Abstract
Background
The genetic cause of primary immunodeficiency disease (PID) carries prognostic information.
Objective
We conducted a whole-genome sequencing study assessing a large proportion of the NIHR BioResource–Rare Diseases cohort.
Methods
In the predominantly European study population of principally sporadic unrelated PID cases (n = 846), a novel Bayesian method identified nuclear factor κB subunit 1 (NFKB1) as one of the genes most strongly associated with PID, and the association was explained by 16 novel heterozygous truncating, missense, and gene deletion variants. This accounted for 4% of common variable immunodeficiency (CVID) cases (n = 390) in the cohort. Amino acid substitutions predicted to be pathogenic were assessed by means of analysis of structural protein data. Immunophenotyping, immunoblotting, and ex vivo stimulation of lymphocytes determined the functional effects of these variants. Detailed clinical and pedigree information was collected for genotype-phenotype cosegregation analyses.
Results
Both sporadic and familial cases demonstrated evidence of the noninfective complications of CVID, including massive lymphadenopathy (24%), unexplained splenomegaly (48%), and autoimmune disease (48%), features prior studies correlated with worse clinical prognosis. Although partial penetrance of clinical symptoms was noted in certain pedigrees, all carriers have a deficiency in B-lymphocyte differentiation. Detailed assessment of B-lymphocyte numbers, phenotype, and function identifies the presence of an increased CD21low B-cell population. Combined with identification of the disease-causing variant, this distinguishes between healthy subjects, asymptomatic carriers, and clinically affected cases.
Conclusion
We show that heterozygous loss-of-function variants in NFKB1 are the most common known monogenic cause of CVID, which results in a temporally progressive defect in the formation of immunoglobulin-producing B cells.
Key words: B cells, common variable immunodeficiency, nuclear factor κB1
Abbreviations used: CFSE, Carboxyfluorescein succinimidyl ester; CVID, Common variable immunodeficiency; LOF, Loss-of-function; NF-κB, Nuclear factor κB; NFKB1, Nuclear factor κB subunit 1; NIHRBR-RD, NIHR BioResource–Rare Diseases; PID, Primary immunodeficiency disease; PML, Progressive multifocal leukoencephalopathy; RHD, Rel homology domain; VEP, Variant effect predictor
Graphical abstract
Common variable immunodeficiency (CVID; MIM 607594), which occurs in approximately 1:25,000 persons1, 2, 3 is a clinically and genetically heterogeneous disorder characterized by susceptibility to sinopulmonary infections, hypogammaglobulinemia, and poor vaccine responses. CVID is the most common primary immune deficiency, requiring lifelong clinical follow-up, and the clinical course is highly variable, with substantial excess mortality. Affected subjects present frequently with recurrent respiratory tract infections, as well as immune dysregulatory features. The antibody deficiency is often not as marked as the agammaglobulinemia seen in patients with genetically defined conditions leading to B-lymphocyte aplasia.4, 5 Conversely, although patients with B-lymphocyte aplasia have a favorable prognosis on adequate replacement immunoglobulin treatment, the response of patients with CVID is highly variable.
Past studies focused on familial cases with CVID and used techniques ranging from traditional linkage analysis to more recent exome sequencing to characterize the genetic cause. This has revealed that monogenic gene dysfunction accounts for 10% of cases.4, 5 Several of the variants in these genes have been characterized as partially penetrant; it remains unclear whether genetic or environmental factors determine disease onset. Multiple recent studies identified variants in nuclear factor κB subunit 1 (NFKB1) as a monogenic cause of CVID and reported on the clinical features of these cases.6, 7, 8, 9, 10, 11
As part of this NIHR BioResource–Rare Diseases study, we sequenced the genomes of 846 unrelated patients with predominantly sporadic primary immunodeficiency disease (PID) who were recruited from across the United Kingdom and by European collaborators. Application of the recently developed statistical method BeviMed12 to the 846 PID cases and more than 5000 control genomes identified NFKB1 as the gene with the highest probability of association with the disease and with the largest number of cases explained by variants in that gene. Further investigations revealed a series of 16 heterozygous loss-of-function (LOF) variants in NFKB1 as the most common genetic cause of CVID.
Mutations in genes that affect nuclear factor κB (NF-κB)–dependent signaling are associated with a number of immunodeficiencies.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 NF-κB is a ubiquitous transcription factor member of the Rel proto-oncogene family. NF-κB regulates the expression of several genes involved in inflammatory and immune responses. The classical activated form of NF-κB consists of a heterodimer of the p50/p65 protein subunits. The NF-κB family of transcription factors comprises 5 related proteins, c-Rel, p65 (RelA), RelB, p50 (NF-κB1) and p52 (NF-κB2), which interact to form homodimers and heterodimers with distinct gene regulatory functions.13, 27, 28, 29 Each Rel NF-κB protein has a conserved 300-amino-acid N-terminal Rel homology domain (RHD) that encompasses sequences needed by NF-κB proteins to bind DNA motifs (κB elements), form dimers, interact with regulatory inhibitor IκB proteins, and enter the nucleus. The 10 different NF-κB dimers identified have distinct transcriptional properties.28 In most cells NF-κB is retained in the cytosol in a latent state through interaction with the IκB proteins (eg, IκBα, IκBβ, and IκBε), a family of proteins with ankyrin repeats that mediate IκB interaction with the RHD of NF-κB, masking the nuclear localization sequence and DNA-binding domains. Signal-dependent activation of an IκB kinase complex comprising catalytic (α and β) and regulatory (NF-κB essential modulator) subunits induces the phosphorylation and degradation of IκB,29 which permits NF-κB factors to enter the nucleus and regulate gene expression.
We show that variants in NFKB1 culminate in a progressive humoral immunodeficiency indistinguishable from CVID, with a highly variable penetrance. We demonstrate the utility of an in silico protein prediction model for validating the predicted disease-causing substitutions, and we report on the clinical spectrum, immunologic phenotype, and functional consequences of heterozygous NFKB1 variants.
Methods
Cohort
The NIHR BioResource–Rare Diseases (NIHRBR-RD) study was established in the United Kingdom to further the clinical management of patients with rare diseases by providing a national resource of whole-genome sequence data. All participants provided written informed consent, and the study was approved by the East of England–Cambridge South national institutional review board (13/EE/0325). At the time of our analysis, the NIHRBR-RD study included whole-genome sequence data from 8066 subjects, of whom 1299 were part of the PID cohort. These were predominantly singleton cases, but additional affected and/or unaffected family members of some of the patients were also sequenced; in total, there were 846 unrelated index cases.
Patients with PIDs were recruited by specialists in clinical immunology (either trained in pediatrics or internal medicine) from 26 hospitals in the United Kingdom and a smaller number came from The Netherlands, France, and Italy. The recruitment criteria included the following: clinical diagnosis of CVID according to the European Society for Immunodeficiencies ESID criteria (ESID Registry–Working Definitions for Clinical Diagnosis of PID, 2014, latest version: April 25, 2017), extreme autoimmunity, or recurrent (and/or unusual) infections suggestive of severely defective innate or cell-mediated immunity. Exclusion of known causes of PID was encouraged, and some of the patients were screened for 1 or more PID genes before enrollment in the PID cohort. The ethnic makeup of the study cohort represented that of the general United Kingdom population: 82% were European, 6% were Asian, 2% were African, and 10% were of mixed ethnicity based on the patients’ whole-genome data.
Given that PID is a heterogeneous disease, with overlap in phenotypes and genetic causes across different diagnostic categories, we decided to perform an unbiased genetic analysis of all 846 unrelated index cases. Whole-genome sequence data were additionally available for 63 affected and 345 unaffected relatives. Within a broad range of phenotypes, CVID is the most common disease category, comprising 46% of the NIHRBR-RD PID cohort (n = 390 index cases; range, 0-93 years of age).
Sequencing and variant filtering
Whole-genome sequencing of paired-end reads was performed by Illumina on their HiSeq X Ten system (Illumina, San Diego, Calif). Reads of 100, 125, or 150 bp in length were aligned to the GRCh37 genome build by using the Isaac aligner, variants across the samples were jointly called with the AGG tool, and large deletions were identified by using Canvas and Manta algorithms (all software by Illumina), as described previously.30 Average read depth was 35, with 95% of the genome covered by at least 20 reads.
Single nucleotide variants and small insertions/deletions were filtered based on the following criteria: passing standard Illumina quality filters in greater than 80% of the genomes sequenced by the NIHRBR-RD study, having a variant effect predictor (VEP)31 effect of either moderate or high, having a minor allele frequency less than 0.001 in the Exome Aggregation Consortium data set, and having a minor allele frequency of less than 0.01 in the NIHRBR-RD cohort. Large deletions called by both Canvas and Manta algorithms, passing standard Illumina quality filters, overlapping at least 1 exon absent from control data sets,32 and having a frequency of less than 0.01 in the NIHRBR-RD genomes were included in the analysis.
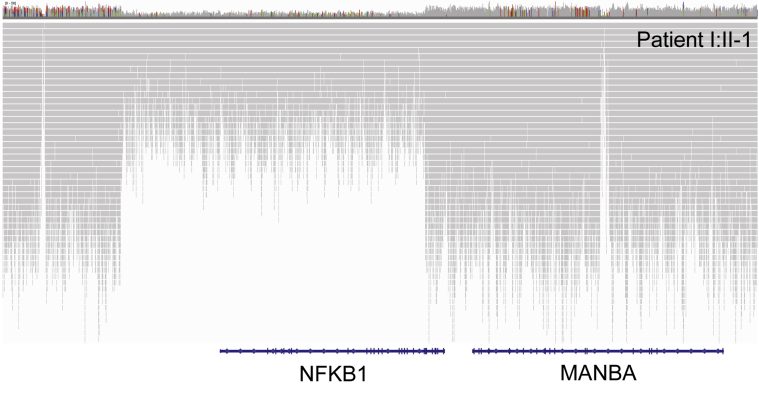
All variants reported as disease causing in this study were confirmed by using Sanger sequencing with standard protocols. Large deletions were inspected in the Integrative Genomics Viewer plot (see Fig E1 in this article's Online Repository at www.jacionline.org), and breakpoints were confirmed by sequencing the PCR products spanning each deletion.
Gene and variant pathogenicity estimation
To evaluate genes for their association with PID, we applied the BeviMed inference procedure12 to the NIHRBR-RD whole-genome data set. BeviMed (https://CRAN.R-project.org/package=BeviMed) evaluates the evidence for association between case/control status of unrelated subjects and allele counts at rare variant sites in a given locus. The method infers the posterior probabilities of association under dominant and recessive inheritance and, conditional on such an association, the posterior probability of pathogenicity of each considered variant in the locus. BeviMed was applied to rare variants and large rare deletions in each gene, treating the 846 unrelated PID index cases as cases and the 5097 unrelated subjects from the rest of the NIHRBR-RD cohort as control subjects. All genes were assigned the same prior probability of association with the disease of .01, regardless of their previously published associations with an immune deficiency phenotype. Variants with a VEP effect labeled as high were assigned higher prior probabilities of pathogenicity than variants with a moderate effect, as described previously.12
Immunophenotyping and B- and T-cell functional assays
PBMCs were isolated by using standard density gradient centrifugation techniques with Lymphoprep (Nycomed, Oslo, Norway). Absolute numbers of lymphocytes, T cells, B cells, and natural killer cells were determined with Multitest 6-color reagents (BD Biosciences, San Jose, Calif), according to the manufacturer's instructions. For PBMC immunophenotyping, we refer to the Methods section in this article's Online Repository at www.jacionline.org.
