Abstract
Exercise is a simple intervention that profoundly benefits cognition. In rodents, running increases neurogenesis in the hippocampus, a brain area important for memory. We describe the dynamic changes in new neuron number and afferent connections throughout their maturation. We highlight the effects of exercise on the neurotransmitter systems involved, with a focus on the role of glutamate and acetylcholine in the initial development of new neurons in the adult brain.
The mammalian brain can modify itself in response to new stimuli, behaviors, diet, and environment. Exercise elicits a robust effect on neural plasticity. In rodents, voluntary wheel running results in structural and functional changes in brain regions important for cognition, such as the hippocampus and cortex (34, 138, 139). In humans, physical activity benefits hippocampus-dependent memory and prefrontal cortex-mediated executive function, and may maintain neural gray and white matter volume over the lifespan (34, 141). The onset of effects of exercise on the brain and behavior is rapid (6) and evolves over time. Initially, neurotransmitter levels and blood flow are changed, followed by an upregulation of growth factors in the brain [e.g., brain-derived nerve growth factor (BDNF)] and the genesis of new neurons in the hippocampus (6, 24, 26, 31, 94, 138). In this review, we evaluate how 1 wk and 1 mo of wheel running in rodents influences adult hippocampal cell genesis, neuronal morphology, neural circuitry, and synaptic plasticity. We also discuss the effects of exercise on other brain areas and the potential implications for human brain function.
Adult Hippocampal Neurogenesis
The hippocampus is essential for the acquisition of new memories (109) and includes three subfields: area CA1, area CA3, and the dentate gyrus that may mediate select aspects of memory function. Area CA1 is deemed to encode memories (91); area CA3 is considered to mediate retrieval of complete memories from partial information (pattern completion) (92, 93); the dentate gyrus is important for spatial pattern separation, the process by which similar incoming information or stimuli are transformed into distinct non-overlapping events (58, 70, 81). There are many more neurons in dentate gyrus (DG) than in entorhinal cortex (EC), the main projection to the DG. The divergence from a smaller to a larger number of cells may promote pattern separation processes (90, 126). In the dentate gyrus, the V-shaped granule cell layer (GCL) consists of densely packed granule cells (4, 145), with round cell bodies that have spiny apical dendrites that extend into the molecular layer. The DG molecular layer receives axons from the septum, entorhinal cortex, mossy cells, and GABAergic interneurons. Between the two blades of the “V” of the DG is the hilus, which contains inhibitory GABAergic interneurons and excitatory glutamatergic mossy cells. At the interface of the GCL and the hilus is the subgranular zone, the location of neural stem cells from which new neurons develop in the adult mammalian brain (FIGURE 1).
FIGURE 1.
The development of adult-born neurons in the dentate gyrus over time
Photomicrographs show the gradual development of adult-born granule cells (green) from 7 to 30 days of age in coronal brain sections derived from a sedentary young adult male C57Bl/6 mouse. Dividing cells in the dentate gyrus were labeled with retrovirus-expressing green fluorescent protein (GFP) and imaged 7, 14, or 30 days later. Nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI), blue.
Adult hippocampal neurogenesis is observed in mammalian species, including humans (1, 3), and is considered to have functional relevance for synaptic plasticity, memory function, and pattern separation processes (42, 47, 138). In the DG subgranular zone, stem cells consist of quiescent type 1 radial glia-like cells that express markers such as GFAP, Nestin, and Sox2, which give rise to type 2 proliferating neuronal precursor cells (36, 74). The progenitor cells differentiate into DG granule cells over several weeks, expressing markers such as PSA-NCAM and doublecortin transiently, followed by mature neuronal markers such as NeuN and calbindin. The morphological development of new neurons follows the expression of these markers. During the first days, the cells do not have clear dendritic and axonal processes. Gradually, the cells extend dendrites, and, after ~2 wk, their axons, called mossy fibers, form synapses with area CA3 pyramidal cells (148). During the third week, the dendritic processes of the new DG cells reach the outer molecular layer, and spine density increases. However, overall cell growth and integration into the dentate granule cell layer and associated neural network continues over several months (FIGURE 1).
Adult neurogenesis is upregulated by voluntary wheel running (FIGURE 2). Running increases cell proliferation, survival, and neuronal differentiation in correlation with improved synaptic plasticity and memory function (21, 27, 40, 79, 89, 128, 130–133, 138, 141). Although neurogenesis declines with aging, running can stimulate this process throughout life in rodent models (57, 65, 79, 130, 141, 146), with the exception of very old (>2 yr old) mice (27). Exercise increases neurogenesis in both female and male rodents in a strain-dependent manner (20, 83), regardless of whether activity is voluntary or forced (12, 29, 40, 67, 95, 112, 127, 131–133, 138, 141). In addition, voluntary resistance wheel running, resulting in shorter distances but higher work levels, has been evaluated. Four weeks of voluntary wheel running with a resistance of 30% of body weight in 19-wk-old rats increases adult neurogenesis and BDNF levels, in correlation with improved spatial memory (68, 69). Moderate treadmill activity (8 m/min twice a day, total daily distance 930 m), also enhances neurogenesis and memory function (29), whereas high-intensity training (30 m/min) may be less beneficial (112). In mice bred for high levels of voluntary wheel running (>20 km/night), neurogenesis is increased but dissociated from distance run and spatial memory (103). Thus running is a strong neurogenic stimulus; however, optimal network integration of the added new neurons (105, 137, 139, 140) may determine functional outcome.
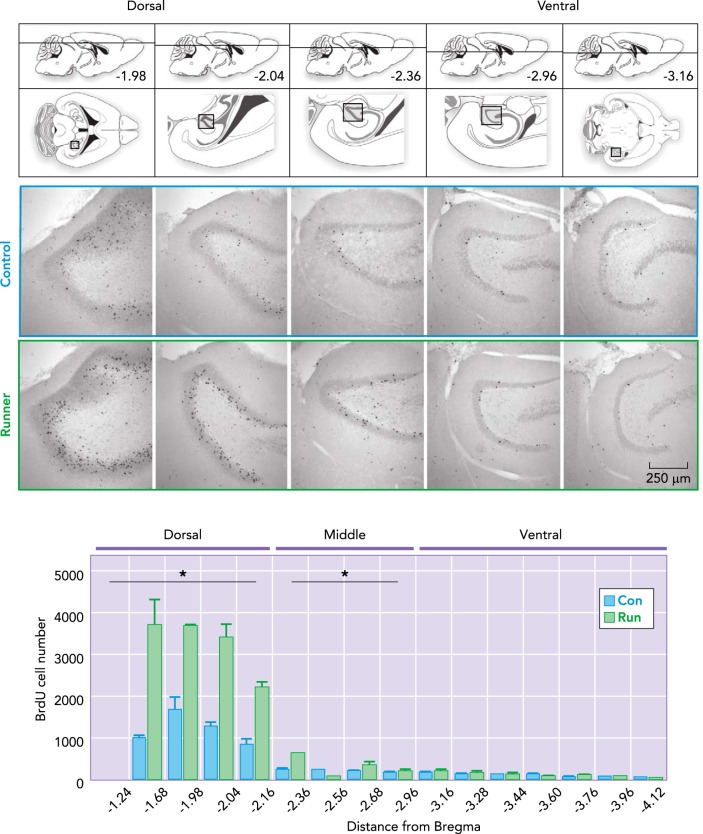
FIGURE 2.
Voluntary wheel running increases cell genesis in the dorsal dentate gyrus of young adult male mice
One month of running increases the number of new adult-born granule cells. Top: illustrations of a mouse brain through the dorso-ventral axes (see also Ref. 43): dorso-ventral depth (distance from Bregma) of horizontal sections below. Boxed areas in the illustrations correspond to the dentate gyrus in the photomicrographs. Photomicrographs show bromodeoxyuridine (BrdU+) cells in horizontal sections throughout the dorso-ventral dentate gyrus, derived from 10-wk-old male C57Bl/6 mice after housing with or without a running wheel for 1 mo. Running significantly increases the number of BrdU+ cells in the dorsal and middle, but not the ventral dentate gyrus (modified and reproduced from Ref. 139 with permission from Neuroimage).
Early Development and Running-Induced Plasticity
The initial development of newly born neurons is thought to be regulated by GABA, the main inhibitory neurotransmitter in the brain (30, 38, 44, 97, 113, 114, 125). GABAergic signaling determines whether radial-glial stem cells remain quiescent or give rise to rapidly amplifying type 2 progenitor cells. Specifically, tonic activation of the gamma 2 receptor by GABA, released from Parvalbumin expressing interneurons, keeps radial-glia stem cells quiet, whereas knockdown of the receptor in the stem cells promotes type 2 cell proliferation (113). Under voluntary running conditions, it has been suggested that GABA-mediated quiescence of radial-glia stem cells is inhibited by a diazepam binding inhibitor (DBI)-dependent mechanism to increase cell proliferation (33).
