Abstract
Objective:
The Fagan Test of Infant Intelligence (FTII) uses longer gaze length for unfamiliar versus familiar human faces to gauge visual-spatial encoding, attention, and working memory in infants. Our objective was to establish the feasibility of automated eye-tracking with the FTII in HIV-exposed Ugandan infants.
Method:
The FTII was administered to 31 perinatally HIV exposed non-infected (HEU) Ugandan children 6 to 12 months of age (11 boys; M=0.69 yrs, SD=0.14; 19 girls; M=0.79, SD=0.15). A series of 10 different faces were presented (familiar face exposure for 25 seconds followed by a gaze preference trial of 15 seconds with both the familiar and unfamiliar faces). Tobii X2–30 infrared camera for pupil detection provided automated eye-tracking measures of gaze location and length during presentation of Ugandan faces selected to correspond to the gender, age (adult, child), face expression, and orientation of the original FTII. Eye-tracking gaze length for unfamiliar faces was correlated with performance on the Mullen Scales of Early Learning (MSEL).
Results:
Infants gazed longer at the novel picture compared to familiar across 10 novelty preference trials. Better MSEL cognitive development was correlated with proportionately longer time spent looking at the novel faces (r(30)=0.52,P=0.004); especially for the Fine Motor cognitive sub-scale (r(30)=0.54,P=0.002).
Conclusion:
Automated eye tracking in a human face recognition test proved feasible and corresponded to the MSEL composite cognitive development in HEU infants in a resource-constrained clinical setting. Eye tracking may be a viable means of enhancing the validity and accuracy of other neurodevelopmental measures in at-risk children in sub-Saharan Africa.
Keywords: eye tracking, Mullen Scales of Early Learning, child development, HIV, Fagan test, memory, human faces, infants, Uganda, Africa
INTRODUCTION
Dr. Joseph Fagan pioneered the use of length of infant gaze to novel human faces in his development of the Fagan Test of Infant Intelligence (FTII) in the early 1980s (Plomin, 2006). Fagan and colleagues demonstrated the predictive validity of the Fagan test to other widely used developmental assessments for mental development, such as the Bayley Scales of Infant Development and the Wechsler Scale Intelligence of Children performance in middle childhood (Fagan, 2000; Fagan & Detterman, 1992; Fagan & Haiken-Vasen, 1997). Since then, infant gaze length as a measure of novelty preference is a well-established principle in developmental psychology (Judge, Chang, & Lammi-Keefe, 2015).
Since the pioneering work for Fagan and colleagues with the FTII in the 1980s and 1990s, the sensitivity of this test to neurodevelopmental risk and disorders in infants and very young children has been further established with outcomes such as premature birth and low birth weight infants (Guzzetta et al., 2006), malnutrition and developmental delay (Nelson, Goldenberg, Hoffman, & Cliver, 1997), polyunsaturated fat oils and other micronutrient interventions for under-nutrition (vitamin A, iron) (O’Connor et al., 2001), environmental toxic exposures (e.g., methyl-mercury, lead, PCBs) (Davidson et al., 1999; Emory, Ansari, Pattillo, Archibold, & Chevalier, 2003; Jedrychowski et al., 2008; Myers et al., 1995), congenital and early childhood diseases (e.g., Rett’s Syndrome) (von Tetzchner et al., 1996), and gestational exposure to cocaine and other maternal drug and alcohol use (Chiriboga, Kuhn, & Wasserman, 2007; Jacobson, Chiodo, Sokol, & Jacobson, 2002).
Although the FTII is sensitive to perinatal risk and toxic exposure (Davidson et al., 1999; Emory et al., 2003; Jedrychowski et al., 2008; Myers et al., 1995), and has strong predictive validity for measures of cognitive ability in later childhood, it has proven less sensitive when applied to gestational and developmental risk in low resource settings (Drotar et al., 1999). To illustrate, the Bayley scales were more sensitive than the Fagan test to early developmental deficits from HIV disease in Ugandan infants in the first two years of life (Drotar et al., 1997). Drotar et al. (1997) applied the FTII to HIV-infected children along with non-infected comparison groups, using the Bayley Scales of Infant Development (BSID) as the gold standard. With the BSID at 12 months, 30% of HIV-infected children showed significant developmental delay, as compared to 5% of the non-infected. The differences in developmental delay were even more dramatic by 24 months (Drotar et al., 1997). However, significant gaze length differences between infected and non-infected Ugandan infants were not observed with the FTII.
We propose that the limited validity of the Fagan test in such low-resource settings was likely due to the high level of training and quality assurance monitoring needed to measure infant gaze length manually from behind a viewing eye slot for the face panels being presented (see Figure 1, upper right photograph). This explanation regarding the validity of the FTII compared to the BSID in documenting developmental delay in Ugandan children with HIV is further supported the results of other attempts to use the FTII in low and middle income countries (LMICs). Attempts to validate the Fagan test in Laos in 1992 in order to gauge developmental risk factors in that setting also met with very limited success (Karen Olness, personal communication). During this period, Fagan and colleagues’ attempts to automate the process with early automated eye gaze tracking ( i-scan) technology proved cumbersome and expensive, and the instrumentation of use only in a highly controlled laboratory setting (Fagan & Detterman, 1992; Fagan & Haiken-Vasen, 1997).
Figure 1 Caption.