PBMCs were resuspended in PBS at a concentration of 5 to 10 × 106 cells/mL and labeled with 0.5 μmol/L carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, Ore), as described previously33 and in the Methods section in this article's Online Repository, to analyze the ex vivo activation of T and B cells. Proliferation of B and T cells was assessed by measuring CFSE dilution in combination with the same mAbs used for immunophenotyping. Analysis of cells was performed with a FACSCanto II flow cytometer (BD Biosciences) and FlowJo software (TreeStar, Ashland, Ore). Patient samples were analyzed simultaneously with PBMCs from healthy control subjects.
ELISA
Secretion of immunoglobulins by mature B cells was assessed by testing supernatants for secreted IgM, IgG, and IgA with an in-house ELISA using polyclonal rabbit anti-human IgM, IgG, and IgA reagents and a serum protein calibrator, all from Dako (Glostrup, Denmark), as described previously.33
SDS-PAGE and Western blot analysis
Blood was separated into neutrophils and PBMCs. Neutrophils (5 × 106) were used for protein lysates, separated by means of SDS-PAGE, and transferred onto a nitrocellulose membrane. Individual proteins were detected with antibodies against NF-κB p50 (mouse mAb E-10; Santa Cruz Biotechnology, Dallas, Tex), against IκBα (rabbit polyclonal antiserum C-21; Santa Cruz Biotechnology), and against human glyceraldehyde-3-phosphate dehydrogenase (mouse mAb; Merck Millipore, Darmstadt, Germany).
Secondary antibodies were either goat anti-mouse-IgG IRDye 800CW, goat anti-rabbit IgG IRDye 680CW or goat anti-mouse IgG IRDye 680LT (LI-COR Biosciences, Lincoln, Neb). Relative fluorescence quantification of bound secondary antibodies was performed on an Odyssey Infrared Imaging system (LI-COR Biosciences) and normalized to glyceraldehyde-3-phosphate dehydrogenase.
NF-κB1 protein structure
A previously resolved crystal structure of the p50 homodimer (A43-K353) bound to DNA was used to gain structural information on the NF-κB1 RHD.34 Ankyrin repeats of NF-κB1 (Q498-D802) were modeled by using comparative homology modeling (Modeller 9v16) with the ankyrin repeats crystal structure of NF-κB2 as a template.35, 36 There is no structural information on the region between the sixth and seventh ankyrin repeats (F751-V771),36 and therefore these were omitted in the model.
Statistical analysis of lymphocyte data
Differences between groups with 1 variable were calculated with a nonpaired Student t test or 1-way ANOVA with the Bonferroni post hoc test, differences between groups with 2 or more variables were calculated with 2-way ANOVA with the Bonferroni post hoc test by using GraphPad Prism 6 software (GraphPad Software, La Jolla, Calif). A P value of less than .05 was considered significant.
Results
Pathogenic variants in NFKB1 are the most common monogenic cause of CVID
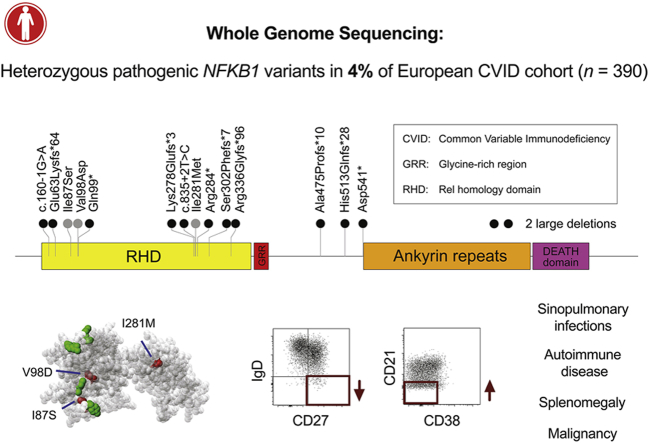
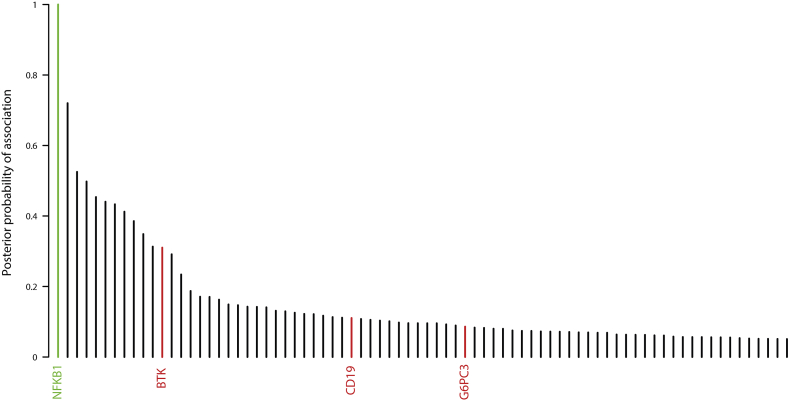
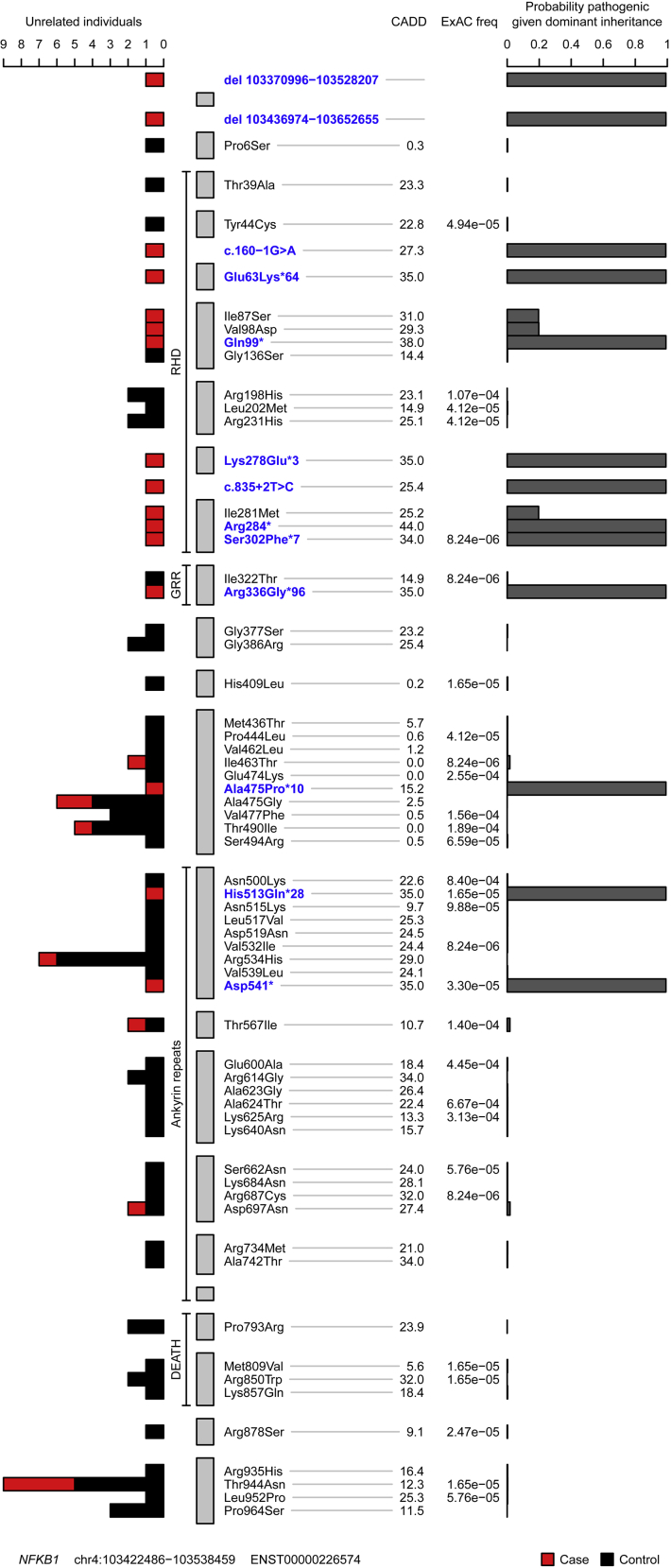
In an unbiased approach to analysis, we obtained BeviMed posterior probabilities of association with PID for every individual gene in all 848 unrelated patients with PID in the NIHRBR-RD study. Genes with posterior probabilities of greater than .05 are shown in Fig 1, showing that NFKB1 has the strongest prediction of association with disease status (1.000). All 13 high-effect variants (large deletion, nonsense, frameshift, and splice site variants) in NFKB1 were observed in cases only, resulting in the very high posterior probabilities of pathogenicity (mean, 0.99) for this class of variants (Fig 2). On the other hand, moderate-effect variants (missense substitutions) were observed both in cases and control subjects. The majority had near-zero probability of pathogenicity, but 3 substitutions were observed in the patients with PID only and had posterior probabilities of greater than 0.15 (Fig 2), suggesting their potential involvement in the disease. Genomic variants with a high Combined Annotation Dependent Depletion score were found within both the PID and control cohorts, suggesting that this commonly used metric of variant deleteriousness cannot reliably distinguish disease-causing from benign variants in NFKB1. All 16 predicted likely pathogenic variants were private to the PID cohort, and further investigation revealed that all 16 subjects were within the diagnostic criteria of CVID (Table I).
Fig 1.
Overall BeviMed results showing that NFKB1 has the highest posterior probability of association with disease in the NIHRBR-RD PID cohort. Genes with variants previously reported to cause PIDs are highlighted in red. Genes with posterior probabilities of greater than .05 are shown.
Fig 2.
Plot of rare missense, truncating, and gene deletion NFKB1 variants identified in the NIHRBR-RD genomes of unrelated subjects and their location relative to NFKB1 domains. Tracks from left to right show the following: number of unrelated case (red) and control (black) subjects in whom each variant was observed; the 4 major NFKB1 domains; each exon in transcript ENST00000226574 (gray bars); variant annotation relative to transcript ENST00000226574 and genomic location of large deletions, with VEP high-effect variants and large deletions highlighted in blue; Combined Annotation Dependent Depletion (CADD) scores of all nonsense, frameshift, splice and missense variants; Exome Aggregation Consortium (ExAC) allele frequencies; and conditional probability of variant pathogenicity inferred by using BeviMed. Only variants labeled as being of moderate or high effect relative to the canonical transcript ENST00000226574 are shown. The initial inference that formed part of the genome-wide analysis included variant chr4:103423325G>A, which was observed in 1 control sample. This variant is intronic (low effect) relative to ENST00000226574 but is a splice variant (high effect) relative to the minor transcript ENST00000505458. Because variants were filtered based on the highest-effect variant annotation against any Ensembl transcript, this variant was originally included in the inference. For this plot, the inference was rerun, including only missense, truncating, and gene deletion variants relative to the canonical transcript.
