | 59 ± 17 | 26 ± 17 | 0.0004 |
| HDL inflammatory index | 2.1 ± 1.9 | 0.5 ± 0.1 | 0.046 |
| MPO activity (ng/ml) | 74.5 ± 48.3 | 0.9 ± 0.6 | 0.008 |
| Aryl activity (μmol/min/ml) | 412 ± 150 | 122 ± 129 | 0.001 |
| PON1 activity (nmol/min/ml) | 87 ± 37 | 39 ± 19 | 0.004 |
| 5HETE (ng/ml SF) | 0.44 ± 0.33 | 0.57 ± 0.68 | 0.97 |
| 12HETE (ng/ml SF) | 0.12 ± 0.07 | 0.12 ± 0.05 | 0.86 |
| 15HETE (ng/ml SF) | 0.37 ± 0.07 | 0.26 ± 0.11 | 0.02 |
| 9HODE (ng/ml SF) | 1.31 ± 1.70 | 0.63 ± 0.36 | 0.27 |
| 13HODE (ng/ml SF) | 4.15 ± 4.20 | 2.02 ± 1.20 | 0.096 |
SF synovial fluid, WBC white blood cell, HDL high density lipoprotein, MPO myeloperoxidase, Aryl arylesterase, PON1 paraoxonase, HETE hydroxyeicosatetraenoic acid, HODE hydroxyoctadecadienoic acid
SF MPO activity and 15-HETE levels were significantly higher in RA SF compared to OA SF (Table 4) and were correlated with SF monocyte/macrophage cell counts (r values = 0.7 and 0.6, respectively, p = 0.04 and 0.01). Higher levels of MPO activity and 15-HETE showed trends for association with worse HDL function (r = 0.5 and 0.3, respectively, p values = 0.2). 9-HODE and 13-HODE were higher in RA SF compared to OA SF but the results did not reach statistical significance (Table 4).
Total and HDL cholesterol levels were higher in SF from RA patients compared to OA patients (Table 4). SF cholesterol levels were strongly correlated with SF WBC counts (r = 0.8 (TC) and r = 0.5 (HDL-C), p = 0.0009 and 0.04, respectively), suggesting that more inflammation in the joint is associated with higher joint cholesterol levels. PON1 activity measured by both paraoxonase and arylesterase assays was significantly increased in RA synovial fluid, consistent with higher SF HDL-C levels.
Discussion
HETES and HODES are biologically active lipids formed via the lipoxygenase oxygenation of arachidonic and linoleic acid, respectively [23]. These oxidized fatty acids have been implicated as important mediators of immune responses, including involvement in macrophage differentiation [24] and dendritic cell maturation [25]. They have also been directly implicated in the pathogenesis of atherosclerosis [8, 9]. ApoE mice lacking 12/15-lipoxygenase enzymes develop significantly reduced atherosclerotic lesions when compared to apoE deficient mice with 12/15-lipoxygenase enzymes [26]. HETES and HODES contribute to the oxidation of LDL, and their accumulation in HDL has been proposed to inhibit HDL function, increasing atherosclerotic risk [8, 9].
Patients with active RA suffer significantly increased cardiovascular morbidity and mortality when compared to the general population [2, 3, 27]. High levels of systemic inflammation in active RA patients are strongly associated with cardiovascular death and have been associated with abnormal HDL function [2, 4, 18, 28]. Because dysfunctional HDL has been associated with CV events and death in the general population [29–31], impairment in HDL function has been proposed as a mechanism by which active RA increases CV risk.