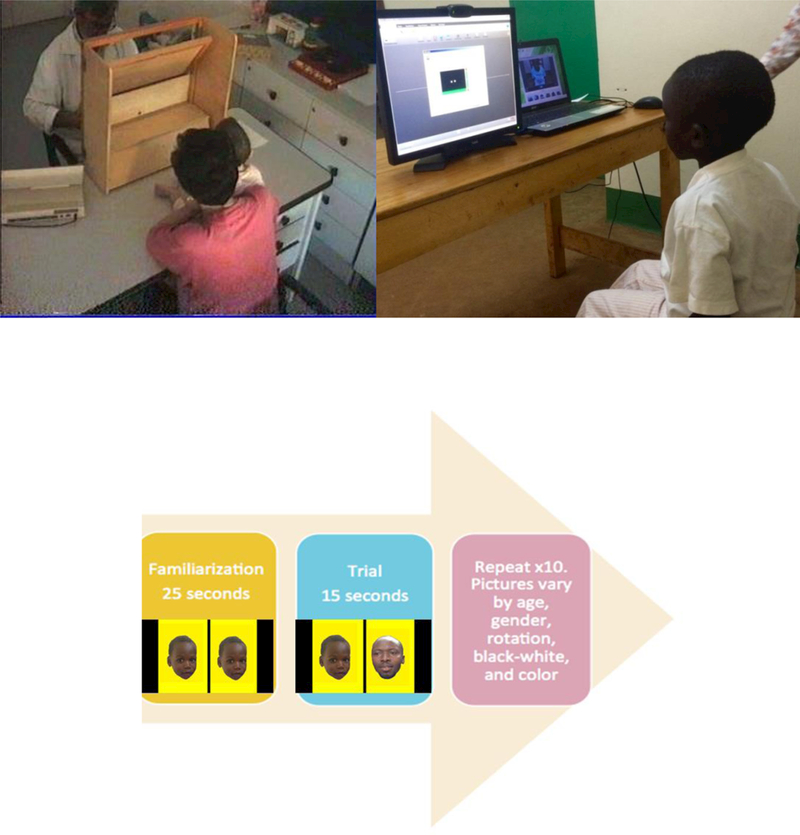
The upper left photograph shows the manual face image and eye gaze measurement apparatus for the original Fagan test. The upper right photograph shows the initial calibration of Tobii X2–30 infrared camera (mounted on bottom of laptop) for calibrating pupil gaze to the modified Fagan Test picture presentations on the large monitor screen. The bottom figure shows the familiarization trial presentation of the same pair of Ugandan faces for 25 seconds, followed by the gaze preference trial with the presentation of both the familiar (prior) face and the new (unfamiliar) face for 15 seconds.
In recent years, eye tracking technology and instrumentation has become very precise, powerful, affordable, and portable as a measurement tool (Sandgren, Andersson, van de Weijer, Hansson, & Sahlen, 2013; Seligman & Giovannetti, 2015). Because of this, eye-tracking instruments have been extensively validated as a sensitive measure of child attention, working memory, and learning in laboratory-based cognitive neurodevelopmental research related to language learning and literacy development (Caruana, Brock, & Woolgar, 2015; Ellis, Borovsky, Elman, & Evans, 2015).
However, we know of only two published reports where eye tracking instrumentation has been used to evaluate neurocognitive response with children in the African setting. The first is a study by our group in which Tobii eye tracking was compared to webcam-based observer scoring on an animation viewing measure of attention (Early Childhood Vigilance Test or ECVT) to evaluate the feasibility of automating Tobii eye tracking measurement and scoring, with the same instrumentation used in the present study (Michael J. Boivin et al., 2017). For HIV-exposed Ugandan children 44 to 65 months from our present study site, Tobii eye tracking measures of gaze length duration for a six minute animation cartoon were compared performance measures from the Mullen Scales of Early Learning (MSEL), Color-Object Association Test (COAT), and Behavior Rating Inventory of Executive Function for preschool children (BRIEF-P). Children watched 78% of the cartoon, and this measure of ECVT attention was significantly correlated with COAT memory and learning. This established the feasibility of Tobii eye tracking technology as a measure of attention with HIV-affected toddlers in our present study setting.
The other published report was by Forssman and colleagues (2017), in which they presented a visual tracking and visual switching task to a sample of both Finnish (N=39) and Malawian (N=40) nine-month old infants in order to establish the feasibility of Tobii-based eye-tracking measures in a cross-cultural study (Forssman et al., 2017). Both groups of infants had a very high completion rate for the visual testing procedure while providing valid eye tracking data (> 90%), and parents in both cultures had a high acceptance rate of this kind of evaluation for their children. However, compared with Finnish infants, Malawian infants had a lower rate of successful visual search responses, longer attention visual tracking shift times (indicative of processing speed), and a lower rate of gaze disengagements from the presentation of human faces on the computer monitor.
Forssman and colleagues concluded that in addition to risk factors for brain/behavior development, a number of other reasons could explain the difference, such as a heightened sensitivity of the Malawian infants to stressful environments that could disrupt their social responsiveness, or responsiveness to computer monitor displays. Irrespective, both our group with Ugandan HIV-affected children and Forssman and colleagues in their study with Malawian children were able to establish that the use of eye tracking technologies in infant cognitive testing is feasible and acceptable in the African context (i.e., proof of concept).
The present study also seeks to establish the feasibility of eye tracking as part of a neurocognitive assessment in African context, only with infants and very young children in a visual-spatial encoding working memory task for human faces. Furthermore, we seek to do so with a widely used and cross-culturally well-validated test of infant intelligence (the FTII) and to do so with African children perinatally exposed to HIV. It is time to re-consider the application of eye-tracking measures as a sensitive and precise way to measure infant and toddler development (clinic and field settings) in low-resource settings at risk for disease, malnutrition, and neglect (Olness, 2003). Such measures can also gauge the benefits of maternal and child health interventions for such risk factors, by establishing a sensitive developmental trajectory for brain/behavior function and integrity throughout the first year of life (Andersson, 1996; Guzzetta et al., 2006; Nelson et al., 1997; Thompson, Fagan, & Fulker, 1991).
Principal Study Objective and Plan.