Table I.
Summary of the CVID patients' clinical presentation and NFKB1 variants
| Case ID | Sporadic/familial | Infections | Autoimmunity | Malignancy | Chromosome 4 position (GRCh37) | Nucleotide change | Type of variant | cDNA (NM_003998.3); Amino acid |
|---|---|---|---|---|---|---|---|---|
| A | Familial | ● | 103504037 | C>T | Nonsense | c.850C>T;Arg284* | ||
| B | Familial | ● | ● | ○ | 103518717 | delCATGC | Frameshift | c.1539_1543del; His513Glnfs*28 |
| C | Familial | ● | ● | ●○ | 103459014 | G>A | Splice acceptor | c.160-1G>A;? |
| D | Familial | ● | ● | 103518801 | delGA | Nonsense | c.1621_1622del; Asp541* | |
| E | Sporadic | ● | ● | ○ | 103504030 | C>G | Missense | c.843C>G; Ile281Met |
| F | Sporadic | ● | ● | 103488178 | T>A | Missense | c.293T>A; Val98Asp | |
| G | Sporadic | ● | 103488145 | T>G | Missense | c.260T>G; Ile87Ser | ||
| H | Familial | ● | ● | 103501798 | T>C | Splice donor | c.835+2T>C;? | |
| I | Sporadic | ● | 103370996-103528207 | — | Large deletion | — | ||
| J | Familial | ● | 103517415 | delG | Frameshift | c.1423del; Ala475Profs*10 | ||
| K | Sporadic | ● | ● | ● | 103436974-103652655 | — | Large deletion | — |
| L | Familial | ● | ● | 103459041 | delG | Frameshift | c.187del; Glu63Lysfs*64 | |
| M | Sporadic | ● | ● | 103501790 | insA | Frameshift | c.830dup; Lys278Glufs*3 | |
| N | Sporadic | ● | ● | ● | 103504086 | insT | Frameshift | c.904dup; Ser302Phefs*7 |
| O | Sporadic | ● | 103488180 | C>T | Nonsense | c.295C>T; Gln99* | ||
| P | Sporadic | ● | ● | 103505914 | delG | Frameshift | c.1005del; Arg336Glyfs*96 |
●, Presence of symptoms in index patient; ○, presence of symptoms in family member of index patient.
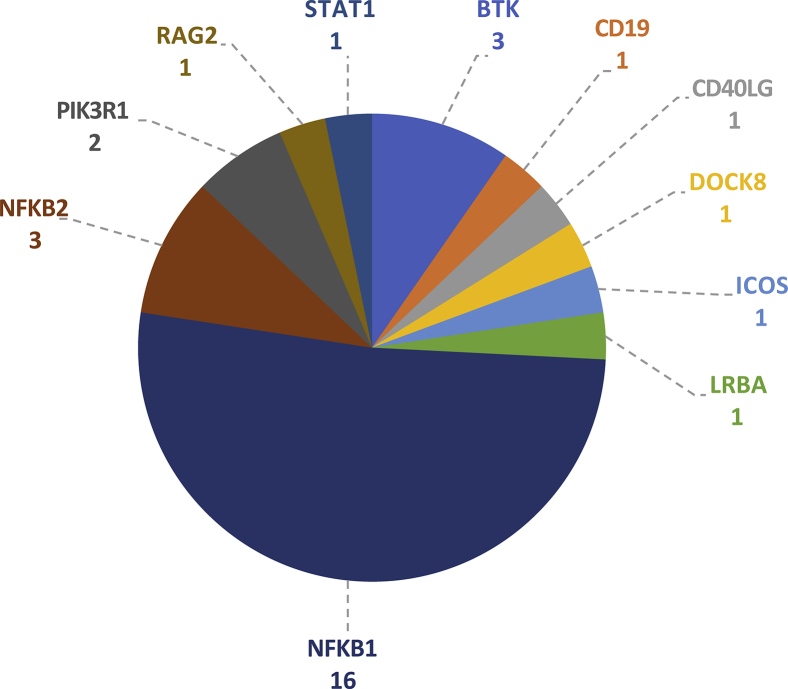
Assessment of all 390 CVID cases in our cohort for pathogenic variants showed that the next most commonly implicated genes are NKFB2 and Bruton tyrosine kinase (BTK), with 3 explained cases each (see Fig E2 in this article's Online Repository at www.jacionline.org). Importantly, based on the gnomAD data set of 135,000 predominantly healthy subjects, none of the NFKB1 variants reported here are observed in a single gnomAD subject, even though 90% of our CVID cohort and all of the NFKB1-positive cases had European ancestry. Therefore our results suggest that LOF variants in NFKB1 are the most commonly identified monogenic cause of CVID in the European population, with 16 of 390 patients with CVID explaining up to 4.1% of our cohort. None of the variants identified here had been reported in the previously described NFKB1 cases.6, 7, 8, 9, 10, 11
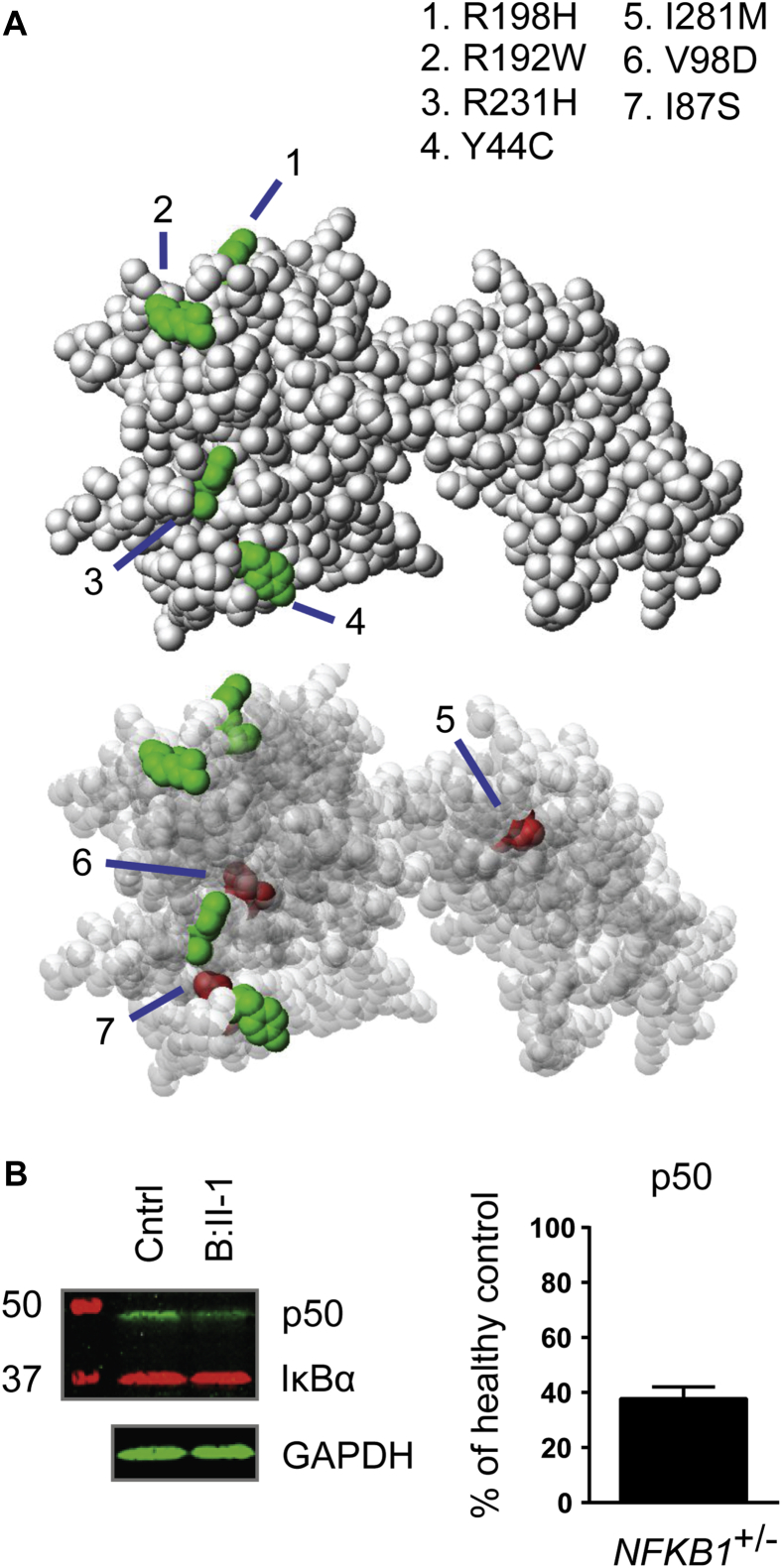
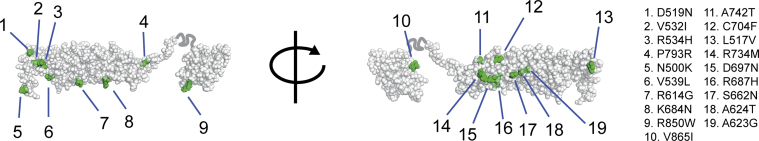
The NFKB1 gene encodes the p105 protein, which is processed to produce the active DNA-binding p50 subunit.13 The 16 potentially pathogenic variants we identified were all located in the N-terminal p50 part of the protein (Fig 2). The effects of the 3 rare substitutions on NF-κB1 structure were less clear than those of the truncating and gene deletion variants, and therefore we assessed their position in the crystal structure of the p50 protein. Their location in the inner core of the RHD (Fig 3, A) suggested a potential effect on protein stability, whereas other rare substitutions in the NIHRBR-RD cohort were found in locations less likely to affect this (Figs 2 and 3, A, and see Fig E3 in this article's Online Repository at www.jacionline.org).
Fig 3.