The effect of GABA on adult-born proliferating neural progenitors is considered similar to that observed during embryogenesis and early postnatal development. In immature neurons, GABA regulates cell proliferation and neurite outgrowth through an excitatory, depolarizing activation, due to the high intracellular concentration of chloride [Cl−] in immature neurons (9, 64). As the cells mature, there are changes in the expression of chloride transporters that control Cl− homeostasis in neurons [Na+-K+-2Cl− co-transporter (NKCC1) and K+-Cl− co-transporter (KCC2)]. NKCC1 (Cl− importer) is predominantly expressed in progenitors and immature neurons, resulting in high intracellular [Cl−] and a GABA-mediated-excitatory action (9). During maturation, neurons downregulate NKCC1 expression and upregulate KCC2 (Cl− exporter) expression. This results in low intracellular [Cl−] in the majority of neurons and shifts the GABA-mediated excitation to inhibition (9). Adult-born cells initially exhibit excitatory tonic GABA currents, followed by an excitatory response to GABAergic synaptic transmission. As the cells mature, expression of KCC2 increases, and at ~2 wk GABA switches from being excitatory to inhibitory (44).
The earliest input to new granule cells is reportedly from GABAergic interneurons (38, 45, 97, 125). Around 2 wk, the cells also begin to receive glutamatergic inputs from mossy cells and entorhinal cortex (17, 66, 71, 137). Thus there is virtually no role for glutamate or other neurotransmitters in the early development of new neurons, even though N-methyl-D-aspartate (NMDA) and acetylcholine (ACh) receptors are already expressed at this stage (28, 54, 56, 85, 121). Indeed, NMDA receptors (NMDAR) are important for the regulation of prenatal neuronal development and connectivity (23, 110). In vivo, knockout of NMDAR1 in individual newborn neurons impaired new neuron survival (121), and, in tissue culture, glutamate application to adult neural progenitor cells increases neuronal differentiation and excitatory synaptic connectivity (28). In addition, application of NMDA in acute hippocampal slices results in dendritic beading, indicative of functional NMDAR, in immature neurons (121). Based on these studies, we recently investigated whether glutamatergic innervation may contribute to the initial wiring of 1-wk-old, adult-born neurons and whether voluntary wheel running may induce modifications in their function, morphology, and circuitry (FIGURE 3).
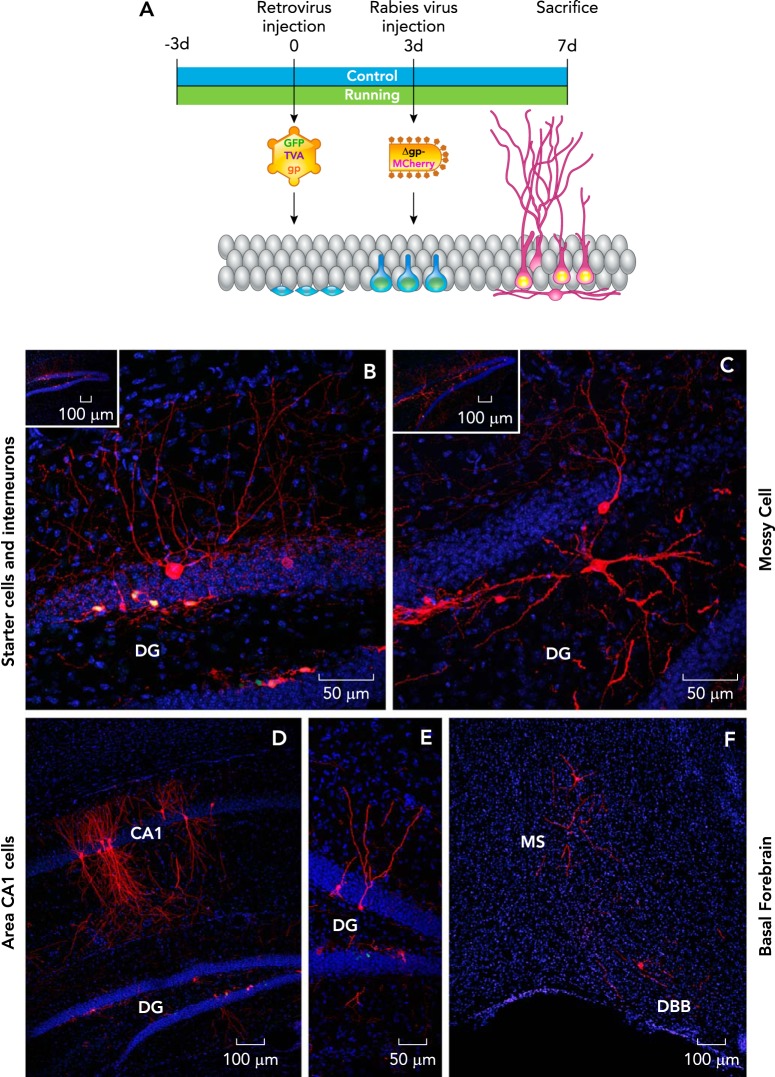
FIGURE 3.
Trans-synaptic tracing of inputs to 1-wk-old new granule cells
A: top shows the timeline of the virus-based neuroanatomical tracing experiment in 8-wk-old male C57Bl/6 mice. B: starter cells (new granule cells) labeled with retrovirus expressing GFP and rabies virus expressing MCh (yellow nuclei) and traced presynaptic interneurons expressing MCh (red) in the dentate gyrus. In addition, traced presynaptic mossy cells (C), pyramidal cells (D), mature GCs (E), and basal forebrain cells (F) expressing MCh are shown. DG, dentate gyrus; MS, medial septum; DBB, diagonal band of Broca. Nuclei were stained with DAPI (blue). Figure modified and reproduced from Ref. 105 with permission from Scientific Reports.
To address these questions, we applied a selective virus-based neuroanatomical retrograde tracing approach to delineate the circuitry of very young neurons (1-wk-old) in control and running mice. Specifically, we combined retrovirus to label dividing cells (129) with rabies virus as a retrograde tracer (137, 139), utilizing the monosynaptic TVA-EnvA tracing method (14, 144). The retrovirus was modified to express the avian TVA receptor, rabies glycoprotein (Rgp), and green fluorescent protein (GFP), and injected to label dividing cells in the dentate gyrus. In previous studies, we applied this approach to study the connectivity of the new neurons from 3 wk to 3 mo of age (FIGURE 4). However, in this study, after an interval of 3 days, avian envelope EnvA pseudotyped rabies virus was injected, and 4 days thereafter the animals were deeply anesthetized and perfused. This method has allowed us to determine the direct inputs to 1-wk-old newly born hippocampal neurons (137, 140). Interestingly, we observed that 1-wk-old immature granule cells receive inputs not only from GABAergic interneurons but also robust innervation from multiple intra-hippocampal glutamatergic cell types, including mossy cells, mature granule cells and area CA1–3 pyramidal cells (FIGURES 3 AND 4). Consistent with the circuitry tracing, patch-clamp recordings showed that these granule cells are activated tonically by ambient NMDA. Furthermore, both electrical and optogenetic stimulation evoked NMDA-mediated synaptic responses (105).
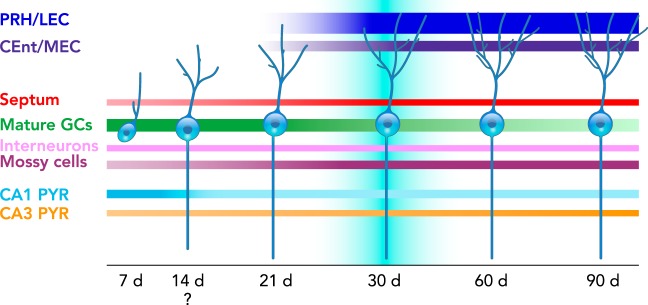
FIGURE 4.
Time course of afferent innervation of newborn neurons
Schematic representation of monosynaptic inputs to the newborn dentate granule cells (GCs) of young adult C57Bl/6 male mice at 7–90 days after retroviral infection, (please note that the 14-day time point was not experimentally tested). At 7 days (d), newborn GCs mainly receive connections from mature GCs, area CA 1–3 pyramidal cells (PYR), mossy cells, interneurons, and the basal forebrain, including the septum. The area CA1 and mGC input diminishes over time, whereas entorhinal cortex [perirhinal (PRH) and lateral entorhinal cortex (LEC), caudo-medial and medial entorhinal cortex (CEnt/MEC)], and hilar cell (mossy cells and interneurons) innervation increase over time. Convergence of excitatory afferents onto the newborn GCs at ~30 days correlates with transiently enhanced new neuron excitability and plasticity.