We completed a randomized controlled trial in an impoverished rural area of Sub-Saharan Africa where we trained caregivers of children exposed to HIV. We enrolled mothers of 31 infants for this Tobii eye-tracking feasibility study. Each infant underwent a single modified Fagan assessment (using photographs of Ugandan faces; these have been matched to the gender, age, and expression attributes of the mostly Caucasian human faces in the standard Fagan test) along with a Mullen Scales of Early Learning (MSEL) developmental assessment.
We gauged the correspondence validity of our Fagan test infant eye-tracking measure to more global developmental outcomes assessed by the MSEL (Gross Motor, Fine Motor, Visual Reception, Receptive Language, and Expressive Language). The MSEL has been used extensively in this setting with young children, and it has been found to have good construct validity in the measurement of the effects of HIV exposure and quality of caregiving in very early childhood (M. J. Boivin, Bangirana, Nakasuja, et al., 2013; M. J. Boivin, Bangirana, Nakasujja, et al., 2013).
This purpose of the present pilot study is to validate a modified-Fagan Test that utilizes eye-tracking technology in a low-resource setting. This is the first study to establish a portable, accessible, and affordable technology that can be applied to any range of neurocognitive testing in a low-resource area. By validating the use of portable eye-tracking technology with this test, early detection and intervention can be made possible. Furthermore, we can quantify the effect of exposures, not limited to HIV, on infant cognitive development.
METHOD
Study recruitment population.
IRB approval for this study was obtained from Michigan State University (FWA #0000 4556) and the Makerere University School of Medicine Research Ethics Committee ( FWA #0000 1293). A research permit was also obtained from the Ugandan National Council of Science and Technology. Participating households with eligible children were staying within a 20 km area of Tororo town, and were referred to our study by the AIDS Support Network (TASO) Tororo community center and the Infectious Disease Research Collaboration (IDRC) at Tororo District Hospital. Our population of HIV-infected mothers had previously enrolled a child in an early childhood malaria-treatment program (Kamya et al., 2014). Study children came from the same mothers and were siblings of children in the malaria chemoprophylaxis treatment program. The study children included 31 infants ranged from 6 months to 12 months of age at enrollment (see Table 1), with 11 boys (M=0.69, SD=0.14) and 20 girls (M=0.79, SD=0.15). Written consent was obtained from all of the mothers after the consent form was explained in the mother’s local language.
Table 1.
Descriptive statistics (N, Mean, Standard Deviation, Minimum, Maximum score) are presented for all of the descriptive and test performance measures for the present study.
| Variable | N | Mean | Std Dev | Minimum | Maximum |
|---|---|---|---|---|---|
| Age at testing (yrs) | 30 | 0.76 | 0.16 | 0.51 | 1.01 |
| Birth Weight (kgs) | 25 | 3.22 | 0.64 | 2.00 | 4.50 |
| Tobii Eye Tracking Measures for the Modified Fagan Test of Infant Intelligence (seconds) | |||||
| Mean Time to First Fixation Full Screen | 30 | 1.22 | 1.72 | 0 | 7.10 |
| Mean Time to First Fixation Non-Familiar Face | 30 | 26.76 | 1.62 | 25.71 | 33.62 |
| Mean Time to First Fixation Familiar Face | 30 | 28.92 | 3.86 | 25.37 | 39.58 |
| Mean Gaze Fixation Duration Unfamiliar-Face Side | 30 | 55.67 | 11.64 | 35.40 | 77.94 |
| Mean Gaze Fixation Duration Familiar-Face Side | 30 | 38.55 | 10.77 | 22.42 | 59.86 |
| Mean Gaze Duration Unfamiliar-Face Side | 30 | 62.04 | 10.45 | 39.18 | 79.32 |
| Mean Gaze Duration Familiar-Face Side | 30 | 43.37 | 10.46 | 25.55 | 63.76 |
| Proportion of Gaze Duration to Unfamiliar Face | 30 | 0.59 | 0.06 | 0.49 | 0.71 |
| Gaze Duration Full Screen | 30 | 242.16 | 35.19 | 165.48 | 295.46 |
| Mullen Scales of Early Learning (MSEL) (Standardized for age by gender using USA norms) | |||||
| Composite Cognitive Score | 30 | 96.42 | 13.19 | 69 | 122 |
| Receptive Language scale | 30 | 44.03 | 9.65 | 25 | 65 |
| Expressive Language scale | 30 | 51.74 | 8.64 | 37 | 66 |
| Fine Motor scale | 30 | 48.48 | 11.72 | 28 | 68 |
| Gross Motor scale | 30 | 51.35 | 9.23 | 23 | 68 |
| Visual Reception scale | 30 | 48.35 | 10.93 | 20 | 68 |
Inclusion criteria.
Only non-infected HIV-exposed children from mothers participating in the IDRC malaria treatment program, ages 6 to 12 months, were included.
Exclusion criteria.
A child was excluded from the study if he or she had a medical history of serious birth complications, severe malnutrition, bacterial meningitis, encephalitis, cerebral malaria, or other known brain injury or disorder requiring hospitalization. A clinical medical officer using the Ten Question Questionnaire screened each eligible child for neurodisability (Durkin, Gottlieb, Maenner, Cappa, & Loaiza, 2008), excluding that child if any was verified.
Modified Fagan Test of Infant Intelligence (FTII).
The presentation sequence of faces from the original Fagan Test was preserved. However, instead of using the stock Caucasian photos, pictures of 11 local Ugandans were taken to preserve cultural appropriateness. The photos taken corresponded to the approximate age range (baby, child, adult), gender, facial expression, and orientation of the image, in accordance with the original photos (see Figure 2). Written and informed consent was received from all of these individuals, none of whom had any relation or known interaction with study participants in order to preserve novelty.
Figure 2 Caption.

The presentation sequence of faces from the original Fagan Test was preserved. However, instead of using the stock Caucasian photos, pictures of 11 local Ugandans were taken to preserve cultural sensitivity. The photos taken corresponded to the approximate age range (baby, child, adult), gender, and facial expression of the original photos.