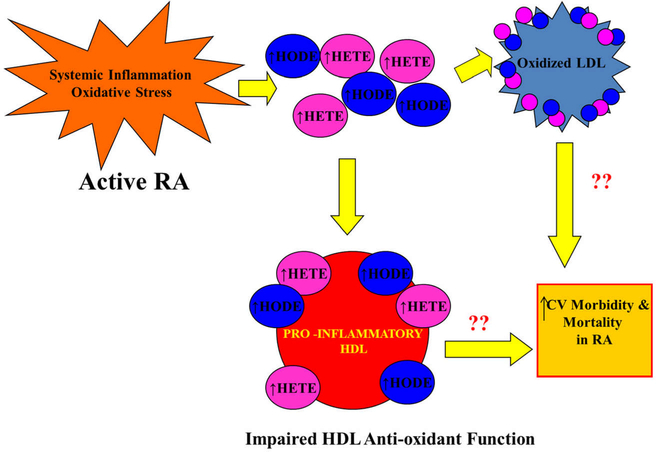
In the current work, 5-HETE, 15-HETE, 9-HODE, and 13-HODE levels were significantly elevated in HDL and LDL from active RA patients as compared to matched healthy controls. In addition, higher levels of systemic inflammation were strongly correlated with higher levels of free oxidized fatty acids in lipoproteins. The levels of HETES and HODES were particularly elevated in HDL as compared to LDL, and higher levels of HETES and HODES in HDL were significantly associated with worse anti-oxidant function of HDL. This data suggests a potential mechanism by which active RA patients with high levels of inflammation and oxidative stress have increased oxidized fatty acid accumulation in lipoproteins, leading to impairment in HDL function, and increased CV risk (Fig. 1).
Fig. 1.
Increased oxidized fatty acids in lipoproteins of patients with active RA: possible link to cardiovascular risk? High levels of systemic inflammation and oxidative stress in active RA patients are associated with increases in free oxidized fatty acids referred to as hydroxyeicosatetraenoic acids (HETES) and hydroxyoctadecadienoic acids (HODES). HETES and HODES are increased in LDL of active RA patients which may contribute to formation of oxidized LDL and predispose to atherosclerosis [32]. HETES and HODES are also significantly increased in HDL of active RA patients and these increases are associated with impaired HDL anti-oxidant function. Further work is needed to evaluate whether these lipoprotein changes are associated with the increased cardiovascular morbidity and mortality in active RA patients
HETES and HODES in LDL fractions were lower than the levels in HDL fractions. These findings must be confirmed in larger studies given the small numbers and pilot nature of the current study. However, Newman et al. reported similar findings in a rat model of proteinuria, a condition linked strongly to increased cardiovascular disease, even in patients without diabetes or hypertension. In the latter study, HETES and HODES were also differentially distributed among the lipo-protein density fractions. Concentrations were significantly greater in HDL fractions compared to LDL fractions in controls as well as in proteinuric animals. The HETES and HODES were also markedly elevated in HDL from protein-uric animals as compared to controls, with lesser differences noted in LDL, which is consistent with the current work [26]. This data supports the hypothesis that the inflammatory properties of HDL in particular may be important in understanding the increased CV risk associated with active RA.
Because abnormal function of HDL has been closely associated with RA disease activity [18], we further examined the anti-oxidant function of HDL isolated from RA synovial fluid as compared to HDL isolated from OA SF. HDL from RA SF had significantly worse anti-oxidant, anti-inflammatory function compared to HDL from OA SF. A modest association between HDL function and both MPO activity and 15-HETE levels in synovial fluid was noted, suggesting that higher SF MPO activity and 15-HETE may be associated with worse function of HDL. Previous work has shown that MPO directly binds HDL, targeting its major protein, apolipoprotein A-I, for oxidative modifications, which lead to functional impairment of HDL [33].
15-HETE levels were significantly higher in RA SF compared to OA SF in the current work. 15-HETE is produced non-enzymatically as well as enzymatically from arachidonic acid by 15-lipoxygenase (15-LOX). Gheorghe et al. previously reported that RA synovium has increased expression of 15 LOX compared to OA synovium and 15-LOX-1 expression was identified in lining macrophages [34]. The current work supports this data, showing higher levels of 15-HETE in RA SF compared to OA SF, which were strongly correlated with SF monocyte/macrophage counts.
The current data is the first work to examine the anti-inflammatory function of HDL isolated from individual RA SF as compared to HDL isolated from OA SF. RA SF HDL demonstrated impaired, pro-inflammatory properties compared to OA SF HDL.