One week of voluntary wheel running does not change the amplitude of evoked NMDAR-mediated synaptic response in 1-wk-old granule cells. However, running increased the stimulus intensity needed to evoke NMDA-mediated responses in these cells (105). This difference between controls and runners may be due to an increase in synaptic contacts onto dendrites of more arborized 1-wk-old granule cells in running mice by redistribution of NMDARs over the dendrites (101). Indeed, our results showed that running increases cell body area and branching of 1-wk-old adult-born neurons, which correlates with an increase in membrane capacitance. These findings are consistent with research at later time points showing that voluntary wheel running stimulates maturation of adult-born granule cells by increasing spine density, dendritic length, and complexity (102, 116, 148). Altogether, the greater complexity and possible differential distribution of synaptic contacts onto these young neurons may already result in enhanced plasticity.
Previous research in mice that were housed in an enriched environment containing running wheels reported that changes in afferent connectivity to adult-born neurons are restricted to the so-called “critical period” of newborn neurons (10). This critical period is a time window between the fourth and sixth week of age, when the new cells exhibit heightened excitability and synaptic plasticity (45, 78, 107). However, our research indicates that voluntary wheel running impacts new neuron networks as early as 1 wk of age. Comparable to our observations in more mature, 1-mo-old granule cells, we observe a trend toward a reduction in the ratio of traced cells to starter cells (105, 139). Detailed analysis shows that running reduced innervation from area CA1 pyramidal cells onto immature neurons (FIGURE 5). The input from area CA1 may be a transient source of glutamate or neurotrophins, such as BDNF, to new granule cells. Voluntary wheel running may promote new neuron maturation processes by elevating BDNF and the TrkB receptor (40, 86, 94). Indeed, CA1 cells may be anatomically well-positioned to provide the DG suprapyramidal blade with neurogenic substrates (55, 79). Similarly, in hippocampal slice cultures, axons of area CA1 pyramidal cells project to the suprapyramidal layer of the DG to form functional connections with granule cells (7, 8). In addition, a recent study showed that there are back-projecting interneurons from area CA1 to the DG (119). Interestingly, this connectivity (CA1-DG) is increased by epilepsy (32). Thus the decrease over time in area CA1 input to new neurons (139) may reflect normal circuitry development (FIGURES 1, 4, AND 5).
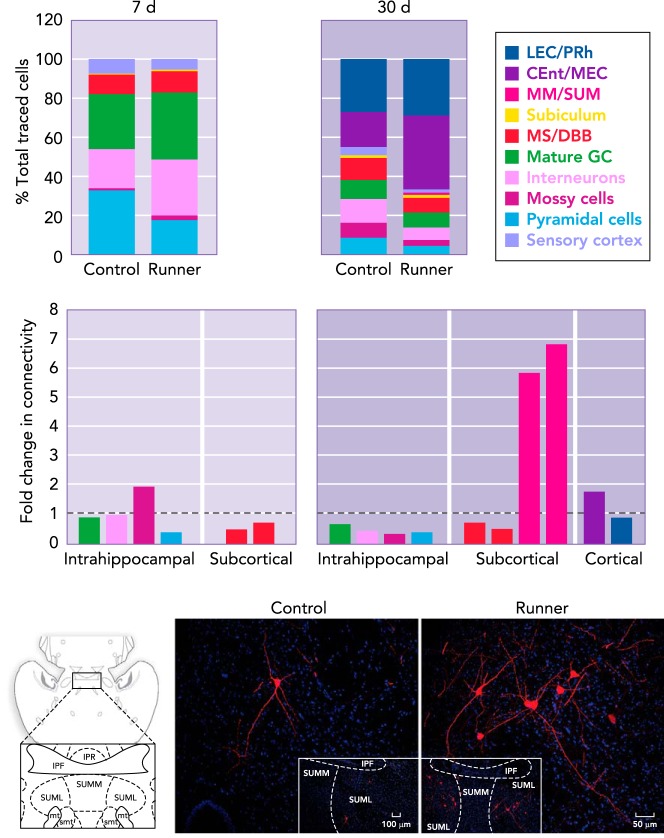
FIGURE 5.
Running modifies the connectivity of 1-wk-old and 1-mo-old new neurons
Running shifts the contributions of presynaptic cell populations (percentage of total traced cells) to the wiring of new dentate granule cells (GC) at 7 days (d) and 30 d of age. At 7 d, running reduces the input and connectivity from area CA1 pyramidal cells, whereas hilar cells (interneurons and mossy cells) show a trend toward an increased contribution. At the 30-d time-point, running results in recruitment of afferents from entorhinal cortex [perirhinal (PRH) and lateral entorhinal cortex (LEC), caudo-medial and medial entorhinal cortex (CEnt/MEC)], whereas hilar input (interneurons and mossy cells) is reduced. The largest fold change in connectivity was observed in the medial mammillary (MM) and supramammillary (SUM) neurons. The photomicrographs depict traced SUM cells expressing MCherry (red) in horizontal sections derived from control and running mice. Nuclei were stained with DAPI (blue). Figure was modified and reproduced from Ref. 139, with permission from Neuroimage.
In addition to GABAergic and glutamatergic inputs, 1-wk-old adult-born granule cells receive innervation from cholinergic basal forebrain cells. Voluntary wheel running did not change the number of cholinergic cells innervating adult-born neurons; however, the exercise-induced increase in cell proliferation can be blocked by the ablation of the septal cholinergic system (54). It remains to be determined whether exercise modifies acetylcholine receptor expression on adult-born neurons. Their activation can regulate the proliferation and survival of adult-born neurons (25, 54, 56) and may play a key role in the integration of very young neurons into the adult hippocampal network, even before dendritic maturation. Overall, the view that GABA drives adult hippocampal neurogenesis may need to be expanded to include other transmitter systems such as acetylcholine and glutamate. Indeed, the development of new neurons in the adult brain may not be a slow-motion replay of the events that take place during embryogenesis.
Effects of Running on 1-Mo-Old Adult-Born Granule Cells
After 1 mo of voluntary wheel running, there are about twice as many new neurons in the dentate gyrus compared with sedentary control mice (FIGURE 2). The running-induced increase in neurogenesis is observed predominantly in the dorsal aspect of the DG (12, 139). The dorsal hippocampus is considered important for spatial navigation, whereas the ventral sector is related to emotion (39, 88). Consistently, running is associated with improved synaptic plasticity and memory function (99, 131, 138). Running also improves another aspect of cognitive function, the ability to discriminate between highly similar stimuli and contexts in rodents (12, 27, 147) and humans (118). Interestingly, ablation of new neurons impairs pattern separation, whereas enhanced neurogenesis results in improvement (2, 27, 106).
Running induces morphological changes in newly born neurons. Several studies have followed the development of dendrites and spines in new neurons over time. A general consensus has emerged that running accelerates new neuron maturation by increasing dendritic complexity and spine density but does not result in overall changes by the time the neurons are 1-mo-old (102, 116, 148, 149). In addition, live imaging analysis of mice living in an enriched environment, including running wheels, has shown a differential pattern of dendritic branch addition and pruning during development that does not result in increased complexity (47). In a very detailed analysis, it has been observed that, although running does not enhance overall dendritic spine density, there is a selective increase in mushroom spine density in 2-mo-old new neurons in outer molecular layer dendrites (149), the location of incoming lateral perforant pathway projections from entorhinal cortex. Changes in fine dendritic morphology following exercise may also be due to the modifications in the presynaptic inputs. Indeed, a local increase in new neurons in the dentate gyrus may underlie, to some extent, improved cognition (27, 106, 141); however, plasticity of adult-born neuron circuitry may play an equally important role.
Under control sedentary conditions, the adult-born neurons integrate into the established hippocampal circuitry gradually over time (137, 140). The ratio of connectivity between presynaptic cells to adult-born neurons increases threefold between 3 wk and 3 mo of age (137). As described above, the early presynaptic inputs to new neurons are from local hippocampal cells, such as mature granule cells, pyramidal cells, mossy cells, and interneurons, and basal forebrain cells. At 3 wk of age, sparse innervation from entorhinal cortex is observed (137). At 1 mo of age, the cells are closer to full integration into subcortical and cortical circuits (FIGURE 4). The new neurons receive particularly substantial input from perirhinal and lateral entorhinal cortex. Lesion of these areas reduces pattern separation ability in mice, supporting their important role in adult-born granule cell circuitry (27, 137). In addition, new neurons receive sparse presynaptic input from medial and supramammillary nucleus (FIGURE 5).