As a result of initial pilot testing with several Ugandan infants who were 12 months of age, we changed the presentation of the Ugandan faces so that they were presented against a yellow background to make them more visually salient for the infants. A chime also sounded whenever the face presentations were changed. Finally, we changed the original human face presentation format so that the presentation of the human faces to the infant was done while a recorded Ugandan instrumental traditional folk music (slow paced/serene) played in the background. This seemed to calm the children so that they could attend through the entire sequence of face presentations. By modifying the FTII to enhance the child’s propensity to attend to the presentation screen, we were better able to use the Tobii eye tracking instrumentation to automate the measurement of gaze duration for the pictures of the faces that were presented on our presentation video. The total presentation time of the video presenting the human faces was approximately 6 minutes.
Tobii Studio-Enterprise software was used for programming and administration of the modified FTII. A series of faces are presented in a repeated pattern throughout a six-minute video. First, unknown Face 1 is presented on both sides of the screen for 25 seconds during a familiarization period. On the next screen, now-familiar Face 1 is presented alongside unfamiliar Face 2 for 15 seconds. On the subsequent screen, the same faces are presented for 15 seconds but right-left orientation is switched. One trial comprises this whole sequence and there are 10 trials throughout the test (see Figure 1, lower portion).
We measured the ratio of time the infant spent looking at the unfamiliar picture to the familiar picture and used this level of novelty (unfamiliar face) preference as a principal outcome measure for the study children. Total time gazing at the familiar face was also measured, as well as total time spent viewing the presentation video screen across all faces (familiar and unfamiliar). Response time gaze latency to the unfamiliar face was also measured, which could be as low as zero seconds if the child was already looking at that side of the monitor screen when it was presented.
Tobii Professional Studio Eye Tracking Programming.
We programmed a Tobii X2–30 portable infrared camera to monitor the child’s pupil direction during the presentation of pictures of human faces (Figure 1). Although the Tobii infrared camera was operated by the laptop on battery charge, an additional independent battery-operated universal power stabilizer in the testing room provided a stable source of AC 120 volt power in the event of power outages. This power source allowed us to operate the 22 inch computer monitor attached to the laptop computers, so that the pictures of human faces could be very visible to the child when presented through the laptop by the Tobii Professional Studio eye tracking software for data collection. A diesel fuel generator for our study clinic was also available for recharging the universal power stabilizer in the event of extended power outages, and to maintain adequate fluorescent bulb lighting for pupil detection by the Tobii infrared camera. After successful calibration of pupillary gaze direction at the start of each testing session using (using the Tobii Studio Professional calibration instrumentation verification program), presentation of pictures of human faces was started and total time spent looking at a given human face was automatically measured using the following program features. The software does not allow the video to play until the automatic calibration tool has sufficiently tracked the participants eye gaze across the entire screen. An area of interest (AOI) is a pre-determined area, or zone, of the screen that is of interest. In this study, for example, an AOI would be the area on the computer screen monitor for the image of a human face. AOIs are set in order to determine total gaze length on that area throughout the test.
A fixation is defined as the time that both eyes are statically locked (i.e. not perceptibly moving) on an area of the screen. Fixation duration measures are the sum of fixation time while looking within the AOI. This measure does not include the time it takes for the eyes to move between points A and B on the computer screen for the human face pictures that were presented.
Total time spent looking at the familiar and the unfamiliar faces in children trials was automatically measured using the following program features. AOIs are set in order to determine total gaze length on that area throughout the test. Fixation duration measures the sum of fixations, which measures the time that both eyes are statically locked (i.e. not perceptibly moving) on the faces within our AOIs. Since human face pictures were presented in pairs (an identical pair or else a familiar (previously presented) and unfamiliar (novel) pair), this measure does not include the time it takes for the eyes to move between points A (human face on one side of the computer screen) and B (human face on the other side) (see lower portion of Figure 1).
A first-visit duration was defined as the interval of time between the first fixation outside of the AOI (not looking at a newly presented human face picture) and the first gaze inside of the AOI (looking at the newly presented face picture). This measure could be as low as zero seconds if the child was already looking inside of the AOI of the monitor screen when the human face picture was presented. Total visit duration measures the duration of each individual visit within an AOI (looks at a given human face picture).
Mullen Scales of Early Learning.
The MSEL is intended for use in assessing children from birth to 68 months and provides assessment scores for the developmental domains of Gross Motor (up to 36 months), Visual Reception, Fine Motor, Receptive Language, and Expressive Language (Mullen, 1995). The Early Learning Composite provides a measure of g, the general measure of fluid intelligence thought to underlie cognitive ability in general. It is derived from the standardized T scores of the four scales, all of which have memory and learning items as part of the assessment (Visual Reception, Fine Motor, Receptive Language, & Expressive Language). In validation studies, the Early Learning Composite has a correlation coefficient of 0.70 with the Bayley Mental Development Index measure (Bradley-Johnson, 2001).
Statistical analysis:
The distributions of and correlations among the principal measures of the developmental test (MSEL, Modified Fagan Test Tobii eye tracking indicators: fixation duration and total visit duration) were evaluated. The associations between the Fagan Test eye tracking indicators and the MSEL composite cognitive development score was also depicted as a scatterplots. Analyses were completed using SAS 9.4
RESULTS
Two of the 30 children tested had to be rescheduled for a 2nd evaluation because valid eye tracking data was not obtained in the initial Fagan test assessment. The remaining children were successfully evaluated on the initial assessment. The left-most column in Table 2 presents the correlation between age and all Tobii eye tracking measures. The Mullen scale scores are already adjusted for age using American-based norms, since no normative data are available for Uganda for this measure. Since age is not significantly correlated with any of the Tobii eye tracking measures (Table 2), the significant correlations in this Table are not compromised when adjusting for age. This was verified by comparing age-adjusted partial to the unadjusted correlations for the significant correlation coefficients in Table 2.