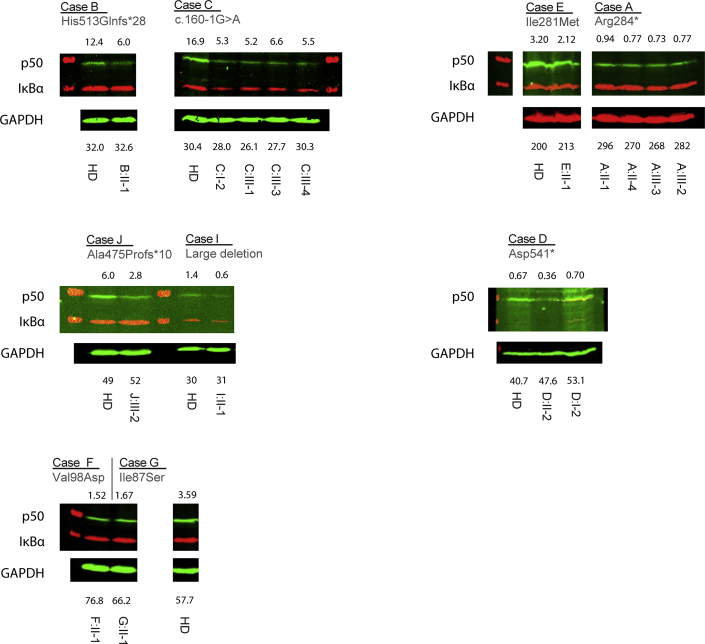
NFKB1 LOF variants lead to haploinsufficiency of the p50 protein. A, Localization of RHD substitutions with a high Combined Annotation Dependent Depletion (CADD) score (>20) within the structure of the NF-κB p50 monomer. Shown is a solid (top panel) and a transparent (bottom panel) sphere representation of the NF-κB p50 monomer. Perturbed residues indicated in green were observed in a control data set and are located on the outside of the structure, whereas residues shown in red were perturbed exclusively in the PID cohort and are buried inside the structure. B, Western blot analysis targeting p50, IκBα, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) of NFKB1 variant carriers. Left, Representative blot of a healthy control subject and patient B-II:1; right, summary of 16 NFKB1 variant carriers showing haploinsufficiency expressed as a percentage of healthy control subjects on the same blot corrected for GAPDH (mean ± SEM).
NFKB1 LOF as the disease mechanism
Twelve patients with truncating variants (Arg284*, His513Glnfs*28, c.160-1G>A, and Asp451*), 1 patient with gene deletion (del 103370996-103528207), and 3 patients with putative protein destabilizing missense variants (Ile281Met, Val98Asp, and Ile87Ser) were investigated for evidence of reduced protein level. Assessment of the NF-κB1 protein level in PBMCs or neutrophils in 9 index cases and 7 NFKB1 variant–carrying relatives demonstrated a reduction in all subjects (Fig 3, B, and see Fig E4 in this article's Online Repository at www.jacionline.org). Relative fluorescence quantification of the bands confirmed this and demonstrated a protein level of 38% ± 4.3% (mean ± SEM) compared with healthy control subjects. There was no difference between clinically affected and clinically unaffected subjects (36% ± 4.4% vs 42% ± 10.1%, n = 11 vs n = 5, P = .50). Our observations indicate that the pathogenic NFKB1 variants result in LOF of the NF-κB1 p50 subunit because reduction in protein levels was seen in all carriers regardless of their clinical phenotype and was absent in family members who were noncarriers.
Variable disease manifestations in NFKB1 LOF subjects
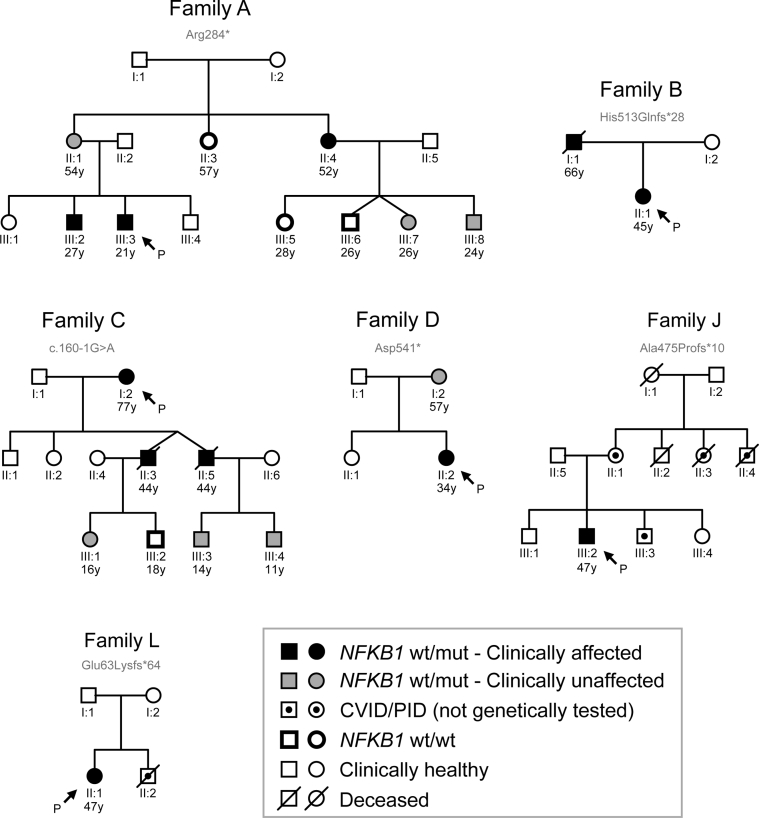
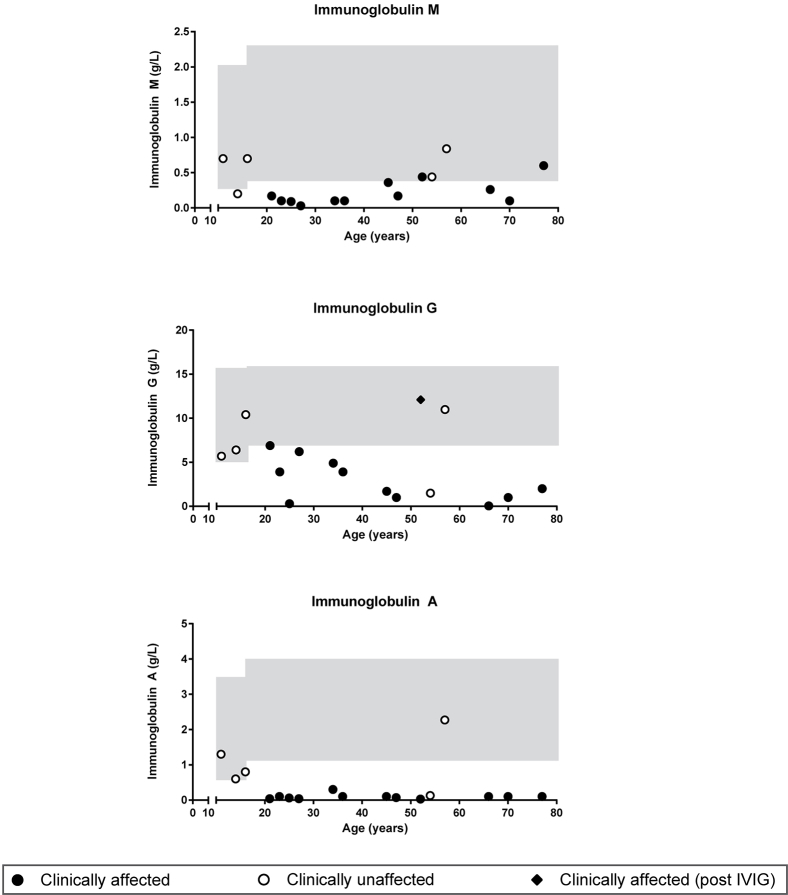
Seven subjects had evidence of familial disease (Table I), prompting us to investigate genotype-phenotype cosegregation and disease penetrance in cases for which pedigree information and additional family members were available (Fig 4 and see Tables E1 to E3 in this article's Online Repository at www.jacionline.org). The age at which hypogammaglobulinemia becomes clinically overt is highly variable (see Fig E5 in this article's Online Repository at www.jacionline.org), as shown by pedigree C in which grandchildren carrying the c.160-1G>A splice-site variant had IgG subclass deficiency (C:III-3 and C:III-4), in one case combined with an IgA deficiency (C:III-3). Although not yet overtly immunodeficient, the clinical courses of their fathers (C:II-3 and C:II-5) and grandmother (C:I-2) predict this potential outcome and warrant long-term clinical follow-up of these children.
Fig 4.
Pedigrees of familial NFKB1 cases. Six affected families for which pedigree information and additional family members were available. P, Proband/index cases.
We also observed variants in subjects who were clinically asymptomatic. Pedigree A highlights variable disease penetrance: the healthy mother (A:II-1) carries the same Arg284* variant as 2 of her clinically affected children (A:III-2 and A:III-3). Identification of this nonsense variant prompted clinical assessment of the extended kindred and demonstrated that her sister (A:II-4) had recurrent sinopulmonary disease and nasal polyps with serum hypogammaglobulinemia consistent with a CVID diagnosis. Overall, based on the clinical symptoms observed at the time of this study across 6 pedigrees, the penetrance of NFKB1 variants with respect to the clinical manifestation of CVID is incomplete (about 60% in our cohort, 11 affected subjects among 18 variant carriers), with varied expressivity not only of age at disease onset but also of specific disease manifestations, even within the same pedigree.
The clinical disease observed among the NFKB1 variant carriers is characteristic of progressive antibody deficiency associated with recurrent sinopulmonary infections (100% of clinically affected subjects) by encapsulated microbes, such as Streptococcus pneumoniae and Haemophilus influenzae (see Table E1). The clinical spectrum of NFKB1 LOF includes massive lymphadenopathy (24%), unexplained splenomegaly (48%), and autoimmune disease (48%), either organ-specific and/or hematologic in nature (mainly autoimmune hemolytic anemia and idiopathic thrombocytopenic purpura, see Tables E1 and E2). The percentage of autoimmune complications is based on the presence of autoimmune cytopenias (autoimmune hemolytic anemia, idiopathic thrombocytopenic purpura [<50-75 × 106/mL], autoimmune neutropenia, and Evans syndrome), alopecia areata/totalis, vitiligo, and Hashimoto thyroiditis among the clinically affected cases. Granulomatous-lymphocytic interstitial lung disease and splenomegaly were considered lymphoproliferation. Enteropathy, liver disease, colitis, and a mild decrease in platelet count (>100 × 106/mL) were neither included in those calculations nor scored separately. Histologic assessment of liver disease found in 3 patients showed no evidence of autoimmune or granulomatous liver disease, although fibrosis and cirrhosis were observed in these male patients. Finally, the number of oncological manifestations, predominantly hematologic, was noticeable. There were 2 cases with solid tumors (parathyroid adenoma and breast cancer) and 4 cases with hematologic malignancies (B-cell non-Hodgkin lymphoma, diffuse large B-cell lymphoma, follicular lymphoma, and peripheral T-cell lymphoma), which add up to 6 (28.6%) of 21 cases.