Recently, we showed that 1 mo of voluntary wheel running in male mice differentially affects specific presynaptic inputs to the adult-born granule cells (139). Within the hippocampus, the ratio of connectivity from presynaptic inhibitory interneurons and glutamatergic mossy cells to adult-born neurons is reduced, whereas innervation from entorhinal cortex (lateral and caudo-medial) and septum is augmented, in proportion to the running-induced enhancement of adult neurogenesis. However, the overall ratio of connectivity between presynaptic cells to adult-born neurons showed a trend toward a decrease (139), similar to our observations after short-term running (105). A reduction in presynaptic inputs converging onto individual neurons throughout their development may be conducive to pattern separation by facilitating sparse activation.
One month of voluntary wheel running induced a striking change in the inputs from the supramammilary nucleus (SUM) pars lateralis and the medial mammillary nuclei (MM). These small populations of cells exhibited a 13-fold increase in the number of projecting cells and a 7-fold increase in their connectivity with newborn neurons (ratio of presynaptic cells to newborn neurons; FIGURE 5). The functional properties of these nuclei, and in particular the specific role of their projections onto newborn neurons, is unclear. Fibers from the lateral SUM predominantly target the dorsal dentate gyrus and CA2/CA3 areas (135). In the dentate gyrus, SUM fibers terminate within the upper third of the granule cell layer and inner molecular layer (135). SUM neurons fire in synchrony with hippocampal theta (136) and may be directly involved in the generation of the theta rhythm (124). It has been suggested that SUM activation may amplify signals from the entorhinal cortex to the dentate gyrus, which may serve to promote the transfer and encoding of information (135). Therefore, increased integration of the SUM cells into adult-born granule cell circuitry may contribute to the running-induced improvement of memory function. Interestingly, MM projections to the hippocampus have not been described previously. Future studies will be needed to elucidate the specific role of these mammillary nuclei in new neuron networks (FIGURE 5).
Running and Neurotransmission
The enhancement of memory function by running may result from increased neurogenesis in conjunction with modifications to the adult-born neuron network. Circuitry analysis reveals that multiple neurotransmitter systems (glutamatergic, GABAergic, cholinergic) connect to adult-born neurons and that these are modified by running. Although the precise role of each neurotransmitter in the context of exercise-induced neurogenesis remains to be determined, we describe those systems that may be directly relevant to the plasticity of adult-born neuron circuitry.
Excitatory Neurotransmission
Running increases long-term potentiation (LTP), considered a physiological model of certain forms of learning and memory (11, 76), in the dentate gyrus (40, 72, 133, 134). Field recordings show a significantly greater LTP in the dentate gyrus in acute hippocampal slices derived from voluntary running mice compared with controls (133, 134). In vivo recordings have shown similarly enhanced LTP in the dentate gyrus of anesthetized rats subjected to either voluntary (40) or forced treadmill running (96). Weak theta-patterned stimulation, which does not produce LTP in the dentate gyrus of control subjects, produces a robust and long-lasting LTP in voluntary running rats, suggesting that running may reduce the LTP induction threshold (40). The mechanisms underlying running-induced enhanced synaptic plasticity in the dentate gyrus remain unclear. LTP induction in control and runner subjects depends on activation of NMDA receptors (40, 134). Running also increases NR2B subunits of NMDA receptor levels in the dentate gyrus (40). Previous studies have shown that alterations in the NR2B subunit of NMDAR affect LTP. Overexpression of this subunit results in increased LTP induction (15, 120), whereas ablation of neurogenesis or deletion of the NR2B subunit in adult-born dentate neurons prevents the induction of LTP in the dentate gyrus (59, 111).
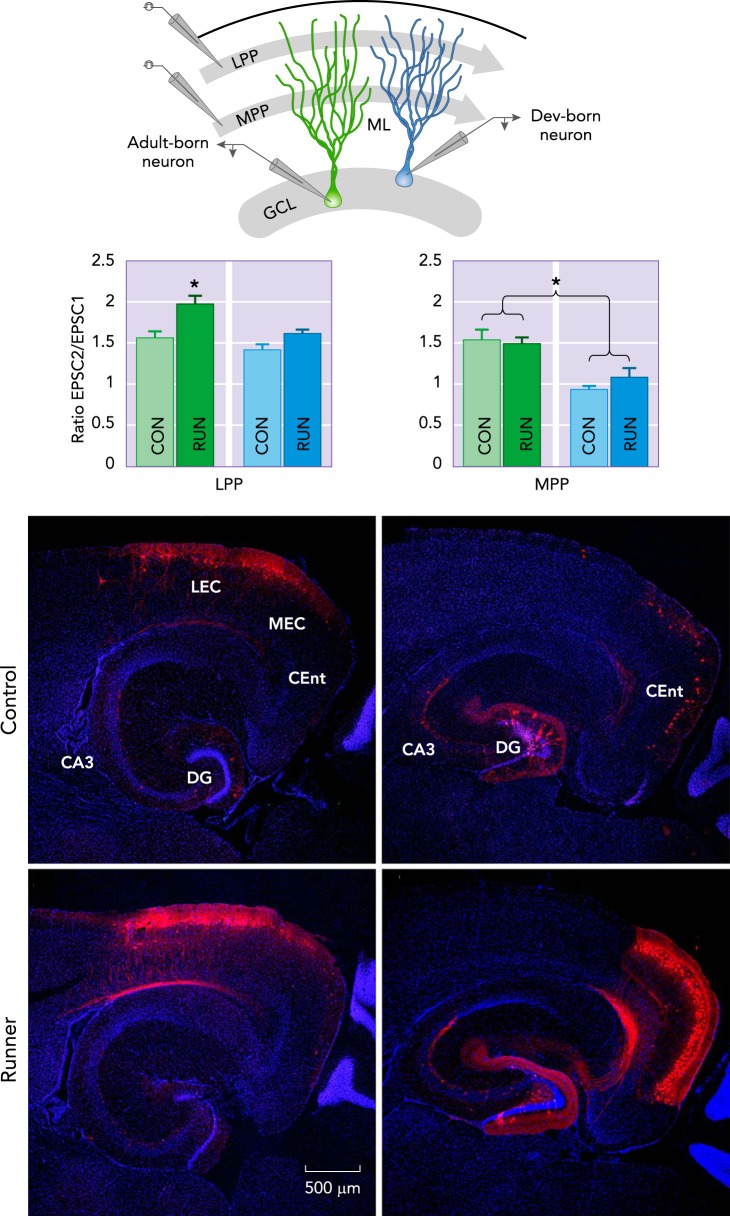
The running-induced enhancement of LTP in the dentate gyrus may result from the increase in new neuron number and the modifications in their neural circuitry (139). Although the adult-born dentate granule cells are a small percentage of the total population of granule cells, they exhibit enhanced excitability, have a lower LTP induction threshold, and enhanced LTP compared with developmentally born dentate granule cells (45, 78, 107, 111). This supports the hypothesis that adult-born dentate neurons may mediate, in part, increased synaptic plasticity. However, the effect of running on LTP in these two populations of granule cells has not yet been directly studied in individual neurons. Even so, short-term synaptic plasticity evaluated by paired-pulse stimulation of either the lateral perforant pathway (LPP) or medial perforant pathway (MPP) projections from entorhinal cortex onto new neurons showed that voluntary running increases paired-pulse facilitation evoked by LPP stimulation but not MPP in adult-born granule cells (FIGURE 6). Conversely, exercise does not change the paired-pulse ratio evoked by either LPP or MPP stimulation on developmentally born granule cells (139).
FIGURE 6.
Running increases entorhinal cortex input and short-term synaptic plasticity of new neurons
Running modifies glutamatergic synaptic transmission onto 1-mo-old dentate gyrus (DG) granule cells (GCs). Top: a schematic representation of the experimental design used to determine the running-induced changes in synaptic plasticity of entorhinal cortex-dentate gyrus synapses in acute hippocampal slices derived from C57Bl/6 male mice. Newborn GCs (green cells) and developmentally (dev) born GCs (blue cells) were recorded, and synaptic responses were evoked by paired-pulse stimulation of lateral (LPP) and medial (MPP) perforant pathway in the molecular layer (ML) of the dentate gyrus. Running significantly increases LPP paired-pulse facilitation onto new GCs compared with all other groups. These findings are complemented by enhanced input to new neurons from lateral entorhinal cortex (LEC). The input from caudomedial entorhinal cortex (CEnt) was also strongly upregulated but not accompanied by an increase in short-term plasticity with running. Traced cells expressing MCherry (red). Nuclei were stained with DAPI (blue). Figure was modified and reproduced from Ref. 139, with permission from Neuroimage.