Table 2.
The Pearson product-moment correlation coefficient between the Tobii eye tracking measures for the modified Fagan test of infant development, and the Mullen Scales of Early Learning (MSEL) standardized composite cognitive and individual scale scores (N=31). The upper value in each cell is the correlation coefficient, and just below it is the corresponding probability value for that coefficient. Statistically significant coefficients are denoted with an asterisk.
| Tobii Eye Tracking Measures for the Modified Fagan Test of Infant Intelligence | Testing Age | Birth Weight | MSEL Composite Cognitive Score |
MSEL Receptive Language Scale |
MSEL Expressive Language Scale |
MSEL Fine Motor Scale |
MSEL Gross Motor Scale |
MSEL Visual Reception Scale |
|---|---|---|---|---|---|---|---|---|
| Mean Time to First Fixation Full Screen | −0.19 | −0.31 | −0.18 | −0.09 | −0.16 | −0.16 | −0.43* | −0.05 |
| 0.312 | 0.155 | 0.341 | 0.652 | 0.411 | 0.426 | 0.021 | 0.788 | |
| Mean Time to First Fixation Non-Familiar Face | −0.09 | −0.20 | 0.15 | 0.20 | 0.03 | 0.06 | −0.03 | 0.12 |
| 0.652 | 0.357 | 0.439 | 0.300 | 0.888 | 0.770 | 0.874 | 0.520 | |
| Mean Time to First Fixation Familiar Face | 0.02 | 0.18 | −0.08 | −0.03 | −0.20 | −0.09 | 0.25 | 0.12 |
| 0.912 | 0.424 | 0.687 | 0.882 | 0.292 | 0.648 | 0.184 | 0.541 | |
| Mean Gaze Fixation Duration Unfamiliar-Face Side | 0.10 | 0.12 | 0.15 | 0.05 | 0.02 | 0.26 | −0.22 | 0.03 |
| 0.621 | 0.570 | 0.426 | 0.797 | 0.900 | 0.175 | 0.254 | 0.875 | |
| Mean Gaze Fixation Duration Familiar-Face Side | −0.13 | 0.02 | 0.32 | 0.16 | 0.22 | 0.32 | −0.11 | 0.12 |
| 0.514 | 0.928 | 0.095 | 0.402 | 0.240 | 0.096 | 0.558 | 0.536 | |
| Mean Gaze Duration Unfamiliar-Face Side | 0.09 | −0.01 | 0.28 | 0.12 | −0.03 | 0.40* | −0.12 | 0.18 |
| 0.659 | 0.966 | 0.146 | 0.540 | 0.871 | 0.032 | 0.542 | 0.345 | |
| Mean Gaze Duration Familiar-Face Side | −0.12 | −0.13 | 0.41 | 0.22 | 0.33 | 0.38 | −0.16 | 0.16 |
| 0.528 | 0.551 | 0.028 | 0.251 | 0.083 | 0.044 | 0.401 | 0.406 | |
| Proportion of Gaze Duration to Unfamiliar Face | −0.26 | −0.03 | 0.52** | 0.24 | 0.28 | 0.54** | 0.15 | 0.27 |
| 0.171 | 0.896 | 0.004 | 0.204 | 0.144 | 0.002 | 0.438 | 0.162 | |
P<0.05
P<0.01
Infants spent significantly more time gazing at the novel pictures than familiar pictures in over 10 trials (t(30)=9.17,P<0.001) (see Table 2). The MSEL composite cognitive ability score was correlated with overall proportion of time spent looking at the novel faces as compared to the familiar faces when they were presented together (r(30)=0.52,P=0.004). The scatterplot of this relationship is in Figure 3. This correlation remained significant when adjusted for the number of coefficients computed within the present study analyses. The MSEL Fine Motor tasks, which measures visuospatial working memory, was also significantly correlated with proportion of gaze length to novel faces (r(30)=0.54,P=0.002), overall gaze length to familiar faces (r(30)=0.38,P=0.04), and overall gaze length to novel faces (r(30)=0.40,P=0.03).
Figure 3 Caption.
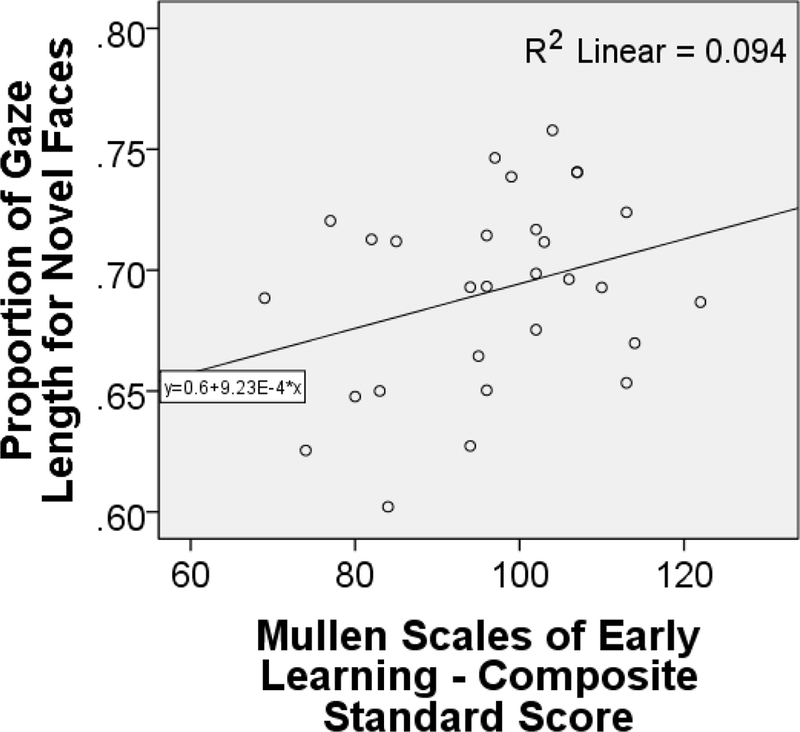
This is a scatterplot with a least-squares fit line depicting the relationship between the Fagan Test Tobii eye tracking measure of proportion of face viewing time viewing the novel face presentations, and the Mullen Scales of Early Learning (MSEL) standardized composite score for the cognitive scales (Visual Reception, Fine Motor, Receptive Language, and Expressive Language
DISCUSSION
The eye-tracking instrument used, Tobii X2–30 compact, was feasibly implemented in our research-setting as it connected to a laptop to measure the length of infant eye gaze to a modified version of the FTII, adapted to the Ugandan context. The software was programmed to evaluate an infant’s visual-spatial encoding working memory for familiar and novel human faces, as well as fixation duration and total visit duration on pre-defined AOI. As hypothesized, we obtained longer gaze length to unfamiliar faces in Ugandan HEU infants 6 to 12 months of age. Furthermore, Tobii measurements of gaze length with the modified FTII were associated with MSEL cognitive development indicators. These results suggest that eye tracking with a locally-modified FTII can be used with the MSEL to evaluate the neurodevelopmental effects of other risk factors and clinical interventions for children in resource-limited settings such as our present study site (e.g., HIV exposure and treatment, malaria exposure and treatment, malnutrition, quality of caregiving).