Scanu et al. reported that a standard, “normal” HDL can inhibit production of CCL2 induced by monosodium urate crystals in fibroblast-like synoviocytes in vitro, suggesting a potential anti-inflammatory role for HDL in acute joint inflammation [35]. Bresnihan et al. also suggested a potential regulatory role of HDL in the joint by showing that apoA-I, the major protein of HDL, is present in the perivascular cellular infiltrates of inflamed RA synovial tissue, but absent from patients in remission [36]. These findings are remarkably similar to those in the artery wall where apoA-I is seen to accumulate in athero-sclerotic lesions but not in normal artery tissue [37].
The current data suggests that while normally HDL may have a regulatory role in joint inflammation similar to its anti-inflammatory role in the artery wall, this function is abnormal in RA SF, perhaps due to similar mechanisms, which oxidatively modify HDL and impair its function in the atherosclerotic plaque. Total and HDL cholesterol levels were higher in RA SF compared to OA SF in the current work, and were correlated with SF white blood cell counts. This data is consistent with past studies [38–40], and suggests increased permeability of the inflammatory RA synovium to lipoproteins despite lower levels in circulation.
There are some limitations to the current work. While these data are interesting, they must be considered hypothesis generating, since they are limited by both sample size and selection bias. Future studies will evaluate levels of HETES and HODES in lipoproteins from larger RA cohorts in order to better understand how HDL is altered in the setting of active RA and to determine the effect of specific treatments and other RA patient characteristics on free oxidized fatty acid levels in lipoproteins. In addition, further work is warranted to determine whether these types of lipoprotein assessments are more useful markers of CV risk in RA patients than traditional cholesterol levels which may have paradoxical relationships to CV risk in RA patients with active disease [41]. Further mechanistic work is also needed to determine the specific pathways leading to HDL dysfunction in the RA joint, as well as to determine whether HDL modulation in the RA joint contributes to dysfunctional HDL in circulation of active RA patients.
In summary, oxidation products of arachidonic acid and linoleic acid are increased in HDL and LDL from patients with active RA compared to healthy controls and are strongly correlated with RA disease activity as measured by levels of systemic inflammation, particularly in HDL, as well as impaired HDL anti-oxidant function. These results suggest a potential mechanism by which active inflammation from RA increases free oxidized fatty acids in circulating lipoproteins, promoting LDL oxidation and HDL dysfunction, thereby increasing atherosclerotic risk. Understanding the mechanisms which promote high cardiovascular risk in active RA patients is particularly important for those patients with active disease despite currently available RA treatments. Further investigation in these patients of targeted therapeutics such as apoA-I mimetic peptides, which bind oxidized fatty acids and reduce atherosclerosis in animal models [42] is warranted.
Acknowledgments
Funding Dr. Charles-Schoeman received support from the NHLBI (5K23HL094834, R01HL123064). Dr. Reddy received support from NHLBI (HL-82823 and HL-71776).
Footnotes
Compliance with ethical standards
Disclosures None.
References
- 1.Nurmohamed MT, Heslinga M, Kitas GD (2015) Cardiovascular comorbidity in rheumatic diseases. Nat Rev Rheumatol 11(12): 693–704 [DOI] [PubMed] [Google Scholar]
- 2.Van Doornum S, McColl G, Wicks IP (2002) Accelerated atherosclerosis: an extraarticular feature of rheumatoid arthritis? Arthritis Rheum 46(4):862–873 [DOI] [PubMed] [Google Scholar]
- 3.Roman MJ, Moeller E, Davis A, Paget SA, Crow MK, Lockshin MD et al. (2006) Preclinical carotid atherosclerosis in patients with rheumatoid arthritis. Ann Intern Med 144(4):249–256 [DOI] [PubMed] [Google Scholar]
- 4.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE (2005) Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum 52(3):722–732 [DOI] [PubMed] [Google Scholar]