Analysis of the synaptic glutamatergic transmission onto developmentally and adult-born granule cells measured by miniature excitatory postsynaptic currents (mEPSC) shows that exercise significantly reduces the amplitude of mEPSCs without modifying their frequency onto adult-born granule cells, whereas, in developmentally born granule cells, both amplitude and frequency of mEPSCs are increased (139). These results suggest that exercise has a differential effect on the short-term plasticity and neurotransmission onto these populations of dentate granule cells. Further research is needed to elucidate the contributions of developmentally and adult-born granule cells to short-term synaptic plasticity and LTP under running conditions (FIGURE 6).
Inhibitory Transmission
In our retrograde tracing studies, we showed that 1 mo of voluntary running reduced the ratio of inhibitory interneuron innervation onto adult-born neurons (139). However, inhibitory synaptic transmission onto newborn neurons was not affected, whereas developmentally born granule cells, in acute hippocampal slices derived from mice housed with a running wheel, became strongly inhibited (139). Interestingly, in studies where all mice were housed in cages that contained running wheels (78, 107, 123), adult-born neurons were activated by weak entorhinal input, whereas developmentally born neurons required strong afferent activation. However, upon GABAergic inhibition blockade, both granule cell neuron populations were easily activated (78). Similarly, stimulation of hilar interneurons strongly inhibited developmentally born neurons, compared with adult-born neurons, in acute hippocampal slices derived from runners (123). Thus increased excitability of adult-born neurons may be, in part, attributable to running.
Modifications in the GABAergic system extend throughout the hippocampus. Exercise changes the expression of various GABAA receptor subunits as well as the GABA-synthesizing enzyme glutamic acid decarboxylase-67 (GAD67) and the vesicular GABA transporter in the hippocampal subfields (49, 108). Specifically, 4 wk of voluntary running increases the mRNA levels of GABA β1 subunits in CA1, CA2, and dentate gyrus, δ1 subunit levels only in CA2, whereas α5 levels are increased in all the hippocampal areas. Similarly, GAD67 mRNA levels are increased in all subfields, suggesting enhanced GABA synthesis capacity in hippocampal GABAergic interneurons. The running-induced changes in the α5 subunit combined with increased GABA synthesis may impact hippocampal neuron excitability, since this subunit is associated with extrasynaptic GABAA receptors, which are subject to tonic activation (16). These changes may contribute to anxiolytic and pro-cognitive effects of running.
Basal Forebrain Cholinergic System
Acetylcholine (ACh) is important for learning and memory (122). The medial septum (MS) and diagonal band of Broca (DBB) supply ACh to the hippocampus (84) and modulate synaptic plasticity through cholinergic receptor activation (nicotinic and muscarinic) (75, 143). The formation of ACh in cholinergic cells requires the transport of choline (a direct precursor of ACh) into cells from the extracellular space and choline acetyltransferase (ChAT) activity. Long-term physical activity (12–14 wk) increases muscarinic receptor density, high-affinity choline uptake in the hippocampus (41), and the number of cells expressing ChAT in the horizontal DB but not in MS or vertical DB (5). The basal forebrain cholinergic system plays an important role in adult hippocampal neurogenesis, possibly by activation of cholinergic receptors on newly born neurons. Neural progenitor cells and adult-born granule cells express ACh receptors, including nicotinic α7 and β2 and muscarinic m1 and m4 subunits (54, 56, 85). Administration of the cholinergic agonist physostigmine increases new neuron number (54, 85), whereas selective lesioning of the cholinergic basal forebrain, using mu p75-saporin immunotoxin injection, reduces both basal (85) and running-induced neurogenesis (50, 54). Furthermore, our tracing studies showed that 1-wk-old granule cells already receive innervation from the basal forebrain and that 1 mo of voluntary wheel running upregulates the input from medial septum to new neurons, proportionate to the increase in hippocampal neurogenesis (105, 139).
The Serotonergic System
Our circuitry analysis did not reveal substantial input from serotonergic neurons onto new neurons. However, this does not preclude input from the raphe nuclei to new neurons, which may be mediated by volume transmission. Serotonin [5-hydroxytryptamine (5-HT)] plays an important role in synaptic plasticity, neurogenesis, neural repair, and mood (19, 61, 80, 142). In the mammalian brain, 5-HT is produced in neurons that are located mainly in the brain stem raphe nuclei; these cells project to the hippocampus with a very dense plexus of serotonergic fibers. Hippocampal 5-HT levels are influenced by running (46, 61, 82). Measurement of 5-HT in the ventral hippocampus by microdialysis, during acute treadmill running, indicates that 5-HT levels increase significantly after 90 min of exercise, with a maximal level in the first 30 min of recovery, lasting over 90 min (46). However, 4 wk of treadmill running reduces hippocampal 5-HT without affecting its metabolism or 5-HT1A, 5-HT1B receptors, and serotonin transporter (SERT) levels (18). In the raphe nuclei, 6 wk of running decreases the levels of 5-HT and SERT mRNA but increases 5-HT1A receptor mRNA expression (48). A reduction in 5-HT resulting from ablation of 95% of serotoninergic neurons was recently shown to increase hippocampal neurogenesis (115). Conversely, tryptophan hydroxylase (TPH) 2-deficient as well as 5-HT3 receptor knockout mice show normal baseline neurogenesis but impaired running-induced neurogenesis (60, 62).
Running Modifies Multiple Brain Areas
Accumulating evidence for exercise effects on the brain led to evaluation of multiple regions. Voluntary wheel running affects cortex, amygdala, striatum, hypothalamus, and raphe nuclei (5, 13, 48, 49, 51, 73, 104, 117). Two months of voluntary wheel running increases the dendritic spine density in hippocampal area CA1 pyramidal cells and the entorhinal cortex (117). In addition, 12 days of voluntary wheel running increases dendritic spine number in medial prefrontal cortex cells and the expression of synaptic plasticity markers such as synaptophysin and PSD-95 in the prefrontal, orbitofrontal, and perirhinal cortex (13). Four weeks of running in rats increases BDNF levels in the perirhinal cortex (51). In addition, gliogenesis is enhanced in the medial prefrontal cortex after 1 mo of voluntary wheel running in mice (35) and rats (77). Altogether, these changes may contribute to exercise-induced benefits for memory and mood.
Translational Effects of Exercise In Humans
There is increasing evidence that exercise benefits brain function and may prevent or delay the onset of neurodegeneration-associated memory loss in humans (34). Research into the underlying neuroanatomical and physiological correlates of physical activity has focused primarily on the prefrontal cortex and hippocampus (6, 22). Recently, it was shown in a 4-mo exercise intervention study in young adults (19–34 yr old) that there was an association between the novel myokine cathepsin B, a muscle secretory factor, and fitness, as well as improved performance on the hippocampus-dependent complex figure recall task. In this study, the training group received three sessions per week of moderate to intense aerobic exercise interval training at 70–90% of maximum heart rate for 45–75 min, whereas the control group walked twice a week for 10–15 min at 50% of maximum heart rate (87). In older adults, a 1-yr walking intervention of 40 min/day (after week 7 of the experiment, target heart rate zone was 60–75% of the maximum heart rate reserve) resulted in a 1–2% increase in hippocampal volume in association with enhanced spatial memory accuracy (37). In addition, 3 mo of aerobic exercise (four times a week for 1 h) in adults (21–45 yr old) increased dentate gyrus blood volume in correlation with improved cognitive function (100). In rodents, neurogenesis is closely related to the vasculature (98), raising the hypothesis that changes in cerebro-vasculature may be an indirect measure of exercise effects on adult neurogenesis in humans (100). Indeed, directly imaging and analyzing a small subpopulation of dentate granule cells deep in the human brain is not yet feasible. An informative approach might be to focus the analyses on brain areas such as the entorhinal cortex, mammillary nuclei, and basal forebrain that project to new neurons and are modified by running in rodents (137, 139). Interestingly, those structures are considered vulnerable to aging-related neurodegenerative diseases in humans (52, 53, 63). Running-induced network expansion of these brain areas may delay or prevent the onset of such conditions.
Conclusions
Altogether, our recent findings show that adult-born neurons have unique anatomical and physiological properties that can be continuously changed by voluntary running from the progenitor cell stage throughout the process of integration into the hippocampal circuitry. Indeed, running not only increases the number of new neurons but it also affects their inputs and synaptic plasticity. These modifications involve a complex and dynamic network of neurotransmitter systems that impinge onto the new neurons, including a previously unappreciated contribution of glutamatergic and cholinergic signaling, very early in their development. Future studies are needed to understand the precise nature of these running-induced changes in adult-born neuron networks, the role of growth factors, metabolism, (epi)genetics, and blood flow, and ultimately their functional relevance for mammalian learning and memory processes.