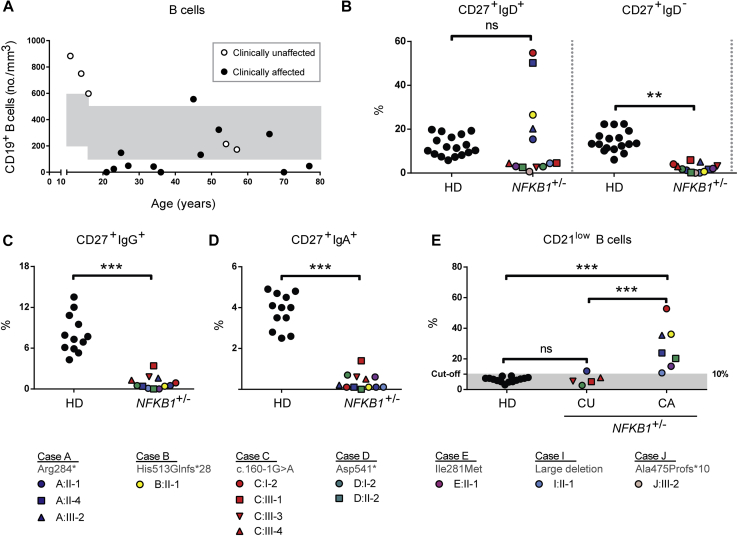
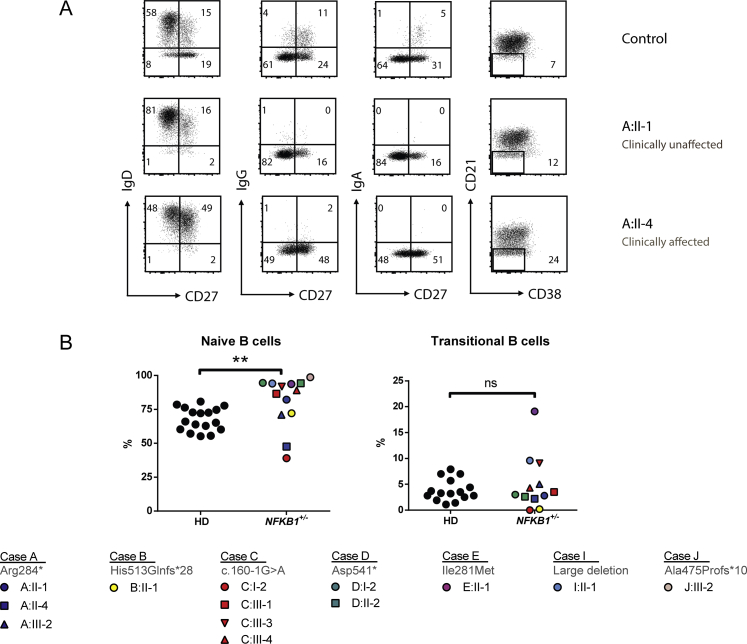
B-cell phenotype in subjects with NFKB1 LOF mutations and immune cell activation
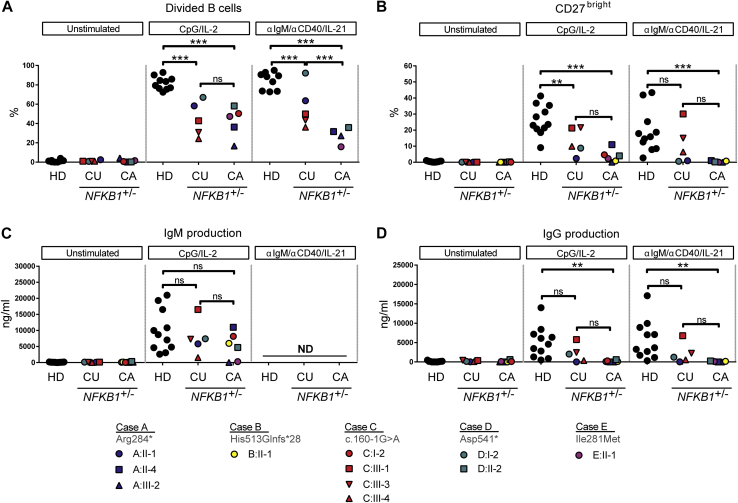
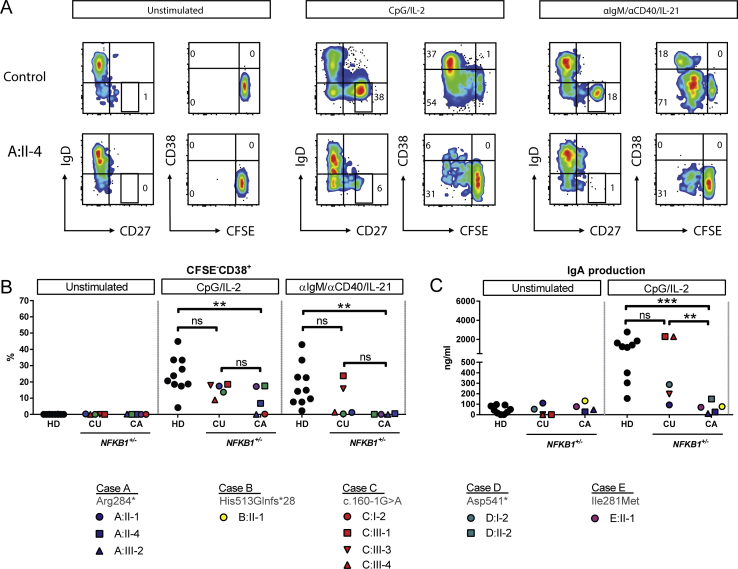
Index cases and family members carrying NFKB1 variants were approached for repeat venipuncture for further functional assessment. In clinically affected subjects, B-cell numbers and phenotypes were indistinguishable from those described for patients with CVID (Fig 5 and see Fig E6 in this article's Online Repository at www.jacionline.org).37 However, in clinically unaffected subjects the absolute B-cell count was often normal or increased (Fig 5, A). In all subjects with NFKB1 LOF variants, the numbers of switched memory B cells were reduced (Fig 5, B-D), whereas a broad range of nonswitched memory B cells was observed. This demonstrates that although the clinical phenotype of NFKB1 LOF variants is partially penetrant, all carriers have a deficiency in class-switched memory B-cell generation. The presence of increased numbers of the CD21low population described in patients with CVID discriminates between clinically affected and unaffected subjects with NFKB1 LOF variants (Fig 5, E). B cells from subjects with NFKB1 LOF variants demonstrated impaired proliferative responses to anti-IgM/anti-CD40/IL-21 and CpG/IL-2 (Fig 6, A); this corresponded with the inability to generate plasmablasts (CD38+/CD27++), which was most pronounced in the more extreme phenotypes (ie, clinically affected cases; Fig 6, B, and see Fig E7, B, in this article's Online Repository at www.jacionline.org). Similarly, ex vivo IgG production was reduced in subjects with LOF variants, whereas IgM levels in the supernatants were normal (Fig 6, C and D, and see Fig E7, C), which is compatible with hypogammaglobulinemia.
Fig 5.
Decreased class-switched memory B-cell and increased CD21low B-cell counts in NFKB1 LOF variant carriers. A, Absolute numbers of CD19+ B cells; each dot represents a single subject and his or her age. Age-dependent reference values are shown in gray. B-E, Percentages within CD19+CD20+ B lymphocytes of CD27+IgD+ (nonswitched memory or marginal-zone B cells) and CD27+IgD− (switched memory B cells; Fig 5, B) or CD27+IgG+ (Fig 5, C), CD27+IgA+ (Fig 5, D), or CD21lowCD38low/dim (Fig 5, E). CA, Clinically affected subjects with an LOF variant in NFKB1; CU, clinically unaffected subjects with an LOF variant in NFKB1; HD, healthy donor. The gating strategy is shown in Fig E6, A. Only subjects with sufficient B cells could be analyzed. P values were determined by using 1-way (Fig 5, E) or 2-way (Fig 5, B) ANOVA with the Bonferroni post hoc test or unpaired Student t test (Fig 5, C and D). ns, Not significant. **P ≤ .01 and ***P ≤ .001.
Fig 6.
Ex vivo class-switch recombination defect of subjects carrying NFKB1 LOF variants is linked to the more extreme phenotype. Six-day culture of CFSE-labeled lymphocytes normalized for B-cell numbers: unstimulated, CpG/IL-2 stimulated (T cell–independent activation), or anti-IgM/anti-CD40/IL-21 stimulated (T cell–dependent activation). A, Percentage of divided B cells, as measured based on CFSE dilution. B, Percentage of CD27++ plasmablasts. The gating strategy is shown in Fig E7, A. C and D, IgM and IgG production in supernatants of 6-day cultures. CA, Clinically affected subjects with an LOF variant in NFKB1; CU, clinically unaffected subject; HD, healthy donor. Only subjects with sufficient B cells could be analyzed. P values were determined by using 2-way ANOVA with the Bonferroni post hoc test. ns, Not significant. **P ≤ .01 and ***P ≤ .001.