- 5.Del Rincon I, Williams K, Stern MP, Freeman GL, O’Leary DH, Escalante A (2003) Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum 48(7):1833–1840 [DOI] [PubMed] [Google Scholar]
- 6.Hitchon CA, El-Gabalawy HS (2004) Oxidation in rheumatoid arthritis. Arthritis Res Ther 6(6):265–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakinuma T, Yasuda T, Nakagawa T, Hiramitsu T, Akiyoshi M, Akagi M et al. (2004) Lectin-like oxidized low-density lipoprotein receptor 1 mediates matrix metalloproteinase 3 synthesis enhanced by oxidized low-density lipoprotein in rheumatoid arthritis cartilage. Arthritis Rheum 50(11):3495–3503 [DOI] [PubMed] [Google Scholar]
- 8.Imaizumi S, Grijalva V, Navab M, Van Lenten BJ, Wagner AC, Anantharamiah GM et al. (2010) L-4F differentially alters plasma levels of oxidized fatty acids resulting in more anti-inflammatory HDL in mice. Drug Metab Lett 4(3):139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgantini C, Natali A, Boldrini B, Imaizumi S, Navab M, Fogelman AM et al. (2011) Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes 60(10):2617–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berliner JA, Watson ADA (2005) Role for oxidized phospholipids in atherosclerosis. N Engl J Med 353(1):9–11 [DOI] [PubMed] [Google Scholar]
- 11.Morgantini C, Meriwether D, Baldi S, Venturi E, Pinnola S, Wagner AC et al. (2014) HDL lipid composition is profoundly altered in patients with type 2 diabetes and atherosclerotic vascular disease. Nutr Metab Cardiovasc Dis 24(6):594–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma S, Umar S, Potus F, Iorga A, Wong G, Meriwether D et al. (2014) Apolipoprotein A-I mimetic peptide 4F rescues pulmonary hypertension by inducing microRNA-193–3p. Circulation 130(9): 776–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross DJ, Hough G, Hama S, Aboulhosn J, Belperio JA, Saggar R et al. (2015) Proinflammatory high-density lipoprotein results from oxidized lipid mediators in the pathogenesis of both idiopathic and associated types of pulmonary arterial hypertension. Pulm Circ 5(4):640–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD et al. (2000) Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res 41(9):1495–1508 [PubMed] [Google Scholar]
- 15.Navab M, Hama SY, Cooke CJ, Anantharamaiah GM, Chaddha M, Jin L et al. (2000) Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J Lipid Res 41(9):1481–1494 [PubMed] [Google Scholar]
- 16.Oram JF, Yokoyama S (1996) Apolipoprotein-mediated removal of cellular cholesterol and phospholipids. J Lipid Res 37(12):2473–2491 [PubMed] [Google Scholar]
- 17.Charles-Schoeman C, Lee YY, Grijalva V, Amjadi S, Fitzgerald J, Ranganath VK et al. (2012) Cholesterol efflux by high density lipo-proteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis 71(7):1157–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charles-Schoeman C, Watanabe J, Lee YY, Furst DE, Amjadi S, Elashoff D et al. (2009) Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis Rheum 60(10):2870–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, Fogelman AMA (2001) Cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J Lipid Res 42(8):1308–1317 [PubMed] [Google Scholar]
- 20.Charles-Schoeman C, Khanna D, Furst DE, McMahon M, Reddy ST, Fogelman AM et al. (2007) Effects of high-dose atorvastatin on Antiinflammatory properties of high density lipoprotein in patients with rheumatoid arthritis: a pilot study. J Rheumatol 34(7):1459–1464 [PubMed] [Google Scholar]
- 21.Charles-Schoeman C, Lee YY, Shahbazian A, Gorn AH, Fitzgerald J, Ranganath VK et al. (2013) Association of paraoxonase 1 gene polymorphism and enzyme activity with carotid plaque in rheumatoid arthritis. Arthritis Rheum 65(11):2765–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aviram M, Rosenblat M (2008) Paraoxonases (PON1, PON2, PON3) analyses in vitro and in vivo in relation to cardiovascular diseases. Methods Mol Biol 477:259–276 [DOI] [PubMed] [Google Scholar]
- 23.Kang LT, Phillips TM, Vanderhoek JY (1999) Novel membrane target proteins for lipoxygenase-derived mono(S)hydroxy fatty acids. Biochim Biophys Acta 1438(3):388–398 [DOI] [PubMed] [Google Scholar]