Acknowledgments
This work was supported in part by the National Institute on Aging, Intramural Research Program, and by Consejo Nacional de Ciencia y Tecnología INFR‐2016 268247 (to C.V). We are most grateful to Linda R. Kitabayashi for assistance in preparing the photomicrographs.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: C.V. and H.v.P. prepared figures; C.V. and H.v.P. drafted manuscript; C.V. and H.v.P. edited and revised manuscript; C.V. and H.v.P. approved final version of manuscript.
References
- 1.Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev 94: 991–1026, 2014. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron 70: 589–596, 2011. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature 207: 953–956, 1965. doi: 10.1038/207953a0. [DOI] [PubMed] [Google Scholar]
- 4.Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog Brain Res 163: 3–22, 2007. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang ET, Dawe GS, Wong PT, Moochhala S, Ng YK. Alterations in spatial learning and memory after forced exercise. Brain Res 1113: 186–193, 2006. doi: 10.1016/j.brainres.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Basso J, Suzuki WA. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: a review. Br Plast 2: 127–152, 2017. doi: 10.3233/BPL-160040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bausch SB, McNamara JO. Synaptic connections from multiple subfields contribute to granule cell hyperexcitability in hippocampal slice cultures. J Neurophysiol 84: 2918–2932, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Bausch SB, McNamara JO. Contributions of mossy fiber and CA1 pyramidal cell sprouting to dentate granule cell hyperexcitability in kainic acid-treated hippocampal slice cultures. J Neurophysiol 92: 3582–3595, 2004. doi: 10.1152/jn.01028.2003. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci 3: 728–739, 2002. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 10.Bergami M, Masserdotti G, Temprana SG, Motori E, Eriksson TM, Göbel J, Yang SM, Conzelmann KK, Schinder AF, Götz M, Berninger B. A critical period for experience-dependent remodeling of adult-born neuron connectivity. Neuron 85: 710–717, 2015. doi: 10.1016/j.neuron.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39, 1993. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 12.Bolz L, Heigele S, Bischofberger J. Running improves pattern separation during novel object recognition. Br Plast 1: 129–141, 2015. doi: 10.3233/BPL-150010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brockett AT, LaMarca EA, Gould E. Physical exercise enhances cognitive flexibility as well as astrocytic and synaptic markers in the medial prefrontal cortex. PLoS One 10: e0124859, 2015. doi: 10.1371/journal.pone.0124859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callaway EM, Luo L. Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. J Neurosci 35: 8979–8985, 2015. doi: 10.1523/JNEUROSCI.0409-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao X, Cui Z, Feng R, Tang YP, Qin Z, Mei B, Tsien JZ. Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur J Neurosci 25: 1815–1822, 2007. doi: 10.1111/j.1460-9568.2007.05431.x. [DOI] [PubMed] [Google Scholar]
- 16.Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA 101: 3662–3667, 2004. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chancey JH, Poulsen DJ, Wadiche JI, Overstreet-Wadiche L. Hilar mossy cells provide the first glutamatergic synapses to adult-born dentate granule cells. J Neurosci 34: 2349–2354, 2014. doi: 10.1523/JNEUROSCI.3620-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen HI, Lin LC, Yu L, Liu YF, Kuo YM, Huang AM, Chuang JI, Wu FS, Liao PC, Jen CJ. Treadmill exercise enhances passive avoidance learning in rats: the role of down-regulated serotonin system in the limbic system. Neurobiol Learn Mem 89: 489–496, 2008. doi: 10.1016/j.nlm.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Chennaoui M, Drogou C, Gomez-Merino D, Grimaldi B, Fillion G, Guezennec CY. Endurance training effects on 5-HT(1B) receptors mRNA expression in cerebellum, striatum, frontal cortex and hippocampus of rats. Neurosci Lett 307: 33–36, 2001. doi: 10.1016/S0304-3940(01)01901-2. [DOI] [PubMed] [Google Scholar]
- 20.Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience 155: 1048–1058, 2008. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 21.Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Brzezinska WJ, Rhodes JS. Genetic influences on exercise-induced adult hippocampal neurogenesis across 12 divergent mouse strains. Genes Brain Behav 10: 345–353, 2011. doi: 10.1111/j.1601-183X.2010.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 14: 125–130, 2003. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 23.Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci 13: 129–154, 1990. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- 24.Cooper C, Moon HY, van Praag H. On the run for hippocampal plasticity. Cold Spring Harbor Lab Perspectives in Medicine. In: The Biology of Exercise, edited by Zierath JR, Joyner MJ, Hawley MA. Cold Spring Harbor, NY: CSHL Press, 2017, p. 207–237. doi: 10.1101/cshperspect.a029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper-Kuhn CM, Winkler J, Kuhn HG. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J Neurosci Res 77: 155–165, 2004. doi: 10.1002/jnr.20116. [DOI] [PubMed] [Google Scholar]
- 26.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30: 464–472, 2007. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci USA 107: 2367–2372, 2010. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron 42: 535–552, 2004. doi: 10.1016/S0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 29.Diederich K, Bastl A, Wersching H, Teuber A, Strecker JK, Schmidt A, Minnerup J, Schäbitz WR. Effects of different exercise strategies and intensities on memory performance and neurogenesis. Front Behav Neurosci 11: 47, 2017. doi: 10.3389/fnbeh.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dieni CV, Chancey JH, Overstreet-Wadiche LS. Dynamic functions of GABA signaling during granule cell maturation. Front Neural Circuits 6: 113, 2013. doi: 10.3389/fncir.2012.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci 24: 1265–1276, 2006. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- 32.Du X, Zhang H, Parent JM. Rabies tracing of birthdated dentate granule cells in rat temporal lobe epilepsy. Ann Neurol 81: 790–803, 2017. doi: 10.1002/ana.24946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dumitru I, Neitz A, Alfonso J, Monyer H. Diazepam binding inhibitor promotes stem cell expansion controlling environment-dependent neurogenesis. Neuron 94: 125–137.e5, 2017. doi: 10.1016/j.neuron.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Duzel E, van Praag H, Sendtner M. Can physical exercise in old age improve memory and hippocampal function? Brain 139: 662–673, 2016. doi: 10.1093/brain/awv407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex 13: 845–851, 2003. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- 36.Encinas JM, Sierra A, Valcárcel-Martín R, Martín-Suárez S. A developmental perspective on adult hippocampal neurogenesis. Int J Dev Neurosci 31: 640–645, 2013. doi: 10.1016/j.ijdevneu.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 108: 3017–3022, 2011. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci 25: 10074–10086, 2005. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65: 7–19, 2010. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 124: 71–79, 2004. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 41.Fordyce DE, Farrar RP. Physical activity effects on hippocampal and parietal cortical cholinergic function and spatial learning in F344 rats. Behav Brain Res 43: 115–123, 1991. doi: 10.1016/S0166-4328(05)80061-0. [DOI] [PubMed] [Google Scholar]
- 42.França TFA, Bitencourt AM, Maximilla NR, Barros DM, Monserrat JM. Hippocampal neurogenesis and pattern separation: a meta-analysis of behavioral data. Hippocampus 27: 937–950, 2017. doi: 10.1002/hipo.22746. [DOI] [PubMed] [Google Scholar]
- 43.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates (3rd ed.). Cambridge, MA: Academic Press, 2007. [Google Scholar]
- 44.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439: 589–593, 2006. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54: 559–566, 2007. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomez-Merino D, Béquet F, Berthelot M, Chennaoui M, Guezennec CY. Site-dependent effects of an acute intensive exercise on extracellular 5-HT and 5-HIAA levels in rat brain. Neurosci Lett 301: 143–146, 2001. doi: 10.1016/S0304-3940(01)01626-3. [DOI] [PubMed] [Google Scholar]
- 47.Gonçalves JT, Bloyd CW, Shtrahman M, Johnston ST, Schafer ST, Parylak SL, Tran T, Chang T, Gage FH. In vivo imaging of dendritic pruning in dentate granule cells. Nat Neurosci 19: 788–791, 2016. doi: 10.1038/nn.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S, Fleshner M. Wheel running alters serotonin (5-HT) transporter, 5-HT1A, 5-HT1B, and alpha 1b-adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry 57: 559–568, 2005. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 49.Hill LE, Droste SK, Nutt DJ, Linthorst AC, Reul JM. Voluntary exercise alters GABA(A) receptor subunit and glutamic acid decarboxylase-67 gene expression in the rat forebrain. J Psychopharmacol 24: 745–756, 2010. doi: 10.1177/0269881108096983. [DOI] [PubMed] [Google Scholar]