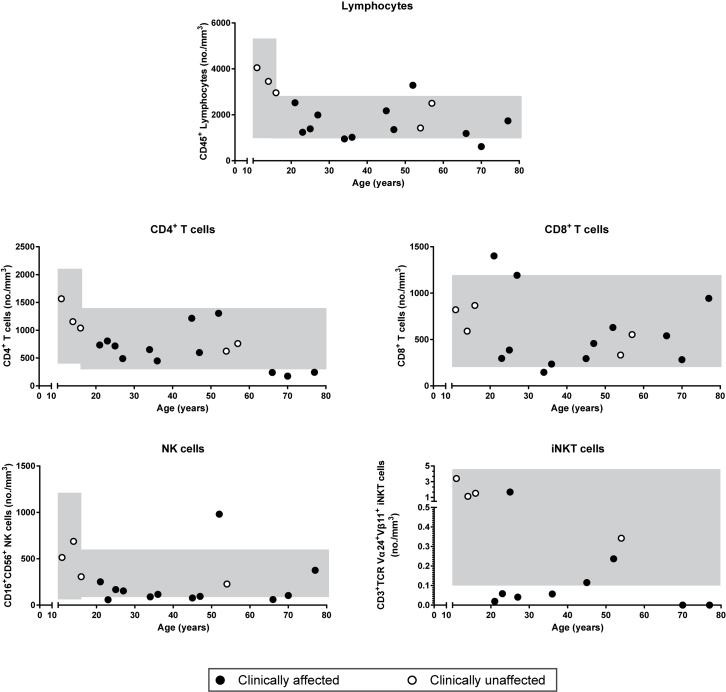
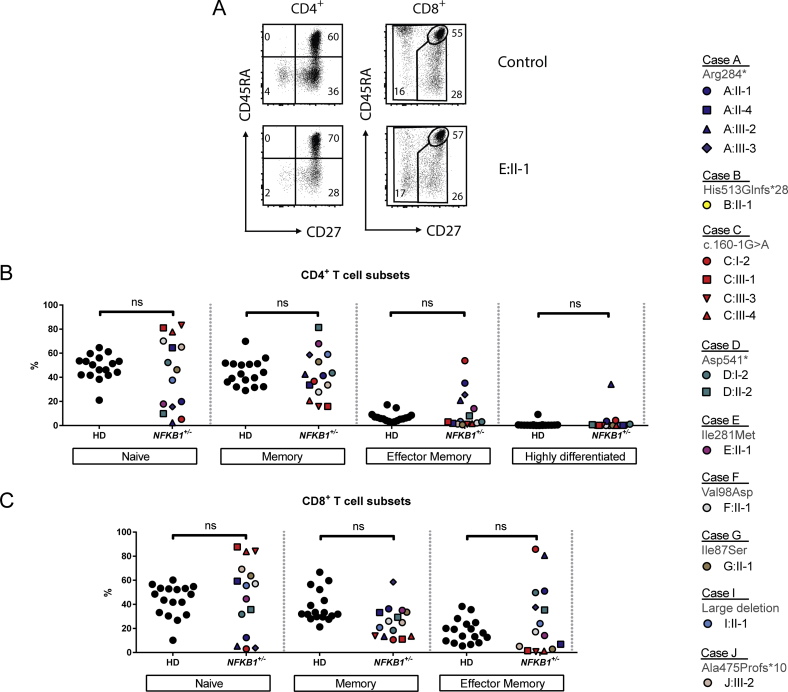
T-cell phenotype in subjects NFKB1 LOF variants
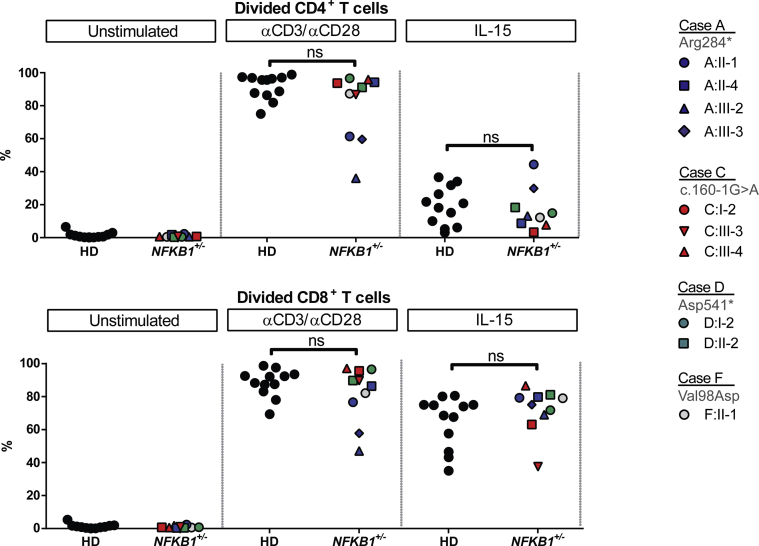
The T-cell phenotype was largely normal in the subset distribution (see Figs E8 and E9 in this article's Online Repository at www.jacionline.org). Similar to the knockout mouse model,38 we found an aberrant number of invariant natural killer T cells in clinically affected subjects (see Fig E8). T-cell proliferation was intact on anti-CD3/anti-CD28 or IL-15 activation (see Fig E10 in this article's Online Repository at www.jacionline.org). Because invariant natural killer T cells have been implicated in diverse immune reactions,39 this deficiency might contribute to the residual disease burden in immunoglobulin replacement–treated patients, some of whom had acute or chronic relapsing infection with herpes virus and, in one case, JC virus.
Discussion
In our study we show that LOF variants in NFKB1 are present in 4% of our cohort of patients with CVID, being the most commonly identified genetic cause of CVID. Furthermore, we highlight specific features of these patients that distinguish them within the diagnostic category of CVID, which otherwise applies to an indiscrete phenotype acquired over time that is termed common and variable. The majority of the genetic variants we report here truncate or delete 1 copy of the gene; together with pedigree cosegregation analyses, protein expression, and B-cell functional data, we conclude that NFKB1 LOF variants cause autosomal dominant haploinsufficiency. This has now been recognized as the genetic mode of inheritance for at least 17 known PIDs, including those associated with previously reported variants in NFKB1.6, 40, 41, 42 In monogenic causes of PID, incomplete penetrance has been more frequently described in haploinsufficient relative to dominant negative PID disease, having been reported in more than half of the monogenic autosomal dominant haploinsufficient immunologic conditions described.40 This might be because dominant negative gain-of-function mutations cause disease by expression of an abnormal protein at any level, whereas, as seen in this study, haploinsufficiency is predicted to lead to 50% residual function of the gene product. By definition, incomplete penetrance of a genetic illness will be associated with substantial variation in the clinical spectrum of disease, and the spectrum seen in this study is consistent with prior reports; in 3 pedigrees with 20 subjects6 harboring heterozygous mutant NFKB1 alleles, the age of onset varied from 2 to 64 years, with a high variety of disease severities, including 2 mutation carriers who were completely healthy at the ages of 2 and 43 years.
It is important to temper skepticism of partial penetrance of immune genetic lesions with our knowledge that individual immune genes might have evolved in response to selection pressure for host protection against specific pathogens.43 Consequently, within the relatively pathogen-free environment of developed countries, the relevant pathogen for triggering disease might be scarce, and reports documenting partial penetrance of the clinical phenotype will increase. This makes the traditional approaches to genetics for determining causality difficult. The BeviMed algorithm used in this study prioritized both the gene NFKB1 and individual variants within NFKB1 for contribution to causality; the power of methods like this will increase with greater data availability. Identification of a number of rare NFKB1 variants with high Combined Annotation Dependent Depletion scores in both the PID and control data sets highlights the potential for false attribution of disease causality when the genetics of an individual case are considered outside the context of relevant control data.
Currently healthy family members carrying the same NFKB1 LOF variant demonstrated similar reductions in p50 expression and low numbers of switched memory B cells as their relatives with CVID. The longitudinal research investigation of these subjects could help identify the additional modifiers, including epigenetic or environmental factors, that influence the clinical penetrance of these genetic lesions. The similarity of results seen in patients with large heterozygous gene deletions and in those with more discrete substitutions is consistent with haploinsufficiency as the shared disease mechanism.
In patients with mild antibody deficiency, it is often difficult to decide when to initiate replacement immunoglobulin therapy; this might be the case for subjects and their family members identified with LOF NFKB1 variants. Two measures seem to correlate well with clinical disease.
First, the class-switch defect and lower IgG and IgA production ex vivo was examined. Immunoglobulin class-switching is known to be regulated by NF-κB. Mutations in NF-κB essential modulator cause a class-switch–defective hyper-IgM syndrome in human subjects,20 as well as in p50 knockout mice.13, 44, 45 Haploinsufficiency of NF-κB might result in defective class-switch recombination because of poor expression of activation-induced cytidine deaminase, a gene regulated by NF-κB, which, when absent, is also associated with immunodeficiency.46
Second, the ability to measure the CD21low B-cell population is widespread in diagnostic immunology laboratories, and our study identifies this marker to correlate with NF-κB disease activity. Although the function of these cells remain to be fully elucidated,47 this laboratory test might be useful for longitudinal assessment of clinically unaffected subjects identified with LOF NFKB1 variants.
Apart from having recurrent and severe infections (including viral disease) for which these patients had been given a diagnosis of PID in the first place, autoimmunity and unexplained splenomegaly are very common manifestations in our patient cohort similar to the other heterozygous NFKB1 cases described.6, 7, 8, 9, 10, 11 Although autoimmunity has been subject to variable percentages per cohort study,3, 48, 49 it seems that these complications occur more frequently in NFKB1-haploinsufficient patients compared with unselected CVID cohorts. In contrast to IKAROS defects but similar to cytotoxic T lymphocyte–associated protein 4 (CTLA4) haploinsufficiency, we observed that NFKB1 haploinsufficiency can also result in chronic and severe viral disease, as noted for cytomegalovirus and JC virus infections in 3 of our patients. In the study of Maffucci et al,11 one of the NFKB1-affected patients also experienced Pneumocystis jirovecii infection and progressive multifocal leukoencephalopathy (PML), which is suggestive for JC virus infection. Whether the B-cell defect in NFKB1 haploinsufficiency is responsible for these nonbacterial infections is unclear.50, 51 PML is most often discovered in the context of an immune reconstitution inflammatory syndrome, as seen in patients with HIV receiving antiretroviral therapy and in patients with multiple sclerosis after natalizumab discontinuation.52 Although the exact contribution of B-cell depletion in PML pathogenesis is unknown, the increased PML risk in rituximab-treated patients53 suggests a protective role for B cells.
Three subjects in this cohort had liver failure, and an additional 3 had transaminitis. Although autoimmunity is suspected, a nonhematopoietic origin of liver disease cannot be excluded in the absence of autoantibodies and nodular regenerative disease. Mouse models have suggested a nonimmune role for NF-κB signaling in patients with liver failure.13, 54, 55, 56
In the cohort of patients with NFKB1 variants, we identified a number of malignancies. Malignancies in patients with PIDs have been cited as the second-leading cause of death after infection,57, 58 and murine models have demonstrated that haploinsufficiency of NF-κB1 is a risk factor for hematologic malignancy.59 In a large CVID registry study of 2212 patients, 9% had malignancies, with one third being lymphomas, some presenting before their CVID diagnosis.49 Despite the fact that our cohort is relatively small, we found oncologic manifestations in 29% of our cases (two thirds being lymphoma), suggesting that malignancies in patients with NFKB1 haploinsufficiency can occur more often than in unselected patients with CVID. In a study in 176 patients with CVID, among the 626 relatives of patients with CVID, no increase in cancer risk was observed,60 suggesting that when this does occur, as in this study (3/7), it might be due to a shared genetic lesion. Therefore in a pedigree with an LOF variant in NFKB1, any relatives with cancer should be suspected of sharing the same pathogenic variant.
In conclusion, previous publications61, 62 have suggested that CVID is largely a polygenic disease. Our results provide further evidence that LOF variants in NFKB1 are the most common monogenic cause of disease to date, even in seemingly sporadic cases. In these patients there is a clear association with complications, such as malignancy, autoimmunity, and severe nonimmune liver disease; this is important because the excess mortality seen in patients with CVID occurs in this group.48 The screening for defined pathogenic NFKB1 variants accompanied by B-cell phenotype assessment has prognostic value and is effective in stratifying these patients.
Key messages.
-
•
Pathogenic variants in NFKB1 are currently the most common known monogenic cause of CVID.
-
•
There is a clear association with complications, such as autoimmunity and malignancy, features associated with worse prognosis.
-
•
These patients can be stratified by NFKB1 protein level and B-cell phenotype.
Acknowledgments
We thank the patients and their family members for participation in our study.