- 24.Vangaveti V, Baune BT, Kennedy RL (2010) Hydroxyoctadecadienoic acids: novel regulators of macrophage differentiation and atherogenesis. Ther Adv Endocrinol Metab 1(2):51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothe T, Gruber F, Uderhardt S, Ipseiz N, Rossner S, Oskolkova O et al. (2015) 12/15-Lipoxygenase-mediated enzymatic lipid oxidation regulates DC maturation and function. J Clin Invest 125(5): 1944–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF et al. (1999) Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J Clin Invest 103(11):1597–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boers M, Dijkmans B, Gabriel S, Maradit-Kremers H, O’Dell J, Pincus T (2004) Making an impact on mortality in rheumatoid arthritis: targeting cardiovascular comorbidity. Arthritis Rheum 50(6):1734–1739 [DOI] [PubMed] [Google Scholar]
- 28.Watanabe J, Charles-Schoeman C, Miao Y, Elashoff D, Lee YY, Katselis G et al. (2012) Proteomic profiling following immunoaffinity capture of HDL: association of acute phase proteins and complement factors with pro-inflammatory HDL in rheumatoid arthritis. Arthritis Rheum [DOI] [PMC free article] [PubMed]
- 29.Ansell BJ, Navab M, Hama S, Kamranpour N, Fonarow G, Hough G et al. (2003) Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 108(22):2751–2756 [DOI] [PubMed] [Google Scholar]
- 30.Khera AV, Cuchel M, de Llera-Moya M, Rodrigues A, Burke MF, Jafri K et al. (2011) Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364(2):127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D et al. (2008) Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 299(11):1265–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jira W, Spiteller G, Carson W, Schramm A (1998) Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients. Chem Phys Lipids 91(1):1–11 [DOI] [PubMed] [Google Scholar]
- 33.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M et al. (2004) Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest 114(4):529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gheorghe KR, Korotkova M, Catrina AI, Backman L, af KE, Claesson HE et al. (2009) Expression of 5-lipoxygenase and 15-lipoxygenase in rheumatoid arthritis synovium and effects of intraarticular glucocorticoids. Arthritis Res Ther 11(3):R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scanu A, Oliviero F, Gruaz L, Sfriso P, Pozzuoli A, Frezzato F et al. (2010) High-density lipoproteins downregulate CCL2 production in human fibroblast-like synoviocytes stimulated by urate crystals. Arthritis Res Ther 12(1):R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bresnihan B, Gogarty M, FitzGerald O, Dayer JM, Burger D, Apolipoprotein A-I (2004) Infiltration in rheumatoid arthritis syno-vial tissue: a control mechanism of cytokine production? Arthritis Res Ther 6(6):R563–R566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackness B, Hunt R, Durrington PN, Mackness MI (1997) Increased immunolocalization of paraoxonase, clusterin, and apolipoprotein A-I in the human artery wall with the progression of atherosclerosis. Arterioscler Thromb Vasc Biol 17(7):1233–1238 [DOI] [PubMed] [Google Scholar]
- 38.Ananth L, Prete PE, Kashyap ML, Apolipoproteins A-I (1993) B and cholesterol in synovial fluid of patients with rheumatoid arthritis. Metabolism 42(7):803–806 [DOI] [PubMed] [Google Scholar]
- 39.Oliviero F, Sfriso P, Baldo G, Dayer JM, Giunco S, Scanu A et al. (2009) Apolipoprotein A-I and cholesterol in synovial fluid of patients with rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Clin Exp Rheumatol 27(1):79–83 [PubMed] [Google Scholar]
- 40.Prete PE, Gurakar-Osborne A, Kashyap ML (1993) Synovial fluid lipoproteins: review of current concepts and new directions. Semin Arthritis Rheum 23(2):79–89 [DOI] [PubMed] [Google Scholar]
- 41.Myasoedova E, Crowson CS, Kremers HM, Roger VL, Fitz-Gibbon PD, Therneau TM et al. (2011) Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis 70(3):482–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G et al. (2002) Oral administration of an Apo A-I mimetic peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation 105(3):290–292 [DOI] [PubMed] [Google Scholar]