- 50.Ho NF, Han SP, Dawe GS. Effect of voluntary running on adult hippocampal neurogenesis in cholinergic lesioned mice. BMC Neurosci 10: 57, 2009. doi: 10.1186/1471-2202-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hopkins ME, Nitecki R, Bucci DJ. Physical exercise during adolescence versus adulthood: differential effects on object recognition memory and brain-derived neurotrophic factor levels. Neuroscience 194: 84–94, 2011. doi: 10.1016/j.neuroscience.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hornberger M, Wong S, Tan R, Irish M, Piguet O, Kril J, Hodges JR, Halliday G. In vivo and post-mortem memory circuit integrity in frontotemporal dementia and Alzheimer’s disease. Brain 135: 3015–3025, 2012. doi: 10.1093/brain/aws239. [DOI] [PubMed] [Google Scholar]
- 53.Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science 225: 1168–1170, 1984. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 54.Itou Y, Nochi R, Kuribayashi H, Saito Y, Hisatsune T. Cholinergic activation of hippocampal neural stem cells in aged dentate gyrus. Hippocampus 21: 446–459, 2011. doi: 10.1002/hipo.20761. [DOI] [PubMed] [Google Scholar]
- 55.Jinno S. Topographic differences in adult neurogenesis in the mouse hippocampus: a stereology-based study using endogenous markers. Hippocampus 21: 467–480, 2011. doi: 10.1002/hipo.20762. [DOI] [PubMed] [Google Scholar]
- 56.Kaneko N, Okano H, Sawamoto K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells 11: 1145–1159, 2006. doi: 10.1111/j.1365-2443.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 57.Kannangara TS, Lucero MJ, Gil-Mohapel J, Drapala RJ, Simpson JM, Christie BR, van Praag H. Running reduces stress and enhances cell genesis in aged mice. Neurobiol Aging 32: 2279–2286, 2011. doi: 10.1016/j.neurobiolaging.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kesner RP, Rolls ET. A computational theory of hippocampal function, and tests of the theory: new developments. Neurosci Biobehav Rev 48: 92–147, 2015. doi: 10.1016/j.neubiorev.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 59.Kheirbek MA, Tannenholz L, Hen R. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci 32: 8696–8702, 2012. doi: 10.1523/JNEUROSCI.1692-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klempin F, Beis D, Mosienko V, Kempermann G, Bader M, Alenina N. Serotonin is required for exercise-induced adult hippocampal neurogenesis. J Neurosci 33: 8270–8275, 2013. doi: 10.1523/JNEUROSCI.5855-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kondo M, Shimada S. Serotonin and exercise-induced brain plasticity. Neurotransmitter 2: e793, 2015. 10.14800/nt.793. [DOI] [Google Scholar]
- 62.Kondo M, Nakamura Y, Ishida Y, Shimada S. The 5-HT3 receptor is essential for exercise-induced hippocampal neurogenesis and antidepressant effects. Mol Psychiatry 20: 1428–1437, 2015. doi: 10.1038/mp.2014.153. [DOI] [PubMed] [Google Scholar]
- 63.Kopelman MD. The Korsakoff syndrome. Br J Psychiatry 166: 154–173, 1995. doi: 10.1192/bjp.166.2.154. [DOI] [PubMed] [Google Scholar]
- 64.Kriegstein AR. GABA puts the brake on stem cells. Nat Neurosci 8: 1132–1133, 2005. doi: 10.1038/nn0905-1132. [DOI] [PubMed] [Google Scholar]
- 65.Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging 27: 1505–1513, 2006. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 66.Kumamoto N, Gu Y, Wang J, Janoschka S, Takemaru K, Levine J, Ge S. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat Neurosci 15: 399–405, 2012. doi: 10.1038/nn.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience 156: 456–465, 2008. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 68.Lee MC, Okamoto M, Liu YF, Inoue K, Matsui T, Nogami H, Soya H. Voluntary resistance running with short distance enhances spatial memory related to hippocampal BDNF signaling. J Appl Physiol (1985) 113: 1260–1266, 2012. doi: 10.1152/japplphysiol.00869.2012. [DOI] [PubMed] [Google Scholar]
- 69.Lee MC, Inoue K, Okamoto M, Liu YF, Matsui T, Yook JS, Soya H. Voluntary resistance running induces increased hippocampal neurogenesis in rats comparable to load-free running. Neurosci Lett 537: 6–10, 2013. doi: 10.1016/j.neulet.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 70.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315: 961–966, 2007. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 71.Li L, Sultan S, Heigele S, Schmidt-Salzmann C, Toni N, Bischofberger J. Silent synapses generate sparse and orthogonal action potential firing in adult-born hippocampal granule cells. eLife 6: e23612, 2017. doi: 10.7554/eLife.23612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu HL, Zhao G, Cai K, Zhao HH, Shi LD. Treadmill exercise prevents decline in spatial learning and memory in APP/PS1 transgenic mice through improvement of hippocampal long-term potentiation. Behav Brain Res 218: 308–314, 2011. doi: 10.1016/j.bbr.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 73.Lloyd BA, Hake HS, Ishiwata T, Farmer CE, Loetz EC, Fleshner M, Bland ST, Greenwood BN. Exercise increases mTOR signaling in brain regions involved in cognition and emotional behavior. Behav Brain Res 323: 56–67, 2017. doi: 10.1016/j.bbr.2017.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Götz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 6: 445–456, 2010. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 75.Luo L, Chen WH, Wang M, Zhu DM, She JQ, Ruan DY. Modulation of long-term potentiation by individual subtypes of muscarinic acetylcholine receptor in the rat dentate gyrus. Hippocampus 18: 989–995, 2008. doi: 10.1002/hipo.20461. [DOI] [PubMed] [Google Scholar]
- 76.Lynch MA. Long-term potentiation and memory. Physiol Rev 84: 87–136, 2004. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 77.Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci 27: 11442–11450, 2007. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marín-Burgin A, Mongiat LA, Pardi MB, Schinder AF. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science 335: 1238–1242, 2012. doi: 10.1126/science.1214956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marlatt MW, Potter MC, Lucassen PJ, van Praag H. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev Neurobiol 72: 943–952, 2012. doi: 10.1002/dneu.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci 27: 589–594, 2004. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 81.McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317: 94–99, 2007. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 82.Meeusen R, Piacentini MF, De Meirleir K. Brain microdialysis in exercise research. Sports Med 31: 965–983, 2001. doi: 10.2165/00007256-200131140-00002. [DOI] [PubMed] [Google Scholar]
- 83.Merritt JR, Rhodes JS. Mouse genetic differences in voluntary wheel running, adult hippocampal neurogenesis and learning on the multi-strain-adapted plus water maze. Behav Brain Res 280: 62–71, 2015. doi: 10.1016/j.bbr.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience 10: 1185–1201, 1983. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- 85.Mohapel P, Leanza G, Kokaia M, Lindvall O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol Aging 26: 939–946, 2005. doi: 10.1016/j.neurobiolaging.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 86.Molteni R, Ying Z, Gómez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci 16: 1107–1116, 2002. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- 87.Moon HY, Becke A, Berron D, Becker B, Sah N, Benoni G, Janke E, Lubejko ST, Greig NH, Mattison JA, Duzel E, van Praag H. Running-induced systemic cathepsin b secretion is associated with memory function. Cell Metab 24: 332–340, 2016. doi: 10.1016/j.cmet.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci USA 92: 9697–9701, 1995. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience 219: 62–71, 2012. doi: 10.1016/j.neuroscience.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Myers CE, Scharfman HE. Pattern separation in the dentate gyrus: a role for the CA3 backprojection. Hippocampus 21: 1190–1215, 2011. doi: 10.1002/hipo.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci 5: 361–372, 2004. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- 92.Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron 38: 305–315, 2003. doi: 10.1016/S0896-6273(03)00165-X. [DOI] [PubMed] [Google Scholar]