Two of our 30 study children did have to be re-tested due to a loss of Tobii infrared camera calibration during the Fagan test assessment, resulting in initially invalid eye tracking data. Both children were successfully retested on the 2nd attempt, but this illustrates one of the key issues that holds impact on the feasibility of eye tracking assessment with infants and very young children in such settings. Movement or a sudden change in lighting for example due to an undisciplined tester or a power outage, can readily invalidate the testing. Parameters like poor calibration accuracy, flicker, child movement, and data loss due to software mismanagement can occur in any clinical setting which attempts to complement neurodevelopmental assessment with eye tracking assessment. We were able to establish the feasibility of such assessments in a clinical setting at a small African hospital, but these are important considerations that can affect the quality of research data, or the clinical utility of such measures in resource-constrained settings. As such, the successful roll-out of this technology for the assessment of neurodevelopmental outcomes in the African context should be cautiously considered.
As did Boivin et al (2017) with Ugandan HIV-exposed toddlers and Forssman et al (2017) with Malawian infants, who both used Tobii eye tacking instrumentation and a cognitive visual tracking task, the present study has also established the feasibility of eye tracking measures, only this time with infant children in a medical clinic setting. We also established ways in which the Fagan test, modified for the Ugandan context, produces working memory outcomes that significantly correlated with other cognitive and neurodevelopmental assessments in HIV-affected children in an African context. In a recent review of a variety of pediatric neurodevelopmental and neuropsychological assessments used in low and middle income countries, the authors noted that many of the assessments adapted for use have not been well validated in these contexts (Semrud-Clikeman et al., 2016). Furthermore, in order to administer these assessments in a valid and reliable matter, significant training and monitoring of the assessment personnel is necessary. This is especially the case for test measures which depend on observer evaluation of a child’s behavior, even if preserved within a video record. Because of this, observer variability in evaluating video-based scoring of behavioral outcomes has proven to be a serious limitation for such measures (Dickerson, Gerhardstein, Zack, & Barr, 2013).
When enhanced with the latest tools in eye tracking measurement, our findings support the use of infant gaze length for novel human faces as a sensitive behavioral indicator of working memory in infancy and early childhood development in the African context. We have also provided evidence to support the sensitivity of this measure to risk factors compromising gestational and early childhood brain development in resource-constrained settings. This is because infant gaze length preference for novelty is related to attention and visual-spatial encoding and working memory processes that are foundational to neuropsychological functions as they mature and expand through middle and late childhood (Thompson et al., 1991).
Our principal study objective was to apply more recent technical advances in eye-tracking instrumentation and technology in order to make the FTII more reliable and sensitive to neurodevelopmental risk and disorders in very young children in resource-constrained settings. Our present findings support the proposal that advances in the sophistication, affordability, and portability of eye-tracking technology to make feasible an infant eye gaze test in low-resource settings.
Eye-tracking technology provides an accurate, portable solution to the limitations of rural sub-Saharan Africa. These trackers have very accurate temporal and spatial measurements, gathering information on the order of milliseconds and accurate to 0.4 degree. Another advantage of modern eye trackers, which use an infrared light source and corneal reflection to precisely measure the direction and duration of pupil movements, is an increased tolerance to infant head movement than past equipment. Eye-tracking equipment thus has potential for the assessment of a broad range of attention and cognitive processes in infants.
In the only previous attempt to use the FTII in the African context, no significant difference demonstrated between HIV infected and non-infected infants through 2 years of age (Drotar et al., 1997). The results of the study were surprising to the authors, as there is extensive predictive validity and cross-cultural data in support of the FTII (Drotar et al., 1997). Applying the Fagan Test manually takes a lot of training, motivation, and attention to be accurate. Experimenters are attempting to gauge rapid and brief adjustments of infantile gaze with a stopwatch, even in perfect study conditions. However, studies are even more susceptible to measurement error with an unpredictable environment that is under-resourced and inundated with patients, such as the rural clinical environments of sub-Saharan Africa. Although computers and digital technology have changed the landscape and given us increasingly accurate ways to measure neurodevelopment, the challenge of utilizing this technology in low-resource areas remains.