Consortia
The members of the NIHRBR-RD PID Consortium are Zoe Adhya, Hana Alachkar, Ariharan Anantharachagan, Richard Antrobus, Gururaj Arumugakani, Chiara Bacchelli, Helen Baxendale, Claire Bethune, Shahnaz Bibi, Barbara Boardman, Claire Booth, Michael Browning, Mary Brownlie, Siobhan Burns, Anita Chandra, Hayley Clifford, Nichola Cooper, Sophie Davies, John Dempster, Lisa Devlin, Rainer Doffinger, Elizabeth Drewe, David Edgar, William Egner, Tariq El-Shanawany, Bobby Gaspar, Rohit Ghurye, Kimberley Gilmour, Sarah Goddard, Pavel Gordins, Sofia Grigoriadou, Scott Hackett, Rosie Hague, Lorraine Harper, Grant Hayman, Archana Herwadkar, Stephen Hughes, Aarnoud Huissoon, Stephen Jolles, Julie Jones, Peter Kelleher, Nigel Klein, Taco Kuijpers (principal investigator), Dinakantha Kumararatne, James Laffan, Hana Lango Allen, Sara Lear, Hilary Longhurst, Lorena Lorenzo, Jesmeen Maimaris, Ania Manson, Elizabeth McDermott, Hazel Millar, Anoop Mistry, Valerie Morrisson, Sai Murng, Iman Nasir, Sergey Nejentsev, Sadia Noorani, Eric Oksenhendler, Mark Ponsford, Waseem Qasim, Ellen Quinn, Isabella Quinti, Alex Richter, Crina Samarghitean, Ravishankar Sargur, Sinisa Savic, Suranjith Seneviratne, Carrock Sewall, Fiona Shackley, Ilenia Simeoni, Kenneth G. C. Smith (principal investigator), Emily Staples, Hans Stauss, Cathal Steele, James Thaventhiran, Moira Thomas, Adrian Thrasher (principal investigator), Steve Welch, Lisa Willcocks, Sarita Workman, Austen Worth, Nigel Yeatman, and Patrick Yong.
The members of the NIHRBR-RD Management Team are Sofie Ashford, John Bradley, Debra Fletcher, Tracey Hammerton, Roger James, Nathalie Kingston, Willem Ouwehand, Christopher Penkett, F Lucy Raymond, Kathleen Stirrups, Marijke Veltman, and Tim Young.
The members of the NIHRBR-RD Enrolment and Ethics Team are Sofie Ashford, Matthew Brown, Naomi Clements-Brod, John Davis, Eleanor Dewhurst, Marie Erwood, Amy Frary, Rachel Linger, Jennifer Martin, Sofia Papadia, and Karola Rehnstrom.
The members of the NIHRBR-RD Bioinformatics Team are William Astle, Antony Attwood, Marta Bleda, Keren Carss, Louise Daugherty, Sri Deevi, Stefan Graf, Daniel Greene, Csaba Halmagyi, Matthias Haimel, Fengyuan Hu, Roger James, Hana Lango Allen, Vera Matser, Stuart Meacham, Karyn Megy, Christopher Penkett, Olga Shamardina, Kathleen Stirrups, Catherine Titterton, Salih Tuna, Ernest Turro, Ping Yu, and Julie von Ziegenweldt.
The members of the Cambridge Translational GenOmics Laboratory are Abigail Furnell, Rutendo Mapeta, Ilenia Simeoni, Simon Staines, Jonathan Stephens, Kathleen Stirrups, Deborah Whitehorn, Paula Rayner-Matthews, and Christopher Watt.
Footnotes
Supported by The National Institute for Health Research England (grant no. RG65966) and the Center of Immunodeficiencies Amsterdam (CIDA). J.E.T. is supported by an MRC Clinician Scientist Fellowship (MR/L006197/1). A.J.T. is supported by both the Wellcome Trust (104807/Z/14/Z) and the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London.
Disclosure of potential conflict of interest: S. O. Burns has received grants from HEFCE, University College London, the National Institute of Health Research (NIHR), UCLH III BRC, and GOSH/ICH BRC and has received personal fees from CSL Behring, Immunodeficiency Canada/IAACI, CSL Behring, Baxalta, and Octagam. A. Chandra has received grants from the Wellcome Trust and GlaxoSmithKline. H. J. Longhurst has received personal fees from BioTest, CSL Behring, and Baxalta; has received grants from CSL Behring; has received nonfinancial support from BioTest and CSL Behring; and has research collaborations with CSL Behring and GlaxoSmithKline. E. Oksenhendler has received personal fees from CSL Behring and MSD. A. J. Thrasher has received personal fees from Orchard Therapeutics, Rocket Pharmaceuticals, and Torus Therapeutics. The rest of the authors declare that they have no relevant conflicts of interest.
Contributor Information
James E. Thaventhiran, Email: jedt2@cam.ac.uk.
Taco W. Kuijpers, Email: t.w.kuijpers@amc.nl.
NIHR BioResource–Rare Diseases Consortium:
Zoe Adhya, Hana Alachkar, Ariharan Anantharachagan, Richard Antrobus, Gururaj Arumugakani, Chiara Bacchelli, Helen Baxendale, Claire Bethune, Shahnaz Bibi, Barbara Boardman, Claire Booth, Michael Browning, Mary Brownlie, Siobhan Burns, Anita Chandra, Hayley Clifford, Nichola Cooper, Sophie Davies, John Dempster, Lisa Devlin, Rainer Doffinger, Elizabeth Drewe, David Edgar, William Egner, Tariq El-Shanawany, Bobby Gaspar, Rohit Ghurye, Kimberley Gilmour, Sarah Goddard, Pavel Gordins, Sofia Grigoriadou, Scott Hackett, Rosie Hague, Lorraine Harper, Grant Hayman, Archana Herwadkar, Stephen Hughes, Aarnoud Huissoon, Stephen Jolles, Julie Jones, Peter Kelleher, Nigel Klein, Taco Kuijpers, Dinakantha Kumararatne, James Laffan, Hana Lango Allen, Sara Lear, Hilary Longhurst, Lorena Lorenzo, Jesmeen Maimaris, Ania Manson, Elizabeth McDermott, Hazel Millar, Anoop Mistry, Valerie Morrisson, Sai Murng, Iman Nasir, Sergey Nejentsev, Sadia Noorani, Eric Oksenhendler, Mark Ponsford, Waseem Qasim, Ellen Quinn, Isabella Quinti, Alex Richter, Crina Samarghitean, Ravishankar Sargur, Sinisa Savic, Suranjith Seneviratne, Carrock Sewall, Fiona Shackley, Ilenia Simeoni, Kenneth G.C. Smith, Emily Staples, Hans Stauss, Cathal Steele, James Thaventhiran, Moira Thomas, Adrian Thrasher, Steve Welch, Lisa Willcocks, Sarita Workman, Austen Worth, Nigel Yeatman, Patrick Yong, Sofie Ashford, John Bradley, Debra Fletcher, Tracey Hammerton, Roger James, Nathalie Kingston, Willem Ouwehand, Christopher Penkett, F Lucy Raymond, Kathleen Stirrups, Marijke Veltman, Tim Young, Sofie Ashford, Matthew Brown, Naomi Clements-Brod, John Davis, Eleanor Dewhurst, Marie Erwood, Amy Frary, Rachel Linger, Jennifer Martin, Sofia Papadia, Karola Rehnstrom, William Astle, Antony Attwood, Marta Bleda, Keren Carss, Louise Daugherty, Sri Deevi, Stefan Graf, Daniel Greene, Csaba Halmagyi, Matthias Haimel, Fengyuan Hu, Roger James, Hana Lango Allen, Vera Matser, Stuart Meacham, Karyn Megy, Christopher Penkett, Olga Shamardina, Kathleen Stirrups, Catherine Titterton, Salih Tuna, Ernest Turro, Ping Yu, Julie von Ziegenweldt, Abigail Furnell, Rutendo Mapeta, Ilenia Simeoni, Simon Staines, Jonathan Stephens, Kathleen Stirrups, Deborah Whitehorn, Paula Rayner-Matthews, and Christopher Watt
Supplementary data
Fig E1.
Fig E2.
Fig E3.
Fig E4.
Fig E5.
Fig E6.
Fig E7.
Fig E8.
Fig E9.
Fig E10.
References
- 1.Primary immunodeficiency diseases. Report of a WHO scientific group. Clin Exp Immunol. 1997;109(Suppl 1):1–28. [PubMed] [Google Scholar]
- 2.Oksenhendler E., Gerard L., Fieschi C., Malphettes M., Mouillot G., Jaussaud R. Infections in 252 patients with common variable immunodeficiency. Clin Infect Dis. 2008;46:1547–1554. doi: 10.1086/587669. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham-Rundles C., Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 4.Bousfiha A., Jeddane L., Al-Herz W., Ailal F., Casanova J.L., Chatila T. The 2015 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol. 2015;35:727–738. doi: 10.1007/s10875-015-0198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picard C., Al-Herz W., Bousfiha A., Casanova J.L., Chatila T., Conley M.E. Primary immunodeficiency diseases: an update on the Classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol. 2015;35:696–726. doi: 10.1007/s10875-015-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fliegauf M., Bryant V.L., Frede N., Slade C., Woon S.T., Lehnert K. Haploinsufficiency of the NF-kappaB1 subunit p50 in common variable immunodeficiency. Am J Hum Genet. 2015;97:389–403. doi: 10.1016/j.ajhg.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boztug H., Hirschmugl T., Holter W., Lakatos K., Kager L., Trapin D. NF-kappaB1 haploinsufficiency causing immunodeficiency and EBV-driven lymphoproliferation. J Clin Immunol. 2016;36:533–540. doi: 10.1007/s10875-016-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lougaris V., Moratto D., Baronio M., Tampella G., van der Meer J.W., Badolato R. Early and late B-cell developmental impairment in nuclear factor kappa B, subunit 1-mutated common variable immunodeficiency disease. J Allergy Clin Immunol. 2017;139:349–352.e1. doi: 10.1016/j.jaci.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 9.Kaustio M., Haapaniemi E., Goos H., Hautala T., Park G., Syrjanen J. Damaging heterozygous mutations in NFKB1 lead to diverse immunologic phenotypes. J Allergy Clin Immunol. 2017;140:782–796. doi: 10.1016/j.jaci.2016.10.054. [DOI] [PubMed] [Google Scholar]
- 10.Schipp C., Nabhani S., Bienemann K., Simanovsky N., Kfir-Erenfeld S., Assayag-Asherie N. Specific antibody deficiency and autoinflammatory disease extend the clinical and immunological spectrum of heterozygous NFKB1 loss-of-function mutations in humans. Haematologica. 2016;101:e392–e396. doi: 10.3324/haematol.2016.145136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maffucci P., Filion C.A., Boisson B., Itan Y., Shang L., Casanova J.L. Genetic diagnosis using whole exome sequencing in common variable immunodeficiency. Front Immunol. 2016;7:220. doi: 10.3389/fimmu.2016.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene D., BioResource N., Richardson S., Turro E. A fast association test for identifying pathogenic variants involved in rare diseases. Am J Hum Genet. 2017;101:104–114. doi: 10.1016/j.ajhg.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q., Lenardo M.J., Baltimore D. 30 Years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshioka T., Nishikomori R., Hara J., Okada K., Hashii Y., Okafuji I. Autosomal dominant anhidrotic ectodermal dysplasia with immunodeficiency caused by a novel NFKBIA mutation, p.Ser36Tyr, presents with mild ectodermal dysplasia and non-infectious systemic inflammation. J Clin Immunol. 2013;33:1165–1174. doi: 10.1007/s10875-013-9924-z. [DOI] [PubMed] [Google Scholar]