- 93.Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297: 211–218, 2002. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature 373: 109, 1995. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 95.Nokia MS, Lensu S, Ahtiainen JP, Johansson PP, Koch LG, Britton SL, Kainulainen H. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J Physiol 594: 1855–1873, 2016. doi: 10.1113/JP271552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O’Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res 176: 362–366, 2007. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 97.Overstreet Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol 94: 4528–4532, 2005. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- 98.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425: 479–494, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 99.Patten AR, Yau SY, Fontaine CJ, Meconi A, Wortman RC, Christie BR. The benefits of exercise on structural and functional plasticity in the rodent hippocampus of different disease models. Br Plast 1: 97–127, 2015. doi: 10.3233/BPL-150016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA 104: 5638–5643, 2007. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Petralia RS. Distribution of extrasynaptic NMDA receptors on neurons. Sci World J 2012: 267120, 2012. doi: 10.1100/2012/267120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Piatti VC, Davies-Sala MG, Espósito MS, Mongiat LA, Trinchero MF, Schinder AF. The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J Neurosci 31: 7715–7728, 2011. doi: 10.1523/JNEUROSCI.1380-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T Jr, Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci 117: 1006–1016, 2003. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- 104.Ruegsegger GN, Toedebusch RG, Will MJ, Booth FW. Mu opioid receptor modulation in the nucleus accumbens lowers voluntary wheel running in rats bred for high running motivation. Neuropharmacology 97: 171–181, 2015. doi: 10.1016/j.neuropharm.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 105.Sah N, Peterson B, Lubejko ST, Vivar C, van Praag H. Running reorganizes the circuitry of one-week-old adult-born hippocampal neurons. Sci Rep 7: 10903, 2017. doi: 10.1038/s41598-017-11268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472: 466–470, 2011. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429: 184–187, 2004. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 108.Schoenfeld TJ, Rada P, Pieruzzini PR, Hsueh B, Gould E. Physical exercise prevents stress-induced activation of granule neurons and enhances local inhibitory mechanisms in the dentate gyrus. J Neurosci 33: 7770–7777, 2013. doi: 10.1523/JNEUROSCI.5352-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. 1957. J Neuropsychiatry Clin Neurosci 12: 103–113, 2000. doi: 10.1176/jnp.12.1.103-a. [DOI] [PubMed] [Google Scholar]
- 110.Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron 5: 745–756, 1990. doi: 10.1016/0896-6273(90)90333-B. [DOI] [PubMed] [Google Scholar]
- 111.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol 85: 2423–2431, 2001. [DOI] [PubMed] [Google Scholar]
- 112.So JH, Huang C, Ge M, Cai G, Zhang L, Lu Y, Mu Y. Intense Exercise Promotes Adult Hippocampal Neurogenesis But Not Spatial Discrimination. Front Cell Neurosci 11: 13, 2017. doi: 10.3389/fncel.2017.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, Deisseroth K, Luscher B, Christian KM, Ming GL, Song H. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 489: 150–154, 2012. doi: 10.1038/nature11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Song J, Sun J, Moss J, Wen Z, Sun GJ, Hsu D, Zhong C, Davoudi H, Christian KM, Toni N, Ming GL, Song H. Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat Neurosci 16: 1728–1730, 2013. doi: 10.1038/nn.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Song NN, Jia YF, Zhang L, Zhang Q, Huang Y, Liu XZ, Hu L, Lan W, Chen L, Lesch KP, Chen X, Xu L, Ding YQ. Reducing central serotonin in adulthood promotes hippocampal neurogenesis. Sci Rep 6: 20338, 2016. doi: 10.1038/srep20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Steib K, Schäffner I, Jagasia R, Ebert B, Lie DC. Mitochondria modify exercise-induced development of stem cell-derived neurons in the adult brain. J Neurosci 34: 6624–6633, 2014. doi: 10.1523/JNEUROSCI.4972-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus 17: 1017–1022, 2007. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suwabe K, Hyodo K, Byun K, Ochi G, Yassa MA, Soya H. Acute moderate exercise improves mnemonic discrimination in young adults. Hippocampus 27: 229–234, 2017. doi: 10.1002/hipo.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Szabo GG, Du X, Oijala M, Varga C, Parent JM, Soltesz I. Extended interneuronal network of the dentate gyrus. Cell Reports 20: 1262–1268, 2017. doi: 10.1016/j.celrep.2017.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature 401: 63–69, 1999. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 121.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 442: 929–933, 2006. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 122.Teles-Grilo Ruivo LM, Mellor JR. Cholinergic modulation of hippocampal network function. Front Synaptic Neurosci 5: 2, 2013. doi: 10.3389/fnsyn.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Temprana SG, Mongiat LA, Yang SM, Trinchero MF, Alvarez DD, Kropff E, Giacomini D, Beltramone N, Lanuza GM, Schinder AF. Delayed coupling to feedback inhibition during a critical period for the integration of adult-born granule cells. Neuron 85: 116–130, 2015. doi: 10.1016/j.neuron.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thinschmidt JS, Kinney GG, Kocsis B. The supramammillary nucleus: is it necessary for the mediation of hippocampal theta rhythm? Neuroscience 67: 301–312, 1995. doi: 10.1016/0306-4522(95)00045-K. [DOI] [PubMed] [Google Scholar]
- 125.Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47: 803–815, 2005. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 126.Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus 2: 189–199, 1992. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- 127.Uda M, Ishido M, Kami K, Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Res 1104: 64–72, 2006. doi: 10.1016/j.brainres.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 128.Van der Borght K, Havekes R, Bos T, Eggen BJ, Van der Zee EA. Exercise improves memory acquisition and retrieval in the Y-maze task: relationship with hippocampal neurogenesis. Behav Neurosci 121: 324–334, 2007. doi: 10.1037/0735-7044.121.2.324. [DOI] [PubMed] [Google Scholar]
- 129.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature 415: 1030–1034, 2002. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 25: 8680–8685, 2005. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med 10: 128–140, 2008. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- 132.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2: 266–270, 1999a. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 133.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 96: 13427–13431, 1999b. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vasuta C, Caunt C, James R, Samadi S, Schibuk E, Kannangara T, Titterness AK, Christie BR. Effects of exercise on NMDA receptor subunit contributions to bidirectional synaptic plasticity in the mouse dentate gyrus. Hippocampus 17: 1201–1208, 2007. doi: 10.1002/hipo.20349. [DOI] [PubMed] [Google Scholar]
- 135.Vertes RP. Major diencephalic inputs to the hippocampus: supramammillary nucleus and nucleus reuniens. Circuitry and function. Prog Brain Res 219: 121–144, 2015. doi: 10.1016/bs.pbr.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vertes RP, Hoover WB, Viana Di Prisco G. Theta rhythm of the hippocampus: subcortical control and functional significance. Behav Cogn Neurosci Rev 3: 173–200, 2004. doi: 10.1177/1534582304273594. [DOI] [PubMed] [Google Scholar]
- 137.Vivar C, Potter MC, Choi J, Lee JY, Stringer TP, Callaway EM, Gage FH, Suh H, van Praag H. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun 3: 1107, 2012. doi: 10.1038/ncomms2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vivar C, Potter MC, van Praag H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci 15: 189–210, 2013. doi: 10.1007/7854_2012_220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vivar C, Peterson BD, van Praag H. Running rewires the neuronal network of adult-born dentate granule cells. Neuroimage 131: 29–41, 2016. doi: 10.1016/j.neuroimage.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vivar C, van Praag H. Functional circuits of new neurons in the dentate gyrus. Front Neural Circuits 7: 15, 2013. doi: 10.3389/fncir.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci 17: 525–544, 2013. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang J, Chen X, Zhang N, Ma Q. Effects of exercise on stress-induced changes of norepinephrine and serotonin in rat hippocampus. Chin J Physiol 56: 245–252, 2013. doi: 10.4077/CJP.2013.BAB097. [DOI] [PubMed] [Google Scholar]
- 143.Welsby PJ, Rowan MJ, Anwyl R. Intracellular mechanisms underlying the nicotinic enhancement of LTP in the rat dentate gyrus. Eur J Neurosci 29: 65–75, 2009. doi: 10.1111/j.1460-9568.2008.06562.x. [DOI] [PubMed] [Google Scholar]
- 144.Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53: 639–647, 2007. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Witter MP. The perforant path: projections from the entorhinal cortex to the dentate gyrus. Prog Brain Res 163: 43–61, 2007. doi: 10.1016/S0079-6123(07)63003-9. [DOI] [PubMed] [Google Scholar]
- 146.Wu CW, Chang YT, Yu L, Chen HI, Jen CJ, Wu SY, Lo CP, Kuo YM. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J Appl Physiol (1985) 105: 1585–1594, 2008. doi: 10.1152/japplphysiol.90775.2008. [DOI] [PubMed] [Google Scholar]
- 147.Wu MV, Luna VM, Hen R. Running rescues a fear-based contextual discrimination deficit in aged mice. Front Syst Neurosci 9: 114, 2015. doi: 10.3389/fnsys.2015.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao C, Teng EM, Summers RG Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 26: 3–11, 2006. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao C, Jou J, Wolff LJ, Sun H, Gage FH. Spine morphogenesis in newborn granule cells is differentially regulated in the outer and middle molecular layers. J Comp Neurol 522: 2756–2766, 2014. doi: 10.1002/cne.23581. [DOI] [PMC free article] [PubMed] [Google Scholar]