We found that eye-tracking technology allowed for a more objective and automated means of monitoring developmental progress. The application of portable technology that can be used in low resource settings to provide a more objective and automated measure of cognitive development is multifold. The use of this modified Fagan Test, using eye-tracking technology, is not limited to the scope of this study. This portable technology can be used to assess neurodevelopmental outcomes from any exposure, such as malaria or malnutrition (Knox et al., 2016).
Having validated the measure using non-infected exposed infants in this study based on correlation with MSEL, a comparison can now be made between infected, exposed, and non-exposed infants. If there is a strong correlation and difference between these three groups, this evidence would yield support to a causal link between HIV and cognitive development. After making these comparisons, the FTII can used to identify at risk infants early in their development. By identifying these infants, intervention can be targeted to children in whom it will yield a large positive trajectory.
Conclusion.
Adapting and improving the sensitivity of the Fagan test in a low-resource setting in rural Uganda by using eye-tracking technology has validated its use in this setting. This technology is not limited to the measurement of working-memory using faces. Rural and low-resource settings provide a challenge for researchers in implementing useful technology for a host of reasons, not limited to electricity, portability, and skill. This Tobii eye-tracker served as a portable device that did not require excessive training or skill to administer. Furthermore, it was powered through the laptop computer itself. Therefore, it did not require a large power source, and it can provide the basis for brain and behavior assessments of infants globally. In this case, the exposure investigated was HIV. Whether malaria, meningitis, malnutrition, or any other exposure is being investigated, this test and technology can be diversely and widely applied.
ACKNOWLEDGEMENTS
This study was supported by RO1 HD070723 (A.S., M.J.B.) and funding from the Michigan State University College of Human Medicine and by the University of Michigan School of Public Health. The study sponsors had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Dr. Itziar Familiar-Lopez provided scientific oversight for the study site and support for the student research internship program; Julius Caesar Ojuka (on-site study coordinator) led the field team responsible for the intervention training of the testers, caregiver and child assessments, and translation into the local languages, along with providing ongoing clinical care. George Okwakol, Claire Apayi, Moses Ejula, Sylvia Adongo, and Anthony Ekisa served as the research assistants for this study, which would not have been possible without their efforts. Their efforts are greatly appreciated.
Funding Sources: This study was supported by RO1 HD070723 (A.S., M.J.B.) and funding from the Michigan State University College of Human Medicine and by the University of Michigan School of Public Health.
Abbreviations:
- AOI
Area of Interest in Tobii eye tracking
- BSID
Bayley Scales of Infant Development
- FTII
Fagan Test of Infant Intelligence
- HAART
Highly Active Anti-Retroviral Therapy
- HEU
perinatally HIV-exposed uninfected children
- HIV
Human Immunodeficiency Virus
- HOME
Caldwell Home Observation for Measurement of the Environment
- HIV
Human Immunodeficiency Virus
- IRB
Institutional Review Board
- MSEL
Mullen Scales of Early Learning
- SAS
Statistics Analysis Software
- UNCST
Ugandan National Council for Science and Technology
- WAZ
Weight-for-Age adjusted Z score
- WHO
World Health Organization
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest to disclose.
REFERENCES
- Andersson H (1996). The Fagan Test of Infant Intelligence: Predictive Validity in a Random Sample. Psychological Rep, 78, 1015–1026. [DOI] [PubMed] [Google Scholar]
- Boivin MJ, Bangirana P, Nakasuja N, Page CF, Shohet C, Givon D, … Klein PS. (2013). A year-long caregiver training program to improve neurocognition in preschool Ugandan HIV-exposed children. J Dev Behav Pediatr, 34(2), 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, Bangirana P, Nakasujja N, Page CF, Shohet C, Givon D, … Klein PS (2013). A year-long caregiver training program improves cognition in preschool Ugandan children with human immunodeficiency virus. J Pediatr, 163, 1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, Weiss J, Chhaya R, Seffren V, Awadu J, Sikorskii A, & Giordani B (2017). The feasibility of automated eye tracking with the Early Childhood Vigilance Test of Attention in younger HIV-exposed Ugandan children. Neuropsychology, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley-Johnson S (2001). Cognitive assessment for the youngest children: A critical review of tests. Journal of Psychoeducational Assessment, 19(1), 19–44. [Google Scholar]
- Caruana N, Brock J, & Woolgar A (2015). A frontotemporoparietal network common to initiating and responding to joint attention bids. Neuroimage, 108, 34–46. doi: 10.1016/j.neuroimage.2014.12.041 [DOI] [PubMed] [Google Scholar]
- Chiriboga CA, Kuhn L, & Wasserman GA (2007). Prenatal cocaine exposures and dose-related cocaine effects on infant tone and behavior. Neurotoxicol Teratol, 29(3), 323–330. doi: 10.1016/j.ntt.2006.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PW, Myer GJ, Shamlaye C, Cox C, Gao P, Axtell C, … Clarkson TW (1999). Association between prenatal exposure to methylmercury and developmental outcomes in Seychellois children: effect modification by social and environmental factors. Neurotoxicology, 20(5), 833–841. [PubMed] [Google Scholar]
- Dickerson K, Gerhardstein P, Zack E, & Barr R (2013). Age-related changes in learning across early childhood: a new imitation task. Dev Psychobiol, 55(7), 719–732. doi: 10.1002/dev.21068 [DOI] [PubMed] [Google Scholar]