- 15.Courtois G., Smahi A., Reichenbach J., Doffinger R., Cancrini C., Bonnet M. A hypermorphic IkappaBalpha mutation is associated with autosomal dominant anhidrotic ectodermal dysplasia and T cell immunodeficiency. J Clin Invest. 2003;112:1108–1115. doi: 10.1172/JCI18714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns S.O., Plagnol V., Gutierrez B.M., Al Zahrani D., Curtis J., Gaspar M. Immunodeficiency and disseminated mycobacterial infection associated with homozygous nonsense mutation of IKKbeta. J Allergy Clin Immunol. 2014;134:215–218. doi: 10.1016/j.jaci.2013.12.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mousallem T., Yang J., Urban T.J., Wang H., Adeli M., Parrott R.E. A nonsense mutation in IKBKB causes combined immunodeficiency. Blood. 2014;124:2046–2050. doi: 10.1182/blood-2014-04-571265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pannicke U., Baumann B., Fuchs S., Henneke P., Rensing-Ehl A., Rizzi M. Deficiency of innate and acquired immunity caused by an IKBKB mutation. N Engl J Med. 2013;369:2504–2514. doi: 10.1056/NEJMoa1309199. [DOI] [PubMed] [Google Scholar]
- 19.Doffinger R., Smahi A., Bessia C., Geissmann F., Feinberg J., Durandy A. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27:277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 20.Jain A., Ma C.A., Liu S., Brown M., Cohen J., Strober W. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol. 2001;2:223–228. doi: 10.1038/85277. [DOI] [PubMed] [Google Scholar]
- 21.Greil J., Rausch T., Giese T., Bandapalli O.R., Daniel V., Bekeredjian-Ding I. Whole-exome sequencing links caspase recruitment domain 11 (CARD11) inactivation to severe combined immunodeficiency. J Allergy Clin Immunol. 2013;131:1376–1383.e3. doi: 10.1016/j.jaci.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Stepensky P., Keller B., Buchta M., Kienzler A.K., Elpeleg O., Somech R. Deficiency of caspase recruitment domain family, member 11 (CARD11), causes profound combined immunodeficiency in human subjects. J Allergy Clin Immunol. 2013;131:477–485.e1. doi: 10.1016/j.jaci.2012.11.050. [DOI] [PubMed] [Google Scholar]
- 23.Torres J.M., Martinez-Barricarte R., Garcia-Gomez S., Mazariegos M.S., Itan Y., Boisson B. Inherited BCL10 deficiency impairs hematopoietic and nonhematopoietic immunity. J Clin Invest. 2014;124:5239–5248. doi: 10.1172/JCI77493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jabara H.H., Ohsumi T., Chou J., Massaad M.J., Benson H., Megarbane A. A homozygous mucosa-associated lymphoid tissue 1 (MALT1) mutation in a family with combined immunodeficiency. J Allergy Clin Immunol. 2013;132:151–158. doi: 10.1016/j.jaci.2013.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinnon M.L., Rozmus J., Fung S.Y., Hirschfeld A.F., Del Bel K.L., Thomas L. Combined immunodeficiency associated with homozygous MALT1 mutations. J Allergy Clin Immunol. 2014;133:1458–1462. doi: 10.1016/j.jaci.2013.10.045. e1-e7. [DOI] [PubMed] [Google Scholar]
- 26.Punwani D., Wang H., Chan A.Y., Cowan M.J., Mallott J., Sunderam U. Combined immunodeficiency due to MALT1 mutations, treated by hematopoietic cell transplantation. J Clin Immunol. 2015;35:135–146. doi: 10.1007/s10875-014-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann A., Natoli G., Ghosh G. Transcriptional regulation via the NF-kappaB signaling module. Oncogene. 2006;25:6706–6716. doi: 10.1038/sj.onc.1209933. [DOI] [PubMed] [Google Scholar]
- 28.Oeckinghaus A., Hayden M.S., Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 29.Vallabhapurapu S., Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 30.Carss K.J., Arno G., Erwood M., Stephens J., Sanchis-Juan A., Hull S. Comprehensive rare variant analysis via whole-genome sequencing to determine the molecular pathology of inherited retinal disease. Am J Hum Genet. 2017;100:75–90. doi: 10.1016/j.ajhg.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLaren W., Gil L., Hunt S.E., Riat H.S., Ritchie G.R., Thormann A. The Ensembl Variant Effect Predictor. Genome Biol. 2016;17:122. doi: 10.1186/s13059-016-0974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarrei M., MacDonald J.R., Merico D., Scherer S.W. A copy number variation map of the human genome. Nat Rev Genet. 2015;16:172–183. doi: 10.1038/nrg3871. [DOI] [PubMed] [Google Scholar]
- 33.aan de Kerk D.J., Jansen M.H., ten Berge I.J., van Leeuwen E.M., Kuijpers T.W. Identification of B cell defects using age-defined reference ranges for in vivo and in vitro B cell differentiation. J Immunol. 2013;190:5012–5019. doi: 10.4049/jimmunol.1201807. [DOI] [PubMed] [Google Scholar]
- 34.Muller C.W., Rey F.A., Sodeoka M., Verdine G.L., Harrison S.C. Structure of the NF-kappa B p50 homodimer bound to DNA. Nature. 1995;373:311–317. doi: 10.1038/373311a0. [DOI] [PubMed] [Google Scholar]
- 35.Webb B., Sali A. Comparative protein structure modeling using MODELLER. Curr Protoc Bioinformatics. 2014;47:5.6.1–5.6.32. doi: 10.1002/0471250953.bi0506s47. [DOI] [PubMed] [Google Scholar]
- 36.Tao Z., Fusco A., Huang D.B., Gupta K., Young Kim D., Ware C.F. p100/IkappaBdelta sequesters and inhibits NF-kappaB through kappaBsome formation. Proc Natl Acad Sci U S A. 2014;111:15946–15951. doi: 10.1073/pnas.1408552111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wehr C., Kivioja T., Schmitt C., Ferry B., Witte T., Eren E. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 38.Sivakumar V., Hammond K.J., Howells N., Pfeffer K., Weih F. Differential requirement for Rel/nuclear factor kappa B family members in natural killer T cell development. J Exp Med. 2003;197:1613–1621. doi: 10.1084/jem.20022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brennan P.J., Brigl M., Brenner M.B. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 40.Rieux-Laucat F., Casanova J.L. Immunology. Autoimmunity by haploinsufficiency. Science. 2014;345:1560–1561. doi: 10.1126/science.1260791. [DOI] [PubMed] [Google Scholar]
- 41.Kuehn H.S., Boisson B., Cunningham-Rundles C., Reichenbach J., Stray-Pedersen A., Gelfand E.W. Loss of B cells in patients with heterozygous mutations in IKAROS. N Engl J Med. 2016;374:1032–1043. doi: 10.1056/NEJMoa1512234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen K., Coonrod E.M., Kumanovics A., Franks Z.F., Durtschi J.D., Margraf R.L. Germline mutations in NFKB2 implicate the noncanonical NF-kappaB pathway in the pathogenesis of common variable immunodeficiency. Am J Hum Genet. 2013;93:812–824. doi: 10.1016/j.ajhg.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casanova J.L. Severe infectious diseases of childhood as monogenic inborn errors of immunity. Proc Natl Acad Sci U S A. 2015;112:E7128–E7137. doi: 10.1073/pnas.1521651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sha W.C., Liou H.C., Tuomanen E.I., Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 45.Snapper C.M., Zelazowski P., Rosas F.R., Kehry M.R., Tian M., Baltimore D. B cells from p50/NF-kappa B knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J Immunol. 1996;156:183–191. [PubMed] [Google Scholar]
- 46.Revy P., Muto T., Levy Y., Geissmann F., Plebani A., Sanal O. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 47.Rakhmanov M., Keller B., Gutenberger S., Foerster C., Hoenig M., Driessen G. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci U S A. 2009;106:13451–13456. doi: 10.1073/pnas.0901984106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Resnick E.S., Moshier E.L., Godbold J.H., Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119:1650–1657. doi: 10.1182/blood-2011-09-377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gathmann B., Mahlaoui N., Ceredih Gerard L., Oksenhendler E., Warnatz K., Schulze I. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134:116–126. doi: 10.1016/j.jaci.2013.12.1077. [DOI] [PubMed] [Google Scholar]
- 50.Durali D., de Goer de Herve M.G., Gasnault J., Taoufik Y. B cells and progressive multifocal leukoencephalopathy: search for the missing link. Front Immunol. 2015;6:241. doi: 10.3389/fimmu.2015.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan C.S., Koralnik I.J. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9:425–437. doi: 10.1016/S1474-4422(10)70040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palazzo E., Yahia S.A. Progressive multifocal leukoencephalopathy in autoimmune diseases. Joint Bone Spine. 2012;79:351–355. doi: 10.1016/j.jbspin.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Carson K.R., Evens A.M., Richey E.A., Habermann T.M., Focosi D., Seymour J.F. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka M., Fuentes M.E., Yamaguchi K., Durnin M.H., Dalrymple S.A., Hardy K.L. Embryonic lethality, liver degeneration, and impaired NF-kappa B activation in IKK-beta-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- 55.Pasparakis M., Luedde T., Schmidt-Supprian M. Dissection of the NF-kappaB signalling cascade in transgenic and knockout mice. Cell Death Differ. 2006;13:861–872. doi: 10.1038/sj.cdd.4401870. [DOI] [PubMed] [Google Scholar]
- 56.Geisler F., Algul H., Paxian S., Schmid R.M. Genetic inactivation of RelA/p65 sensitizes adult mouse hepatocytes to TNF-induced apoptosis in vivo and in vitro. Gastroenterology. 2007;132:2489–2503. doi: 10.1053/j.gastro.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 57.Mueller B.U., Pizzo P.A. Cancer in children with primary or secondary immunodeficiencies. J Pediatr. 1995;126:1–10. [PubMed] [Google Scholar]
- 58.Vajdic C.M., Mao L., van Leeuwen M.T., Kirkpatrick P., Grulich A.E., Riminton S. Are antibody deficiency disorders associated with a narrower range of cancers than other forms of immunodeficiency? Blood. 2010;116:1228–1234. doi: 10.1182/blood-2010-03-272351. [DOI] [PubMed] [Google Scholar]
- 59.Voce D.J., Schmitt A.M., Uppal A., McNerney M.E., Bernal G.M., Cahill K.E. Nfkb1 is a haploinsufficient DNA damage-specific tumor suppressor. Oncogene. 2015;34:2807–2813. doi: 10.1038/onc.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mellemkjaer L., Hammarstrom L., Andersen V., Yuen J., Heilmann C., Barington T. Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: a combined Danish and Swedish study. Clin Exp Immunol. 2002;130:495–500. doi: 10.1046/j.1365-2249.2002.02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Orange J.S., Glessner J.T., Resnick E., Sullivan K.E., Lucas M., Ferry B. Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol. 2011;127:1360–1367.e6. doi: 10.1016/j.jaci.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Schouwenburg P.A., Davenport E.E., Kienzler A.K., Marwah I., Wright B., Lucas M. Application of whole genome and RNA sequencing to investigate the genomic landscape of common variable immunodeficiency disorders. Clin Immunol. 2015;160:301–314. doi: 10.1016/j.clim.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.