- Drotar D, Olness K, Wiznitzer M, Guay L, Marum L, Svilar G, … Kiziri-Mayengo R (1997). Neurodevelopmental outcomes of Ugandan infants with human immunodeficiency virus type 1 infection. Pediatrics, 100(1), E5. [DOI] [PubMed] [Google Scholar]
- Drotar D, Olness K, Wiznitzer M, Schatschneider C, Marum L, Guay L, … Mayengo RK. (1999). Neurodevelopmental outcomes of Ugandan infants with HIV infection: an application of growth curve analysis. Health Psychol, 18(2), 114–121. [DOI] [PubMed] [Google Scholar]
- Durkin MS, Gottlieb CA, Maenner MJ, Cappa C, & Loaiza E (2008). Monitoring child disability in developing countries: results from the Multiple Indicator Cluster Surveys. Retrieved from New York: [Google Scholar]
- Ellis EM, Borovsky A, Elman JL, & Evans JL (2015). Novel word learning: An eye-tracking study. Are 18-month-old late talkers really different from their typical peers? J Commun Disord, 58, 143–157. doi: 10.1016/j.jcomdis.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emory E, Ansari Z, Pattillo R, Archibold E, & Chevalier J (2003). Maternal blood lead effects on infant intelligence at age 7 months. Am J Obstet Gynecol, 188(4), S26–32. [DOI] [PubMed] [Google Scholar]
- Fagan JF (2000). Theory of Intelligence as Processing Implications for Society, Psychology, Public Policy and Law, 6(168–179). [Google Scholar]
- Fagan JF, & Detterman DK (1992). The fagan Test of Infant Intelligence: A Technical Summary. Journal of Applied Developmental Psychology, 13, 173–193. [Google Scholar]
- Fagan JF, & Haiken-Vasen J (1997). Selective Attention to Novelty as a Measure of Information Processing Across the Lifespan. Attention, Development, and Psychopathology, 55–73. [Google Scholar]
- Forssman L, Ashorn P, Ashorn U, Maleta K, Matchado A, Kortekangas E, & Leppanen JM (2017). Eye-tracking-based assessment of cognitive function in low-resource settings. Arch Dis Child, 102(4), 301–302. doi: 10.1136/archdischild-2016-310525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzetta A, Mazzotti S, Tinelli F, Bancale A, Ferretti G, Battini R, … Cioni G. (2006). Early assessment of visual information processing and neurological outcome in preterm infants. Neuropediatrics, 37(5), 278–285. doi: 10.1055/s-2006-955929 [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, & Jacobson JL (2002). Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics, 109(5), 815–825. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Perera F, Jankowski J, Rauh V, Flak E, Caldwell KL, … Lisowska-Miszczyk I (2008). Prenatal low-level lead exposure and developmental delay of infants at age 6 months (Krakow inner city study). Int J Hyg Environ Health, 211(3–4), 345–351. doi: 10.1016/j.ijheh.2007.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge MP, Chang L, & Lammi-Keefe CJ (2015). Evidence of developmental continuity from birth to 1 year: sleep, temperament, problem solving, and recognition memory. Adv Neonatal Care, 15(2), 125–133. doi: 10.1097/ANC.0000000000000143 [DOI] [PubMed] [Google Scholar]
- Kamya MR, Kapisi J, Bigira V, Clark TD, Kinara S, Mwangwa F, … Dorsey G (2014). Efficacy and safety of three regimens for the prevention of malaria in young HIV-exposed Ugandan children: a randomized controlled trial. Aids, 28(18), 2701–2709. doi: 10.1097/QAD.0000000000000497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox PC, MacCormick IJ, Mbale E, Malewa M, Czanner G, & Harding SP (2016). Longitudinal Visuomotor Development in a Malaria Endemic Area: Cerebral Malaria and Beyond. PLoS ONE, 11(10), e0164885. doi: 10.1371/journal.pone.0164885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen Scales of Early Learning:AGS Edition. Minneapolis, MN: American Guidance Services. [Google Scholar]
- Myers GJ, Marsh DO, Davidson PW, Cox C, Shamlaye CF, Tanner M, … Clarkson TW (1995). Main neurodevelopmental study of Seychellois children following in utero exposure to methylmercury from a maternal fish diet: outcome at six months. Neurotoxicology, 16(4), 653–664. [PubMed] [Google Scholar]
- Nelson KG, Goldenberg RL, Hoffman HJ, & Cliver SP (1997). Growth and development during the first year in a cohort of low income term-born American children. Acta Obstet Gynecol Scand Suppl, 165, 87–92. [PubMed] [Google Scholar]
- O’Connor DL, Hall R, Adamkin D, Auestad N, Castillo M, Connor WE, … Ross Preterm Lipid S (2001). Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics, 108(2), 359–371. [DOI] [PubMed] [Google Scholar]
- Olness K (2003). Effects on brain development leading to cognitive impairment: a worldwide epidemic. J Dev Behav Pediatr, 24(2), 120–130. [DOI] [PubMed] [Google Scholar]
- Plomin R (2006). Nature and Nurture During Infancy and Early Childhood: Oxford University Press. [Google Scholar]
- Sandgren O, Andersson R, van de Weijer J, Hansson K, & Sahlen B (2013). Impact of cognitive and linguistic ability on gaze behavior in children with hearing impairment. Front Psychol, 4, 856. doi: 10.3389/fpsyg.2013.00856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman SC, & Giovannetti T (2015). The Potential Utility of Eye Movements in the Detection and Characterization of Everyday Functional Difficulties in Mild Cognitive Impairment. Neuropsychol Rev, 25(2), 199–215. doi: 10.1007/s11065-015-9283-z [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Romero RA, Prado EL, Shapiro EG, Bangirana P, & John CC (2016). Selecting measures for the neurodevelopmental assessment of children in low- and middle-income countries. Child Neuropsychol, 1–42. doi: 10.1080/09297049.2016.1216536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LA, Fagan JF, & Fulker DW (1991). Longitudinal prediction of specific cognitive abilities from infant novelty preference. Child Dev, 62(3), 530–538. [PubMed] [Google Scholar]
- von Tetzchner S, Jacobsen KH, Smith L, Skjeldal OH, Heiberg A, & Fagan JF (1996). Vision, cognition and developmental characteristics of girls and women with Rett syndrome. Dev Med Child Neurol, 38(3), 212–225. [DOI] [PubMed] [Google Scholar]


